Hydrated Metal Vanadate Heterostructures as Cathode Materials for Stable Aqueous Zinc-Ion Batteries
Abstract
1. Introduction
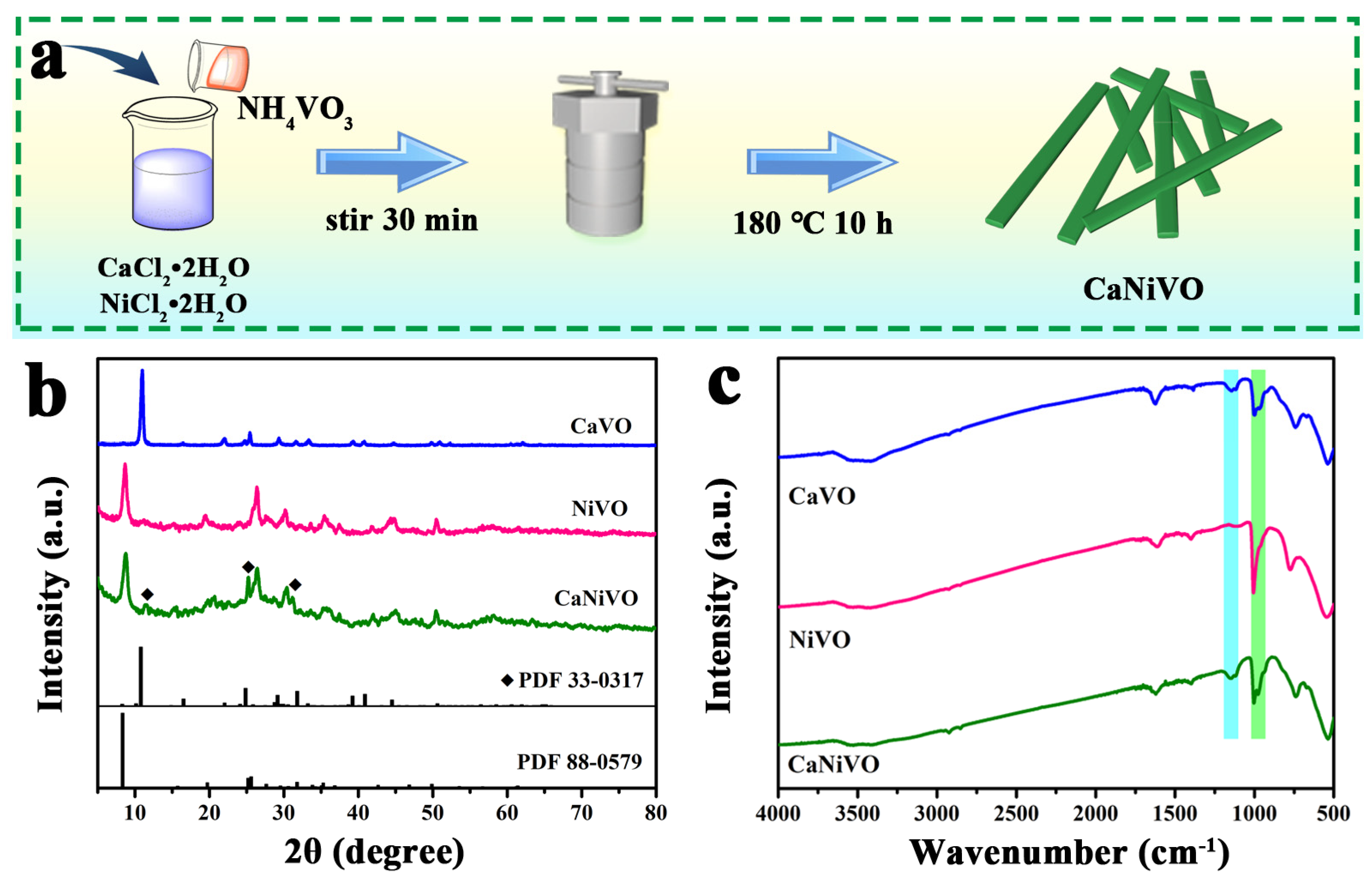
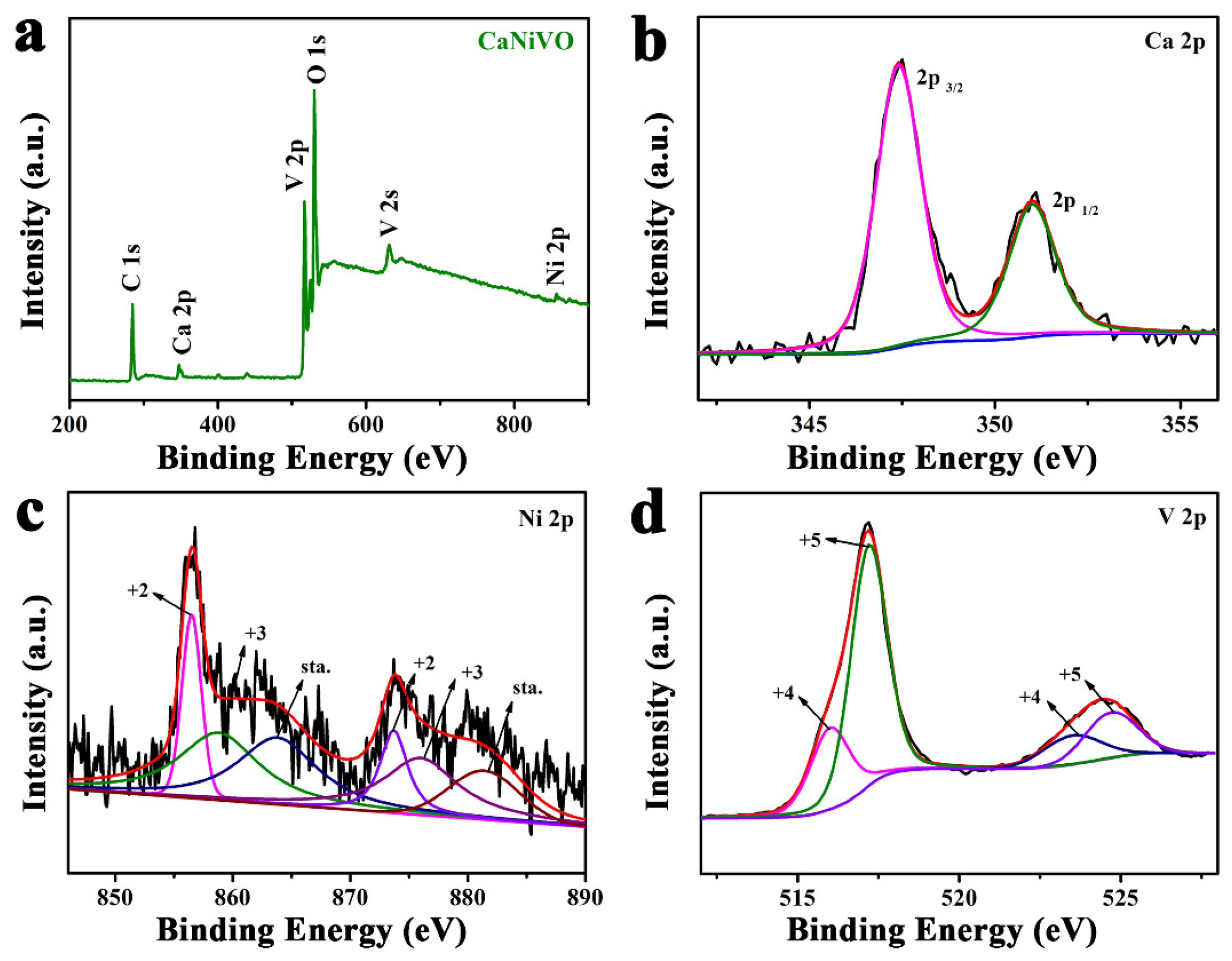
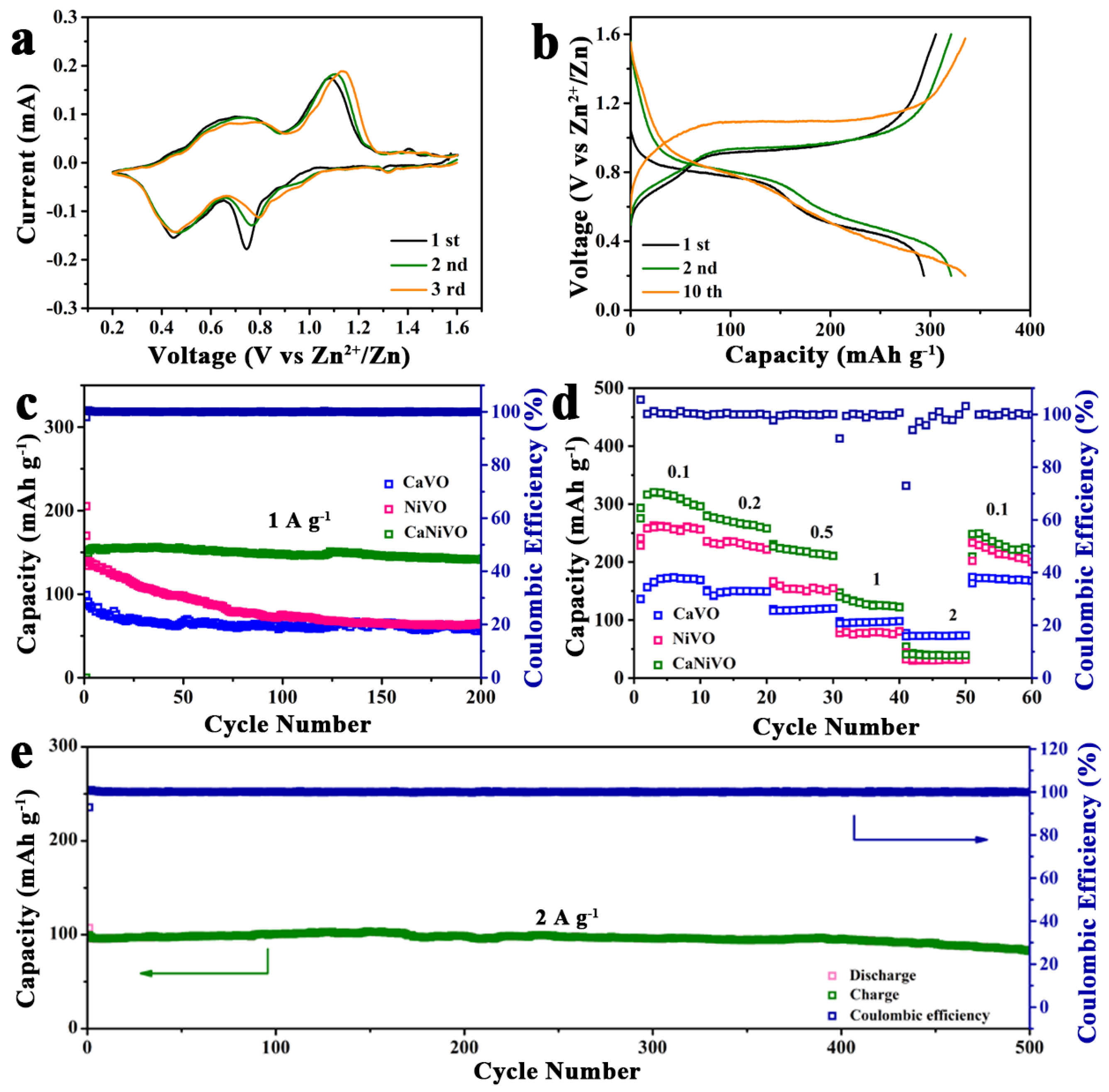
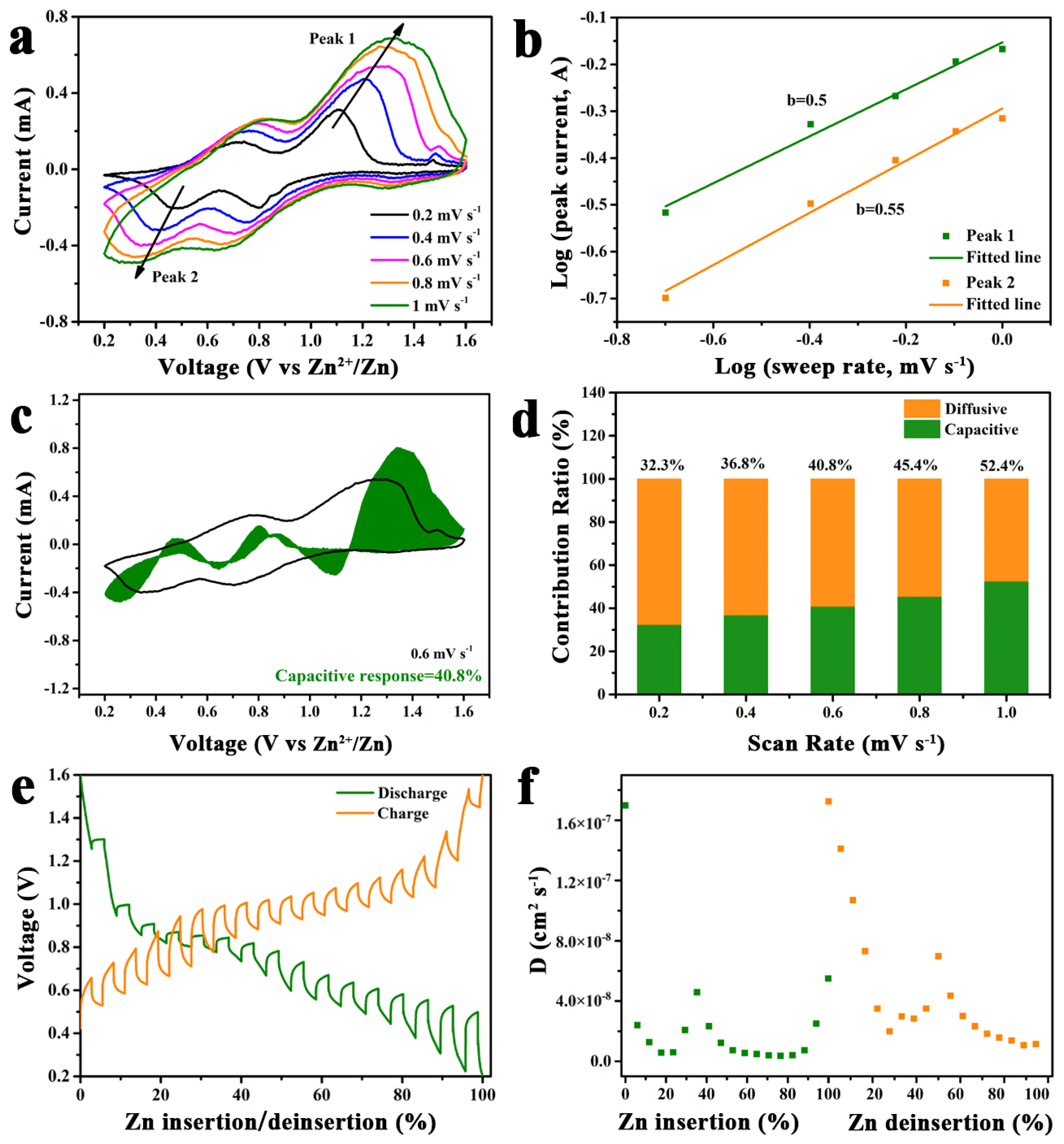
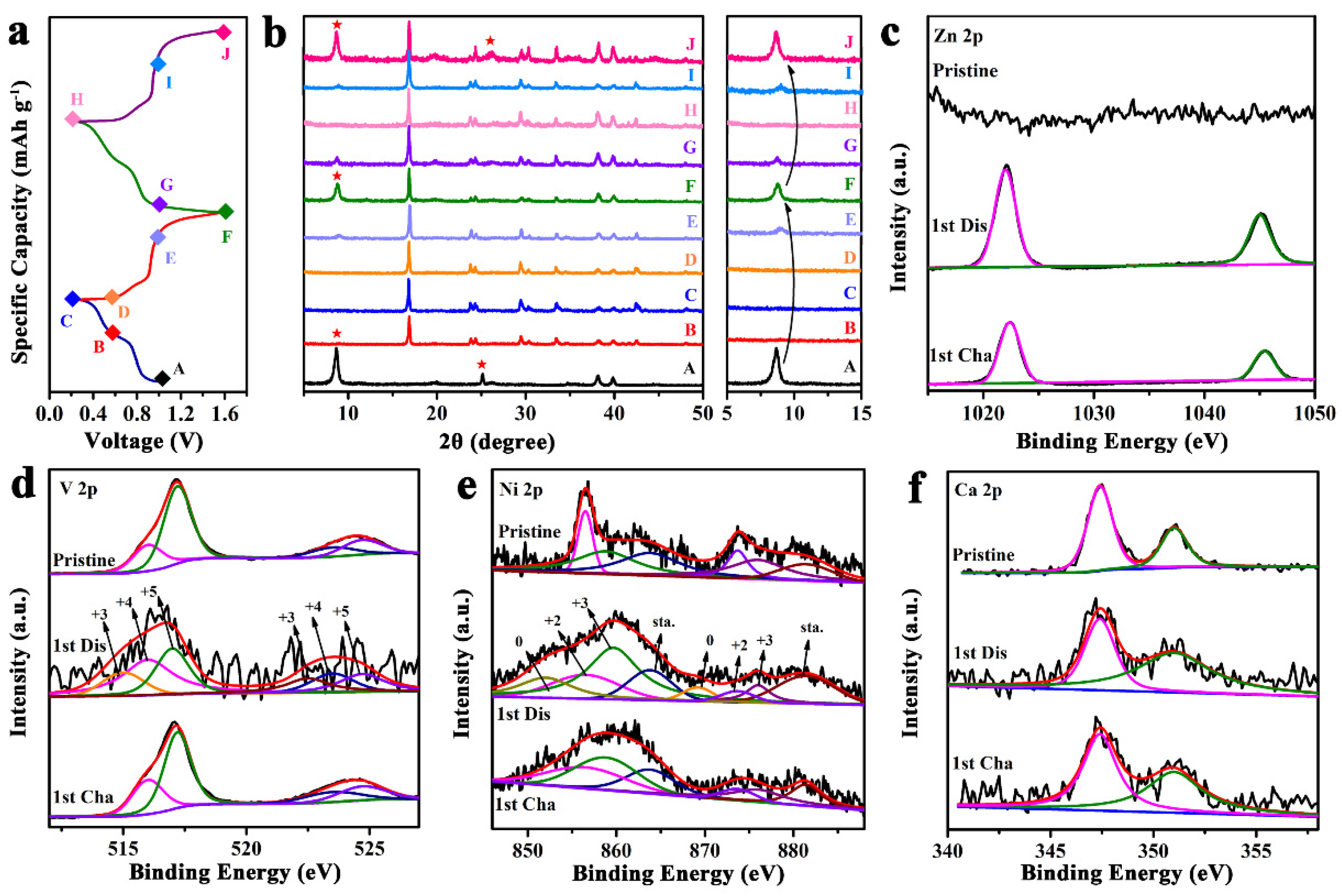
2. Results and Discussion
3. Materials and Methods
3.1. Materials
3.2. Material Synthesis
3.3. Characterizations
3.4. Electrochemical Measurements
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Jia, X.; Liu, C.; Neale, Z.G.; Yang, J.; Cao, G. Active materials for aqueous Zinc ion batteries: Synthesis, crystal structure, morphology, and electrochemistry. Chem. Rev. 2020, 120, 7795–7866. [Google Scholar] [CrossRef]
- Zhang, D.; Wang, W.; Li, S.; Shen, X.; Xu, H. Design strategies and energy storage mechanisms of MOF-based aqueous Zinc ion battery cathode materials. Energy Storage Mater. 2024, 69, 103436. [Google Scholar] [CrossRef]
- Liang, Y.; Yao, Y. Designing modern aqueous batteries. Nat. Rev. Mater. 2023, 8, 109–122. [Google Scholar] [CrossRef]
- Zhou, T.; Gao, G. Pre-intercalation strategy in vanadium oxides cathodes for aqueous Zinc ion batteries: Review and prospects. J. Energy Storage 2024, 84, 110808. [Google Scholar] [CrossRef]
- Du, W.; Ang, E.H.; Yang, Y.; Zhang, Y.; Ye, M.; Li, C. Challenges in the material and structural design of Zinc anode towards high-performance aqueous zinc-ion batteries. Energy Environ. Sci. 2020, 13, 3330–3360. [Google Scholar] [CrossRef]
- Deng, S.; Yuan, Z.; Tie, Z.; Wang, C.; Song, L.; Niu, Z. Electrochemically induced metal-organic-framework-derived amorphous V2O5 for superior rate aqueous Zinc-ion batteries. Angew. Chem. Int. Ed. 2020, 59, 22002–22006. [Google Scholar] [CrossRef] [PubMed]
