Response Properties of Electrorheological Composite Hydrophilic Elastomers Based on Different Morphologies of Magnesium-Doped Strontium Titanate
Abstract
1. Introduction
2. Results and Discussion
2.1. ICP Analyses of Mg-STO
2.2. XRD and Raman Analyses of Mg-STO
2.3. FI-IR Analyses of Mg-STO
2.4. Hydrophilicity Measurement and BET Analyses of Mg-STO
2.5. Dielectric Measurement of Mg-STO
2.6. Analyses of EFR Performance of Mg-STO
3. Materials and Methods
3.1. Preparation of Mg-STO
3.1.1. Preparation of Spherical and Pinecone-Shaped Mg-STO
3.1.2. Preparation of Dendritic and Flake-like Mg-STO
3.2. Preparation of Electrorheological Composite Hydrophilic Elastomers
3.3. Characterizations
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Lavalle, P.; Voegel, J.C.; Vautier, D.; Senger, B.; Schaaf, P. Dynamic aspects of films prepared by a sequential deposition of species: Perspectives for smart and responsive materials. Adv. Mater. 2011, 23, 1191–1221. [Google Scholar] [CrossRef]
- Yarimaga, O.; Jaworski, J.; Yoon, B.; Kim, J.M. Polydiacetylenes: Supramolecular smart materials with a structural hierarchy for sensing, imaging and display applications. Chem. Commun. 2012, 48, 2469–2485. [Google Scholar] [CrossRef] [PubMed]
- Niu, X.; Zhang, M.; Wu, J.; Wen, W.; Sheng, P. Generation and manipulation of “smart” droplets. Soft Matter. 2009, 5, 576–581. [Google Scholar] [CrossRef]
- Ohko, Y.; Tatsuma, T.; Fujii, T.; Naoi, K.; Niwa, C.; Kubota, Y.; Fujishima, A. Multicolour photochromism of TiO2 films loaded with silver nanoparticles. Nat. Matter. 2003, 2, 29–31. [Google Scholar] [CrossRef]
- Varga, Z.; Filipcsei, G.; Zrinyi, M. Magnetic field sensitive functional elastomers with tuneable elastic modulus. Polymer 2006, 47, 227–233. [Google Scholar] [CrossRef]
- Adrian Koh, S.J.; Li, T.-F.; Zhou, J.-X.; Zhao, X.-H.; Hong, W.; Zhu, J.; Suo, Z.-G. Mechanisms of large actuation strain in dielectric elastomers. J. Polym. Sci. 2011, 49, 504–515. [Google Scholar]
