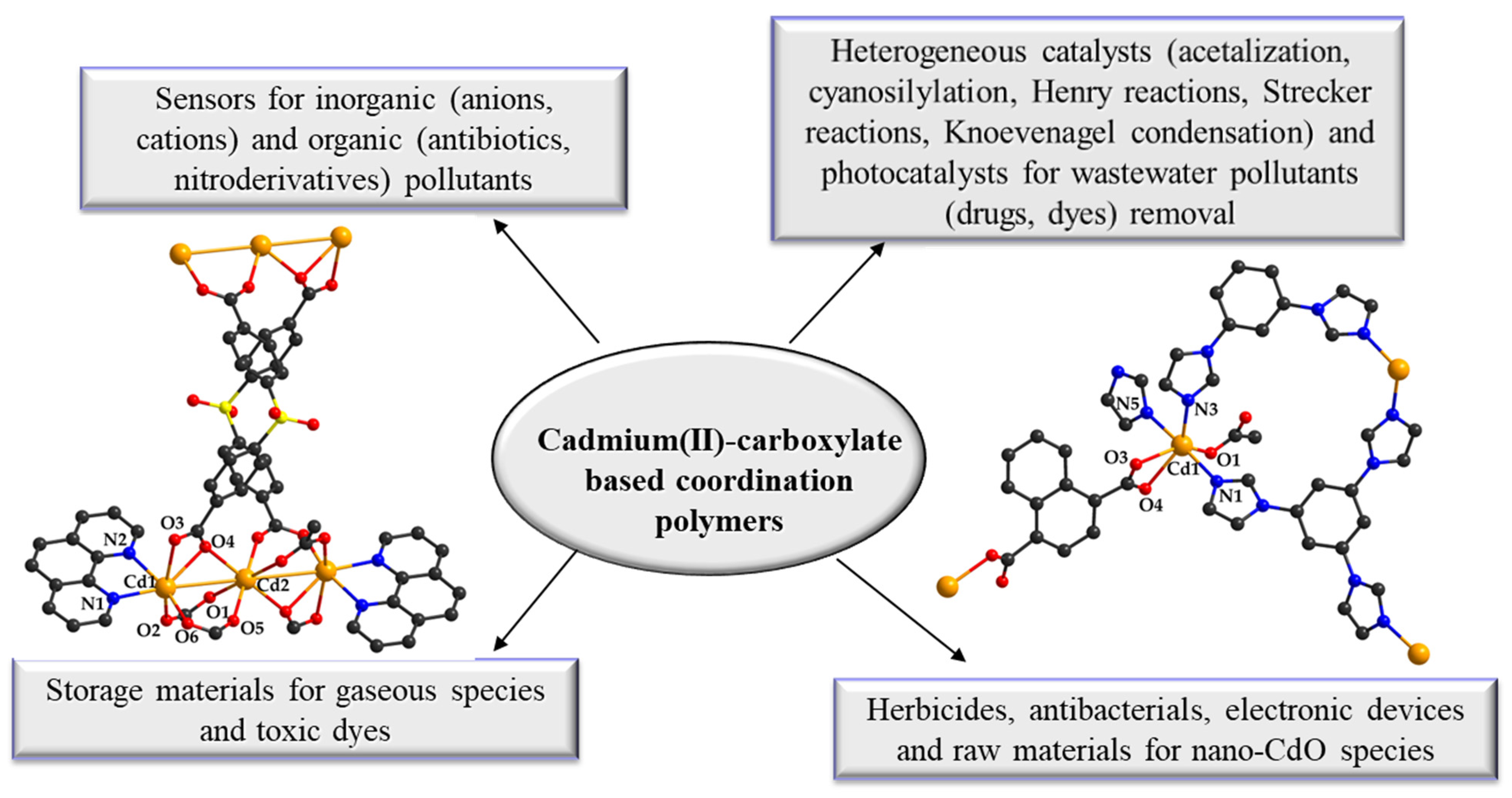
An Overview of Various Applications of Cadmium Carboxylate Coordination Polymers
Abstract
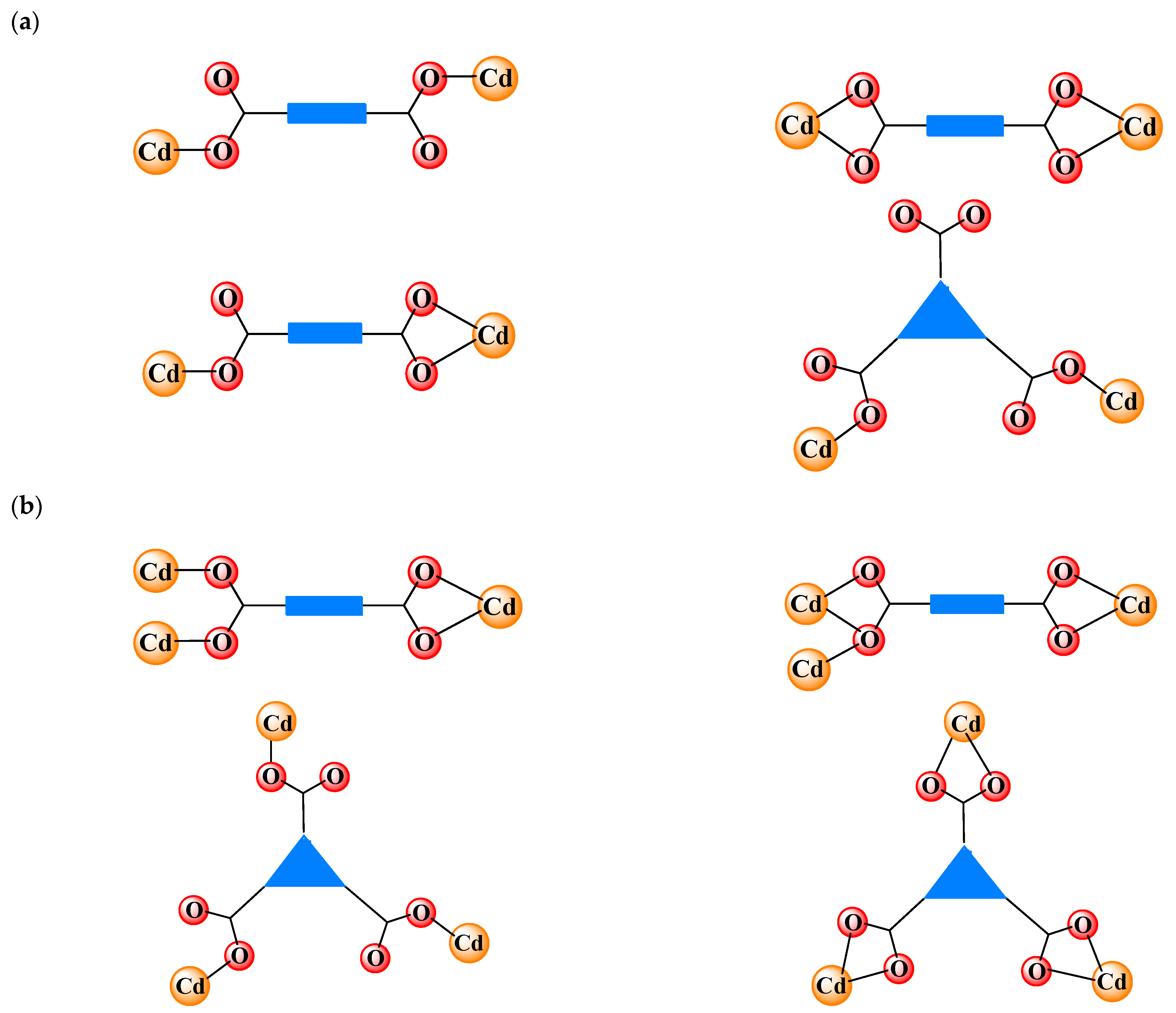
1. Introduction
2. Basic Applications of Cadmium (II) Carboxylate Coordination Polymers
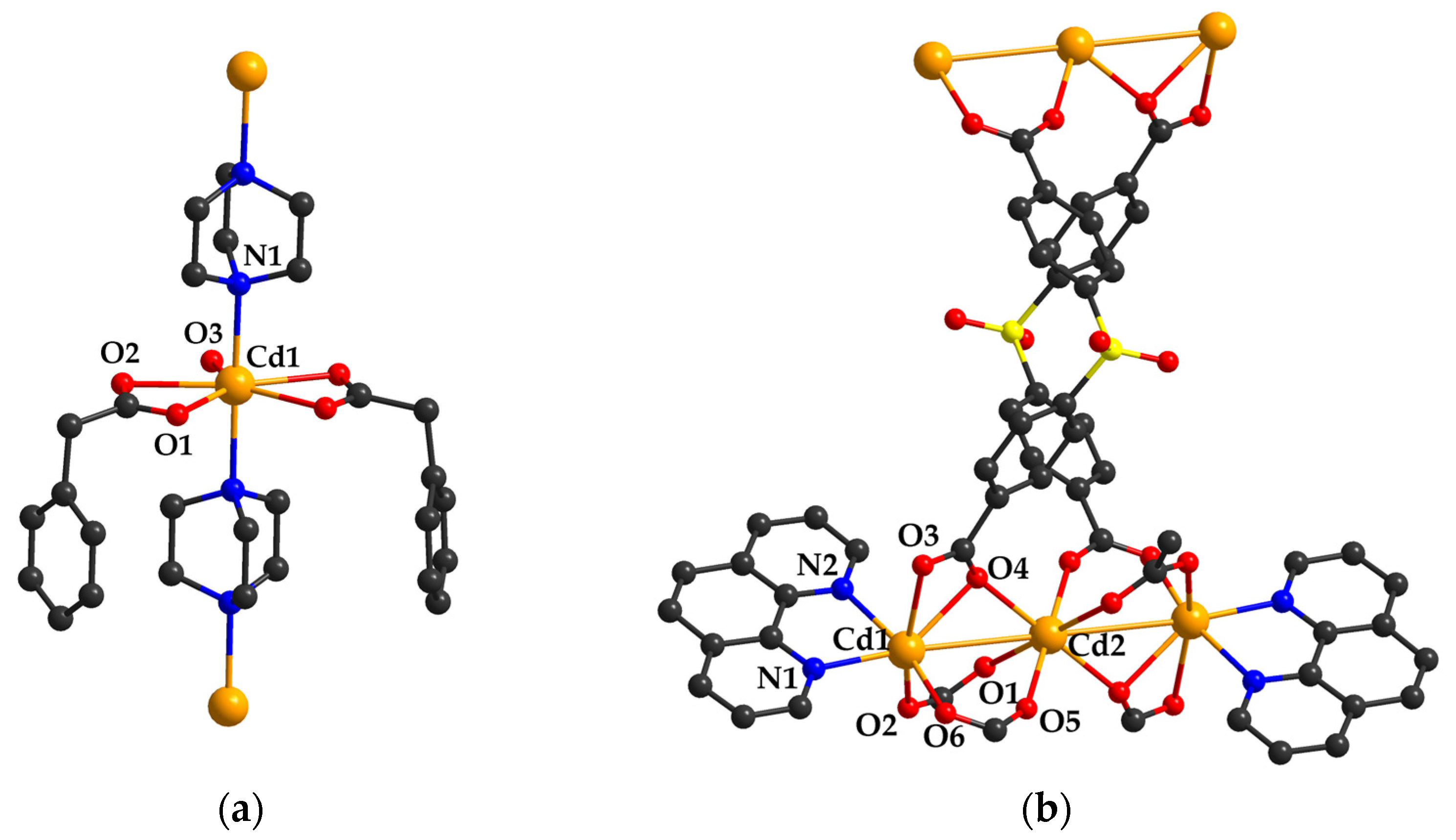
2.1. Sensors Based on Cadmium (II) Carboxylate Coordination Polymers

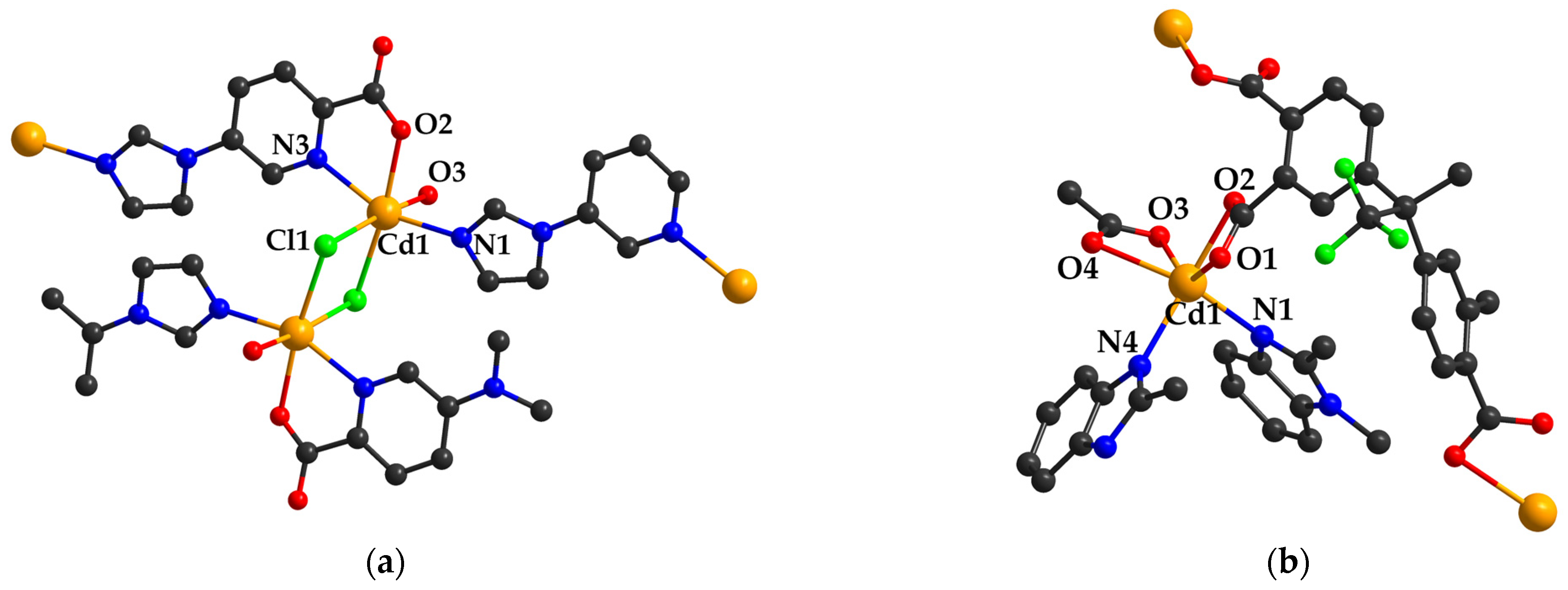
2.2. Catalysts Based on Cadmium (II) Carboxylate Coordination Polymers
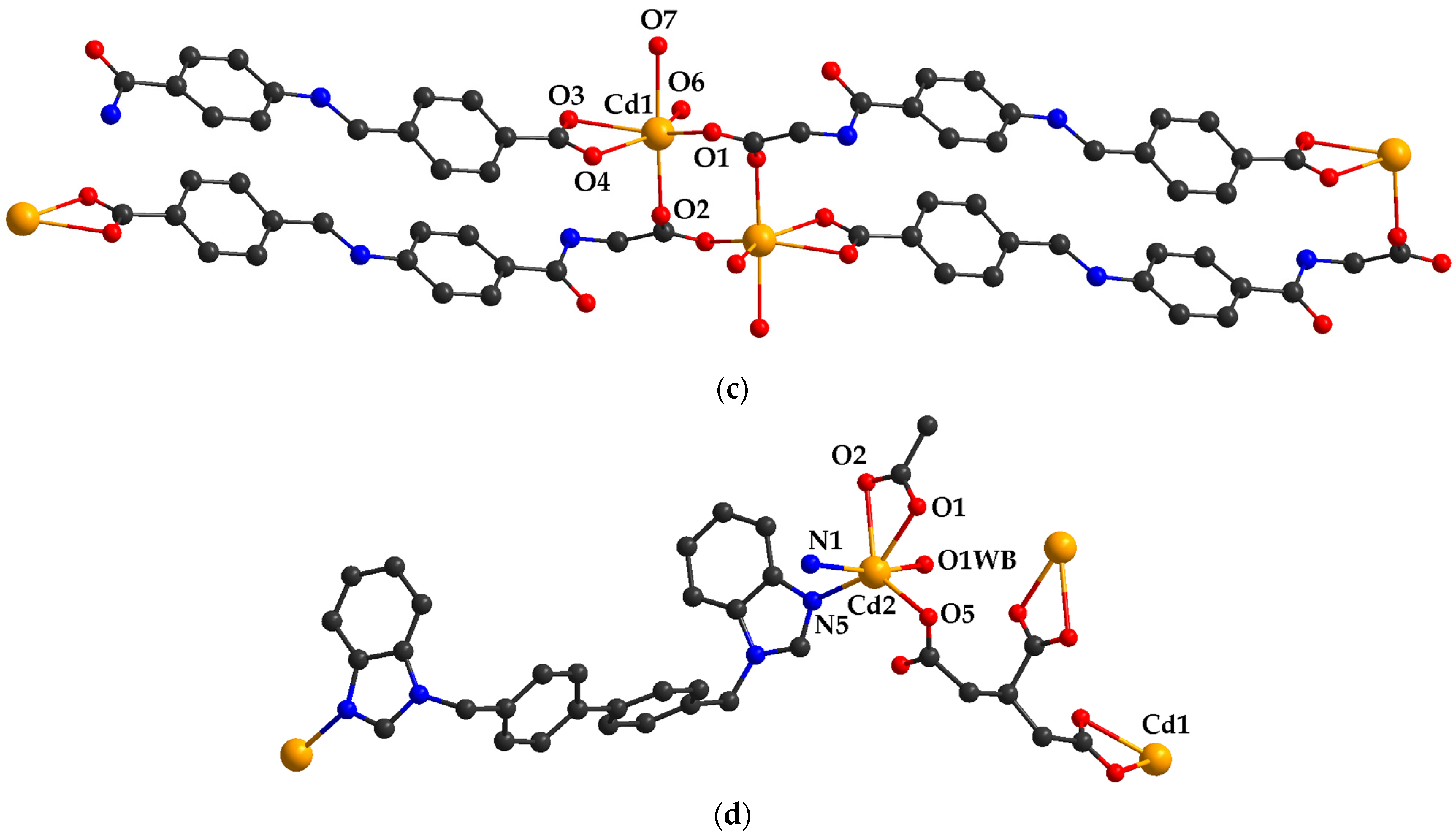
2.3. Adsorbent Materials Based on Porous Cadmium (II) Carboxylate Coordination Polymers
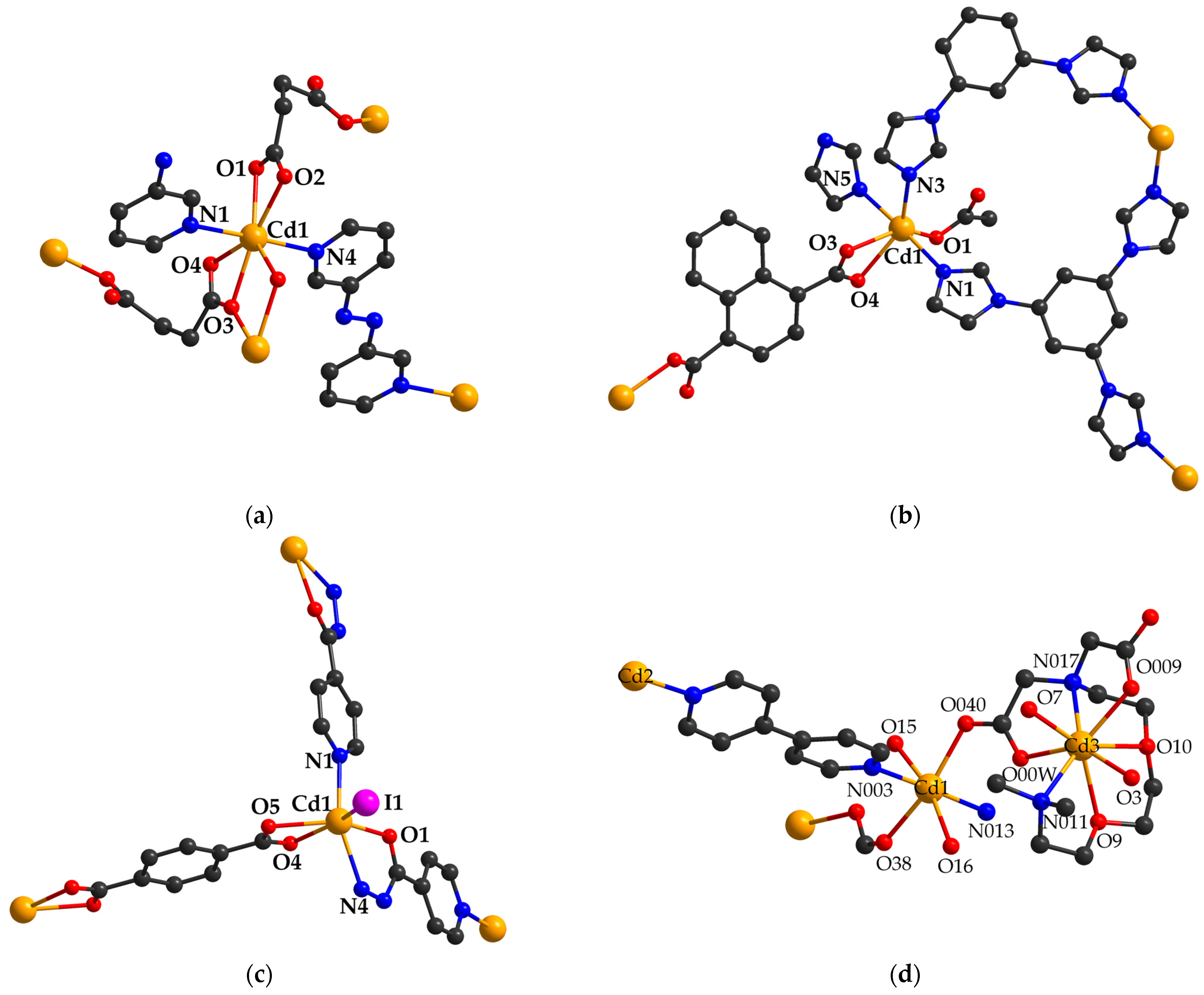
2.4. Cadmium (II) Carboxylate Coordination Polymers with Miscellaneous Applications
| Complex | Carboxylate Linker (Bridge Type)/Ancillary Ligand | Synthesis Method/ Characteristics | Application | Ref. |
|---|---|---|---|---|
| Species developed as sensors | ||||
| [Cd(bpmta)0.5(1,2-bdc)(H2O)]n (1) | 1,2-benzenedicarboxylate (μ2)/N,N′-bis(pyridin-3-ylmethyl)-terephthalamide) | Hydrothermal/EW a 335 nm | Fe(III), CrO42−, Cr2O72− and 2,6-DC-4-NA detection in mM limits | [45] |
| [Cd3(bpy)3(cia)2]n (2) | 5-((4-carboxybenzyl) oxy) isophthalate (μ3)/2,2′-bipyridine | Hydrothermal/EW 308 nm | NBZ detection in μM limits | [46] |
| {[Cd(H4pbitb)]⋅2DMF⋅8H2O}n (3) | 4,4′,4″,4‴-(1,4-phenylenebis (1H-imidazole-2,4,5- triyl))tetrabenzoic acid (μ4) | Solvothermal/EW 361 nm | Fe(III), MnO4− and TNP detection in μM limits | [47] |
| {[Cd2(1,3-bdc)2(H2O)4(hdn)]2H2O}n (4) | 1,3-benzenedicarboxylate (μ2)/N,N′-(hexane-1,6-diyl)dinicotinamide | ydrothermal/EW 400 nm | NBZ detection in mM limits | [48] |
| {[Cd(mbdc)(hdn)]H2O}n (5) | 5-methy-1,3-benzenedicarboxylate (μ2)/N,N′-(hexane-1,6-diyl)dinicotinamide | hHydrothermal/EW 400 nm | NBZ detection in mM limits | [48] |
| [Cd(bzimip)(DMF)]n (6) | 5-(benzimidazole-1-yl)isophthalic acid (μ2)/DMF | Solvothermal | STZ, NFZ, and SDZ detection in μM limits | [49] |
| [Cd4(Hbdcpb)2(H2O)5]n (7) | 2,3-bis(3,5-dicarboxylphenxoy)benzoate (μ7)/water | Solvothermal | SDZ detection in μM limits | [50] |
| {[Cd2(Hbdcpb)(2,2′-bpy)2(H2O)]·6H2O}n (8) | 2,3-bis(3,5-dicarboxylphenxoy)benzoate (μ5)/2,2′-bipyridine | Hydrothermal | NFT detection | [50] |
| [Cd4(2,2′-bpy)4(Hddpb)2·H2O]n (9) | 2,5ʹ-di(3,5-dicarboylphenoxy)benzoic acid (μ6)/2,2′-bipyridine | Hydrothermal/EW 330, 390 nm | NFZ detection in μM limits | [51] |
| {[Cd2(btdb)2(4,4-bpy)]·DMF}n (10) | 4,4′-(benzo[c][1,2,5]thiadiazole-4,7-diyl)dibenzoic acid (μ2)/4,4′-bipyridine | Solvothermal | L-His | [52] |
| [Cd(cphi)(Hbpz)]n (11) | 5-(4-carboxyphenoxy)isophtalate (μ2)/3,3′5,5′-tetramethyl-4,4′-bipyrazole | Solvothermal/EW 300 nm | Fe(III) detection in μM limits | [53] |
| [Cd2(dtta)2]n (12) | 2,5-di(1H-1,2,4-triazol-1-yl)terephthalate (μ6) | Solvothermal/EW 300 nm | Cu(II) detection in mM limits | [54] |
| [Cd(3,3′-dmg)(dpam)]n (13) | Dimethyl glutarate (μ2)/4,4′-dipyridylamine | Hydrothermal/EW 300, 390 nm | NBZ detection in mM limits | [55] |
| [Cd(4-tkpvb)(5-tert-bipa)]n (14) | 5-tert-butylisophthalate (μ2)/1,2,4,5-tetrakis(4-pyridylvinyl)benzene | Solvothermal/EW 380 nm | Hg(II), CrO42−, and Cr2O72− detection in Ag(I); Al(III) and Cr(III) detection in μM limits | [56] |
| [Cd(dpttz)(oba)]n (15) | 4,4′-oxybis(benzoate) (μ3)/2,5-di(pyridine-4-yl)thiazolo [5,4-d]thiazole | Solvothermal/EW 380 nm | 4-NA and CrO42− detection in Ag(I); Al(III) and Cr(III) detection in μM limits | [57] |
| [Cd3(btc)2(phen)3(H2O)2]n (16) | 1,3,5-benzentricarboxylate (μ2)/1,10-phenantroline, water | Hydrothermal/EW 425 nm | Cu(II) and Ni(II) ion detection in Ag(I), Al(III) and Cr(III) detection in μM limits without interferences | [58] |
| [Cd3(cpota)2(phen)3]n·5nH2O (17) | 2-(4-carboxyphenoxy)terephthalate (μ5)/1,10-phenanthroline | Hydrothermal/EW 290 nm | volatile organic ketones and (CrO42−/Cr2O72−) detection in Ag(I); Al(III) and Cr(III) detection in μM limits | [59] |
| {[Cd2(edda)(phen)2]∙H2O}n (18) | 5,5′(ethane-1,2-diylbis(oxy)) diisophthalate (μ6)/1,10-phenanthroline | Hydrothermal/EW 310 nm | MnO4− and Cr(VI) ions in μM, AA in nM, and Hacac in μM limits | [60] |
| [Cd3(dcpb)2(datrz)(H2O)3]n (19) | 4-(2′,3′-dicarboxylphenoxy)benzoate (μ5 + μ6)/3,5-diamino-1,2,4-triazole | Hydrothermal/EW 390 nm | ClO− and Hacac detection in μM limits | [61] |
| [Cd(bibt)(3,4-tdc)]n (20) | 3,4-thiophenedicarboxylate/4,7-bi(1H-imidazol-1-yl)benzo-[2,1,3]thiadiazole | Solvothermal | SA detection in μM limits | [62] |
| {[Cd2(ddb)(Hbimb)]∙3H2O}n (21) | 3,5-di(2′,4′-dicarboxylphenyl)benzoate (μ2 + μ7)/ortho-bis(imidazole-1-ylmethyl)benzene | Solvothermal | NF selective detection in μM limits | [63] |
| {[Cd(1,2-pda)(tib)]·H2O}n (22) | 1,2-phenylenediacetate (μ2)/1,3,5-tris(1-imidazolyl)benzene | Hydrothermal/EW 280 nm, BET b 171.72 m2 g−1 | TEC detection in μM limits | [64] |
| {[Cd4(1,3-pda)4(tib)2(dib)2]·7H2O}n (23) | 1,3-phenylenediacetate (μ2)/1,3,5-tris(1-imidazolyl)benzene; 1,3-di(1-imidazolyl)benzene | Hydrothermal/EW 333 nm | TEC detection in μM limits | [64] |
| {[Cd(1,4-pda)(tib)]·4H2O}n (24) | 1,4-phenylenediacetate (μ2)/1,3,5-tris(1-imidazolyl)benzene | Hydrothermal/EW 333 nm | TEC detection in μM limits | [64] |
| [Cd(tdc)(tbb)]n (25) | 2,5-thiophenedicarboxylate (μ2)/1,4-bis(thiabendazole-1-yl)-2-butene | Hydrothermal/EW 345 nm | Fe(III), Hacac, and NOR detection in μM limits | [65] |
| {[Cd(tpca)(tbb)]·H2O}n (26) | 2,3,5,6-tetrabromoterephtalate (μ2)/1,4-bis(thiabendazole-1-yl)-2-butene | Hydrothermal/EW 345 nm | Fe(III), Hacac, and NOR detection in μM limits | [65] |
| [Cd3(bptc)2(H2O)4]n (27) | Biphenyl-2,4′,5-tricarboxylate (μ2)/water | hydrothermal | Fe(III) and acetone detection | [66] |
| {[Cd(dint)(1,4-bdc)(H2O)]·ACN}n (28) | 1,4-benzenedicarboxylate (μ2)/1,4-di(imidazol-1-yl)naphthalene | Solvothermal/EW of 230 and 290 nm | Vitamin B2 detection in mM limits, nitenpyram and imidacloprid detection in μM limits | [67] |
| [Cd(pddb)H2O]n (29) | 4,4′-(pyridine-2,6-diyl)-dibenzoic acid (μ4)/water | Hydrothermal/EW 340 nm | MEAA detection in μM limits | [68] |
| {Cd3(btc)2(btd-bpy)2]·1.5MeOH·4H2O}n (30) | Benzene-1,3,5-tricarboxylate (μ5)/bis(pyridin-4-yl)benzothiadiazole | Hydrothermal/EW 320, 335 nm | Ag(I), Al(III) and Cr(III) detection in μM limits | [69] |
| [Cd2(1,4-ndc)2(btd-bpy)2]n (31) | Naphthalene-1,4-dicarboxylate (μ3)/bis(pyridin-4-yl)benzothiadiazole | Solvothermal/EW 335, 365 nm | Ag(I), Al(III) and Cr(III) detection in μM limits | [69] |
| Species developed as catalysts | ||||
| [Cd(ipc)(Cl)(H2O)]n (32) | 5-imidazol-1-yl-2-pyridine carboxylate (μ2)/chloride, water | Hydrothermal/four RR c | Catalyst for acetalization reaction | [71] |
| {[Cd(cbdcp)(H2O)4]∙(H2O)}n (33) | 1-(3,5-dicarboxybenzyl)-4,4′-bipyridinium ion (μ2)/water | Hydrothermal/five RR | Catalyst for cyanosilylation reaction | [72] |
