Identification of Biomarkers Associated with Phyllosticta citricarpa Tolerance
Abstract
1. Introduction
2. Results and Discussion
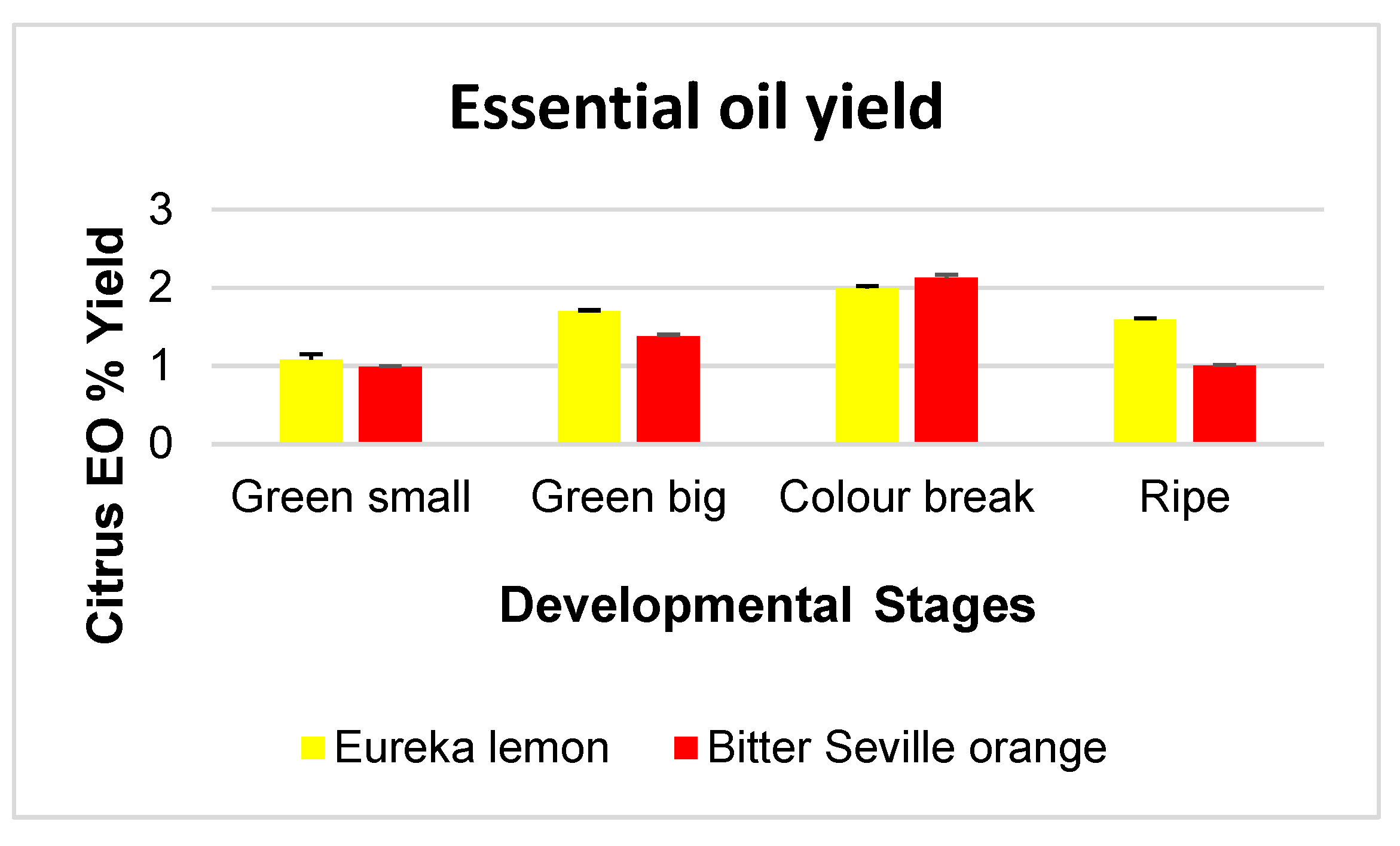
2.1. Essential Oil Yield at Different Developmental Stages
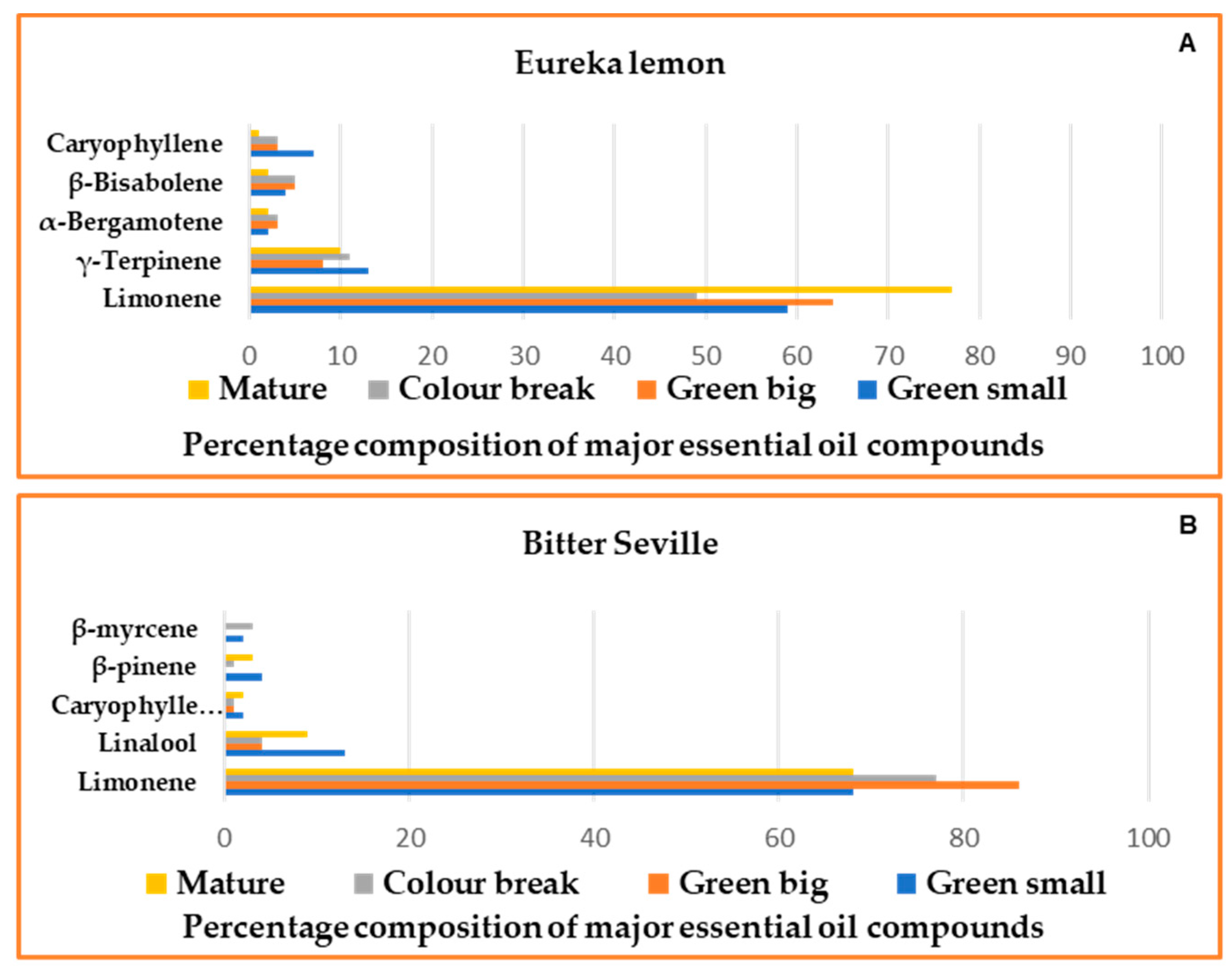
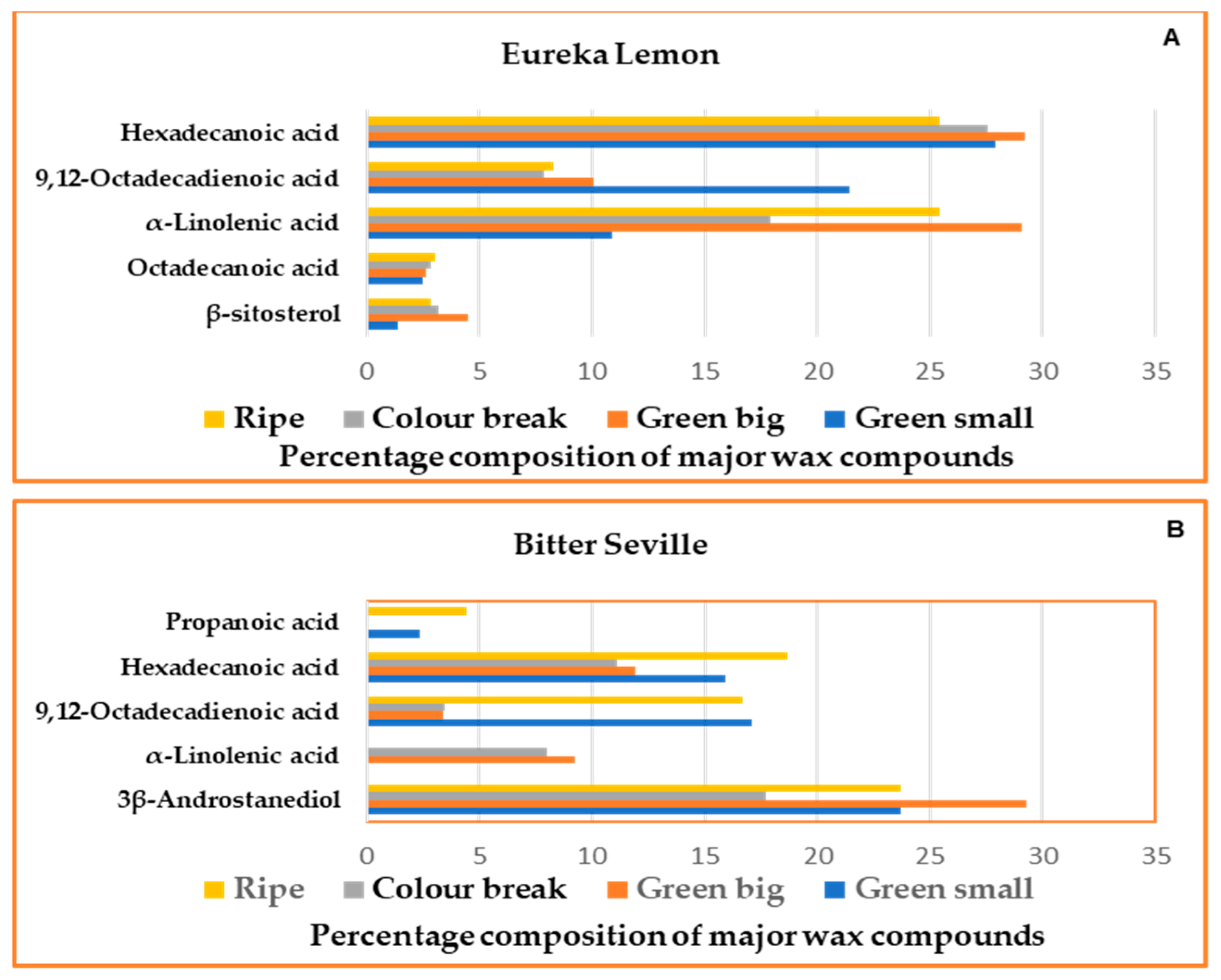
2.2. Gas Chromatography–Mass Spectrometry
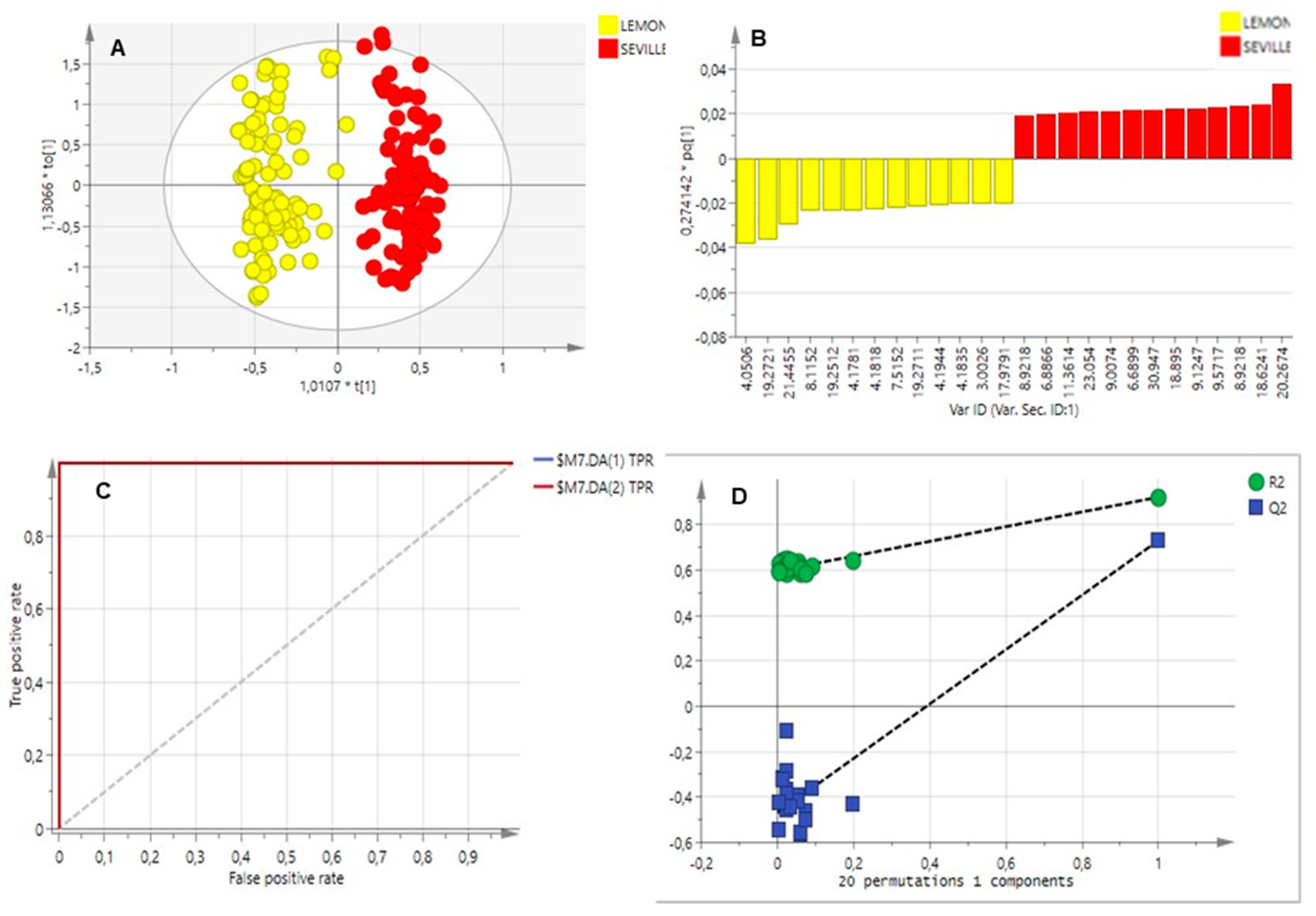
2.3. Multivariate Data Analysis
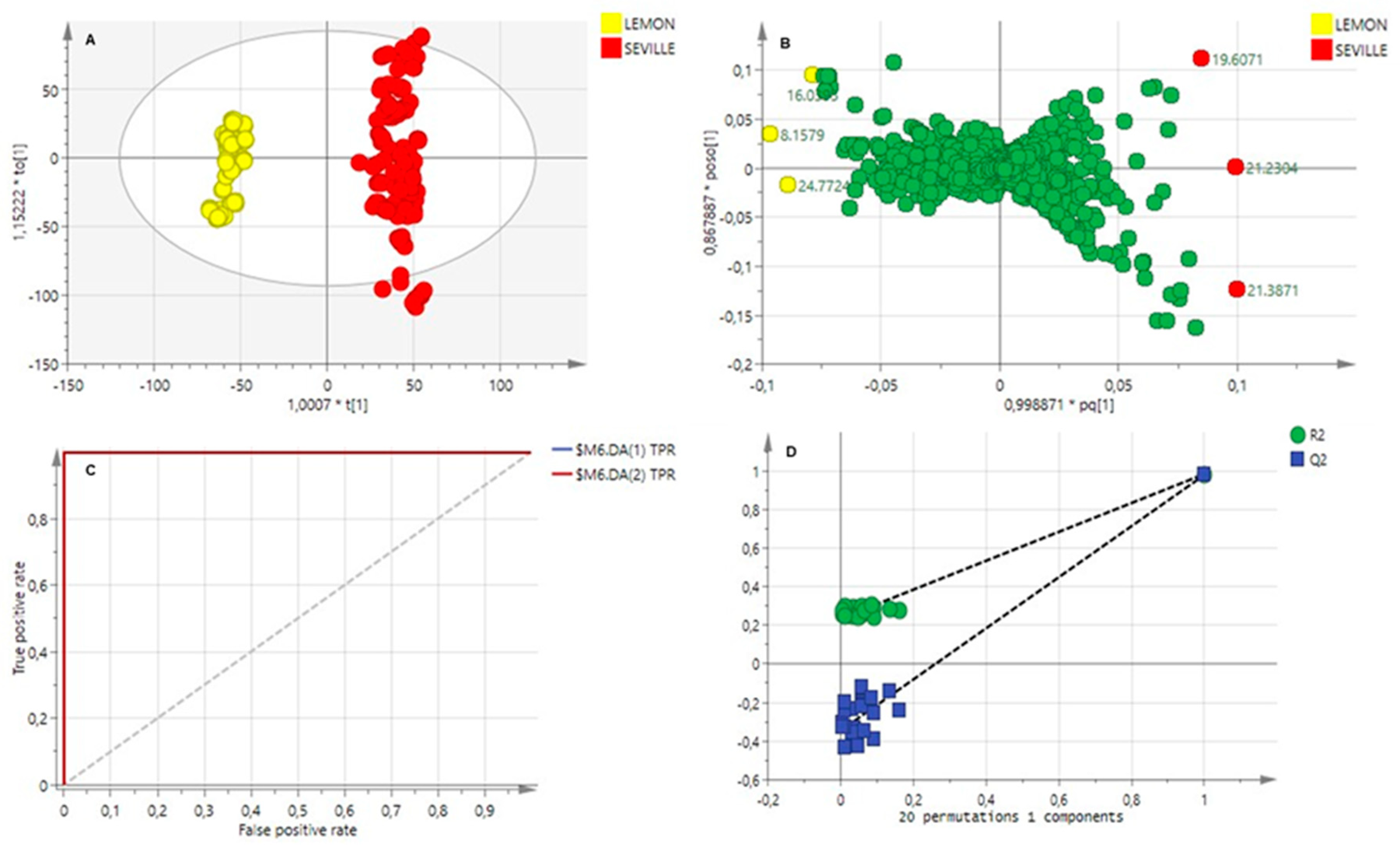
2.3.1. Essential Oils Analysis
2.3.2. Cuticular Wax Analysis
2.4. In Vitro Investigation of the Biochemical Activity of Secondary Metabolites against the Pathogen
3. Materials and Methods
3.1. Sampling
3.2. Sample Extraction
3.2.1. Hydro-Distillation of Essential Oils
3.2.2. Cuticular Wax Extraction
3.3. Gas Chromatography–Mass Spectrometry Analysis
3.4. Multivariate Data Analysis
3.5. Antifungal Properties
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Rehman, A.; Deyuan, Z.; Hussain, I.; Iqbal, M.S.; Yang, Y.; Jingdong, L. Prediction of Major Agricultural Fruits Production in Pakistan by Using an Econometric Analysis and Machine Learning Technique. Int. J. Fruit Sci. 2018, 18, 445–461. [Google Scholar] [CrossRef]
- Matheyambath, A.C.; Padmanabhan, P.; Paliyath, G. Citrus Fruits. In Encyclopedia of Food and Health; Caballero, B., Finglas, P.M., Toldrá, F., Eds.; Academic Press: Oxford, UK, 2016; pp. 136–140. [Google Scholar]
- Dlikilili, X.; Van Rooyen, J. Measuring the Competitive Trends of the South African Citrus Industry. Southern Africa-Towards Inclusing Growth; SA-TIED Working Paper 11; International Food Policy Research Institute: Washington, DC, USA, 2018. [Google Scholar]
- Mattos, D.; Kadyampakeni, D.M.; Oliver, A.Q.; Boaretto, R.M.; Morgan, K.T.; Quaggio, J.A. Chapter 15-Soil and nutrition interactions. In The Genus Citrus; Talon, M., Caruso, M., Gmitter, F.G., Eds.; Woodhead Publishing: Sawston, UK, 2020; pp. 311–331. [Google Scholar]
- Sikuka, W. South Africa-Republic of Citrus Annual Strong Growth of South African Citrus Production Continues. Global Agricultural Information Network; 2019. Available online: https://apps.fas.usda.gov/newgainapi/api/report/downloadreportbyfilename?filename=Citrus%20Annual_Pretoria_South%20Africa%20-%20Republic%20of_2-5-2019.pdf (accessed on 24 July 2024).
- Basfar, A.A.; Mohamed, K.A.; Al-Saqer, O.A. De-contamination of pesticide residues in food by ionizing radiation. Radiat. Phys. Chem. 2012, 81, 473–478. [Google Scholar] [CrossRef]
- Palou, L.; Valencia-Chamorro, S.A.; PÉREZ-GAGO, M.B. Antifungal edible coatings for fresh citrus fruit: A review. Coatings 2015, 5, 962–986. [Google Scholar] [CrossRef]
