Study of the Optical and Acoustic Parameters and Surface Tensions of 3,4,4′-Trichlorodiphenylurea in Binary Mixtures with Different Organic Solvents between (293.15 and 323.15) K
Abstract
1. Introduction
2. Results
2.1. Tables and Figures
2.2. Formatting of Mathematical Components
- Sn—solvation number of binary mixture;
- n2—the number of moles of solvent in the sample;
- n1—the number of moles of solute in the sample;
- κ—isentropic compressibility of binary mixture;
- κ0—isentropic compressibility of pure solvent.
- ρ—the density (kg·m−3) in the mixture;
- u—the speed of sound (m·s−1) in the mixture.
- u—the speed of sound in the experimental solution;
- uct—a constant with a value of 1600 m∙s−1 [99].
- K′—a temperature dependent constant, with the name Jacobson’s constant [101]:
- K′ = (93.875 + 0.375 T) 10−8
- T—absolute temperature;
- κS—the isentropic compressibility in binary mixtures.
- ρ—density;
- u—speed of sound;
- T—absolute temperature;
- s—solubility from prepared samples, measured in [mol L−1];
- tm—the TCC melting point; tm = 250 °C;
- MTCC—the TCC molecular mass; MTCC = 313.58 [g mol−1].
3. Discussion
4. Materials and Methods
4.1. Materials
4.2. Apparatus and Measurement Procedure
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Yun, H.; Liang, B.; Kong, D.; Li, X.; Wang, A. Fate, risk and removal of triclocarban: A critical review. J. Hazard. Mater. 2020, 387, 121944. [Google Scholar] [CrossRef] [PubMed]
- Gaviria-Castillo, A.C.; Artunduaga-Tole, J.D.; Rodríguez-Rubiano, J.D.; Zuñiga-Andrade, J.A.; Delgado, D.R.; Jouyban, A.; Martínez, F. Solution thermodynamics and preferential solvation of triclocarban in {1,4-dioxane (1) + water (2)} mixtures at 298.15 K. Phys. Chem. Liq. 2019, 57, 55–56. [Google Scholar] [CrossRef]
- Holguín, A.R.; Delgado, D.R.; Martínez, F. Thermodynamic study of the solubility of triclocarban in ethanol + propylene glycol mixtures. Química Nova 2012, 35, 280–285. [Google Scholar] [CrossRef]
- Delgado, D.R.; Holguin, A.R.; Martínez, F. Solution thermodynamics of triclosan and triclocarban in some volatile organic solvents. Vitae 2012, 19, 79–92. [Google Scholar] [CrossRef]
- Breneman, D.L.; Hanifin, J.M.; Berge, C.A.; Kewick, B.H.; Neumann, P.B. The effect of antibacterial soap with 1.5% triclocarban on Staphylococcus aureus in patients with atopic dermatitis. Cutis 2000, 66, 296–300. [Google Scholar]
- Luby, S.; Agboatwalla, M.; Feikin, D.; Painter, J.; Billhimer, W.; Altaf, A.; Hoekstra, R.M. Effect of handwashing on child health: A randomised controlled trial. Lancet 2005, 366, 225–233. [Google Scholar] [CrossRef]
- Vimalkumar, K.; Seethappan, S.; Pugazhendhi, A. Fate of Triclocarban (TCC) in aquatic and terrestrial systems and human exposure. Chemosphere 2019, 230, 201–209. [Google Scholar] [CrossRef]
- Delgado, D.R.; Sosnik, A.; Martínez, F. Transfer thermodynamics of triclosan from water to organic solvents with different hydrogen bonding capability. Lat. Am. J. Pharm 2011, 30, 459–466. [Google Scholar]
- Dar, O.I.; Aslam, R.; Pan, D.; Sharma, S.; Andotra, M.; Kaur, A.; Jia, A.-Q.; Faggio, C. Source, bioaccumulation, degradability and toxicity of triclosan in aquatic environments: A review. Environ. Technol. Innov. 2022, 25, 10212. [Google Scholar] [CrossRef]
- Agredo-Collazos, J.J.; Ortiz, C.P.; Cerquera, N.E.; Cardenas-Torres, R.E.; Delgado, D.R.; Peña, M.Á.; Martínez, F. Equilibrium solubility of triclocarban in (cyclohexane + 1,4-dioxane) mixtures: Determination, correlation, thermodynamics and preferential solvation. J. Solut. Chem. 2022, 51, 1603–1625. [Google Scholar] [CrossRef]
- Cruz-González, A.M.; Vargas-Santana, M.S.; Polania-Orozco, S.d.J.; Ortiz, C.P.; Cerquera, N.E.; Martínez, F.; Delgado, D.R.; Jouyban, A.; Acree, W.E., Jr. Thermodynamic analysis of the solubility of triclocarban in ethylene glycol + water mixtures. J. Mol. Liq. 2021, 325, 115222. [Google Scholar] [CrossRef]
- Munoz-Ortiz, C.A.; Cerquera, N.E.; Camacho, J.K.C.; Osorio-Gallego, J.; Cárdenas-Torres, R.E.; Herrera, M.; Delgado, D.R. Preferential solvation of triclocarban in N-methyl-2-pyrrolidone + water cosolvent mixtures according to the Inverse Kirkwood-Buff Integrals (IKBI) method and correlation of solubility by means of some mathematical models. Rev. Colomb. Cienc. Químico-Farm. 2024, 53, 219–243. [Google Scholar]
- Delgado, D.R.; Mogollon-Waltero, E.M.; Ortiz, C.P.; Peña, M.; Almanza, O.A.; Martínez, F.; Jouyban, A. Enthalpy-entropy compensation analysis of the triclocarban dissolution process in some {1,4-dioxane (1) + water (2)} mixtures. J. Mol. Liq. 2018, 271, 522–529. [Google Scholar] [CrossRef]
- Montoya Bautista, C.V.; Mohamed, B.A.; Li, L.Y. Sludge-based activated carbon from two municipal sewage sludge precursors for improved secondary wastewater-treatment discharge-effluent. J. Environ. Chem. Eng. 2022, 10, 108704. [Google Scholar] [CrossRef]
- Aolin, H.; Quin, L.; Zhu, S.; Hu, X.; Yin, D. Combined effects of pH and dissolved organic matter on the availability of pharmaceuticals and personal care products in aqueous environment. Sci. Total Environ. 2024, 929, 172637. [Google Scholar] [CrossRef]
- Lu, S.; Wang, N.; Ma, S.; Hu, X.; Kang, L.; Yu, Y. Parabens and triclosan in shellfish from Shenzhen coastal waters: Bioindication of pollution and human health risks. Environ. Pollut. 2019, 246, 257–263. [Google Scholar] [CrossRef]
- Crini, E.; Lichtfouse, G.; Wilson, L.D.; Morin Crini, N. Conventional and non conventional adsorbents for wastewater treatment. Environ. Chem. Lett. 2019, 17, 195–213. [Google Scholar] [CrossRef]
- Snyder, E.H.; O’Connor, G.A.; McAvoy, D.C. Measured physicochemical characteristics and biosolids-borne concentrations of the antimicrobial Triclocarban (TCC). Sci. Total Environ. 2010, 408, 2667–2673. [Google Scholar] [CrossRef]
- Volesky, B. Biosorption of Metals; CRC Press: Boca Raton, FL, USA, 1990. [Google Scholar]
- Harvey, P.J.; Campanella, B.F.; Castro, P.M.; Harms, H.; Lichtfouse, E.; Schäfner, A.R.; Smrcek, S.; Werck-Reichhart, D. Phytoreme-diation of polyaromatic hydrocarbons, anilines and phenols. Environ. Sci. Pollut. Res. Int. 2002, 9, 29–47. [Google Scholar] [CrossRef]
- Crini, G.; Badot, P.M. Traitement Et Épuration Des Eaux Industrielles Polluées; PUFC: Besançon, France, 2007. [Google Scholar]
- Cox, M.; Négré, P.; Yurramendi, L. Industrial Liquid Efuents; INASMET Tecnalia: San Sebastian, Spain, 2007. [Google Scholar]
- Sharma, S.K. Green Chemistry for Dyes Removal from Wastewater; Scrivener Publishing LLC Wiley: Beverley, UK, 2015. [Google Scholar]
- Morin-Crini, N.; Crini, G. Eaux Industrielles Contaminées; PUFC: Besançon, France, 2017. [Google Scholar]
- Miller, T.R.; Colquhoun, D.R.; Halden, R.U. Identification of wastewater bacteria involved in the degradation of triclocarban and its non-chlorinated congener. J. Hazard. Mater. 2010, 183, 766–772. [Google Scholar] [CrossRef]
- Aragón, D.M.; Sosnik, A.; Martínez, F. Solution thermodynamics of triclocarban in organic solvents of different hydrogen bonding capability. J. Sol. Chem. 2009, 38, 1493–1503. [Google Scholar] [CrossRef]
- Lim, J.; Jang, S.; Kim, H.; Cho, H.K.; Shin, M.S. Solubility of triclocarban in pure alkanols at different temperatures. Korean J. Chem. Eng. 2013, 30, 181–186. [Google Scholar] [CrossRef]
- Weise, K.; Beil, S.; Schwanebeck, K.; Ion, A.C.; Berendonk, T.U.; Jungmann, D. An informative short-term study on the impacts of a triclocarban/weathered multi-walled carbon nanotube-adsorbed complex to benthic organisms. Environ. Sci. Pollut. Res. 2024, 31, 19917–19926. [Google Scholar] [CrossRef] [PubMed]
- Sou, T.; Bergström, C.A.S. Automated assays for thermodynamic (equilibrium) solubility determination. Drug Discov. Today Technol. 2018, 27, 11–19. [Google Scholar] [CrossRef]
