Mechanisms of Flavonoids and Their Derivatives in Endothelial Dysfunction Induced by Oxidative Stress in Diabetes
Abstract
1. Introduction
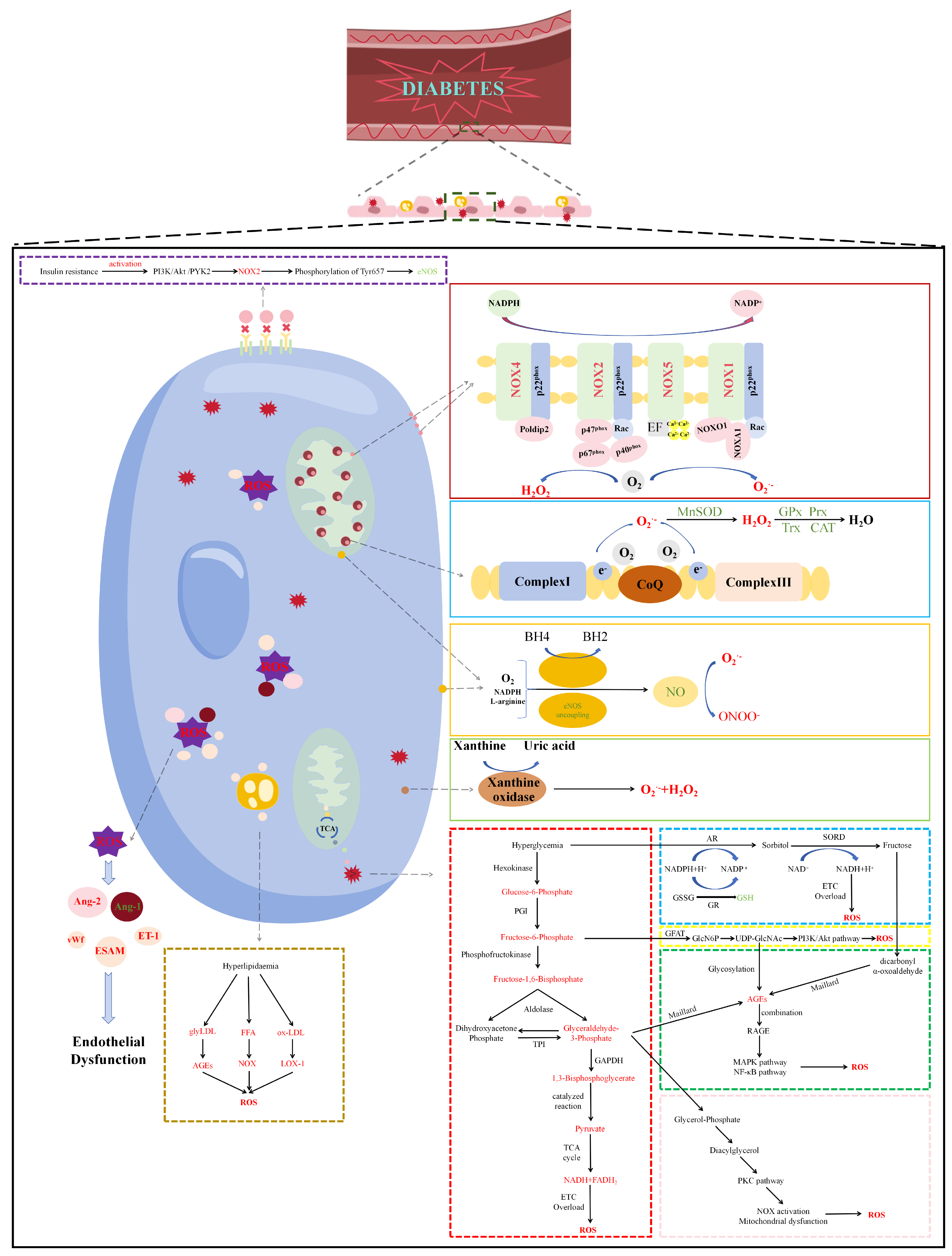
2. The Generation of Diabetes-Induced Oxidative Stress in Endothelial Cells
2.1. Mitochondrial-Derived Reactive Oxygen Species in Diabetes
2.2. NADPH Oxidase-Derived Reactive Oxygen Species in Diabetes
2.3. Uncoupled eNOS-Derived Reactive Oxygen Species in Diabetes
2.4. Reactive Oxygen Species Derived from other Enzymes in Diabetes
2.5. Attenuation of Antioxidant Defense Systems in Diabetes
3. Mechanisms of Oxidative Stress-Induced Endothelial Dysfunction in Diabetes
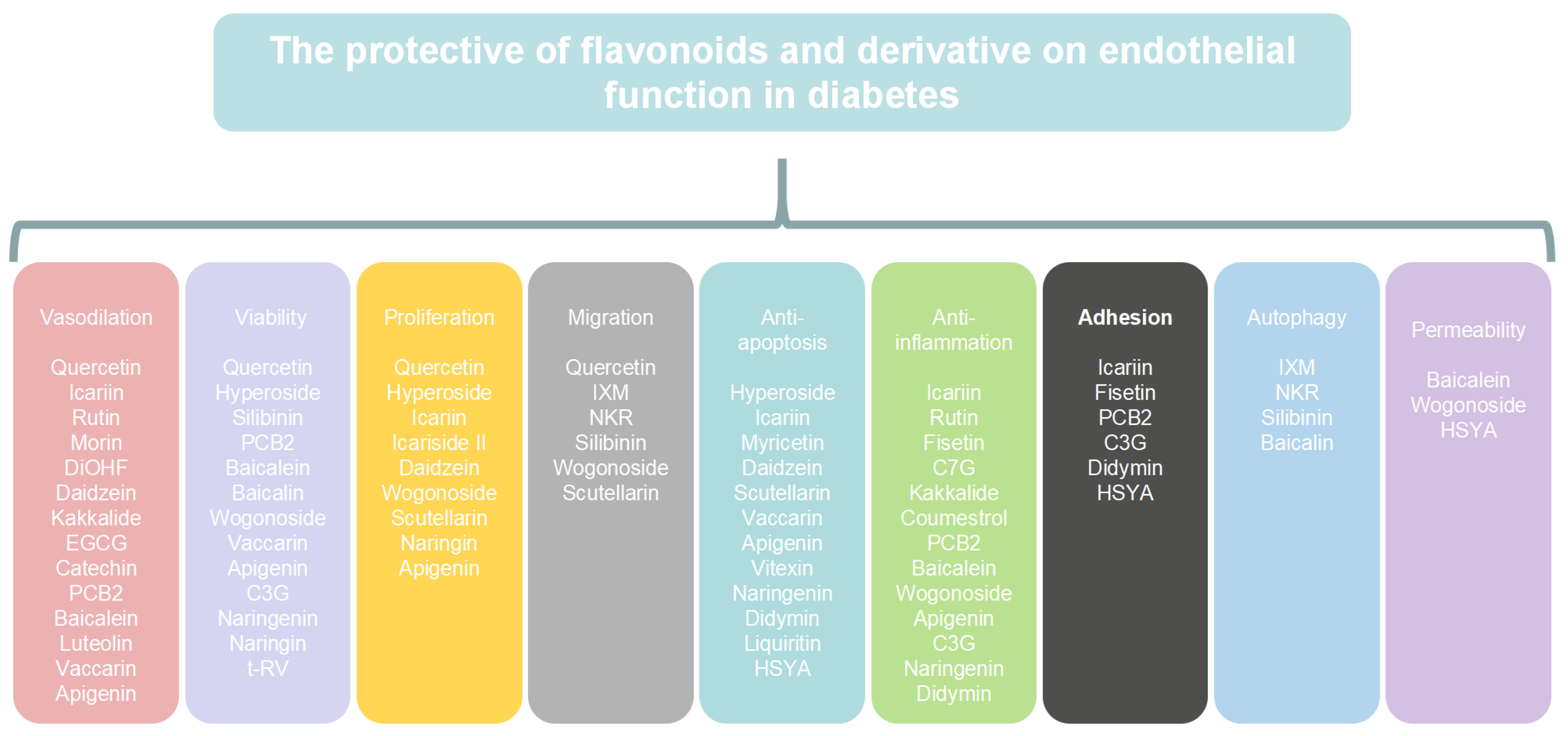
4. The Mechanism of Flavonoids and Their Derivatives in Endothelial Dysfunction in Diabetes
4.1. Flavonols
4.1.1. Quercetin
4.1.2. Hyperoside
4.1.3. Icariin
4.1.4. Myricetin
4.1.5. Rutin
4.1.6. Fisetin
4.1.7. Morin