- Li, G.; Sun, L.; Zhang, S.; Zhang, C.; Jin, H.; Davey, K.; Liang, G.; Liu, S.; Mao, J.; Guo, Z. Developing cathode materials for aqueous Zinc ion batteries: Challenges and practical prospects. Adv. Funct. Mater. 2024, 34, 2301291. [Google Scholar] [CrossRef]
- Zeng, Y.; Luan, D.; Lou, X.W. Recent advances in electrode engineering strategies for aqueous Zn-based batteries. Chem 2023, 9, 1118–1146. [Google Scholar] [CrossRef]
- Chen, X.; Zhang, H.; Liu, J.H.; Gao, Y.; Cao, X.; Zhan, C.; Wang, Y.; Wang, S.; Chou, S.L.; Dou, S.X.; et al. Vanadium-based cathodes for aqueous Zinc-ion batteries: Mechanism, design strategies and challenges. Energy Storage Mater. 2022, 50, 21–46. [Google Scholar] [CrossRef]
- Zhang, N.; Wang, J.; Guo, Y.; Wang, P.; Zhu, Y.; Yi, T. Insights on rational design and energy storage mechanism of Mn-based cathode materials towards high performance aqueous Zinc-ion batteries. Coordin. Chem. Rev. 2023, 479, 215009. [Google Scholar] [CrossRef]
- Zeng, Y.; Lu, X.F.; Zhang, S.; Luan, D.; Li, S.; Lou, X. Construction of Co-Mn prussian blue analog hollow spheres for efficient aqueous Zn-ion batteries. Angew. Chem. Int. Ed. 2021, 60, 22189–22194. [Google Scholar] [CrossRef] [PubMed]
- Chu, J.; Liu, Z.; Yu, J.; Cheng, L.; Wang, H.G.; Cui, F.; Zhu, G. Boosting H+ storage in aqueous Zinc ion batteries via integrating redox-active sites into hydrogen-bonded organic frameworks with strong π-π stacking. Angew. Chem. Int. Ed. 2024, 15, e202314411. [Google Scholar]
- Kundu, D.; Adams, B.D.; Duffort, V.; Vajargah, S.H.; Nazar, L.F. A high-capacity and long-life aqueous rechargeable Zinc battery using a metal oxide intercalation cathode. Nat. Energy 2016, 1, 16119. [Google Scholar] [CrossRef]
- Zhu, K.; Wu, T.; Huang, K. NaCa0.6V6O16·3H2O as an ultra-stable cathode for Zn-ion batteries: The roles of pre-inserted dual-cations and structural water in V3O8 layer. Adv. Energy Mater. 2019, 9, 1901968. [Google Scholar] [CrossRef]
