Green Synthesis and Antifungal Activities of Novel N-Aryl Carbamate Derivatives
Abstract
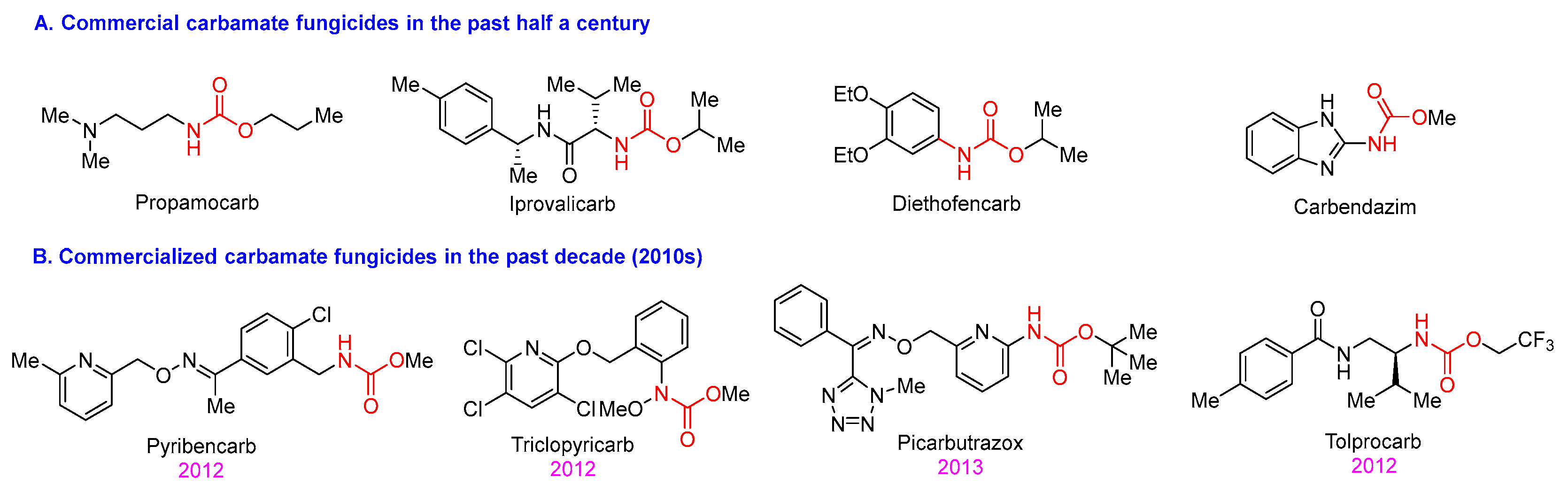
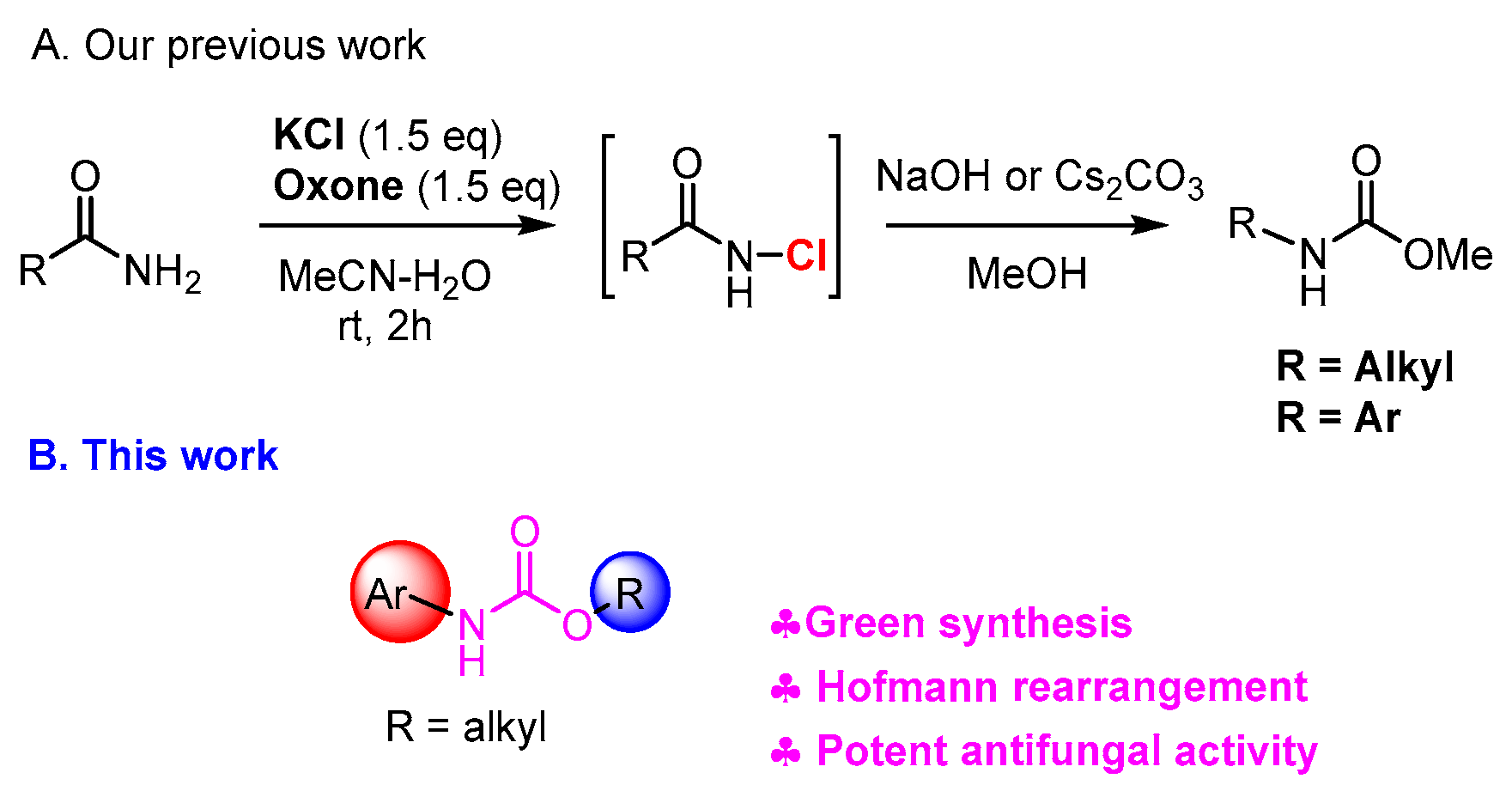
1. Introduction
2. Results
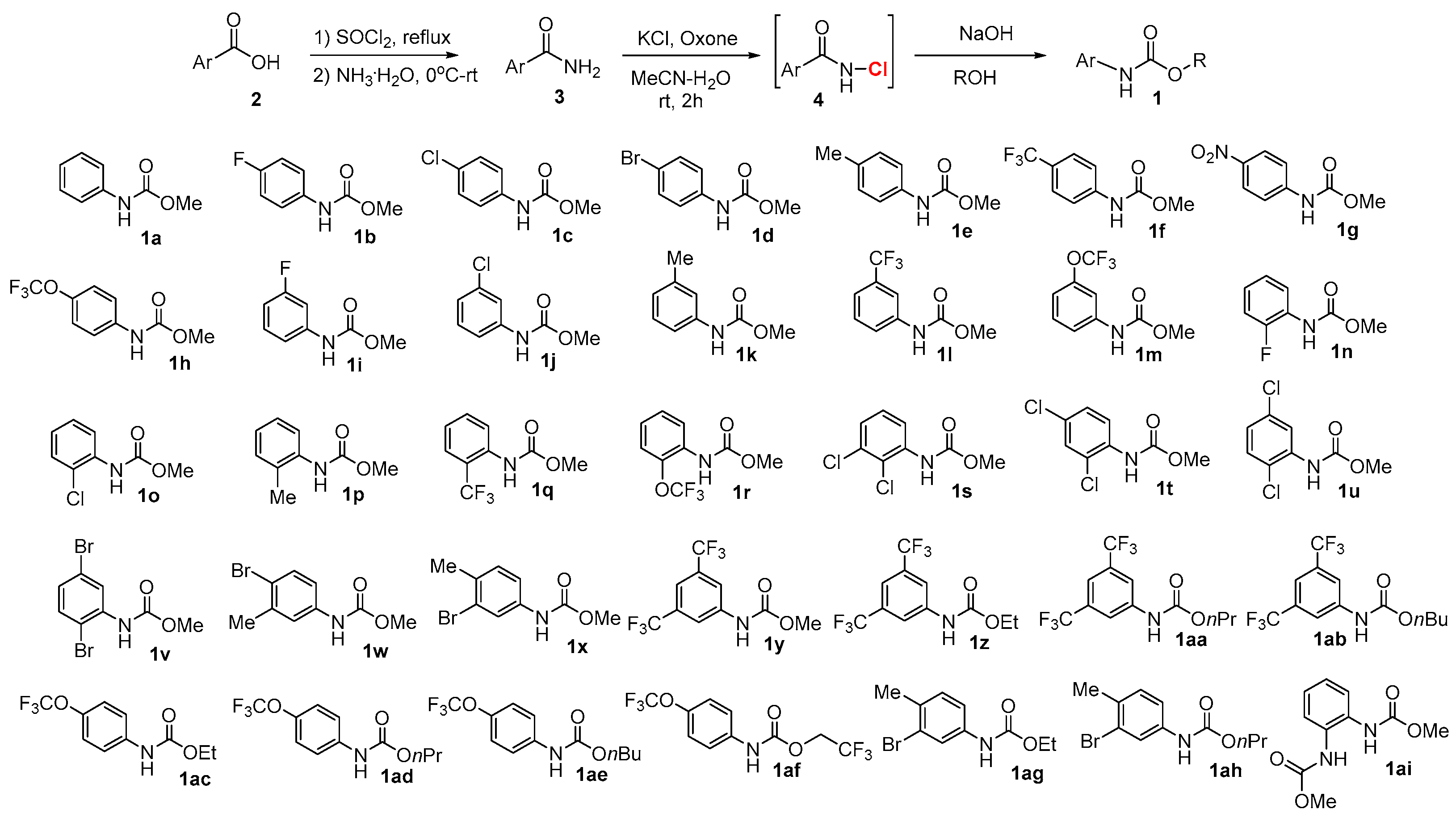
2.1. Chemistry
2.2. In Vitro Antifungal Activity
3. Discussion
4. Materials and Methods
4.1. General Information
4.2. Synthetic Procedures
4.2.1. Synthesis of Amides 3
4.2.2. Synthesis of Carbamates 1
4.3. Bioassays
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kelman, A.; Peterson, P.D. Contributions of plant scientists to the development of the germ theory of disease. Microbes Infect. 2002, 4, 257–260. [Google Scholar] [CrossRef]
- Dean, R.; Van Kan, J.A.L.; Pretorius, Z.A.; Hammond-Kosack, K.E.; Di Pietro, A.; Spanu, P.D.; Rudd, J.J.; Dickman, M.; Kahmann, R.; Ellis, J.; et al. The Top 10 fungal pathogens in molecular plant pathology. Mol. Plant Pathol. 2012, 13, 414–430. [Google Scholar] [CrossRef]
- Horsfall, J.G. Fungi and fungicides: The story of a nonconformist. Ann. Rev. Phytopathol. 1975, 13, 1–14. [Google Scholar] [CrossRef]
- Fisher, M.C.; Henk, D.A.; Briggs, C.J.; Brownstein, J.S.; Madoff, L.C.; McCraw, S.L.; Gurr, S.J. Emerging fungal threats to animal, plant and ecosystem health. Nature 2012, 484, 186–194. [Google Scholar] [CrossRef]
- Strange, R.N.; Scott, P.R. Plant Disease: A Threat to Global Food Security. Annu. Rev. Phytopathol. 2005, 43, 83–116. [Google Scholar] [CrossRef]
- Dorigan, A.F.; Moreira, S.I.; Guimarães, S.S.C.; Cruz-Magalhães, V.; Alves, E. Target and non-target site mechanisms of fungicide resistance and their implications for the management of crop pathogens. Pest Manag. Sci. 2023, 79, 4731–4753. [Google Scholar] [CrossRef]
