Prenyl Pterocarpans from Algerian Bituminaria bituminosa and Their Effects on Neuroblastoma
Abstract
1. Introduction
2. Results
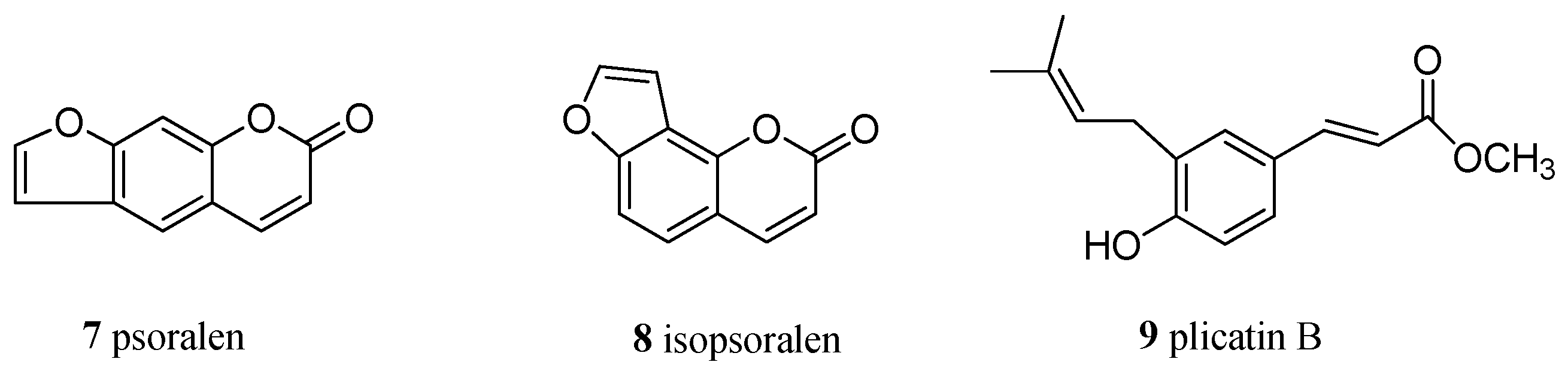
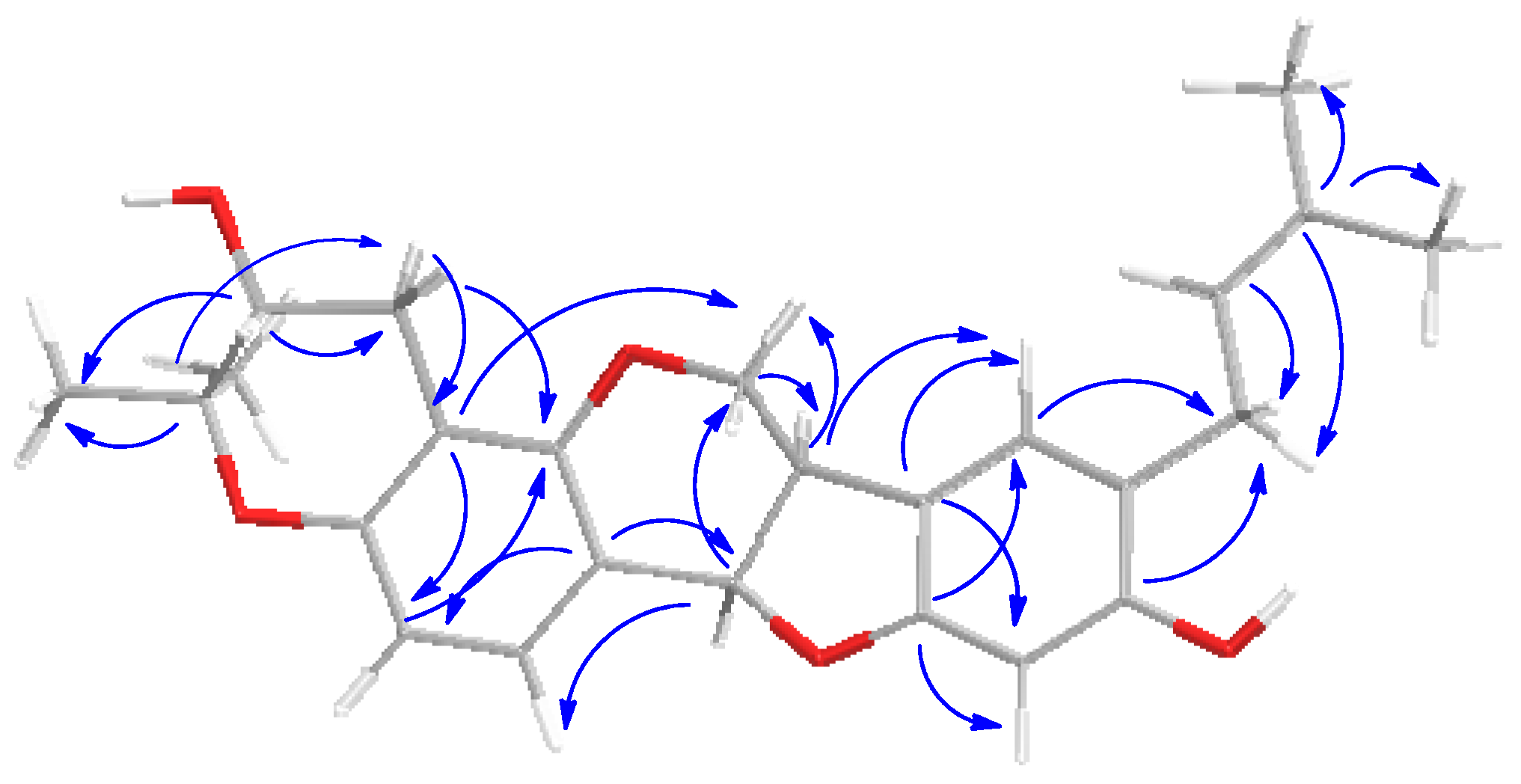
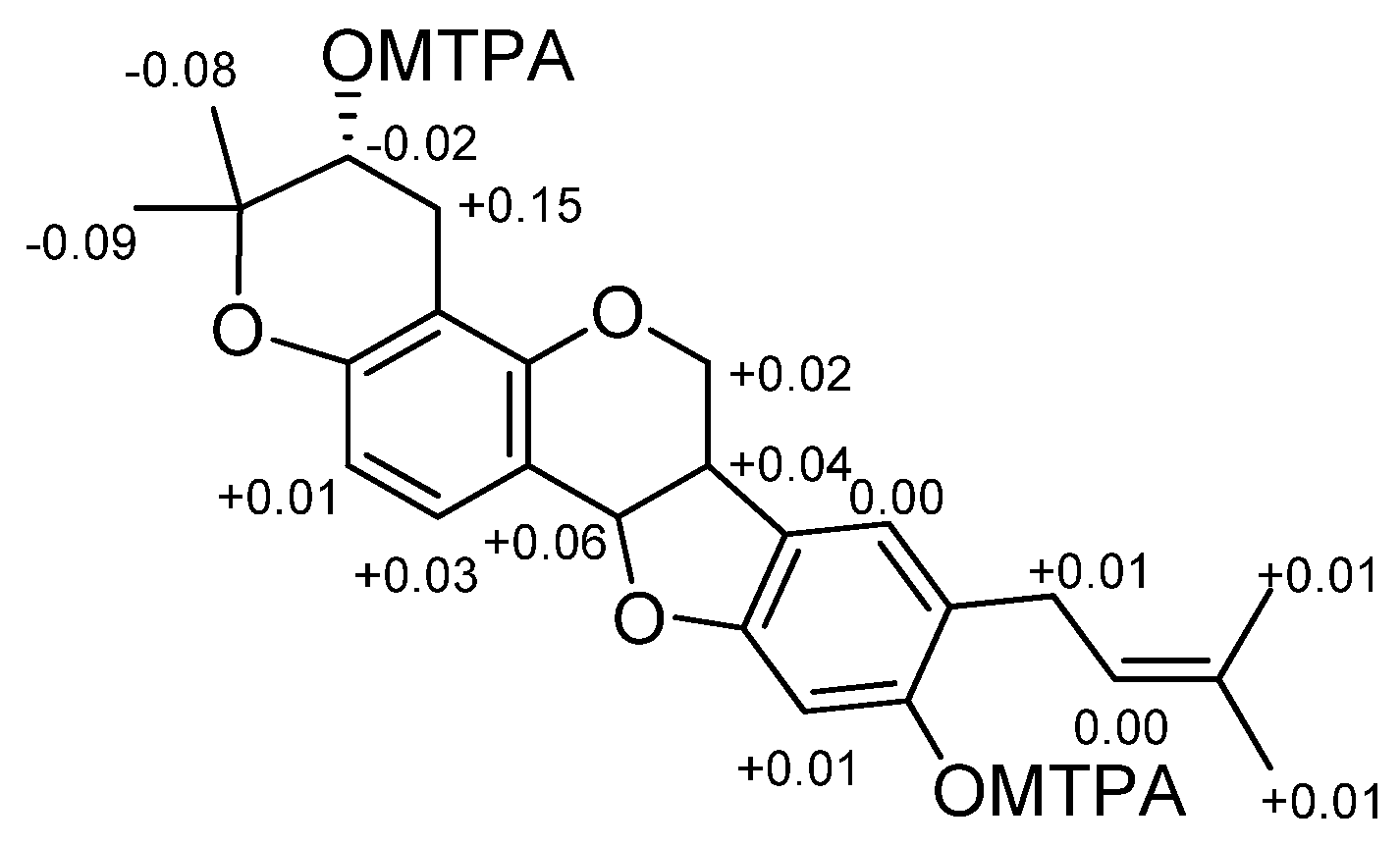
2.1. Chemistry: Structural Elucidation
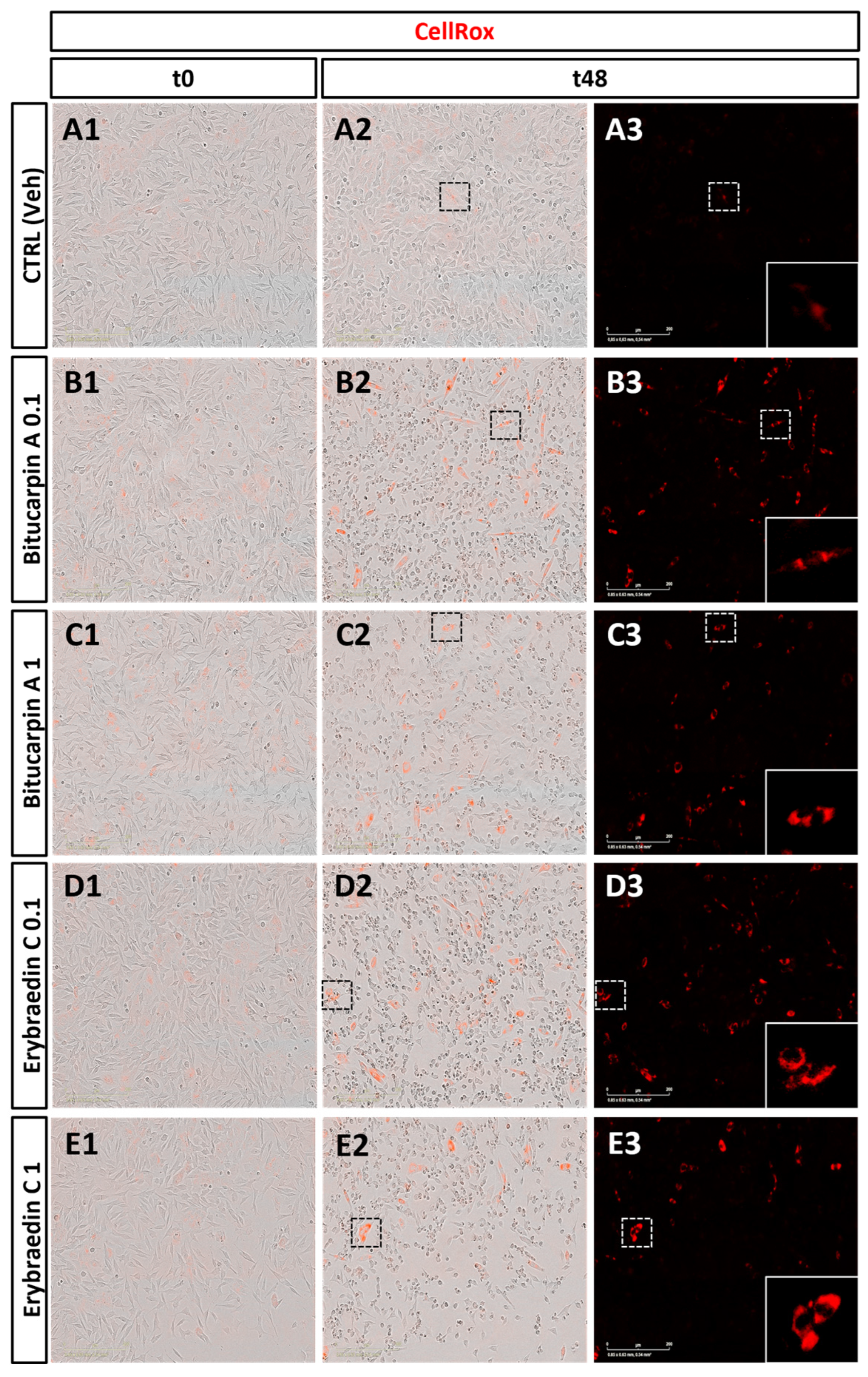
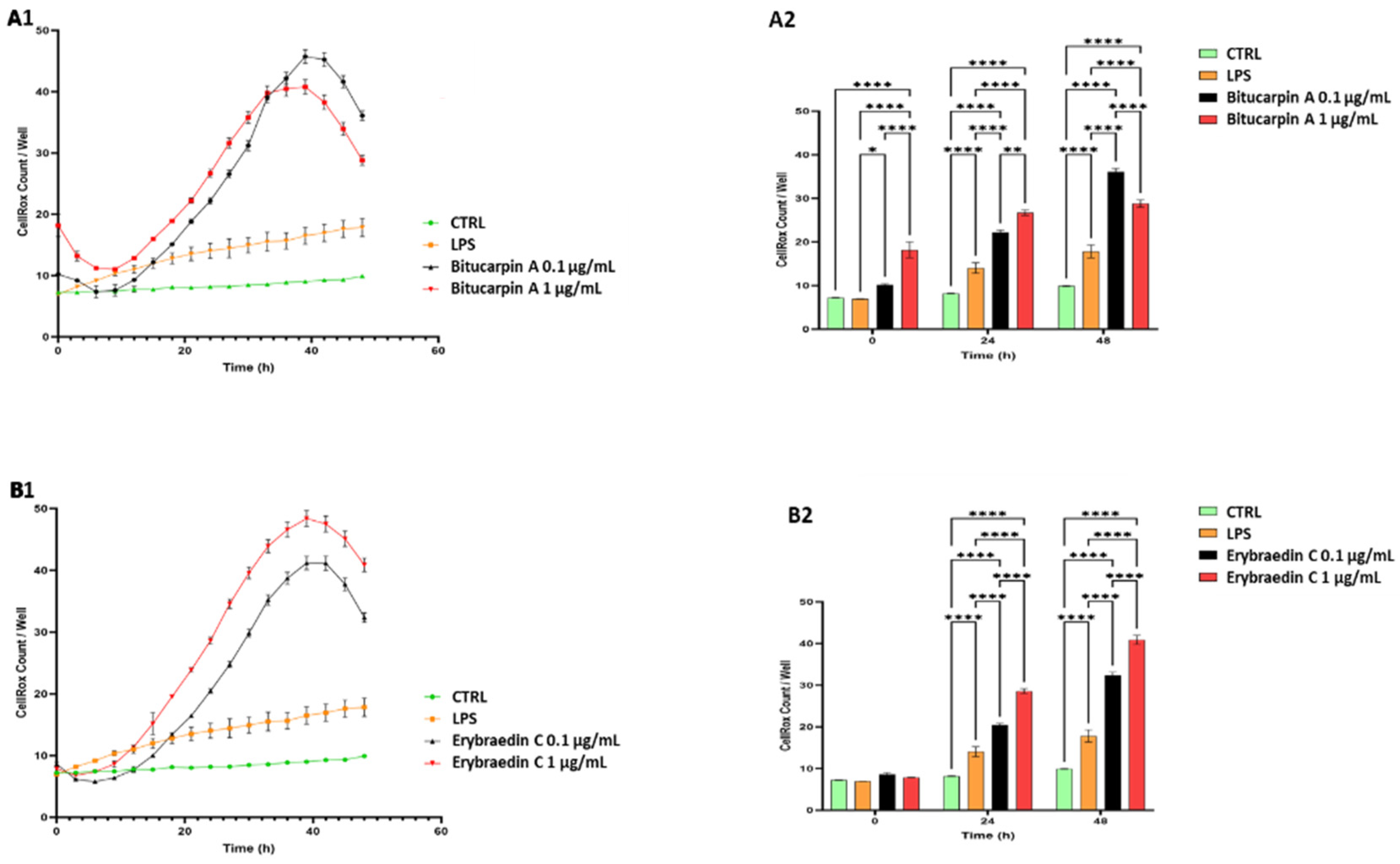
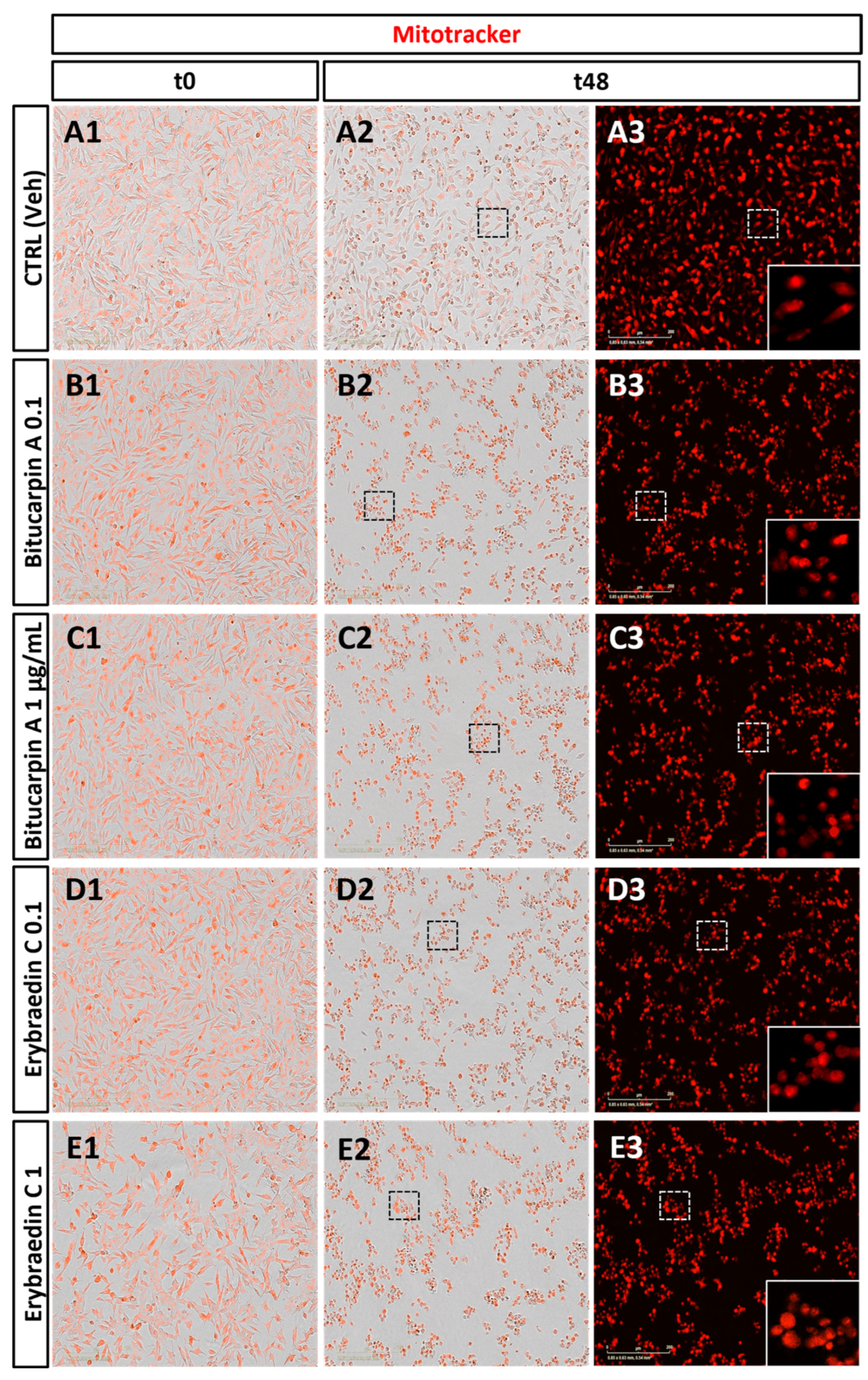
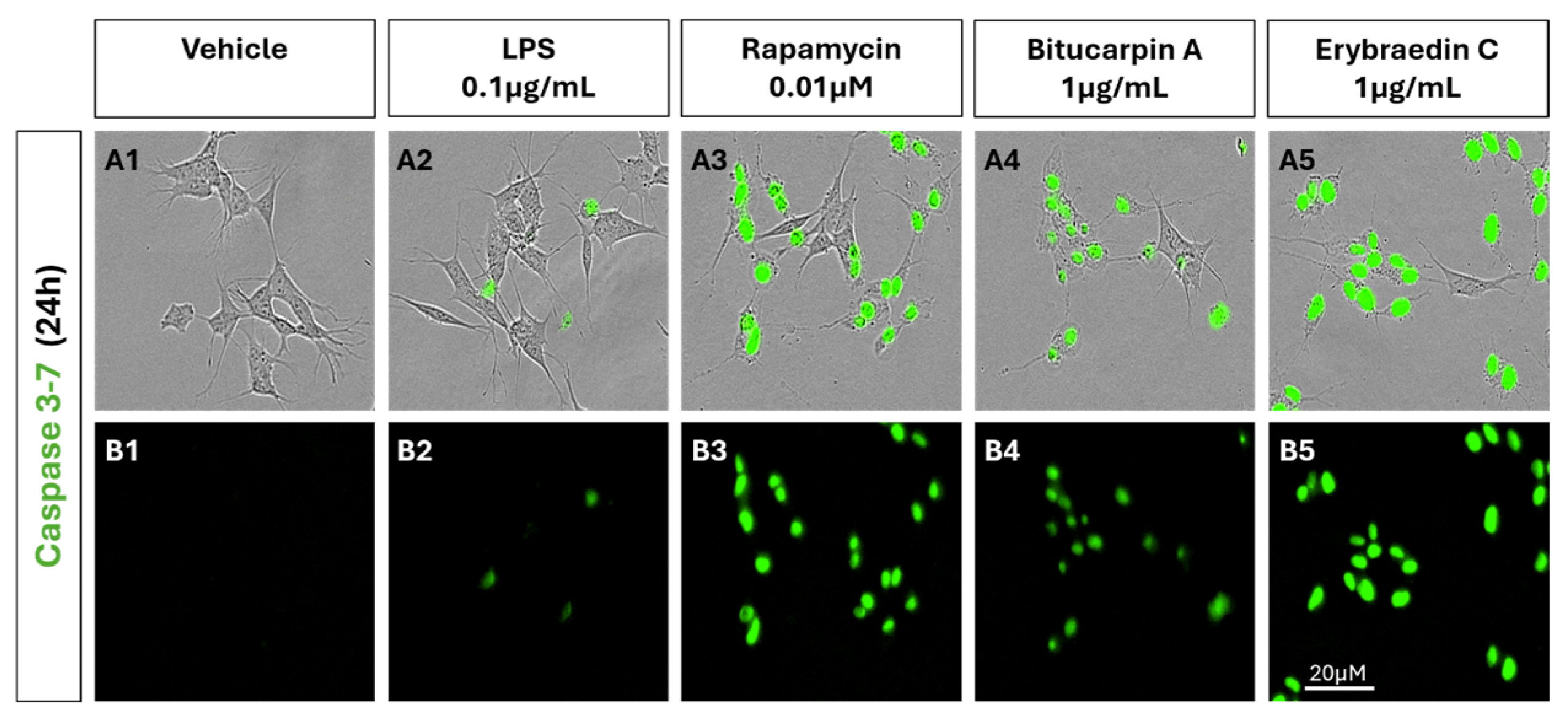
2.2. Biological Activity: Antiproliferative Activity of Bitucarpin A (4) and Erybraedin C (5) on Neuroblastoma SH-SY5Y Cells
3. Discussion and Conclusions
4. Materials and Methods
4.1. General Experimental Procedures
4.2. Plant Material
4.3. Extraction and Isolation
- Bituminarin A (1)
- Bituminarin B (2)
- Bituminarin C (3)
4.4. Acetylation of Bituminarins A–C
4.5. Preparation of MTPA Esters of 1
4.6. Biological Activity
4.6.1. Cell Cultures
4.6.2. Immunofluorescence
4.6.3. Incucyte Live Cell Scanning
4.6.4. Reagents
4.6.5. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- WFO Plant List. Available online: https://wfoplantlist.org/plant-list/ (accessed on 1 February 2024).
- Ventura, M.R.; Mendez, P.; Flores, M.P.; Rodriguez, R.; Castanon, J.I.R. Energy and protein content of Tedera (Biturninaria bituminosa). Cha. Opt. Med. 2000, 45, 219. [Google Scholar]
- Ventura, M.R.; Flores, M.P.; Castanon, J.I.R. Nutritive value of forage shrubs: Bituminaria bituminosa, Acacia salicina and Medicago arborea. Cah. Options Méditerranéennes 1999, 39, 171–173. [Google Scholar]
- Pistelli, L.; Noccioli, C.; Appendino, G.; Bianchi, F.; Sterner, O.; Ballero, M. Pterocarpans from Bituminaria morisiana and Bituminaria bituminosa. Phytochemistry 2003, 64, 595–598. [Google Scholar] [CrossRef] [PubMed]
