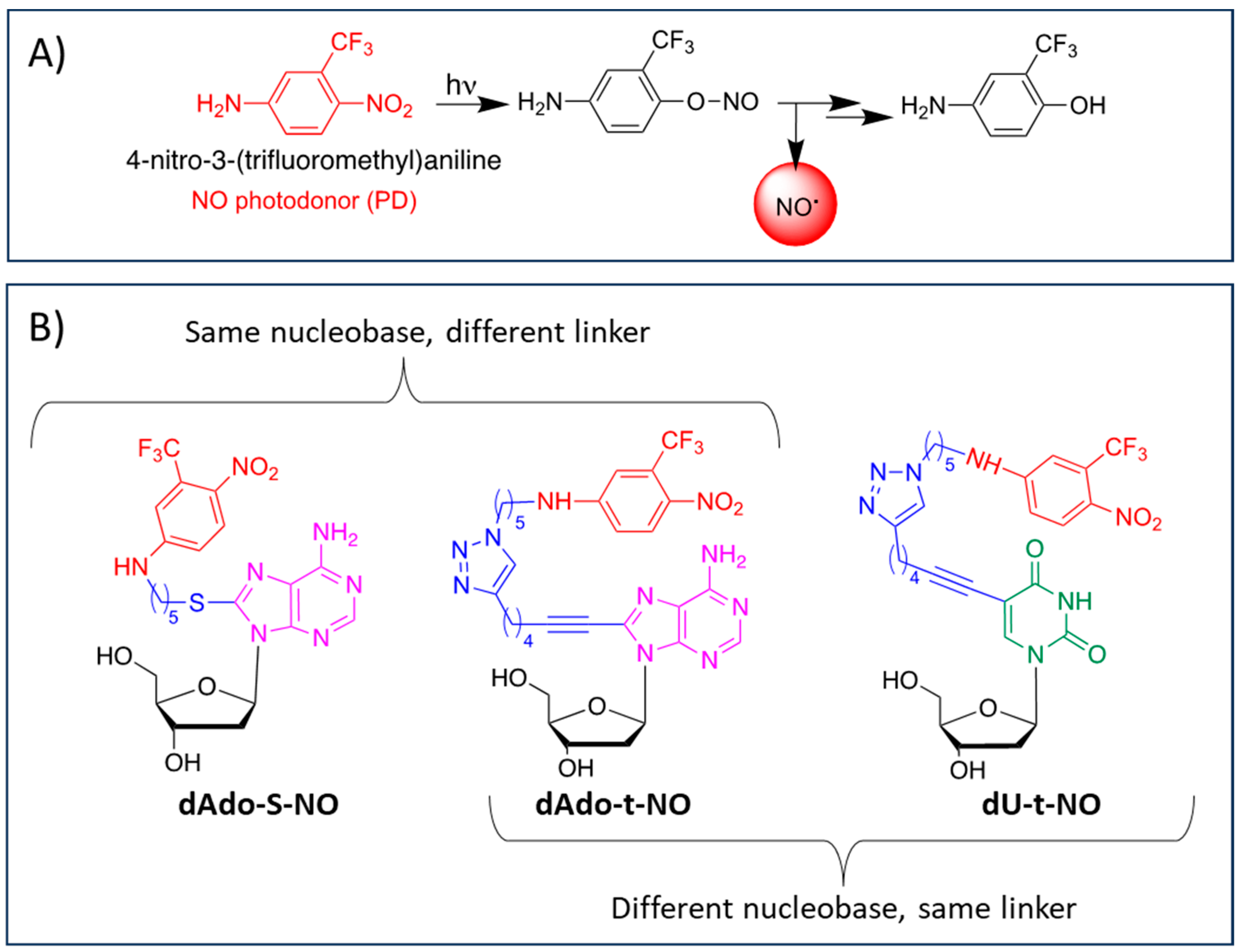
Evaluation of Anticancer Activity of Nucleoside–Nitric Oxide Photo-Donor Hybrids
Abstract
1. Introduction
2. Results
2.1. Chemistry
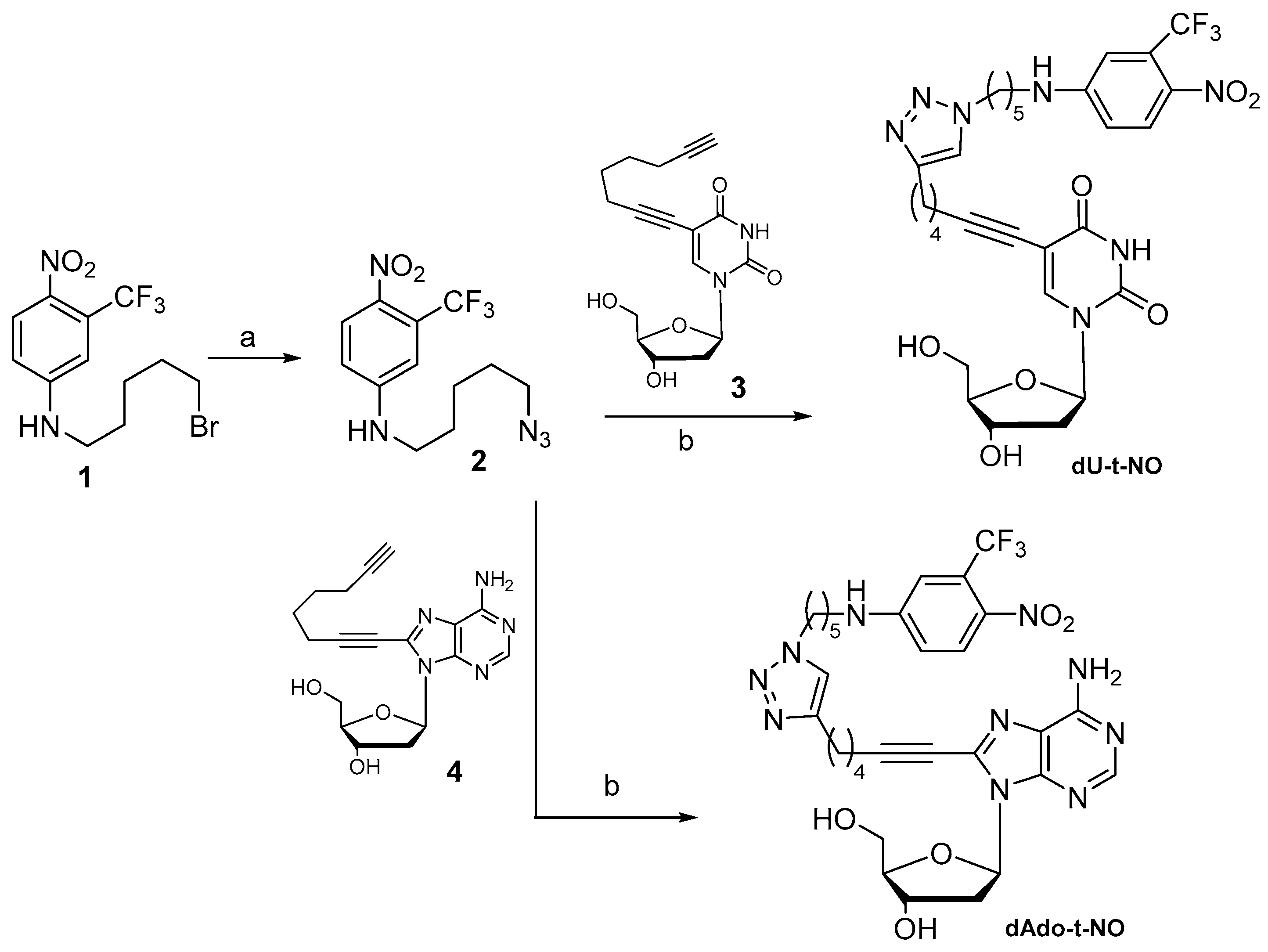
2.1.1. Synthesis of Nucleoside–NO Photo-Donor Hybrids
2.1.2. Chemical Stability in Cell Culture Medium
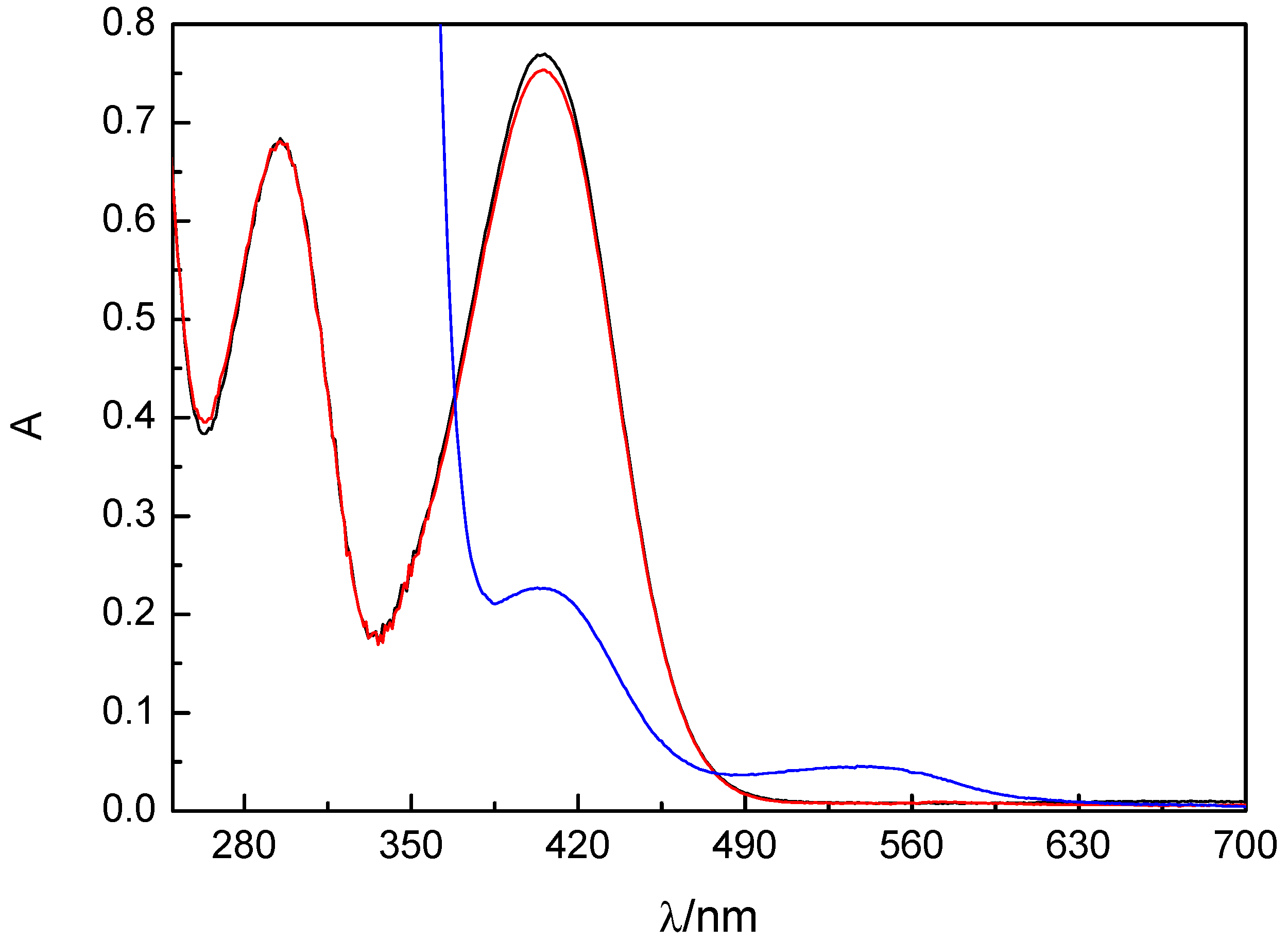
2.2. Photochemistry
2.3. Biological Evaluation
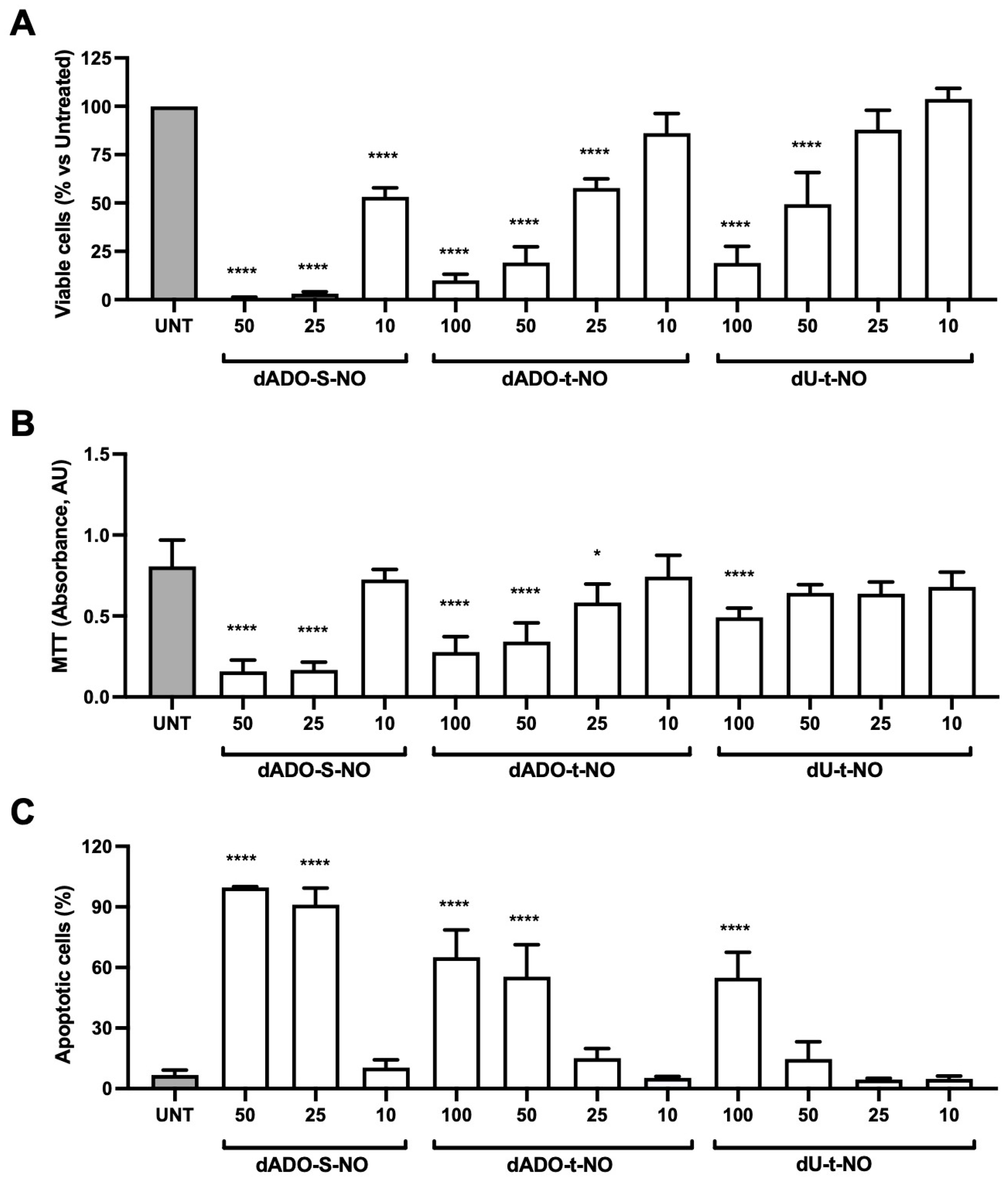
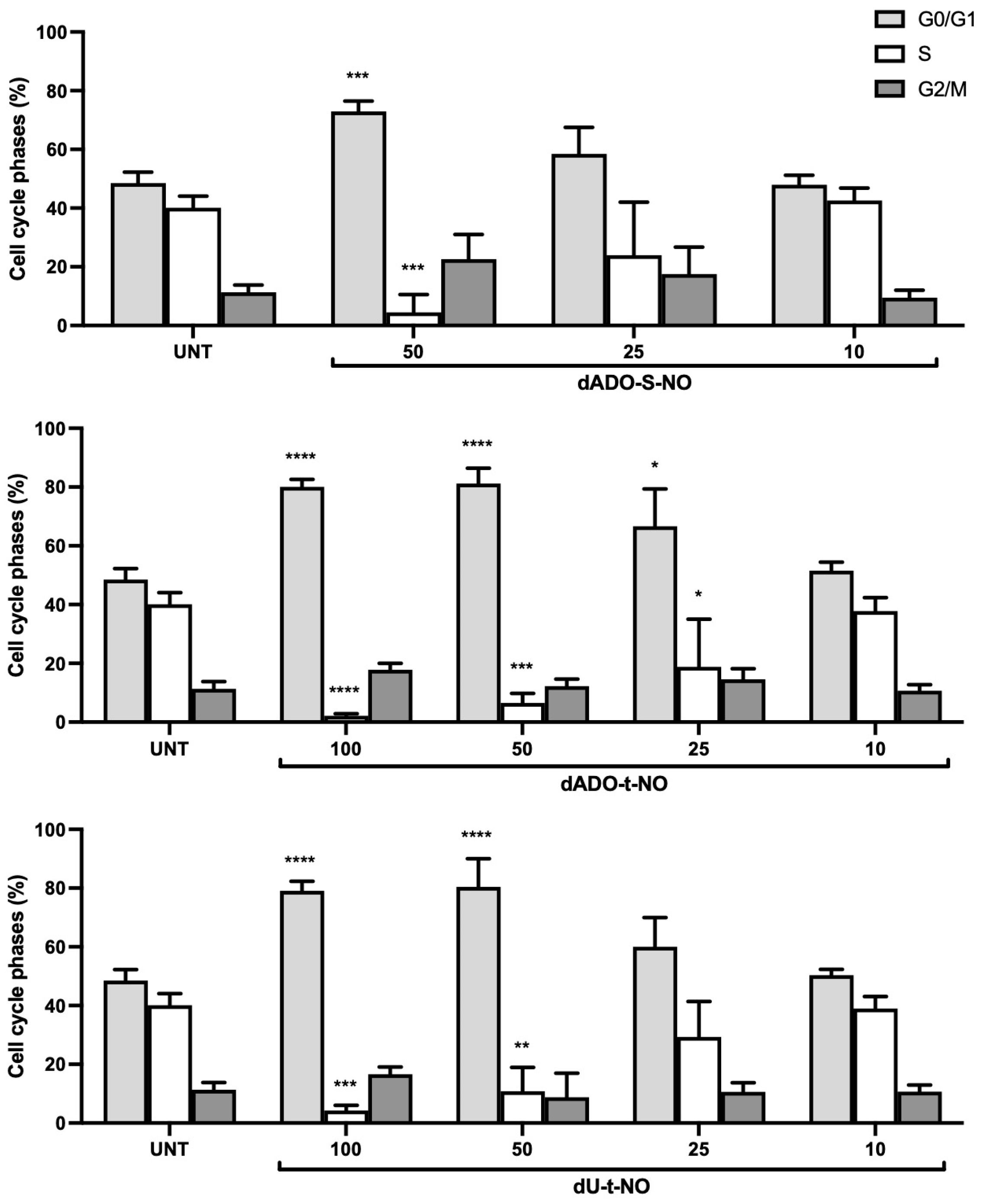
2.3.1. Evaluation of the Biological Activity of dAdo-S-NO, dAdo-t-NO, and dU-t-NO on Colon and Hepatocarcinoma Cancer Cells in the Dark
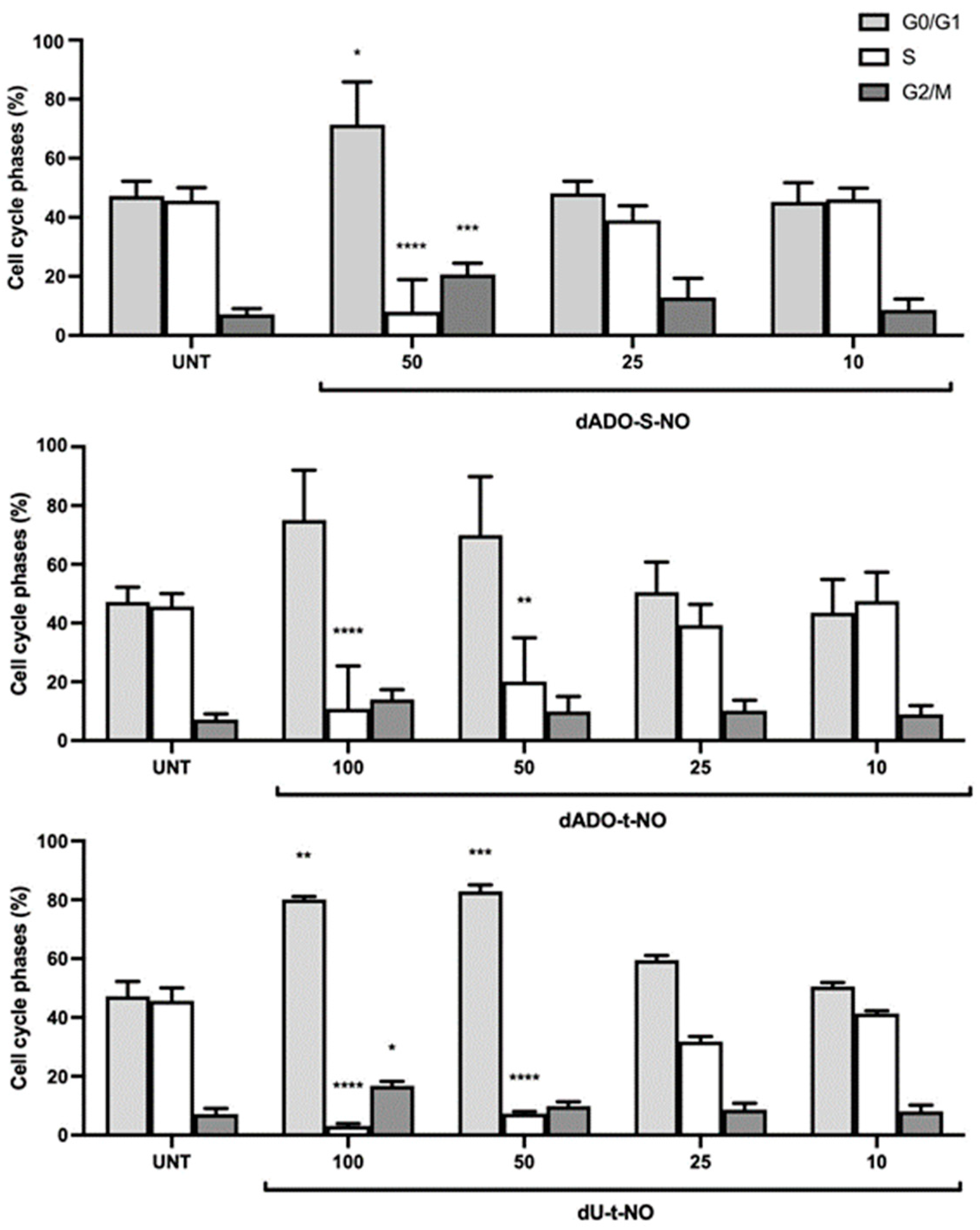
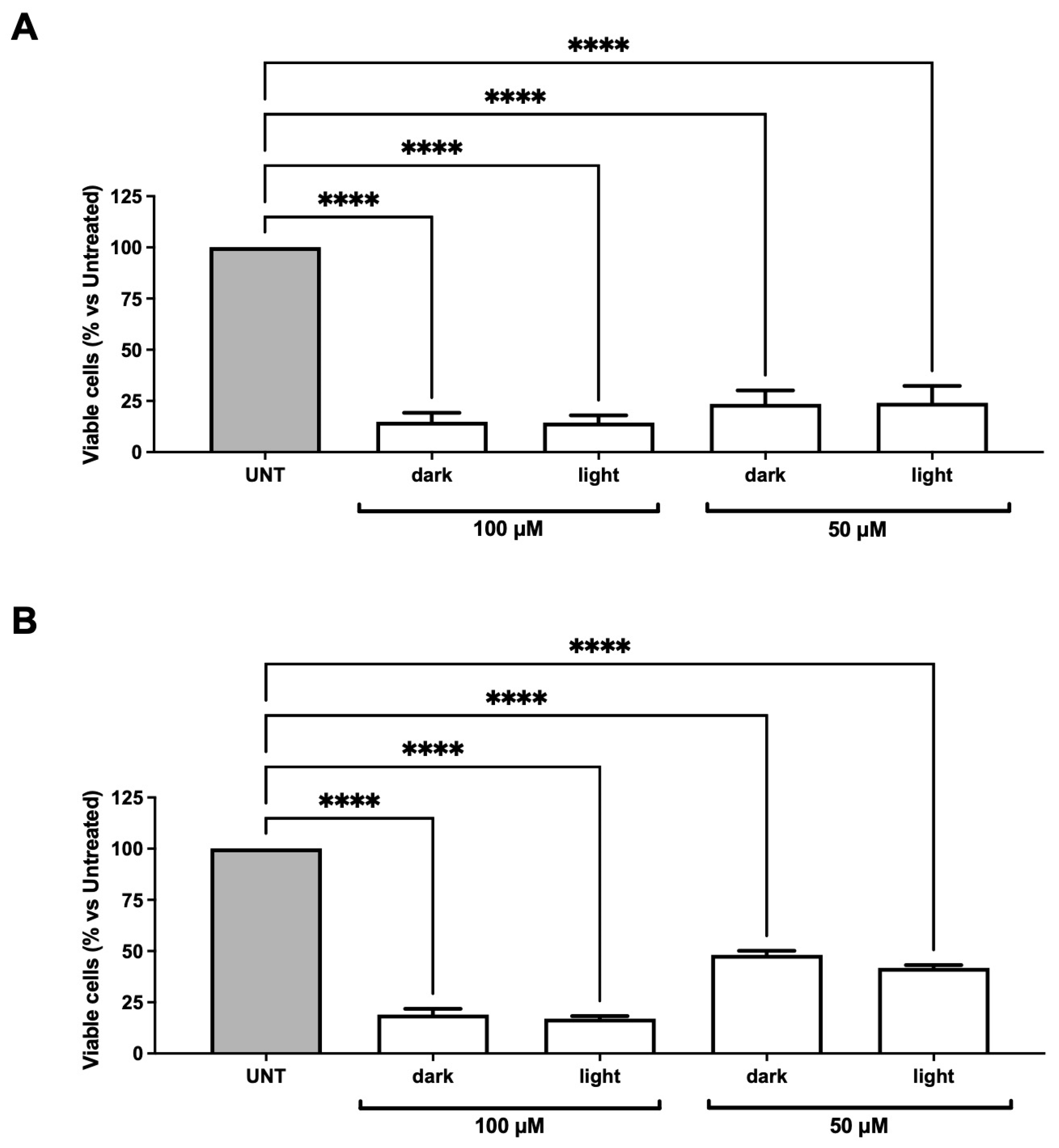
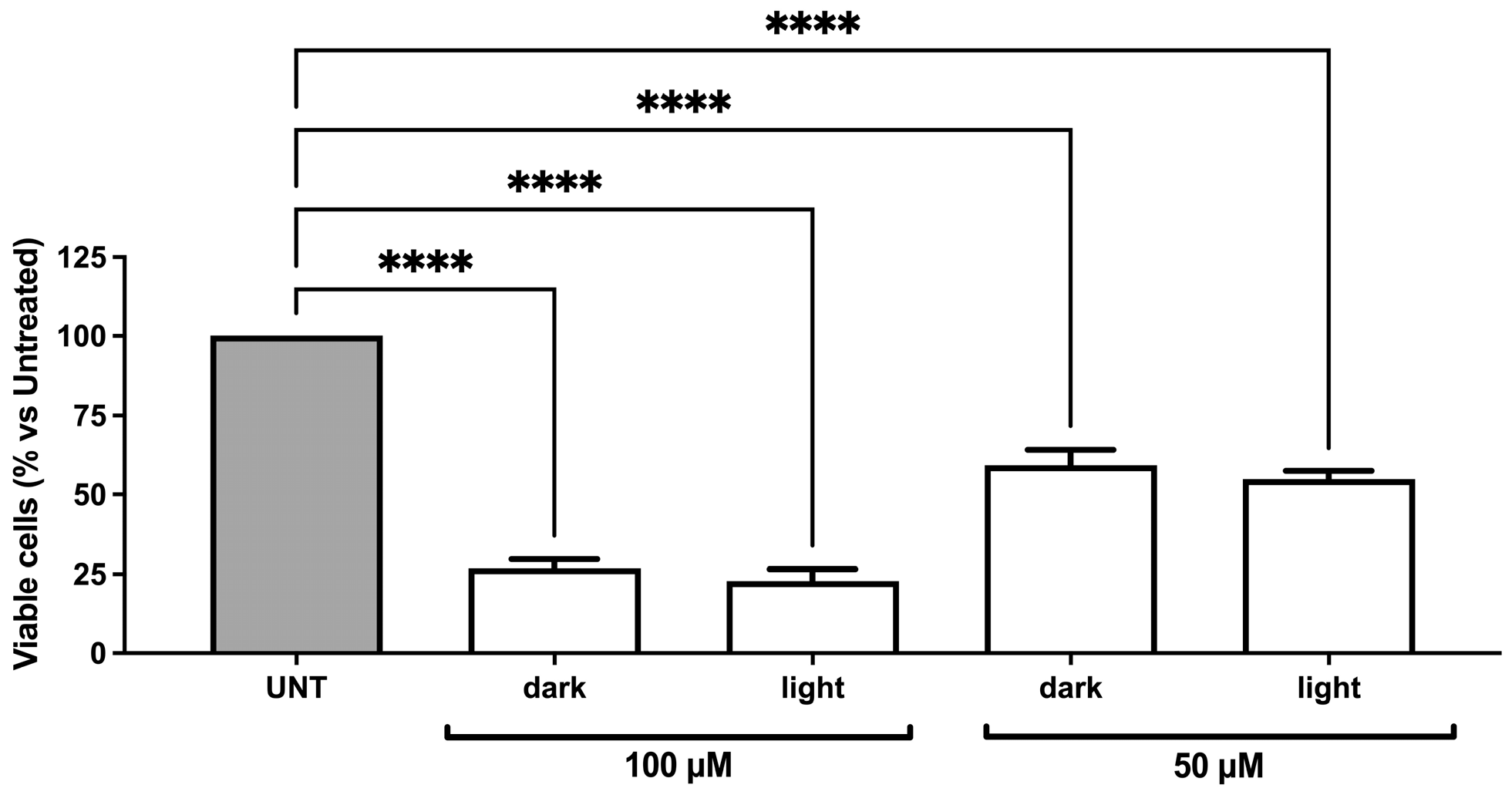
2.3.2. Evaluation of the Biological Activity of dU-t-NO on RKO Colon Carcinoma and dAdo-t-NO on RKO Colon Carcinoma and Hep3B2.1-7 Hepatocarcinoma Cell Lines after Irradiation
3. Discussion
4. Materials and Methods
4.1. Synthesis and Characterization
4.1.1. General
4.1.2. Synthesis of N-(5-Azidopentyl)-4-Nitro-3-(trifluoromethyl)aniline (2)
4.1.3. General Procedure for the Click Reaction
4.1.4. Chemical Stability of Hybrids
4.2. Photochemistry
4.2.1. Photochemical Quantum Yield Measurements
4.2.2. Irradiation Apparatus for In Vitro Experiments
4.2.3. Griess Assay
4.3. Biological Evaluation
4.3.1. Cell Lines
4.3.2. Evaluation of the Cytotoxic and Cytostatic Effects of the Hybrids
4.3.3. Evaluation of the Effects of Irradiation on dAdo-t-NO and dU-t-NO Effectiveness
4.3.4. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef] [PubMed]
