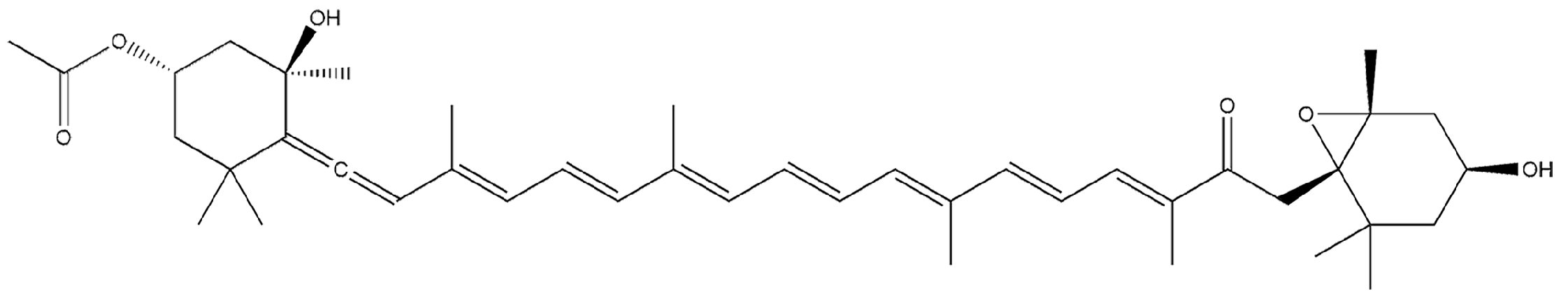
Fucoxanthin Inhibits the Proliferation and Metastasis of Human Pharyngeal Squamous Cell Carcinoma by Regulating the PI3K/Akt/mTOR Signaling Pathway
Abstract
1. Introduction
2. Results
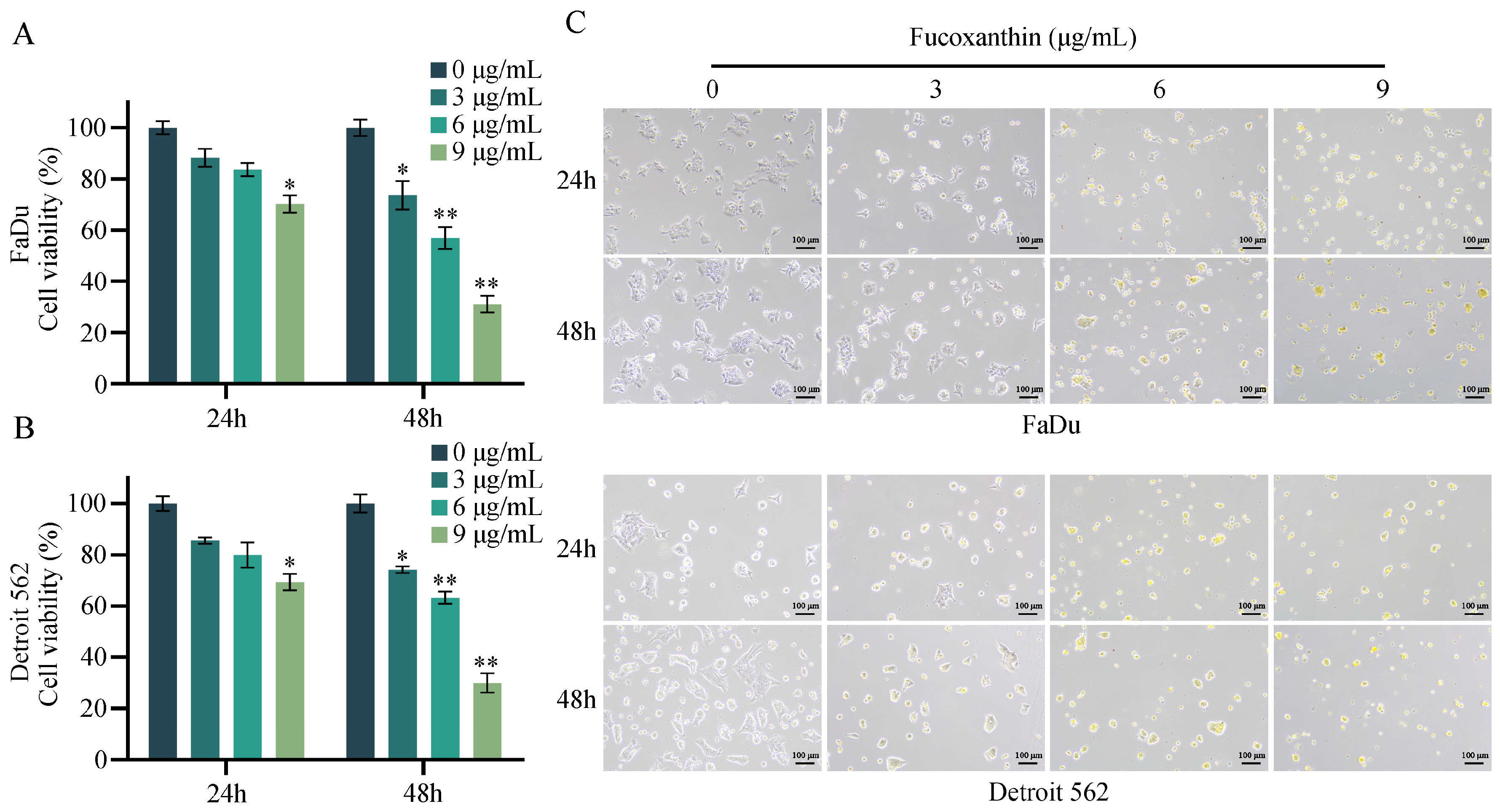
2.1. Inhibitory Effects of Fucoxanthin on HPSCC Cells
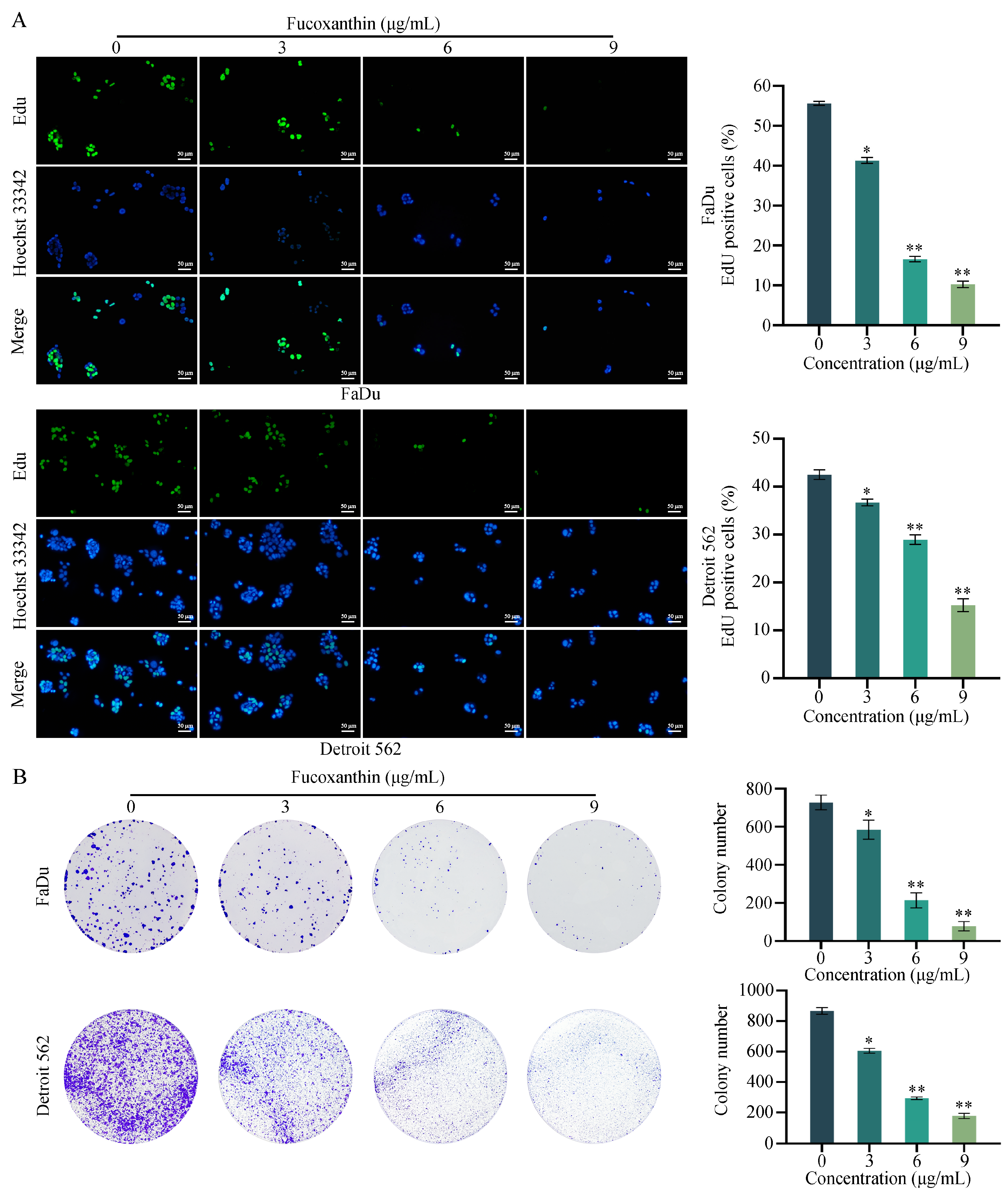
2.2. Fucoxanthin Inhibits the Proliferation of HPSCC Cells
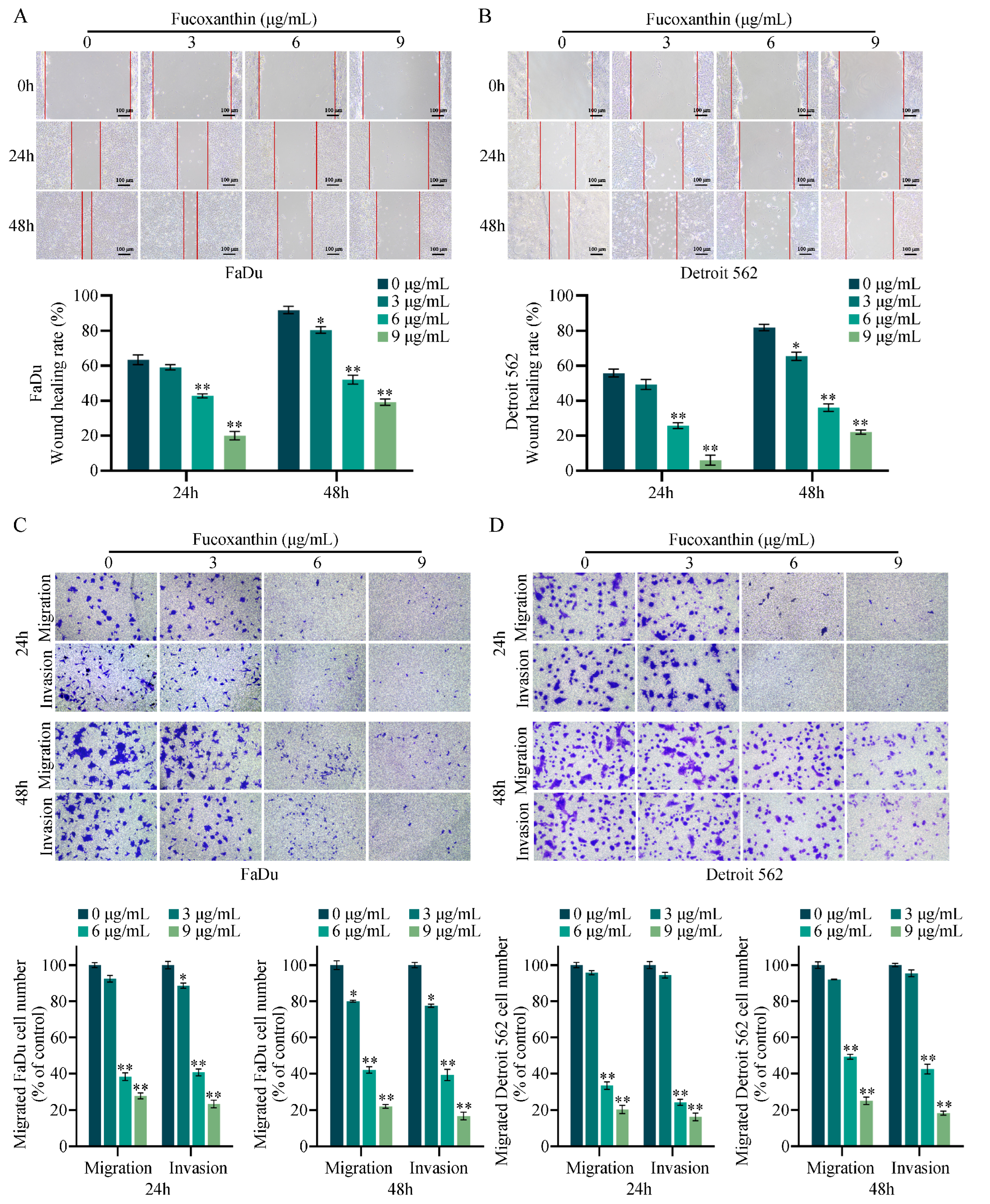
2.3. Fucoxanthin Inhibits the Migration and Invasion of FaDu and Detroit 562 Cells
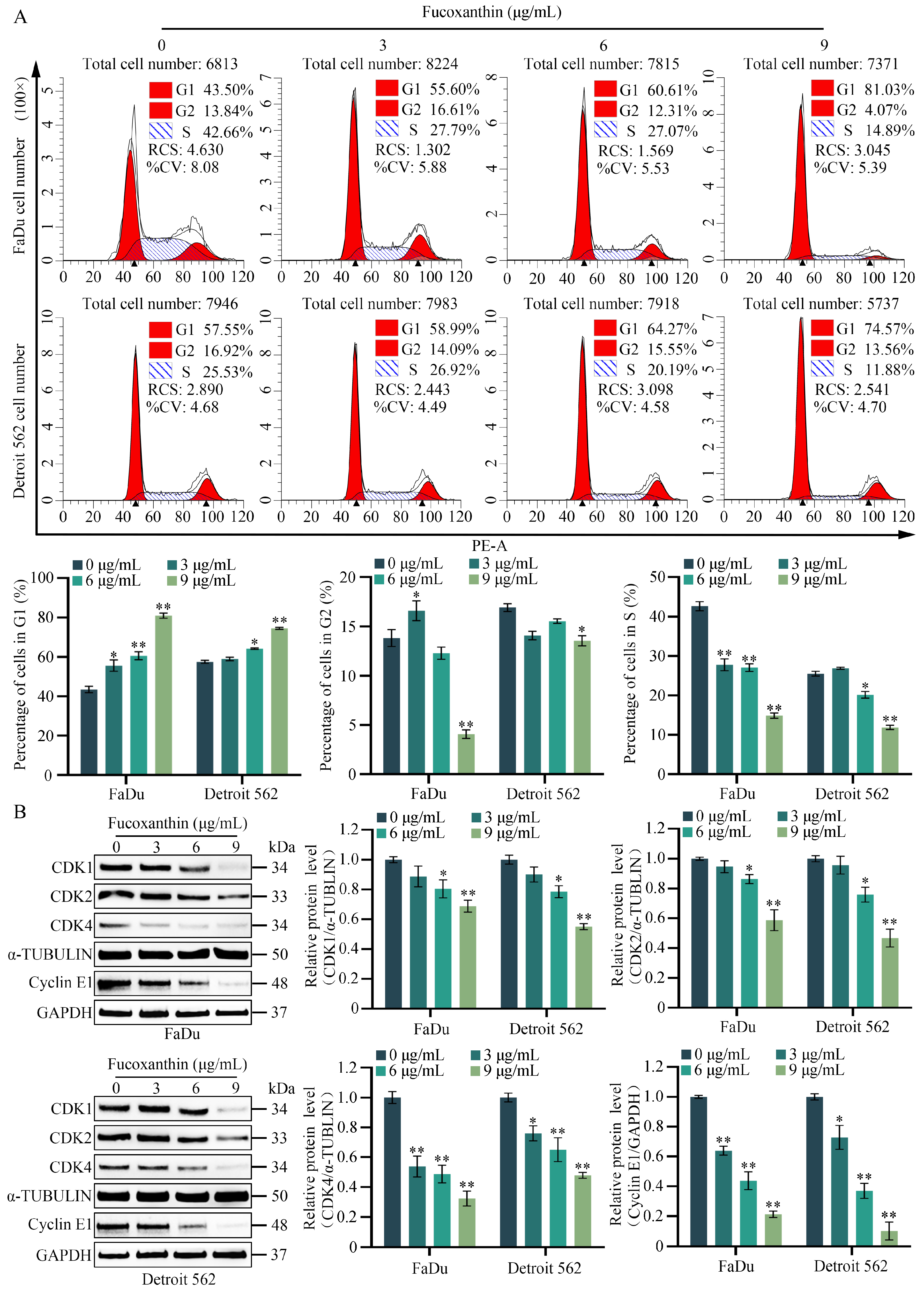
2.4. Fucoxanthin Induces Cell Cycle Arrest in HPSCC Cells
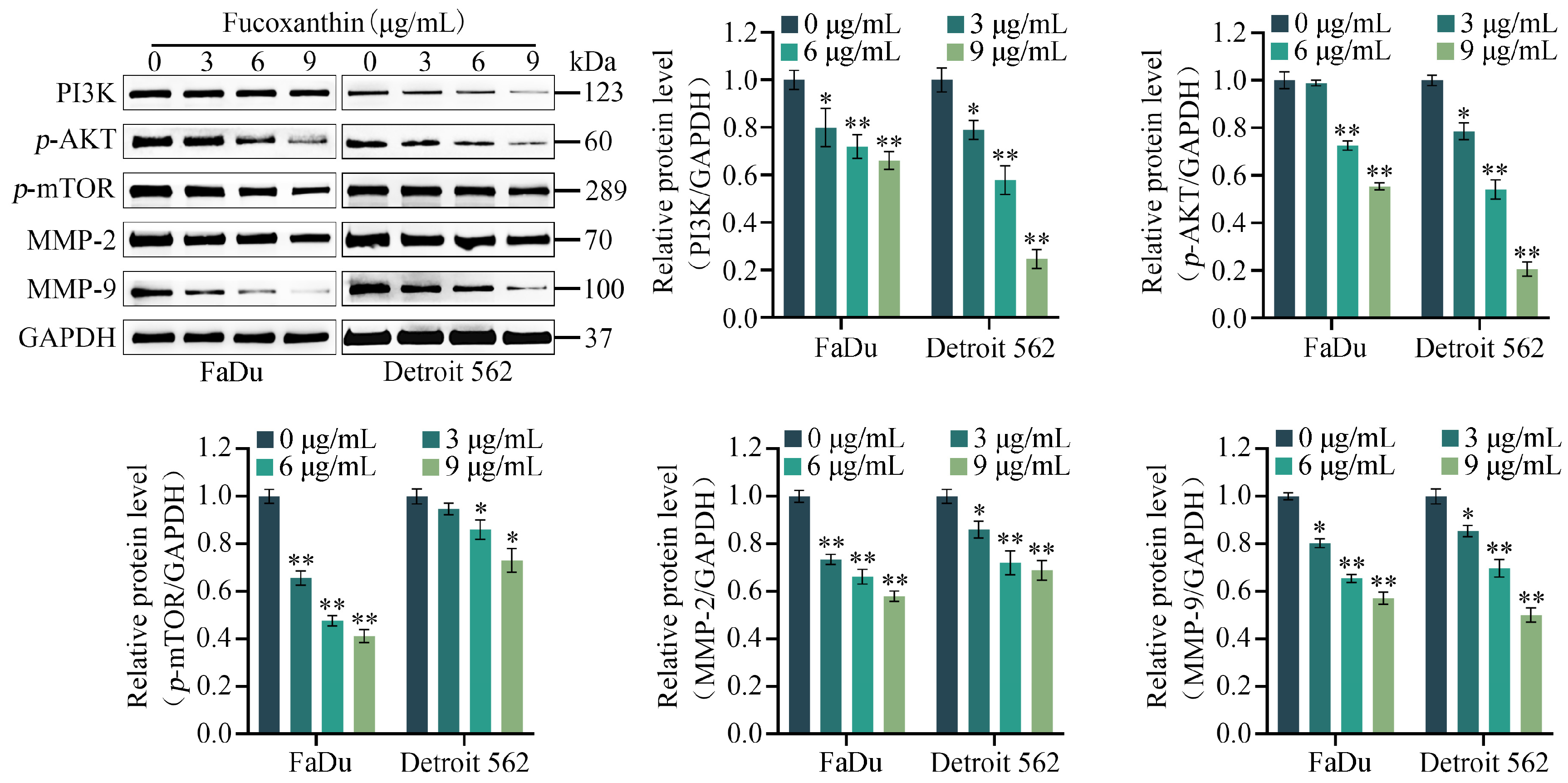
2.5. Fucoxanthin Downregulates the Expression Levels of MMP−2, MMP−9, and PI3K/AKT/mTOR Signaling Pathway Components
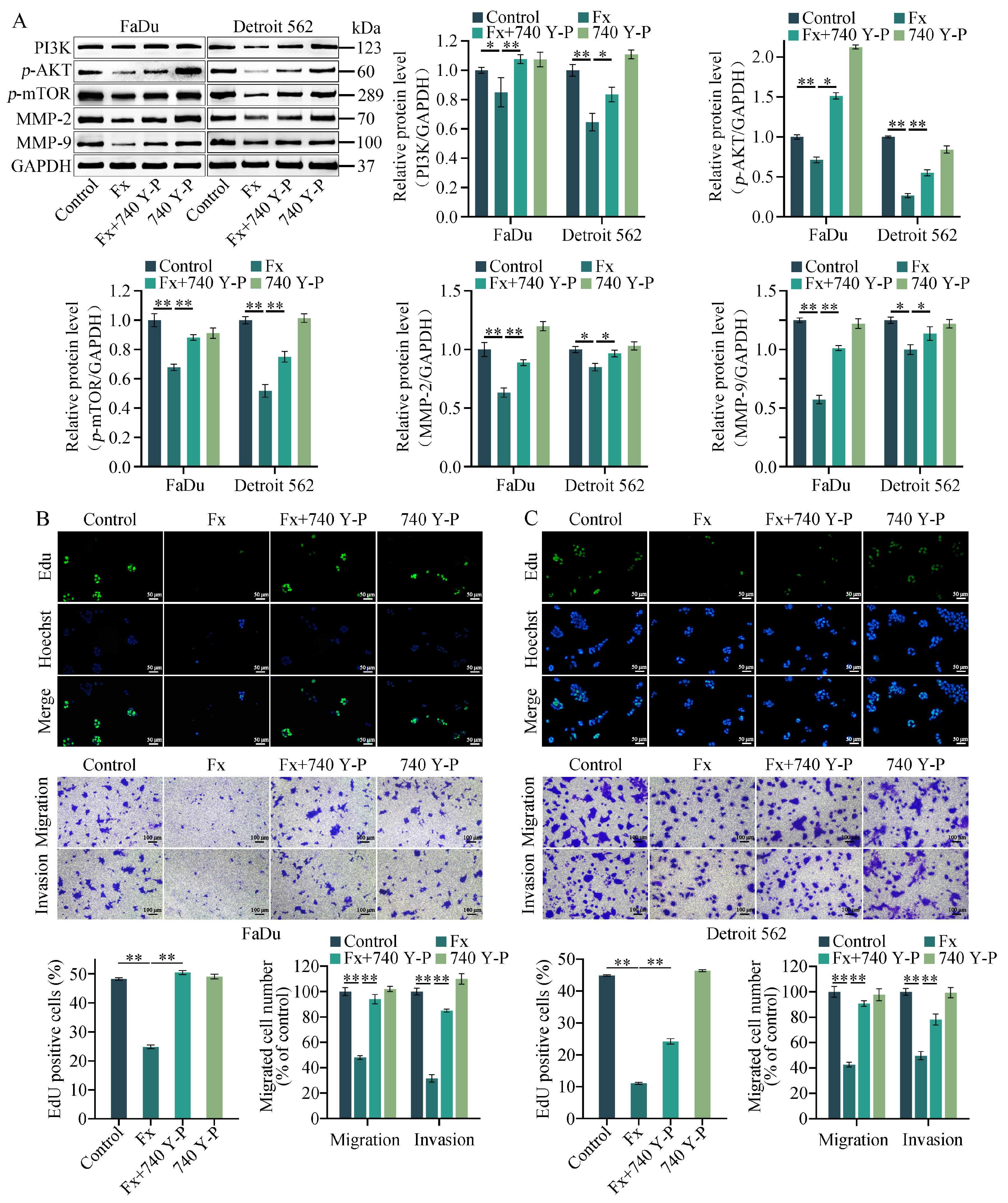
2.6. 740 Y−P Reversed the Fucoxanthin-Induced Downregulation of PI3K/AKT/mTOR, MMP−2, and MMP−9 Protein Levels
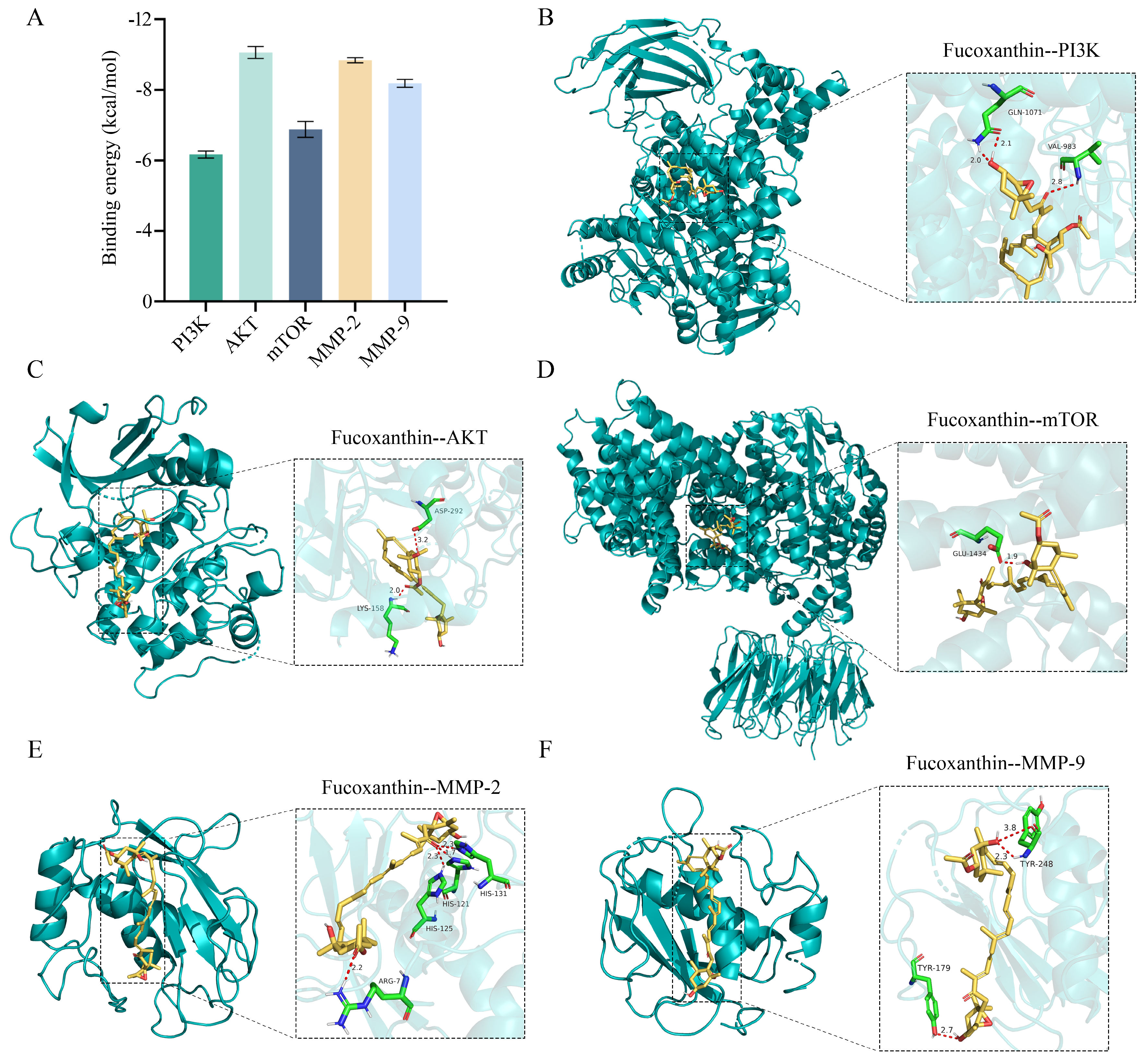
2.7. Interactions of Fucoxanthin with Target Proteins
3. Discussion
4. Materials and Methods
4.1. Cell Source and Culture
4.2. Cell Viability and Observation of Cell Morphology
4.3. EdU Fluorescent Staining Assay
4.4. Cell Colony Formation Assay
4.5. Wound Healing Assay
4.6. Transwell Migration and Invasion Assays
4.7. Cell Cycle Analysis
4.8. Western Blotting Analysis
4.9. Molecular Docking
4.10. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Johnson, D.E.; Burtness, B.; Leemans, C.R.; Lui, V.W.Y.; Bauman, J.E.; Grandis, J.R. Head and neck squamous cell carcinoma. Nat. Rev. Dis. Primers 2020, 6, 92. [Google Scholar] [CrossRef] [PubMed]