- Umetsu, N.; Shirai, Y. Development of novel pesticides in the 21st century. J. Pestic. Sci. 2020, 45, 54–74. [Google Scholar] [CrossRef]
- Ghosh, A.K.; Brindisi, M. Organic Carbamates in Drug Design and Medicinal Chemistry. J. Med. Chem. 2015, 58, 2895–2940. [Google Scholar] [CrossRef]
- World Health Organization (WHO). Carbamate Pesticides: A General Introduction; World Health Organization (WHO): Geneva, Switzerland, 1986. [Google Scholar]
- Müller, F. Fungicides. In Agrochemicals: Composition, Production, Toxicology, Applications; Wiley-VCH: Weinheim, NY, USA, 2000; pp. 383–494. [Google Scholar]
- Takagaki, M.; Ozaki, M.; Fujimoto, S.; Fukumoto, S. Development of a novel fungicide, pyribencarb. J. Pestic. Sci. 2014, 39, 177–178. [Google Scholar] [CrossRef]
- Li, H.; Hu, S.; Sun, F.; Sun, Q.; Wang, N.; Li, B.; Zou, N.; Lin, J.; Mu, W.; Pang, X. Residual analysis of QoI fungicides in multiple (six) types of aquatic organisms by UPLC-MS/MS under acutely toxic conditions. Environ. Sci. Pollut. Res. 2023, 30, 12075–12084. [Google Scholar] [CrossRef]
- Ichinari, D.; Nagaki, A.; Yoshida, J. Generation of hazardous methyl azide and its application to synthesis of a key-intermediate of picarbutrazox, a new potent pesticide in flow. Bioorg. Med. Chem. 2017, 25, 6224–6228. [Google Scholar] [CrossRef]
- Hagiwara, H.; Ezaki, R.; Hamada, T.; Tsuda, M.; Ebihara, K. Development of a novel fungicide, tolprocarb. J. Pestic. Sci. 2019, 44, 208–213. [Google Scholar] [CrossRef]
- Wan, F.-X.; Wang, J.-H.; Shi, Y.-H.; Niu, L.-Z.; Jiang, L. Design, Synthesis and Antifungal Activity of Novel Benzoylcarbamates Bearing a Pyridine Moiety. Appl. Sci. 2018, 8, 2577. [Google Scholar] [CrossRef]
