Compositional Characterization of Syngas-Based Glycolide Using Gas Chromatogram-Mass Spectrometry and Electrospray Ionization High-Resolution Mass Spectrometry
Abstract
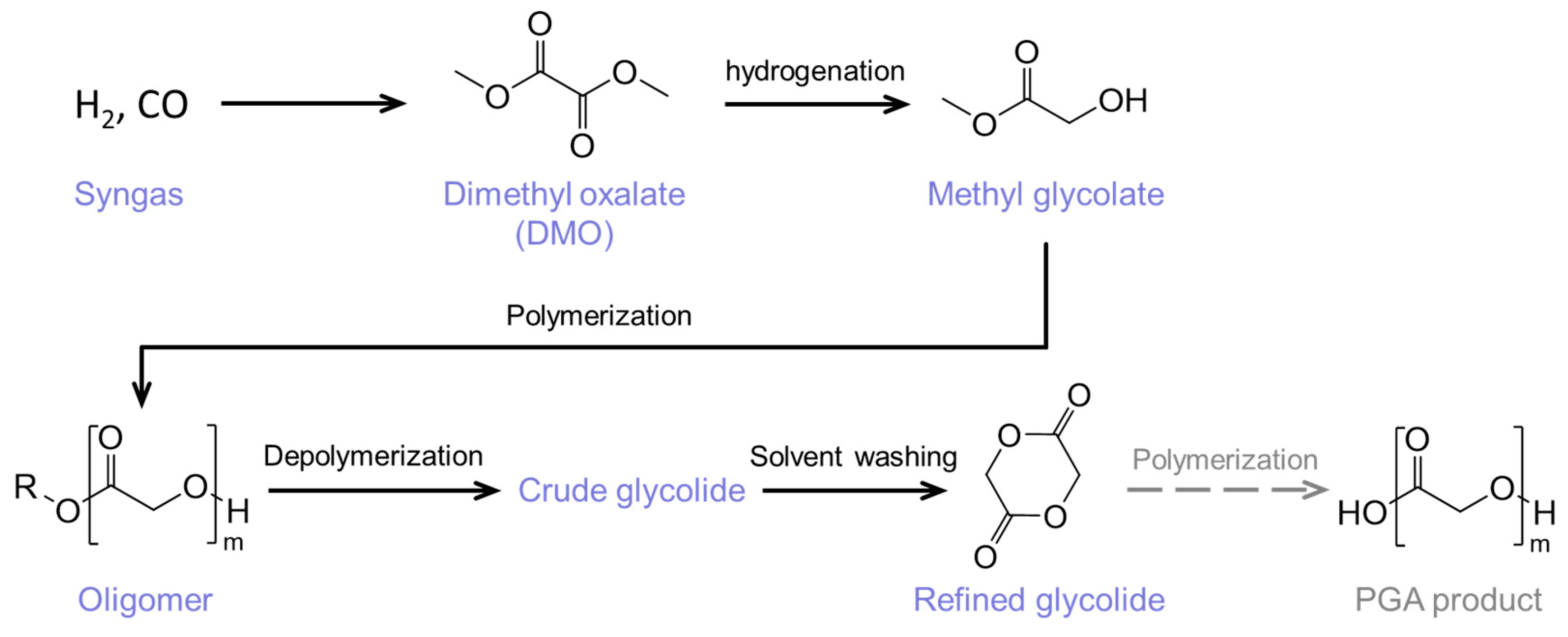
1. Introduction
2. Results and Discussion
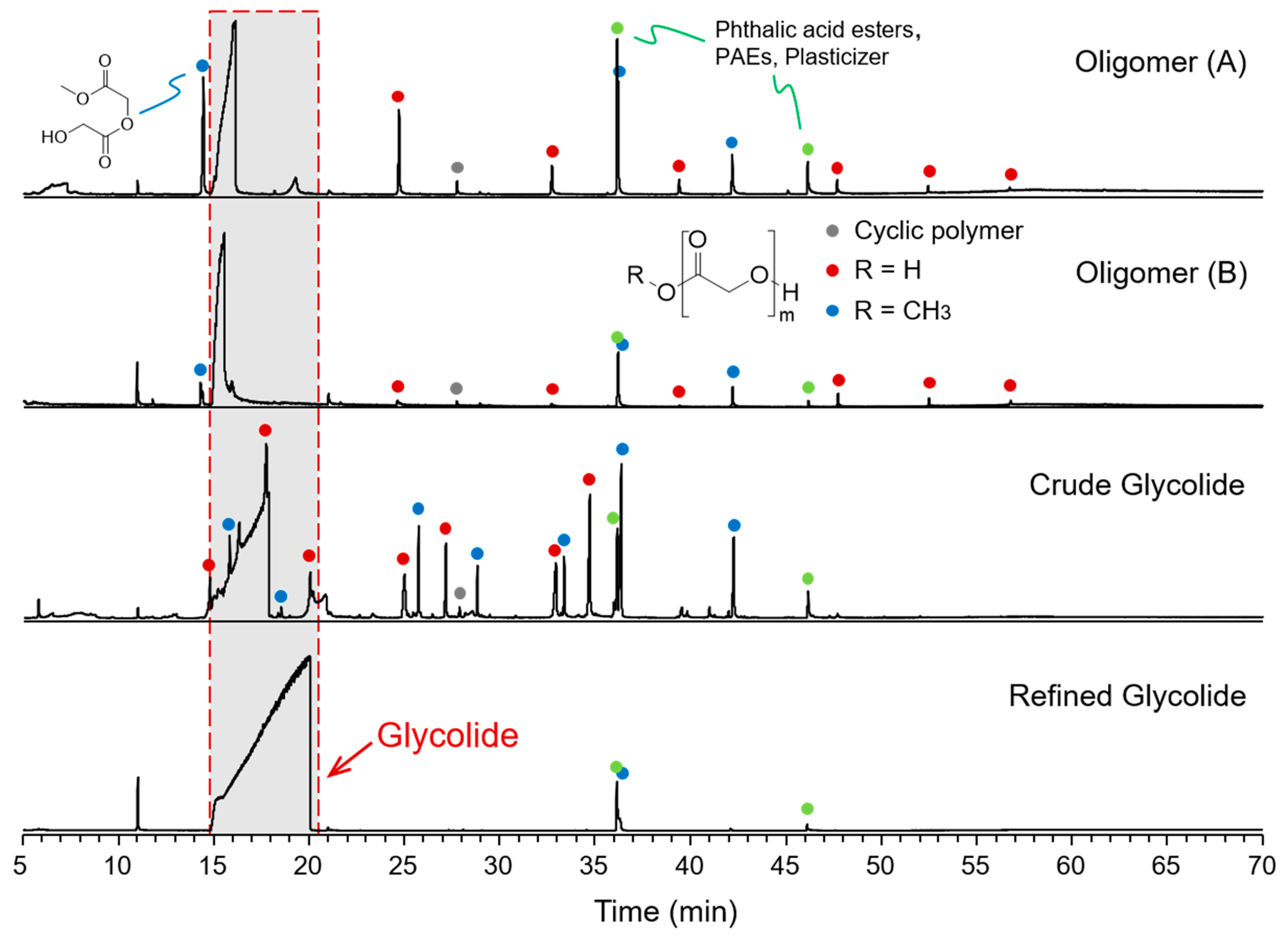
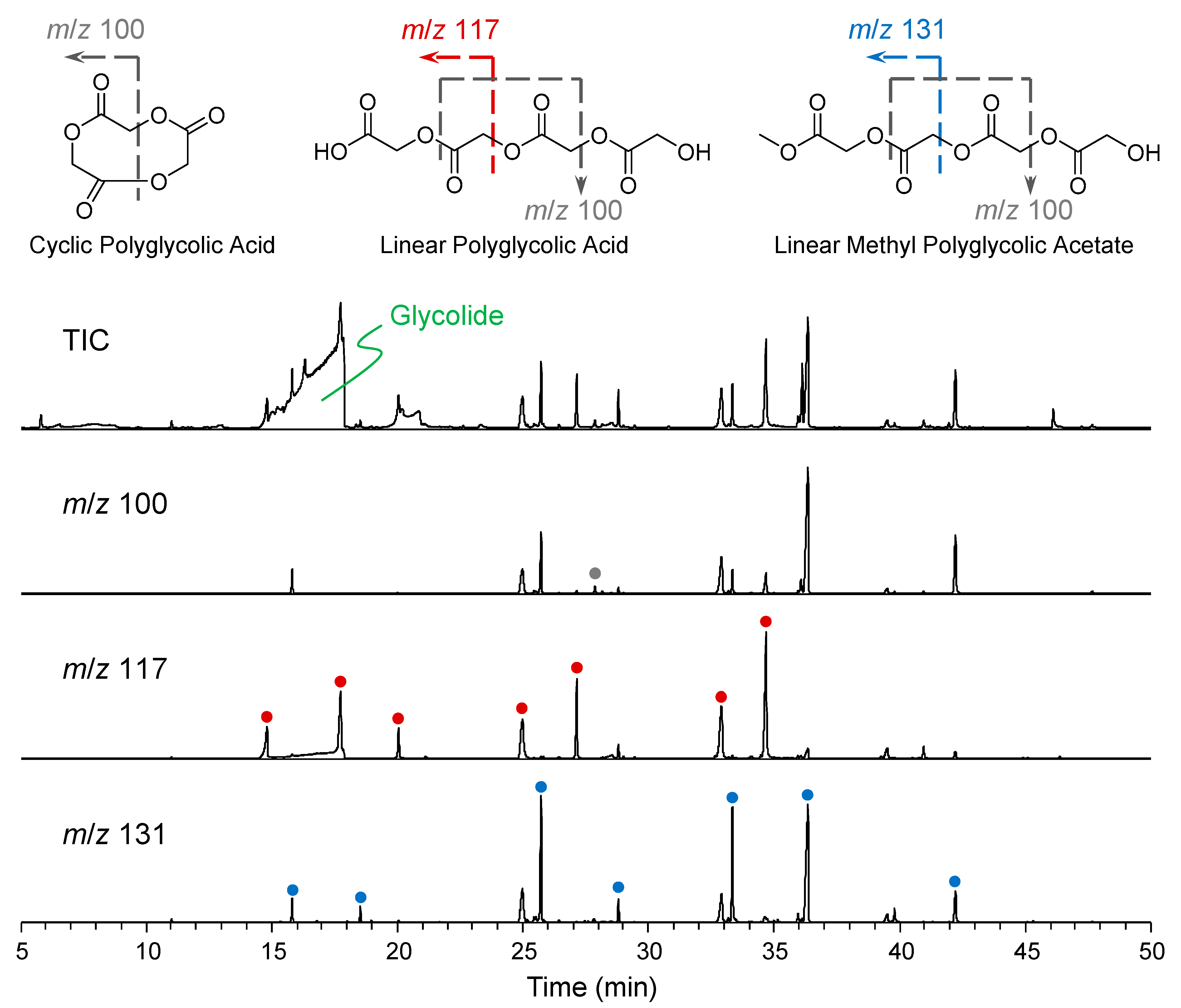
2.1. GC-MS Analysis of the Oligomer and Glycolide Samples
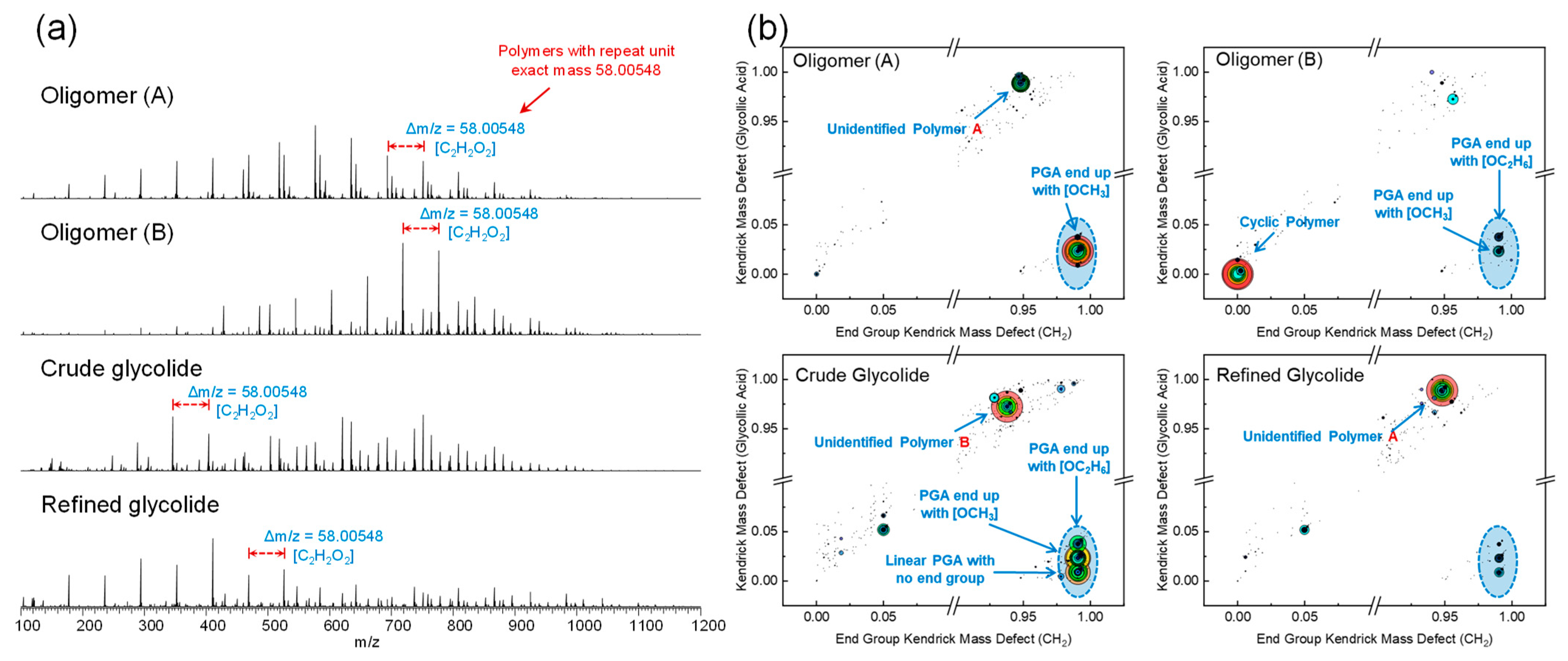
2.2. ESI(+) Orbitrap MS Analysis
2.3. Composition of the Impurities in the Samples from Different Process Units
3. Experimental Section
3.1. Samples and Regents
3.2. GC-MS Analysis
3.3. Orbitrap MS Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Benatti, A.C.B.; Pattaro, A.F.; Rodrigues, A.A.; Xavier, M.V.; Kaasi, A.; Barbosa, M.I.R.; Jardini, A.L.; Filho, R.M.; Kharmandayan, P. Chapter 4—Bioreabsorbable polymers for tissue engineering: PLA, PGA, and their copolymers. In Materials for Biomedical Engineering; Holban, A.-M., Grumezescu, A.M., Eds.; Elsevier: Amsterdam, The Netherlands, 2019; pp. 83–116. [Google Scholar]
- Middleton, J.C.; Tipton, A.J. Synthetic biodegradable polymers as orthopedic devices. Biomaterials 2000, 21, 2335–2346. [Google Scholar] [CrossRef]
