Recent Advances in Alkaloids from Papaveraceae in China: Structural Characteristics and Pharmacological Effects
Abstract
1. Introduction
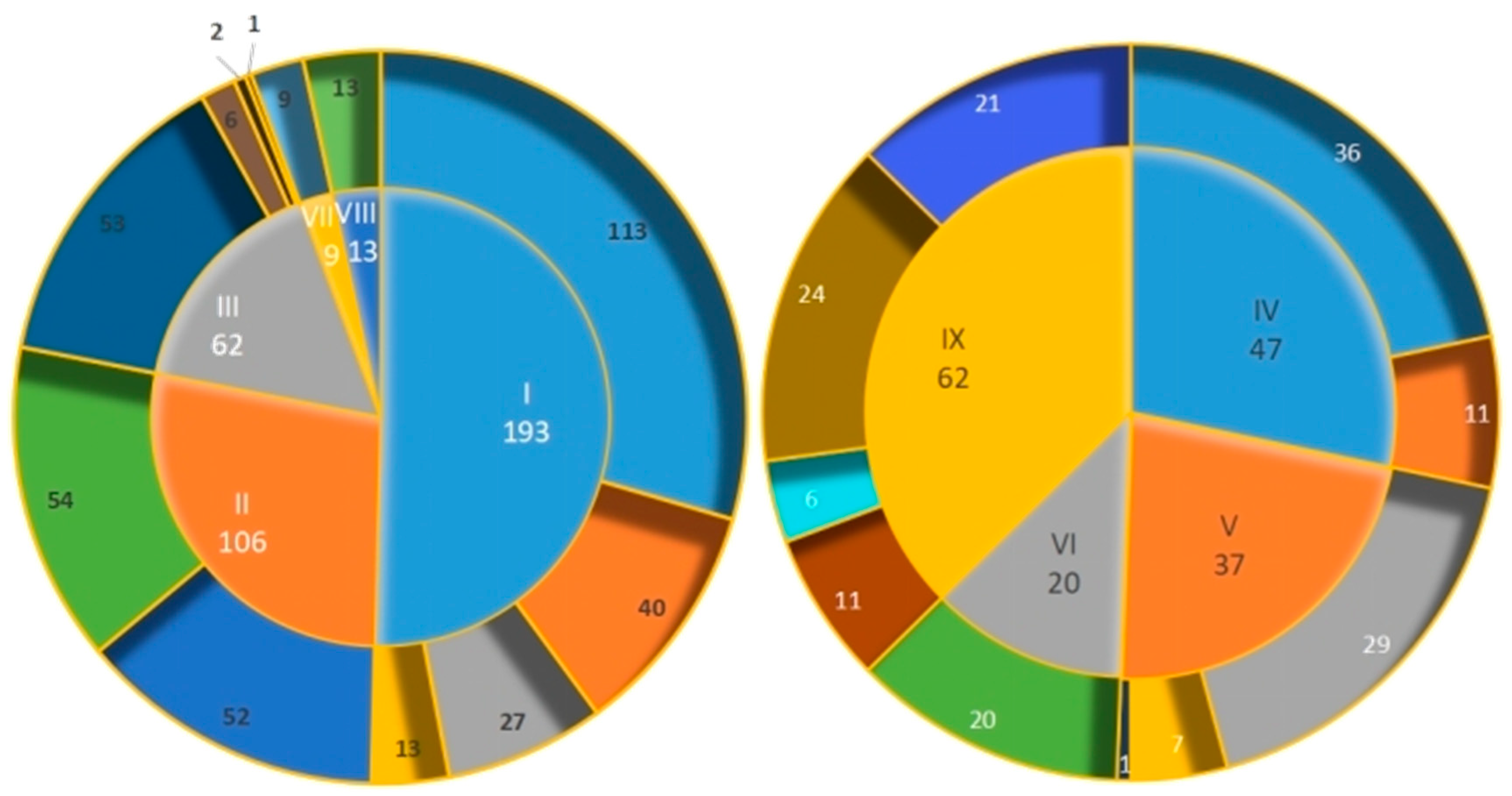
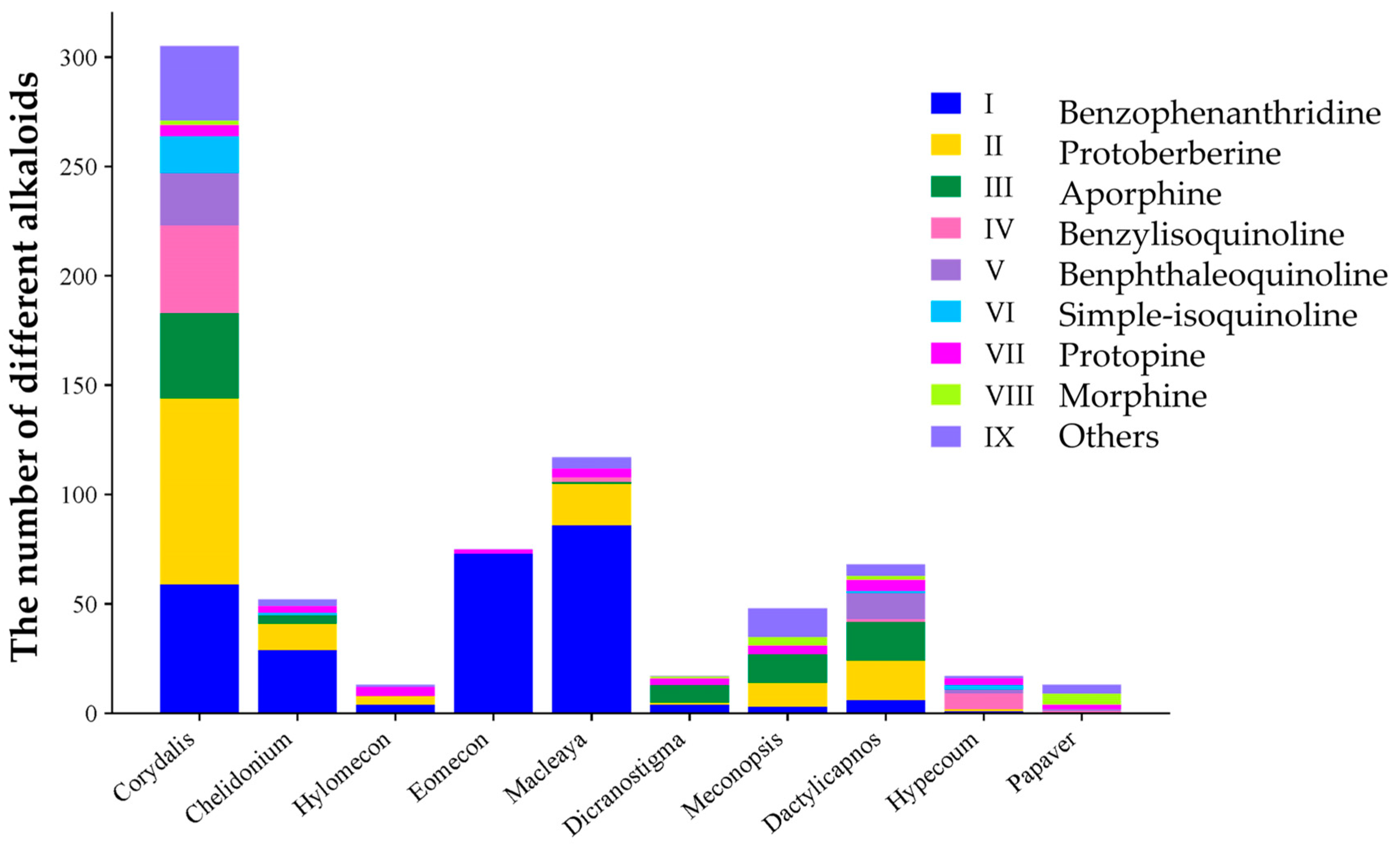
2. Alkaloids
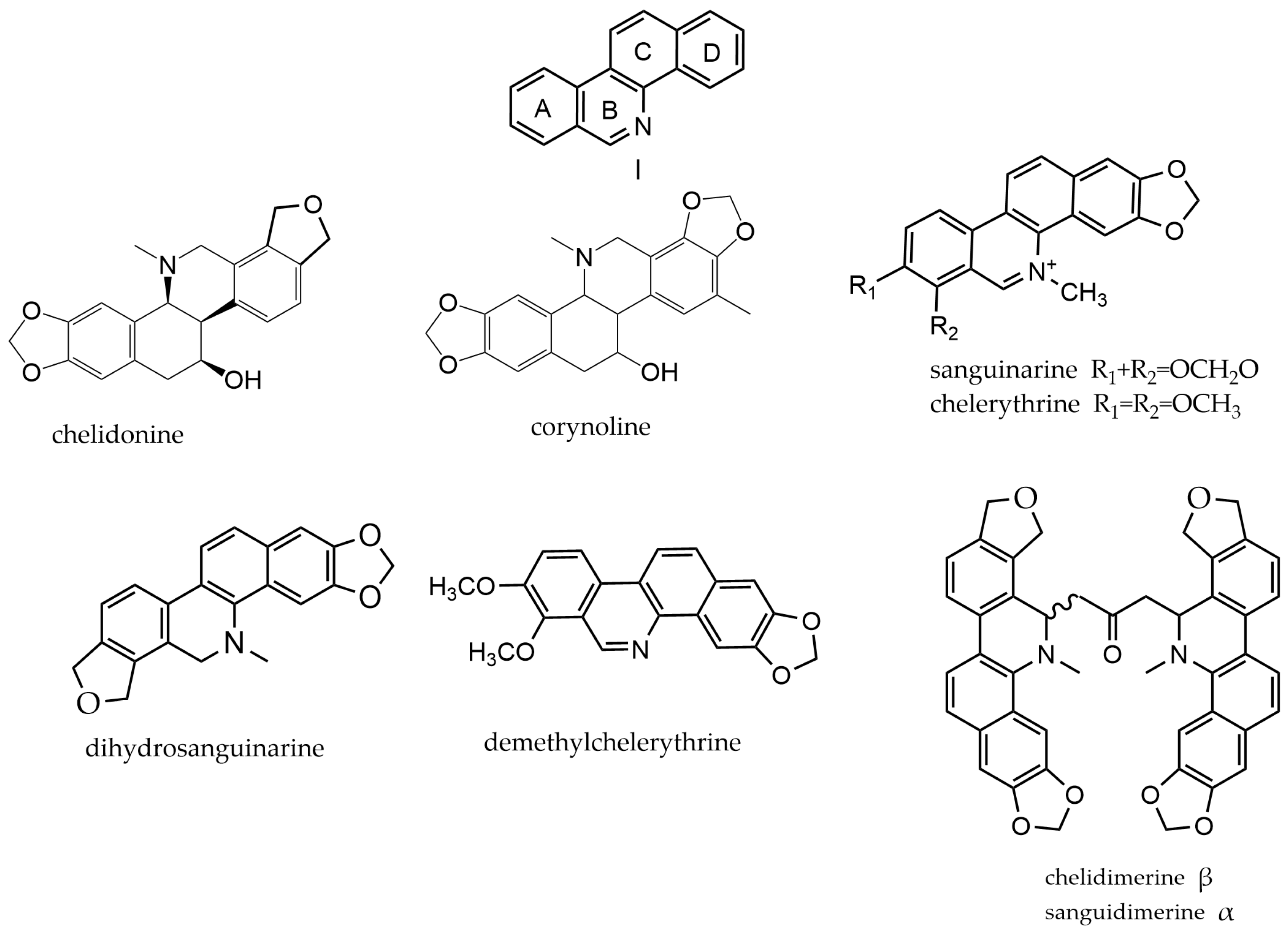
2.1. Benzophenanthridine Alkaloids
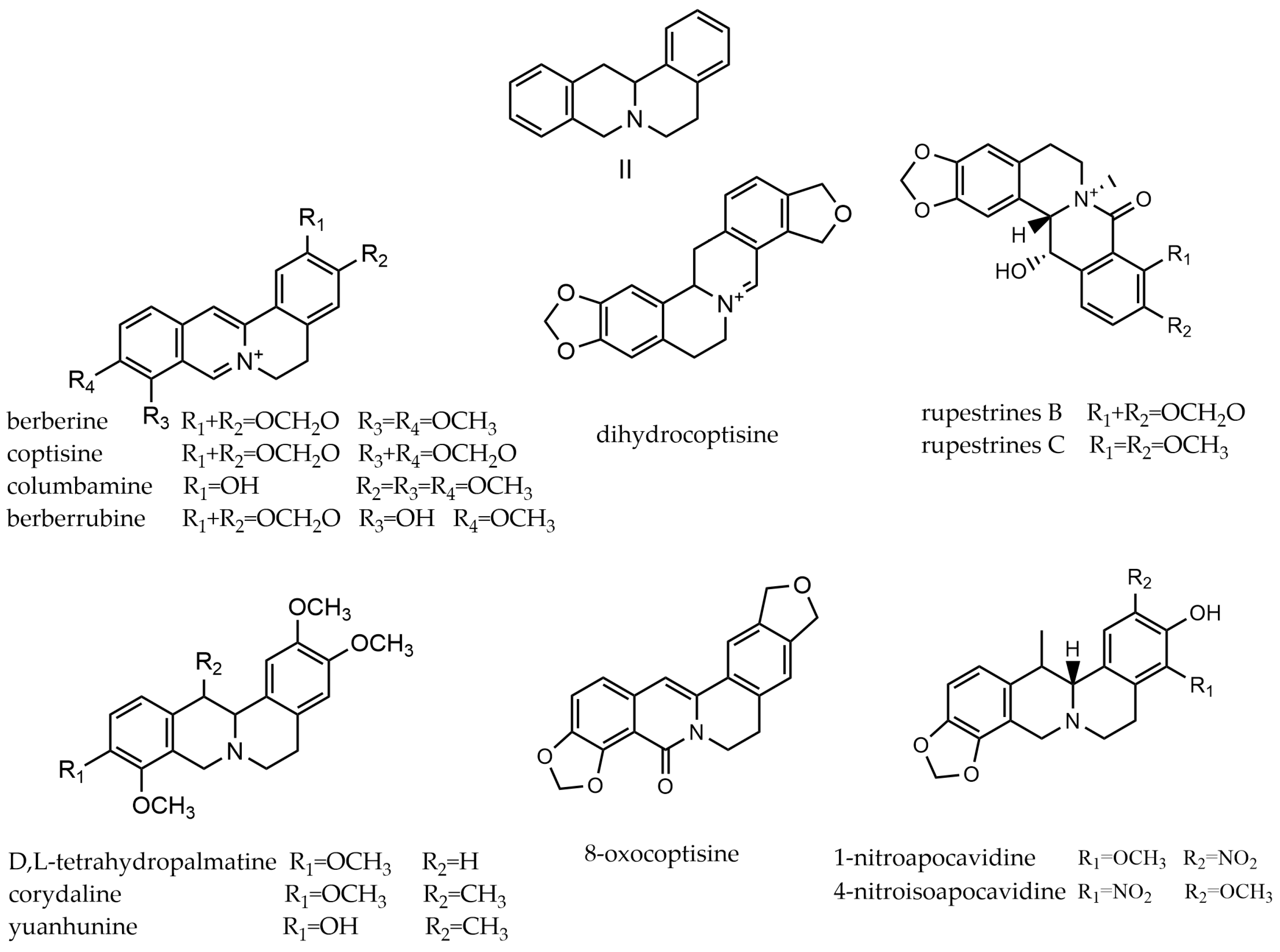
2.2. Protoberberine Alkaloids
2.3. Aporphine Alkaloids
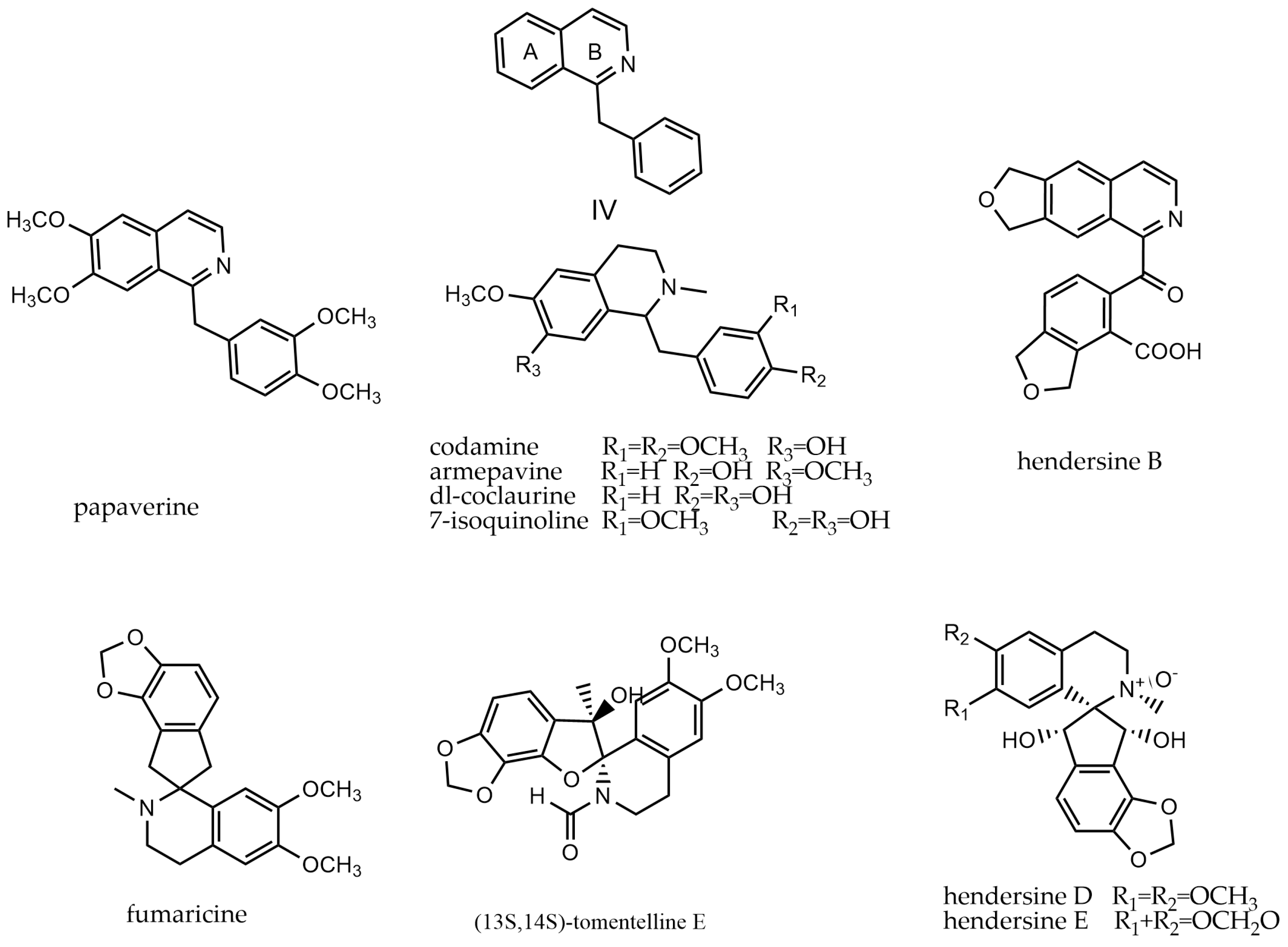
2.4. Benzylisoquinoline Alkaloids
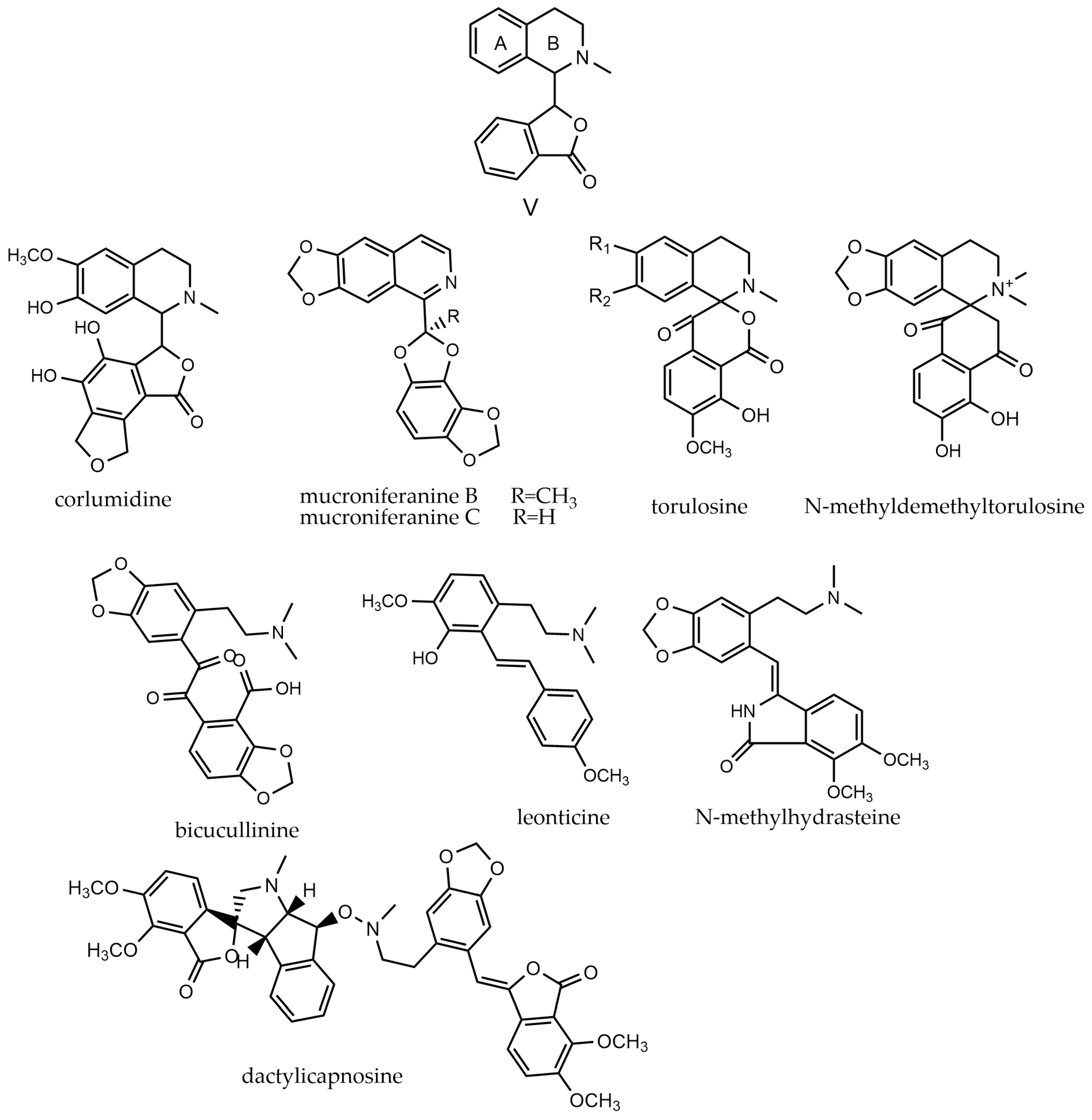
2.5. Benphthaleoquinoline Alkaloids
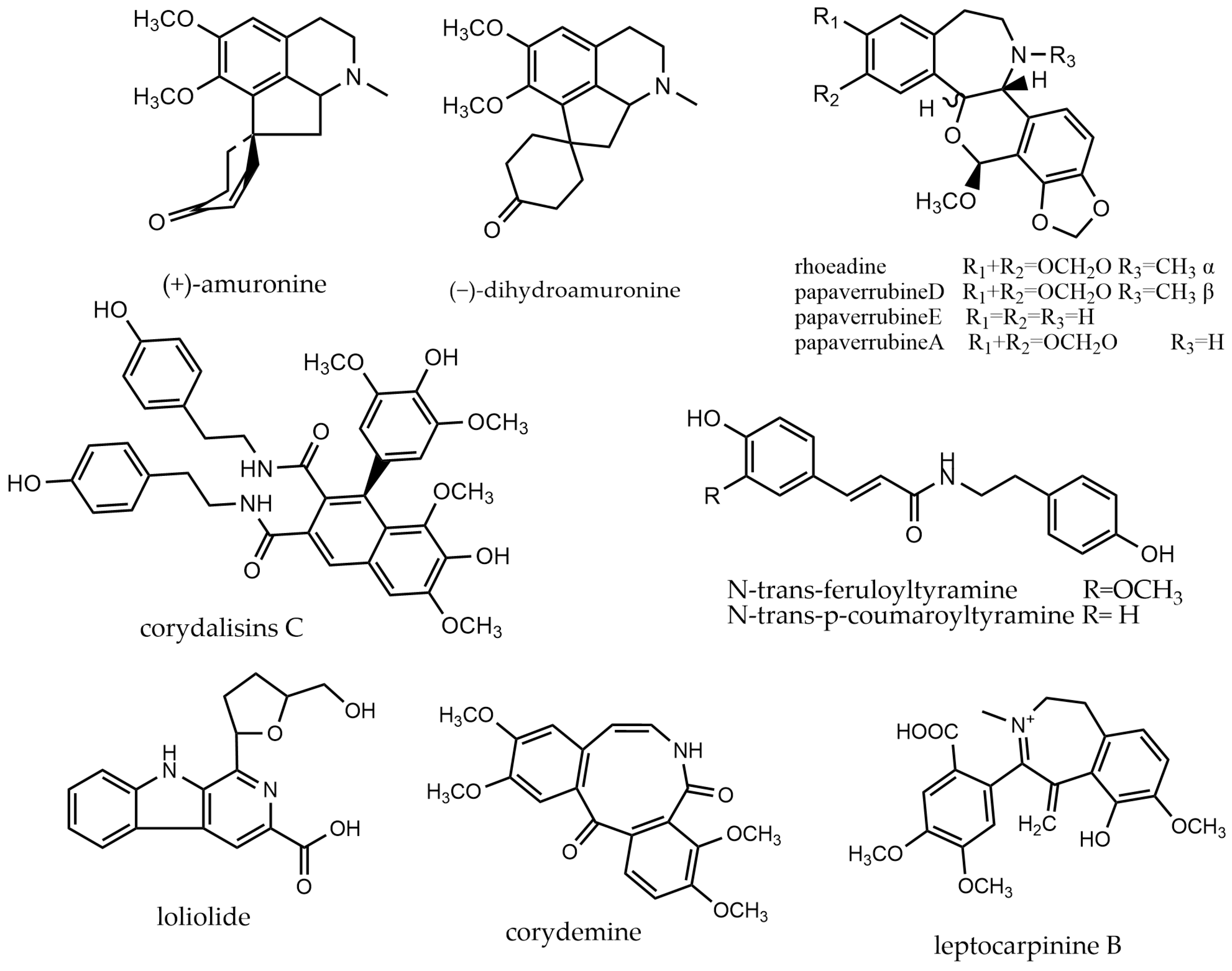
2.6. Simple-Isoquinoline Alkaloids
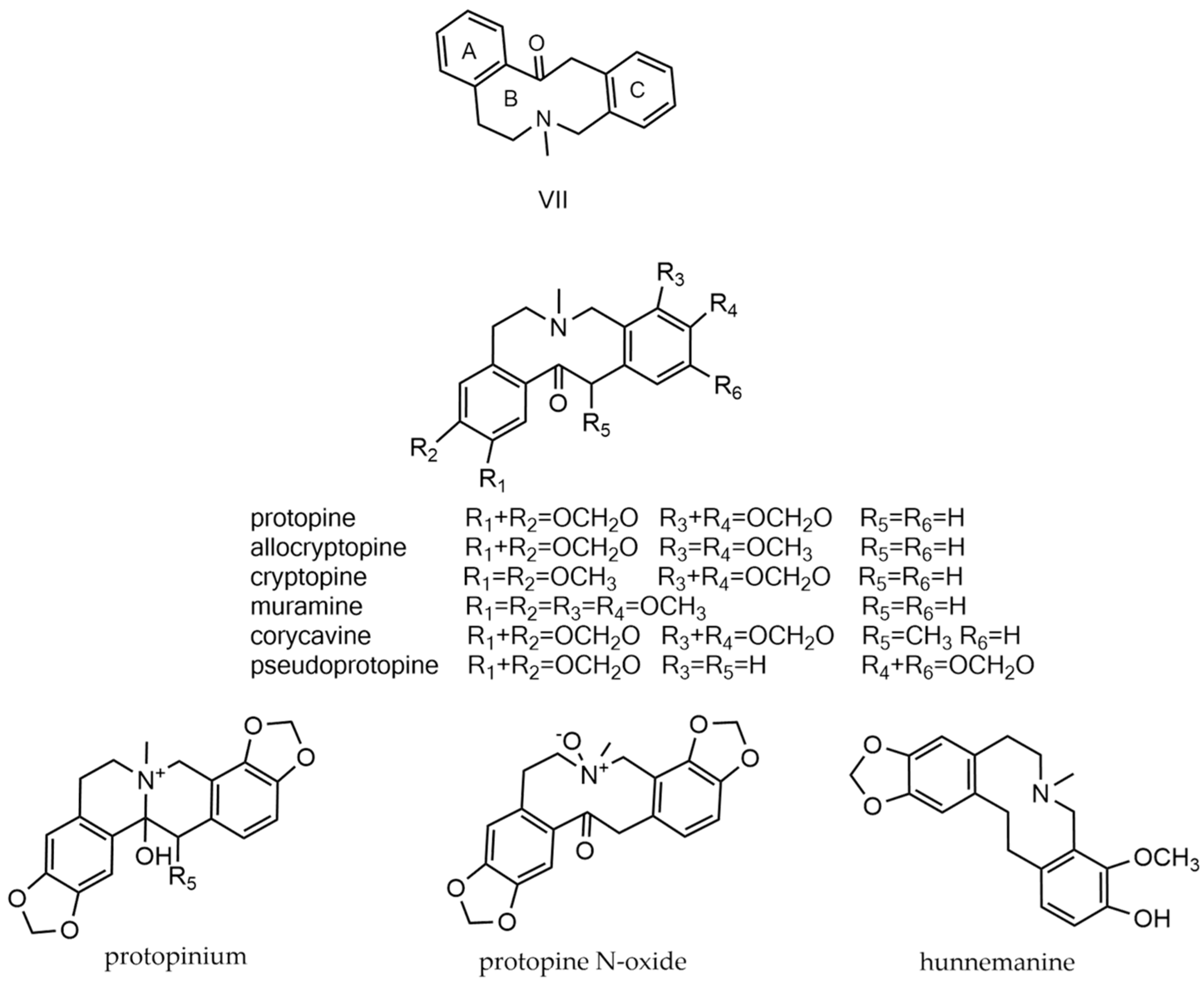
2.7. Protopine Alkaloids
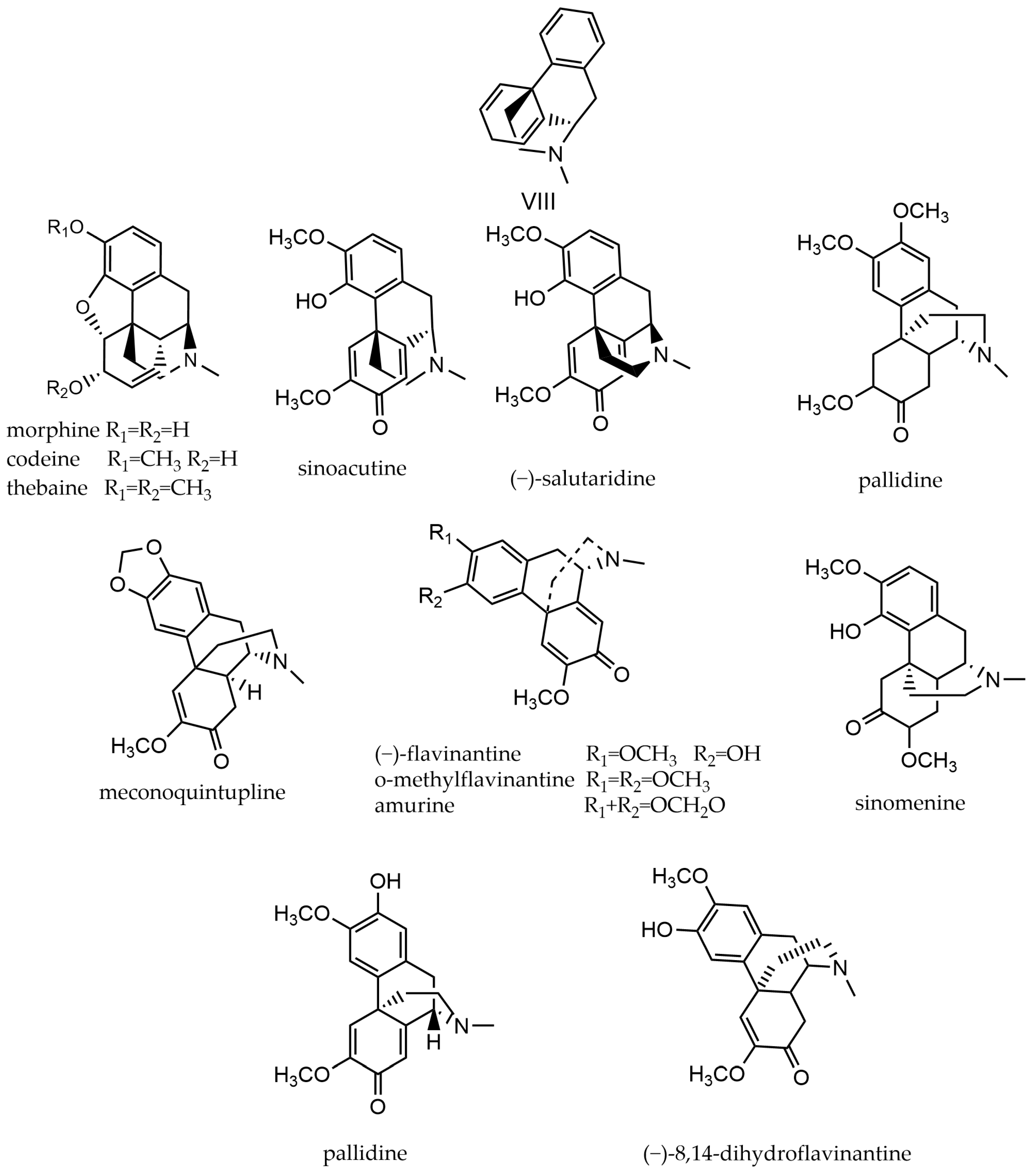
2.8. Morphine Alkaloids
2.9. Other Alkaloids
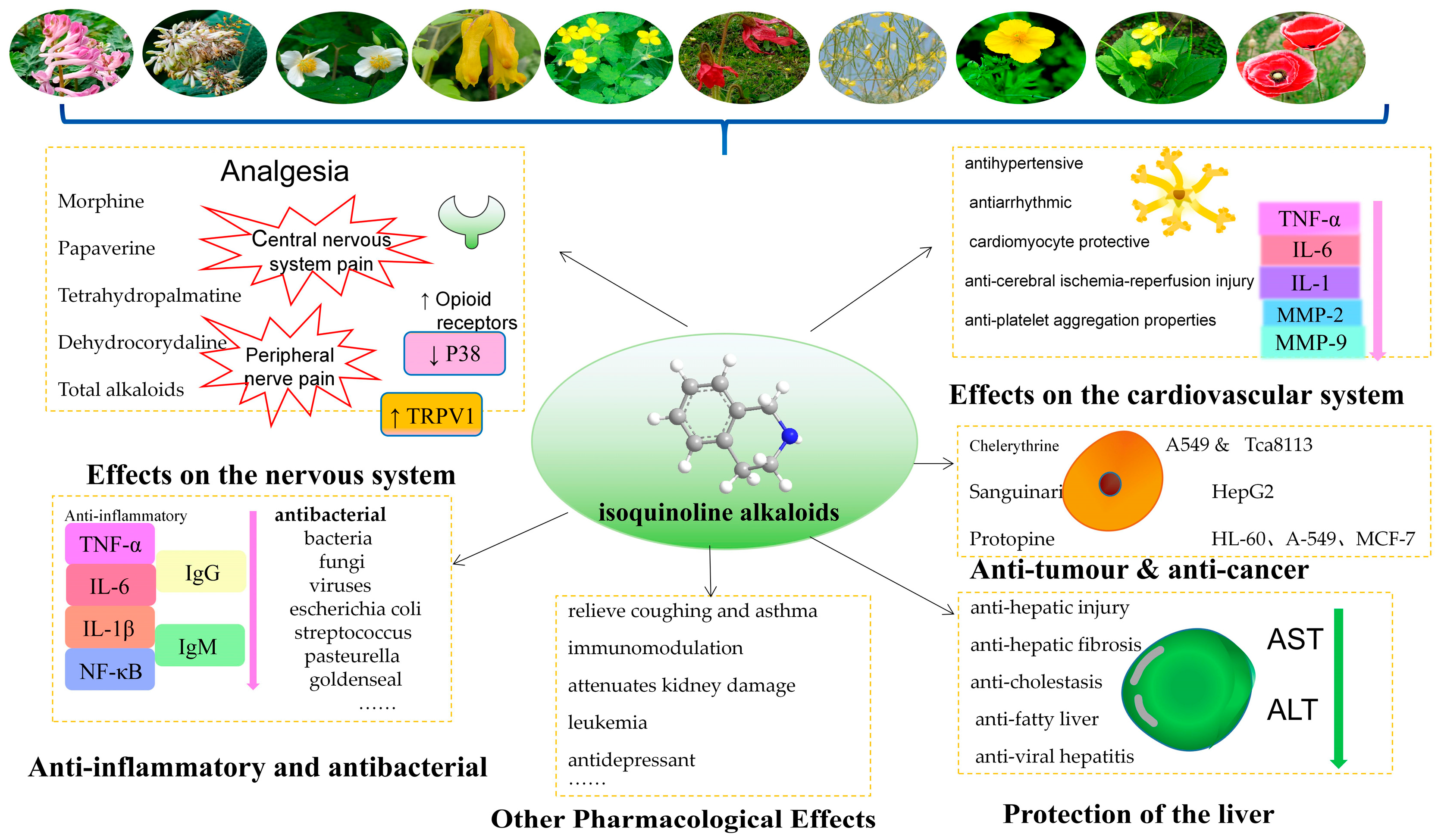
3. Pharmacological Actions and Clinical Applications
3.1. Effects on the Nervous System
3.2. The Importance of the Cardiovascular System
3.3. Anti-Inflammatory and Antibacterial Effects
3.4. Anti-Tumor and Anti-Cancer Effects
3.5. Protection of the Liver
3.6. Antitussive Effects and Other Pharmacological Effects
4. Conclusions and Prospects
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Beaudoin, G.A.W.; Facchini, P.J. Benzylisoquinoline alkaloid biosynthesis in opium poppy. Planta 2014, 240, 19–32. [Google Scholar] [CrossRef]
- Liu, Y.-Y.; Kan, S.-L.; Wang, J.-L.; Cao, Y.-N.; Li, J.-M. Complete chloroplast genome sequences of Corydalis edulis and Corydalis shensiana (Papaveraceae). Mitochondrial DNA Part B 2021, 6, 257–258. [Google Scholar] [CrossRef]
- Jiang, L.; Li, M.; Zhao, F.; Chu, S.; Zha, L.; Xu, T.; Peng, H.; Zhang, W. Molecular Identification and Taxonomic Implication of Herbal Species in Genus Corydalis (Papaveraceae). Molecules 2018, 23, 1393. [Google Scholar] [CrossRef]
- Editorial Committee of Chinese Plants, Chinese Academy of Sciences. Flora of China; Beijing Science Press: Beijing, China, 2004; Volume 32. [Google Scholar]
- Butnariu, M.; Quispe, C.; Herrera-Bravo, J.; Pentea, M.; Sarac, I.; Küşümler, A.S.; Özçelik, B.; Painuli, S.; Semwal, P.; Imran, M.; et al. Papaver Plants: Current Insights on Phytochemical and Nutritional Composition Along with Biotechnological Applications. Oxidative Med. Cell. Longev. 2022, 2022, 2041769. [Google Scholar] [CrossRef] [PubMed]