- Zhao, F.-H.; Zhang, H.; Sun, C.-X.; Li, P.-H.; Jiang, L. Synthesis and fungicidal activity of 2-(methylthio)-4-methylpyrimidine carboxamides bearing a carbamate moiety. Phosphorus Sulfur Silicon Relat. Elem. 2023, 198, 655–658. [Google Scholar]
- Li, Z.; Wu, Z.; Luo, F. Synthesis and Antifungal Activities of Alkyl N-(1,2,3-Thiadiazole-4-Carbonyl) Carbamates and S-Alkyl N-(1,2,3-Thiadiazole-4-Carbonyl) Carbamothioates. J. Agric. Food Chem. 2005, 53, 3872–3876. [Google Scholar] [CrossRef]
- Ning, L.; Wang, S.; Du, L.; Guo, B.; Zhang, J.; Lu, H.; Dong, Y. Synthesis, bioactivity and 3D-QSAR of azamacrolide compounds with a carbamate or urea moiety as potential fungicides and inhibitors of quorum sensing. New J. Chem. 2021, 45, 3048–3058. [Google Scholar] [CrossRef]
- Lu, Y.; Cui, Y.; Yang, W.; Meng, F. Design and synthesis of novel totarol derivatives bearing carbamate moiety as potential fungicides. Monatsh. Chem. 2023, 154, 915–923. [Google Scholar] [CrossRef]
- Jia, C.; Yang, D.; Che, C.; Ma, Y.; Rui, C.; Yan, X.; Qin, Z. Synthesis, Structural Characterization, Insecticidal and Fungicidal Activity of (1H-1,2,4-Triazol-5-yl) carbamates. Chem. J. Chin. Univ. 2016, 37, 892–901. [Google Scholar]
- Liu, C. Synthesis and fungicidal activity of (2-chloropyridin-5-yl) methyl carbamates. Chin. J. Pestic. Sci. 2015, 17, 97–100. [Google Scholar]
- You, J.; Gao, Y.; Zhou, P.; Guo, Q.; Xu, Z. Synthesis and biological activity of N-substituted phenyl-1-1(2,4-difluorophenyl)-2-(1H-1,2,4-triazol-1-yl)ethylcarbamate. Chin. J. Pestic. Sci. 2022, 24, 723–731. [Google Scholar]
- Chaturvedi, D.; Mishra, N.; Mishra, V. Various approaches for the synthesis of organic carbamates. Curr. Org. Synth. 2007, 4, 308–320. [Google Scholar] [CrossRef]