- Chamoli, A.; Gosavi, A.S.; Shirwadkar, U.P.; Wangdale, K.V.; Behera, S.K.; Kurrey, N.K.; Kalia, K.; Mandoli, A. Overview of oral cavity squamous cell carcinoma: Risk factors, mechanisms, and diagnostics. Oral Oncol. 2021, 121, 105451. [Google Scholar] [CrossRef] [PubMed]
- Magnes, T.; Wagner, S.; Kiem, D.; Weiss, L.; Rinnerthaler, G.; Greil, R.; Melchardt, T. Prognostic and predictive factors in advanced head and neck squamous cell carcinoma. Int. J. Mol. Sci. 2021, 22, 4981. [Google Scholar] [CrossRef] [PubMed]
- Ou, X.M.; He, X.Y.; Yu, W.; Lu, X.G.; Du, C.R.; Ji, D.M.; Ji, Q.H.; Hu, C.S. Induction chemotherapy and toripalimab for larynx preservation in resectable locally advanced laryngeal/hypopharyngeal carcinoma: Preliminary results of INSIGHT study. J. Clin. Oncol. 2023, 41, 6068. [Google Scholar] [CrossRef]
- Gong, H.L.; Shen, T.; Ding, H.; Tao, L.; Wang, L.F.; Wang, J.; Wang, T.; Zhang, M.H.; Shi, Y.; Xu, C.Q. 934P Antitumor activity and safety profile of camrelizumab plus docetaxel, cisplatin, and capecitabine for induction therapy in advanced stage hypopharyngeal carcinoma. Ann. Oncol. 2023, 34, 588. [Google Scholar] [CrossRef]
- Pereira, A.G.; Otero, P.; Echave, J.; Carreira-Casais, A.; Chamorro, F.; Collazo, N.; Jaboui, A.; Lourenço-Lopes, C.; Simal-Gandara, C.; Prieto, M.A. Xanthophylls from the sea: Algae as source of bioactive carotenoids. Mar. Drugs 2021, 19, 188. [Google Scholar] [CrossRef]
- Winarto, J.; Song, D.G.; Pan, C.H. The Role of Fucoxanthin in Non-Alcoholic Fatty Liver Disease. Int. J. Mol. Sci. 2023, 24, 8023. [Google Scholar] [CrossRef]