- Pecetti, L.; Tava, A.; Pagnotta, M.A.; Russi, L. Variation in forage quality and chemical composition among Italian accessions of Bituminaria bituminosa (L.) Stirt. J. Sci. Food Agric. 2007, 87, 985–991. [Google Scholar] [CrossRef]
- Pecetti, L.; Mella, M.; Tava, A. Variation in Herbage Biochemical Composition among Pitch Trefoil (Bituminaria bituminosa) Populations from Elba Island, Italy. J. Agric. Food Chem. 2016, 64, 195–203. [Google Scholar] [CrossRef] [PubMed]
- Koul, B.; Taak, P.; Kumar, A.; Kumar, A.; Sanyal, I. Genus Psoralea: A review of the traditional and modern uses, phytochemistry and pharmacology. J. Ethnopharm. 2019, 232, 201–226. [Google Scholar] [CrossRef] [PubMed]
- Tava, A.; Pecetti, L.; Ricci, M.; Pagnotta, M.A.; Russi, L. Volatile compounds from leaves and flowers of Bituminaria bituminosa (L.) Stirt. (Fabaceae) from Italy. Flav. Fragr. J. 2007, 22, 363–370. [Google Scholar] [CrossRef]
- Innocenti, G.; Piovan, A.; Filippini, R.; Caniato, R.; Cappelletti, E.M. Quantitative recovery of furanocoumarins from Psoralea bituminosa. Phytochem. Anal. 1997, 8, 84–86. [Google Scholar] [CrossRef]
- Azzouzi, S.; Zaabat, N.; Medjroubi, K.; Akkal, S.; Benlabed, K.; Smati, F.; Dijoux-Franca, M.G. Phytochemical and biological activities of Bituminaria bituminosa L. (Fabaceae). Asian Pac. J. Trop. Med. 2014, 7, S481. [Google Scholar] [CrossRef] [PubMed]
- Llorent-Martinez, E.J.; Spinola, V.; Gouveia, S.; Castilho, P.P. HPLC-ESI-MSn characterization of phenolic compounds, terpenoid saponins, and other minor compounds in Bituminaria bituminosa. Ind. Crop Prod. 2015, 69, 80–90. [Google Scholar] [CrossRef]
- Selvam, C.; Jordan, B.C.; Prakash, S.; Mutisya, D.; Thilagavathi, R. Pterocarpan scaffold: A natural lead molecule with diverse pharmacological properties. Eur. J. Med. Chem. 2017, 128, 219–236. [Google Scholar] [CrossRef] [PubMed]