- Wei, L.; Ma, S.; Hao, M.; Ma, L.; Lin, X. Modifying Anti-Compression Property and Water-Soluble Ability of Polyglycolic Acid via Melt Blending with Polyvinyl Alcohol. Polymers 2022, 14, 3375. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Wang, C.; Deng, X.; Cai, R.; Cao, L.; Cao, C.; Zheng, L.; Zhao, P.; Huang, Q. Preparation of Thifluzamide Polylactic Acid Glycolic Acid Copolymer Microspheres and Its Effect on the Growth of Cucumber Seedlings. Int. J. Mol. Sci. 2023, 24, 10121. [Google Scholar] [CrossRef]
- Budak, K.; Sogut, O.; Aydemir Sezer, U. A review on synthesis and biomedical applications of polyglycolic acid. J. Polym. Res. 2020, 27, 208. [Google Scholar] [CrossRef]
- Sanko, V.; Sahin, I.; Aydemir Sezer, U.; Sezer, S. A versatile method for the synthesis of poly(glycolic acid): High solubility and tunable molecular weights. Polym. J. 2019, 51, 637–647. [Google Scholar] [CrossRef]
- Zheng, H.; Xue, Y.; Niu, Y.; Gao, X.; Wang, Y.; Ding, G.; Zhu, Y. Synthesis of methyl glycolate via low-temperature hydrogenation of dimethyl oxalate over an efficient and stable Ru/activated carbon catalyst. J. Chem. Technol. Biotechnol. 2022, 97, 2572–2580. [Google Scholar] [CrossRef]