- Xi, Y.; Xu, P. Global Colorectal Cancer Burden in 2020 and Projections to 2040. Transl. Oncol. 2021, 14, 101174. [Google Scholar] [CrossRef] [PubMed]
- Chidambaranathan-Reghupaty, S.; Fisher, P.B.; Sarkar, D. Hepatocellular Carcinoma (HCC): Epidemiology, Etiology and Molecular Classification. In Advances in Cancer Research; Academic Press Inc.: Cambridge, MA, USA, 2021; Volume 149, pp. 1–61. ISBN 9780128240304. [Google Scholar]
- Rumgay, H.; Arnold, M.; Ferlay, J.; Lesi, O.; Cabasag, C.J.; Vignat, J.; Laversanne, M.; McGlynn, K.A.; Soerjomataram, I. Global Burden of Primary Liver Cancer in 2020 and Predictions to 2040. J. Hepatol. 2022, 77, 1598–1606. [Google Scholar] [CrossRef]
- Longley, D.B.; Johnston, P.G. Molecular Mechanisms of Drug Resistance. J. Pathol. 2005, 205, 275–292. [Google Scholar] [CrossRef]
- Mokhtari, R.B.; Homayouni, T.S.; Baluch, N.; Morgatskaya, E.; Kumar, S.; Das, B.; Yeger, H. Combination Therapy in Combating Cancer. Oncotarget 2017, 8, 38022–38043. [Google Scholar] [CrossRef] [PubMed]
- Jin, H.; Wang, L.; Bernards, R. Rational Combinations of Targeted Cancer Therapies: Background, Advances and Challenges. Nat. Rev. Drug Discov. 2023, 22, 213–234. [Google Scholar] [CrossRef]
- Doostmohammadi, A.; Jooya, H.; Ghorbanian, K.; Gohari, S.; Dadashpour, M. Potentials and Future Perspectives of Multi-Target Drugs in Cancer Treatment: The next Generation Anti-Cancer Agents. Cell Commun. Signal. 2024, 22, 228. [Google Scholar] [CrossRef]
- Meunier, B. Hybrid Molecules with a Dual Mode of Action: Dream or Reality? Acc. Chem. Res. 2008, 41, 69–77. [Google Scholar] [CrossRef]