4.1.8. 3′,4′Dihydroxyflavonol
4.2. Isoflavones
4.2.1. Daidzein
4.2.2. Puerarin
4.2.3. Calycosin-7-O-β-D-Glucopyranoside
4.2.4. Kakkalide
4.2.5. Coumestrol
4.3. Flavanols
4.3.1. Dihydromyricetin
4.3.2. Silibinin
4.3.3. (–)-Epicatechin
4.3.4. Catechin
4.3.5. Proanthocyanidis
4.4. Flavones
4.4.1. Baicalein
4.4.2. Luteolin
4.4.3. Vaccarin
4.4.4. Apigenin
4.5. Anthocyanins
4.5.1. Cyanidin
4.5.2. Malvidin
4.6. Flavanones
4.6.1. Naringenin
4.6.2. Trans-Resveratrol
4.6.3. Didymin
4.6.4. Liquiritin
4.6.5. Isoxanthohumol and Norkurarinone
4.7. Chalcones
Hydroxysafflor Yellow A
4.8. Deficiencies and Suggestions Regarding the above Study
5. Challenges and Perspectives Using Flavonoids and Their Derivatives to Treat Diabetic Complications
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- International Diabetes Federation IDF Diabetes Atlas|Tenth Edition. Available online: https://diabetesatlas.org/ (accessed on 1 May 2023).