- Chakraborti, P.; Karahan Toprakci, H.A.; Yang, P.; Spigna, N.D.; Franzon, P.; Ghosh, T. A compact dielectric elastomer tubular actuator for refreshable braille displays. Sens. Actuators A 2012, 179, 151–157. [Google Scholar] [CrossRef]
- Xia, H.; Takasaki, M.; Hirai, T. Actuation mechanism of plasticized PVC by electric field. Sens. Actuators A 2010, 157, 307–312. [Google Scholar] [CrossRef]
- Gao, L.-X.; Zhan, L.-J.; Liu, W.; Zhang, Y.-L.; Xie, Z.-Y.; Ren, J. Preparation and electro responsive properties of Mg-doped BaTiO3 with novel morphologies. J. Mater. Sci.-Mater. Electron. 2019, 30, 12107–12112. [Google Scholar] [CrossRef]
- Hao, L.; Shi, Z.; Zhao, X. Mechanical behavior of starch/silicone oil/silicone rubber hybrid electric elastomer. React. Funct. Polym. 2009, 69, 165–169. [Google Scholar] [CrossRef]
- Li, Y.-Q.; Li, X.-N.; Chen, C.; Su, Z.-G.; Ma, G.-H.; Yu, R. Sol-gel transition characterization of thermosensitive hydrogels based on water mobility variation provided by low field NMR. J. Polym. Res. 2017, 24, 1–10. [Google Scholar] [CrossRef]
- Dashtimoghadam, E.; Mirzadeh, H.; Taromi, F.A.; Nyström, B. Thermoresponsive biopolymer hydrogels with tunable gel characteristics. RSC Adv. 2014, 4, 39386–39393. [Google Scholar] [CrossRef]
- Dong, X.-F.; Niu, C.-G.; Qi, M. Enhancement of electrorheological performance of electrorheological elastomers by improving TiO2 particles/silicon rubber interface. J. Mater. Chem. C 2016, 4, 6806–6815. [Google Scholar] [CrossRef]
- Chen, J.; Li, H.-F.; Han, H.-J.; Sun, R.-Y.; Xie, M.-R. Multiple polarizations and nanostructure of double-stranded conjugated block copolymer for enhancing dielectric performance. Mater. Lett. 2017, 208, 95–97. [Google Scholar] [CrossRef]
- Liu, W.; Xie, Z.-Y.; Lu, Y.-P.; Gao, M.-X.; Zhang, W.-Q.; Gao, L.-X. Fabrication and excellent electroresponsive properties of ideal PMMA@BaTiO3 composite particles. RSC Adv. 2019, 9, 12404–12414. [Google Scholar] [CrossRef]