- Al-Maharik, N. Isolation of naturally occurring novel isoflavonoids: An update. Nat. Prod. Rep. 2019, 36, 1156–1195. [Google Scholar] [CrossRef] [PubMed]
- Veitch, N.C. Isoflavonoids of the Leguminosae. Nat. Prod. Rep. 2013, 30, 988–1027. [Google Scholar] [CrossRef] [PubMed]
- Smadi, A.; Bitam, F.; Ciavatta, M.L.; Carbone, M.; Bertella, A.; Gavagnin, M. Chemical constituents of the aerial parts of Algerian Galium brunneum: Isolation of new hydroperoxy sterol glucosyl derivatives. Phytochem. Lett. 2020, 38, 39–45. [Google Scholar] [CrossRef]
- Kebbi, S.; Ciavatta, M.L.; Mahmoud, A.M.; Carbone, M.; Ligresti, A.; Seghiri, R.; Gavagnin, M. Sesquiterpene lactones with the 12,8-guaianolide skeleton from Algerian Centaurea omphalotricha. Biomolecules 2021, 11, e1053. [Google Scholar] [CrossRef] [PubMed]
- Bensaid, S.O.; Carbone, M.; Palomba, L.; Bicha, S.; Bentamene, A.; Carannante, A.; Gavagnin, M.; Ciavatta, M.L. First occurrence of megastigmane glucosides in a plant of Retama genus. Chem. Biodivers. 2022, 19, e202200675. [Google Scholar]
- Mitscher, L.A.; Okwute, S.K.; Gollapudi, S.R.; Drake, S.; Avona, E. Antimicrobial pterocarpans of Nigerian Erythrina mildbraedii. Phytochemistry 1988, 27, 3449–3452. [Google Scholar] [CrossRef]
- Nkengfack, A.E.; Vardamides, J.C.; Fomum, Z.T.; Meyers, M. Prenylated isoflavanone from Erythrina eriotricha. Phytochemistry 1995, 40, 1803–1808. [Google Scholar] [CrossRef]
- Mitscher, L.A.; Okwute, S.K.; Gollapudi, S.R.; Keshavarz-Shokri, A. Antimicrobial agents from higher plants. The isolation and structural characterization of two additional pterocarpan antimicrobial agents from Nigerian Erythrina mildbraedii. Heterocycles 1988, 27, 2517–2522. [Google Scholar] [CrossRef]