| [Cd2(1,4-ndc)2(DMF)2]n (34) | 1,4-naphtalenedicarboxylate (μ4)/N,N′-dimethylformamide | Solvothermal | Catalyst for cyanosilylation reaction of aromatic aldehydes | [73] |
| [Cd(3,3′-dbdc)(2,2′-dipy)(H2O)]n (35) | 3,3′-dihydroxy-(1,1′-biphenyl)-4,4′-dicarboxylate (μ2)/2,2′-dipyridine | Hydrothermal | Catalyst for Henry reaction | [74] |
| [Cd(4,4′-dbdc)(phen)]n (36) | 4,4′-dihydroxy-(1,1′-biphenyl)-3,3′-dicarboxylate (μ3)/1,10-phenantroline | Hydrothermal | Catalyst for Henry reaction | [74] |
| [Cd(bdc-OH)(DMF)2·DMF]n (37) | 2-hydroxyterephtalate (μ2)/N,N′-dimethylformamide | Solvothermal/four RR | Heterogeneous catalyst for Knoevenagel condensation | [77] |
| {(H2O)2[Cd3(2,7-cdc)4]∙3DMF∙4H2O}n (38) | 9H-carbazole-2,7-dicarboxylate (μ2 + μ3 + μ4) | Solvothermal/two RR | Catalyst for Knoevenagel condensation | [78] |
| {[Cd3(bidc)2(DMF)2(H2O)2]·H2O}n (39) | 1,3-bisbenzyl-2-imidazolidine-4,5-dicarboxylate (μ3)/N,N′-dimethylformamide, water | One pot at heating | Catalyst for aldehyde coupling reaction | [79] |
| [Cd(paip)(NMF)2]n (40) | 5-{pyren-1-ylmethyl)amino}isophtalate (μ3)/N-methylformamide | Solvothermal/four RR | Catalysts for solvent-free microwave-assisted cyanation of acetals | [80] |
| {[Cd(aaip)(DMF)(H2O)2]·H2O}n (41) | 5-{anthracen-9-ylmethyl)amino}isophtalate (μ2)/N,N′-dimethylformamide | Solvothermal/four RR | atalysts for solvent-free microwave-assisted cyanation of acetals | [80] |
| {[Cd(hipamifba)(H2O)2∙2H2O}n (42) | 4-(((4-((carboxymethyl)carbamoyl)-phenyl)amino)methyl)benzoate (μ2)/water | One pot at rt by stirring/five RR | hHeterogeneous catalyst for Strecker reaction | [81] |
| {[Cd3(pyzdca)6(H2O)4]·8H2O}n (43) | Pyrazine-2-carboxylate (μ2)/water | One pot at rt by stirring/active in presence of H2O2 | Catalyst for degradation of AB-92 | [83] |
| [Cd(dctp)(bix)]n (44) | 2,5-dichloroterephtalate (μ2)/1,4-bis(imidazol-1-ylmethyl)benzene | Hydrothermal and sonochemical/EW 365 nm | Photocatalytic activity for degradation of MB under UV irradiation | [84] |
| [Cd(bpdc)(bix)2]n (45) | Biphenyl-4,4′-dicarboxylate (μ2)/1,4-bis(imidazol-1-ylmethyl)benzene | Hydrothermal and sonochemical/UV irradiation | Photocatalytic activity for degradation of MB under UV irradiation | [84] |
| [Cd2(hfpd)(mbp)2]n (46) | 4,4′-(hexafluoroisopropylidene)diphtalate (μ4)/1,5-bis(2-methylbenzimidazol-1-yl)pentane | Hydrothermal and sonochemical/EW 365 nm | Photocatalytic activity for degradation of MB under UV irradiation | [85] |
| {[Cd2(btc)(bmi)2]·4H2O·DMF}n (47) | 1,2,4,5-benzen-tetracarboxylate (μ4)/1,5-bis(2-Methylimidazolil-1-yl)pentane, water, N,N′-dimethylformamide | Solvothermal/EW 365 nm | Photocatalyst for MV degradation | [86] |
| [Cd(1,2-bdc)(bip)(H2O)]n (48) | 1,2-benzen-dicarboxylate (μ2)/1,3-bis(2-methyl-imidazol-1-yl)propane, water | Solvothermal/EW 365 nm | Photocatalyst for degradation of MV and RhB | [87] |
| [Cd2(tca)(htca)0.5(bbmb)2(H2O)]n (49) | Tricarballylic acid anion (μ2+μ3)/4,4′-bis(benzimidazol-1-ylmethyl)biphenyl | Hydrothermal/active in presence of H2O2 | Catalyst for degradation of MO in Fenton-like process | [88] |
| {[Cd2(suc)2(bbmb)2(H2O)]∙H2O}n (50) | Succinate (μ2)/4,4′-bis(benzimidazol-1-ylmethyl)biphenyl, water | Hydrothermal/active in presence of H2O2 | Catalyst for degradation of MO in Fenton-like process | [88] |
| [Cd2(4-cpa)4(bip)2]n (51) | 4-chlorophenylacetate/1,3-bis(2-methyl-imidazol-1-yl)propane | Hydrothermal/active in presence of Na2S2O8 | Catalyst for degradation of MO in Fenton-like process | [89] |
| {[Cd(Hipa)(Hiz)(H2O)2]⋅3H2O}n (52) | 5-hydroxy isophthalic acid (μ2)/imidazole | Solvothermal | Photocatalysts in MB, MO, and CV degradation | [90] |
| Species developed as adsorbent materials | ||||
| {[Cd(1,3-bdc)(bc)(H2O)]∙2H2O}n (53) | 1,3-benzendicarboxylate (μ2)/1,1′-(4-nitro-1,3-phenylene)bis(1H-benzo[d]imidazole, water | Solvothermal/type I gas sorption isotherm | Adsorption of CO2 | [92] |
| {[Cd(suc)(3,3′-azbpy)]∙(MeOH)}n (54) | Succinate (μ3)/3,3-azobispyridene | Slow diffusion/surface adsorption | Adsorption of CO2 | [93] |
| [Cd(msuc)(3,3′-azbpy)]n (55) | Methyl succinate (μ3)/3,3-azobispyridene | Slow diffusion/surface adsorption | Adsorption of CO2 | [93] |
| {[Cd(2,2′-dmglut)(3,3′-azbpy)(H2O)]∙2H2O}n (56) | 2,2′-dimethylglutarate (μ2)/3,3-azobispyridene, water | Slow diffusion/surface adsorption | Adsorption of CO2 | [93] |
| [Cd(ndc)0.5(pca)]n (57) | 2,6-naphtalenedicarboxylate (μ2)/4-pyridincarboxylate | Solvothermal/type I gas sorption isotherm | Adsorption of CO2 | [94] |
| {[Cd(Hbtc)(bpp)]∙1.5DMF∙2H2O}n (58) | 1,3,5-benzentricarboxylate (μ2)/1,3-bis(4-pyridyl)propane | Solvothermal/surface adsorption | Adsorption of CO2 | [95] |
| {[Cd(1,4-ndc)(tib)]∙3H2O}n (59) | 1,4-naphtalenedicarboxylate (μ2)/1,3,5-tris(1-imidazolyl)benzene | Solvothermal/BET 421.05 m2 g−1 | Adsorption of CO2 | [96] |
| {[Cd(bpydb)]·6H2O}n (60) | 4,4′-(4,4′-bipyridine-2,6-diyl) dibenzoate (μ2) | Solvothermal/BET 346 m2 g−1, reversible type I gas sorption isotherm for N2 and H2 | Adsorption of N2, H2, and CH4 | [97] |
| {[Cd4(bcpbp)3Cl6][CdCl4]}n (61) | 1,1′-bis(4-carboxyphenyl)-4,4′-bipyridinium (μ2)/chloride | Solvothermal/complex shape of gas sorption isotherms | Adsorption of CO2, MeOH, H2O, and NH3 | [98] |
| {[Cd2I2(1,4-bdc)2(inh)2]∙2DMF·H2O)}n (62) | 1,4-benzendicarboxylate (μ2)/isoniazid, iodine | Slow diffusion/BET 611 m2 g−1 | Adsorption of I2, and N2 | [99] |
| {(Me2NH2)3[Cd5(atnc)6]·18DMA·2H2O}n (63) | 1-amino-2,4,6-tris(5-naphtalenecarboxylate) (μ6 + μ7) | Hydrothermal/BET 1193 m2 g−1, type I gas sorbtion isoterm | Adsorption of C2/C1 hydrocarbons and C2/CO2 separation | [100] |
| {[Cd(bdc-NO2)(bmip)]·3DMF}n (64) | 2-nitro-1,4-benzenedicarboxylic acid (μ2)/bis(2-methylimidazolyl)propane | Solvothermal/BET of 103 m2·g−1 | Adsorption of ethylene, benzene, cyclohexane, lower alcohols (methanol, ethanol, isopropyl alcohol) | [101] |
| {[Cd(bdc-Br)(bmip)]·3DMF}n (65) | 2-brom-1,4-benzenedicarboxylic acid (μ2)/bis(2-methylimidazolyl)propane | Solvothermal/BET of 283 m2 g−1 | Adsorption of ethylene, benzene, cyclohexane, lower alcohols (methanol, ethanol, isopropyl alcohol) | [101] |
| [Cd(btca)(ppz)]n (66) | 1,2,3,4-benzentetracarboxylate (μ4)/piperazine | One pot at rt by stirring/BET of 5.45 m2 g−1 | Adsorption of CSB and MB dyes | [102] |
| [Cd(pda)(abpy)0.5(H2O)]n (67) | 1,2-phenylenediacetate (μ4)/4,4′-azobis(pyridine), water | Hydrothermal | adsorption of MB | [103] |
| {[Cd4(CH3COO)(μ-OH)4]·C2H5OH}n (68) | Acetate (μ2)/hydroxide | Solvothermal/type I gas sorption isotherm | Adsorption of MB and MO dyes | [104] |
| {[Cd5(egta)2(4,4′-bipy)4(H2O)4](NO3)2·4,4′-bpy·8H2O}n (69) | Ethylene bis(oxyethylenenitrilo) tetracetate (μ2)/4,4′-bipyridine, water | Solvothermal/ | Adsorption of MO and CR dyes | [105] |