- Neumann, U.; Derwenskus, F.; Flaiz Flister, V.; Schmid-Staiger, U.; Hirth, T.; Bischoff, S.C. Fucoxanthin, a carotenoid derived from phaeodactylum tricornutum exerts antiproliferative and antioxidant activities In Vitro. Antioxidants 2019, 8, 183. [Google Scholar] [CrossRef]
- Li, S.Y.; Ren, X.M.; Wang, Y.D.; Hu, J.N.; Wu, H.T.; Song, S.; Yan, C.H. Fucoxanthin alleviates palmitate-induced inflammation in RAW 264.7 cells through improving lipid metabolism and attenuating mitochondrial dysfunction. Food Funct. 2020, 11, 3361–3370. [Google Scholar] [CrossRef]
- Terasaki, M.; Kubota, A.; Kojima, H.; Maeda, H.; Miyashita, K.; Kawagoe, C.; Mutoh, M.; Tanaka, T. Fucoxanthin and colorectal cancer prevention. Cancers 2021, 13, 2379. [Google Scholar] [CrossRef]
- Beppu, F.; Niwano, Y.; Sato, E.; Kohno, M.; Tsukui, T.; Hosokawa, M.; Miyashita, K. In vitro and in vivo evaluation of mutagenicity of fucoxanthin (FX) and its metabolite fucoxanthinol (FXOH). J. Toxicol. Sci. 2009, 34, 693–698. [Google Scholar] [CrossRef]
- Beppu, F.; Niwano, Y.; Tsukui, T.; Hosokawa, M.; Miyashita, K. Single and repeated oral dose toxicity study of fucoxanthin (FX), a marine carotenoid, in mice. J. Toxicol. Sci. 2009, 34, 501–510. [Google Scholar] [CrossRef]
- Garg, S.; Afzal, S.; Elwakeel, A.; Sharma, D.; Radhakrishnan, N.; Dhanjal, J.K.; Sundar, D.; Kaul, S.C.; Wadhwa, R. Marine carotenoid fucoxanthin possesses anti-metastasis activity: Molecular evidence. Mar. Drugs 2019, 17, 338. [Google Scholar] [CrossRef]
- Wang, J.; Ma, Y.H.; Yang, J.S.; Jin, L.; Gao, Z.X.; Xue, L.Y.; Hou, L.; Sui, L.L.; Liu, J.; Zou, X.Y. Fucoxanthin inhibits tumour-related lymphangiogenesis and growth of breast cancer. J. Cell Mol. Med. 2019, 23, 2219–2229. [Google Scholar] [CrossRef]