- Truter, M. Epidemiology of Citrus Black Spot Disease in South Africa and Its Impact on Phytosanitary Trade Restrictions); University of Pretoria: Pretoria, South Africa, 2010. [Google Scholar]
- Paul, I.; van Jaarsveld, A.S.; Korsten, L.; Hattingh, V. The potential global geographical distribution of Citrus Black Spot caused by Guignardia citricarpa (Kiely): Likelihood of disease establishment in the European Union. Crop Prot. 2005, 24, 297–308. [Google Scholar] [CrossRef]
- Hendricks, K.E.; Christman, M.C.; Roberts, P.D. A statistical evaluation of methods of in-vitro growth assessment for Phyllosticta citricarpa: Average colony diameter vs. area. PLoS ONE 2017, 12, e0170755. [Google Scholar] [CrossRef]
- Sposito, M.B.; Amorim, L.; Bassanezi, R.B.; Filho, A.B.; Hau, B. Relative importance of inoculum sources of Guignardia citricarpa on the citrus black spot epidemic in Brazil. Crop Prot. 2011, 30, 1546–1552. [Google Scholar] [CrossRef]
- Fonseca, A.E.; Goes, A.D.; Pereira, F.D. Copper oxychloride applied alone to control citrus black spot. Rev. Caatinga 2019, 32, 616–624. [Google Scholar] [CrossRef]
- Vitanovic, E. Use of Cu fungicides in Vineyards and Olive Groves; INTECH Open Access Publisher: London, UK, 2012. [Google Scholar]
- Al-Jumaili, A.; Kumar, A.; Bazaka, K.; Jacob, M.V. Plant Secondary Metabolite-Derived Polymers: A Potential Approach to Develop Antimicrobial Films. Polymers 2018, 10, 515. [Google Scholar] [CrossRef] [PubMed]
- Pang, Z.; Chen, J.; Wang, T.; Gao, C.; Li, Z.; Guo, L.; Xu, J.; Cheng, Y. Linking Plant Secondary Metabolites and Plant Microbiomes: A Review. Front. Plant Sci. 2021, 12, 621276. [Google Scholar] [CrossRef]
- Jeppesen, M.J.; Powers, R. Multiplatform untargeted metabolomics. Magn. Reson. Chem. 2023, 61, 628–653. [Google Scholar] [CrossRef]
- Chandran, H.; Meena, M.; Sharma, K. Microbial Biodiversity and Bioremediation Assessment Through Omics Approaches. Front. Environ. Chem. 2020, 1, 570326. [Google Scholar] [CrossRef]
- Tugizimana, F.; Piater, L.; Dubery, I. Plant metabolomics: A new frontier in phytochemical analysis. South Afr. J. Sci. 2013, 109, 1–11. [Google Scholar] [CrossRef]
- Cheng, J.; Chen, C.; Kristopher, K.W.; Manna, S.K.; Scerba, M.; Friedman, F.K.; Luecke, H.; Idle, J.R.; Gonzalez, F.J. Identification of 2-Piperidone as a Biomarker of CYP2E1 Activity Through Metabolomic Phenotyping. Toxicol. Sci. 2013, 135, 37–47. [Google Scholar] [CrossRef] [PubMed]