- Ran, Y.; Ren, J.; Yang, Z.C.; Zhao, H.; Wang, Y.; Le, Y. The 3D flower-like MnV12O31·10H2O as a high-capacity and long-lifespan cathode material for aqueous Zinc-ion batteries. Small Struct. 2023, 4, 2300136. [Google Scholar] [CrossRef]
- Deng, W.; Zhou, Z.; Li, Y.; Zhang, M.; Yuan, X.; Hu, J.; Li, Z.; Li, C.; Li, R. High-capacity layered magnesium vanadate with concentrated gel electrolyte toward high-performance and wide temperature Zinc-ion battery. ACS Nano 2020, 14, 15776–15785. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Neale, Z.; Zheng, J.; Jia, X.; Huang, J.; Yan, M.; Tian, M.; Wang, M.; Yang, J.; Cao, G. Expanded hydrated vanadate for high-performance aqueous Zinc-ion batteries. Energy Environ. Sci. 2019, 12, 2273–2285. [Google Scholar] [CrossRef]
- Hu, P.; Zhu, T.; Wang, X.; Wei, X.; Yan, M.; Li, J.; Luo, W.; Yang, W.; Zhang, W.; Zhou, L.; et al. Highly Durable Na2V6O16·1.63H2O nanowire cathode for aqueous Zinc-ion battery. Nano Lett. 2018, 18, 1758–1763. [Google Scholar] [CrossRef]
- Chen, S.; Li, K.; Hui, K.S.; Zhang, J. Regulation of lamellar structure of vanadium oxide via polyaniline intercalation for high-performance aqueous Zinc-ion battery. Adv. Funct. Mater. 2020, 30, 2003890. [Google Scholar] [CrossRef]
- Yi, T.F.; Qiu, L.; Qu, J.P.; Liu, H.; Zhang, J.H.; Zhu, Y.R. Towards high-performance cathodes: Design and energy storage mechanism of vanadium oxides-based materials for aqueous Zn-ion batteries. Coordin. Chem. Rev. 2021, 446, 214124. [Google Scholar] [CrossRef]
- Liu, S.; Zhu, H.; Zhang, B.; Li, G.; Zhu, H.; Ren, Y.; Geng, H.; Yang, Y.; Liu, Q.; Li, C.C. Tuning the kinetics of Zinc-ion insertion/extraction in V2O5 by in situ polyaniline intercalation enables improved aqueous Zinc-ion storage performance. Adv. Mater. 2022, 32, 2001113. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; McColl, K.; Lu, X.; Sathasivam, S.; Dong, H.; Kang, L.; Li, Z.; Zhao, S.; Kafizas, A.G.; Wang, R.; et al. Multi-scale investigations of δ-Ni0.25V2O5·nH2O cathode materials in aqueous Zinc-ion batteries. Adv. Energy Mater. 2020, 10, 2000058. [Google Scholar] [CrossRef]