- Ming, J.X.; Wang, Z.C.; Huang, Y.; Ohishi, H.; Wu, R.J.; Shao, Y.; Wang, H.; Qin, M.Y.; Wu, Z.L.; Li, Y.Y.; et al. Fucoxanthin extracted from Laminaria Japonica inhibits metastasis and enhances the sensitivity of lung cancer to Gefitinib. J. Ethnopharmacol. 2021, 265, 113302. [Google Scholar] [CrossRef]
- Domingo-Vidal, M.; Whitaker-Menezes, D.; Martos-Rus, C.; Tassone, P.; Snyder, C.M.; Tuluc, M.; Philp, N.; Curry, J.; Martinez-Outschoorn, U. Cigarette smoke induces metabolic reprogramming of the tumor stroma in head and neck squamous cell carcinoma. Mol. Cancer Res. 2019, 17, 1893–1909. [Google Scholar] [CrossRef]
- Ferlay, J.; Colombet, M.; Soerjomataram, I.; Mathers, C.; Parkin, D.M.; Piñeros, M.; Znaor, A.; Bray, F. Estimating the global cancer incidence and mortality in 2018: Globocan sources and methods. Int. J. Cancer 2019, 144, 1941–1953. [Google Scholar] [CrossRef]
- Bray, F.; Ferlay, J.; Soerjomataram, I.; Siegel, R.L.; Torre, L.A.; Jemal, A. Global cancer statistics 2018: Globocan estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA. Cancer J. Clin. 2018, 68, 394–424. [Google Scholar] [CrossRef]
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: Globocan estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA. Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef]
- Shirasu, H.; Yokota, T.; Hamauchi, S.; Onozawa, Y.; Ogawa, H.; Onoe, T.; Onitsuka, T.; Yurikusa, T.; Mori, K.; Yasui, H. Risk factors for aspiration pneumonia during concurrent chemoradiotherapy or bio-radiotherapy for head and neck cancer. BMC. Cancer 2020, 20, 182. [Google Scholar] [CrossRef]