- Yun, Z.; Gao, H.; Liu, P.; Liu, S.; Luo, T.; Jin, S.; Xu, Q.; Xu, J.; Cheng, Y.; Deng, X. Comparative proteomic and metabolomic profiling of citrus fruit with enhancement of disease resistance by postharvest heat treatment. BMC Plant Biol. 2013, 13, 44. [Google Scholar] [CrossRef] [PubMed]
- Esfahani, R.E.; Moradi, P. The effect of different development stages on the quantity and quality of the essential oil of Citrus aurantifolia (Christm.) Swingle in Iran. Herba Pol. 2017, 64, 32–42. [Google Scholar] [CrossRef]
- Wu, Z.; Li, H.; Yang, Y.; Zhan, Y.; Tu, D. Variation in the components and antioxidant activity of Citrus medica L. var. sarcodactylis essential oils at different stages of maturity. Ind. Crops Prod. 2013, 46, 311–316. [Google Scholar] [CrossRef]
- Moghaddam, M.; Mehdizadeh, L. Chapter 13—Chemistry of Essential Oils and Factors Influencing Their Constituents. In Soft Chemistry and Food Fermentation; Grumezescu, A.M., Holban, A.M., Eds.; Academic Press: Cambridge, MA, USA, 2017; pp. 379–419. [Google Scholar]
- Korf, H.; Schutte, G.; Kotzé, J.J.A.P.P. Effect of packhouse procedures on the viability of Phyllosticta citricarpa, anamorph of the citrus black spot pathogen. Afr. Plant Prot. 2001, 7, 103–109. [Google Scholar]
- Guarnaccia, V.; Gehrmann, T.; Silva-Junior, G.J.; Fourie, P.H.; Haridas, S.; Vu, D.; Spatafora, J.; Martin, F.M.; Robert, V.; Grigoriev, I.V.; et al. Phyllosticta citricarpa and sister species of global importance to Citrus. Mol. Plant Pathol. 2019, 20, 1619–1635. [Google Scholar] [CrossRef] [PubMed]
- Anjali; Kumar, S.; Korra, T.; Thakur, R.; Arutselvan, R.; Kashyap, A.S.; Nehela, Y.; Chaplygin, V.; Minkina, T.; Keswani, C. Role of plant secondary metabolites in defence and transcriptional regulation in response to biotic stress. Plant Stress 2023, 8, 100154. [Google Scholar] [CrossRef]
- Vekiari, S.A.; Protopapadakis, E.E.; Papadopoulou, P.; Papanicolaou, D.; Panou, C.; Vamvakias, M. Composition and Seasonal Variation of the Essential Oil from Leaves and Peel of a Cretan Lemon Variety. J. Agric. Food Chem. 2002, 50, 147–153. [Google Scholar] [CrossRef]
- Lavergne, F.D.; Broeckling, C.D.; Cockrell, D.M.; Haley, S.D.; Peairs, F.B.; Jahn, C.E.; Heuberger, A.L. GC-MS Metabolomics to Evaluate the Composition of Plant Cuticular Waxes for Four Triticum aestivum Cultivars. Int. J. Mol. Sci. 2018, 19, 249. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Hao, H.; Liu, R.; Ma, Q.; Xu, J.; Chen, F.; Cheng, Y.; Deng, X. Comparative analysis of surface wax in mature fruits between Satsuma mandarin (Citrus unshiu) and ‘Newhall’ navel orange (Citrus sinensis) from the perspective of crystal morphology, chemical composition and key gene expression. Food Chem. 2014, 153, 177–185. [Google Scholar] [CrossRef] [PubMed]