- Singh, A.K.; Kumar, A.; Singh, H.; Sonawane, P.; Paliwal, H.; Thareja, S.; Pathak, P.; Grishina, M.; Jaremko, M.; Emwas, A.-H.; et al. Concept of Hybrid Drugs and Recent Advancements in Anticancer Hybrids. Pharmaceuticals 2022, 15, 1071. [Google Scholar] [CrossRef]
- Gediya, L.K.; Njar, V.C. Promise and Challenges in Drug Discovery and Development of Hybrid Anticancer Drugs. Expert Opin. Drug Discov. 2009, 4, 1099–1111. [Google Scholar] [CrossRef]
- Szumilak, M.; Wiktorowska-Owczarek, A.; Stanczak, A. Hybrid Drugs—A Strategy for Overcoming Anticancer Drug Resistance? Molecules 2021, 26, 2601. [Google Scholar] [CrossRef] [PubMed]
- Zimmermann, G.R.; Lehár, J.; Keith, C.T. Multi-Target Therapeutics: When the Whole Is Greater than the Sum of the Parts. Drug Discov. Today 2007, 12, 34–42. [Google Scholar] [CrossRef] [PubMed]
- Shalini; Kumar, V. Have Molecular Hybrids Delivered Effective Anti-Cancer Treatments and What Should Future Drug Discovery Focus On? Expert Opin. Drug Discov. 2021, 16, 335–363. [Google Scholar] [CrossRef] [PubMed]
- Ignarro, L.J. Preface to This Special Journal Issue on Nitric Oxide Chemistry and Biology. Arch. Pharm. Res. 2009, 32, 1099–1101. [Google Scholar] [CrossRef] [PubMed]
- Korde Choudhari, S.; Chaudhary, M.; Bagde, S.; Gadbail, A.R.; Joshi, V. Nitric Oxide and Cancer: A Review. World J. Surg. Oncol. 2013, 11, 118. [Google Scholar] [CrossRef] [PubMed]
- Mintz, J.; Vedenko, A.; Rosete, O.; Shah, K.; Goldstein, G.; Hare, J.M.; Ramasamy, R.; Arora, H. Current Advances of Nitric Oxide in Cancer and Anticancer Therapeutics. Vaccines 2021, 9, 94. [Google Scholar] [CrossRef] [PubMed]