- Zhou, T.; Xie, L.; Han, Q.; Yang, X.; Zhu, L.; Cao, X. Investigation of Na6V10O28 as a promising rechargeable aqueous zinc-ion batteries cathode. Chem. Eng. J. 2022, 445, 136789. [Google Scholar] [CrossRef]
- Sun, Q.; Cheng, H.; Yuan, Y.; Liu, Y.; Nie, W.; Zhao, K.; Wang, K.; Yao, W.; Lu, X.; Lu, J. Uncovering the fundamental role of interlayer water in charge storage for bilayered V2O5·nH2O xerogel cathode materials. Adv. Energy Mater. 2023, 13, 2202515. [Google Scholar] [CrossRef]
- Dai, Y.; Liao, X.; Yu, R.; Li, J.; Li, J.; Tan, S.; He, P.; An, Q.; Wei, Q.; Chen, L.; et al. Quicker and more Zn2+ storage predominantly from the interface. Adv. Mater. 2021, 33, 2100359. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Liu, Y.; Wu, X. Pre-intercalation strategies to layered vanadium-based electrode materials for aqueous Zinc batteries. Batter. Supercaps 2023, 6, e202200461. [Google Scholar] [CrossRef]
- Wan, F.; Niu, Z. Design strategies for Vanadium-based aqueous Zinc-ion batteries. Angew. Chem. Int. Ed. 2019, 58, 16358–16367. [Google Scholar] [CrossRef]
- Xu, J.; Zhang, Y.; Liu, C.; Cheng, H.; Cai, X.; Jia, D.; Lin, H. Al3+ introduction hydrated vanadium oxide induced high performance for aqueous Zinc-ion batteries. Small 2022, 18, 2204180. [Google Scholar] [CrossRef]
- Wan, F.; Huang, S.; Cao, H.; Niu, Z. Freestanding potassium vanadate/carbon nanotube films for ultralong-life aqueous Zinc-ion batteries. ACS Nano 2020, 14, 6752–6760. [Google Scholar] [CrossRef]
- Hu, H.; Zhao, P.; Li, X.; Liu, J.; Liu, H.; Sun, B.; Pan, K.; Song, K.; Cheng, H. Heterojunction tunnelled vanadium-based cathode materials for high-performance aqueous Zinc ion batteries. J. Colloid Interface Sci. 2023, 665, 564–572. [Google Scholar] [CrossRef]