- Song, L.; Meng, Y.; Zhao, T.; Liu, L.; Pan, X.; Huang, B.; Yao, H.; Lin, R.; Tong, R. Unified and green oxidation of amides and aldehydes for the Hofmann and Curtius rearrangements. Green Chem. 2024, 26, 428–438. [Google Scholar] [CrossRef]
- Zhao, T.; Sun, Y.; Meng, Y.; Liu, L.; Dai, J.; Yan, G.; Pan, X.; Guan, X.; Song, L.; Lin, R. Design, Synthesis and Antifungal Activities of Novel Pyrazole Analogues Containing the Aryl Trifluoromethoxy Group. Molecules 2023, 28, 6279. [Google Scholar] [CrossRef]
- Si, Y.; Tang, P. Development and Application of Trifluoromethoxylating Reagents. Chin. J. Chem. 2023, 41, 2179–2196. [Google Scholar] [CrossRef]
- Xiao, H.; Zhang, Z.; Fang, Y.; Zhu, L.; Li, C. Radical trifluoromethylation. Chem. Soc. Rev. 2021, 50, 6308–6319. [Google Scholar] [CrossRef]
- Moghaddam, F.M.; Tavakoli, G.; Saeednia, B.; Langer, P.; Jafari, B. Palladium-Catalyzed Carbamate-Directed Regioselective Halogenation: A Route to Halogenated Anilines. J. Org. Chem. 2016, 81, 3868–3876. [Google Scholar] [CrossRef]
- Finger, G.C.; Dickerson, D.R.; Orlopp, D.E.; Ehrmantraut, J.W. Aromatic Fluorine Compounds. XII. N-(Fluorophenyl) Carbamates. J. Med. Chem. 1964, 7, 572–573. [Google Scholar] [CrossRef]
- 2,2,2-Trifluoroethyl N-[4-(Trifluoromethoxy)Phenyl]Carbamate. Available online: https://pubchem.ncbi.nlm.nih.gov/compound/39871375 (accessed on 5 July 2024).
| Compound | Average Inhibition Rate ± SD (%) (n = 3) a | ||||||
|---|---|---|---|---|---|---|---|
| No. | B. c. | M. o. | P. a. | F. g. | V. m. | C. s. | F. o. |
| 1a | 0 | 0.44 ± 1.38 | 2.96 ± 3.35 | 16.86 ± 1.76 | 2.36 ± 2.9 | 11.92 ± 9.83 | 25.2 ± 0.92 |
| 1b | 1.33 ± 1.52 | 3.31 ± 7.28 | 3.27 ± 2.15 | 4.98 ± 0.64 | 9.36 ± 0.83 | 9.09 ± 0.71 | 25.32 ± 1.56 |