- Sirbu, F.; Dragoescu, D.; Shchamialiou, A.A.; Khasanshin, T. Densities, speeds of sound, refractive indices, viscosities and their related thermodynamic properties for n-hexadecane + two aromatic hydrocarbons binary mixtures at temperatures from 298.15 K to 318.15 K. J. Chem. Thermodyn. 2019, 128, 383–393. [Google Scholar] [CrossRef]
- Sirbu, F.; Gheorghe, I. Study on thermophysical properties in the ternary mixture of N-methylglycine solute with (D-glucose+water) binary solvent at temperatures of 298.15, 308.15, and 318.15 K. J. Mol. Liq. 2018, 253, 149–159. [Google Scholar] [CrossRef]
- Ion, I.; Bogdan, D.; Mincu, M.M.; Ion, A.C. Modified Exfoliated Carbon Nanoplatelets as Sorbents for Ammonium from Natural Mineral Waters. Molecules 2021, 26, 3541. [Google Scholar] [CrossRef]
- Ivan, G.R.; Ion, I.; Capra, L.; Oprea, O.; Ion, A.C. The influence of the chemical composition of natural waters about the triclocarban sorption on pristine and irradiated MWCNTs. Separations 2023, 10, 46. [Google Scholar] [CrossRef]
- Ion, I.; Sirbu, F.; Ion, A.C. Thermophysical investigations of exfoliated graphite nanoplatelets and active carbon in binary aqueous environments at different temperatures. J. Mater. Sci. 2015, 50, 587–598. [Google Scholar] [CrossRef]
- Ratina, K.; Umadevi, M.; Senthamil, S.C.; Ramalatha, M. Research on Ion-Solvent Interactions in the Inorganic Liquid Mixtures by Ultrasonic Technique. Int. J. Eng. Adv. Technol. 2019, 8, 151–159. [Google Scholar] [CrossRef]
- Rathina, K.; Umadevi, M.; Senthamil selvi, C.; Marimuthu, R.; Mrad, S.; Lafuente, C.; Hichri, M.; Khattech, I. Density, Speed of Sound, Refractive Index, and Viscosity of the Binary Mixtures of N,N dimethylacetamide with Methanol and Ethanol. J. Chem. Eng. Data 2016, 61, 2946–2953. [Google Scholar]
- Gonzalez, E.J.; Alonso, L.; Domínguez, A. Physical Properties of Binary Mixtures of the Ionic Liquid 1-Methyl-3-octylimidazolium Chloride with Methanol, Ethanol, and 1-Propanol at T = (298.15, 313.15, and 328.15) K and at p = 0.1 MPa. J. Chem. Eng. Data 2006, 51, 1446–1452. [Google Scholar] [CrossRef]
- Rodríguez, A.; Canosa, J.; Tojo, J. Physical Properties of Binary Mixtures (Dimethyl Carbonate + Alcohols) at Several Temperatures. J. Chem. Eng. Data 2001, 46, 1476–1486. [Google Scholar] [CrossRef]
- Goncalves, F.A.M.M.; Trindade, A.R.; Costa, C.S.M.F.; Bernardo, J.C.S.; Johnson, I.; Fonseca, I.M.A.; Ferreira, A.G.M. Viscosity, and Surface Tension of Ethanol: New Measurements and Literature Data Evaluation. J. Chem. Thermodyn. 2010, 42, 1039–1049. [Google Scholar] [CrossRef]
- Salinas, R.; Pla-Franco, J.; Lladosa, E.; Monton, J.B. Density, Speed of Sound, Viscosity, and Excess Properties of Binary Mixtures Formed by Ethanol and Bis(trifluorosulfonyl)imide-Based Ionic Liquids. J. Chem. Eng. Data 2015, 60, 525–540. [Google Scholar] [CrossRef]
- Ortega, J. Densities and Refractive Indices of Pure Alcohols as a Function of Temperature. J. Chem. Eng. Data 1982, 27, 312–317. [Google Scholar] [CrossRef]
- Khirade, P.W.; Chaudhari, A.; Shinde, J.B.; Helambe, S.N.; Methrotra, S.C. Static Dielectric Constant and Relaxation Time Measurements on Binary Mixtures of Dimethyl Sulfoxide with Ethanol, 2-Ethoxyethanol, and Propan-1-ol at 293, 303, 313, and 323 K. J. Chem. Eng. Data 1999, 44, 879–881. [Google Scholar] [CrossRef]
- Benkelfat-Seladji, N.L.; Ouaar, F.; Hernandez, A.; Bahadur, I.; Munoz-Rujas, N.; Singh, S.K.; Montero, E.; Chiali-Baba Ahmed, N.; Negadi, L. Density, speed of sound, refractive index of binary mixtures containing 2-ethoxyethanol and some alcohols: Measurement and correlation. J. Chem. Thermodyn. 2022, 170, 106762. [Google Scholar] [CrossRef]
- Aralaguppi, M.; Jadar, C.V.; Aminabhavi, T.M. Density, refractive index, viscosity, and speed of sound in binary mixtures of 2-ethoxyethanol with dioxane, acetonitrile, and tetrahydrofuran at (298.15, 303.15, and 308.15) K. J. Chem. Eng. Data 1996, 41, 1307–1310. [Google Scholar] [CrossRef]
- Belhadj, D.; Bahadur, I.; Negadi, A.; Munoz-Rujas, N.; Montero, E.; Negadi, L. Thermodynamic, Ultrasonic, and Transport Study of Binary Mixtures Containing 2-(2-Methoxyethoxy)ethanol and Alcohols at (293.15–323.15) K. J. Chem. Eng. Data 2020, 65, 5192–5209. [Google Scholar] [CrossRef]
- Das, M.; Roy, M.N. Volumetric, viscosimetric and acoustic studies of binary mixtures of 2-ethoxyethanol with 1-alkanols at 298.15 K. Phys. Chem. Liq. 2006, 44, 663–685. [Google Scholar] [CrossRef]
- Makhlouf, H.; Muñoz-Rujas, N.; Aguilar, F.; Belhachemi, B.; Montero, E.A.; Bahadur, I.; Negadi, L. Density, speed of sound and re-fractive index of mixtures containing 2-phenoxyethanol with propanol or butanol at various temperatures. J. Chem. Thermodyn. 2019, 25, 394–405. [Google Scholar] [CrossRef]
- Rodríguez, A.; Canosa, J.; Tojo, J. Density, refractive index, and speed of sound of binary mixtures (diethyl carbonate + alcohols) at several temperatures. J. Chem. Eng. Data 2001, 46, 1506–1515. [Google Scholar] [CrossRef]
- Pang, F.-M.; Seng, C.-E.; Teng, T.-T.; Ibrahim, M.H. Densities and viscosities of aqueous solutions of 1-propanol and 2-propanol at temperatures from 293.15 K to 333.15 K. J. Mol. Liq. 2007, 136, 71–78. [Google Scholar] [CrossRef]
- Paramo, R.; Alonso, V.; Gonzalez, J.A.; de la Fuente, I.G.; Casanova, C.; Carlos, J. Thermodynamics of mixtures containing amines. XIV. CpmE of benzylamine with heptane at 293.15K or with methanol, 1-propanol or 1-pentanol at 293.15–308.15 K. Thermochim. Acta 2014, 586, 75–79. [Google Scholar] [CrossRef]
- Aminabhavi, T.M.; Gopalakrishna, B. Density, Viscosity, Refractive Index, and Speed of Sound in Binary Mixtures of 2-Ethoxyethanol with n-Alkanes (C6 to C12), 2,2,4-Trimethylpentane, and Cyclohexane in the Temperature Interval 298.15–313.15 K. J. Chem. Eng. Data 1995, 40, 632–641. [Google Scholar] [CrossRef]
- Resa, J.M.; González, C.; Goenaga, J.M. Density, refractive index, speed of sound at 298.15 K, and vapor-liquid equilibria at 101.3 kPa for binary mixtures of propanol + 2-methyl-1-butanol and propanol + 3-methyl-l-butanol. J. Chem. Eng. Data 2006, 51, 73–78. [Google Scholar] [CrossRef]
- Benkelfat-Seladji, N.L.; Ouaar, F.; Hernandez, A.; Munoz-Rujas, N.; Bahadur, I.; Chiali-Baba Ahmed, N.; Montero, E.; Negadi, L. Intermolecular Interactions of Binary Mixtures Comprising 2-Benzylaminoethanol with Alcohols (C1–C3) at Different Temperatures: Experiments and Modelling. J. Chem. Eng. Data 2021, 66, 3397–3416. [Google Scholar] [CrossRef]
- Mrad, S.; Hichri, M.; Khattech, I.; Lafuente, C. Thermophysical study of the binary mixtures of N,N-dimethylacetamide with 1-propanol and 1-butanol. J. Mol. Liq. 2017, 231, 168–173. [Google Scholar] [CrossRef]
- Aralaguppi, M.I.; Baragi, J.G. Physico-chemical and excess properties of the binary mixtures of methylcyclohexane + ethanol, + pro-pan-1-ol, + propan-2-ol, + butan-1-ol, + 2-methyl-1-propanol, or 3-methyl-1-butanol at T = (298.15, 303.15, and 308.15) K. J. Chem. Thermodyn. 2006, 38, 434–442. [Google Scholar] [CrossRef]
- Mokhtarani, B.; Sharifi, A.; Mortaheb, H.R.; Mirzaei, M.; Mafi, M.; Sadeghian, F. Density and viscosity of 1-butyl-3-methylimidazolium nitrate with ethanol, 1-propanol, or 1-butanol at several temperatures. J. Chem. Thermodyn. 2009, 41, 1432–1438. [Google Scholar] [CrossRef]
- Ritzoulis, G.; Fidantsi, A. Relative Permittivities, Refractive Indices, and Densities for the Binary Mixtures N,N′-Dimethylacetamide with Methanol, Ethanol, 1-Butanol, and 2-Propanol at 298.15 K. J. Chem. Eng. Data 2000, 45, 207–209. [Google Scholar] [CrossRef]