- Wang, W.; Liu, D.; Jiang, Y.; Zhang, D.; Shen, X.; Li, S.; Liang, J.; Xu, H. Mechanism enhancement of V3O7/V6O13 heterostructures to achieve high-performance aqueous Zn-ion batteries. Chem. Eng. J. 2023, 463, 142309. [Google Scholar] [CrossRef]
- Wang, Z.; Song, Y.; Wang, J.; Lin, Y.; Meng, J.; Cui, W.; Liu, X.X. Vanadium oxides with amorphous-crystalline heterointerface network for aqueous Zinc-ion batteries. Angew. Chem. Int. Ed. 2023, 135, e202216290. [Google Scholar]
- Zhang, G.; Yang, H.; Zhou, H.; Huang, T.; Yang, Y.; Zhu, G.; Zhang, Y.; Pang, H. MXene-mediated interfacial growth of 2D-2D heterostructured nanomaterials as cathodes for Zn-based aqueous batteries. Angew. Chem. Int. Ed. 2024, 63, e202401903. [Google Scholar] [CrossRef] [PubMed]
- Shan, L.; Wang, Y.; Liang, S.; Tang, B.; Yang, Y.; Wang, Z.; Lu, B.; Zhou, J. Interfacial adsorption–insertion mechanism induced by phase boundary toward better aqueous Zn-ion battery. InfoMat 2021, 3, 1028–1036. [Google Scholar] [CrossRef]
- Zhang, X.; Yang, W.; Liu, J.; Zhou, Y.; Feng, S.; Yan, S.; Yao, Y.; Wang, G.; Wan, L.; Fang, C.; et al. Ultralong metahewettite CaV6O16·3H2O nanoribbons as novel host materials for lithium storage: Towards high-rate and excellent long-term cyclability. Nano Energy 2016, 22, 38–47. [Google Scholar] [CrossRef]
- Xu, N.; Lian, X.; Huang, H.; Ma, Y.; Li, L.; Peng, S. CaV6O16·3H2O nanorods as cathode for high-performance aqueous Zinc-ion battery. Mater. Lett. 2021, 287, 129285. [Google Scholar] [CrossRef]
- Wei, M.; Luo, W.; Yu, D.; Liang, X.; Wei, W.; Gao, M.; Sun, S.; Zhu, Q.; Liu, G. Layered Ni0.22V2O5·nH2O as high-performance cathode material for aqueous Zinc-ion batteries. Ionics 2021, 27, 4801–4809. [Google Scholar] [CrossRef]
- Londoño-Calderón, C.L.; Vargas-Hernández, C.; Jurado, J.F. Desorption influence of water on structural, electrical properties and molecular order of vanadium pentoxide xerogel films. Rev. Mex. Fis. 2010, 56, 411–415. [Google Scholar]