- Augustyn, W.A.; Regnier, T.; Combrinck, S.; Botha, B.M. Metabolic profiling of mango cultivars to identify biomarkers for resistance against Fusarium infection. Phytochem. Lett. 2014, 10, civ. [Google Scholar] [CrossRef]
- Wishart, D.S. Metabolomics: Applications to food science and nutrition research. Trends Food Sci. Technol. 2008, 19, 482–493. [Google Scholar] [CrossRef]
- Iacomi-Vasilescu, B.; Avenot, H.; Bataille-Simoneau, N.; Laurent, E.; Guénard, M.; Simoneau, P.J.C.P. In vitro fungicide sensitivity of Alternaria species pathogenic to crucifers and identification of Alternaria brassicicola field isolates highly resistant to both dicarboximides and phenylpyrroles. Crop Prot. 2004, 23, 481–488. [Google Scholar] [CrossRef]
- Mohammedi, Z.; Atik, F. Fungitoxic effect of natural extracts on mycelial growth, spore germination and aflatoxin B1 production of Aspergillus flavus. Aust. J. Crop Sci. 2013, 7, 293–298. [Google Scholar]
- Marques, J.P.; Amorim, L.; Silva-Junior, G.J.; Sposito, M.B.; Appezzato-da Gloria, B. Structural and biochemical characteristics of citrus flowers associated with defence against a fungal pathogen. AoB PLANTS 2014, 7, plu090. [Google Scholar] [CrossRef] [PubMed]
- Yamasaki, Y.; Kunoh, H.; Yamamoto, H.; Akimitsu, K. Biological roles of monoterpene volatiles derived from rough lemon (Citrus jambhiri Lush) in citrus defense. J. Gen. Plant Pathol. 2007, 73, 168–179. [Google Scholar] [CrossRef]
- Ullah, A.; Orij, R.; Brul, S.; Smits Gertien, J. Quantitative Analysis of the Modes of Growth Inhibition by Weak Organic Acids in Saccharomyces cerevisiae. Appl. Environ. Microbiol. 2012, 78, 8377–8387. [Google Scholar] [CrossRef]
- Brock, M.; Buckel, W. On the mechanism of action of the antifungal agent propionate. Eur. J. Biochem. 2004, 271, 3227–3241. [Google Scholar] [CrossRef]
- Semchyshyn, H.M.; Abrat, O.B.; Miedzobrodzki, J.; Inoue, Y.; Lushchak, V.I. Acetate but not propionate induces oxidative stress in bakers’ yeast Saccharomyces cerevisiae. Redox Rep. 2011, 16, 15–23. [Google Scholar] [CrossRef] [PubMed]
- Yun, J.; Lee, D.G. A novel fungal killing mechanism of propionic acid. FEMS Yeast Res. 2016, 16, fow089. [Google Scholar] [CrossRef] [PubMed]
- Micalizzi, E.W.; Golshani, A.; Smith, M.L. Propionic acid disrupts endocytosis, cell cycle, and cellular respiration in yeast. BMC Res. Notes 2021, 14, 335. [Google Scholar] [CrossRef] [PubMed]