- Calixto, B.; Campos, M.M.; Santos, A.R.S. Botanical analgesic and anti-inflammatory drugs. In Ethnopharmacology; Elisabetsky, E., Etkin, N.L., Eds.; Eolss Publishers: Oxford, UK, 2009; Volume II. [Google Scholar]
- Gracz-Bernaciak, J.; Mazur, O.; Nawrot, R. Functional Studies of Plant Latex as a Rich Source of Bioactive Compounds: Focus on Proteins and Alkaloids. Int. J. Mol. Sci. 2021, 22, 12427. [Google Scholar] [CrossRef] [PubMed]
- Cui, Z.; Qi, M.L.; Yin, J.; Zhu, G. Chemical constituents of Corydalis yanhusuo and phytochemical taxonomy of related plants. Chinese Botanical Society. In Proceedings of the Abstracts of the 75th Anniversary Annual Meeting of the Chinese Botanical Society, Lanzhou, China, 12–15 July 2008; Lanzhou University Press: Lanzhou, China, 2008; Volume 2. [Google Scholar]
- Cheng, C.; Tian, R.N. Survey and Analysis of Corydalis Plants’ Prospects in the Nanjing Purple Mountain. Chin. J. Wild Plant Res. 2010, 29, 13–16. [Google Scholar]
- Yu, R.M.; Wang, C.S.; Song, L.Y. Progress on the Study of Chemical Constituents and Pharmacological Action for the Plants of Papaveraceae. Acad. J. Shanghai Univ. Tradit. Chin. Med. 2004, 38, 59–61. [Google Scholar]
- Zhang, X.P.; Shi, X.F.; Zhang, H.Y.; Zhu, R.Y.; Zhang, C.X. Establishment of HPLC Fingerprint and Content Determination of 5 Components in Papaveris Pericarpium. J. China Pharm. 2021, 32, 2755–2760. [Google Scholar]
- Yuan, Y.Z.; Huang, X.Y.; Zhang, X.; Li, D.H.; Bai, J.; Li, Z.L.; Hua, M.H. lsolation and identification of chemical constituents from Chelidonium majus L. Chin. J. Med. Chem. 2022, 32, 454–461. [Google Scholar]
- Zhao, T.Y. Studies on Separation and Characterization of Alkaloids in Chelidonium majus L. Master’s Thesis, Dalian Jiaotong University, Dalian, China, 2020. [Google Scholar]
- Zhang, W.-J.; You, C.-X.; Wang, C.-F.; Fan, L.; Wang, Y.; Su, Y.; Deng, Z.-W.; Du, S.-S. One new alkaloid from Chelidonium majus L. Nat. Prod. Res. 2014, 28, 1873–1878. [Google Scholar] [CrossRef]
- Li, W.B. Bioactive Compounds from Chelidonium majus L. Master’s Thesis, Northwest A&F University, Xianyang, China, 2015. [Google Scholar]
- Tian, B.; Tian, M.; Huang, S.-M. Advances in phytochemical and modern pharmacological research of Rhizoma corydalis. Pharm. Biol. 2020, 58, 265–275. [Google Scholar] [CrossRef] [PubMed]