| {[Cd3(egta)(1,2-dpe)1.5(H2O)3](NO3)2·6H2O}n (70) | Ethylene bis(oxyethylenenitrilo) tetracetate (μ2 + μ4)/1,2-di(4-pyridyl)ethane, water | Solvothermal/ | Adsorption of MO and CR dyes | [105] |
| {[Cd2(egta)(dpe)(H2O)]·4H2O}n (71) | Ethylene bis(oxyethylenenitrilo) tetracetate (μ2)/dpe = 1,2-di(4-pyridyl)ethylene, water | Solvothermal/ | Adsorption of MO and CR dyes | [105] |
| [Cd(amoip)(FA)2]n (72) | 5-{(anthracen-9-ylmethyl)amino}isophthalic acid (μ3)/formamide | Hydrothermal/three RR, Langmuir isotherm | Adsorption of CR, MB, MV, RhB, and Rh6G | [106] |
| [Cd2(pmoip)2(MeOH)2]n (73) | 5-{(pyren-1-ylmethyl)amino}isophthalic acid (μ4)/methanol | Hydrothermal/three RR, Langmuir isotherm | Adsorption of CR, MB, MV, RhB, and Rh6G | [106] |
| [Cd3(dpa)(HCO2)(bpp)3]n (74) | Diphenic acid dianion (μ4)/formate, 1,3-bis(4-pyridyl)propane | Hydrothermal/Langmuir isotherm | Adsorbtion of Cr2O72− anion | [107] |
| Species with miscellaneous applications | ||||
| {[Cd(4-cpha)(bpp)2(H2O)]∙(4-cpha)∙(H2O)}n (75) | 4-chlorophenoxyacetate (μ2)/1,3-bis(4-pyridyl)propane, water | Hydrothermal | Phytogrowth-inhibitory activity against Brassica campestris L., Echinochloa utilis Ohwi et Yabuno | [108] |
| [Cd(5-meo-ip)(bip)]n (76) | 5-methoxyisophtalate (μ3)/1,3-bis(2-methyl-imidazol-1-yl)propane | Hydrothermal | Uterine fibroid treatment | [109] |
| [Cd(phac)2(dabco)(H2O)]n (77) | Phenylacetate (μ2)/1,4-diazabicyclo [2.2.2]octane | Solvothermal | Antibacterial activity against S. aureus and E. coli | [110] |
| {[Cd3(sba)2(phen)2(CH3COO)2]∙2DMA}n (78) | 4,4′-sulfonyldibenzoate (μ4)/1,10-phenantroline, acetate | Solvothermal/dielectric constant 20.0 (1 kHz) | Gate dielectrics in electronic devices | [111] |
| {[Cd(fba)(phen)]∙DMA}n (79) | 4,4′-(hexafluoroisopropylidene)bisbenzoate (μ3)/1,10-phenantroline | Solvothermal/dielectric constant 32.0 (1 kHz) | Gate dielectrics in electronic devices | [111] |
| {[Cd(cbia)2(H2O)4]∙2H2O}n (80) | 2-(1-carboxymethyl)-1H-benzo[d]imidazol-3-ium-3-yl)acetate (μ3)/water | Solvothermal/proton conductivity 5.09 × 10−3 S cm−1 | Material with high proton conductivity | [112] |
| {[Cd2(cbia)2(4,4′-bipy)2(H2O)2]∙(cbia)(OH)∙2H2O}n (81) | 2-(1-carboxymethyl)-1H-benzo[d]imidazol-3-ium-3-yl)acetate (μ2)/4,4′-bipyridine, water | Solvothermal/proton conductivity 3.41 × 10−3 S cm−1 | Material with high proton conductivity | [112] |
| [Cd(3-pbi)(DMF)]n (82) | 5-(3-pyridin-3-yl)benzamido)isophtalate (μ2)/N,N′-dimethylformamide | Solvothermal | hHost for lithium-selenium batteries | [113] |
| [Cd2(ptpy)2.5(atc)4]n (83) | Anthracene-9-carboxylic acid/4′-(4-pyridyl)-2,2′:6′,2″-terpyridine (ptpy) | Sonochemical | OLED fabrications | [114] |
| {[Cd(CH3COO)2(pip)2]∙H2O}n (84) | Acetate (μ2)/2-phenylimidazo [4,5-f][1,10]phenantroline | Solvothermal | loculant for CR | [115] |
| [Cd(4-pyc)2(H2O)4]n (85) | 4-pyridincarboxylate anion (μ2)/water | Hydrothermal/average particle diameter 77 nm | Raw material for CdO nanoparticles | [117] |
| {[Cd3(3-pyc)4(N3)2(H2O)]n (86) | Pyridine-3-carboxylate (μ2)/azido, water | Sonochemical/average particle diameter 780 nm | Raw material for CdO nanoparticles | [118] |
3. Conclusions
4. Further Perspectives
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| AA | Ascorbic acid |
| H2aaip | 5-{anthracen-9-ylmethyl)amino}isophtalic acid |
| AB-92 | Acid blue 92 dye |
| abpy | 4,4′-azobis(pyridine) |
| Hacac | Acetylacetone |
| ACN | Acetonitrile |
| H2amoip | 5-{(anthracen-9-ylmethyl)amino}isophthalic acid |
| Hatc | Anthracene-9-carboxylic acid |
| 3,3′-azbpy | 3,3′-azobispyridene |
| bbi | 1,4-bis(imidazol-1-yl)butane |
| bbmb | 4,4′-bis(benzimidazol-1-ylmethyl)biphenyl |
| bmip | Bis(2-methylimidazolyl)propane |
| H2bdc | Benzendicarboxylic acid |
| H2bdc-Br | 2-brom-1,4-benzenedicarboxylic acid acid |
| H2bdc-NO2 | 2-nitro-1,4-benzenedicarboxylic acid |
| bcpbp | 1,1′-bis(4-carboxyphenyl)-4,4′-bipyridinium |
| H5bdcpb | 2,3-bis(3,5-dicarboxylphenxoy)benzoic acid |
| BET | Brunauer–Emmett–Teller |
| H2bidc | 1,3-bisbenzyl-2-imidazolidine-4,5-dicarboxylic acid |
| bibt | 4,7-bi(1H-imidazol-1-yl)benzo-[2,1,3]thiadiazole |
| bimb | Ortho-bis(imidazole-1-ylmethyl)benzene |
| bip | 1,3-bis(2-methyl-imidazol-1-yl)propane |
| bix | 1,4-bis(imidazol-1-ylmethyl)benzene |
| bpp | 1,3-bi(4-pyridyl)propane |
| 2,2′-bpy | 2,2′-bipyridine |
| 4,4′-bpy | 4,4′-bipyridine |
| bix | 1,4-bis(imidazol-1-ylmethyl)benzene |
| bmi | 1,5-bis(2-methylimidazolil-1-yl)pentane |
| H2bpdc | Biphenyl-4,4′-dicarboxylic acid |
| bpmta | N,N′-bis(pyridin-3-ylmethyl)-terephthalamide |
| bpp | 1,3-bis(4-pyridyl)propane |
| H3bptc | Biphenyl-2,4′,5-tricarboxylic acid |
| H2bpydb | 4,4′-(4,4′-bipyridine-2,6-diyl) dibenzoic acid |
| Hbpz | 3,3′5,5′-tetramethyl-4,4′-bipyrazole |
| H3btc | 1,3,5-benzentricarboxylic acid |
| H4btca | 1,2,3,4-benzene tetracarboxylic acid |
| btd-bpy | Bis(pyridin-4-yl)benzothiadiazole |
| H2bzimip | 5-(benzimidazole-1-yl)isophthalic acid |
| H3cbdcpBr | 1-(3,5-dicarboxybenzyl)-4,4′-bipyridinium bromide |
| Hcbia | 2-(1-carboxymethyl)-1H-benzo[d]imidazol-3-ium-3-yl)acetate |
| 2,7-H2cdc | 9H-carbazole-2,7-dicarboxylic acid |
| 5-H3cia | 5-((4-carboxybenzyl) oxy) isophthalic acid |
| 4-Hcpa | 4-chlorophenylacetic acid |
| 4-Hcpha | 4-chlorophenoxyacetic acid |
| H3cphi | 5-(4-carboxyphenoxy)isopthalic acid |
| H3cpota | 2-(4-carboxyphenoxy)terephthalic |
| CR | Congo Red |
| CSB | Chicago Sky Blue |
| dabco | 1,4-diazabicyclo[2.2.2]octane |
| datrz | 3,5-diamino-1,2,4-triazole |
| H4(3,3′-dbdc) | 3,3′-dihydroxy-(1,1′-biphenyl)-4,4′-dicarboxylic acid |
| H4(4,4′-dbdc) | 4,4′-dihydroxy-(1,1′-biphenyl)-3,3′-dicarboxylic acid |
| 2,6-DC-4-NA | 2,6-dichloro-4-nitroaniline |
| H3dcpb | 4-(2′,3′-dicarboxylphenoxy) benzoic acid |
| H2dctp | 2,5-dichloroterephtalic acid |
| H5ddb | 3,5-di(2′,4′-dicarboxylphenyl)benzoic acid |
| H5ddpb | 2,5ʹ-di(3,5-dicarboylphenoxy)benzoic acid |
| dib | 1,3-di(1-imidazolyl)benzene |
| dint | 1,4-di(imidazol-1-yl)naphthalene |
| DMA | N,N′-dimethylacetamide |
| DMF | N,N′-dimethylformamide |
| H2dmg | 2,2′-dimethyl glutaric acid |
| H2dpa | Diphenic acid/dibenzoic acid |
| dpam | 4,4′-dipyridylamine |
| 1,2-dpe | 1,2-di(4-pyridyl)ethane |
| dpe | 1,2-di(4-pyridyl)ethylene |
| dpttz | 2,5-di(pyridine-4-yl)thiazolo[5,4-d]thiazole |
| H4edda | 5,5′(ethane-1,2-diylbis(oxy)) diisophthalic acid |
| EW | Excitation wavelength |
| H4egta | Ethylene bis(oxyethylenenitrilo)tetraacetic acid |
| FA | Formamide |
| H2fba | 4,4′-(hexafluoroisopropylidene)bisbenzoic acid |
| H2glut | Glutaric acid |
| HER | Hydrogen evolution reaction |
| hdn | N,N′-(hexane-1,6-diyl)dinicotinamide |
| H4hfpd | 4,4′-(hexafluoroisopropylidene)diphtalic acid |