| 1c | 18.42 ± 2.46 | 12.67 ± 1.38 | 27.86 ± 1.4 | 37.16 ± 0.81 | 31.24 ± 0.93 | 20.05 ± 0.53 | 31.24 ± 8.07 |
| 1d | 42.58 ± 1.72 | 43.46 ± 0.28 | 43.14 ± 0.67 | 51.58 ± 0.72 | 52.94 ± 1.19 | 29.1 ± 1.62 | 42.03 ± 0.49 |
| 1e | 13.32 ± 3.08 | 11.22 ± 0.29 | 16.68 ± 2.73 | 22.4 ± 3.46 | 13.47 ± 5.24 | 15.71 ± 0.73 | 27.66 ± 1.64 |
| 1f | 55.98 ± 2.19 | 53.03 ± 0.85 | 55.77 ± 1.24 | 64.1 ± 2.2 | 68.91 ± 1.56 | 41.1 ± 1.29 | 43.26 ± 1.09 |
| 1g | 0 | 0 | 0 | 0.88 ± 0.94 | 3.15 ± 2.95 | 11.88 ± 8.81 | 27.03 ± 1.79 |
| 1h | 64.44 ± 0.95 | 64.82 ± 1.15 | 64.33 ± 0.81 | 54.27 ± 0.66 | 61.69 ± 0.77 | 24.79 ± 1.35 | 20.46 ± 0.28 |
| 1i | 10.52 ± 1.79 | 7.79 ± 1.57 | 15.9 ± 1.69 | 39.17 ± 2.39 | 24.73 ± 1.29 | 18.65 ± 1.62 | 18.69 ± 7.94 |
| 1j | 32.70 ± 1.37 | 28.77 ± 0.87 | 29.93 ± 1.34 | 45.89 ± 1.58 | 37.38 ± 1.57 | 37.07 ± 0.99 | 42.09 ± 0.71 |
| 1k | 4.09 ± 0.68 | 0 | 4.33 ± 1.33 | 4.6 ± 0.83 | 6.82 ± 1 | 23.22 ± 1.65 | 20.74 ± 1.01 |
| 1l | 43.64 ± 1.59 | 40.56 ± 0.75 | 49.18 ± 2 | 56.44 ± 1.63 | 42.43 ± 1.14 | 47.72 ± 0.93 | 36.78 ± 1.04 |
| 1m | 57.94 ± 0.73 | 52.05 ± 1.16 | 49.64 ± 4.23 | 52.52 ± 0.82 | 34.78 ± 1.35 | 30.01 ± 0.99 | 26.55 ± 0.51 |
| 1n | 0 | 0 | 3.27 ± 2.7 | 13.25 ± 1.75 | 0 | 0 | 25.84 ± 1.02 |
| 1o | 7.12 ± 0.86 | 1.33 ± 1.33 | 0.11 ± 0.65 | 0 | 0 | 0 | 0 |
| 1p | 5.28 ± 3.11 | 0 | 0 | 16.73 ± 22.9 | 7.53 ± 7.73 | 0.89 ± 4.54 | 1.25 ± 0.48 |
| 1q | 0 | 0 | 14.6 ± 2.24 | 22.25 ± 4.3 | 5.01 ± 4.77 | 2.6 ± 6.49 | 0 |
| 1r | 0.6 ± 2.45 | 0 | 0.22 ± 0.8 | 0 | 0 | 0 | 3.31 ± 1.26 |
| 1s | 55.76 ± 1.26 | 55.16 ± 1.32 | 60.37 ± 3.15 | 53.41 ± 3.88 | 52.08 ± 0.56 | 52.67 ± 1.35 | 59.07 ± 3.2 |