- Liu, C.; Tian, M.; Wang, M.; Zheng, J.; Wang, S.; Yan, M.; Wang, Z.; Yin, Z.; Yang, J.; Cao, G. Catalyzing Zinc-ion intercalation in hydrated vanadates for aqueous Zinc-ion batteries. J. Mater. Chem. 2020, 8, 7713–7723. [Google Scholar] [CrossRef]
- Ji, S.; Jiang, T.; Xu, K.; Li, S. FTIR study of the adsorption of water on ultradispersed diamond powder surface. Appl. Surf. Sci. 1998, 133, 231–238. [Google Scholar] [CrossRef]
- Huang, S.; He, S.; Qin, H.; Hou, X. Oxygen defect hydrated vanadium dioxide/graphene as a superior cathode for aqueous Zn batteries. ACS Appl. Mater. Interfaces 2021, 13, 44379–44388. [Google Scholar] [CrossRef]
- Yi, T.F.; Qiu, L.Y.; Mei, J.; Qi, S.Y.; Cui, P.; Luo, S.; Zhu, Y.R.; Xie, Y.; He, Y.B. Porous spherical NiO@NiMoO4@PPy nanoarchitectures as advanced electrochemical pseudocapacitor materials. Sci. Bull. 2020, 65, 546–556. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Tang, Y.; Fang, G.; Shan, L.; Guo, J.; Zhang, W.; Wang, C.; Wang, L.; Zhou, J.; Liang, S. Li+ intercalated V2O5·nH2O with enlarged layer spacing and fast ion diffusion as an aqueous Zinc-ion battery cathode. Energy Environ. Sci. 2018, 11, 3157–3162. [Google Scholar] [CrossRef]
- Hu, B.; Cheng, H.; Huang, C.; Aslam, M.K.; Liu, L.; Xu, C.; Chen, P.; Yu, D.; Chen, C. The controlled study of surfactants on the morphologies of three-dimensional turbine-like V2O5 for the application of high performance lithium ion storage. Solid State Ionics 2019, 342, 115059. [Google Scholar] [CrossRef]
- Hu, B.; Xu, C.; Aslam, M.K.; Cen, Y.; Hu, J.; Li, Y.; Liu, Y.; Guo, C.; Yu, D.; Chen, C. La-doped V2O5·nH2O@OAB and flexible Fe2O3@rGO as binder-free thin film electrodes for asymmetric supercapacitors. Chem. Eng. J. 2020, 389, 123534. [Google Scholar] [CrossRef]