| H2hipamifba | 4-(((4-((carboxymethyl)carbamoyl)-phenyl)amino)methyl)benzoate |
| HOMO | Highest occupied molecular orbital |
| inh | Isoniazid |
| H3ipa | 5-hydroxy isophthalic acid |
| Hipc | 5-imidazol-1-yl)-2-pyridine carboxylic acid |
| Hiz | Imidazole |
| LUMO | Lowest unoccupied molecular orbital |
| MB | Methylene blue |
| H2mbdc | 5-methy-1,3- benzenedicarboxylic acid |
| mbp | 1,5-bis(2-methylbenzimidazol-1-yl)pentane |
| MeOH | Methanol |
| 5-meo-H2ip | 5-methoxyisophtalic acid |
| MO | Methyl orange |
| H2msuc | Methyl succinic acid |
| MV | Methyl violet |
| 4-NA | 4-nitroaniline |
| NBZ | Nitrobenzene |
| H2ndc | Naphtalenedicarboxylic acid |
| NF | Nitrofuran |
| NFT | Nitrofurantoin |
| NFZ | Nitrofurazone |
| NMF | N-methylformamide |
| NOR | Norfloxacin |
| NPBI | 1,1′-(4-nitro-1,3-phenylene)bis(1H-benzo[d]imidazole |
| H2oba | 4,4′-oxybis(benzoic acid) |
| OLED | Organic light-emitting diode |
| H2pda | 1,2-phenylenediacetic acid |
| H2pa | Pamoic acid |
| H2paip | 5-{pyren-1-ylmethyl)amino}isophtalic acid |
| 3-H2pbi | 5-(3-pyridin-3-yl)benzamido)isophtalic acid |
| pc1 | 1,1′-bis(4-carboxyphenyl)-4,4′-bipyridinium |
| Hpca | 4-pyridincarboxylic acid |
| Hphac | Phenylacetic acid |
| phen | 1,10-phenantroline |
| pip | 2-phenylimidazo[4,5-f][1,10]phenantroline |
| H2pmoip | 5-{(pyren-1-ylmethyl)amino}isophthalic acid |
| Ppz | Piperazine |
| ptpy | 4′-(4-pyridyl)-2,2′:6′,2″-terpyridine |
| 3-Hpyc | 3-pyridincarboxylic acid |
| Hpyzca | Pyrazine-2-carboxylic acid |
| RH | Relative humidity |
| RhB/6G | Rhodamine B/6G |
| RR | Recycling runs |
| H2SBA | 4,4′-sulfonyldibenzoic acid |
| H2suc | Succinic acid |
| SDZ | Sulfadiazine |
| STZ | Sulfathiazole |
| tbb | 1,4-bis(thiabendazole-1-yl)-2-butene |
| H3tca | Tricarballylic acid |
| H3tcpb | 1,3,5-tris(4-carboxyphenoxy)benzene |
| H2tdc | Thiophenedicarboxylic acid |
| TEC | Tetracycline |
| 5-tert-H2bipa | 5-tert-butylisophthalic acid |
| tib | 1,3,5-tris(1-imidazolyl)benzene |
| 4-tkpvb | 1,2,4,5-tetrakis(4-pyridylvinyl)benzene |
| H2tpca | 2,3,5,6-tetrabromoterephtalic acid |
| TNP | 2,4,6-trinitrophenol |
References
- World Health Organization. 10 Chemicals of Public Health Concern. Available online: https://www.who.int/news-room/photo-story/photo-story-detail/10-chemicals-of-public-health-concern (accessed on 16 August 2023).
- International Agency for Research on Cancer (IARC). Agents Classifed by the IARC Monographs, Volume 1–131. Available online: https://monographs.iarc.who.int/agents-classified-by-the-iarc/ (accessed on 16 August 2023).
- Sheikh Kulsum, P.G.P.; Khanam, R.; Das, S.; Nayak, A.K.; Tack, F.M.G.; Meers, E.; Vithanage, M.; Shahid, M.; Kumar, A.; Chakraborty, S.; et al. A state-of-the-art review on cadmium uptake, toxicity, and tolerance in rice: From physiological response to remediation process. Environ. Res. 2023, 220, 115098. [Google Scholar] [CrossRef]
- Howard, J.A.; Kuznietsova, H.; Dziubenko, N.; Aigle, A.; Natuzzi, M.; Thomas, E.; Lysenko, V.; David, L.; Brichart, T.; Lux, F.; et al. Combating lead and cadmium exposure with an orally administered chitosan-based chelating polymer. Sci. Rep. 2023, 13, 2215. [Google Scholar] [CrossRef]
- Saedi, S.; Watson, S.E.; Young, J.L.; Tan, Y.; Wintergerst, K.A.; Cai, L. Does maternal low-dose cadmium exposure increase the risk of offspring to develop metabolic syndrome and/or type 2 diabetes? Life Sci. 2023, 315, 121385. [Google Scholar] [CrossRef]
- Peana, M.; Pelucelli, A.; Chasapis, C.T.; Perlepes, S.P.; Bekiari, V.; Medici, S.; Zoroddu, M.A. Biological Effects of Human Exposure to Environmental Cadmium. Biomolecules 2023, 13, 36. [Google Scholar] [CrossRef]
- Xiong, L.; Zhou, B.; Young, J.L.; Xu, L.; Wintergerst, K.; Cai, L. Effects of whole-life exposure to low-dose cadmium with post-weaning high-fat diet on offspring testes in a male mouse model. Chem. Biol. Interact. 2022, 353, 109797. [Google Scholar] [CrossRef] [PubMed]
- Ruczaj, A.; Brzóska, M.M. Environmental exposure of the general population to cadmium as a risk factor of the damage to the nervous system: A critical review of current data. J. Appl. Toxicol. 2023, 43, 66–88. [Google Scholar] [CrossRef] [PubMed]
- Nordberg, M.; Nordberg, G.F. Metallothionein and Cadmium Toxicology-Historical Review and Commentary. Biomolecules 2022, 12, 360. [Google Scholar] [CrossRef] [PubMed]
- de Frémont, P.; Adet, N.; Parmentier, J.; Xu, X.; Jacques, B.; Dagorne, S. Cationic organometallic complexes of group 12 metals: A decade of progress toward the quest of novel Lewis acidic catalysts. Coord. Chem. Rev. 2022, 469, 214647. [Google Scholar] [CrossRef]
- Vasile Scaeteanu, G.; Maxim, C.; Badea, M.; Olar, R. Zinc (II) Carboxylate Coordination Polymers with Versatile Applications. Molecules 2023, 28, 1132. [Google Scholar] [CrossRef]
- Parmar, B.; Bisht, K.K.; Rachuri, Y.; Suresh, E. Zn (II)/Cd (II) based mixed ligand coordination polymers as fluorosensors for aqueous phase detection of hazardous pollutants. Inorg. Chem. Front. 2020, 7, 1082. [Google Scholar] [CrossRef]
- Jeong, A.R.; Shin, J.W.; Jeong, J.H.; Jeoung, S.; Moon, H.R.; Kang, S.; Min, K.S. Porous and Nonporous Coordination Polymers Induced by Pseudohalide Ions for Luminescence and Gas Sorption. Inorg. Chem. 2020, 59, 15987–15999. [Google Scholar] [CrossRef] [PubMed]
- Erxleben, A. Structures and properties of Zn (II) coordination polymers. Coord. Chem. Rev. 2003, 246, 203–228. [Google Scholar] [CrossRef]
- Kreno, L.E.; Leong, K.; Farha, O.K.; Allendorf, M.; Van Duyne, R.P.; Hupp, J.T. Metal–Organic Framework Materials as Chemical Sensors. Chem. Rev. 2012, 112, 1105–1125. [Google Scholar] [CrossRef] [PubMed]
- Dutta, A.; Pan, Y.; Liu, J.-Q.; Kumar, A. Multicomponent isoreticular metal–organic frameworks: Principles, current status and challenges. Coord. Chem. Rev. 2021, 445, 214074. [Google Scholar] [CrossRef]
- Ding, M.; Flaig, R.W.; Jiang, H.; Yaghi, O.M. Carbon capture and conversion using metal–organic frameworks and MOF-based materials. Chem. Soc. Rev. 2019, 48, 2783–2828. [Google Scholar] [CrossRef] [PubMed]
- Allendorf, M.D.; Stavila, V.; Witman, M.; Brozek, C.K.; Hendon, C.H. What Lies beneath a Metal–Organic Framework Crystal Structure? New Design Principles from Unexpected Behaviors. J. Am. Chem. Soc. 2021, 143, 6705–6723. [Google Scholar] [CrossRef] [PubMed]
- Li, J.-R.; Sculley, J.; Zhou, H.-C. Metal–Organic Frameworks for Separations. Chem. Rev. 2012, 112, 869–932. [Google Scholar] [CrossRef]
- Ghorai, P.; Hazra, A.; Mandal, J.; Malik, S.; Brandão, P.; Banerjee, P.; Saha, A. Selective Low-Level Detection of a Perilous Nitroaromatic Compound Using Tailor-Made Cd (II)-Based Coordination Polymers: Study of Photophysical Properties and Effect of Functional Groups. Inorg. Chem. 2023, 62, 98–113. [Google Scholar] [CrossRef]