- Avis, T.J.; Bélanger, R.R. Specificity and Mode of Action of the Antifungal Fatty Acid cis-9-Heptadecenoic Acid Produced by Pseudozyma flocculosa. Appl. Environ. Microbiol. 2001, 67, 956–960. [Google Scholar] [CrossRef] [PubMed]
- Membré, J.-M.; Kubaczka, M.; Chèné, C. Growth rate and growth–no-growth interface of Penicillium brevicompactum as functions of pH and preservative acids. Food Microbiol. 2001, 18, 531–538. [Google Scholar] [CrossRef]
- Desbois, A.P.; Smith, V.J. Antibacterial free fatty acids: Activities, mechanisms of action and biotechnological potential. Appl. Microbiol. Biotechnol. 2010, 85, 1629–1642. [Google Scholar] [CrossRef] [PubMed]
- Sun, C.Q.; O’Connor, C.J.; Roberton, A.M. Antibacterial actions of fatty acids and monoglycerides against Helicobacter pylori. FEMS Immunol. Med. Microbiol. 2003, 36, 9–17. [Google Scholar] [CrossRef] [PubMed]
- Asikin, Y.; Maeda, G.; Tamaki, H.; Mizu, M.; Oku, H.; Wada, K. Cultivation line and fruit ripening discriminations of Shiikuwasha (Citrus depressa Hayata) peel oils using aroma compositional, electronic nose, and antioxidant analyses. Food Res. Int. 2015, 67, 102–110. [Google Scholar] [CrossRef]
- Li, Y.; Yin, Y.; Chen, S.; Bi, Y.; Ge, Y. Chemical composition of cuticular waxes during fruit development of Pingguoli pear and their potential role on early events of Alternaria alternata infection. Funct. Plant Biol. 2013, 41, 313–320. [Google Scholar] [CrossRef]
- Kumirska, J.; Plenis, A.; Łukaszewicz, P.; Caban, M.; Migowska, N.; Białk-Bielińska, A.; Czerwicka, M.; Stepnowski, P. Chemometric optimization of derivatization reactions prior to gas chromatography–mass spectrometry analysis. J. Chromatogr. A 2013, 1296, 164–178. [Google Scholar] [CrossRef]
- Cook, D.W.; Rutan, S.C. Chemometrics for the analysis of chromatographic data in metabolomics investigations. J. Chemom. 2014, 28, 681–687. [Google Scholar] [CrossRef]
- Peres, N.A.; Harakava, R.; Carroll, G.C.; Adaskaveg, J.E.; Timmer, L.W. Comparison of Molecular Procedures for Detection and Identification of Guignardia citricarpa and G. mangiferae. Plant Dis. 2007, 91, 525–531. [Google Scholar] [CrossRef] [PubMed]
- Regnier, T.; Combrinck, S. In vitro and in vivo screening of essential oils for the control of wet bubble disease of Agaricus bisporus. South Afr. J. Bot. 2010, 76, 681–685. [Google Scholar] [CrossRef]
| RT | Compound Name | % Relative Content | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Eureka Lemon | Bitter Seville | ||||||||
| Green Small | Green Big | Colour Break | Mature | Green Small | Green Big | Colour Break | Mature | ||