- Aminabhavi, T.M.; Banerjee, K. Density, Viscosity, Refractive Index, and Speed of Sound in Binary Mixtures of 2-Chloroethanol with Alkanols (C1–C6) at 298.15, 303.15, and 308.15 K. J. Chem. Eng. Data 1998, 43, 509–513. [Google Scholar] [CrossRef]
- Giner, B.; Artigas, H.; Carrion, A.; Lafuente, C.; Royo, F. Excess thermodynamic properties of isomeric butanols with 2-methyl-tetrahydrofuran. J. Mol. Liq. 2003, 108, 303–311. [Google Scholar] [CrossRef]
- Bahadur, I.; Deenadayalu, N.; Tywabi, Z.; Sen, S.; Hofman, T. Volumetric properties of ternary (IL + 2-propanol or 1-butanol or 2-butanol + ethyl acetate) systems and binary (IL + 2-propanol or 1-butanol or 2-butanol) and (1-butanol or 2-butanol + ethyl acetate) systems. J. Chem. Thermodyn. 2012, 49, 24–38. [Google Scholar] [CrossRef]
- Troncoso, J.; Carballo, E.; Cerdeiriña, C.A.; González, D.; Romaní, L. Systematic Determination of Densities and Speeds of Sound of Nitroethane+ isomers of Butanol in the Range (283.15–308.15) K. J. Chem. Eng. Data 2000, 45, 594–599. [Google Scholar] [CrossRef]
- Jiménez, E.; Cabanas, M.; Segade, L.; García-Garabal, S.; Casas, H. Excess volume, changes of refractive index and surface tension of binary 1,2-ethanediol + 1-propanol or 1-butanol mixtures at several several temperatures. Fluid Phase Equilibria 2001, 180, 151–164. [Google Scholar] [CrossRef]
- Dubey, G.P.; Sharma, M. Temperature and Composition Dependence of the Densities, Viscosities, and Speeds of Sound of Binary Liquid Mixtures of 1-Butanol with Hexadecane and Squalane. J. Chem. Eng. Data 2008, 53, 1032–1038. [Google Scholar] [CrossRef]
- Varfolomeev, M.A.; Zaitseva, K.V.; Rakipov, I.T.; Solomonov, B.N.; Marczak, W. Speed of sound, density, and related thermodynamic excess properties of binary mixtures of butan-2-one with C1–C4 n-alkanols and chloroform. J. Chem. Eng. Data 2014, 59, 4118–4132. [Google Scholar] [CrossRef]
- Wandschneider, A.; Lehmann, J.K.; Heintz, A. Surface Tension and Density of Pure Ionic Liquids and Some Binary Mixtures with 1-Propanol and 1-Butanol. J. Chem. Eng. Data 2008, 53, 596–599. [Google Scholar] [CrossRef]
- Al-Kandary, J.A.; Al-Jimaz, A.S.; Abdul-Latif, A.M. Densities, Viscosities, Speeds of Sound and Refractive Indices of Binary Mixtures of Tetrahydrofuran with 1-Hexanol, 1-Heptanol, 1-Octanol, 1-Nonanol and 1-Decanol at 298.15, 303.15, 308.15 and 313.15 K. Phys. Chem. Liq. 2009, 47, 210–224. [Google Scholar] [CrossRef]
- Sirbu, F.; Ion, A.C.; Capra, L.; Ion, I. A Thermodynamics Study on the Tetrahydrofuran Effect in Exfoliated Graphite Nanoplatelets and Activated Carbon Mixtures at Temperatures between 293.15 and 308.15 K. Adv. Mater. Sci. Eng. 2018, 2018, 9106043. [Google Scholar] [CrossRef]
- Iloukhani, H.; Zoorasna, Z.; Soleimani, R. Excess molar volumes and speeds of sound of tetrahydrofuran with chloroethanes or chloroethenes at 298.15 K. Phys. Chem. Liq. 2005, 43, 391–401. [Google Scholar] [CrossRef]
- Arce, A.; Blanco, A.; Soto, A.; Vidal, I. Densities, refractive indices, and excess molar volumes of the ternary systems water + methanol + 1-octanol and water + ethanol + 1-octanol and their binary mixtures at 298.15 K. J. Chem. Eng. Data 1993, 38, 336–340. [Google Scholar] [CrossRef]
- Wankhede, D.S. Refractive indices for binary mixtures of propylene carbonate. Int. J. Chem. Res. 2011, 2, 23–26. [Google Scholar]
- Dubey, G.P.; Kumar, R. Densities, speeds of sound and viscosities of binary mixtures of tetrahydrofuran with 1-hexanol, 1-octanol and 1-decanol at T = (298.15 to 313.15) K. J. Chem. Thermodyn. 2014, 71, 27–36. [Google Scholar] [CrossRef]
- Alkhaldi, K.H.A.E.; Al-Jimaz, A.S.; AlTuwaim, M.S. Densities, ultrasonic speeds and refractive indices of phenetole with N-methyl-2-pyrrolidone, N,N-dimethylformamide and tetrahydrofuran binary mixtures at different temperatures. J. Chem. Thermodyn. 2016, 103, 249–256. [Google Scholar] [CrossRef]
- Hoga, H.E.; Olivieri, G.V.; Torres, R.B. Experimental Measurements of Volumetric and Acoustic Properties of Binary Mixtures of 1-Butyl-3-methylimidazolium Hexafluorophosphate with Molecular Solvents. J. Chem. Eng. Data 2020, 65, 3406–3419. [Google Scholar] [CrossRef]
- Fattahi, M.; Iloukhani, H. Excess molar volume, viscosity, and refractive index study for the ternary mixture {2-methyl-2-butanol (1) + tetrahydrofuran (2) + propylamine (3)} at different temperatures. Application of the ERAS-model and Peng–Robinson–Stryjek–Vera equation of state. J. Chem. Thermodyn. 2010, 42, 1335–1345. [Google Scholar] [CrossRef]
- AlTuwaim, M.S.; Alkhaldi, K.H.; Al-Jimaz, A.S.; Mohammad, A.A. Physico-chemical properties of binary mixtures of N,N-dimethylformamide with 1-octanol, 1-nonanol and 1-decanol at different temperatures. J. Chem. Thermodyn. 2013, 58, 367–376. [Google Scholar] [CrossRef]
- Acree, W.E., Jr. Thermodynamic Properties of Non Electrolyte Solutions; Academic Press: Cambridge, MA, USA, 1984. [Google Scholar]
- Ion, I.; Sirbu, F.; Ion, A.C. Density, Refractive Index, and Ultrasound Speed in Mixtures of Active Carbon and Exfoliated Graphite Na-noplatelets Dispersed in N,N Dimethylformamide at Temperatures from (293.15 to 318.15) K. J. Chem. Eng. Data 2013, 58, 1212–1222. [Google Scholar] [CrossRef]
- Zhang, S.; Zhao, L.; Yue, X.; Li, B.; Zhang, J. Density, viscosity, surface tension and spectroscopic studies for the liquid mixture of tetra-ethylene glycol + N,N-dimethylformamide at six temperatures. J. Mol. Liq. 2018, 264, 451–457. [Google Scholar] [CrossRef]
- Aminabhavi, T.M.; Patil, V.B. Density, Viscosity, Refractive Index, and Speed of Sound in Binary Mixtures of Ethenylbenzene with N,N-Dimethylacetamide, Tetrahydrofuran, N,N-Dimethylformamide, 1,4-Dioxane, Dimethyl Sulfoxide, Chloroform, Bromoform, and 1-Chloronaphthalene in the Temperature Interval (298.15–308.15) K. J. Chem. Eng. Data 1998, 43, 497–503. [Google Scholar]
- Riddick, J.A.; Bunger, W.B.; Sakano, T.K. Organic Solvents Physical Properties and Methods of Purifications. In Techniques in Chemistry; Wiley: New York, NY, USA, 1986; Volume II. [Google Scholar]
- El-Dossoki, F.I. Refractive index and density measurements for selected binary protic-protic, aprotic-aprotic, and aprotic-protic systems at temperatures from 298.15 to 308.15 K. J. Chin. Chem. Soc. 2007, 54, 1129–1137. [Google Scholar] [CrossRef]
- Zarei, H.; Keley, V. Density and Speed of Sound of Binary Mixtures of Ionic Liquid 1-Ethyl-3-methylimidazolium Tetrafluorobo-rate,N,N-Dimethylformamide, and N,N-Dimethylacetamide at Temperature Range of 293.15–343.15 K: Measurement and PC-SAFT Modeling. J. Chem. Eng. Data 2017, 62, 913–923. [Google Scholar] [CrossRef]
- Garcia, B.; Alcalde, R.; Leal, J.M.; Trenzado, J.L. Volumetric behaviour of N-methylformamide–(C1–C10)alkan-1-ol and N,N-dimethylformamide–(C1–C10)alkan-1-ol solvent systems. J. Phys. Org. Chem. 1997, 10, 138–144. [Google Scholar] [CrossRef]
- Shukla, R.K.; Kumar, A.; Awasthi, N.; Srivastava, U.; Gangwar, V.S. Density, viscosity and refractive index of binary liquid mixtures at 293.15, 298.15, 303.15, 308.15 and 313.15 K. Exp. Therm. Fluid Sci. 2012, 37, 1–11. [Google Scholar] [CrossRef]
- Chen, F.; Wu, J.; Wang, Z. Volumetric properties of the binary liquid mixtures of N,N-dimethylacetamide + benzene, +toluene, or +ethylbenzene at different temperatures and atmospheric pressure. J. Mol. Liq. 2008, 140, 6–9. [Google Scholar] [CrossRef]
- Cobos, A.; González, J.A.; Hevia, F.; De La Fuente, I.G.; Tristán, C.A. Thermodynamics of amide + ketone mixtures. Volumetric, speed of sound and refractive index data for N,N-dimethylacetamide + 2-alkanone systems at several temperatures. Application of Flory’s model to tertiary amide + n-alkanone systems. J. Mol. Liq. 2017, 248, 286–301. [Google Scholar] [CrossRef]