- Jois, H.S.; Manjunath, B.L.; Rao, S.V. Chemische untersuchung der samen von Psoralea corylifolia, Linn. I. Chem. Zentralblatt. 1933, 104, 77. [Google Scholar]
- Jois, H.S.; Manjunath, B.L.; Rao, S.V. Chemical examination of seeds of Psoralea coryfolia. J. Ind. Chem. Soc. 1933, 10, 41. [Google Scholar]
- Xiao, G.; Li, G.; Chen, L.; Zhang, Z.; Yin, J.J.; Wu, T.; Cheng, Z.; Wei, X.; Wang, Z. Isolation of antioxidants from Psoralea corylifolia fruits using high-speed counter-current chromatography guided by thin layer chromatography-antioxidant autographic assay. J. Chromat. A 2010, 1217, 5470–5476. [Google Scholar] [CrossRef] [PubMed]
- Späth, E.; Pesta, O. Die constitution des angelicin. Ber. Dtsch. Chem. Ges. 1934, 67, 853. [Google Scholar] [CrossRef]
- Schmitt, A.; Telikepalli, H.; Mitscher, L.A. Plicatin B, the antimicrobial principle of Psoralea juncea. Phytochemistry 1991, 30, 3569–3570. [Google Scholar] [CrossRef]
- Eventi, A.; Lubrano, V.; Scarpato, R.; Turchi, G. Protective effects of plicatin B on micronucleus induction in cultured human lymphocytes by different mutageno. Food Chem. Toxicol. 2009, 47, 124–128. [Google Scholar] [CrossRef] [PubMed]
- Cottiglia, F.; Casu, L.; Bonsignore, L.; Casu, M.; Floris, C.; Leonti, M.; Gertsch, J.; Heilmann, J. New cytotoxic prenylated isoflavonoids from Bituminaria morisiana. Planta Med. 2005, 71, 254–260. [Google Scholar] [CrossRef]
- Tesauro, C.; Fiorani, P.; D’Annessa, I.; Chillemi, G.; Turchi, G.; Desideri, A. Erybraedin C, a natural compound from the plant Bituminaria bituminosa, inhibits both the cleavage and religation activities of human topoisomerase I. Biochem. J. 2010, 425, 531–539. [Google Scholar] [CrossRef]
- Sakurai, Y.; Sakurai, N.; Taniguchi, M.; Naganishi, Y.; Bastow, K.F.; Wang, X.; Cragg, G.M.; Lee, K.-H. Rautandiols A and B, pterocarpans and cytotoxic constituents from Neorautanenia mitis. J. Nat. Prod. 2006, 69, 397–399. [Google Scholar] [CrossRef]