- Duan, C.; Yu, Y.; Xiao, J.; Li, Y.; Yang, P.; Hu, F.; Xi, H. Recent advancements in metal–organic frameworks for green applications. Green Energy Environ. 2021, 6, 33–49. [Google Scholar] [CrossRef]
- Shivam; Megha, R.; Lakhani, V.; Vala, S.; Dharaskar, S.; Paluvai, N.R.; Sinha, M.K.; Jampa, S.S. Removal of heavy metals and dyes from its aqueous solution utilizing metal organic Frameworks (MOFs): Review. Mater. Today Proc. 2023, 77, 188–200. [Google Scholar] [CrossRef]
- Cao, T.; Peng, Y.; Liu, T.; Wang, S.; Dou, J.; Li, Y.; Zhou, C.; Li, D.; Bai, J. Assembly of a series of d10 coordination polymers of pamoic acid through a mixed-ligand synthetic strategy: Syntheses, structures and fluorescence properties. CrystEngComm 2014, 16, 10658–10673. [Google Scholar] [CrossRef]
- Liu, Q.-F.; Liu, W.; Cao, Y.-P.; Dong, Y.-L.; Liu, H.-M. Synthesis, structure, and luminescent property of a new Cd (II) coordination polymer with (4, 8)-connected topology. Inorg. Nano-Met. Chem. 2016, 47, 65–68. [Google Scholar] [CrossRef]
- Li, Z.-H.; Zhang, J.; Qin, Q.-P. Synthesis, structure and luminescence properties of a three-dimensional Cd (II) coordination polymer with (3, 7)-connected topology. J. Sulfur Chem. 2020, 41, 508–516. [Google Scholar] [CrossRef]
- Xu, M.; Liang, G.; Wang, S.; Ma, X.; Liang, G.; Ni, Q. Structural variability, topology and luminescent properties of three new cadmium (II) coordination polymers based on 4′,4′,4′-[(trimethylamino)]-tris[(1,1′-biphenyl)-2-carboxylate]. J. Mol. Struct. 2020, 1217, 128411. [Google Scholar] [CrossRef]
- Narea, P.; Cisterna, J.; Cárdenas, A.; Amo-Ochoa, P.; Zamora, F.; Climent, C.; Alemany, P.; Conejeros, S.; Llanos, J.; Brito, I. Crystallization induced enhanced emission in two new Zn (II) and Cd (II) supramolecular coordination complexes with the 1-(3,4-dimethylphenyl)-5-methyl-1H-1,2,3-triazole-4-carboxylate ligand. Polymers 2020, 12, 1756. [Google Scholar] [CrossRef] [PubMed]
- Bai, Y.-T.; Zheng, H.; Tong, K.-W.; Feng, S.-S.; Zhu, M.-L. Construction of a one-dimensional cadmium coordination polymer based on a triangle flexible multicarboxylate linker. Inorg. Nano-Met. Chem. 2021, 51, 919–924. [Google Scholar] [CrossRef]
- Wang, N.; Long, B.-F.; Yin, X.-H.; Huang, Z.-j.; Mi, Y.; Hu, F.-L.; Young, D.J. New structurally diverse photoactive cadmium coordination polymers. Dalton Trans. 2021, 50, 18194–18201. [Google Scholar] [CrossRef]
- Lozovan, V.; Kravtsov, V.C.H.; Costriucova, N.V.; Siminel, A.V.; Kulikova, O.V.; Fonari, M.S. Tunability in dimension, metal and ligand coordination modes and emission properties in Cd (II) and Zn (II) coordination networks based on 4,4′-(hydrazine-1,2-diyilidenebis (methanylylidene)) dibenzoic acid linker. J. Solid State Chem. 2022, 310, 123021. [Google Scholar] [CrossRef]
- Lin, H.; Deng, X.; Sun, Y.; Chen, S.; Zhou, T. Effect of N-donor ancillary ligand on zinc/cadmium-organic arsonates: Structural analysis and photoluminescence. J. Solid State Chem. 2022, 311, 123148. [Google Scholar] [CrossRef]
- Cui, S.; Zhang, W.; Zhu, B.; Yuan, N.; Yu, J.; Sun, Z.; Li, J.; Zuo, M. Two novel cadmium coordination polymers bearing viologen-derived ligand: Structure and photochromism properties. Inorg. Chim. Acta 2022, 534, 120818. [Google Scholar] [CrossRef]
- Li, Z.-Y.; Chang, H.; Zhao, J.-J.; Zhang, C.; Wu, D.-Q.; Zhai, B. Tunable structures and magnetic/optical properties of six Cd (II)-based coordination polymers by introducing different para- or dia-magnetic metal ions. J. Mol. Struct. 2023, 1283, 135270. [Google Scholar] [CrossRef]
- Liu, Y.-H.; Liao, T.-T.; Lin, S.-Y.; Zhong, S.-Y.; Chen, T.-R.; Chen, J.-D. Cd (II) and Zn (II) coordination polymers constructed from bis-pyridyl-bis-amide and dicarboxylic or tetracarboxylic acid: Synthesis, structures and luminescent properties. Inorg. Chim. Acta 2023, 556, 121641. [Google Scholar] [CrossRef]
- He, S.-S.; Jiang, T.; Fu, W.-W.; Chen, H.; Li, S.; Zhou, P.; Shen, J.-R.; Liu, Y.; Chen, M.-S. Six novel Zn (II)/Cd (II) coordination polymers based on a rigid tridentate imidazolyl terpyridine ligand fine-tuned by different carboxylates: Syntheses, structural diversities and luminescence properties. Polyhedron 2023, 242, 116497. [Google Scholar] [CrossRef]
- Lozovan, V.; Kravtsov, V.C.H.; Chumakov, Y.M.; Costriucova, N.V.; Siminel, N.; Petuhov, O.; Vlase, T.; Vlase, G.; Barba, A.; Fonari, M.S. Zn (II) and Cd (II) metal–organic frameworks with azine-functionalized pores: Crystal structures, photoluminescence, solvent exchange, and molecular simulations of carbon dioxide binding sites. Cryst. Growth Des. 2023, 23, 3171–3185. [Google Scholar] [CrossRef]
- Zhang, S.; Lu, K.; Shen, X.; Ye, Y.; Sun, G.; Xu, J.; Han, G. Two new alkali/cadmium bimetallic coordination polymers: Luminescent properties and cancer prevention by regulating the interaction between high mobility group protein B1 and immune cells. J. Clust. Sci. 2023, 34, 1215–1225. [Google Scholar] [CrossRef]
- Liu, L.; Li, J.-M.; Zhang, M.-D.; Wang, H.-J.; Li, Y.; Zhang, Z.-B.; Zhao, Z.-F.; Xi, Y.; Huang, Y.-Y.; Xu, J.; et al. Cd (II)/Mn (II)/Co (II)/Ni (II)/Zn (II) coordination polymers built from dicarboxylic acid/tetracarboxylic acid ligands: Their structural diversity and fluorescence properties. Polymers 2023, 15, 1803. [Google Scholar] [CrossRef]
- Li, Z.; Bu, J.; Zhang, R.; Zhang, C.; Dongqing, W.; Zhai, B. Two temperature-dependent 2D heterometallic Cd (II)–Dy (III) coordination polymers exhibiting slow magnetic relaxation and luminescence properties. J. Rare Earths 2023, 41, 613–620. [Google Scholar] [CrossRef]
- Wu, Y.-Q.; Wu, F.F.; Wang, Z.-X.; He, X.; Xing, F.-F.; Li, M.-X. Syntheses, crystal structures, luminescent and magnetic properties of six 5,5′-(1,2-phenylenebis (methoxy)) diisophthalate coordination polymers. Inorg. Chim. 2023, 547, 121357. [Google Scholar] [CrossRef]
- Yang, F.; Chen, J.; Wang, J.; Liu, J. Structure and photochromic properties of two cadmium coordination polymers derived from viologen ligands. Z. Anorg. Allg. Chem. 2023, 649, e202300051. [Google Scholar] [CrossRef]
- Zhao, Y.; Zhao, D.D.; Liu, H.L.; Zhou, W.Y.; Li, G. Imidazole Multi-Carboxylate-Based 2D Cd (II) MOF: Preparation, Crystal Structure, and Properties. Russ. J. Coord. Chem. 2020, 46, 283–289. [Google Scholar] [CrossRef]
- Ye, Y.; Lin, S.; Wu, X.; Zhang, X.; Wang, A. Solvothermal synthesis and luminescence properties of a novel Cd(II) coordination polymer containing 3-pyridinecarboxylate and phen ligands and [TaF6]− anion. J. Mol. Struct. 2020, 1202, 127311. [Google Scholar] [CrossRef]
- Xu, B.; Yao, W.; Yu, X.; Fedin, V.P.; Gao, E. Cadmium (II)-Organic Coordination Polymer Containing Carboxyl Groups: Solvothermal Synthesis, Structure, and Properties. Russ. J. Coord. Chem. 2023, 49, 771–776. [Google Scholar] [CrossRef]