- Chang, S.; Liu, Z.H.; Han, N.; Yang, Z.Y.; Wang, P.P.; Li, F.; Yin, J. Alkaloids from the tubers of Corydalis ambigua var. amurensis and their antitumor activities. Chin. Tradit. Pat. Med. 2022, 44, 3507–3513. [Google Scholar]
- Yan, L.P.; Xie, Z.H.; Sheng, W.S.; Huang, G.T.; Li, Q.L.; Wang, A.G.; Chen, Q.; Zhou, S.; Li, L.Y. Research Progress in Corydalis decumbens. J. Anhui Agric. Sci. 2022, 50, 5–7+10. [Google Scholar]
- Liao, H.P.; Ou, Y.H.; Huang, L.Q.; Huang, W.P.; Feng, Y.L.; Li, Z.F.; Wu, B.; Yang, S.L. Chemical constituents of Corydalis decumbens. Chin. Tradit. Herb. Drugs 2014, 45, 3067–3070. [Google Scholar]
- Yuan, J.S.; Gu, G.H.; Zeng, W.; Wang, S.Y.; Yang, D.J.; Guo, D.A. Simultaneous determination of 11 alkaloids in Corydalis decumbens by HPLC. J. Chin. Med. Mater. 2013, 36, 1283–1287. [Google Scholar]
- Liang, S.Y. Constituents and pharmacological studies of Corydalis decumbentis Rhizoma. J. Acta Med. Sin. 2007, 02, 419–421. [Google Scholar] [CrossRef]
- Zeng, W.L. Chemical Constituents and Their Biological Activity of the Corydalis decumbentis. Master’s Thesis, Shandong University, Jinan, China, 2005. [Google Scholar]
- Gao, C.; Ma, Y.Y.; Ni, B.; Guo, Y.C.; Wei, G.L. Research progress on chemical constituents and pharmacological effects of Corydalis bungeanae. J. Chin. Med. Mater. 2022, 45, 1780–1786. [Google Scholar]
- Xiang, Y.; Li, M.Z.; Xie, Y.P.; Qu, X.Y.; Tan, X.B.; Cui, G.Q.; Zhu, F.X. LC-MS identification of chemical constituents and metabolites of Corydalis bungeana. Chin. Tradit. Herb. Drugs 2022, 53, 1949–1963. [Google Scholar]
- Li, M.Z.; Zhang, H.; Zhu, F.X. Study on the chemical constituents of Corydalis bungeana. West China J. Pharm. Sci. 2019, 34, 120–126. [Google Scholar]
- Zheng, J.F.; Qin, M.J.; Zheng, Y.; Chen, C.L. Alkaloids from Corydalis bungeana. J. China Pharm. Univ. 2007, 38, 112–114. [Google Scholar]
- Zhang, Z.J.; Du, Q.; Wang, Q.; Zhou, X.J. Research progress of benzophenanthridine alkaloids in Eomecon chionantha. Chin. Tradit. Pat. Med. 2022, 44, 1562–1570. [Google Scholar]
- Peng, W.A.; Liao, H.Q.; Yi, C.Y. Study on Isolation and identification of Alkaloids from Eomecn chionantha. Guiding J. Tradit. Chin. Med. Pharm. 2018, 24, 64–65+69. [Google Scholar]
- Zhu, P.H.; Li, Y.Y.; Gong, H.; Cao, H.; Xiao, Y.H. Chemical constituents from Eomecn chionantha. Chin. Tradit. Pat. Med. 2017, 39, 980–983. [Google Scholar]