| 2.93 | α-pinene | 0.80 ± 0.05 | 0.64 ± 0.08 | 0.53 ± 0.4 | 1.3 ± 0.05 | 1.2 ± 0.7 | 0.28 ± 0.01 | 0.67 ± 0.2 | 0.49 ± 0.1 |
| 4.26 | β-pinene | 0.82 ± 0.3 | 0.84 ± 0.08 | 5.2 ± 0.8 | 2.6 ± 0.7 | 3.8 ± 2 | 0.25 ± 0.07 | 1.1 ± 0.6 | 3.3 ± 0.4 |
| 5.32 | β-myrcene | n.d | 0.35 ± 0.04 | 0.29 ± 0.04 | 0.60 ± 0.1 | 2.2 ± 1 | 0.38 ± 0.3 | 2.69 ± 2 | n.d |
| 7.60 | Limonene | 59 ± 0.6 | 64 ± 1.2 | 49 ± 0.7 | 77 ± 2 | 68 ± 0.2 | 86 ± 2 | 77 ± 2 | 68 ± 0.01 |
| 7.91 | Ocimene | n.d | 0.030 ± 0.01 | 0.030 ± 0.01 | n.d | 3.3 ± 2 | n.d | n.d | 0.15 ± 2 |
| 8.12 | γ-Terpinene | 13 ± 0.04 | 7.8 ± 0.3 | 11 ± 0.3 | 9.9 ± 2 | n.d | n.d | n.d | n.d |
| 8.92 | Linalool oxide | n.d | n.d | n.d | n.d | 1.9 ± 0.6 | 0.67 ± 0.4 | 0.070 ± 2 | 0.45 ± 2 |
| 9.08 | cis-Linaloloxide | n.d | n.d | n.d | n.d | 0.85 ± 0.2 | 0.36 ± 0.4 | n.d | n.d |
| 9.57 | (+)-4-Carene | 0.62 ± 1 | 0.18 ± 0.02 | 0.48 ± 0.08 | 0.14 ± 1 | n.d | 0.090 ± 0.1 | 0.13 ± 0.05 | n.d |
| 10.38 | Linalool | 4.0 ± 0.03 | 0.25 ± 0.04 | 0.20 ± 0.07 | 0.81 ± 0.01 | 13 ± 0.2 | 3.8 ± 0.6 | 4.4 ± 3 | 8.5 ± 2 |
| 12.64 | Terpinen-4-ol | 0.68 ± 0.2 | 1.8 ± 0.1 | 0.98 ± 0.1 | 0.38 ± 0.01 | 0.14 ± 0.2 | 0.65 ± 0.08 | 0.47 ± 0.2 | 0.64 ± 0.02 |
| 13.38 | α-Terpineol | 1.8 ± 0.10 | 4.4 ± 0.4 | 1.3 ± 0.1 | 0.29 ± 0.01 | 0.25 ± 0.01 | 0.96 ± 0.5 | 0.85 ± 0.4 | 1.1 ± 0.01 |
| 14.89 | Linalyl acetate | n.d | n.d | n.d | n.d | 0.040 ± 0.04 | n.d | 0.67 ± 0.4 | 1.0 ± 1 |
| 14.65 | β-Citral | 0.45 ± 0.6 | 0.010 ± 0.01 | 0.030 ± 0.01 | n.d | n.d | n.d | n.d | n.d |
| 15.70 | α-Citral | 0.25 ± 0.1 | 0.030 ± 0.01 | 0.010 ± 0.05 | n.d | n.d | n.d | n.d | n.d |
| 15.98 | cis Farnesol | 0.090 ± 0.1 | 0.090 ± 0.8 | 0.090 ± 0.8 | 0.040 ± 0.01 | n.d | n.d | n.d | n.d |
| 16.01 | Camphene | n.d | n.d | n.d | n.d | 0.020 ± 0.03 | n.d | n.d | n.d |
| 16.29 | γ-Elemene | 0.54 ± 0.04 | 0.16 ± 0.05 | 0.59 ± 0.2 | 0.29 ± 0.2 | 0.14 ± 0.06 | n.d | 0.12 ± 0.01 | n.d |
| 16.50 | δ-Elemene | n.d | n.d | n.d | n.d | n.d | 0.11 ± 0.05 | 0.44 ± 0.02 | 0.49 ± 0.4 |
| 18.00 | β-Elemene | n.d | n.d | n.d | n.d | 0.14 ± 0.06 | n.d | 0.28 ± 0.2 | 0.30 ± 0.8 |
| 18.03 | Farnesol acetate | 0.13 ± 0.06 | 0.89 ± 0.1 | 0.27 ± 0.1 | 0.090 ± 0.02 | n.d | n.d | n.d | n.d |
| 18.62 | Caryophyllene | 6.9 ± 0.04 | 2.8 ± 0.1 | 2.6 ± 0.4 | 1.1 ± 0.04 | 1.9 ± 0.7 | 0.35 ± 0.04 | 0.93 ± 0.06 | 1.5 ± 0.60 |
| 18.83 | Germacrene D | n.d | n.d | n.d | n.d | 1.6 ± 1 | n.d | 0.82 ± 0.5 | n.d |
| 19.27 | α-Bergamotene | 2.4 ± 1 | 2.7 ± 0.2 | 2.9 ± 0.3 | 1.5 ± 0.1 | n.d | n.d | n.d | n.d |
| 19.42 | α-Caryophyllene | 0.67 ± 0.03 | 0.35 ± 0.05 | 0.29 ± 0.07 | 0.16 ± 0.06 | 0.34 ± 0.2 | 0.080 ± 0.01 | n.d | 0.020 ± 0.5 |