- Liu, G.; Han, S.; Gao, Y.; Xu, N.; Wang, X.; Chen, B. Multifunctional fluorescence responses of phenyl-amide-bridged d10 coordination polymers structurally regulated by dicarboxylates and metal ions. CrystEngComm 2020, 22, 7952–7961. [Google Scholar] [CrossRef]
- Song, X.; Dong, W.; Hou, X.; Zhao, Q.; Zhang, Z.; Ren, Y. The high fluorescence sensitivity property and quenching mechanism of one-dimensional Cd-HCIA-1 sensor for nitrobenzene. Phys. Chem. Chem. Phys. 2023, 25, 14907–14917. [Google Scholar] [CrossRef]
- Zhao, Y.-Y.; Chen, L.; Xu, Z.; Zhu, C.-Y.; Li, P.; Gao, W.; Li, J.-Y.; Zhang, X.-M. Microporous Cd-MOF as multifunctional material for rapid and visual luminescence sensing of Fe3+, MnO4− and TNP in water and efficient CO2 catalytic conversion. Micropor. Mesopor. Mater. 2023, 362, 112764. [Google Scholar] [CrossRef]
- Meyers, A.E.; Randolph, R.K.; LaDuca, R.L. Divergent topologies in luminescent zinc and cadmium substituted isophthalate coordination polymers constructed from long-spanning dipyridylamide ligand precursors. Inorg. Chim. Acta 2017, 467, 330–342. [Google Scholar] [CrossRef]
- Li, B.; Duan, W.X.; Liu, S.S.; Jin, Y.-J.; Wang, L.-Y. Zinc (II) and cadmium (II) coordination polymers constructed from 5-(benzimidazole-1-yl) isophthalic acid ligand: Syntheses, structures and detection of antibiotics in aqueous medium. J. Clust. Sci. 2023, 34, 479–485. [Google Scholar] [CrossRef]
- Zhao, Y.Y.; Zhou, Y.; Li, R.; Li, B. Synthesis, characterization and efficient detection of antibiotics of two Cd (II)-based coordination polymers. J. Clust. Sci. 2023, 34, 2791–2797. [Google Scholar] [CrossRef]
- Du, Y.; Ghosh, M.K.; Lu, L.; Wang, J.; Mohanty, A.; Ghorai, T.K.; Afzal, M.; Alarifi, A. Synthesized and characterization of a new Cd (II)-based coordination polymer: Sensing activity and photocatalytic activity against antibiotic. Polyhedron 2023, 241, 116468. [Google Scholar] [CrossRef]
- Li, J.; Yao, S.-L.; Zheng, T.-F.; Xu, H.; Li, J.-Y.; Peng, Y.; Chen, J.-L.; Liu, S.-J.; Wen, H.-R. Turn-on and blue-shift fluorescence sensor toward l-histidine based on stable Cd (II) metal–organic framework with tetranuclear cluster units. Dalton Trans. 2022, 51, 5983–5988. [Google Scholar] [CrossRef]
- Cai, S.-L.; Lu, L.; Wu, W.-P.; Wang, J.; Sun, Y.-C.; Ma, A.-Q.; Singh, A.; Kumar, A. A new mixed ligand based Cd (II) 2D coordination polymer with functional sites: Photoluminiscence and photocatalytic properties. Inorg. Chim. Acta 2019, 484, 291–296. [Google Scholar] [CrossRef]
- Cai, H.; Li, N.; Li, Y.; An, D.-M. New three-dimensional Zn (II)/Cd (II)-based coordination polymers as luminescent sensor for Cu2+. Inorg. Chim. Acta 2020, 512, 119886. [Google Scholar] [CrossRef]
- Contejean, Z.I.; LaDuca, R.L. Nitroaromatic detecting zinc and cadmium coordination polymers with methyl-substituted aliphatic dicarboxylate and 4,4′-dipyridylamine ligands and diverse topologies. J. Solid State Chem. 2018, 266, 44–53. [Google Scholar] [CrossRef]
- Gong, W.-J.; Yao, R.; Li, H.-X.; Ren, Z.-G.; Zhang, J.-G.; Lang, J.-P. 2017. Luminescent cadmium (II) coordination polymers of 1,2,4,5-tetrakis (4-pyridylvinyl) benzene used as efficient multi-responsive sensors for toxic metal ions in water. Dalton Trans. 2017, 46, 16861–16871. [Google Scholar] [CrossRef] [PubMed]
- Karbalaee Hosseini, A.; Tadjarodi, A. Luminescent Cd coordination polymer based on thiazole as a dual-responsive chemosensor for 4-nitroaniline and CrO42− in water. Sci. Rep. 2023, 13, 269. [Google Scholar] [CrossRef] [PubMed]
- Kabak, B.; Kendüzler, E. Synthesis, characterization and adsorption/sensing applications of novel cadmium (II) based coordination polymer. J. Environ. Chem. Eng. 2022, 10, 107989. [Google Scholar] [CrossRef]
- Li, S.; Lu, L.; Zhu, M.; Yuan, C.; Feng, S. A bifunctional chemosensor for detection of volatile ketone or hexavalent chromate anions in aqueous solution based on a Cd (II) metal–organic framework. Sens. Actuators B Chem. 2018, 258, 970–980. [Google Scholar] [CrossRef]
- Wang, Y.-N.; Xu, H.; Wang, S.-D.; Zhang, M.-H.; Wang, Y.-T.; Qiu, Q.-C.; Bai, J.-T.; Mo, Y.; Feng, W.-Y.; Yang, Q.-F. Multifunctional Cd-CP for fluorescence sensing of Cr (VI), MnO4−, acetylacetone and ascorbic acid in aqueous solutions. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2023, 291, 122369. [Google Scholar] [CrossRef]
- Wang, Y.-N.; Xu, H.; Wang, S.-D.; Mao, R.-Y.; Wen, L.-M.; Wang, S.-Y.; Liu, L.-J.; Sun, Y.; Lu, S.-Q.; Wang, F.; et al. A water-stable dual-responsive Cd-CP for fluorometric recognition of hypochlorite and acetylacetone in aqueous media. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2023, 286, 121952. [Google Scholar] [CrossRef]
- Yan, X.-L.; Cao, X.-Q.; Deng, C.-R.; Zheng, T.-F.; Yao, S.-L.; Liu, S.-J. A highly stable and efficient benzothiadiazole-based fluorescence sensor for salicylaldehyde in aqueous solution. CrystEngComm 2023, 25, 2366–2371. [Google Scholar] [CrossRef]
- Li, W.; Zhao, D.; Lei, N.; Wen, R.; Li, W.; Dou, M.; Fan, L. Luminiscence sensing and electrocatalytic redox performances of a new stable cadmium (II) coordination polymer. J. Solid State Chem. 2023, 317, 123649. [Google Scholar] [CrossRef]
- Zhang, M.-L.; Lu, X.-Y.; Ren, Y.-X.; Wang, J.-J.; Yang, X.-G. Three Cd (II) coordination polymers containing phenylenediacetate isomers: Luminescence sensing and adsorption antibiotics performance in water. Dye. Pigm. 2022, 202, 110172. [Google Scholar] [CrossRef]
- Wu, Y.-B.; Ren, L.; Dong, G.-Y. Syntheses, crystal structures, luminiscent sensing and photocatalytic properties of two 2D cadmium (II) coordination polymers constructed from mixed ligands. Inorg. Chim. Acta 2022, 520, 120703. [Google Scholar] [CrossRef]
- Chang, J.-L.; Wu, J.-F.; Zhang, J.-W.; Cui, K.; Liu, Z.-Q. Crystal structure and fluorescence-based sensor properties of a Metal-Organic Framework. Z. Naturforsch. B 2024, 79, 81–87. [Google Scholar] [CrossRef]
- Wang, Z.-P.; Wang, Y.; Li, X.-Y.; Jia, L.-F.; Yang, A.-Z.; Zhao, W.-T.; Jia, Y.; Yu, B.-Y.; Zhao, H.-Q. Water-stable mixed-ligand Cd (II) metal–organic frameworks as bis-color excited fluorescent sensors for the detection of vitamins and pesticides in aqueous solutions. J. Mol. Struct. 2024, 1305, 137699. [Google Scholar] [CrossRef]
- Liu, T.-Y.; Qu, X.-L.; Zhang, Y.; Yan, B. A Stable Cd (II)-Based Metal–Organic Framework: Synthesis, Structure, and Its Eu3+ Functionalization for Ratiometric Sensing on the Biomarker 2-(2-Methoxyethoxy) Acetic Acid. Inorg. Chem. 2021, 60, 8613–8620. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Q.-J.; Chuang, P.-M.; Wu, J.-Y. Fluorescence-Responsive Detection of Ag (I), Al (III), and Cr (III) Ions Using Cd (II) Based Pillared-Layer Frameworks. Int. J. Mol. Sci. 2023, 24, 369. [Google Scholar] [CrossRef] [PubMed]