| 1t | 71.93 ± 1.74 | 74.18 ± 0.58 | 69.96 ± 3.93 | 93.61 ± 0.21 | 70.72 ± 0.43 | 69.97 ± 1.86 | 67.89 ± 0.28 |
| 1u | 13.05 ± 2.42 | 13.12 ± 1.06 | 14.05 ± 0.34 | 36.79 ± 0.35 | 16.09 ± 0.56 | 6.98 ± 2.58 | 18.22 ± 1.25 |
| 1v | 25.57 ± 1.42 | 29.71 ± 3.35 | 36.6 ± 0.51 | 28.15 ± 0.93 | 23.03 ± 1.9 | 20.38 ± 1.52 | 33.14 ± 0.48 |
| 1w | 67.49 ± 1.58 | 67.49 ± 0.67 | 69.78 ± 1.62 | 49.82 ± 2.84 | 71.99 ± 0.42 | 65.22 ± 1.69 | 56.92 ± 0.96 |
| 1x | 70.16 ± 1.52 | 70.25 ± 0.65 | 72.48 ± 0.12 | 48.89 ± 2.01 | 72.06 ± 0.52 | 68.08 ± 1.62 | 61.92 ± 0.42 |
| 1y | 47.88 ± 0.81 | 54.82 ± 1.34 | 57.02 ± 0.6 | 74.55 ± 1.18 | 53.1 ± 0.23 | 43.13 ± 2.77 | 52.73 ± 0.21 |
| 1z | 61.42 ± 0.49 | 63.38 ± 0.63 | 64.12 ± 0.46 | 77.6 ± 2.6 | 73.77 ± 1.21 | 58.76 ± 0.6 | 66.35 ± 0.55 |
| 1aa | 27.31 ± 0.76 | 32.29 ± 1.29 | 32.28 ± 0.35 | 46.44 ± 0.49 | 38.05 ± 0.79 | 22.23 ± 0.82 | 32.12 ± 0.95 |
| 1ab | 6.77 ± 0.41 | 3.03 ± 0.45 | 7.35 ± 0.54 | 19.8 ± 4.98 | 5.48 ± 0.47 | 0.26 ± 0.44 | 6.92 ± 1.77 |
| 1ac | 54.48 ± 0.57 | 54.67 ± 0.6 | 55.51 ± 1.14 | 77.85 ± 4.53 | 55.12 ± 0.3 | 51.3 ± 0.61 | 40.86 ± 0.73 |
| 1ad | 44.31 ± 0.24 | 46.56 ± 1.14 | 47.3 ± 0.56 | 70.07 ± 0.57 | 46.4 ± 0.13 | 40.42 ± 0.25 | 24.15 ± 4.07 |
| 1ae | 31.89 ± 0.75 | 31.73 ± 1.15 | 30.76 ± 1.02 | 55.45 ± 1.42 | 36.96 ± 1.34 | 27.13 ± 0.81 | 11.8 ± 0.55 |
| 1af | 49.05 ± 0.45 | 52.80 ± 0.58 | 50.00 ± 0.69 | 85.83 ± 1.55 | 52.73 ± 1.75 | 45.51 ± 0.51 | 34.03 ± 0.82 |
| 1ag | 73.94 ± 0.61 | 72.72 ± 0.96 | 75.4 ± 0.45 | 73.37 ± 2.76 | 76.78 ± 0.47 | 72.12 ± 0.66 | 68.42 ± 0.48 |
| 1ah | 62.07 ± 0.54 | 55.04 ± 0.14 | 61.56 ± 1.01 | 69.09 ± 0.88 | 63.91 ± 1.05 | 56.09 ± 4.16 | 50.58 ± 0.95 |
| 1ai | 10.05 ± 0.67 | 5.67 ± 1.1 | 19.67 ± 6.49 | 16.77 ± 7.16 | 6.24 ± 1.73 | 16.49 ± 1.17 | 11.1 ± 0.89 |