- Zhu, L.J.; Wang, S.R.; Li, X.B.; Yin, Q.Q.; Luo, Z.Y. Thermodynamic Analysis of Indirect Ethanol Synthesis from Syngas. Adv. Mater. Res. 2012, 433–440, 457–462. [Google Scholar] [CrossRef]
- Flieger, M.; Kantorová, M.; Prell, A.; Řezanka, T.; Votruba, J. Biodegradable plastics from renewable sources. Folia Microbiol. 2003, 48, 27–44. [Google Scholar] [CrossRef]
- Ellis, G.; Marco, C.; Gómez, M. Highly resolved transmission infrared microscopy in polymer science. Infrared Phys. Technol. 2004, 45, 349–364. [Google Scholar] [CrossRef]
- Ellis, G.; Santoro, G.; Gómez, M.A.; Marco, C. Synchrotron IR microspectroscopy: Opportunities in polymer science. IOP Conf. Ser. Mater. Sci. Eng. 2010, 14, 012019. [Google Scholar] [CrossRef]
- Soccio, M.; Lotti, N.; Finelli, L.; Gazzano, M.; Munari, A. (2-Hydroxy isobutyric) acid containing poly(glycolic acid): Structure–properties relationship. J. Polym. Sci. Part B Polym. Phys. 2010, 48, 1901–1910. [Google Scholar] [CrossRef]
- Zhao, N.; Ma, Z.; Xiong, C. Effect of Poly(L-lactic acid) on Hydrolytic Degradation of Poly(glycolic acid). Int. J. Polym. Mater. Polym. Biomater. 2012, 61, 587–595. [Google Scholar] [CrossRef]
- Ren, H.; Hu, H.; Yu, B.; Du, Y.; Wu, T. Identification of polymer building blocks by Py–GC/MS and MALDI-TOF MS. Int. J. Polym. Anal. Charact. 2018, 23, 9–17. [Google Scholar] [CrossRef]