- Zhu, P.H. Study on the Chemical Components and Bioactivity of Eomecon chionantha Hance. Master’s Thesis, Wuhan Institute of Technology, Wuhan, China, 2017. [Google Scholar]
- Gui, Z.J.; Shen, K.; Huang, M.H.; Li, G.W.; Huang, T.Q.; Jin, W.H. Alkaloid constituents in Corydalis from different habitats. Nat. Prod. Res. Dev. 2023, 35, 410–419. [Google Scholar]
- Jin, S.Y.; Li, H.T.; Jiang, W.L.; Qin, M.J.; Xie, G.Y. Research progress on chemical constituents and pharmacological effects of Corydalis saxicola Bunting. Chin. Tradit. Pat. Med. 2022, 44, 1545–1552. [Google Scholar]
- Li, K.; Zhang, D.J.; Li, Z.Q.; Lu, D.X. Alkaloids in the Corydalis plants and their biological activities: Research advances. Int. J. Pharm. Res. 2018, 45, 748–757. [Google Scholar]
- Huang, Y.; Liu, Y.Y.; Aga, E.B.; Xie, H.J.; Xiong, H.; Ye, B.G. Study on chemical constituents of Meconopsis punicea. W. China J. Pharm. Sci. 2023, 38, 246–250. [Google Scholar]
- Gu, X.Y.; Geng, B.R.; Chen, H.G.; Duan, H.J.; Zhang, J.B.; Zhao, W.L. Systematic Research Progress on Resources, Chemistry and Pharmacology of Tibetan Medicine Meconopsis punicea Maxim. J. Chin. Wild Plant Res. 2022, 41, 50–55. [Google Scholar]
- Wu, H.F.; Ding, L.S.; Wang, H.; Zhang, X.F. Advances in the Research of Phytochemistry and Pharmacology of Meconopsis Vig. Nat. Prod. Res. Dev. 2011, 23, 163–168. [Google Scholar]
- Wang, Z.M. Research progress on chemical constituents and biological activities of Meconopsis Vig. West China J. Pharm. Sci. 2010, 25, 759–761. [Google Scholar]
- Yang, M.; Shi, X.B.; Min, K.; Sun, Q.L.; Chen, Y.G. Advances in the Research of Phytochemistry and Pharmacology of Meconopsis Vig. Chin. Tradit. Pat. Med. 2010, 32, 279–283. [Google Scholar]
- Chen, X.L.; Li, S.X.; Zhang, H.W.; Jiang, Y.; Wang, W.; Zhang, D.D.; Song, X.M.; Li, Y.Z. Research progress on chemical constituents and pharmacological effects of Hylomecon japonica. J. Shaanxi Univ. Chin. Med. 2023, 46, 19–25. [Google Scholar]
- Sun, B. Studies on the Chemical Constituents o Tibetan Medicine Hypecoum leptocarpum Hook.f.et Thoms. Master’s Thesis, Southeast University, Nanjing, China, 2021. [Google Scholar]
- Sun, L.Y.; Wang, S.L. Extraction of effective components from Macleaya cordata and its application in animal husbandry production. J. Agric. Sci. Eng. Chin. 2022, 34, 63–70. [Google Scholar] [CrossRef] [PubMed]