- Xue, B.-X.; He, F.; Zhao, X.-P.; Yin, J.-B. Electro-responsive electrorheological effect and dielectric spectra analysis of topological self-crosslinked poly (ionic liquid)s. Eur. Polym. J. 2022, 170, 111160. [Google Scholar] [CrossRef]
- Gao, L.-X.; Gao, S.-J.; Wei, W.-X.; Zhang, W.-Q. Morphology modification of micron-sized barium strontium titanate by hydrothermal growth. J. Mater. Sci.-Mater. Electron. 2015, 26, 1354–1362. [Google Scholar] [CrossRef]
- Dong, W.-J.; Li, X.-Y.; Yu, J.; Guo, W.-C.; Li, B.-J.; Tan, L.; Li, C.-R.; Shi, J.-J. Porous SrTiO3 spheres with enhanced photocatalytic performance. Mater. Lett. 2012, 67, 131–134. [Google Scholar] [CrossRef]
- Huang, Y.; Zhao, X.-P.; Wang, L.-S.; Luo, C.-R. Tunable left-handed metamaterial based on electrorheological fluids. Prog. Nat. Sci.-Mater. 2008, 18, 907–911. [Google Scholar] [CrossRef]
- Tang, H.; Zhao, X.-P.; Wang, B.-X.; Zhao, Y. Response characteristics of a viscoelastic gel under the co-action of sound waves and an electric field. Smart Mater. Struct. 2006, 15, 86–92. [Google Scholar] [CrossRef]
- Chen, H.; Cheng, R.; Zhao, X.; Zhang, Y.; Tam, A.; Yan, Y. An injectable self-healing coordinative hydrogel with antibacterial and angiogenic properties for diabetic skin wound repair. NPG Asia. Mater. 2019, 11, 1427–1429. [Google Scholar] [CrossRef]
- Shiga, T.; Ohta, T.; Hirose, Y.; Okada, A.; Kurauchi, T. Electroviscoelastic effect of polymeric composites consisting of polyelectrolyte particles and polymer gel. J. Mater. Sci. 1993, 28, 1293–1299. [Google Scholar] [CrossRef]
- Hanaoka, R.; Takata, S.; Nakazawa, Y.; Fukami, T.; Sakurai, K. Effect of electric field on viscoelastic properties of a disperse system in silicone gel. Electr. Eng. Jpn. 2003, 142, 1–9. [Google Scholar] [CrossRef]
- An, Y.; Shaw, T.M. Actuating properties of soft gels with ordered iron particles: Basis for a shear actuator. Smart Mater. Struct. 2003, 12, 157–163. [Google Scholar] [CrossRef]
- Chen, H.-M.; Koh, J.-J.; Long, C.-J.; Liu, S.-Q.; Shi, H.-H.; Min, J.-K.; Zhou, L.-L.; He, C.-B. Speed-induced extensibility elastomers with good resilience and high toughness. Macromolecules 2021, 54, 3358–3365. [Google Scholar] [CrossRef]