- Ren, F.-C.; Wang, L.-X.; Lv, Y.-F.; Hu, J.-M.; Zhou, J. Structure revision of four classes of prenylated aromatic natural products based on a rule for diagnostic 13C NMR chemical shifts. J. Org. Chem. 2021, 86, 10982–10990. [Google Scholar] [CrossRef]
- Goel, A.; Kumar, A.; Raghuvanshi, A. Synthesis, Stereochemistry, Structural Classification, and Chemical Reactivity of Natural Pterocarpans. Chem. Rev. 2013, 113, 1614–1640. [Google Scholar] [CrossRef]
- Tarbeeva, D.V.; Pislyagin, E.A.; Menchinskaya, E.S.; Berdyshev, D.V.; Kalinovskiy, A.I.; Grigorchuk, V.P.; Mishchenko, N.P.; Aminin, D.L.; Fedoreyev, S.A. Polyphenolic compounds from Lespedeza bicolor protect neuronal cells from oxidative stress. Antioxidants 2022, 11, e11040709. [Google Scholar] [CrossRef] [PubMed]
- Haga, N.; Fujita, N.; Tsuruo, T. Mitochondrial aggregation precedes cytochrome c release from mitochondria during apoptosis. Oncogene 2003, 22, 5579–5585. [Google Scholar] [CrossRef] [PubMed]
- Zamzami, N.; Marchetti, P.; Castedo, M.; Zanin, C.; Vayssiere, J.L.; Petit, P.X.; Kroemer, G. Reduction in mitochondrial potential constitutes an early irreversible step of programmed lymphocyte death in vivo. J. Exp. Med. 1995, 181, 1661–1672. [Google Scholar] [CrossRef] [PubMed]
- Bock, F.J.; Tait, S.W.J. Mitochondria as multifaceted regulators of cell death. Nat. Rev. Mol. Cell Biol. 2020, 2, 85–100. [Google Scholar] [CrossRef] [PubMed]
- Karbowski, M.; Youle, R. Dynamics of mitochondrial morphology in healthy cells and during apoptosis. Cell Death Differ. 2003, 10, 870–880. [Google Scholar] [CrossRef] [PubMed]