- Warminska, D.; Placzek, A.; Koziel, H.; Grzybkowski, W. Adiabatic Compressibilities of Divalent Transition-Metal Perchlorates and Chlorides in N,N-Dimethylacetamide and Dimethylsulfoxide. J. Chem. Eng. Data 2009, 54, 745–751. [Google Scholar] [CrossRef]
- Iloukhani, H.; Rakhshi, M. Excess molar volumes, viscosities, and refractive indices for binary and ternary mixtures of {cyclohexanone (1) + N,N-dimethylacetamide (2) + N,N-diethylethanolamine (3)} at (298.15, 308.15, and 318.15) K. J. Mol. Liq. 2009, 149, 86–95. [Google Scholar] [CrossRef]
- Ivanov, E.V.; Smirnov, V.I. Water as a solute in aprotic dipolar solvents: D2O–H2O solute isotope effects on the enthalpy of water dissolution in dimethylsulphoxide, N,N-dimethylformamide and N,N-dimethylacetamide at 298.15 K. Thermochim. Acta 2011, 526, 257–261. [Google Scholar] [CrossRef]
- Aminabhavi, T.M.; Gopalakrishna, B. Density, Viscosity, Refractive Index, and Speed of Sound in Aqueous Mixtures of N,N-Dimethylformamide, Dimethyl Sulfoxide, N,N-Dimethylacetamide, Acetonitrile, Ethylene Glycol, Diethylene Glycol, 1,4-Dioxane, Tetrahydrofuran, 2-Methoxyethanol, and 2-Ethoxyethanol at 298.15 K. J. Chem. Eng. Data 1995, 40, 856–861. [Google Scholar]
- Papamatthaiakis, D.; Aroni, F.; Havredaki, V. Isentropic Compressibilities of (Amide + Water) Mixtures: A comparative Study. J. Chem. Thermodyn. 2008, 40, 107–118. [Google Scholar] [CrossRef]
- Baragi, J.G.; Aralaguppi, M.I.; Aminabhavi, T.M.; Kariduraganavar, M.Y.; Kulkarni, S.S. Density, viscosity, refractive index, and speed of sound for binary mixtures of 1,4-dioxane with different organic liquids at (298.15, 303.15, and 308.15) K. J. Chem. Eng. Data 2005, 50, 917–923. [Google Scholar] [CrossRef]
- Rakini Chandrasekaran, J.H.; Nithiyanantham, S. Solvation number, thermochemical parameter, and viscosity study of sweeteners in aqueous and non-aqueous media through ultrasonic measurements. Chem. Phys. Mater. 2023, 2, 303–314. [Google Scholar] [CrossRef]
- Gonzalez, C.; Resa, J.M.; Lanz, J.; Iglesias, M. Intermolecular interactions in soybean oil + different organic solvents by ultrasonic velocity measurements. J. Food Eng. 2006, 77, 152–161. [Google Scholar] [CrossRef]
- Rowlinson, J.S.; Swinton, F.L. Liquid and Liquid Mixtures, 3rd ed.; Butterworths: London, UK, 1982. [Google Scholar]
- Gerecze, N.G. Ultrasonic studies in solutions of polyethylene glycol. Acta Acust. United Acust. 1977, 38, 51–57. [Google Scholar]
- Spierings, G.A.C.M.; Melis, G.P. Refractive index and density of alkali lime borogermanosilicate glasses. J. Mater. Sci. 1981, 16, 1059–1062. [Google Scholar] [CrossRef]
- Reddy, V.N.; Rao, K.S.W.K.; Subha, M.C.S.; Rao, K.C. Miscibility behaviour of dextrin/PVA blends in water at 35 °C. In Proceedings of the International Conference on Advances in Polymer Technology, Berhampur, India, 26–27 February 2010; pp. 356–368. [Google Scholar]
- Allen, R.D. A new equation relating index of refraction and specific gravity. Am. Mineral. 1956, 41, 245–257. [Google Scholar]
- Jacobson, B. Ultrasonic Velocity in Liquids and Liquid Mixtures. J. Chem. Phys. 1952, 20, 927–928. [Google Scholar] [CrossRef]
- Jacobson, B. Intermolecular free lengths in the liquid state. I. Adiabatic and isothermal compressibilities. Acta Chem. Scand 1952, 6, 1485–1498. [Google Scholar] [CrossRef]
- Kumar Sharma, D.; Agarwal, S.; KhanDer, A. Intermolecular Free Length and Molar Volume of Binary Liquid Mixtures of Ethyl Acetate with 1-Alkanol at 303.15K. Pharma Chem. 2022, 14, 1–9. [Google Scholar]
- Altenberg, K. Ultraschallgeschwindigkeit und Molekülstruktur. Z. Phys. Chem. 1950, 195, 145–164. [Google Scholar] [CrossRef]
- Lomesh, S.K.; Bala, M.; Nathan, V. A study of enhancing the solubility of streptomycin sulphate in sorbitol using volumetric, acoustic and viscometric properties. J. Mol. Liq. 2024, 404, 124997. [Google Scholar] [CrossRef]
- Tewari, Y.B.; Miller, M.M.; Wasik, S.P. Calculation of Aqueous Solubility of Organic Compounds. J. Res. Natl. Bur. Stand. 1982, 87, 155–158. [Google Scholar] [CrossRef]
- Ilyas, H.; Masih, I.; van Hullebusch, E.D. Prediction of the removal efficiency of emerging organic contaminants in constructed wetlands based on their physicochemical properties. J. Environ. Manag. 2021, 294, 112916. [Google Scholar] [CrossRef]
- Baldevraj, P.P.; Rajendran, V. Science and Technology of Ultrasonics; Narosa publishing House: Delhi, India, 2006. [Google Scholar]
- Ikhe, S.; Narwade, M. Ultrasonic studies of substituted izoxazoles and pyrazolines in dioxane and dioxane-water mixtures at different temperatures. Ind. J. Chem. 2005, 44, 1203–1205. [Google Scholar]
- Sinha, A.; Roy, M.N. Densities, Viscosities, and Sound Speeds of Some Acetate Salts in Binary Mixtures of Tetrahydrofuran and Methanol at (303.15, 313.15, and 323.15) K. J. Chem. Eng. Data. 2006, 51, 1415–1423. [Google Scholar] [CrossRef]
- Dikko, A.B.; Ezike, S.C.; Ike, E. Ultrasonic velocity and some acoustical and thermodynamic parameters of multi-component liquid mixture at different temperatures. Int. J. Sci. Eng. Appl. Sci. 2015, 1, 454–458. [Google Scholar]
- Kaur, P.; Tarsikka, P.S. Intercorrelations of Ultrasonic Velocity with Density and Viscosity in Adulterated Mustard Oil. J. Agric. Phys. 2020, 20, 191–198. [Google Scholar]
- Guan, J.; Yan, X.; Zhao, Y.; Lu, J.; Sun, Y.; Peng, X. Investigation of the molecular interactions of triclocarban with human serum albumin using multispectroscopies and molecular modeling. J. Biomol. Struct. Dyn. 2019, 37, 3550–3565. [Google Scholar] [CrossRef] [PubMed]
- Guan, J.; Yan, X.; Zhao, Y.; Sun, Y.; Peng, X. Binding studies of triclocarban with bovine serum albumin: Insights from multi-spectroscopy and molecular modeling methods. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2018, 202, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Brausch, J.M.; Rand, G.M. A review of personal care products in the aquatic environment: Environmental concentrations and toxicity. Chemosphere 2011, 82, 1518–1532. [Google Scholar] [CrossRef]
- Schmitz, G.S.; Santana, E.R.; Baumgarten, L.G.; Winiarskia, J.P.; Colaçob, M.C.; Caramorib, G.F.; Spinellic, A.; Vieira, I.C. A simple and reliable electrochemical method employing an unmodified boron-doped diamond electrode for the determination of triclocarban. Electrochim. Acta 2024, 486, 144093. [Google Scholar] [CrossRef]
- Yang, H.; Sanidad, K.Z.; Wang, W.; Xie, M.; Gu, M.; Cao, X.; Xiao, H.; Zhang, G. Triclocarban exposure exaggerates colitis and colon tumorigenesis: Roles of gut microbiota involved. Gut Microbes 2020, 12, 1690364. [Google Scholar] [CrossRef]
- Ameta, R.K.; Singh, M.; Kale, R.K. Comparative study of density, sound velocity and refractive index for (water + alkali metal) phosphates aqueous systems at T = (298.15, 303.15, 308.15) K. J. Chem. Thermodyn. 2013, 60, 159–168. [Google Scholar] [CrossRef]
 ), X1 = 0.0663; TCC (1) + nB (2) (■), X1 = 0.0697; TCC (1) + THF (2) (▲), X1 = 0.0390; TCC(1) + DMF (2) (♦), X1 = 0.1261; and TCC (1) + DMA (2) (
), X1 = 0.0663; TCC (1) + nB (2) (■), X1 = 0.0697; TCC (1) + THF (2) (▲), X1 = 0.0390; TCC(1) + DMF (2) (♦), X1 = 0.1261; and TCC (1) + DMA (2) ( ), X1 = 0.0564 mixtures. The lines in the figure are presented only to visualize the Sn variation.
), X1 = 0.0564 mixtures. The lines in the figure are presented only to visualize the Sn variation.
 ), X1 = 0.0663; TCC (1) + nB (2) (■), X1 = 0.0697; TCC (1) + THF (2) (▲), X1 = 0.0390; TCC(1) + DMF (2) (♦), X1 = 0.1261; and TCC (1) + DMA (2) (
), X1 = 0.0663; TCC (1) + nB (2) (■), X1 = 0.0697; TCC (1) + THF (2) (▲), X1 = 0.0390; TCC(1) + DMF (2) (♦), X1 = 0.1261; and TCC (1) + DMA (2) ( ), X1 = 0.0564 mixtures. The lines in the figure are presented only to visualize the Sn variation.