- Sinha, B.K. Can Nitric Oxide-Based Therapy Be Improved for the Treatment of Cancers? A Perspective. Int. J. Mol. Sci. 2023, 24, 13611. [Google Scholar] [CrossRef]
- Wink, D.A.; Mitchell, J.B. Chemical Biology of Nitric Oxide: Insights into Regulatory, Cytotoxic, and Cytoprotective Mechanisms of Nitric Oxide. Free Radic. Biol. Med. 1998, 25, 434–456. [Google Scholar] [CrossRef] [PubMed]
- Miranda, K.M.; Ridnour, L.A.; McGinity, C.L.; Bhattacharyya, D.; Wink, D.A. Nitric Oxide and Cancer: When to Give and When to Take Away? Inorg. Chem. 2021, 60, 15941–15947. [Google Scholar] [CrossRef]
- Poderoso, J.J.; Helfenberger, K.; Poderoso, C. The Effect of Nitric Oxide on Mitochondrial Respiration. Nitric Oxide 2019, 88, 61–72. [Google Scholar] [CrossRef]
- Moncada, S.; Erusalimsky, J.D. Does Nitric Oxide Modulate Mitochondrial Energy Generation and Apoptosis? Nat. Rev. Mol. Cell Biol. 2002, 3, 214–220. [Google Scholar] [CrossRef] [PubMed]
- Wink, D.A.; Vodovotz, Y.; Laval, J.; Laval, F.; Dewhirst, M.W.; Mitchell, J.B. The Multifaceted Roles of Nitric Oxide in Cancer. Carcinogenesis 1998, 19, 711–721. [Google Scholar] [CrossRef] [PubMed]
- Laneri, F.; Graziano, A.C.E.; Seggio, M.; Fraix, A.; Malanga, M.; Béni, S.; Longobardi, G.; Conte, C.; Quaglia, F.; Sortino, S. Enhancing the Anticancer Activity of Sorafenib through Its Combination with a Nitric Oxide Photodelivering β-Cyclodextrin Polymer. Molecules 2022, 27, 1918–1930. [Google Scholar] [CrossRef] [PubMed]
- Thomas, D.D.; Liu, X.; Kantrow, S.P.; Lancaster, J.R. The Biological Lifetime of Nitric Oxide: Implications for the Perivascular Dynamics of NO and O 2. Proc. Natl. Acad. Sci. USA 2001, 98, 355–360. [Google Scholar] [CrossRef] [PubMed]