- Maruyama, F.; Fujimaki, S.; Sakamoto, Y.; Kudo, Y.; Miyagawa, H. Screening of Phthalates in Polymer Materials by Pyrolysis GC/MS. Anal. Sci. 2015, 31, 3–5. [Google Scholar] [CrossRef] [PubMed]
- Marshall, A.G.; Hendrickson, C.L.; Jackson, G.S. Fourier transform ion cyclotron resonance mass spectrometry: A primer. Mass Spectrom. Rev. 1998, 17, 1–35. [Google Scholar] [CrossRef]
- Comisarow, M.B.; Marshall, A.G. Fourier transform ion cyclotron resonance spectroscopy. Chem. Phys. Lett. 1974, 25, 282–283. [Google Scholar] [CrossRef]
- Wallace, W.E.; Guttman, C.M. Data analysis methods for synthetic polymer mass spectrometry: Autocorrelation. J. Res. Natl. Inst. Stand. Technol. 2002, 107, 1. [Google Scholar] [CrossRef]
- Jackson, A.T.; Williams, J.P.; Scrivens, J.H. Desorption electrospray ionisation mass spectrometry and tandem mass spectrometry of low molecular weight synthetic polymers. Rapid Commun. Mass Spectrom. 2006, 20, 2717–2727. [Google Scholar] [CrossRef]
- Cody, R.B.; Fouquet, T.N.J.; Takei, C. Thermal desorption and pyrolysis direct analysis in real time mass spectrometry for qualitative characterization of polymers and polymer additives. Rapid Commun. Mass Spectrom. 2020, 34, e8687. [Google Scholar] [CrossRef]