- Davidson, J.R.; Krebs, H.I. An electrorheological fluid actuator for rehabilitation robotics. IEEE-ASME Trans. Mech. 2018, 23, 2156–2167. [Google Scholar] [CrossRef]
- Gao, L.-X.; Zhao, X.-P. Electrorheological behaviors of barium titanate/gelatin composite hydrogel elastomers. J. Appl. Polym. Sci. 2004, 94, 2517–2521. [Google Scholar] [CrossRef]
- Gao, L.-X.; Liu, Q.-P.; Gao, Z.-W.; Lin, Y. Preparation and characterization of polyimide/silica-barium titanate nanocomposites. Polym. Compos. 2008, 29, 1160–1164. [Google Scholar] [CrossRef]
- Yin, J.-B.; Zhao, X.-P. Preparation and electrorheological characteristic of Y-doped BaTiO3 suspension under dc electric field. J. Solid State Chem. 2004, 177, 3650–3659. [Google Scholar] [CrossRef]
- Liu, B.; Shaw, M.T. Electrorheology of filled silicone elastomers. J. Rheol. 2001, 45, 641–657. [Google Scholar] [CrossRef]
- Liu, B.; Shaw, M.T. Electrorheological effects of ER gels containing iron particles. J. Intell. Mater. Syst. Struct. 2001, 12, 57–63. [Google Scholar] [CrossRef]
- Mitsumata, T.; Sugitani, K.; Koyama, K. Electrorheological response of swollen silicone gels containing barium titanate. Polymer 2004, 45, 3811–3817. [Google Scholar] [CrossRef]
- Fan, Y.-H.; Yu, S.-H.; Sun, R.; Li, L.; Yin, Y.-S.; Wong, K.-W.; Du, R.-X. Microstructure and electrical properties of Mn-doped barium strontium titanate thin films prepared on copper foils. Appl. Surf. Sci. 2010, 256, 6531–6535. [Google Scholar] [CrossRef]
- Fatema, M.; Bajpai, A.; Somvanshi, A.; Manzoor, S.; Arshad, M.; Zarrin, N.; Qahtan, A.A.A.; Khan, W.; Husain, S. Study of structural correlations with temperature dependent dielectric response and ferroelectric behavior for (Sr, Mn) co-doped BaTiO3. J. Mater. Sci.-Mater. Electron. 2022, 33, 6329–6353. [Google Scholar] [CrossRef]