- Roberts, J.D.; Fineman, J.R.; Morin, F.C.; Shaul, P.W.; Rimar, S.; Schreiber, M.D.; Polin, R.A.; Zwass, M.S.; Zayek, M.M.; Gross, I.; et al. Inhaled Nitric Oxide and Persistent Pulmonary Hypertension of the Newborn. N. Engl. J. Med. 1997, 336, 605–610. [Google Scholar] [CrossRef] [PubMed]
- Bloch, K.; Ichinose, F.; Robertsjr, J.; Zapol, W. Inhaled NO as a Therapeutic Agent. Cardiovasc. Res. 2007, 75, 339–348. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Jin, A.; Yang, Z.; Huang, W. Advanced Nitric Oxide Generating Nanomedicine for Therapeutic Applications. ACS Nano. 2023, 17, 8935–8965. [Google Scholar] [CrossRef] [PubMed]
- Huang, Z.; Fu, J.; Zhang, Y. Nitric Oxide Donor-Based Cancer Therapy: Advances and Prospects. J. Med. Chem. 2017, 60, 7617–7635. [Google Scholar] [CrossRef] [PubMed]
- Pieretti, J.C.; Pelegrino, M.T.; Nascimento, M.H.M.; Tortella, G.R.; Rubilar, O.; Seabra, A.B. Small Molecules for Great Solutions: Can Nitric Oxide-Releasing Nanomaterials Overcome Drug Resistance in Chemotherapy? Biochem. Pharmacol. 2020, 176, 113740. [Google Scholar] [CrossRef]
- Alimoradi, H.; Greish, K.; Gamble, A.B.; Giles, G.I. Controlled Delivery of Nitric Oxide for Cancer Therapy. Pharm. Nanotechnol. 2019, 7, 279–303. [Google Scholar] [CrossRef]
- Wimalawansa, S.J. Nitric Oxide: New Evidence for Novel Therapeutic Indications. Expert Opin. Pharmaco. 2008, 9, 1935–1954. [Google Scholar] [CrossRef] [PubMed]
- Sortino, S. Photoactivated Nanomaterials for Biomedical Release Applications. J. Mater. Chem. 2012, 22, 301–318. [Google Scholar] [CrossRef]