- Li, D.; Xu, H.-Q.; Jiao, L.; Jiang, H.-L. Metal–Organic Frameworks for Catalysis: State-of-the-Art, Challenges, and Opportunities. EnergyChem 2019, 1, 100005. [Google Scholar] [CrossRef]
- Liu, S.-H.; Zhang, J.-W.; Wang, X.; Wang, L.-H.; Wang, Z.-H.; Wei, Y.-B. Synthesis, crystal structure and catalytic property of a new cadmium coordinarion polymer. J. Clust. Sci. 2017, 28, 1955–1962. [Google Scholar] [CrossRef]
- Zhang, J.-W.; Cheng, Y.; Liu, S.-H. Synthesis, crystal structure and catalytic property of a cadmium (II) coordination polymer derived from a zwitterionic carboxylate ligand. Inorg. Nano-Met. Chem. 2019, 48, 347–351. [Google Scholar] [CrossRef]
- Choi, I.H.; Kim, Y.; Lee, D.N.; Huh, S. Three-dimensional cobalt (II) and cadmium (II) MOFs containing 1,4-naphtalenedicarboxylate: Catalytic activity of Cd-MOF. Polyhedron 2016, 105, 96–103. [Google Scholar] [CrossRef]
- Cheng, X.; Guo, L.; Wang, H.; Gu, J.; Yang, Y.; Kirillova, M.; Kirillov, A. Coordination polymers from biphenyl-dicarboxylate linkers: Synthesis, structural diversity, interpenetration, and catalytic properties. Inorg. Chem. 2022, 61, 12577–12590. [Google Scholar] [CrossRef] [PubMed]
- Palomo, C.; Oiarbide, M.; Laso, A. Recent advances in the catalytic asymmetric nitroaldol (Henry) reaction. Eur. J. Org. Chem. 2007, 16, 2561–2574. [Google Scholar] [CrossRef]
- Dallesandro, E.; Collin, H.; Guimarães, L.G.; Valle, M.; Pliego, J. Mechanism of the piperidine-catalyzed Knoevenagel condensation reaction in methanol: The role of iminium and enolate ions. J. Phys. Chem. B 2017, 121, 5300–5307. [Google Scholar] [CrossRef] [PubMed]
- Sefidabi, F.; Abbasi, A.; Mortazavi, S.-S.; Masteri-Farahani, M. A new 2D cadmium coordination polymer based on hydroxyl-substituted benzendicarboxylic acid as an effective heterogeneous catalyst for Knoevenagel condensation. Appl. Organomet. Chem. 2020, 34, e5890. [Google Scholar] [CrossRef]
- Yi, X.-C.; Huang, M.-X.; Qi, Y.; Gao, E.-Q. Synthesis, structure, luminescence and catalytic properties of cadmium (II) coordination polymers with 9H-carbazole-2,7-dicarboxylic acid. Dalton Trans. 2014, 43, 3691–3697. [Google Scholar] [CrossRef]
- Ji, Z.X.; Li, P.F. Crystal structure and catalytic activity of a novel Cd (II) coordination polymer formed by dicarboxylic ligand. Bull. Chem. React. Eng. 2018, 13, 220–226. [Google Scholar] [CrossRef]
- Karmakar, A.; Paul, A.; Guedes da Silva, M.F.C.; Pombeiro, A.J.L. Polyaromatic group embedded Cd (II)-coordination polymers for microwave-assisted solvent-free Strecker-type cyanation of acetals. Molecules 2023, 28, 945. [Google Scholar] [CrossRef] [PubMed]
- Khan, S.; Markad, D.; Mandal, S. Two Zn (II)/Cd (II) coordination polymers as recyclable heterogeneous catalysts for an efficient room-temperature synthesis of α-aminonitriles via the solvent-free Strecker reaction. Inorg. Chem. 2023, 62, 275–284. [Google Scholar] [CrossRef] [PubMed]
- Gutić, S.J.; Dobrota, A.S.; Fako, E.; Skorodumova, N.V.; López, N.; Pašti, I.A. Hydrogen Evolution Reaction—From Single Crystal to Single Atom Catalysts. Catalysts 2020, 10, 290. [Google Scholar] [CrossRef]
- Etaiw, S.; El-Bendary, M. Cd (II) supramolecular coordination polymer incorporating pyrazine-2-carboxylic acid: Crystal structure, spectral characteristics and catalytic activity. J. Lumin. 2018, 199, 232–239. [Google Scholar] [CrossRef]
- Hao, S.Y.; Li, Y.H.; Zhu, J.; Cui, G.H. Synthesis, structures, luminiscence and photocatalytic properties of two nanostructured cadmium (II) coordination polymers synthesized by sonochemical process. Ultrason. Sonochem. 2018, 40, 68–77. [Google Scholar] [CrossRef] [PubMed]
- Zhao, X.-X.; Qin, Z.-B.; Li, Y.-H.; Cui, G.-H. New Cd (II) and Zn (II) coordination polymers showing luminescent sensing for Fe (III) and photocatalytic degrading methylene blue. Polyhedron 2018, 153, 197–204. [Google Scholar] [CrossRef]
- Lu, L.; Wang, J.; Zhou, Y.; Sun, Y.; Wu, X.; Singh, A.; Kumar, A. Two new coordination polymers driven by polycarboxylate and N-donor spacers: Photocatalytic performance and theoretical analysis. Inorg. Chim. Acta 2020, 508, 119647. [Google Scholar] [CrossRef]
- Wang, J.; Lu, L.; He, J.-R.; Wu, W.-P.; Gong, C.; Fang, L.; Pan, Y.; Singh, A.K.; Kumar, A. Photocatalytic performances of two new Cd (II) and Zn (II)-based coordination polymers. J. Mol. Struct. 2019, 1182, 79–86. [Google Scholar] [CrossRef]
- Zhang, X.; Blatov, V.; Zhao, Y.-Q.; Hao, Z.-C.; Cui, G.-H. Synthesis, structures, and properties of cadmium (II) and nickel (II) coordination polymers based on a 4,4′-biphenyl-containing ligand and aliphatic carboxylic acids. Z. Anorg. Allg. Chem. 2016, 642, 1184–1190. [Google Scholar] [CrossRef]
- Feng, Z.-Q.; Yang, X.-L.; Ye, Y.-F. Synthesis, crystal structures, luminescence, biological and catalytic properties of two d10 metal-organic coordination polymers constructed from mixed ligands. J. Inorg. Organomet. Polym. Mater. 2014, 24, 684–693. [Google Scholar] [CrossRef]
- Muslim, M.; Ahmad, M.; Alam, M.J.; Ahmad, S. Experimental and density functional theory investigation on one- and two-dimensional coordination polymers and their ZnO-doped nanocomposite materials for wastewater remediation. Sep. Purif. Technol. 2023, 315, 123598. [Google Scholar] [CrossRef]
- Zheng, M.; Chen, J.; Zhang, L.; Cheng, Y.; Lu, C.; Liu, Y.; Singh, A.; Trivedi, M.; Kumar, A.; Liu, J. Metal organic frameworks as efficient adsorbents for drugs from wastewater. Mater. Today Commun. 2022, 31, 103514. [Google Scholar] [CrossRef]
- Agarwal, R.; Mukherjee, S. One dimensional coordination polymers of Cd (II) and Zn (II): Synthesis, structure, polar packing through strong inter-chain hydrogen bonding and gas adsorption studies. Polyhedron 2016, 106, 163–170. [Google Scholar] [CrossRef]
- Haque, F.; Halder, A.; Ghosh, S.; Ghoshal, D. Five coordination polymers of Cd (II) and Co(II) using 3,3′-azobispyridine and different carboxylates: Synthesis, structures and adsorption properties. Polyhedron 2019, 161, 289–297. [Google Scholar] [CrossRef]
- Nagarkar, S.; Chaudhari, A.; Ghosh, S. Selective CO2 adsorption in a robust and water-stable porous coordination polymer with a new network topology. Inorg. Chem. 2012, 51, 572–576. [Google Scholar] [CrossRef] [PubMed]
- Somnath; Tyagi, L.; Lama, R.; Siddiqui, K.A. Gas sorption and luminiscence properties of activated forms of a Cd (II)-coordination polymer. J. Coord. Chem. 2021, 74, 2227–2238. [Google Scholar] [CrossRef]
- Hua, J.; Wang, M.; Zhang, D.; Pei, X.; Zhao, X.; Ma, X. A three-dimensional cadmium mixed ligands coordination polymer with CO2 adsorption ability. J. Struct. Chem. 2022, 63, 2045–2053. [Google Scholar] [CrossRef]
