Investigating the Discoloration of Leaves of Dioscorea polystachya Using Developed Atomic Absorption Spectrometry Methods for Manganese and Molybdenum
Abstract
1. Introduction
2. Results and Discussion
2.1. Selection of the Wavelengths
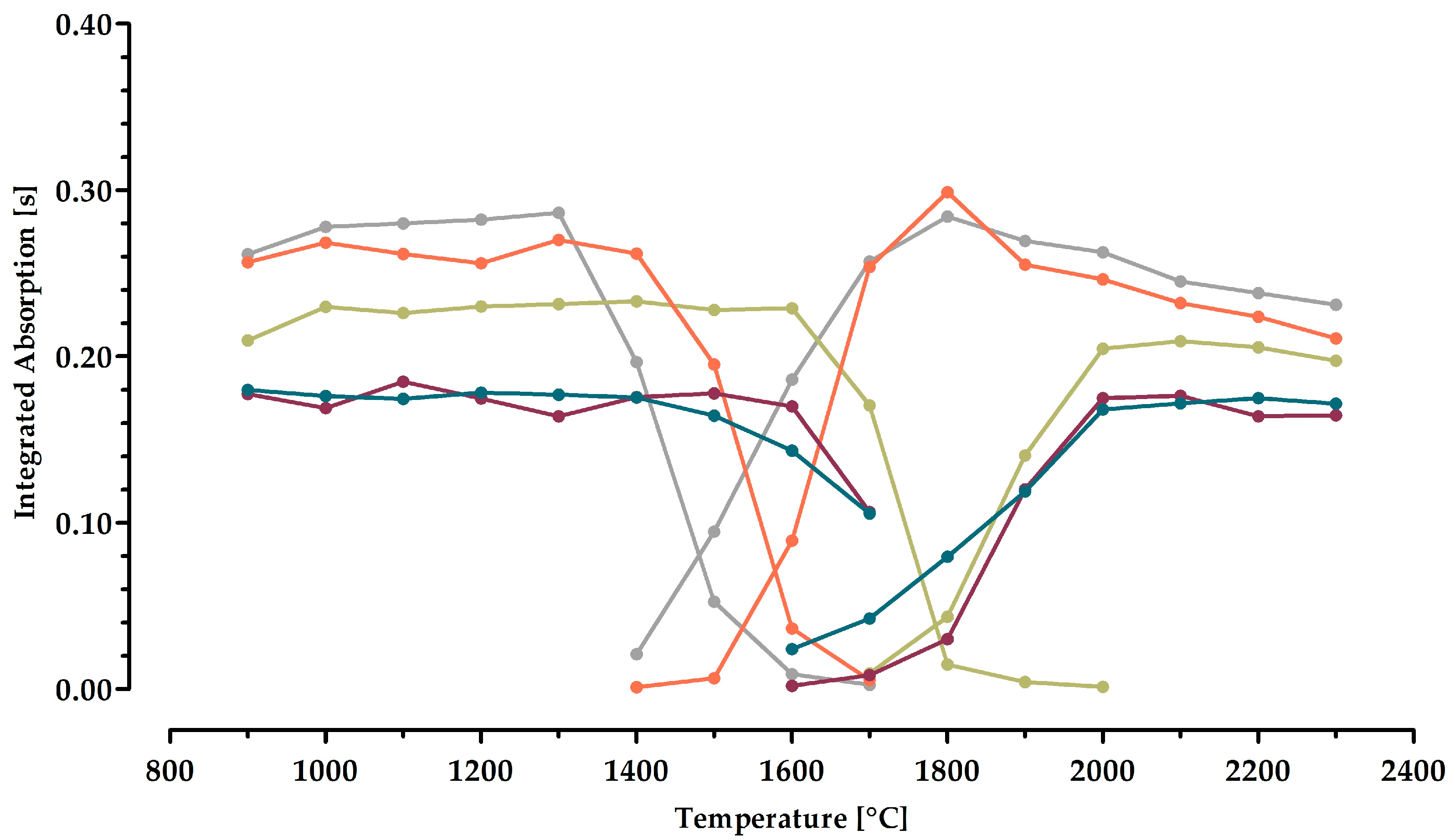
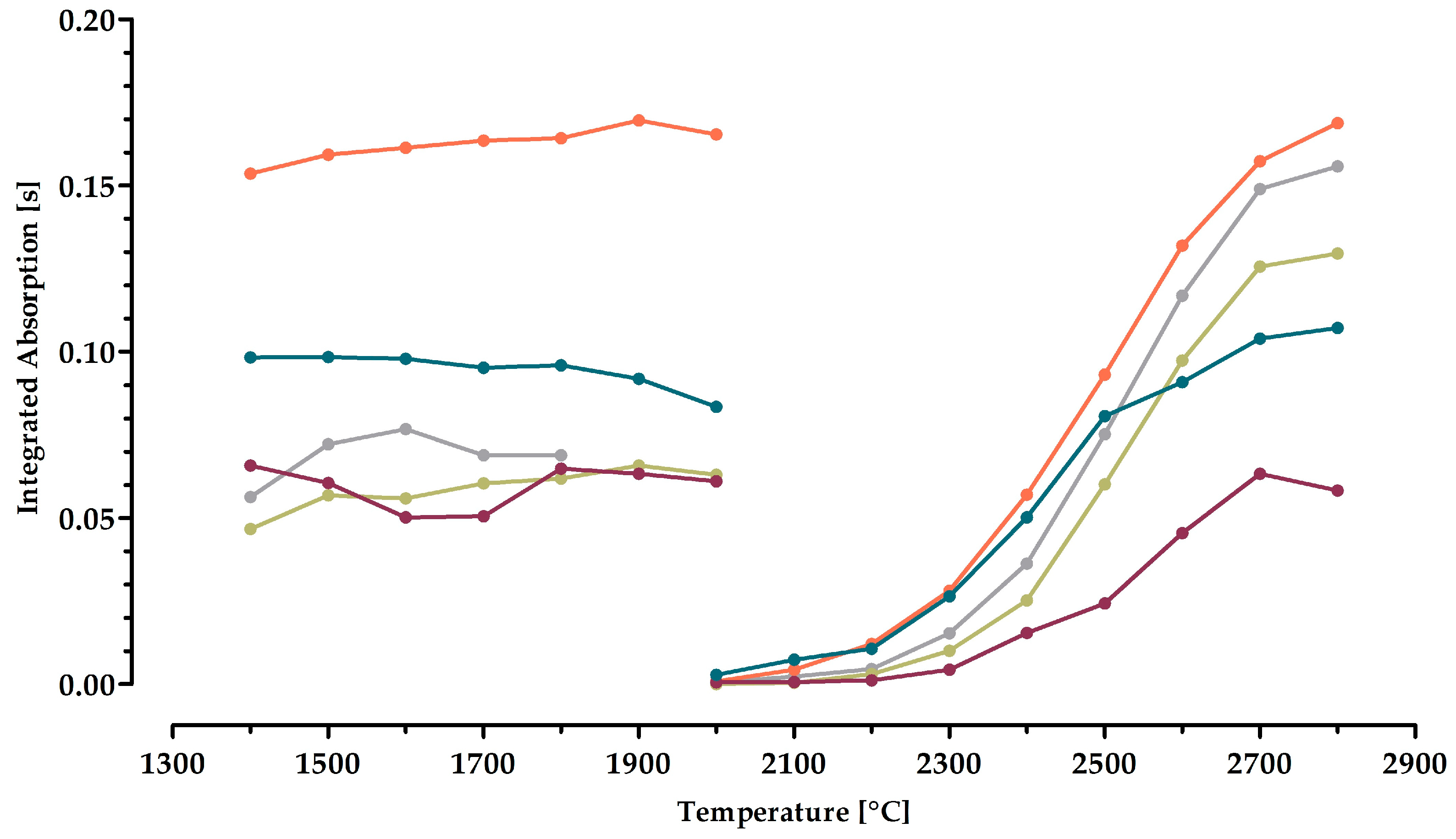
2.2. Optimization of the Time–Temperature Program for Manganese
2.3. Optimization of the Time–Temperature Program for Molybdenum
2.4. Analytical Parameters of the Manganese Method
2.5. Analytical Parameters of the Molybdenum Method
2.6. Quantification of the Extracts Regarding Manganese and Molybdenum
3. Materials and Methods
3.1. Chemicals
3.2. Plant Collection and Extraction
3.3. Atomic Absorption Spectrometer and Procedure
3.4. Statistical Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Epping, J.; Laibach, N. An underutilized orphan tuber crop—Chinese yam: A review. Planta 2020, 252, 58. [Google Scholar] [CrossRef]
- Li, Y.; Ji, S.; Xu, T.; Zhong, Y.; Xu, M.; Liu, Y.; Li, M.; Fan, B.; Wang, F.; Xiao, J.; et al. Chinese yam (Dioscorea): Nutritional value, beneficial effects, and food and pharmaceutical applications. Trends Food Sci. Technol. 2023, 134, 29–40. [Google Scholar] [CrossRef]