- Sharma, N.; Dhyani, A.K.; Marepally, S.; Jose, D.A. Nanoscale Lipid Vesicles Functionalized with a Nitro-Aniline Derivative for Photoinduced Nitric Oxide (NO) Delivery. Nanoscale Adv. 2020, 2, 463–469. [Google Scholar] [CrossRef] [PubMed]
- Navacchia, M.L.; Fraix, A.; Chinaglia, N.; Gallerani, E.; Perrone, D.; Cardile, V.; Graziano, A.C.E.; Capobianco, M.L.; Sortino, S. NO Photoreleaser-Deoxyadenosine and -Bile Acid Derivative Bioconjugates as Novel Potential Photochemotherapeutics. ACS Med. Chem. Lett. 2016, 7, 939–943. [Google Scholar] [CrossRef] [PubMed]
- Guinan, M.; Benckendorff, C.; Smith, M.; Miller, G.J. Recent Advances in the Chemical Synthesis and Evaluation of Anticancer Nucleoside Analogues. Molecules 2020, 25, 2050. [Google Scholar] [CrossRef]
- Perrone, D.; Marchesi, E.; Preti, L.; Navacchia, M.L. Modified Nucleosides, Nucleotides and Nucleic Acids via Click Azide-Alkyne Cycloaddition for Pharmacological Applications. Molecules 2021, 26, 3100. [Google Scholar] [CrossRef]
- Navacchia, M.L.; Marchesi, E.; Mari, L.; Chinaglia, N.; Gallerani, E.; Gavioli, R.; Capobianco, M.L.; Perrone, D. Rational Design of Nucleoside-Bile Acid Conjugates Incorporating a Triazole Moiety for Anticancer Evaluation and SAR Exploration. Molecules 2017, 22, 1710. [Google Scholar] [CrossRef]
- Perrone, D.; Bortolini, O.; Fogagnolo, M.; Marchesi, E.; Mari, L.; Massarenti, C.; Navacchia, M.L.; Sforza, F.; Varani, K.; Capobianco, M.L. Synthesis and in Vitro Cytotoxicity of Deoxyadenosine-Bile Acid Conjugates Linked with 1,2,3-Triazole. New J. Chem. 2013, 37, 3559–3567. [Google Scholar] [CrossRef]