), X1 = 0.0564 mixtures. The lines in the figure are presented only to visualize the Sn variation.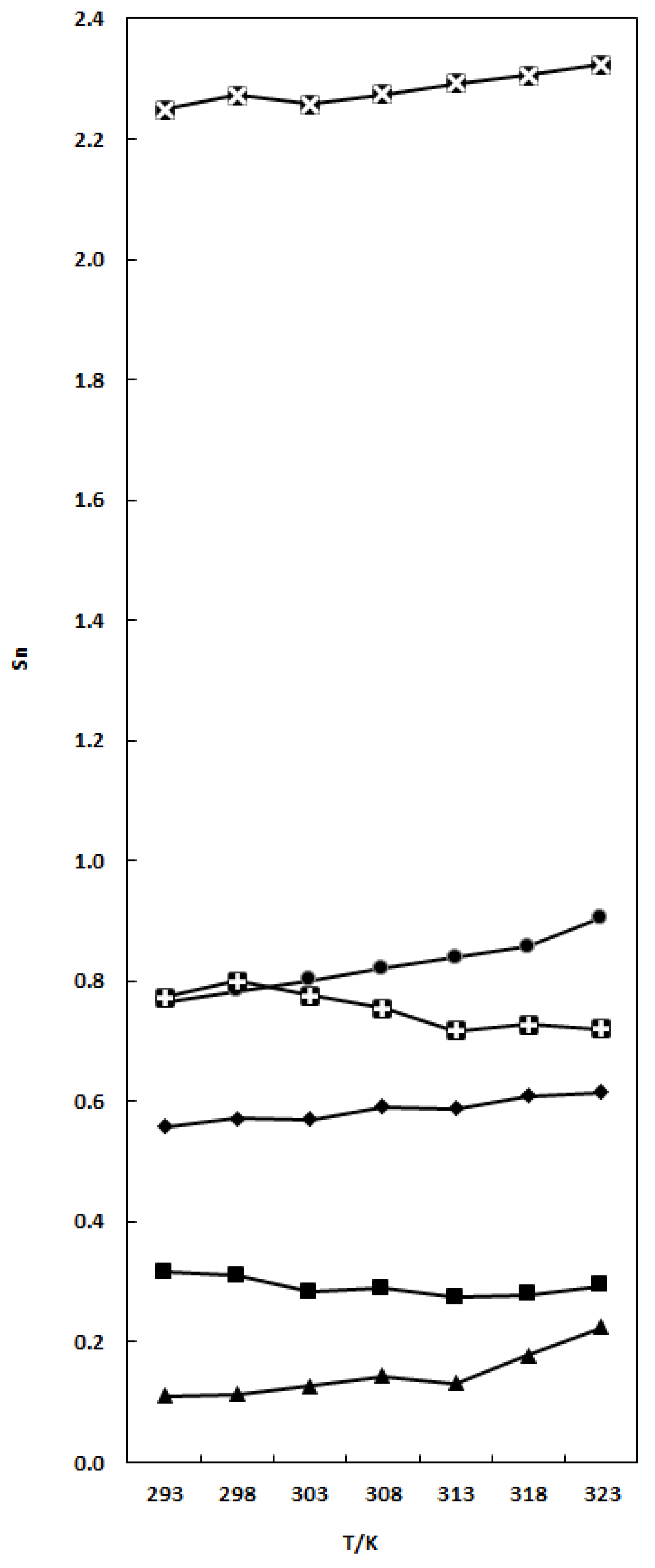
 ), X1 = 0.0663; TCC (1) + nB (2) (■), X1 = 0.0697; TCC (1) + THF (2) (▲), X1 = 0.0390; TCC (1) + DMF (2) (♦), X1 = 0.1261; and TCC (1) + DMA (2) (
), X1 = 0.0663; TCC (1) + nB (2) (■), X1 = 0.0697; TCC (1) + THF (2) (▲), X1 = 0.0390; TCC (1) + DMF (2) (♦), X1 = 0.1261; and TCC (1) + DMA (2) ( ), X1 = 0.0564 mixtures. – – linear correlation for pure solvents; ⎯⎯⎯ linear correlation for binary mixtures.
), X1 = 0.0564 mixtures. – – linear correlation for pure solvents; ⎯⎯⎯ linear correlation for binary mixtures.
 ), X1 = 0.0663; TCC (1) + nB (2) (■), X1 = 0.0697; TCC (1) + THF (2) (▲), X1 = 0.0390; TCC (1) + DMF (2) (♦), X1 = 0.1261; and TCC (1) + DMA (2) (
), X1 = 0.0663; TCC (1) + nB (2) (■), X1 = 0.0697; TCC (1) + THF (2) (▲), X1 = 0.0390; TCC (1) + DMF (2) (♦), X1 = 0.1261; and TCC (1) + DMA (2) ( ), X1 = 0.0564 mixtures. – – linear correlation for pure solvents; ⎯⎯⎯ linear correlation for binary mixtures.
), X1 = 0.0564 mixtures. – – linear correlation for pure solvents; ⎯⎯⎯ linear correlation for binary mixtures.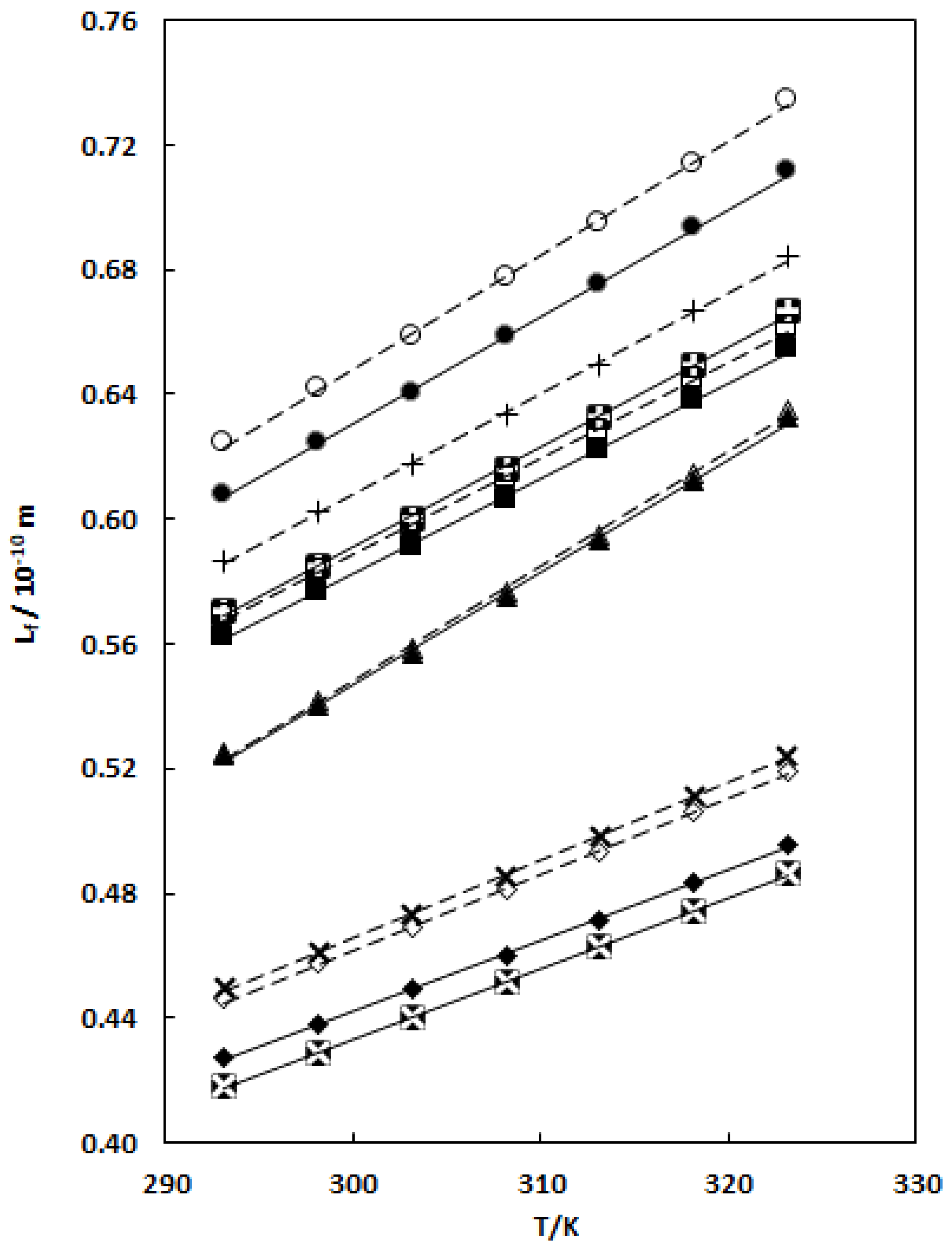
 ), X1 = 0.0663; TCC (1) + nB (2) (■), X1 = 0.0697; TCC (1) + THF (2) (▲), X1 = 0.0390; TCC(1) + DMF (2) (♦), X1 = 0.1261; and TCC (1) + DMA (2) (
), X1 = 0.0663; TCC (1) + nB (2) (■), X1 = 0.0697; TCC (1) + THF (2) (▲), X1 = 0.0390; TCC(1) + DMF (2) (♦), X1 = 0.1261; and TCC (1) + DMA (2) ( ), X1 = 0.0564 mixtures. – – linear correlation for pure solvents; ⎯⎯⎯ linear correlation for binary mixtures.
), X1 = 0.0564 mixtures. – – linear correlation for pure solvents; ⎯⎯⎯ linear correlation for binary mixtures.
 ), X1 = 0.0663; TCC (1) + nB (2) (■), X1 = 0.0697; TCC (1) + THF (2) (▲), X1 = 0.0390; TCC(1) + DMF (2) (♦), X1 = 0.1261; and TCC (1) + DMA (2) (
), X1 = 0.0663; TCC (1) + nB (2) (■), X1 = 0.0697; TCC (1) + THF (2) (▲), X1 = 0.0390; TCC(1) + DMF (2) (♦), X1 = 0.1261; and TCC (1) + DMA (2) ( ), X1 = 0.0564 mixtures. – – linear correlation for pure solvents; ⎯⎯⎯ linear correlation for binary mixtures.