- Gilkerson, R.W.; Margineantu, D.H.; Capaldi, R.A.; Selker, J.M.L. Mitochondrial DNA depletion causes morphological changes in the mitochondrial reticulum of cultures human cells. FEBS Lett. 2000, 474, 1–4. [Google Scholar] [CrossRef] [PubMed]
- Westrate, L.M.; Drocco, J.A.; Martin, K.R.; Hlavacek, W.S.; MacKeigan, J.P. Mitochondrial morphological features are associated with fission and fusion events. PLoS ONE 2014, 9, e0095265. [Google Scholar] [CrossRef] [PubMed]
- Ngo, J.; Choi, D.W.; Stanley, I.A.; Stiles, L.; Molina, A.J.A.; Chen, P.H.; Lako, A.; Sung, I.C.H.; Goswami, R.; Kim, M.Y.; et al. Mitochondrial morphology controls fatty acid utilization by changing CPT1 sensitivity to malonyl-CoA. EMBO J. 2023, 42, e111901. [Google Scholar] [CrossRef] [PubMed]
- Nassief, S.M.; Amer, M.E.; Shawky, E.; Sishtla, K.; Mas-Claret, E.; Muniyandi, A.; Corson, T.W.; Mulholland, D.A.; Sawsan El-Masry, S. Antiangiogenic pterocarpan and flavonoid constituents of Erythrina lysistemon. J. Nat. Prod. 2023, 86, 759–766. [Google Scholar] [CrossRef]
- D’Angiolillo, F.; Pistelli, L.; Noccioli, C.; Ruffoni, B.; Piaggi, S.; Scarpato, R.; Pistelli, L. In vitro Cultures of Bituminaria bituminosa: Pterocarpan, furanocoumarin and isoflavone production and cytotoxic activity evaluation. Nat. Prod. Commun. 2014, 9, 477–480. [Google Scholar] [CrossRef]
- Dewick, M. The Flavonoids: Advances in Research; Harborne, J.B., Ed.; Chapman and Hill: London, UK, 1988; pp. 125–149. [Google Scholar]
- Wu, J.; Gu, L.; Geacintov, N.E.; Li, G.-M. Mismatch repair processing of carcinogen-DNA adducts triggers apoptosis. Mol. Cell. Biol. 1999, 19, 8292–8302. [Google Scholar] [CrossRef] [PubMed]
- Kaufmann, S.H. Cell death induced by topoisomerase-targeted drugs—More questions than answers. Biochem. Biophys. Acta 1998, 1400, 1009–1014. [Google Scholar] [CrossRef] [PubMed]