- Xu, Z.; Zhao, S.-J.; Liu, Y. 1,2,3-Triazole-Containing Hybrids as Potential Anticancer Agents: Current Developments, Action Mechanisms and Structure-Activity Relationships. Eur. J. Med. Chem. 2019, 183, 111700. [Google Scholar] [CrossRef]
- Pasieka, A.; Diamanti, E.; Uliassi, E.; Laura Bolognesi, M. Click Chemistry and Targeted Degradation: A Winning Combination for Medicinal Chemists? ChemMedChem 2023, 18, e202300422. [Google Scholar] [CrossRef]
- Gupta, S.; Ameta, C.; Ameta, R.; Punjabi, P.B. Click Chemistry: A Tool for Green Chemical Organic Synthesis. In Green Sustainable Process for Chemical and Environmental Engineering and Science; Elsevier: Amsterdam, The Netherlands, 2020; pp. 13–48. [Google Scholar]
- Melloni, E.; Marchesi, E.; Preti, L.; Casciano, F.; Rimondi, E.; Romani, A.; Secchiero, P.; Navacchia, M.L.; Perrone, D. Synthesis and Biological Investigation of Bile Acid-Paclitaxel Hybrids. Molecules 2022, 27, 471. [Google Scholar] [CrossRef] [PubMed]
- Stochel, G.; Wanat, A.; Kuliś, E.; Stasicka, Z. Light and Metal Complexes in Medicine. Coord. Chem. Rev. 1998, 171, 203–220. [Google Scholar] [CrossRef]
- Bordini, J.; Hughes, D.L.; Da Motta Neto, J.D.; Jorge da Cunha, C. Nitric Oxide Photorelease from Ruthenium Salen Complexes in Aqueous and Organic Solutions. Inorg. Chem. 2002, 41, 5410–5416. [Google Scholar] [CrossRef] [PubMed]
- Fallica, A.N.; Barbaraci, C.; Amata, E.; Pasquinucci, L.; Turnaturi, R.; Dichiara, M.; Intagliata, S.; Gariboldi, M.B.; Marras, E.; Orlandi, V.T.; et al. Nitric Oxide Photo-Donor Hybrids of Ciprofloxacin and Norfloxacin: A Shift in Activity from Antimicrobial to Anticancer Agents. J. Med. Chem. 2021, 64, 11597–11613. [Google Scholar] [CrossRef]