| AZO | 54.39 ± 0.24 | 59.94 ± 0.24 | 56.40 ± 0.87 | 58.17 ± 0.22 | 63.14 ± 0.66 | 54.79 ± 0.67 | 62.50 ± 2.14 |
 .
.| Compound | Regression Equation | R2 | EC50 (μg/mL, 95% CI) a | EC50 (μM, 95% CI) a |
|---|---|---|---|---|
| B. cinerea | ||||
| 1t | Y = 2.04x + 1.86 | 0.972 | 34.05 (32.25–42.39) | 0.1548 (0.1466–0.1927) |
| 1x | Y = 2.89x + 0.48 | 0.994 | 36.55 (31.91–40.25) | 0.1498 (0.1308–0.1650) |
| 1ag | Y = 2.41x + 1.15 | 0.974 | 29.44 (25.66–34.33) | 0.1141 (0.0995–0.1331) |
| azoxystrobin | Y = 0.38x + 4.52 | 0.917 | 18.06 (4.97–37.44) | 0.0448 (0.0123–0.0928) |
| M. grisea | ||||
| 1t | Y = 1.65x + 2.62 | 0.998 | 27.34 (24.54–34.22) | 0.1243 (0.1115–0.1555) |
| 1x | Y = 2.60x + 0.98 | 0.995 | 34.53 (32.70–40.98) | 0.1415 (0.1340–0.1680) |
| 1ag | Y = 2.03x + 1.99 | 0.997 | 29.98 (27.23–36.05) | 0.1162 (0.1055–0.1397) |
| azoxystrobin | Y = 0.32x + 4.70 | 0.917 | 7.90 (0.08–17.84) | 0.0196 (0.0002–0.0443) |
| P. aphanidermatum | ||||
| 1x | Y = 2.22x + 1.73 | 0.974 | 29.22 (27.04–35.04) | 0.1198 (0.1108–0.1436) |
| 1ag | Y = 1.95x + 2.27 | 0.993 | 24.64 (20.27–27.40) | 0.0955 (0.0503–0.1062) |
| azoxystrobin | Y = 0.33x + 4.61 | 0.968 | 13.99 (1.08–30.50) | 0.0347 (0.0027–0.0757) |
| F. graminearum | ||||
| 1t | Y = 2.42x + 1.83 | 0.955 | 20.22 (13.71–30.14) | 0.0919 (0.0623–0.1370) |
| 1ac | Y = 2.23x + 2.03 | 0.977 | 21.17 (19.31–25.15) | 0.0850 (0.0776–0.1010) |
| 1af | Y = 1.78x + 3.04 | 0.993 | 12.50 (10.38–14.93) | 0.0413 (0.0343–0.0493) |
| azoxystrobin | Y = 0.34x + 4.65 | 0.971 | 10.36 (0.48–21.80) | 0.0257 (0.0012–0.0541) |
| V. mali | ||||
| 1t | Y = 1.80x + 2.35 | 0.989 | 28.96 (25.63–34.98) | 0.1316 (0.1165–0.1590) |
| 1w | Y = 2.45x + 1.52 | 0.902 | 26.23 (13.61–53.10) | 0.1075 (0.0558–0.2176) |