- Yu, K.; Xiang, F.; Qing, Z.X.; Yang, P.; Zeng, J.G. Separation of Alkaloids from the Roots of Macleaya cordata. China Pharm. 2017, 20, 1903–1906. [Google Scholar]
- Yang, P.; Xiang, F.; Qing, Z.X.; Cao, H.L.; Zeng, J.G. Isolation and Identification of Alkaloids from Fresh Leaves of Macleaya cordata. Mod. Chin. Med. 2017, 19, 1371–1375. [Google Scholar]
- Sai, C.M.; Hua, H.M.; Wang, J.A.; Wang, Q.B.; Wang, H.Y. Isolation and identification of the racemic benzophenanthridine alkaloids from Macleaya cordata. J. Jining. Med. Univ. 2017, 40, 256–263. [Google Scholar]
- Yu, K.; Peng, Y.; Qing, Z.X.; Yang, P.; Zuo, Z.; Zeng, J.G. Chemical Constituents from the Roots of Macleaya cordata. J. Chin. Med. Mater. 2016, 39, 1767–1770. [Google Scholar]
- Sai, C.M. Studies on the Alkaloids from Macleaya cordata and Their Biological Activities. Ph.D. Thesis, Shenyang Pharmaceutical University, Shenyang, China, 2016. [Google Scholar]
- Qing, Z.-X.; Xu, Y.-Q.; Yang, P.; Yu, K.; Peng, Y.; Zuo, Z.; Zeng, J.-G. Study on Alkaloids of Fruit from Macleaya cordata. J. Chin. Med. Mater. 2016, 39, 312–314. [Google Scholar]
- Zhou, H.L.; Li, Y.H.; Yu, S.F.; Cheng, M.; Zhou, X.D.; Zhang, Y.; Liu, B.L.; Zhou, G.X. Alkaloids from Macleaya cordata and their cytotoxicity assay. J. Chin. Med. Mater. 2015, 40, 458–462. [Google Scholar]
- Zhou, N.L. Studies on the Chemical Constituents and Bioactivity of the Macleaya cordata Roots. Master’s Thesis, Hebei Normal University of Science & Technology, Qinhuangdao, China, 2012. [Google Scholar]
- Ye, F.Z.; Feng, F.; Liu, W.Y. Alkaloids from Macleaya cordata. J. Chin. Med. Mater. 2009, 34, 1683–1686. [Google Scholar]
- Long, F.L.; Yue, Z.G.; Yu, G.; Liu, H.; Hu, P.H.; Jin, M. Research progress on chemical constituents, pharmacological effects and toxicity of Dicranostigma leptopodum. Hebei Med. J. 2017, 39, 3492–3495. [Google Scholar]
- Liu, D.H.; Zhang, T.C.; Liu, J.X.; Di, D.L.; Dang, Y. Chemical constituents of alkaloids from Dicranostigma leptopodum. Chin. Tradit. Herb. Drugs 2011, 42, 1505–1508. [Google Scholar]
- Gao, W.; Wu, H.; Hu, X.G.; Yu, H.F.; Zhang, R.P.; Ding, C.F. Isoquinoline Alkaloids from the Roots of Dactylicapnos scandens and Their Antibacterial Activities. J. Trop. Subtrop. Bot. 2023, 1–7. Available online: https://link.cnki.net/urlid/44.1374.Q.20230607.1335.002 (accessed on 5 August 2024).