- Khan, A.R.; Goel, R.; Gupta, A.; Tripathi, H.; Kumar, N.; Bhardwaj, S.; Kumar, S.; Sharma, I.; Kumar, G.; Sharma, P. Enhanced structural, magnetodielectric, and multiferroic response in Fe and Co Co-doped Barium strontium titanate ceramics. Phys. Scr. 2024, 99, 055963. [Google Scholar] [CrossRef]
- Kosińska-Klähn, M.; John, Ł.; Drag-Jarzabek, A.; Utko, J.; Petrus, R.; Jerzykiewicz, L.B.; Sobota, P. Transformation of barium–titanium chloro–alkoxide compound to BaTiO3 nanoparticles by BaCl2 elimination. Inorg. Chem. 2014, 53, 1630–1636. [Google Scholar] [CrossRef]
- Mao, Y.-P.; Mao, S.-Y.; Ye, Z.-G.; Xie, Z.-X.; Zheng, L.-S. Size-dependences of the dielectric and ferroelectric properties of BaTiO3/polyvinylidene fluoride nanocomposites. Mater. Chem. Phys. 2010, 108, 014102. [Google Scholar] [CrossRef]
- Huang, K.C.; Huang, T.C.; Hsieh, W.F. Morphology-controlled synthesis of barium titanate nanostructures. Inorg. Chem. 2009, 48, 9180–9184. [Google Scholar] [CrossRef]
- Merz, W.J. The electric and optical behavior of BaTiO3 single-domain crystals. Phys. Rev. 1949, 76, 1221–1225. [Google Scholar] [CrossRef]
- Gao, S.-J. Dielectric properties and synthesis of magnesium doped strontium titanate with annona squamosa-like. Results Phys. 2020, 3, 102884. [Google Scholar] [CrossRef]
- Hirata, T.; Ishioka, K.; Kitajima, M. Vibrational Spectroscopy and X-Ray Diffraction of Perovskite Compounds Sr1−xMxTiO3 (M = Ca, Mg; 0 ≤ x ≤ 1). J. Solid State Chem. 1996, 124, 353–359. [Google Scholar] [CrossRef]
- Noesges, B.A.; Lee, D.; Lee, J.W.; Eom, C.B.; Brillson, L.J. Nanoscale interplay of native point defects near Sr-deficient SrxTiO3/SrTiO3 interfaces. J. Vac. Sci. Technol. A 2022, 40, 043201. [Google Scholar] [CrossRef]
- Zvanut, M.E.; Jeddy, S.; Towett, E.; Janowski, G.M.; Brooks, C.; Schlom, D. An annealing study of an oxygen vacancy related defect in SrTiO3 substrates. J. Appl. Phys. 2008, 104, 064122. [Google Scholar] [CrossRef]
- Naidu, K.C.B.; Sarmash, T.S.; Maddaiah, M.; Reddy, P.S.; Subbarao, T. Synthesis and characterization of MgO doped SrTiO3 ceramics. J. Aust. Ceram. Soc. 2016, 52, 95–101. [Google Scholar]