| 1x | Y = 2.24x + 1.75 | 0.993 | 27.87 (25.61–33.17) | 0.1142 (0.1050–0.1359) |
| 1ag | Y = 2.06x + 2.05 | 0.991 | 26.69 (23.65–31.17) | 0.1034 (0.0917–0.1208) |
| azoxystrobin | Y = 0.5x + 4.42 | 0.958 | 4.71 (0.06–11.32) | 0.0117 (0.0002–0.0281) |
| F. oxysporum | ||||
| 1t | Y = 1.74x + 2.42 | 0.975 | 30.03 (28.35–38.60) | 0.1365 (0.1289–0.1755) |
| 1z | Y = 0.94x + 3.84 | 0.885 | 16.65 (5.71–38.79) | 0.0553 (0.0188–0.1288) |
| 1ag | Y = 1.74x + 2.63 | 0.996 | 22.71 (21.29–29.07) | 0.0880 (0.0825–0.1127) |
| azoxystrobin | Y = 0.50x + 4.42 | 0.977 | 14.14 (5.66–23.54) | 0.0351 (0.0140–0.0584) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, X.; Sun, Y.; Liu, L.; Duan, X.; You, S.; Yu, B.; Pan, X.; Guan, X.; Lin, R.; Song, L. Green Synthesis and Antifungal Activities of Novel N-Aryl Carbamate Derivatives. Molecules 2024, 29, 3479. https://doi.org/10.3390/molecules29153479
Liu X, Sun Y, Liu L, Duan X, You S, Yu B, Pan X, Guan X, Lin R, Song L. Green Synthesis and Antifungal Activities of Novel N-Aryl Carbamate Derivatives. Molecules. 2024; 29(15):3479. https://doi.org/10.3390/molecules29153479
Chicago/Turabian StyleLiu, Xiyao, Yuyao Sun, Lifang Liu, Xufei Duan, Shujun You, Baojia Yu, Xiaohong Pan, Xiong Guan, Ran Lin, and Liyan Song. 2024. "Green Synthesis and Antifungal Activities of Novel N-Aryl Carbamate Derivatives" Molecules 29, no. 15: 3479. https://doi.org/10.3390/molecules29153479
APA StyleLiu, X., Sun, Y., Liu, L., Duan, X., You, S., Yu, B., Pan, X., Guan, X., Lin, R., & Song, L. (2024). Green Synthesis and Antifungal Activities of Novel N-Aryl Carbamate Derivatives. Molecules, 29(15), 3479. https://doi.org/10.3390/molecules29153479