- Weykamp, F.; Seidensaal, K.; Rieken, S.; Green, K.; Mende, S.; Zaoui, K.; Freier, K.; Adeberg, S.; Debus, J.; Welte, S.E. Age-dependent hemato- and nephrotoxicity in patients with head and neck cancer receiving chemoradiotherapy with weekly cisplatin. Strahlenther. Onkol. 2020, 196, 515–521. [Google Scholar] [CrossRef]
- Aung, T.N.; Qu, Z.; Kortschak, R.D.; Adelson, D.L. Understanding the effectiveness of natural compound mixtures in cancer through their molecular mode of action. Int. J. Mol. Sci. 2017, 18, 656. [Google Scholar] [CrossRef]
- Suwanmanee, G.; Tantrawatpan, C.; Kheolamai, P.; Paraoan, L.; Manochantr, S. Fucoxanthin diminishes oxidative stress damage in human placenta-derived mesenchymal stem cells through the PI3K/Akt/Nrf−2 pathway. Sci. Rep. 2023, 13, 22974. [Google Scholar] [CrossRef]
- Su, J.Q.; Guan, B.Y.; Su, Q.F.; Hu, S.; Wu, S.Z.; Tong, Z.Y.; Zhou, F. Fucoxanthin ameliorates sepsis via modulating microbiota by targeting IRF3 activation. Int. J. Mol. Sci. 2023, 24, 13803. [Google Scholar] [CrossRef]
- Yan, J.; Li, Z.H.; Liang, Y.; Yang, C.B.; Ou, W.; Mo, H.Q.; Tang, M.; Chen, D.S.; Zhong, C.B.; Que, D.D.; et al. Fucoxanthin alleviated myocardial ischemia and reperfusion injury through inhibition of ferroptosis via the NRF2 signaling pathway. Food Funct. 2023, 14, 10052–10068. [Google Scholar] [CrossRef]
- Long, Y.; Cao, X.B.; Zhao, R.Q.; Gong, S.M.; Jin, L.J.; Feng, C. Fucoxanthin treatment inhibits nasopharyngeal carcinoma cell proliferation through induction of autophagy mechanism. Environ. Toxicol. 2020, 35, 1082–1090. [Google Scholar] [CrossRef]