| 19.82 | β-Santalene | 0.14 ± 0.08 | 0.14 ± 0.01 | 0.14 ± 0.03 | 0.070 ± 0.01 | n.d | n.d | n.d | n.d |
| 20.12 | β-Farnesene | 0.17 ± 0.01 | 0.22 ± 0.08 | 0.18 ± 0.7 | 0.060 ± 0.2 | n.d | n.d | n.d | n.d |
| 20.27 | β-Cubebene | n.d | n.d | n.d | n.d | 0.61 ± 0.07 | 0.15 ± 0.01 | n.d. | 0.88 ± 0.1 |
| 20.60 | γ-Muurolene | 0.02 ± 0.05 | 0.080 ± 0.66 | 0.060 ± 0.01 | n.d | 0.23 ± 0.1 | n.d | 0.12 ± 0.6 | 0.22 ± 0.1 |
| 21.45 | β-Bisabolene | 4.0 ± 0.20 | 4.9 ± 1.13 | 4.9 ± 0.3 | 2.3 ± 0.01 | 0.11 ± 0.2 | n.d | 0.18 ± 0.08 | 0.070 ± 0.2 |
| 21.48 | α-Cadinene | n.d | n.d | n.d | n.d | 0.030 ± 0.01 | 0.070 ± 0.04 | n.d | 0.21 ± 0.04 |
| 21.77 | β-Sesquiphellandrene | n.d | n.d | n.d | n.d | 0c.10 ± 0.04 | n.d | 0.11 ± 0.2 | 0.09 ± 0.3 |
| 22.85 | Caryophyllene oxide | 0.060 ± 0.01 | 0.16 ± 0.06 | 0.080 ± 0.01 | n.d | n.d | n.d | n.d | n.d |
| 22.97 | Spathulenol | 0.060 ± 0.01 | 0.29 ± 0.05 | 0.25 ± 0.09 | 0.070 ± 0.06 | n.d | n.d | n.d | n.d |
| 24.77 | Azulene | n.d | n.d | n.d | n.d | n.d | n.d | n.d | n.d |
| 25.84 | cis-α-Bisabolene | 0.21 ± 0.08 | 0.15 ± 0.61 | 0.28±0.08 | 0.060 ± 0.02 | n.d | n.d | n.d | n.d |
| RT | Chemical Formula | Compound Name | %Relative Area | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Eureka Lemon | Bitter Seville | |||||||||
| Green Young | Green Big | Colour Break | Ripe | Green Young | Green Big | Colour Break | Ripe | |||
| 3.57 | C10H18O | Geraniol | 0.72 ± 0.2 | n.d | 1.4 ± 1 | n.d | n.d | n.d | n.d | n.d |
| 5.04 | C10H16O2 | 2-Butenoic acid, 3-hexenyl ester | 2.3 ± 2 | n.d | n.d | n.d | n.d | n.d | 0.050 ± 0.3 | 0.050 ± 0.1 |
| 10.84 | C7H6CO2 | Benzoic acid | n.d | 1.5 ± 1 | n.d | 3.1 ± 1 | n.d | n.d | 0.77 ± 1 | 0.65 ± 0.8 |
| 12.45 | C14H28O2 | Tetradecanoic acid | 0.23 ± 0.3 | 0.25 ± 0.2 | 0.82 ± 1 | 0.25 ± 0.9 | 0.17 ± 0.1 | 0.22 ± 0.2 | 0.070 ± 0.9 | 0.24 ± 0.2 |
| 12.97 | C8H8O2 | Benzeneacetic acid | n.d | 1.1 ± 0.6 | n.d | 1.5 ± 0.5 | n.d | n.d | n.d | n.d |
| 13.26 | C20H42 | Eicosane | n.d | n.d | 0.31 ± 0.3 | n.d | n.d | n.d | n.d | n.d |
| 14.22 | C15H30O2 | n-Pentadecanoic acid | 1.2 ± 0.06 | 1.9 ± 1 | 2.2 ± 0.2 | n.d | n.d | n.d | n.d | n.d |
| 15.96 | C16H30O2 | Hexadecanoic acid | 28 ± 0.02 | 29 ± 1 | 29 ± 1 | 25 ± 2 | 16 ± 0.2 | 12 ± 0.3 | 11 ± 1 | 19 ± 1 |
| 16.82 | C9H8O2 | Cinnamic acid | 0.60 ± 0.2 | 2.5 ± 0.7 | n.d | 4.1 ± 0.8 | n.d | n.d | 8.6 ± 1 | 5.0 ± 2 |
| 17.60 | C17H34O2 | Heptadecanoic acid | n.d | 1.2 ± 1 | n.d | 1.4 ± 1 | 0.36 ± 0.3 | 0.31 ± 0.1 | n.d | 1.3 ± 0.3 |