- Cecile, N.M.; Erve, N.A.; Awah, T.M.; Parfait, N.G.; Serge, E.S.; Fotso; Desire, T.V. Growth parameters, mineral distribution, chlorophyll content, biochemical constituents and non-enzymatic antioxidant compounds of white yam (Dioscorea rotundata (L.) var. gana) grown under salinity stress. GSC Biol. Pharm. Sci. 2020, 12, 139–149. [Google Scholar] [CrossRef]
- Jezek, M.; Allan, A.C.; Jones, J.J.; Geilfus, C.-M. Why do plants blush when they are hungry? New Phytol. 2023, 239, 494–505. [Google Scholar] [CrossRef] [PubMed]
- Krüger, D.; Weng, A.; Baecker, D. Development and Application of an Atomic Absorption Spectrometry-Based Method to Quantify Magnesium in Leaves of Dioscorea polystachya. Molecules 2024, 29, 109. [Google Scholar] [CrossRef] [PubMed]
- Alejandro, S.; Höller, S.; Meier, B.; Peiter, E. Manganese in Plants: From Acquisition to Subcellular Allocation. Front. Plant Sci. 2020, 11, 300. [Google Scholar] [CrossRef]
- Yu, M.; Hu, C.-X.; Wang, Y.-H. Effects of Molybdenum on the Intermediates of Chlorophyll Biosynthesis in Winter Wheat Cultivars Under Low Temperature. Agric. Sci. China 2006, 5, 670–677. [Google Scholar] [CrossRef]
- Krüger, D.; Matshwele, J.T.P.; Mukhtar, M.D.; Baecker, D. Insights into the Versatility of Using Atomic Absorption Spectrometry in Antibacterial Research. Molecules 2024, 29, 3120. [Google Scholar] [CrossRef] [PubMed]
- Baecker, D.; Obermoser, V.; Kirchner, E.A.; Hupfauf, A.; Kircher, B.; Gust, R. Fluorination as tool to improve bioanalytical sensitivity and COX-2-selective antitumor activity of cobalt alkyne complexes. Dalton Trans. 2019, 48, 15856–15868. [Google Scholar] [CrossRef]
- Bäcker, D. Atomabsorptionsspektrometrie—Eine analytische Methode im Arzneibuch. PZ Prisma 2016, 23, 175–185. Available online: https://www.ingentaconnect.com/content/govi/pzpr/2016/00000023/00000003/art00007 (accessed on 4 August 2024).
- Welz, B.; Sperling, M. Atomabsorptionsspektrometrie, 4th ed.; Wiley-VHS: Weinheim, Germany, 1997; pp. 393–394. Available online: https://scholar.google.com/scholar_lookup?title=Atomabsorptionsspektrometrie&author=Welz,+B.&author=Sperling,+M.&publication_year=1997 (accessed on 4 August 2024).