- Ramanathan, T.; Skinner, H. Coronary Blood Flow. Contin. Educ. Anaesth. Crit. Care Pain 2005, 5, 61–64. [Google Scholar] [CrossRef]
- Westein, E.; Hoefer, T.; Calkin, A.C. Thrombosis in Diabetes: A Shear Flow Effect? Clin. Sci. Lond. Engl. 1979 2017, 131, 1245–1260. [Google Scholar] [CrossRef] [PubMed]
- Xu, S.; Ilyas, I.; Little, P.J.; Li, H.; Kamato, D.; Zheng, X.; Luo, S.; Li, Z.; Liu, P.; Han, J.; et al. Endothelial Dysfunction in Atherosclerotic Cardiovascular Diseases and Beyond: From Mechanism to Pharmacotherapies. Pharmacol. Rev. 2021, 73, 924–967. [Google Scholar] [CrossRef] [PubMed]
- Incalza, M.A.; D’Oria, R.; Natalicchio, A.; Perrini, S.; Laviola, L.; Giorgino, F. Oxidative Stress and Reactive Oxygen Species in Endothelial Dysfunction Associated with Cardiovascular and Metabolic Diseases. Vascul. Pharmacol. 2018, 100, 1–19. [Google Scholar] [CrossRef] [PubMed]
- Shaito, A.; Aramouni, K.; Assaf, R.; Parenti, A.; Orekhov, A.; Yazbi, A.E.; Pintus, G.; Eid, A.H. Oxidative Stress-Induced Endothelial Dysfunction in Cardiovascular Diseases. Front. Biosci. Landmark Ed. 2022, 27, 105. [Google Scholar] [CrossRef] [PubMed]
- Mittal, M.; Siddiqui, M.R.; Tran, K.; Reddy, S.P.; Malik, A.B. Reactive Oxygen Species in Inflammation and Tissue Injury. Antioxid. Redox Signal. 2014, 20, 1126–1167. [Google Scholar] [CrossRef]
- Al-Ishaq, R.K.; Abotaleb, M.; Kubatka, P.; Kajo, K.; Büsselberg, D. Flavonoids and Their Anti-Diabetic Effects: Cellular Mechanisms and Effects to Improve Blood Sugar Levels. Biomolecules 2019, 9, 430. [Google Scholar] [CrossRef] [PubMed]
- Gandhi, G.R.; Vasconcelos, A.B.S.; Wu, D.-T.; Li, H.-B.; Antony, P.J.; Li, H.; Geng, F.; Gurgel, R.Q.; Narain, N.; Gan, R.-Y. Citrus Flavonoids as Promising Phytochemicals Targeting Diabetes and Related Complications: A Systematic Review of In Vitro and In Vivo Studies. Nutrients 2020, 12, 2907. [Google Scholar] [CrossRef] [PubMed]
- Zibadi, S.; Rohdewald, P.J.; Park, D.; Watson, R.R. Reduction of Cardiovascular Risk Factors in Subjects with Type 2 Diabetes by Pycnogenol Supplementation. Nutr. Res. 2008, 28, 315–320. [Google Scholar] [CrossRef]
- Shen, N.; Wang, T.; Gan, Q.; Liu, S.; Wang, L.; Jin, B. Plant Flavonoids: Classification, Distribution, Biosynthesis, and Antioxidant Activity. Food Chem. 2022, 383, 132531. [Google Scholar] [CrossRef]
- Tabit, C.E.; Chung, W.B.; Hamburg, N.M.; Vita, J.A. Endothelial Dysfunction in Diabetes Mellitus: Molecular Mechanisms and Clinical Implications. Rev. Endocr. Metab. Disord. 2010, 11, 61–74. [Google Scholar] [CrossRef]
- Shenouda, S.M.; Widlansky, M.E.; Chen, K.; Xu, G.; Holbrook, M.; Tabit, C.E.; Hamburg, N.M.; Frame, A.A.; Caiano, T.L.; Kluge, M.A.; et al. Altered Mitochondrial Dynamics Contributes to Endothelial Dysfunction in Diabetes Mellitus. Circulation 2011, 124, 444–453. [Google Scholar] [CrossRef]