- Qu, J.F.; Sun, Y.P.; Yang, L.K.; Niu, X.X.; Li, L.Y. Fucoxanthin prevents cell growth and induces apoptosis in endometrial cancer HEC−1A cells by the inhibition of the PI3K/Akt/mTOR pathway. J. Biochem. Mol. Toxicol. 2022, 36, e23027. [Google Scholar] [CrossRef]
- Lau, T.Y.; Kwan, H.Y. Fucoxanthin is a potential therapeutic agent for the treatment of breast cancer. Mar. Drugs 2022, 20, 370. [Google Scholar] [CrossRef]
- Karpiński, T.M.; Ożarowski, M.; Alam, R.; Łochyńska, M.; Stasiewicz, M. What Do we know about antimicrobial activity of astaxanthin and fucoxanthin? Mar. Drugs 2021, 20, 36. [Google Scholar] [CrossRef]
- Molina, N.; Morandi, A.C.; Bolin, A.; Otton, R. Comparative effect of fucoxanthin and vitamin C on oxidative and functional parameters of human lymphocytes. Int. Immunopharmacol. 2014, 22, 41–50. [Google Scholar] [CrossRef]
- Zheng, J.; Piao, M.J.; Keum, Y.S.; Kim, H.S.; Hyun, J.W. Fucoxanthin protects cultured human keratinocytes against oxidative stress by blocking free radicals and inhibiting apoptosis. Biomol. Ther. 2013, 21, 270–276. [Google Scholar] [CrossRef]
- Glaviano, A.; Foo, A.S.C.; Lam, H.Y.; Yap, K.C.H.; Jacot, W.; Jones, R.H.; Eng, H.; Nair, M.G.; Makvandi, P.; Geoerger, B.; et al. PI3K/AKT/mTOR signaling transduction pathway and targeted therapies in cancer. Mol. Cancer 2023, 22, 138. [Google Scholar] [CrossRef] [PubMed]
- Aguayo, F.; Perez-Dominguez, F.; Osorio, J.C.; Oliva, C.; Calaf, G.M. PI3K/AKT/mTOR signaling pathway in HPV-driven head and neck carcinogenesis: Therapeutic implications. Biology 2024, 12, 672. [Google Scholar] [CrossRef] [PubMed]
- Wiese, W.; Barczuk, J.; Racinska, O.; Siwecka, N.; Rozpedek-Kaminska, W.; Slupianek, A.; Sierpinski, R.; Majsterek, I. PI3K/Akt/mTOR signaling pathway in blood malignancies-new therapeutic possibilities. Cancers 2023, 15, 5297. [Google Scholar] [CrossRef] [PubMed]