- Zeng, W.D.; Rao, G.X. Chemical Constituents of Dactylicapnos lichiangensis. J. Chin. Med. Mater. 2022, 45, 1627–1631. [Google Scholar]
- He, Q.B. Chemical Constituents of Dactylicapnos torulosa and Their Biological Activities. Master’s Thesis, Shandong University, Jinan, China, 2021. [Google Scholar]
- Yu, X.; Gao, X.; Zhu, Z.; Cao, Y.; Zhang, Q.; Tu, P.; Chai, X. Alkaloids from the Tribe Bocconieae (Papaveraceae): A Chemical and Biological Review. Molecules 2014, 19, 13042–13060. [Google Scholar] [CrossRef] [PubMed]
- Zhou, X.-C.; Ma, X.-J.; Ge, F.-X.; Liu, C.-X.; Liang, Y.-N.; Gao, X.-L.; Chai, X.-Y. Two new isoquinoline alkaloids from Corydalis hendersonii. J. Chin. Med. Mater. 2023, 48, 3508–3515. [Google Scholar]
- Yin, X.; Zhao, F.; Feng, X.; Li, J.; Yang, X.; Zhang, H.; Tu, P.; Chai, X. Four new spirobenzylisoquinoline N-oxide alkaloids from the whole plant of Corydalis hendersonii. Fitoterapia 2018, 128, 31–35. [Google Scholar] [CrossRef]
- Wuken, S.N.; Yin, X.; Mi, J.; Jiao, S.G.; Zhang, H.X.G.; Tu, P.F.; Chai, X.Y.; Liu, C.S. Isolation and Elucidation of a Novel Isoquinoline Alkaloid from Corydalis hendersonii. Chin. J. Exp. Tradit. Med. Formulae 2019, 25, 172–175. [Google Scholar]
- Long, X.-N.; Han, F.; Wei, L.; Meng, F.-C.; Qu, S.-Y.; Chen, M. A novel alkaloid from Corydalis tomentella. China J. Chin. Mater. Med. 2021, 46, 5020–5026. [Google Scholar]
- Istatkova, R.; Philipov, S.; Yadamsurenghiin, G.O.; Samdan, J.; Dangaa, S. Alkaloids from Papaver nudicaule L. Nat. Prod. Res. 2008, 22, 607–611. [Google Scholar] [CrossRef]
- Laines-Hidalgo, J.I.; Muñoz-Sánchez, J.A.; Loza-Müller, L.; Vázquez-Flota, F. An Update of the Sanguinarine and Benzophenanthridine Alkaloids’ Biosynthesis and Their Applications. Molecules 2022, 27, 1378. [Google Scholar] [CrossRef]
- Da-Cunha, E.V.; Fechinei, I.M.; Guedes, D.N.; Barbosa-Filho, J.M.; Da-Silva, M.S. Protoberberine alkaloids. Alkaloids Chem. Biol. 2005, 62, 1–75. [Google Scholar]
- Niu, Z.X.; Wang, Y.T.; Wang, J.F. Recent advances in total synthesis of protoberberine and chiral tetrahydroberberine alkaloids. Nat. Prod. Rep. 2024. [Google Scholar] [CrossRef]
- Ge, Y.-C.; Wang, K.-W. New Analogues of Aporphine Alkaloids. Mini Rev. Med. Chem. 2018, 18, 1590–1602. [Google Scholar] [CrossRef]
- Singla, D.; Sharma, A.; Kaur, J.; Panwar, B.; Raghava, G.P. BIAdb: A curated database of benzylisoquinoline alkaloids. BMC Pharmacol. 2010, 10, 4. [Google Scholar] [CrossRef]
- Deng, A.P.; Zhang, Y.; Zhou, L.; Kang, C.Z.; Lv, C.G.; Kang, L.P.; Nan, T.G.; Zhan, Z.L.; Guo, L.P.; Huang, L.Q. Systematic review of the alkaloid constituents in several important medicinal plants of the Genus Corydalis. Phytochemistry 2021, 183, 112644. [Google Scholar] [CrossRef]
- Paul, L.D.; Maurer, H.H. Studies on the metabolism and toxicological detection of the Eschscholtzia californica alkaloids californine and protopine in urine using gas chromatography-mass spectrometry. J. Chromatogr. B 2003, 789, 43–57. [Google Scholar] [CrossRef] [PubMed]
- Guinaudeau, H.; Shamma, M. The Protopine Alkaloids. J. Nat. Prod. 1982, 45, 237–246. [Google Scholar] [CrossRef]
- Odna, M.; Takahashi, H. Chapter 4 Protopine Alkaloids. Alkaloids Chem. Pharmacol. 1989, 34, 181–210. [Google Scholar]
- Shamma, M. The lsoquinoline Alkaloids-chemistry and Pharmacology. Organ. Chem. 1972, 25, 315–343. [Google Scholar]
- Dostál, J. Two Faces of Alkaloids. J. Chem. Educ. 2000, 77, 993–998. [Google Scholar] [CrossRef]
- Iwasa, K.; Sugiura, M.; Takao, N. Stereochemistry of 13-Hydroxyprotoberberines, Their Derivatives, and a Protopine-type Alkaloid. J. Organ. Chem. 1982, 47, 4275–4280. [Google Scholar] [CrossRef]
- Li, Q.; Zhang, H. Research Progress on the Synthesis of Morphine Alkaloids. Chin. J. Org. Chem. 2017, 37, 1629–1652. [Google Scholar] [CrossRef]
- Wicks, C.; Hudlicky, T.; Rinner, U. Morphine alkaloids: History, biology, and synthesis. Alkaloids. Chem. Biol. 2021, 86, 145–342. [Google Scholar]
- Wu, L.; Yang, Y.; Mao, Z.; Wu, J.; Ren, D.; Zhu, B.; Qin, L. Processing and Compatibility of Corydalis yanhusuo: Phytochemistry, Pharmacology, Pharmacokinetics, and Safety. Evid. Based Complement. Altern. Med. 2021, 2021, 1271953. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Chen, C.; Wang, F.-Q.; Li, C.-H.; Zhang, Q.-H.; Hu, Y.-J.; Xia, Z.-N.; Yang, F.-Q. Simultaneous screening and analysis of antiplatelet aggregation active alkaloids from Rhizoma corydalis. Pharm. Biol. 2016, 54, 3113–3120. [Google Scholar] [CrossRef] [PubMed]
- Zielińska, S.; Wójciak-Kosior, M.; Dziągwa-Becker, M.; Gleńsk, M.; Sowa, I.; Fijałkowski, K.; Rurańska-Smutnicka, D.; Matkowski, A.; Junka, A. The Activity of Isoquinoline Alkaloids and Extracts from Chelidonium majus against Pathogenic Bacteria and Candida sp. Toxins 2019, 11, 406. [Google Scholar] [CrossRef] [PubMed]
- Ali, I.; Li, J.; Cui, L.; Zhao, H.; He, Q.; Wang, D. Efficient extraction and purification of benzo[c]phenanthridine alkaloids from Macleaya cordata (Willd) R. Br. by combination of ultrahigh pressure extraction and pH-zone-refining counter-current chromatography with anti-breast cancer activity in vitro. Phytochem. Anal. 2021, 32, 423–432. [Google Scholar] [CrossRef] [PubMed]
- Xue, C.; Liu, S.X.; Huang, F. Research progress on the analgesic effect of poppy, Rhizoma Corydalis and Corydalis saxicola Bunting. J. Pharm. Res. 2019, 38, 290–294. [Google Scholar]
- Zhou, H.-H.; Wu, D.-L.; Gao, L.-Y.; Fang, Y.; Ge, W.-H. L-Tetrahydropalmatine alleviates mechanical hyperalgesia in models of chronic inflammatory and neuropathic pain in mice. NeuroReport 2016, 27, 476–480. [Google Scholar] [CrossRef]
- Wang, J.B.; Mantsch, J.R. L-Tetrahydropalamatine: A potential new medication for the treatment of cocaine addiction. Future Med. Chem. 2012, 4, 177–186. [Google Scholar] [CrossRef]
- Ma, Z.-Z.; Xu, W.; Jensen, N.H.; Roth, B.L.; Liu-Chen, L.-Y.; Lee, D.Y.W. Isoquinoline alkaloids isolated from Corydalis yanhusuo and their binding affinities at the dopamine D1 receptor. Molecules 2008, 13, 2303–2312. [Google Scholar] [CrossRef]
- Dey, P.; Kundu, A.; Kumar, A.; Gupta, M.; Lee, B.M.; Bhakta, T.; Dash, S.; Kim, H.S. Analysis of alkaloids (indole alkaloids, isoquinoline alkaloids, tropane alkaloids). Recent Adv. Nat. Prod. Anal. 2020, 505–567. [Google Scholar] [CrossRef]
- Zhang, Y.; Wang, C.; Wang, L.; Parks, G.S.; Zhang, X.; Guo, Z.; Ke, Y.; Li, K.-W.; Kim, M.K.; Vo, B.; et al. A Novel Analgesic Isolated from a Traditional Chinese Medicine. Curr. Biol. 2014, 24, 117–123. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.; Lu, M.; Pan, Q.; Fichna, J.; Zheng, L.; Wang, K.; Yu, Z.; Li, Y.; Li, K.; Song, A.; et al. Berberine Improves Intestinal Motility and Visceral Pain in the Mouse Models Mimicking Diarrhea-Predominant Irritable Bowel Syndrome (IBS-D) Symptoms in an Opioid-Receptor Dependent Manner. PLoS ONE 2015, 10, e0145556. [Google Scholar] [CrossRef] [PubMed]
- Yang, S.; Yu, Z.; Sun, W.; Jiang, C.; Ba, X.; Zhou, Q.; Xiong, D.; Xiao, L.; Deng, Q.; Hao, Y. The antiviral alkaloid berberine ameliorates neuropathic pain in rats with peripheral nerve injury. Acta Neurol. Belg. 2018, 120, 557–564. [Google Scholar] [CrossRef] [PubMed]
- Eldufani, J.; Blaise, G. The role of acetylcholinesterase inhibitors such as neostigmine and rivastigmine on chronic pain and cognitive function in aging: A review of recent clinical applications. Alzheimers Dement. 2019, 5, 175–183. [Google Scholar] [CrossRef] [PubMed]
- Zou, K.; Li, Z.; Zhang, Y.; Zhang, H.-Y.; Li, B.; Zhu, W.-L.; Shi, J.-Y.; Jia, Q.; Li, Y.-M. Advances in the study of berberine and its derivatives: A focus on anti-inflammatory and anti-tumor effects in the digestive system. Acta Pharmacol. Sin. 2016, 38, 157–167. [Google Scholar] [CrossRef] [PubMed]
- Shen, Y.; Guan, S.; Ge, H.; Xiong, W.; He, L.; Liu, L.; Yin, C.; Liu, H.; Li, G.; Xu, C.; et al. Effects of palmatine on rats with comorbidity of diabetic neuropathic pain and depression. Brain Res. Bull. 2018, 139, 56–66. [Google Scholar] [CrossRef]
- Sun, L. Protective effect of magnoflorine on LPS-induced acute lung injury. World Latest Med. Inf. 2019, 19, 100–101. [Google Scholar] [CrossRef]
- Tao, C.; Hu, S.-Q.; Chen, J.; Chen, Y.-J.; Sun, K.-H.; Cui, G.-Z.; Ma, M.; Wu, Z.-Z. Highly efficient synthesis and monoamine oxidase B inhibitory profile of demethyleneberberine, columbamine and palmatine. Neurochem. Int. 2020, 139, 104807. [Google Scholar] [CrossRef] [PubMed]
- Yin, Z.Y.; Chu, S.S.; Sun, Q. Effects of dehydrocorydaline on mechanical hyperalgesia induced by complete Freund’s adjuvant in mice. Chin. J. Behav. Med. Brain Sci. 2016, 25, 300–303. [Google Scholar]
- Li, H.L.; Pan, G.Q.; You, Z.L.; Jiang, W.; Nan, K.P. Studies on Analgesic Effects of the Total Alkaloids of Tibetan Medicine Saibei Corydalis. Nat. Prod. Res. Dev. 2013, 25, 544–546. [Google Scholar]