), X1 = 0.0564 mixtures. – – linear correlation for pure solvents; ⎯⎯⎯ linear correlation for binary mixtures.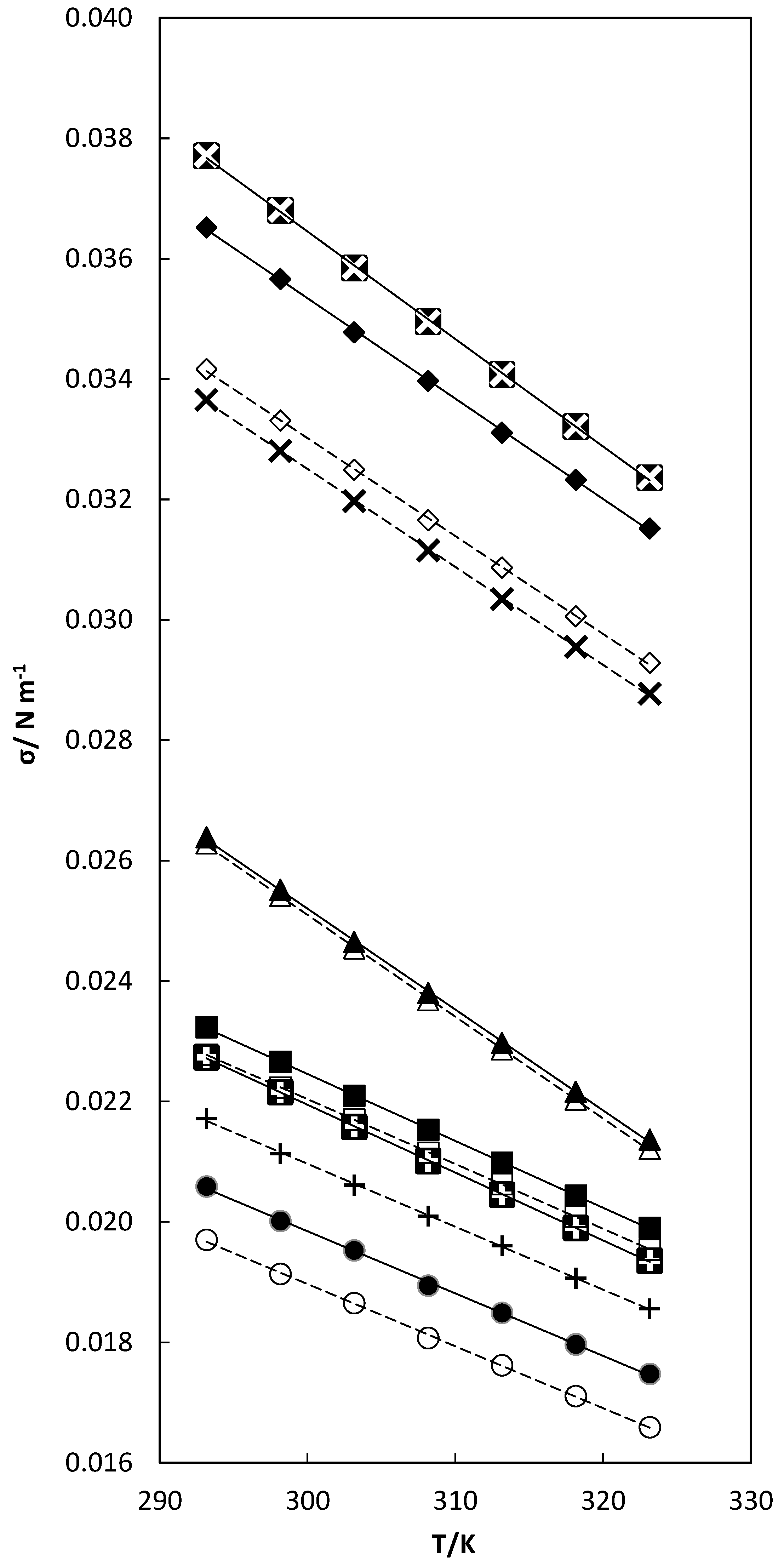
| T, K | ρ, g cm−3 | u, m s−1 | nD |
|---|---|---|---|
| Ethyl alcohol | |||
| 293.15 | 0.789811 | 1161.63 | 1.361447 |
| 298.15 | 0.785489 | 1143.42 | 1.359439 |
| 0.78506 [36] | 1143.17 [36] | 1.359130 [36] | |
| 0.78511 [37] | 1143 [37] | 1.35922 [38] | |
| 0.7855 [39] | 1143.07 [40] | 1.3593 [41] | |
| 0.7893 [42] | 1144 [43] | 1.3605 [44] | |
| 0.786 [45] | 1142 [46] | 1.360 [45] | |
| 303.15 | 0.781122 | 1128.18 | 1.357238 |
| 308.15 | 0.776707 | 1108.97 | 1.355301 |
| 313.15 | 0.772236 | 1094.72 | 1.353645 |
| 318.15 | 0.767711 | 1077.64 | 1.351281 |
| 323.15 | 0.761515 | 1061.53 | 1.349268 |
| n-Propyl alcohol | |||
| 293.15 | 0.804317 | 1224.61 | 1.385059 |
| 298.15 | 0.800413 | 1206.42 | 1.383034 |
| 0.800 [43,45,47] | 1208.03 [45] | 1.38307 [48] | |
| 0.80021 [49] | 1207.3 [47] | 1.38370 [38] | |
| 0.799657 [50] | 1206 [48] | 1.3832 [41] | |
| 0.7996 [51] | 1205.69 [52] | 1.383 [45,47,53] | |
| 0.79977 [54] | 1209.4 [46] | 1.3840 [55] | |
| 303.15 | 0.796251 | 1190.63 | 1.380950 |
| 308.15 | 0.792497 | 1174.36 | 1.378912 |
| 313.15 | 0.788632 | 1158.86 | 1.376804 |
| 318.15 | 0.784748 | 1141.31 | 1.374651 |
| 323.15 | 0.781084 | 1124.35 | 1.372472 |
| n-Butyl alcohol | |||
| 293.15 | 0.809863 | 1258.46 | 1.398898 |
| 298.15 | 0.806857 | 1241.25 | 1.396938 |
| 0.806 [47,56,57] | 1241.8 [47] | 1.397 [47] | |
| 0.8071 [58] | 1241 [59] | 1.3967 [41] | |
| 0.8070 [60] | 1238.99 [61] | 1.3972 [62] | |
| 0.80590 [48,63] | 1240.37 [64] | 1.39702 [48] | |
| 0.8053 [65] | 1240.5 [63] | 1.3983 [55] | |
| 303.15 | 0.802061 | 1226.28 | 1.394814 |
| 308.15 | 0.798782 | 1208.71 | 1.392757 |
| 313.15 | 0.795437 | 1192.14 | 1.390715 |
| 318.15 | 0.791082 | 1175.49 | 1.388654 |
| 323.15 | 0.786328 | 1158.65 | 1.386560 |
| Tetrahydrofuran | |||
| 293.15 | 0.887551 | 1302.54 | 1.407321 |
| 298.15 | 0.882123 | 1278.86 | 1.404612 |
| 0.882322 [66] | 1278.93 [66] | 1.4053 [66] | |
| 0.88216 [67] | 1279.38 [67] | 1.40464 [67] | |
| 0.88207 [68] | 1277.60 [69] | 1.4049 [70] | |
| 0.882150 [71] | 1280.1 [71] | 1.405 [72] | |
| 0.8828 [73] | 1278 [72,73] | 1.4037 [74] | |
| 303.15 | 0.876542 | 1254.62 | 1.40205 |
| 308.15 | 0.871126 | 1230.31 | 1.39938 |
| 313.15 | 0.865659 | 1206.87 | 1.39679 |
| 318.15 | 0.860174 | 1182.26 | 1.39415 |
| 323.15 | 0.854689 | 1157.73 | 1.39136 |
| N,N-Dimethylformamide | |||
| 293.15 | 0.949789 | 1482.82 | 1.430508 |
| 298.15 | 0.945014 | 1462.94 | 1.428251 |
| 0.944290 [75] | 1458 [73] | 1.4305 [76] | |
| 0.94502 [77] | 1457 [72] | 1.429 [72] | |
| 0.9445 [78,79] | 1457.50 [80] | 1.4290 [81] | |
| 0.943978 [82] | 1457.69 [75] | 1.42810 [83] | |
| 0. 95010 [84] | 1463.76 [77] | 1.42805 [77] | |
| 303.15 | 0.940119 | 1443.86 | 1.425988 |
| 308.15 | 0.935320 | 1423.79 | 1.423685 |
| 313.15 | 0.930455 | 1404.95 | 1.421411 |
| 318.15 | 0.925848 | 1384.87 | 1.419096 |
| 323.15 | 0.921060 | 1365.71 | 1.416780 |
| N,N-Dimethylacetamide | |||
| 293.15 | 0.941052 | 1477.02 | 1.438107 |
| 298.15 | 0.936459 | 1456.92 | 1.435740 |
| 0.9364 [85] | 1453.68 [36] | 1.43621 [86] | |
| 0.9365 [80] | 1455.37 [87] | 1.43571 [88] | |
| 0.93639 [86] | 1475.3 [86] | 1.4359 [89] | |
| 0.9366 [90] | 1458 [90] | 1.435794 [36] | |
| 0.93634 [91] | 1478.98 [91] | 1.4364 [92] | |
| 303.15 | 0.931848 | 1436.87 | 1.433288 |
| 308.15 | 0.927228 | 1416.83 | 1.430911 |
| 313.15 | 0.922604 | 1396.87 | 1.428593 |
| 318.15 | 0.917991 | 1377.03 | 1.426357 |
| 323.15 | 0.913344 | 1357.23 | 1.424067 |
| T, K | ρ/g cm−3 | u/m s−1 | nD | Sn | ρ/g cm−3 | u/m s−1 | nD | Sn |
|---|---|---|---|---|---|---|---|---|
| TCC (1) + AE (2), X1 = 0.0626 | TCC (1) + nP (2), X1 = 0.0663 | |||||||
| 293.15 | 0.804476 | 1181.60 | 1.362256 | 0.76546 | 0.815279 | 1251.19 | 1.385218 | 0.77326 |
| 298.15 | 0.800156 | 1163.77 | 1.360200 | 0.78383 | 0.811173 | 1233.97 | 1.383165 | 0.80024 |
| 303.15 | 0.795834 | 1148.91 | 1.358040 | 0.80216 | 0.807021 | 1216.66 | 1.381185 | 0.77601 |
| 308.15 | 0.791388 | 1130.08 | 1.356017 | 0.82146 | 0.802821 | 1199.38 | 1.379087 | 0.75493 |
| 313.15 | 0.786860 | 1116.21 | 1.354388 | 0.83846 | 0.798572 | 1182.13 | 1.377012 | 0.71732 |
| 318.15 | 0.782251 | 1099.5 | 1.352035 | 0.85661 | 0.794268 | 1164.96 | 1.374886 | 0.72788 |
| 323.15 | 0.777548 | 1083.78 | 1.350055 | 0.90447 | 0.789907 | 1147.79 | 1.372540 | 0.72015 |
| TCC (1) + nB (2), X1 = 0.0697 | TCC (1) + THF (2), X1 = 0.0390 | |||||||
| 293.15 | 0.815711 | 1269.05 | 1.399069 | 0.31589 | 0.889290 | 1304.19 | 1.407681 | 0.11042 |
| 298.15 | 0.811823 | 1252.07 | 1.397035 | 0.30989 | 0.883858 | 1280.53 | 1.404894 | 0.11252 |
| 303.15 | 0.807897 | 1235.04 | 1.395032 | 0.28368 | 0.878381 | 1256.54 | 1.402220 | 0.12673 |
| 308.15 | 0.803933 | 1218.06 | 1.392923 | 0.28829 | 0.872861 | 1232.66 | 1.399653 | 0.14271 |
| 313.15 | 0.799931 | 1201.18 | 1.390879 | 0.27397 | 0.867305 | 1208.94 | 1.397029 | 0.13097 |
| 318.15 | 0.795882 | 1184.37 | 1.388827 | 0.27865 | 0.861711 | 1185.49 | 1.394445 | 0.17787 |
| 323.15 | 0.791782 | 1167.56 | 1.386741 | 0.29344 | 0.856069 | 1162.10 | 1.391881 | 0.22448 |
| TCC (1) + DMF (2), X1 = 0.1261 | TCC (1) + DMA (2), X1 = 0.0564 | |||||||
| 293.15 | 0.963793 | 1535.12 | 1.427914 | 0.55827 | 0.962332 | 1569.88 | 1.435618 | 2.24954 |
| 298.15 | 0.959057 | 1515.99 | 1.425728 | 0.57120 | 0.957682 | 1549.70 | 1.433703 | 2.27238 |