- Acar, O. The use of chemical modifiers in electrothermal atomic absorption spectrometry. Appl. Spectrosc. Rev. 2024, 59, 340–354. [Google Scholar] [CrossRef]
- Scancar, J.; Milacic, R.; Falnoga, I.; Cemazar, M.; Bukovec, P. Use of nitric acid in sample pretreatment for determination of trace elements in various biological samples by ETAAS. J. Pharm. Biomed. Anal. 2000, 22, 993–1002. [Google Scholar] [CrossRef]
- Schlemmer, G.; Welz, B. Palladium and magnesium nitrates, a more universal modifier for graphite furnace atomic absorption spectrometry. Spectrochim. Acta Part. B At. Spectrosc. 1986, 41, 1157–1165. [Google Scholar] [CrossRef]
- Pasias, I.N.; Rousis, N.I.; Psoma, A.K.; Thomaidis, N.S. Simultaneous or Sequential Multi-element Graphite Furnace Atomic Absorption Spectrometry Techniques: Advances Within the Last 20 Years. At. Spectrosc. 2021, 42, 310–327. [Google Scholar] [CrossRef]
- Acar, O.; Özvatan, S.; Ilim, M. Determination of Cadmium, Copper, Iron, Manganese, Lead and Zinc in Lichens and Botanic Samples by Electrothermal and Flame Atomic Absorption Spectrometry. Turk. J. Chem. 2005, 29, 335–344. Available online: https://journals.tubitak.gov.tr/chem/vol29/iss4/1 (accessed on 4 August 2024).
- Dahl, K.; Thomassen, Y.; Martinsen, I.; Radziuk, B.; Salbu, B. Thermal stabilization of antimony in electrothermal atomic absorption spectrometry. J. Anal. At. Spectrom. 1994, 9, 1–5. [Google Scholar] [CrossRef]
- Baecker, D.; Guenther, S. General Applicability of High-Resolution Continuum-Source Graphite Furnace Molecular Absorption Spectrometry to the Quantification of Oligopeptides Using the Example of Glutathione. Analytica 2022, 3, 24–35. [Google Scholar] [CrossRef]
- Luna, A.S.; de Campos, R.C. Determination of Mn in Whole Blood and Urine by Graphite Furnace AAS Using Different Modifiers. At. Spectrosc. 1999, 20, 108–112. [Google Scholar]
- Almeida, J.S.; Brandao, G.C.; Dos Santos, G.L.; Teixeira, L.S.G. Fast sequential determination of manganese and chromium in vegetable oil and biodiesel samples by high-resolution continuum source graphite furnace atomic absorption spectrometry. Anal. Methods 2016, 8, 3249–3254. [Google Scholar] [CrossRef]
- Kostova, D.; Kanazirska, V.; Kamburova, M. A comparative analysis of different vegetable crops for content of manganese and molybdenum. Agron. Res. 2008, 6, 477–488. Available online: https://agronomy.emu.ee/vol062/p6205.pdf (accessed on 4 August 2024).
- Wu, S.; Chakrabarti, C.L.; Marcantonio, F.; Headrick, K.L. Mechanisms of atomization of molybdenum in graphite furnace atomic absorption spectrometry. Acta Part. B At. Spectrosc. 1986, 41, 651–667. [Google Scholar] [CrossRef]
- Belonoshko, A.B.; Simak, S.I.; Kochetov, A.E.; Johansson, B.; Burakovsky, L.; Preston, D.L. High-Pressure Melting of Molybdenum. Phys. Rev. Lett. 2004, 92, 195701. Available online: https://journals.aps.org/prl/abstract/10.1103/PhysRevLett.92.195701 (accessed on 4 August 2024). [CrossRef]
- De Babos, D.V.; Bechlin, M.A.; Barros, A.I.; Ferreira, E.C.; Neto, J.A.G.; De Oliveira, S.R. Cobalt internal standard for Ni to assist the simultaneous determination of Mo and Ni in plant materials by high-resolution continuum source graphite furnace atomic absorption spectrometry employing direct solid sample analysis. Talanta 2016, 152, 457–462. [Google Scholar] [CrossRef]