- Handy, D.E.; Loscalzo, J. Redox Regulation of Mitochondrial Function. Antioxid. Redox Signal. 2012, 16, 1323–1367. [Google Scholar] [CrossRef]
- Wang, J.; Toan, S.; Zhou, H. New Insights into the Role of Mitochondria in Cardiac Microvascular Ischemia/Reperfusion Injury. Angiogenesis 2020, 23, 299–314. [Google Scholar] [CrossRef] [PubMed]
- Gerő, D.; Torregrossa, R.; Perry, A.; Waters, A.; Le-Trionnaire, S.; Whatmore, J.L.; Wood, M.; Whiteman, M. The Novel Mitochondria-Targeted Hydrogen Sulfide (H2S) Donors AP123 and AP39 Protect against Hyperglycemic Injury in Microvascular Endothelial Cells in Vitro. Pharmacol. Res. 2016, 113, 186–198. [Google Scholar] [CrossRef]
- Keller, A.; Hull, S.E.; Elajaili, H.; Johnston, A.; Knaub, L.A.; Chun, J.H.; Walker, L.; Nozik-Grayck, E.; Reusch, J.E.B. (−)-Epicatechin Modulates Mitochondrial Redox in Vascular Cell Models of Oxidative Stress. Oxid. Med. Cell. Longev. 2020, 2020, 6392629. [Google Scholar] [CrossRef]
- Xie, X.; Zhao, R.; Shen, G.X. Impact of Cyanidin-3-Glucoside on Glycated LDL-Induced NADPH Oxidase Activation, Mitochondrial Dysfunction and Cell Viability in Cultured Vascular Endothelial Cells. Int. J. Mol. Sci. 2012, 13, 15867–15880. [Google Scholar] [CrossRef] [PubMed]
- Roy Chowdhury, S.K.; Sangle, G.V.; Xie, X.; Stelmack, G.L.; Halayko, A.J.; Shen, G.X. Effects of Extensively Oxidized Low-Density Lipoprotein on Mitochondrial Function and Reactive Oxygen Species in Porcine Aortic Endothelial Cells. Am. J. Physiol. Endocrinol. Metab. 2010, 298, E89–E98. [Google Scholar] [CrossRef] [PubMed]
- Sangle, G.V.; Chowdhury, S.K.R.; Xie, X.; Stelmack, G.L.; Halayko, A.J.; Shen, G.X. Impairment of Mitochondrial Respiratory Chain Activity in Aortic Endothelial Cells Induced by Glycated Low-Density Lipoprotein. Free Radic. Biol. Med. 2010, 48, 781–790. [Google Scholar] [CrossRef]
- Faria, A.; Persaud, S.J. Cardiac Oxidative Stress in Diabetes: Mechanisms and Therapeutic Potential. Pharmacol. Ther. 2017, 172, 50–62. [Google Scholar] [CrossRef]
- Gorin, Y.; Block, K. Nox as a Target for Diabetic Complications. Clin. Sci. 2013, 125, 361–382. [Google Scholar] [CrossRef]
- Huang, X.; Sun, M.; Li, D.; Liu, J.; Guo, H.; Dong, Y.; Jiang, L.; Pan, Q.; Man, Y.; Wang, S.; et al. Augmented NADPH Oxidase Activity and P22phox Expression in Monocytes Underlie Oxidative Stress of Patients with Type 2 Diabetes Mellitus. Diabetes Res. Clin. Pract. 2011, 91, 371–380. [Google Scholar] [CrossRef] [PubMed]
- Taye, A.; Saad, A.H.; Kumar, A.H.; Morawietz, H. Effect of Apocynin on NADPH Oxidase-Mediated Oxidative Stress-LOX-1-eNOS Pathway in Human Endothelial Cells Exposed to High Glucose. Eur. J. Pharmacol. 2010, 627, 42–48. [Google Scholar] [CrossRef] [PubMed]