- Roach, P.J.; Laskin, J.; Laskin, A. Higher-Order Mass Defect Analysis for Mass Spectra of Complex Organic Mixtures. Anal. Chem. 2011, 83, 4924–4929. [Google Scholar] [CrossRef]
- Kendrick, E. A Mass Scale Based on CH2 = 14.0000 for High Resolution Mass Spectrometry of Organic Compounds. Anal. Chem. 1963, 35, 2146–2154. [Google Scholar] [CrossRef]
- Chu, G.; Yang, Q.; Zhao, L.; Zhang, D.; Xie, J. Conceptual Design and Techno-Economic Analysis of a Novel Coal-to-Polyglycolic Acid Process Considering Wind–Solar Energy Complementary Features for Green H2 Production and CO2 Reduction. ACS Sustain. Chem. Eng. 2023, 11, 5937–5952. [Google Scholar] [CrossRef]
- You, Y.; Youk, J.H.; Lee, S.W.; Min, B.-M.; Lee, S.J.; Park, W.H. Preparation of porous ultrafine PGA fibers via selective dissolution of electrospun PGA/PLA blend fibers. Mater. Lett. 2006, 60, 757–760. [Google Scholar] [CrossRef]
- Shi, Q.; Pan, N.; Long, H.; Cui, D.; Guo, X.; Long, Y.; Chung, K.H.; Zhao, S.; Xu, C.; Hsu, C.S. Characterization of Middle-Temperature Gasification Coal Tar. Part 3: Molecular Composition of Acidic Compounds. Energy Fuels 2013, 27, 108–117. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, Y.; Chen, J.; He, J.; Li, S.; Wang, Y.; Zhang, Y.; Shi, Q. Compositional Characterization of Syngas-Based Glycolide Using Gas Chromatogram-Mass Spectrometry and Electrospray Ionization High-Resolution Mass Spectrometry. Molecules 2024, 29, 3759. https://doi.org/10.3390/molecules29163759
Zhang Y, Chen J, He J, Li S, Wang Y, Zhang Y, Shi Q. Compositional Characterization of Syngas-Based Glycolide Using Gas Chromatogram-Mass Spectrometry and Electrospray Ionization High-Resolution Mass Spectrometry. Molecules. 2024; 29(16):3759. https://doi.org/10.3390/molecules29163759
Chicago/Turabian StyleZhang, Yachun, Junyang Chen, Jianhua He, Shuofan Li, Yuanfeng Wang, Yahe Zhang, and Quan Shi. 2024. "Compositional Characterization of Syngas-Based Glycolide Using Gas Chromatogram-Mass Spectrometry and Electrospray Ionization High-Resolution Mass Spectrometry" Molecules 29, no. 16: 3759. https://doi.org/10.3390/molecules29163759
APA StyleZhang, Y., Chen, J., He, J., Li, S., Wang, Y., Zhang, Y., & Shi, Q. (2024). Compositional Characterization of Syngas-Based Glycolide Using Gas Chromatogram-Mass Spectrometry and Electrospray Ionization High-Resolution Mass Spectrometry. Molecules, 29(16), 3759. https://doi.org/10.3390/molecules29163759