- Sharma, M.; Senkovska, I.; Kaskel, S.; Bharadwaj, P. Three-dimensional porous Cd (II) coordination polymer with large one-dimensional hexagonal channels: High pressure CH4 and H2 adsorption studies. Inorg. Chem. 2011, 50, 539–544. [Google Scholar] [CrossRef] [PubMed]
- Leroux, M.; Mercier, N.; Allain, M.; Dul, M.-C.; Dittmer, J.; Kassiba, A.H.; Bellat, J.-P.; Weber, G.; Bezverkhyy, I. Porous coordination polymer based on bipyridinium carboxylate linkers with high and reversible ammonia uptake. Inorg. Chem. 2016, 55, 8587–8594. [Google Scholar] [CrossRef]
- Naskar, K.; Dey, A.; Maity, S.; Bhunia, M.; Ray, P.P.; Sinha, C. Novel porous polycatenated iodo-cadmium coordination polymer for iodine sorption and electrical conductivity measurement. Cryst. Growth Des. 2019, 19, 2206–2218. [Google Scholar] [CrossRef]
- Zhang, T.; Lin, S.; Yan, T.; Li, B.; Liang, Y.; Liu, D.; He, Y. Integrating self-partitioned pore space and amine functionality into an aromatic-rich coordination framework with Ph stability for effective purification of C2 hydrocarbons. Inorg. Chem. 2023, 62, 5593–5601. [Google Scholar] [CrossRef] [PubMed]
- Burlak, P.V.; Samsonenko, D.G.; Kovalenko, K.A.; Fedin, V.P. Series of Cadmium–Organic Frameworks Based on Mixed Flexible and Rigid Ligands: Single-Crystal-to-Single-Crystal Transformations, Sorption, and Luminescence Properties. Inorg. Chem. 2023, 62, 18087–18097. [Google Scholar] [CrossRef]
- Al’Abri, A.M.; Sharhan, O.; Halim, S.N.A.; Bakar, N.K.A.; Sherino, B.; Kamboh, M.A.; Nodeh, H.R.; Mohamad, S. Effect of framework metal ions of analogous magnetic porous coordination polymers on adsorption of cationic and anionic dyes from aqueous solution. Chem. Pap. 2022, 76, 3541–3556. [Google Scholar] [CrossRef]
- Sezer, G.G.; Arici, M.; Erucar, I.; Yeșilel, O.Z.; Özel, H.U.; Gemici, B.T.; Erer, H. Zinc (II) and cadmium (II) coordination polymers containing phenylenediacetate and 4,4′-azobis (pyridine) ligands: Syntheses, structures, dye adsorption properties and molecular dynamics simulations. J. Solid State Chem. 2017, 255, 89–96. [Google Scholar] [CrossRef]
- Ahmed, A.; Ali, A.; Ahmed, M.; Parida, K.; Ahmad, M.; Ahmad, A. Construction and topological studies of a three dimensional (3D) coordination polymer showing selective adsorption of aromatic hazardous dyes. Sep. Purif. Technol. 2021, 265, 118482. [Google Scholar] [CrossRef]
- Gaur, R. Selective anionic dye adsorption, topology and luminescence study of structurally diverse cadmium (II) coordination polymers. Inorg. Chem. Front. 2019, 6, 278. [Google Scholar] [CrossRef]
- Karmakar, A.; Paul, A.; Santos, P.M.R.; Santos, I.R.M.; Guedes da Silva, M.F.C.; Pombeiro, A.J.L. Novel anthracene and pyrene containing Cd (II)-based coordination polymers for adsorptive removal of toxic dyes from aqueous medium. Colloids Surf. A Physicochem. Eng. Asp. 2023, 6705, 131488. [Google Scholar] [CrossRef]
- Luo, B.; Yu, D.; Huo, J. Polynuclear Cd (II) coordination polymer with unique configuration for chromium pollutants removal. J. Solid State Chem. 2020, 283, 121137. [Google Scholar] [CrossRef]
- Ma, D.-Y.; Guo, H.-F.; Qin, L.; Li, Y.; Ruan, Q.-T.; Huang, Y.-W.; Xu, J. Construction of a new 2D cadmium (II) coordination polymer based on N- and O-donor ligands: Synthesis, luminiscence and biological activities. J. Chem. Crystallogr. 2014, 44, 63069. [Google Scholar] [CrossRef]
- Liu, H.-M.; Shang, X.-N. Two new Cd (II)/Zn (II) cordination polymers: Luminiscence properties and synergistic treatment activity with ultrasound therapy on uterine fibroids. Des. Monomers Polym. 2022, 25, 197–204. [Google Scholar] [CrossRef] [PubMed]
- Soltani, S.; Akhbari, K.; White, J. Effect of structural features on the stability and bactericidal potential of two cadmium coordination polymers. CrystEngComm 2021, 23, 7450. [Google Scholar] [CrossRef]
- Balendra; Sanyukta; Mahboob, A.; Sevi, M. Cadmium-based coordination polymers (CPs) constructed from two different V-shaped dicarboxylate ligands: Synthesis, structure and dielectric properties. Inorg. Chem. Commun. 2023, 148, 110280. [Google Scholar] [CrossRef]
- Liu, Y.-R.; Chen, Y.-Y.; Jing, Y.-F.; Xie, L.-X.; Li, G. High water-assisted proton conductivities of two cadmium (II) complexes constructed from zwitterionic ligands. Inorg. Chem. 2022, 61, 19502–19511. [Google Scholar] [CrossRef]
- Cheng, Q.; Qin, L.; Zhou, J.; Lin, J.; Lin, X.; Zhang, G.; Cai, Y. Four new Zn (II) and Cd (II) coordination polymers using two amide-like aromatic multi-carboxylate ligands: Synthesis, structures and lithium-selenium batteries application. RSC Adv. 2019, 9, 14750–14757. [Google Scholar] [CrossRef] [PubMed]
- Najafi, E.; Janghouri, M.; Hashemzadeh, A.; Ng, S.W. Mixed ligand Cd (II) coordination architectures based on bulky anthracene-9-carboxylate ligand: Crystal structures and optical properties. Inorg. Chim. Acta 2023, 552, 121482. [Google Scholar] [CrossRef]
- Lu, Y.F.; He, Y.-H.; Liang, J.-B.; Jin, Q.; Ou, Y.-C.; Wu, J.-Z. First cadmium coordination compound as an efficient floculant for Congo Red. Inorg. Chem. Commun. 2022, 141, 109544. [Google Scholar] [CrossRef]
- Al-Fakeh, M.S.; Alsaedi, R.O.; Amiri, N.; Allazzam, G.A. Synthesis, Characterization, and Antimicrobial of MnO and CdO Nanoparticles by Using a Calcination Method. Coatings 2022, 12, 215. [Google Scholar] [CrossRef]
- Payehghadr, M.; Morsali, A. Thermolysis preparation of cadmium (II) oxide nanoparticles from a new three-dimensional cadmium (II) supramolecular compound. J. Struct. Chem. 2013, 54, 787–791. [Google Scholar] [CrossRef]
- Ramazani, M.; Morsali, A. Sonochemical syntheses of a new nano-plate cadmium (II) coordination polymer as a precursor for the synthesis of cadmium (II) oxide nanoparticles. Ultrason. Sonochem. 2011, 18, 1160–1164. [Google Scholar] [CrossRef] [PubMed]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vasile Scaeteanu, G.; Maxim, C.; Badea, M.; Olar, R. An Overview of Various Applications of Cadmium Carboxylate Coordination Polymers. Molecules 2024, 29, 3874. https://doi.org/10.3390/molecules29163874
Vasile Scaeteanu G, Maxim C, Badea M, Olar R. An Overview of Various Applications of Cadmium Carboxylate Coordination Polymers. Molecules. 2024; 29(16):3874. https://doi.org/10.3390/molecules29163874
Chicago/Turabian StyleVasile Scaeteanu, Gina, Catalin Maxim, Mihaela Badea, and Rodica Olar. 2024. "An Overview of Various Applications of Cadmium Carboxylate Coordination Polymers" Molecules 29, no. 16: 3874. https://doi.org/10.3390/molecules29163874
APA StyleVasile Scaeteanu, G., Maxim, C., Badea, M., & Olar, R. (2024). An Overview of Various Applications of Cadmium Carboxylate Coordination Polymers. Molecules, 29(16), 3874. https://doi.org/10.3390/molecules29163874