- Leiphrakpam, P.D.; Are, C. PI3K/Akt/mTOR signaling pathway as a target for colorectal cancer treatment. Int. J. Mol. Sci. 2024, 25, 3178. [Google Scholar] [CrossRef] [PubMed]
- Yan, H.; Fu, Z.H.; Lin, P.X.; Gu, Y.; Cao, J.; Li, Y. Inhibition of human glioblastoma multiforme cells by 10,11-dehydrocurvularin through the MMP−2 and PI3K/AKT signaling pathways. Eur. J. Pharmacol. 2022, 936, 175348. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Tao, L.S.; Bao, L. Fucoxanthin inhibits cell proliferation and stimulates apoptosis through downregulation of PI3K/AKT/mTOR signaling pathway in human ovarian cancer cells. Pharmacogn. Mag. 2022, 16, 311. [Google Scholar]
- Fang, X.H.; Zhu, Y.; Zhang, T.M.; Li, Q.; Fan, L.H.; Li, X.D.; Jiang, D.S.; Lin, J.; Zou, L.Y.; Ren, J.W. Fucoxanthin inactivates the PI3K/AKT signaling pathway to mediate malignant biological behaviors of non-small cell lung cancer. Nutr. Cancer 2022, 74, 3747–3760. [Google Scholar] [CrossRef]
- Tewari, D.; Patni, P.; Bishayee, A.; Sah, A.N.; Bishayee, A. Natural products targeting the PI3K−Akt−mTOR signaling pathway in cancer: A novel therapeutic strategy. Semin. Cancer Biol. 2022, 80, 1–17. [Google Scholar] [CrossRef]
- Gao, Y.; Guan, Z.F.; Chen, J.Q.; Xie, H.J.; Yang, Z.; Fan, J.H.; Wang, X.Y.; Li, L. CXCL5/CXCR2 axis promotes bladder cancer cell migration and invasion by activating PI3K/AKT-induced upregulation of MMP2/MMP9. Int. J. Oncol. 2015, 47, 690–700. [Google Scholar] [CrossRef]