- Liu, N.T.; Li, R.H.; Jia, T.Z. Study on Analgesic Effect of Vinegar Made Chelidonium majus. Asia Pac. Trad. Med. 2022, 18, 35–38. [Google Scholar]
- Guo, J.; Zhang, X.D.; Huang, W. Selection of Effective parts in Fructus Illcii Veri. J. Shaanxi Univ. Chin. Med. 2006, 29, 58–60. [Google Scholar] [CrossRef]
- Lan, Q.X.; Jiang, Z.B.; Zhang, A.M.; He, R.L.; Zhou, Z.H.; Xu, S.R.; Chen, Y.T. Observation on the curative effect of Corydalis decumbentis intramuscular injection in the treatment of postherpetic neuralgia. Shandong Med. J. 2019, 59, 85–87. [Google Scholar]
- Guo, M.; Zhang, Y.; Wang, Z.W.; Zhao, J.G. Experimental study on analgesic effect of total flavonoids and total alkaloids from Tibetan medicine var. quintuplinervia. J. Gansu Univ. of Chin. Med. 2010, 27, 31–32. [Google Scholar]
- Feng, J.-H.; Chen, K.; Shen, S.-Y.; Luo, Y.-F.; Liu, X.-H.; Chen, X.; Gao, W.; Tong, Y.-R. The composition, pharmacological effects, related mechanisms and drug delivery of alkaloids from Corydalis yanhusuo. Biomed. Pharmacother. 2023, 167, 115511. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Wu, J.; Huang, J.; Cheng, Y.; Wang, D.; Liu, Z. Uncovering the Effect and Mechanism of Rhizoma Corydalis on Myocardial Infarction through an Integrated Network Pharmacology Approach and Experimental Verification. Front. Pharmacol. 2022, 13, 927488. [Google Scholar] [CrossRef]
- Kim, Y.M.; Ha, Y.M.; Jin, Y.C.; Shi, L.Y.; Lee, Y.S.; Kim, H.J.; Seo, H.G.; Choi, J.S.; Kim, Y.S.; Kang, S.S.; et al. Palmatine from Coptidis rhizoma reduces ischemia–reperfusion-mediated acute myocardial injury in the rat. Food Chem. Toxicol. 2009, 47, 2097–2102. [Google Scholar] [CrossRef]
- Li, Y.; Zhang, L.; Zhang, P.; Hao, Z. Dehydrocorydaline Protects Against Sepsis-Induced Myocardial Injury through Modulating the TRAF6/NF-κB Pathway. Front Pharmacol. 2021, 12, 709604. [Google Scholar] [CrossRef]
- He, K.; Gao, J.L.; Zhao, G.S. Advances in studies on chemistry, pharmacology, and quality control of Corydalis yanhusuo. Chin. Tradit. Herb. Drugs 2007, 12, 1909–1912. [Google Scholar] [CrossRef]
- Yu, L.; Li, Q.; Yu, B.; Yang, Y.; Jin, Z.; Duan, W.; Zhao, G.; Zhai, M.; Liu, L.; Yi, D.; et al. Berberine Attenuates Myocardial Ischemia/Reperfusion Injury by Reducing Oxidative Stress and Inflammation Response: Role of Silent Information Regulator 1. Oxidative Med. Cell. Longev. 2016, 2016, 1689602. [Google Scholar] [CrossRef]
- Zhao, G.L.; Yu, L.M.; Gao, W.L.; Duan, W.X.; Jiang, B.; Liu, X.D.; Zhang, B.; Liu, Z.H.; Zhai, M.E.; Jin, Z.X.; et al. Berberine protects rat heart from ischemia/reperfusion injury via activating JAK2/STAT3 signaling and attenuating endoplasmic reticulum stress. Acta Pharmacol. Sin. 2016, 37, 354–367. [Google Scholar] [CrossRef]
- Yuan, Q.H.; Yuan, Y.; Zheng, Y.; Sheng, R.; Liu, L.; Xie, F.; Tan, J. Anti-cerebral ischemia reperfusion injury of polysaccharides: A review of the mechanisms. Biomed Pharmacother. 2021, 137, 111303. [Google Scholar] [CrossRef]
- Tan, C.N.; Zhang, Q.; Li, C.H.; Fan, J.J.; Yang, F.Q.; Hu, Y.J.; Hu, G. Potential target-related proteins in rabbit platelets treated with active monomers dehydrocorydaline and canadine from Rhizoma corydalis. Phytomedicine 2019, 54, 231–239. [Google Scholar] [CrossRef]
- Xiao, X.; Liu, J.; Hu, J.; Li, T.; Zhang, Y. Protective effect of protopine on the focal cerebral ischaemic injury in rats. Basic Clin. Pharmacol. Toxicol. 2007, 101, 85–89. [Google Scholar] [CrossRef]
- Li, B.; Huang, X.N.; Wu, Q.; Yu, L.M.; Sun, A.S.; Shi, J.S. Effect of protopine on rat blood pressure. J. Zunyi Med. Univ. 2003, 3, 209–212. [Google Scholar] [CrossRef]
- Liu, M.H.; Li, Y.; Wen, Y.; Wang, L. The effect of allocryptopine on arrhythmia and monophasic action potential in animal models. Chin. J. Mult. Organ Dis. Elder. 2006, 5, 48–50+65. [Google Scholar]
- Hu, B.; Xu, G.; Zheng, Y.; Tong, F.; Qian, P.; Pan, X.; Zhou, X.; Shen, R. Chelerythrine Attenuates Renal Ischemia/Reperfusion-induced Myocardial Injury by Activating CSE/H2S via PKC/NF-κB Pathway in Diabetic Rats. Kidney Blood Press Res. 2017, 42, 379–388. [Google Scholar] [CrossRef]
- Gao, F.; Guo, L.J.; Wang, Y.F.; Liu, Y.C.; Ma, X.C. Research progress of Corydalis rhizoma in the treatment of cardiovascular diseases. Chin. J. Integr. Med. Cardio-Cerebrovasc. Dis. 2023, 21, 2198–2201. [Google Scholar]
- Yu, L.M.; Wen, G.R.; Deng, J.; Jin, F.; Sun, A.S.; Huang, X.N.; Zhang, J. Protective Effects of Injection of Corydalis Decumbens Pers on Cerebral lschemia and Neurons. Shanghai J. Tradit. Chin. Med. 2006, 40, 70–72. [Google Scholar]
- Zhang, G.X.; Shi, J.S.; Yu, L.M.; Wu, Q.; Deng, J.; Wang, D.Z. The effects of injection of coryadlis decumbens pers on focal cerebral ischemia reperfusion injury and the level of ICAM-1 mRNA in rats. J. Zunyi Med. Univ. 2007, 1, 14–17. [Google Scholar] [CrossRef]
- Liu, L.X.; Lin, Y.B. Clinical Observation of Complex Decumbent Corydalis Rhizome on 46 Cases of Arrhythmia Treated. Mod. J. Integr. Tradit. Chin. West. Med. 2007, 35, 5273. [Google Scholar] [CrossRef]
- Xu, X.Y.; Liu, J.H.; Chen, X.H. Clinical Observation of Complex Decumbent Corydalis Rhizome on 64 Cases of Patients with Syndrome of Blood Stasis in Cerebral Infarction at Acute Stage. Chin. J. Mod. Appl. Pharm. 2013, 30, 904–907. [Google Scholar]
- Gao, J.; Wang, T.Y.; He, X.H.; Gu, Z.L.; Liao, F.L.; Li, W.; Yin, X.J. Effect of Total Alkaloids from Rhizoma Corydalis Decumbeutis on Platelet Aggregation Function. Suzhou Univ. J. Med. Sci. 2004, 24, 137–140. [Google Scholar]
- Shang, W.Q.; Chen, Y.M.; Gao, X.L.; Pu, C.; Tu, P.F.; Chai, X.Y. Phytochemical and pharmacological advance on Tibetan medicinal plants of Corydalis. China J. Chin. Mater. Med. 2014, 39, 1190–1198. [Google Scholar]
- Hou, T.D.; Liu, A.P.; Zhang, J.; Cheng, F.; Gong, Y.Q. Effect of general alkaloid of Corydalis on blood pressure and on the tension of aortic smooth muscle. J. Northwest Norm. Univ. Nat. Sci. 2004, 4, 71–73. [Google Scholar] [CrossRef]
- Bai, R.; Yin, X.; Feng, X.; Cao, Y.; Wu, Y.; Zhu, Z.; Li, C.; Tu, P.; Chai, X. Corydalis hendersonii Hemsl. protects against myocardial injury by attenuating inflammation and fibrosis via NF-κB and JAK2-STAT3 signaling pathways. J. Ethnopharmacol. 2017, 207, 174–183. [Google Scholar] [CrossRef]
- Niu, X.; Fan, T.; Li, W.; Huang, H.; Zhang, Y.; Xing, W. Protective effect of sanguinarine against acetic acid-induced ulcerative colitis in mice. Toxicol. Appl. Pharmacol. 2013, 267, 256–265. [Google Scholar] [CrossRef]
- Li, W.F.; Hao, D.J.; Fan, T.; Huang, H.M.; Yao, H.; Niu, X.F. Protective effect of chelerythrine against ethanol-induced gastric ulcer in mice. Chem. Biol. Interact. 2014, 208, 18–27. [Google Scholar] [CrossRef]
- Fan, L.; Fan, Y.; Liu, L.; Tao, W.; Shan, X.; Dong, Y.; Li, L.; Zhang, S.; Wang, H. Chelerythrine Attenuates the Inflammation of Lipopolysaccharide-Induced Acute Lung Inflammation Through NF-κB Signaling Pathway Mediated by Nrf2. Front. Pharmacol. 2018, 9, 1047. [Google Scholar] [CrossRef]
- Wu, Y.R.; Ma, Y.B.; Zhao, Y.X.; Yao, S.Y.; Zhou, J.; Gong, Q.F.; Chen, J.J. Anti-hepatitis virus constituents from Corydalis saxicola. Chin. Tradit. Herb. Drugs 2012, 43, 32–37. [Google Scholar]
- Xu, X.; Zhang, L.; Zhao, Y.; Xu, B.Y.; Qin, W.X.; Yan, Y.Q.; Yin, B.Q.; Xi, C.Y.; Ma, L.B. Anti-inflammatory mechanism of berberine on lipopolysaccharide-induced IEC-18 models based on comparative transcriptomics. Mol. Med. Rep. 2020, 22, 5163–5180. [Google Scholar] [CrossRef]
- Cheng, J.J.; Ma, X.D.; Ai, G.X.; Yu, Q.X.; Chen, X.Y.; Yan, F.; Li, Y.C.; Xie, J.H.; Su, Z.R.; Xie, Q.F. Palmatine Protects Against MSU-Induced Gouty Arthritis via Regulating the NF-κB/NLRP3 and Nrf2 Pathways. Drug Des. Dev. Ther. 2022, 16, 2119–2132. [Google Scholar] [CrossRef] [PubMed]
- Móricz, A.M.; Fornal, E.; Jesionek, W.; Majer-Dziedzic, B.; Choma, I.M. Effect directed isolation and identification of antibacterial Chelidonium majus L. alkaloids. Chromatographia 2015, 78, 707. [Google Scholar]
- Tavares, L.C.; Zanon, G.; Weber, A.D.; Neto, A.T.; Mostardeiro, C.P.; Da Cruz, I.B.M.; Oliveira, R.M.; Ilha, V.; Dalcol, I.I.; Morel, A.F. Structure-activity relationship of benzophenanthridine alkaloids from Zanthoxylum rhoifolium having antimicrobial activity. PLoS ONE 2014, 9, e97000. [Google Scholar] [CrossRef] [PubMed]
- Zhou, H.; Wang, W.; Cai, L.; Yang, T. Potentiation and Mechanism of Berberine as an Antibiotic Adjuvant Against Multidrug-Resistant Bacteria. Infect. Drug Resist. 2023, 16, 7313–7326. [Google Scholar] [CrossRef]
- Li, L. Experimental Study on Anti-inflammatory Effects of Corydalis saxicola. Chin. J. Ethnomed. Ethnopharm. 2009, 18, 20–21. [Google Scholar]
- Dai, G.; Sun, B.; Wu, L.; Gao, X.; Song, S.; Sun, H.; Ju, W. Comparative pharmacokinetics of three alkaloids in normal and acute hepatitis rats after oral administration of Yanhuanglian total alkaloids extract. Biomed. Chromatogr. 2018, 32, e4329. [Google Scholar] [CrossRef]