| 303.15 | 0.953329 | 1496.72 | 1.423548 | 0.57039 | 0.952894 | 1527.68 | 1.431822 | 2.25815 |
| 308.15 | 0.949526 | 1477.44 | 1.421315 | 0.59063 | 0.948195 | 1507.23 | 1.429601 | 2.27499 |
| 313.15 | 0.943774 | 1458.23 | 1.419066 | 0.58812 | 0.943469 | 1486.86 | 1.427330 | 2.29182 |
| 318.15 | 0.939945 | 1439.05 | 1.416875 | 0.60845 | 0.938710 | 1466.52 | 1.425094 | 2.30651 |
| 323.15 | 0.935123 | 1419.86 | 1.414695 | 0.61512 | 0.933926 | 1446.30 | 1.422831 | 2.32331 |
| T/K | Z/105 kg m−2s−1 | κS/ 10−9m2 N−1 | S | rD/10−3 m3 kg−1 | r | Lf·1010/m | σ/N·m−1 | σmod./N·m−1 |
|---|---|---|---|---|---|---|---|---|
| TCC (1) + AE (2), X1 = 0 | ||||||||
| 293.15 | 9.17468 | 0.93830 | 0.22149 | 0.28044 | 0.47290 | 0.62429 | 0.0197 | 0.0208 |
| 298.15 | 8.98144 | 0.97375 | 0.22039 | 0.28058 | 0.48929 | 0.64183 | 0.0191 | 0.0203 |
| 303.15 | 8.81246 | 1.00583 | 0.21918 | 0.28059 | 0.50282 | 0.65826 | 0.0186 | 0.0199 |
| 308.15 | 8.61345 | 1.04690 | 0.21811 | 0.28081 | 0.51960 | 0.67763 | 0.0181 | 0.0194 |
| 313.15 | 8.45382 | 1.08055 | 0.21719 | 0.28125 | 0.53187 | 0.69460 | 0.0176 | 0.0190 |
| 318.15 | 8.27316 | 1.12164 | 0.21588 | 0.28120 | 0.54636 | 0.71396 | 0.0171 | 0.0185 |
| 323.15 | 8.08371 | 1.16535 | 0.21477 | 0.28203 | 0.55983 | 0.73414 | 0.0166 | 0.0181 |
| TCC (1) + AE (2), X1 = 0.0626 | ||||||||
| 293.15 | 9.50569 | 0.89032 | 0.22194 | 0.27588 | 0.45462 | 0.60812 | 0.0206 | 0.0217 |
| 298.15 | 9.31198 | 0.92276 | 0.22081 | 0.27596 | 0.47095 | 0.62480 | 0.0200 | 0.0212 |
| 303.15 | 9.14342 | 0.95193 | 0.21962 | 0.27596 | 0.48438 | 0.64038 | 0.0195 | 0.0208 |
| 308.15 | 8.94332 | 0.98945 | 0.21850 | 0.27610 | 0.50114 | 0.65878 | 0.0189 | 0.0203 |
| 313.15 | 8.78301 | 1.02002 | 0.21760 | 0.27655 | 0.51331 | 0.67487 | 0.0185 | 0.0199 |
| 318.15 | 8.60085 | 1.05746 | 0.21630 | 0.27651 | 0.52777 | 0.69324 | 0.0180 | 0.0195 |
| 323.15 | 8.42691 | 1.09494 | 0.21520 | 0.27677 | 0.54118 | 0.71162 | 0.0175 | 0.0190 |
| TCC (1) + nP (2), X1 = 0 | ||||||||
| 293.15 | 9.84975 | 0.82904 | 0.23438 | 0.29140 | 0.41419 | 0.58682 | 0.0217 | 0.0229 |
| 298.15 | 9.65634 | 0.85840 | 0.23328 | 0.29145 | 0.43147 | 0.60261 | 0.0211 | 0.0224 |
| 303.15 | 9.48040 | 0.88592 | 0.23215 | 0.29156 | 0.44625 | 0.61778 | 0.0206 | 0.0220 |
| 308.15 | 9.30677 | 0.91496 | 0.23104 | 0.29154 | 0.46128 | 0.63349 | 0.0201 | 0.0215 |
| 313.15 | 9.13914 | 0.94420 | 0.22990 | 0.29152 | 0.47541 | 0.64930 | 0.0196 | 0.0211 |
| 318.15 | 8.95641 | 0.97828 | 0.22873 | 0.29146 | 0.49118 | 0.66678 | 0.0191 | 0.0207 |
| 323.15 | 8.78212 | 1.01274 | 0.22754 | 0.29131 | 0.50619 | 0.68439 | 0.0186 | 0.0202 |
| TCC (1) + nP (2), X1 = 0.0663 | ||||||||
| 293.15 | 10.20069 | 0.78351 | 0.23447 | 0.28759 | 0.38849 | 0.57048 | 0.0227 | 0.0240 |
| 298.15 | 10.00963 | 0.80961 | 0.23335 | 0.28767 | 0.40520 | 0.58524 | 0.0222 | 0.0235 |
| 303.15 | 9.81870 | 0.83710 | 0.23228 | 0.28782 | 0.42177 | 0.60052 | 0.0216 | 0.0230 |
| 308.15 | 9.62887 | 0.86590 | 0.23114 | 0.28791 | 0.43808 | 0.61628 | 0.0210 | 0.0225 |
| 313.15 | 9.44016 | 0.89610 | 0.23001 | 0.28803 | 0.45413 | 0.63254 | 0.0204 | 0.0220 |
| 318.15 | 9.25290 | 0.92771 | 0.22885 | 0.28813 | 0.46987 | 0.64931 | 0.0199 | 0.0216 |
| 323.15 | 9.06647 | 0.96095 | 0.22757 | 0.28810 | 0.48538 | 0.66666 | 0.0194 | 0.0211 |
| TCC (1) + nB (2), X1 = 0 | ||||||||
| 293.15 | 10.19180 | 0.77967 | 0.24183 | 0.29861 | 0.38136 | 0.56908 | 0.0228 | 0.0240 |
| 298.15 | 10.01511 | 0.80442 | 0.24078 | 0.29842 | 0.39816 | 0.58336 | 0.0222 | 0.0236 |
| 303.15 | 9.83551 | 0.82911 | 0.23964 | 0.29878 | 0.41259 | 0.59764 | 0.0217 | 0.0231 |
| 308.15 | 9.65496 | 0.85689 | 0.23853 | 0.29862 | 0.42930 | 0.61306 | 0.0211 | 0.0227 |
| 313.15 | 9.48272 | 0.88459 | 0.23743 | 0.29850 | 0.44484 | 0.62847 | 0.0206 | 0.0222 |
| 318.15 | 9.29909 | 0.91483 | 0.23632 | 0.29873 | 0.46024 | 0.64479 | 0.0201 | 0.0218 |
| 323.15 | 9.11079 | 0.94731 | 0.23519 | 0.29910 | 0.47560 | 0.66191 | 0.0195 | 0.0213 |
| TCC (1) + nB (2), X1 = 0.0697 | ||||||||
| 293.15 | 10.35178 | 0.76121 | 0.24193 | 0.29658 | 0.37090 | 0.56230 | 0.0232 | 0.0245 |
| 298.15 | 10.16459 | 0.78574 | 0.24083 | 0.29666 | 0.38763 | 0.57655 | 0.0227 | 0.0240 |
| 303.15 | 9.97785 | 0.81149 | 0.23976 | 0.29677 | 0.40417 | 0.59126 | 0.0221 | 0.0236 |
| 308.15 | 9.79239 | 0.83838 | 0.23862 | 0.29682 | 0.42044 | 0.60641 | 0.0215 | 0.0231 |
| 313.15 | 9.60861 | 0.86643 | 0.23752 | 0.29693 | 0.43639 | 0.62198 | 0.0210 | 0.0226 |
| 318.15 | 9.42619 | 0.89573 | 0.23642 | 0.29705 | 0.45206 | 0.63802 | 0.0204 | 0.0221 |
| 323.15 | 9.24453 | 0.92648 | 0.23529 | 0.29716 | 0.46750 | 0.65459 | 0.0199 | 0.0217 |
| TCC (1) + THF (2), X1 = 0 | ||||||||
| 293.15 | 11.56071 | 0.66409 | 0.24634 | 0.27755 | 0.33726 | 0.52521 | 0.0263 | 0.0277 |
| 298.15 | 11.28112 | 0.69315 | 0.24489 | 0.27762 | 0.36114 | 0.54151 | 0.0254 | 0.0270 |
| 303.15 | 10.99727 | 0.72477 | 0.24352 | 0.27782 | 0.38513 | 0.55878 | 0.0245 | 0.0262 |
| 308.15 | 10.71755 | 0.75839 | 0.24209 | 0.27791 | 0.40873 | 0.57675 | 0.0237 | 0.0254 |
| 313.15 | 10.44738 | 0.79311 | 0.24070 | 0.27806 | 0.43104 | 0.59508 | 0.0229 | 0.0246 |
| 318.15 | 10.16949 | 0.83174 | 0.23928 | 0.27818 | 0.45401 | 0.61481 | 0.0220 | 0.0239 |
| 323.15 | 9.89499 | 0.87293 | 0.23778 | 0.27821 | 0.47643 | 0.63539 | 0.0212 | 0.0231 |
| TCC (1) + THF (2), X1 = 0.0390 | ||||||||
| 293.15 | 11.59803 | 0.66111 | 0.24653 | 0.27722 | 0.33558 | 0.52403 | 0.0264 | 0.0278 |