- Curtis, P.R.; Grusovin, J. Determination of molybdenum in plant tissue by graphite furnace atomic absorption spectrophotometry (GFAAS). Commun. Soil. Sci. Plan. 1985, 16, 1279–1291. [Google Scholar] [CrossRef]
- Hoenig, M.; Van Elsen, Y.; Van Cauter, R. Factors influencing the determination of molybdenum in plant samples by electrothermal atomic absorption spectrometry. Anal. Chem. 1986, 58, 777–780. [Google Scholar] [CrossRef]
- Pyrzynska, K. Determination of molybdenum in environmental samples. Anal. Chim. Acta 2007, 590, 40–48. [Google Scholar] [CrossRef]
- Ruiz, F.; Benzo, Z.; Quintal, M.; Garaboto, A.; Albornoz, A.; Brito, J.L. X-ray photoelectron spectroscopy study of pyrolytically coated graphite platforms submitted to simulated electrothermal atomic absorption spectrometry conditions. Appl. Surf. Sci. 2006, 252, 8695–8701. [Google Scholar] [CrossRef]
- Bhatla, S.C.; Lal, M.A. Plant Physiology, Development and Metabolism, 2nd ed.; Springer Nature: Singapore, 2023; pp. 25–49. [Google Scholar] [CrossRef]
- Xie, K.; Pan, Y.; Meng, X.; Wang, M.; Guo, S. Critical Leaf Magnesium Thresholds for Growth, Chlorophyll, Leaf Area, and Photosynthesis in Rice (Oryza sativa L.) and Cucumber (Cucumis sativus L.). Agronomy 2024, 14, 1508. [Google Scholar] [CrossRef]
- Chaudhry, A.H.; Hussain, S.B.; Du, W.; Liu, Y.; Peng, S.-A.; Deng, X.; Pan, Z. A novel bud mutant of navel orange (Citrus sinensis) shows tolerance to chlorosis in acidic and magnesium-deficient soils. Plant Physiol. Biochem. 2023, 196, 739–745. [Google Scholar] [CrossRef]
- De Mello Prado, R. Mineral Nutrition of Tropical Plants, 1st ed.; Springer: Cham, Switzerland, 2021; pp. 235–242. [Google Scholar] [CrossRef]
- Uikey, I.; Mitra, N.G.; Chhigarha, J. Effect of Molybdenum on Leaf Chlorophyll and Biomass Yield of Cowpea (Vigna unguiculata). Int. J. Soil. Sci. 2023, 35, 1992–1999. [Google Scholar] [CrossRef]
- Skorka, M.; Sieprawska, A.; Telk, A. The Implication of Manganese Surplus on Plant Cell Homeostasis: A Review. J. Plant Growth Regul. 2023, 42, 1327–1341. [Google Scholar] [CrossRef]
- Üstündag, Ü.; Macar, O.; Macar, T.K.; Yalcin, E.; Cavusoglu, K. Effect of Melissa officinalis L. leaf extract on manganese-induced cyto-genotoxicity on Allium cepa L. Sci. Rep. 2023, 13, 22110. [Google Scholar] [CrossRef]
- Bai, Y.; Zhou, Y.; Gong, J. Physiological mechanisms of the tolerance response to manganese stress exhibited by Pinus massoniana, a candidate plant for the phytoremediation of Mn-contaminated soil. Environ. Sci. Pollut. Res. Int. 2021, 28, 45422–45433. [Google Scholar] [CrossRef] [PubMed]
- Pan, G.; Zhang, H.; Liu, W.; Liu, P. Integrative study of subcellular distribution, chemical forms, and physiological responses for understanding manganese tolerance in the herb Macleaya cordata (papaveraceae). Ecotoxicol. Environ. Saf. 2019, 181, 455–462. [Google Scholar] [CrossRef]