- Xiao, P.; Lin, C.X.; Pan, B.J.; Zhuge, M.L.; Huang, Y.; Zeng, D.Y. Pharmacodynamics of Yanhuanglian suppository for rats with chronic pelvic inflammatory diseases. Cent. South Pharm. 2019, 17, 2052–2058. [Google Scholar]
- Zeng, F.L.; Xiang, Y.F.; Liang, Z.R.; Wang, X.; Huang, D.E.; Zhu, S.N.; Li, M.M.; Yang, D.P.; Wang, D.M.; Wang, Y.F. Anti-hepatitis B virus effects of dehydrocheilanthifoline from Corydalis saxicola. Am. J. Chin. Med. 2013, 41, 119–130. [Google Scholar] [CrossRef]
- Danielewski, M.; Zielińska, S.; Matuszewska, A.; Słupski, W.; Włodarczyk, M.; Jęśkowiak, I.; Wiatrak, B.; Kowalski, K.; Jezierska-Domaradzka, A.; Ziółkowski, P.; et al. Sanguinarine-Chelerythrine Fraction of Coptis chinensis Exerts Anti-inflammatory Activity in Carrageenan Paw Oedema Test in Rats and Reveals Reduced Gastrotoxicity. Oxid. Med. Cell. Longev. 2022, 2022, 1504929. [Google Scholar] [CrossRef]
- Qian, W.D.; Huang, J.; Zhang, J.N.; Li, X.C.; Kong, Y.; Wang, T.; Li, Y.D. Antimicrobial and Antibiofilm Activities and Mechanism of Action of Chelerythrine against Carbapenem-Resistant Serratia marcescens In Vitro. Microb. Drug Resist. 2021, 27, 1105–1116. [Google Scholar] [CrossRef]
- Cruz-Galvez, A.M.; Gómez-Aldapa, C.A.; Villagómez-Ibarra, J.R.; Chavarría-Hernández, N.; Rodríguez-Baños, J.; Rangel-Vargas, E.; Castro-Rosas, J. Antibacterial effect against foodborne bacteria of plants used in traditional medicine in central Mexico: Studies in vitro and in raw beef. Food Control 2013, 32, 289–295. [Google Scholar] [CrossRef]
- Liu, L.Y.; Liu, N.N. Extraction of alkaloids from Chelidonium majus and its bacteriostatic test in vitro. J. Liaoning Agric. Technical. College. 2012, 14, 4–5. [Google Scholar]
- Liu, X.J.; Wei, H.; Yang, J.; Yu, B.Y.; Ji, Y.H. Protection effect of herb of thinfruit hypecoum(Xi Guǒ Jiǎo Huí Xiang) alcohol extract on endotoxin induced inflammatory mice. Suzhou Univ. J. Med. Sci. 2012, 32, 754–759. [Google Scholar]
- Li, M.Z.; Zhang, H.; Cui, G.Q.; Xiang, Y.; Zhu, F.X. Study on anti-inflammatory active components and mechanism of Corydalis bungeanae Herba. China J. Chin. Mater. Med. 2020, 45, 2586–2594. [Google Scholar]
- Zhou, J.; Qiu, Z.D.; Wang, Y.C.; Xu, J.M.; Huang, X.W. Research progress of chelerythrine. J. Ginseng Res. 2022, 34, 46–49. [Google Scholar]
- Sai, C.; Wang, J.; Li, B.; Ding, L.; Wang, H.; Wang, Q.; Hua, H.; Zhang, F.; Ren, Q. Isolation and identification of alkaloids from Macleaya microcarpa by UHPLC-Q-TOF-MS and their cytotoxic activity in vitro, antiangiogenic activity in vivo. BMC Chem. 2020, 14, 5. [Google Scholar] [CrossRef]
- Zhao, F.; Zhang, H.X.G.; Bai, R.F.; Du, B.Z.; Gao, X.L.; Tu, P.F.; Chai, X.Y. Advance of a representative traditional Tibetan medicine Meconopsis horridula on its phytochemical and pharmacological aspects. China J. Chin. Mater. Med. 2017, 42, 3676–3683. [Google Scholar]
- Gong, X.; Chen, Z.; Han, Q.; Chen, C.; Jing, L.; Liu, Y.; Zhao, L.; Yao, X.; Sun, X. Sanguinarine triggers intrinsic apoptosis to suppress colorectal cancer growth through disassociation between STRAP and MELK. BMC Cancer 2018, 18, 578. [Google Scholar] [CrossRef] [PubMed]
- Tang, Z.-H.; Cao, W.-X.; Wang, Z.-Y.; Lu, J.-H.; Liu, B.; Chen, X.; Lu, J.-J. Induction of reactive oxygen species-stimulated distinctive autophagy by chelerythrine in non-small cell lung cancer cells. Redox Biol. 2017, 12, 367–376. [Google Scholar] [CrossRef] [PubMed]
- Xie, Y.-J.; Gao, W.-N.; Wu, Q.-B.; Yao, X.-J.; Jiang, Z.-B.; Wang, Y.-W.; Wang, W.-J.; Li, W.; Hussain, S.; Liu, L.; et al. Chelidonine Selectively Inhibits the Growth of Gefitinib-resistant Non-small Cell Lung Cancer Cells through the EGFR-AMPK Pathway. Pharmacol. Res. 2020, 159, 104934. [Google Scholar] [CrossRef]
- Zhang, Z.; Guo, Y.; Zhang, L.; Zhang, J.; Wei, X. Chelerythrine chloride from Macleaya cordata induces growth inhibition and apoptosis in human gastric cancer BGC-823 cells. Acta Pharm. Sin. B 2012, 2, 464–471. [Google Scholar] [CrossRef]
- Piyanuch, R.; Sukhthankar, M.; Wandee, G.; Baek, S.J. Berberine, a natural isoquinoline alkaloid, induces NAG-1 and ATF3 expression in human colorectal cancer cells. Cancer Lett. 2007, 258, 230–240. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Wang, X.; Cao, S.; Sun, Y.; He, X.; Jiang, B.; Yu, Y.; Duan, J.; Qiu, F.; Kang, N. Berberine represses human gastric cancer cell growth in vitro and in vivo by inducing cytostatic autophagy via inhibition of MAPK/mTOR/p70S6K and Akt signaling pathways. Biomed. Pharmacother. 2020, 128, 110245. [Google Scholar] [CrossRef] [PubMed]
- Poornima, P.; Weng, C.F.; Padma, V.V. Neferine from Nelumbo nucifera induces autophagy through the inhibition of PI3K/Akt/mTOR pathway and ROS hyper generation in A549 cells. Food Chem. 2013, 141, 3598–3605. [Google Scholar] [CrossRef]
- Wu, H.L.; Hsu, C.Y.; Liu, W.H.; Yung, B.Y.M. Berberine-induced apoptosis of human leukemia HL-60 cells is associated with down-regulation of nucleophosmin/B23 and telomerase activity. Int. J. Cancer. 1999, 81, 923–929. [Google Scholar] [CrossRef]
- Zhang, L.; Miao, X.-J.; Wang, X.; Pan, H.-H.; Li, P.; Ren, H.; Jia, Y.-R.; Lu, C.; Wang, H.-B.; Yuan, L.; et al. Antiproliferation of berberine is mediated by epigenetic modification of constitutive androstane receptor (CAR) metabolic pathway in hepatoma cells. Sci. Rep. 2016, 6, 28116. [Google Scholar] [CrossRef]
- Salminen, K.A.; Meyer, A.; Jerabkova, L.; Korhonen, L.E.; Rahnasto, M.; Juvonen, R.O.; Imming, P.; Raunio, H. Inhibition of human drug metabolizing cytochrome P450 enzymes by plant isoquinoline alkaloids. Phytomedicine 2011, 18, 533–538. [Google Scholar] [CrossRef] [PubMed]
- Zeng, Y.Z.; Zhong, H.S.; Su, X.D.; Chen, J.M.; Mai, W.T.; Huang, Q.J.; Ye, Y. Chemical constituents, pharmacological effects and clinical applications of Corydalis saxicola Bunting and predictive analysis on quality marker. Chin. J. New Drugs 2023, 32, 1325–1335. [Google Scholar]
- Gong, P.; Long, H.; Guo, Y.; Wang, Z.; Yao, W.; Wang, J.; Yang, W.; Li, N.; Xie, J.; Chen, F. Chinese herbal medicines: The modulator of nonalcoholic fatty liver disease targeting oxidative stress. J. Ethnopharmacol. 2024, 318 Pt B, 116927. [Google Scholar] [CrossRef] [PubMed]
- Wang, T.; Zhao, L.J.; Li, P.; Jiang, H.; Lu, G.C.; Zhang, W.D.; Li, H.L.; Yuan, B.J. Hepatoprotective effects and mechanisms of dehydrocavidine in rats with carbon tetrachloride-induced hepatic fibrosis. J. Ethnopharmacol. 2011, 138, 76–84. [Google Scholar] [CrossRef] [PubMed]
- Vrba, J.; Vrublova, E.; Modriansky, M.; Ulrichova, J. Protopine and allocryptopine increase mRNA levels of cytochromes P450 1A in human hepatocytes and HepG2 cells independently of AhR. Toxicol. Lett. 2011, 203, 135–141. [Google Scholar] [CrossRef] [PubMed]
- Rathi, A.; Srivastava, A.K.; Shirwaikar, A.; Singh Rawat, A.K.; Mehrotra, S. Hepatoprotective potential of Fumaria indica Pugsley whole plant extracts, fractions and an isolated alkaloid protopine. Phytomedicine 2008, 15, 470–477. [Google Scholar] [CrossRef]
- Li, H.T.; Jin, S.Y.; Han, F.; Luo, C.; Xie, G.Y.; Qin, M.J. Pharmacological Action and Mechanism of Yanhuanglian in the Prevention and Treatment of Hepatopathy. Chin. Wild Plant Res. 2022, 41, 51–54. [Google Scholar]
- Xu, Y.T.; Xu, H.F. Research progress of Meconopsis quintuplinervia. Mod. J. Integr. Tradit. Chin. West. Med. 2014, 23, 451–453. [Google Scholar]
- Li, T.B.; Zhou, Y.D. Preliminary study on ‘Cure long cough’ of Papaveris pericarpium Guangming. J. Chin. Med. 2014, 29, 2172–2173. [Google Scholar]
- Tong, J.M.; Guo, X.M.; Shi, Y.H. Study on relieving cough and eliminating phlegm of total alkloid from Chelidonium majus. Chin. J. Hosp. Pharm. 2004, 24, 20–21. [Google Scholar]
- Cao, Y.; Gao, J.; Gao, X.L.; Zhen, J.; Wang, J.; Tu, P.F.; Chai, X.Y. Research progress on alkaloids from genus Dactylicapnos Wall. and their pharmacological activities. Chin. Tradit. Herb. Drugs 2014, 45, 2556–2563. [Google Scholar]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, M.; Yang, J.; Sun, Y.; Kuang, H. Recent Advances in Alkaloids from Papaveraceae in China: Structural Characteristics and Pharmacological Effects. Molecules 2024, 29, 3778. https://doi.org/10.3390/molecules29163778
Zhang M, Yang J, Sun Y, Kuang H. Recent Advances in Alkaloids from Papaveraceae in China: Structural Characteristics and Pharmacological Effects. Molecules. 2024; 29(16):3778. https://doi.org/10.3390/molecules29163778
Chicago/Turabian StyleZhang, Meixian, Jing Yang, Yanping Sun, and Haixue Kuang. 2024. "Recent Advances in Alkaloids from Papaveraceae in China: Structural Characteristics and Pharmacological Effects" Molecules 29, no. 16: 3778. https://doi.org/10.3390/molecules29163778
APA StyleZhang, M., Yang, J., Sun, Y., & Kuang, H. (2024). Recent Advances in Alkaloids from Papaveraceae in China: Structural Characteristics and Pharmacological Effects. Molecules, 29(16), 3778. https://doi.org/10.3390/molecules29163778