| 298.15 | 11.31807 | 0.68998 | 0.24504 | 0.27724 | 0.35947 | 0.54027 | 0.0255 | 0.0271 |
| 303.15 | 11.03721 | 0.72105 | 0.24361 | 0.27734 | 0.38325 | 0.55734 | 0.0246 | 0.0263 |
| 308.15 | 10.75941 | 0.75399 | 0.24224 | 0.27752 | 0.40646 | 0.57508 | 0.0238 | 0.0255 |
| 313.15 | 10.48520 | 0.78889 | 0.24083 | 0.27768 | 0.42909 | 0.59350 | 0.0230 | 0.0248 |
| 318.15 | 10.21550 | 0.82574 | 0.23944 | 0.27787 | 0.45102 | 0.61259 | 0.0222 | 0.0240 |
| 323.15 | 9.94838 | 0.86498 | 0.23806 | 0.27809 | 0.47247 | 0.63249 | 0.0214 | 0.0233 |
| TCC (1) + DMF (2), X1 = 0 | ||||||||
| 293.15 | 14.08366 | 0.47885 | 0.25859 | 0.27226 | 0.14111 | 0.44598 | 0.0342 | 0.0360 |
| 298.15 | 13.82499 | 0.49443 | 0.25741 | 0.27238 | 0.16399 | 0.45735 | 0.0333 | 0.0353 |
| 303.15 | 13.57400 | 0.51023 | 0.25622 | 0.27254 | 0.18565 | 0.46883 | 0.0325 | 0.0346 |
| 308.15 | 13.31699 | 0.52741 | 0.25501 | 0.27264 | 0.20813 | 0.48097 | 0.0317 | 0.0339 |
| 313.15 | 13.07243 | 0.54448 | 0.25381 | 0.27278 | 0.22895 | 0.49306 | 0.0309 | 0.0333 |
| 318.15 | 12.82179 | 0.56317 | 0.25258 | 0.27281 | 0.25083 | 0.50591 | 0.0301 | 0.0326 |
| 323.15 | 12.57901 | 0.58210 | 0.25136 | 0.27290 | 0.27142 | 0.51886 | 0.0293 | 0.0319 |
| TCC (1) + DMF (2), X1 = 0.1261 | ||||||||
| 293.15 | 14.79538 | 0.44028 | 0.25723 | 0.26689 | 0.07946 | 0.42764 | 0.0365 | 0.0385 |
| 298.15 | 14.53921 | 0.45369 | 0.25608 | 0.26701 | 0.10226 | 0.43810 | 0.0357 | 0.0378 |
| 303.15 | 14.26867 | 0.46825 | 0.25493 | 0.26741 | 0.12493 | 0.44913 | 0.0348 | 0.0371 |
| 308.15 | 14.02868 | 0.48247 | 0.25376 | 0.26725 | 0.14733 | 0.46002 | 0.0340 | 0.0364 |
| 313.15 | 13.76240 | 0.49829 | 0.25257 | 0.26762 | 0.16936 | 0.47169 | 0.0331 | 0.0357 |
| 318.15 | 13.52628 | 0.51374 | 0.25141 | 0.26747 | 0.19107 | 0.48319 | 0.0323 | 0.0350 |
| 323.15 | 13.27744 | 0.53044 | 0.25026 | 0.26762 | 0.21250 | 0.49530 | 0.0315 | 0.0343 |
| TCC (1) + DMA (2), X1 = 0 | ||||||||
| 293.15 | 13.89953 | 0.48709 | 0.26256 | 0.27901 | 0.14782 | 0.44981 | 0.0337 | 0.0355 |
| 298.15 | 13.64346 | 0.50308 | 0.26133 | 0.27906 | 0.17085 | 0.46133 | 0.0328 | 0.0348 |
| 303.15 | 13.38944 | 0.51978 | 0.26005 | 0.27907 | 0.19352 | 0.47320 | 0.0320 | 0.0341 |
| 308.15 | 13.13724 | 0.53725 | 0.25880 | 0.27911 | 0.21586 | 0.48543 | 0.0312 | 0.0334 |
| 313.15 | 12.88758 | 0.55549 | 0.25759 | 0.27920 | 0.23779 | 0.49802 | 0.0303 | 0.0327 |
| 318.15 | 12.64101 | 0.57448 | 0.25641 | 0.27932 | 0.25929 | 0.51096 | 0.0296 | 0.0320 |
| 323.15 | 12.39618 | 0.59437 | 0.25521 | 0.27942 | 0.28044 | 0.52430 | 0.0288 | 0.0313 |
| TCC (1) + DMA (2), X1 = 0.0564 | ||||||||
| 293.15 | 15.10746 | 0.42164 | 0.26127 | 0.27149 | 0.03730 | 0.41849 | 0.0377 | 0.0398 |
| 298.15 | 14.84120 | 0.43479 | 0.26026 | 0.27177 | 0.06189 | 0.42888 | 0.0368 | 0.0390 |
| 303.15 | 14.55717 | 0.44967 | 0.25928 | 0.27210 | 0.08836 | 0.44013 | 0.0358 | 0.0382 |
| 308.15 | 14.29148 | 0.46424 | 0.25812 | 0.27222 | 0.11260 | 0.45125 | 0.0350 | 0.0375 |
| 313.15 | 14.02806 | 0.47944 | 0.25692 | 0.27232 | 0.13642 | 0.46268 | 0.0341 | 0.0367 |
| 318.15 | 13.76637 | 0.49533 | 0.25575 | 0.27245 | 0.15989 | 0.47446 | 0.0332 | 0.0360 |
| 323.15 | 13.50737 | 0.51188 | 0.25456 | 0.27257 | 0.18290 | 0.48656 | 0.0324 | 0.0353 |
| Solvent Name | X1 | Solubility TCC in Solvent | KOW |
|---|---|---|---|
| Ethyl alcohol | 0.062619 | 1.138753 | 0.173696 |
| n-Propyl alcohol | 0.066311 | 0.945736 | 0.184112 |
| n-Butyl alcohol | 0.069708 | 0.815611 | 0.192859 |
| Tetrahydrofuran | 0.038987 | 0.496207 | 0.225380 |
| N,N-Dimethylformamide | 0.126069 | 1.864863 | 0.148805 |
| N,N-Dimethylacetamide | 0.056367 | 0.642128 | 0.207878 |
| Chemical Name | Molar Mass/g mol−1 | Supplier | Mass Fraction Purity/% | Purification Method * |
|---|---|---|---|---|
| 3,4,4′-Trichlorodiphenylurea | 315.58 | Sigma Aldrich | ≤100% | dried in vacuum |
| Ethyl alcohol | 46.070 | Sigma Aldrich | ≥98% | none |
| n-Propyl alcohol | 60.100 | Sigma Aldrich | ≥98% | (G.C.) |
| n-Butyl alcohol | 74.120 | Sigma Aldrich | ≥99% | none |
| Tetrahydrofuran | 72.110 | Fluka Chemie AG | ≥99% | none |
| N,N-Dimetilformamide | 73.090 | E. Merck | 99% | none |
| N,N-Dimethylacetamide | 87.120 | Fluka Chemie AG | >99.5% | none |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sirbu, F.; Ion, A.C.; Ion, I. Study of the Optical and Acoustic Parameters and Surface Tensions of 3,4,4′-Trichlorodiphenylurea in Binary Mixtures with Different Organic Solvents between (293.15 and 323.15) K. Molecules 2024, 29, 4521. https://doi.org/10.3390/molecules29194521
Sirbu F, Ion AC, Ion I. Study of the Optical and Acoustic Parameters and Surface Tensions of 3,4,4′-Trichlorodiphenylurea in Binary Mixtures with Different Organic Solvents between (293.15 and 323.15) K. Molecules. 2024; 29(19):4521. https://doi.org/10.3390/molecules29194521
Chicago/Turabian StyleSirbu, Florinela, Alina Catrinel Ion, and Ion Ion. 2024. "Study of the Optical and Acoustic Parameters and Surface Tensions of 3,4,4′-Trichlorodiphenylurea in Binary Mixtures with Different Organic Solvents between (293.15 and 323.15) K" Molecules 29, no. 19: 4521. https://doi.org/10.3390/molecules29194521
APA StyleSirbu, F., Ion, A. C., & Ion, I. (2024). Study of the Optical and Acoustic Parameters and Surface Tensions of 3,4,4′-Trichlorodiphenylurea in Binary Mixtures with Different Organic Solvents between (293.15 and 323.15) K. Molecules, 29(19), 4521. https://doi.org/10.3390/molecules29194521