- Hafeez, A.; Rasheed, R.; Ashraf, M.A.; Rizwan, M.; Ali, S. Effects of exogenous taurine on growth, photosynthesis, oxidative stress, antioxidant enzymes and nutrient accumulation by Trifolium alexandrinum plants under manganese stress. Chemosphere 2022, 308, 136523. [Google Scholar] [CrossRef] [PubMed]
- Santos, E.F.; Santini, J.M.K.; Paixao, A.P.; Junior, E.F.; Lavres, J.; Campos, M.; Dos Reis, A.R. Physiological highlights of manganese toxicity symptoms in soybean plants: Mn toxicity responses. Plant Physiol. Biochem. 2017, 113, 6–19. [Google Scholar] [CrossRef]
- Millaleo, R.; Reyes-Diaz, M.; Ivanov, A.G.; Mora, M.L.; Alberdi, M. Manganese as essential and toxic element for plants: Transport, accumulation and resistance mechanisms. J. Soil. Sci. Plant Nutr. 2010, 10, 470–481. [Google Scholar] [CrossRef]
- Li, J.; Jia, Y.; Dong, R.; Huang, R.; Liu, P.; Li, X.; Wang, Z.; Liu, G.; Chen, Z. Advances in the Mechanisms of Plant Tolerance to Manganese Toxicity. Int. J. Mol. Sci. 2019, 20, 5096. [Google Scholar] [CrossRef]
- Fernando, D.R.; Lynch, J.P. Manganese phytotoxicity: New light on an old problem. Ann. Bot. 2015, 116, 313–319. [Google Scholar] [CrossRef]
- Huang, H.; Zhao, Y.; Xu, Z.; Zhang, W.; Jiang, K. Physiological responses of Broussonetia papyrifera to manganese stress, a candidate plant for phytoremediation. Ecotox. Environ. Saf. 2019, 181, 18–25. [Google Scholar] [CrossRef]
- Jin, C. Plant vs. Metal: Manganese Uptake in Raphanus sativus Under Hydroponic Conditions. SSRN Electron. J. 2024, 1–11. [Google Scholar] [CrossRef]
- Yang, S.; Ling, G.; Li, Q.; Li, K.; Tang, X.; Zhang, M.; Li, X. Manganese toxicity-induced chlorosis in sugarcane seedlings involves inhibition of chlorophyll biosynthesis. Crop J. 2022, 10, 1674–1682. [Google Scholar] [CrossRef]
- Lawson-Wood, K.; Jaafar, M.; Felipe-Sotelo, M.; Ward, N.I. Investigation of the uptake of molybdenum by plants from Argentinean groundwater. Environ. Sci. Pollut. Res. Int. 2021, 28, 48929–48941. [Google Scholar] [CrossRef]
- Kaiser, B.N.; Gridley, K.L.; Brady, J.N.; Phillips, T.; Tyerman, S.D. The Role of Molybdenum in Agricultural Plant Production. Ann. Bot. 2005, 96, 745–754. [Google Scholar] [CrossRef] [PubMed]
- Mendel, R.R. The History of the Molybdenum Cofactor—A Personal View. Molecules 2022, 27, 4934. [Google Scholar] [CrossRef] [PubMed]
- Brychkova, G.; Xia, Z.; Yang, G.; Yesbergenova, Z.; Zhang, Z.; Davydov, O.; Fluhr, R.; Sagi, M. Sulfite oxidase protects plants against sulfur dioxide toxicity. Plant J. 2007, 50, 696–709. [Google Scholar] [CrossRef]
- Schwarz, G.; Mendel, R.R. Molybdenum cofactor biosynthesis and molybdenum enzymes. Annu. Rev. Plant Biol. 2006, 57, 623–647. [Google Scholar] [CrossRef]
- El-Jaoual, T.; Cox, D.A. Manganese toxicity in plants. J. Plant Nutr. 1998, 21, 353–386. [Google Scholar] [CrossRef]
- Fageria, N.K.; Stone, L.F. Micronutrient Deficiency Problems in South America, in Micronutrient Deficiencies in Global Crop Production. In Micronutrient Deficiencies in Global Crop Production; Alloway, B.J., Ed.; Springer: Dordrecht, The Netherlands, 2008; pp. 245–266. [Google Scholar] [CrossRef]