| 17.61 | C10H20O2 | Decanoic acid | 0.53 ± 0.5 | n.d | n.d | n.d | n.d | 0.61 ± 0.2 | n.d | n.d |
| 18.59 | C18H32O2 | 9,12-Octadecadienoic acid (Z,Z) | 21 ± 2 | 10 ± 0.2 | 7.9 ± 0.8 | 8.3 ± 1 | 17 ± 1 | 3.4 ± 0.9 | 3.4 ± 2 | 17 ± 1 |
| 18.77 | C18H30O2 | α-Linolenic acid | 12 ± 1 | 29 ± 0.9 | 18 ± 0.7 | 25 ± 1 | n.d | 9.3 ± 1 | 8.0 ± 2 | n.d |
| 19.26 | C18H36O2 | Octadecanoic acid | 2.5 ± 1 | 2.6 ± 2 | 2.8 ± 1 | 3.1 ± 1 | 1.7 ± 1 | 0.34 ± 1 | 1.4 ± 0.9 | 2.3 ± 0.8 |
| 19.98 | C3H4O2 | Propanoic acid | n.d | 0.18 ± 0.9 | 1.1 ± 0.9 | n.d | 2.3 ± 1 | n.d | n.d | 4.5 ± 1 |
| 20.06 | C17H36 | Heptadecane | n.d | n.d | 0.44 ± 0.7 | 0.48 ± 0.8 | 0.36 ± 0.3 | 0.31 ± 0.1 | n.d | 1.3 ± 0.3 |
| 21.33 | C19H32O2 | Androstane-3, 17 diol | n.d | n.d | n.d | n.d | 25 ± 1 | 29 ± 1 | 18 ± 1 | 24 ± 0.9 |
| 21.61 | C24H50 | Tetracosane | n.d | n.d | 3.1 ± 0.1 | n.d | n.d | n.d | n.d | n.d |
| 23.17 | C16H34 | Hexadecane | n.d | 0.18 ± 1 | n.d | n.d | n.d | n.d | n.d | n.d |
| 28.70 | C29H60 | Nonacosane | n.d | 0.43 ± 0.6 | n.d | n.d | n.d | n.d | n.d | n.d |
| 29.94 | C28H58 | Octacosane | n.d | n.d | 0.89 ± 0.7 | 0.31 ± 0.9 | n.d | 0.18 ± 1 | 0.15 ± 0.8 | n.d |
| 31.15 | C18H36 | Octadecane | 0.20 ± 1 | n.d | n.d | 0.57 ± 0.8 | n.d | n.d | 0.070 ± 0.18 | 0.080 ± 0.3 |
| 32.03 | C28H48O | Campesterol | 0.25 ± 0.9 | 1.0 ± 26 | 0.80 ± 0.1 | 0.68 ± 0.2 | 0.16 ± 0.3 | 0.21 ± 0.3 | 0.25 ± 0.4 | 0.14 ± 0.2 |
| 32.37 | C29H48O | Stigmasterol | 0.89 ± 0.9 | 1.7 ± 0.3 | 6.7 ± 0.4 | 0.85 ± 0.4 | 0.47 ± 0.3 | 0.51 ± 0.8 | 0.41 ± 0.6 | 0.32 ± 0.9 |
| 33.06 | C29H50O | β-Sitosterol | 1.4 ± 1 | 4.5 ± 0.8 | 3.2 ± 1 | 2.9 ± 1 | 0.97 ± 0.3 | 1.5 ± 0.3 | 1.1 ± 0.2 | 0.76 ± 0.4 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tswaai, P.O.J.; Augustyn, W.A.; Regnier, T.; du Plooy, W. Identification of Biomarkers Associated with Phyllosticta citricarpa Tolerance. Molecules 2024, 29, 3582. https://doi.org/10.3390/molecules29153582
Tswaai POJ, Augustyn WA, Regnier T, du Plooy W. Identification of Biomarkers Associated with Phyllosticta citricarpa Tolerance. Molecules. 2024; 29(15):3582. https://doi.org/10.3390/molecules29153582
Chicago/Turabian StyleTswaai, Puseletso O. J., Wilma A. Augustyn, Thierry Regnier, and Wilma du Plooy. 2024. "Identification of Biomarkers Associated with Phyllosticta citricarpa Tolerance" Molecules 29, no. 15: 3582. https://doi.org/10.3390/molecules29153582
APA StyleTswaai, P. O. J., Augustyn, W. A., Regnier, T., & du Plooy, W. (2024). Identification of Biomarkers Associated with Phyllosticta citricarpa Tolerance. Molecules, 29(15), 3582. https://doi.org/10.3390/molecules29153582