- Takeuchi, T.; Nomura, Y.; Tamita, T.; Nishikawa, R.; Kakinuma, H.; Kojima, N.; Hitaka, K.; Tamura, Y.; Kamitani, M.; Mima, M.; et al. Discovery of TP0597850: A selective, chemically stable, and slow tight-binding matrix metalloproteinase−2 inhibitor with a phenylbenzamide-pentapeptide hybrid scaffold. J. Med. Chem. 2023, 66, 822–836. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Du, H.-F.; Jiang, J.-M.; Wu, S.-H.; Shi, Y.-F.; Liu, H.-T.; Hua, Z.-H.; Wang, C.-S.; Qian, G.-Y.; Ding, H.-M. Fucoxanthin Inhibits the Proliferation and Metastasis of Human Pharyngeal Squamous Cell Carcinoma by Regulating the PI3K/Akt/mTOR Signaling Pathway. Molecules 2024, 29, 3603. https://doi.org/10.3390/molecules29153603
Du H-F, Jiang J-M, Wu S-H, Shi Y-F, Liu H-T, Hua Z-H, Wang C-S, Qian G-Y, Ding H-M. Fucoxanthin Inhibits the Proliferation and Metastasis of Human Pharyngeal Squamous Cell Carcinoma by Regulating the PI3K/Akt/mTOR Signaling Pathway. Molecules. 2024; 29(15):3603. https://doi.org/10.3390/molecules29153603
Chicago/Turabian StyleDu, Hao-Fei, Jia-Min Jiang, Si-Han Wu, Yan-Fang Shi, Hai-Tian Liu, Zheng-Hao Hua, Cai-Sheng Wang, Guo-Ying Qian, and Hao-Miao Ding. 2024. "Fucoxanthin Inhibits the Proliferation and Metastasis of Human Pharyngeal Squamous Cell Carcinoma by Regulating the PI3K/Akt/mTOR Signaling Pathway" Molecules 29, no. 15: 3603. https://doi.org/10.3390/molecules29153603
APA StyleDu, H.-F., Jiang, J.-M., Wu, S.-H., Shi, Y.-F., Liu, H.-T., Hua, Z.-H., Wang, C.-S., Qian, G.-Y., & Ding, H.-M. (2024). Fucoxanthin Inhibits the Proliferation and Metastasis of Human Pharyngeal Squamous Cell Carcinoma by Regulating the PI3K/Akt/mTOR Signaling Pathway. Molecules, 29(15), 3603. https://doi.org/10.3390/molecules29153603