| RKO | Hep 3B2.1-7 | |
|---|---|---|
| Compound | IC50 (μM) | IC50 (μM) |
| dAdo-S-NO | 10.39 ± 0.13 | 17.64 ± 2.19 |
| dAdo-t-NO | 27.61 ± 1.22 | 44.46 ± 3.45 |
| dU-t-NO | 51.74 ± 2.69 | 61.66 ± 5.29 |
| Paclitaxel | 5.01 × 10−3 ± 0.74 × 10−3 | 0.41 ± 0.12 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Marchesi, E.; Melloni, E.; Casciano, F.; Pozza, E.; Argazzi, R.; De Risi, C.; Preti, L.; Perrone, D.; Navacchia, M.L. Evaluation of Anticancer Activity of Nucleoside–Nitric Oxide Photo-Donor Hybrids. Molecules 2024, 29, 3383. https://doi.org/10.3390/molecules29143383
Marchesi E, Melloni E, Casciano F, Pozza E, Argazzi R, De Risi C, Preti L, Perrone D, Navacchia ML. Evaluation of Anticancer Activity of Nucleoside–Nitric Oxide Photo-Donor Hybrids. Molecules. 2024; 29(14):3383. https://doi.org/10.3390/molecules29143383
Chicago/Turabian StyleMarchesi, Elena, Elisabetta Melloni, Fabio Casciano, Elena Pozza, Roberto Argazzi, Carmela De Risi, Lorenzo Preti, Daniela Perrone, and Maria Luisa Navacchia. 2024. "Evaluation of Anticancer Activity of Nucleoside–Nitric Oxide Photo-Donor Hybrids" Molecules 29, no. 14: 3383. https://doi.org/10.3390/molecules29143383
APA StyleMarchesi, E., Melloni, E., Casciano, F., Pozza, E., Argazzi, R., De Risi, C., Preti, L., Perrone, D., & Navacchia, M. L. (2024). Evaluation of Anticancer Activity of Nucleoside–Nitric Oxide Photo-Donor Hybrids. Molecules, 29(14), 3383. https://doi.org/10.3390/molecules29143383