- Wang, A.; Liu, D.H.; Yang, L.; Xu, F.; Luo, D.; Dou, H.; Song, M.; Xu, C.; Zhang, B.; Zheng, J.; et al. Building stabilized Cu0.17Mn0.03V2O5-□2.16H2O cathode enables an outstanding room-/low-temperature aqueous Zn-ion batteries. Carbon Energy 2024, 13, 512. [Google Scholar] [CrossRef]
- Fan, L.; Li, Z.; Kang, W. Spontaneous growth of alkali metal ion-preintercalated vanadium pentoxide for high-performance aqueous Zinc-ion batteries. ACS Sustain. Chem. Eng. 2021, 9, 5095–5104. [Google Scholar] [CrossRef]
- He, P.; Zhang, G.; Liao, X.; Yan, M.; Xu, X.; An, Q.; Liu, J.; Mai, L. Sodium ion stabilized vanadium oxide nanowire cathode for high-performance Zinc-ion batteries. Adv. Energy Mater. 2018, 8, 1702463. [Google Scholar] [CrossRef]
- Cai, Y.; Liu, F.; Luo, Z.; Fang, G.; Zhou, J.; Pan, A.; Liang, S. Pilotaxitic Na1.1V3O7.9 nanoribbons/graphene as high-performance sodium ion battery and aqueous Zinc ion battery cathode. Energy Storage Mater. 2018, 13, 168–174. [Google Scholar] [CrossRef]
- Chen, Z.; Liu, H.; Fan, S.; Zhang, Q.; Yuan, C.; Peng, W.; Li, Y.; Fan, X. Inhibition of vanadium cathode dissolution in Zinc-ion batteries on thermodynamics and kinetics by guest pre-intercalation. Adv. Energy Mater. 2024, 14, 2400977. [Google Scholar] [CrossRef]
- Sun, T.; Nian, Q.; Zheng, S.; Shi, J.; Tao, Z. Layered Ca0.28MnO2·0.5H2O as a high performance cathode for aqueous Zinc-ion battery. Small 2020, 16, 2000597. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Niu, S.; Gong, S.; Ju, N.; Jiang, T.; Wang, Y.; Zhang, X.; Sun, Q.; Sun, H. Fused functional organic material with the alternating conjugation of quinone-pyrazine as cathode for aqueous Zinc ion batteries. Small 2024, 7, 2301301. [Google Scholar] [CrossRef] [PubMed]
- Tan, Y.; An, F.; Liu, Y.; Li, S.; He, P.; Zhang, N.; Li, P.; Qu, X. Reaction kinetics in rechargeable Zinc-ion batteries. J. Power Sources 2021, 492, 229655. [Google Scholar] [CrossRef]