- Chen, F.; Qian, L.-H.; Deng, B.; Liu, Z.-M.; Zhao, Y.; Le, Y.-Y. Resveratrol Protects Vascular Endothelial Cells from High Glucose-Induced Apoptosis through Inhibition of NADPH Oxidase Activation-Driven Oxidative Stress. CNS Neurosci. Ther. 2013, 19, 675–681. [Google Scholar] [CrossRef]
- Williams, C.R.; Lu, X.; Sutliff, R.L.; Hart, C.M. Rosiglitazone Attenuates NF-κB-Mediated Nox4 Upregulation in Hyperglycemia-Activated Endothelial Cells. Am. J. Physiol.-Cell Physiol. 2012, 303, C213–C223. [Google Scholar] [CrossRef]
- Ceolotto, G.; Gallo, A.; Papparella, I.; Franco, L.; Murphy, E.; Iori, E.; Pagnin, E.; Fadini, G.P.; Albiero, M.; Semplicini, A.; et al. Rosiglitazone Reduces Glucose-Induced Oxidative Stress Mediated by NAD(P)H Oxidase via AMPK-Dependent Mechanism. Arterioscler. Thromb. Vasc. Biol. 2007, 27, 2627–2633. [Google Scholar] [CrossRef] [PubMed]
- Christ, M.; Bauersachs, J.; Liebetrau, C.; Heck, M.; Günther, A.; Wehling, M. Glucose Increases Endothelial-Dependent Superoxide Formation in Coronary Arteries by NAD(P)H Oxidase Activation: Attenuation by the 3-Hydroxy-3-Methylglutaryl Coenzyme A Reductase Inhibitor Atorvastatin. Diabetes 2002, 51, 2648–2652. [Google Scholar] [CrossRef] [PubMed]
- Quagliaro, L.; Piconi, L.; Assaloni, R.; Martinelli, L.; Motz, E.; Ceriello, A. Intermittent High Glucose Enhances Apoptosis Related to Oxidative Stress in Human Umbilical Vein Endothelial Cells: The Role of Protein Kinase C and NAD(P)H-Oxidase Activation. Diabetes 2003, 52, 2795–2804. [Google Scholar] [CrossRef]
- Jha, J.C.; Dai, A.; Holterman, C.E.; Cooper, M.E.; Touyz, R.M.; Kennedy, C.R.; Jandeleit-Dahm, K.A.M. Endothelial or Vascular Smooth Muscle Cell-Specific Expression of Human NOX5 Exacerbates Renal Inflammation, Fibrosis and Albuminuria in the Akita Mouse. Diabetologia 2019, 62, 1712–1726. [Google Scholar] [CrossRef]
- Chen, F.; Yu, Y.; Haigh, S.; Johnson, J.; Lucas, R.; Stepp, D.W.; Fulton, D.J.R. Regulation of NADPH Oxidase 5 by Protein Kinase C Isoforms. PLoS ONE 2014, 9, e88405. [Google Scholar] [CrossRef]
- Wendt, M.C.; Daiber, A.; Kleschyov, A.L.; Mülsch, A.; Sydow, K.; Schulz, E.; Chen, K.; Keaney, J.F.; Lassègue, B.; Walter, U.; et al. Differential Effects of Diabetes on the Expression of the Gp91phox Homologues Nox1 and Nox4. Free Radic. Biol. Med. 2005, 39, 381–391. [Google Scholar] [CrossRef] [PubMed]
- Youn, J.Y.; Gao, L.; Cai, H. The P47phox- and NADPH Oxidase Organiser 1 (NOXO1)-Dependent Activation of NADPH Oxidase 1 (NOX1) Mediates Endothelial Nitric Oxide Synthase (eNOS) Uncoupling and Endothelial Dysfunction in a Streptozotocin-Induced Murine Model of Diabetes. Diabetologia 2012, 55, 2069–2079. [Google Scholar] [CrossRef] [PubMed]