- Fofana, S.; Ouédraogo, M.; Esposito, R.C.; Ouedraogo, W.P.; Delporte, C.; Van Antwerpen, P.; Mathieu, V.; Guissou, I.P. Systematic review of potential anticancerous activities of Erythrina senegalensis DC (Fabaceae). Plants 2021, 11, e11010019. [Google Scholar] [CrossRef] [PubMed]






| 1 | 2 | 3 | ||||
|---|---|---|---|---|---|---|
| n. | δC, Type | δH (J in Hz) | δC, Type | δH (J in Hz) | δC, Type | δH (J in Hz) |
| 1 | 130.2, CH | 7.19, d (8.5) | 130.2, CH | 7.19, d (8.6) | 130.2, CH | 7.20, d (8.4) |
| 2 | 111.1, CH | 6.44, d (8.5) | 111.1, CH | 6.44, d (8.6) | 111.2, CH | 6.45, d (8.4) |
| 3 | 155.9 *, C | 154.2 *, C | 155.3 *, C | |||
| 4 | 108.9 *, C | 108.8 *, C | 109.0 *, C | |||
| 4a | 154.5 *, C | 154.4 *, C | 155.2 *, C | |||
| 6 | 67.5, CH2 | 4.31α, dd (10.5, 4.6) 3.57β, dd (10.5, 10.5) | 67.4, CH2 | 4.31 α, dd (10.6, 4.8) 3.60 β, dd (10.6,10.6) | 67.4, CH2 | 4.30 α, dd (10.9, 4.8) 3.62 α, dd (10.9,10.9) |
| 6a | 40.5, CH | 3.52, m | 40.6, CH | 3.51, m | 40.5, CH | 3.53, ddd (10.1, 7.0, 4.8) |
| 6b | 119.2 *, C | 118.8 *, C | 119.1 *, C | |||
| 7 | 126.0, CH | 7.03, s | 126.0, CH | 7.03, s | 127.9, CH | 7.06, s |
| 8 | 120.7 *, C | 120.1 *, C | 120.0 *, C | |||
| 9 | 155.9 *, C | 156.0 *, C | 158.5 *, C | |||
| 10 | 98.2, CH | 6.31, s | 98.3, CH | 6.31, s | 99.1, CH | 6.29, s |
| 10a | 163.7 *, C | 159.5 *, C | 160.2 *, C | |||
| 11a | 79.3, CH | 5.42, d (6.9) | 79.3, CH | 5.43, d (7.0) | 79.4, CH | 5.45, d (7.0) |
| 11b | 113.5 *, C | 112.7, C | 112.8 *, C | |||
| 1′ | 27.1, CH2 | 2.88α, dd (16.9, 5.5) 2.48β, dd (16.9, 7.9) | 27.1, CH2 | 2.86α, dd (17.0, 5.4) 2.52β, dd (17.0, 7.4) | 27.1, CH2 | 2.87α, dd (17.3, 5.6) 2.53β, dd (17.3, 7.4) |
| 2′ | 69.6, CH | 3.74, m | 69.5, CH | 3.75, m | 69.5, CH | 3.75, m |
| 3′ | 78.8 *, C | 77.8 *, C | 79.3 *, C | |||