- Sun, Q.; Cheng, H.; Sun, C.; Liu, Y.; Nie, W.; Zhao, K.; Lu, X.; Zhou, J. Architecting a hydrated Ca0.24V2O5 cathode with a facile desolvation interface for superior-performance aqueous Zinc ion batteries. ACS Appl. Mater. Interfaces 2021, 13, 60035–60045. [Google Scholar] [CrossRef]
- Wang, X.; Naveed, A.; Zeng, T.; Wan, T.; Zhang, H.; Zhou, Y.; Dou, A.; Su, M.; Liu, Y.; Chu, D. Sodium ion stabilized ammonium vanadate as a high-performance aqueous Zinc-ion battery cathode. Chem. Eng. J. 2022, 446, 137090. [Google Scholar] [CrossRef]
- Xia, X.; Yun, J.; Huang, C.; Li, D.; Yang, Z.; Huang, H.; Zhang, W. Ca/Ni codoping enables the integration of high-rate and high-capacity Zn-ion storage performances for layered hydrated vanadate. Eng. Chem. Res. 2022, 61, 4212–4221. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, S.; Wang, Y.; Wu, Y.; Zhang, G.; Chen, Y.; Wang, F.; Fan, L.; Yang, L.; Wu, Q. Hydrated Metal Vanadate Heterostructures as Cathode Materials for Stable Aqueous Zinc-Ion Batteries. Molecules 2024, 29, 3848. https://doi.org/10.3390/molecules29163848
Zhang S, Wang Y, Wu Y, Zhang G, Chen Y, Wang F, Fan L, Yang L, Wu Q. Hydrated Metal Vanadate Heterostructures as Cathode Materials for Stable Aqueous Zinc-Ion Batteries. Molecules. 2024; 29(16):3848. https://doi.org/10.3390/molecules29163848
Chicago/Turabian StyleZhang, Siqi, Yan Wang, Yunyu Wu, Guanlun Zhang, Yanli Chen, Fengyou Wang, Lin Fan, Lili Yang, and Qiong Wu. 2024. "Hydrated Metal Vanadate Heterostructures as Cathode Materials for Stable Aqueous Zinc-Ion Batteries" Molecules 29, no. 16: 3848. https://doi.org/10.3390/molecules29163848
APA StyleZhang, S., Wang, Y., Wu, Y., Zhang, G., Chen, Y., Wang, F., Fan, L., Yang, L., & Wu, Q. (2024). Hydrated Metal Vanadate Heterostructures as Cathode Materials for Stable Aqueous Zinc-Ion Batteries. Molecules, 29(16), 3848. https://doi.org/10.3390/molecules29163848