- Jeon, J.E.; Han, H.S.; Park, K.R.; Hong, Y.R.; Shim, K.B.; Mhin, S. The effect of pH control on synthesis of Sr doped barium titanate nanopowder by oxalate precipitation method. Ceram. Int. 2018, 44, 1420–1424. [Google Scholar] [CrossRef]
- Wang, B.-X.; Zhao, Y.; Zhao, X.-P. The wettability, size effect and electrorheological activity of modified titanium oxide nanoparticles. Colloid Surface. A. 2007, 295, 27–33. [Google Scholar] [CrossRef]
- McHale, G.; Shirtcliffe, N.J.; Newton, M.I. Contact-angle hysteresis on super-hydrophobic surfaces. Langmuir 2004, 20, 10146–10149. [Google Scholar] [CrossRef]
- Yin, J.-B.; He, L.; Gao, Z.-W.; Gao, L.-X.; Wang, B. Facile method for fabricating titania spheres for chromatographic packing. Mater. Lett. 2009, 63, 2191–2193. [Google Scholar] [CrossRef]
- Yin, J.-B.; Zhao, X.-P. Electrorheological properties of titanate nanotube suspensions. Colloid Surface. A. 2008, 329, 153–160. [Google Scholar] [CrossRef]
- Krause, S.; Bohon, K. Electromechanical response of electrorheological fluids and poly (dimethylsiloxane) networks. Macromolecules 2001, 34, 7179–7189. [Google Scholar] [CrossRef]
- See, H.; Sakurai, R.; Saito, T.; Asai, S.; Sumita, M. Relationship between electric current and matrix modulus in electrorheological elastomers. J. Electrostat. 2001, 50, 303–312. [Google Scholar] [CrossRef]
- Wang, Y.-F.; Lin, M.-H.; Dai, W.-Q.; Zhou, Y.-W.; Xie, Z.-Y.; Liu, K.-Q.; Gao, L.-X. Enhancement of Fe(III) to electro-response of starch hydrogel. Colloid Polym. Sci. 2020, 298, 1533–1541. [Google Scholar] [CrossRef]
- Wu, X.-N.; Gao, L.-X.; Liu, Y.; Gao, Z.-W.; Pei, C.-P.; Gao, S.-J. Preparation and electric field response behavior of barium strontium titanate with different controlled shapes. Chem. J. Chin. Univ. 2009, 30, 1066–1070. [Google Scholar]
- He, L.; Gao, L.-X.; Gao, Z.-W.; Zhang, Y.; Wu, X.-N.; Ni, Y.-R. Synthesis and electric field response behavior of 4-Chlorophthalate titanocene complexes. Chem. J. Chin. Univ. 2009, 30, 855–861. [Google Scholar]