- Ma, F.; Wang, R.; Zhu, J.; Zhang, Y.; Wang, Y.; Hu, W.; Bell, A.E.; Liu, X. Characterisation comparison of polysaccharides from Dioscorea opposita Thunb. growing in sandy soil, loessial soil and continuous cropping. Int. J. Biol. Macromol. 2019, 126, 776–785. [Google Scholar] [CrossRef]


| Operation | Temperature (°C) | Heating Rate (°C s−1) | Holding Time (s) | Argon Flow |
|---|---|---|---|---|
| Drying | 90 | 10 | 10 | Maximal |
| Drying | 100 | 5 | 10 | Maximal |
| Drying | 120 | 5 | 15 | Maximal |
| Pyrolysis | 1300 | 150 | 15 | Maximal |
| Auto-zero | 1300 | 0 | 5 | Stop |
| Atomization | 1800 | 1500 | 5 | Stop |
| Cleaning | 2200 | 500 | 5 | Maximal |
| Operation | Temperature (°C) | Heating Rate (°C s−1) | Holding Time (s) | Argon Flow |
|---|---|---|---|---|
| Drying | 90 | 10 | 10 | Maximal |
| Drying | 100 | 5 | 10 | Maximal |
| Drying | 120 | 5 | 15 | Maximal |
| Pyrolysis | 400 | 50 | 20 | Maximal |
| Pyrolysis | 1900 | 500 | 10 | Maximal |
| Auto-zero | 1900 | 0 | 5 | Stop |
| Atomization | 2800 | 1500 | 5 | Stop |
| Cleaning | 2850 | 500 | 5 | Maximal |
| Parameter | Value |
|---|---|
| Linear working range | 2–20 µg L−1 |
| Correlation coefficient (R2) | 0.9995 |
| Characteristic mass (m0) | 0.67 ± 0.02 pg |
| Limit of detection (LOD) | 0.67 µg L−1 |
| Limit of quantification (LOQ) | 1.83 µg L−1 |
| Recovery/Precision (2 µg L−1) | 98.5%/1.9% |
| Parameter | Value |
|---|---|
| Linear working range | 10–100 µg L−1 |
| Correlation coefficient (R2) | 0.9909 |
| Characteristic mass (m0) | 21.4 ± 3.4 pg |
| Limit of detection (LOD) | 9.94 µg L−1 |
| Limit of quantification (LOQ) | 37.64 µg L−1 |
| Recovery/Precision (40 µg L−1) | 100.7%/3.8% |
| Coloration of Leaves | Extract | Manganese Content (mg kg−1) | Mean (mg kg−1) | Molybdenum Content (mg kg−1) | Mean (mg kg−1) |
|---|---|---|---|---|---|
| Normal leaves | NL-1 | 39.09 | 30.85 | 26.59 | 18.41 |
| NL-2 | 45.25 | 12.35 | |||
| NL-3 | 10.62 | 8.84 | |||
| NL-4 | 28.46 | 25.84 | |||
| Discolored Leaves | DL-1 | 33.39 | 37.27 | 14.29 | 15.87 |
| DL-2 | 66.47 | 13.08 | |||
| DL-3 | 35.48 | 20.77 | |||
| DL-4 | 13.74 | 15.35 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Krüger, D.; Weng, A.; Baecker, D. Investigating the Discoloration of Leaves of Dioscorea polystachya Using Developed Atomic Absorption Spectrometry Methods for Manganese and Molybdenum. Molecules 2024, 29, 3975. https://doi.org/10.3390/molecules29163975
Krüger D, Weng A, Baecker D. Investigating the Discoloration of Leaves of Dioscorea polystachya Using Developed Atomic Absorption Spectrometry Methods for Manganese and Molybdenum. Molecules. 2024; 29(16):3975. https://doi.org/10.3390/molecules29163975
Chicago/Turabian StyleKrüger, David, Alexander Weng, and Daniel Baecker. 2024. "Investigating the Discoloration of Leaves of Dioscorea polystachya Using Developed Atomic Absorption Spectrometry Methods for Manganese and Molybdenum" Molecules 29, no. 16: 3975. https://doi.org/10.3390/molecules29163975
APA StyleKrüger, D., Weng, A., & Baecker, D. (2024). Investigating the Discoloration of Leaves of Dioscorea polystachya Using Developed Atomic Absorption Spectrometry Methods for Manganese and Molybdenum. Molecules, 29(16), 3975. https://doi.org/10.3390/molecules29163975