| 4′ | 26.0, CH3 | 1.31α, s | 25.8, CH3 | 1.31α, s | 25.8, CH3 | 1.31 α, s |
| 5′ | 20.3, CH3 | 1.21β, s | 20.8, CH3 | 1.21β, s | 20.8, CH3 | 1.21β, s |
| 1″ | 28.7, CH2 | 3.27, dd (15.0, 7.2) 3.21, dd (15.0, 7.6) | 28.7, CH2 | 3.27, dd (15.8, 7.2) 3.21, dd (15.8, 7.4) | 38.6, CH2 | 2.78 (overlapped) |
| 2″ | 124.5, CH | 5.31, br t (7.0) | 124.4, CH | 5.31, br t (7.0) | 77.4, CH | 4.32, m |
| 3″ | 131.7 *, C | 131.9 *, C | 148.6 *, C | |||
| 4″ | 25.9, CH3 | 1.70, s | 25.9, CH3 | 1.71, s | 110.4, CH2 | 4.94, s 4.77, s |
| 5″ | 17.5, CH3 | 1.70, s | 17.3, CH3 | 1.71, s | 18.5, CH3 | 1.78, s |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Benhabrou, H.; Bitam, F.; Cristino, L.; Nicois, A.; Carbone, M.; Ammar, D.; Gavagnin, M.; Ciavatta, M.L. Prenyl Pterocarpans from Algerian Bituminaria bituminosa and Their Effects on Neuroblastoma. Molecules 2024, 29, 3678. https://doi.org/10.3390/molecules29153678
Benhabrou H, Bitam F, Cristino L, Nicois A, Carbone M, Ammar D, Gavagnin M, Ciavatta ML. Prenyl Pterocarpans from Algerian Bituminaria bituminosa and Their Effects on Neuroblastoma. Molecules. 2024; 29(15):3678. https://doi.org/10.3390/molecules29153678
Chicago/Turabian StyleBenhabrou, Hakim, Fatma Bitam, Luigia Cristino, Alessandro Nicois, Marianna Carbone, Dibi Ammar, Margherita Gavagnin, and Maria Letizia Ciavatta. 2024. "Prenyl Pterocarpans from Algerian Bituminaria bituminosa and Their Effects on Neuroblastoma" Molecules 29, no. 15: 3678. https://doi.org/10.3390/molecules29153678
APA StyleBenhabrou, H., Bitam, F., Cristino, L., Nicois, A., Carbone, M., Ammar, D., Gavagnin, M., & Ciavatta, M. L. (2024). Prenyl Pterocarpans from Algerian Bituminaria bituminosa and Their Effects on Neuroblastoma. Molecules, 29(15), 3678. https://doi.org/10.3390/molecules29153678