- Liu, Q.-P.; Gao, L.-X.; Gao, Z.-W.; Yang, L. Preparation and characterization of polyimide/silica nanocomposite spheres. Mater. Lett. 2007, 61, 4456–4458. [Google Scholar] [CrossRef]
- Zhang, J.; Sun, L.-D.; Yin, J.-L.; Su, H.-L.; Liao, C.-S.; Yan, C.-H. Control of ZnO morphology via a simple solution route. Chem. Mater. 2002, 14, 4172–4177. [Google Scholar] [CrossRef]
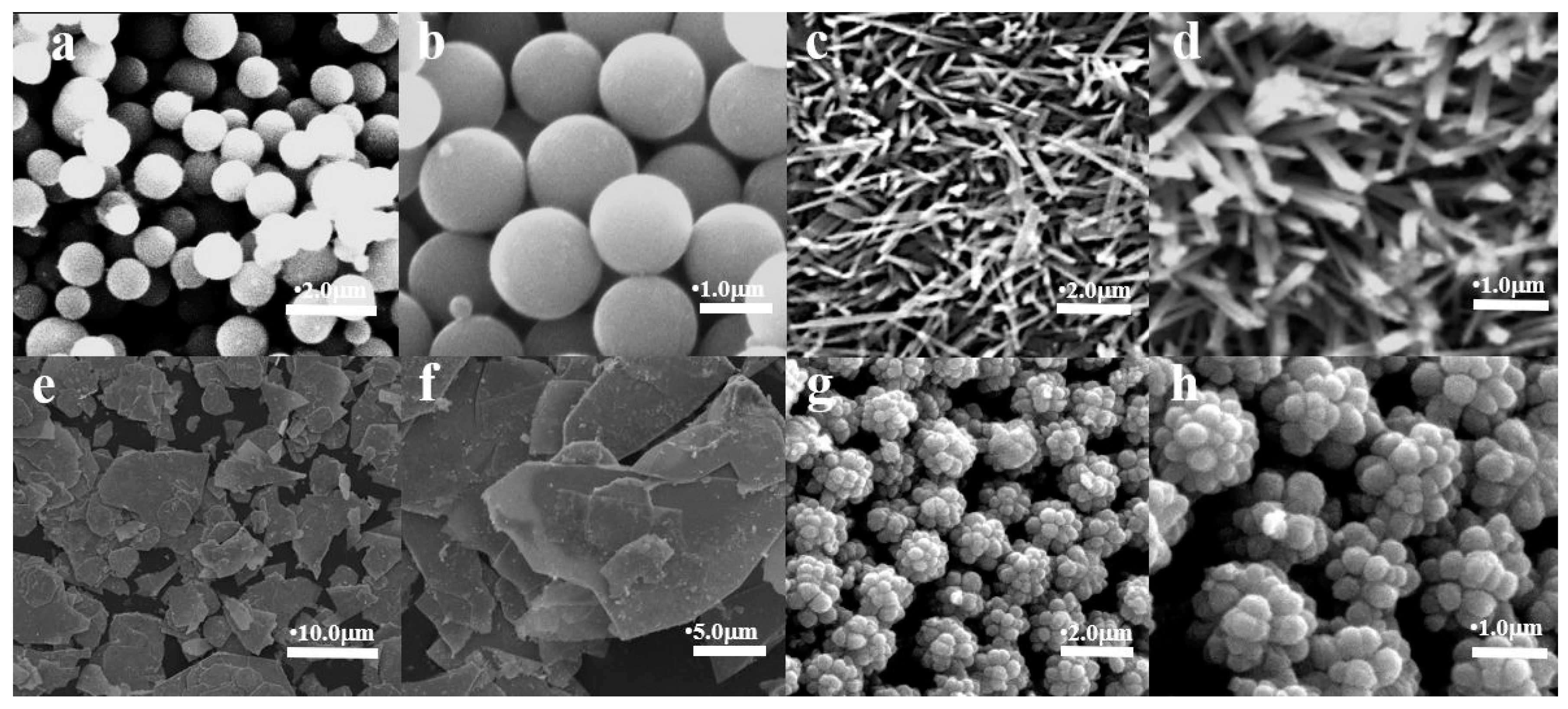


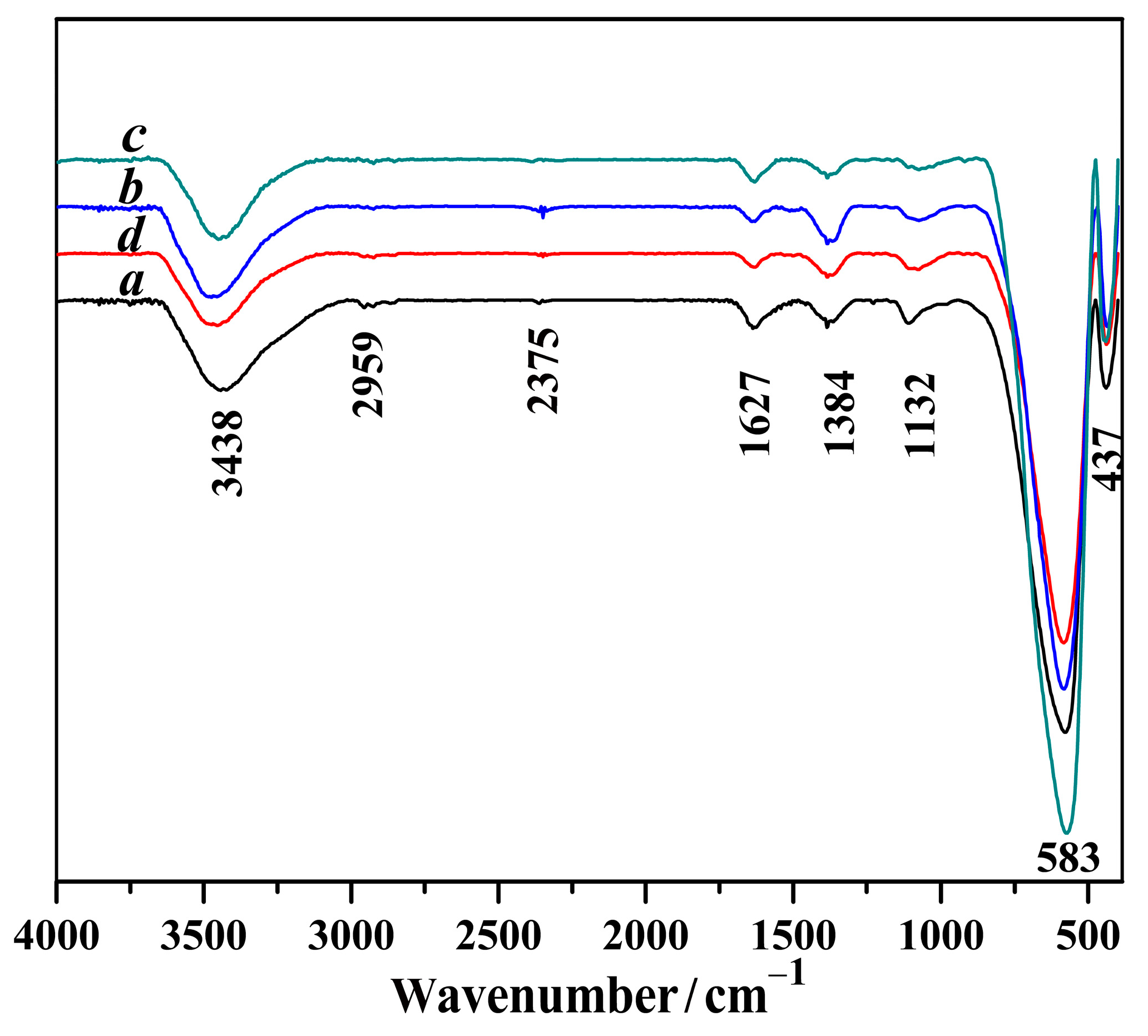


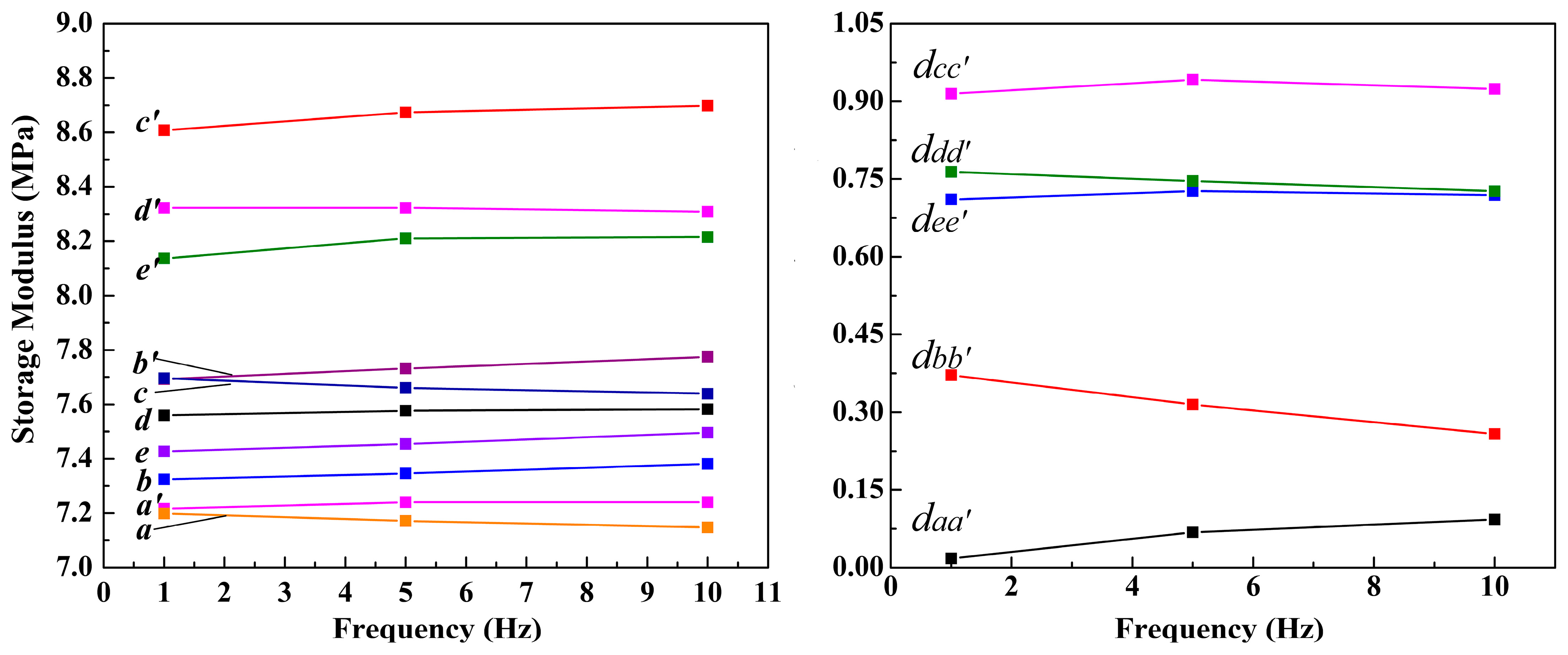
| Sample | Sr/mol | Mg/mol |
|---|---|---|
| M1 | 1.095 ± 0.002 | 0.030 ± 0.002 |
| M2 | 1.013 ± 0.001 | 0.017 ± 0.001 |
| M3 | 1.021 ± 0.001 | 0.023 ± 0.001 |
| M4 | 1.071 ± 0.001 | 0.022 ± 0.001 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gao, S.-J.; Li, L.-Z.; Han, P.-F.; Wang, L.; Li, F.; Yu, T.-L.; Li, Y.-F. Response Properties of Electrorheological Composite Hydrophilic Elastomers Based on Different Morphologies of Magnesium-Doped Strontium Titanate. Molecules 2024, 29, 3462. https://doi.org/10.3390/molecules29153462
Gao S-J, Li L-Z, Han P-F, Wang L, Li F, Yu T-L, Li Y-F. Response Properties of Electrorheological Composite Hydrophilic Elastomers Based on Different Morphologies of Magnesium-Doped Strontium Titanate. Molecules. 2024; 29(15):3462. https://doi.org/10.3390/molecules29153462
Chicago/Turabian StyleGao, Shu-Juan, Lin-Zhi Li, Peng-Fei Han, Ling Wang, Feng Li, Tan-Lai Yu, and Yan-Fang Li. 2024. "Response Properties of Electrorheological Composite Hydrophilic Elastomers Based on Different Morphologies of Magnesium-Doped Strontium Titanate" Molecules 29, no. 15: 3462. https://doi.org/10.3390/molecules29153462
APA StyleGao, S.-J., Li, L.-Z., Han, P.-F., Wang, L., Li, F., Yu, T.-L., & Li, Y.-F. (2024). Response Properties of Electrorheological Composite Hydrophilic Elastomers Based on Different Morphologies of Magnesium-Doped Strontium Titanate. Molecules, 29(15), 3462. https://doi.org/10.3390/molecules29153462