- Sukumar, P.; Viswambharan, H.; Imrie, H.; Cubbon, R.M.; Yuldasheva, N.; Gage, M.; Galloway, S.; Skromna, A.; Kandavelu, P.; Santos, C.X.; et al. Nox2 NADPH Oxidase Has a Critical Role in Insulin Resistance-Related Endothelial Cell Dysfunction. Diabetes 2013, 62, 2130–2134. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Li, X.; Zhang, Y.; Huang, Y.; Zhang, Y.; Ma, Q. Oxymatrine Ameliorates Diabetes-Induced Aortic Endothelial Dysfunction via the Regulation of eNOS and NOX4. J. Cell. Biochem. 2019, 120, 7323–7332. [Google Scholar] [CrossRef] [PubMed]
- Förstermann, U.; Münzel, T. Endothelial Nitric Oxide Synthase in Vascular Disease: From Marvel to Menace. Circulation 2006, 113, 1708–1714. [Google Scholar] [CrossRef] [PubMed]
- Hink, U.; Li, H.; Mollnau, H.; Oelze, M.; Matheis, E.; Hartmann, M.; Skatchkov, M.; Thaiss, F.; Stahl, R.A.; Warnholtz, A.; et al. Mechanisms Underlying Endothelial Dysfunction in Diabetes Mellitus. Circ. Res. 2001, 88, E14–E22. [Google Scholar] [CrossRef] [PubMed]
- Daiber, A.; Chlopicki, S. Revisiting Pharmacology of Oxidative Stress and Endothelial Dysfunction in Cardiovascular Disease: Evidence for Redox-Based Therapies. Free Radic. Biol. Med. 2020, 157, 15–37. [Google Scholar] [CrossRef] [PubMed]
- Chis, I.; Coseriu, A.; Simedrea, R.; Oros, A.; Nagy, A.; Clichici, S. In Vivo Effects of Quercetin in Association with Moderate Exercise Training in Improving Streptozotocin-Induced Aortic Tissue Injuries. Molecules 2015, 20, 21770–21786. [Google Scholar] [CrossRef] [PubMed]
- Taguchi, K.; Hida, M.; Hasegawa, M.; Matsumoto, T.; Kobayashi, T. Dietary Polyphenol Morin Rescues Endothelial Dysfunction in a Diabetic Mouse Model by Activating the Akt/eNOS Pathway. Mol. Nutr. Food Res. 2016, 60, 580–588. [Google Scholar] [CrossRef]
- Taguchi, K.; Tano, I.; Kaneko, N.; Matsumoto, T.; Kobayashi, T. Plant Polyphenols Morin and Quercetin Rescue Nitric Oxide Production in Diabetic Mouse Aorta through Distinct Pathways. Biomed. Pharmacother. 2020, 129, 110463. [Google Scholar] [CrossRef]
- Pacher, P.; Nivorozhkin, A.; Szabó, C. Therapeutic Effects of Xanthine Oxidase Inhibitors: Renaissance Half a Century after the Discovery of Allopurinol. Pharmacol. Rev. 2006, 58, 87–114. [Google Scholar] [CrossRef]
- Hasan, M.; Fariha, K.A.; Barman, Z.; Mou, A.D.; Miah, R.; Habib, A.; Tuba, H.R.; Ali, N. Assessment of the Relationship between Serum Xanthine Oxidase Levels and Type 2 Diabetes: A Cross-Sectional Study. Sci. Rep. 2022, 12, 20816. [Google Scholar] [CrossRef]
- Washio, K.; Kusunoki, Y.; Tsunoda, T.; Osugi, K.; Ohigashi, M.; Murase, T.; Nakamura, T.; Matsuo, T.; Konishi, K.; Katsuno, T.; et al. Xanthine Oxidoreductase Activity Correlates with Vascular Endothelial Dysfunction in Patients with Type 1 Diabetes. Acta Diabetol. 2020, 57, 31–39. [Google Scholar] [CrossRef] [PubMed]
- Okuyama, T.; Shirakawa, J.; Nakamura, T.; Murase, T.; Miyashita, D.; Inoue, R.; Kyohara, M.; Togashi, Y.; Terauchi, Y. Association of the Plasma Xanthine Oxidoreductase Activity with the Metabolic Parameters and Vascular Complications in Patients with Type 2 Diabetes. Sci. Rep. 2021, 11, 3768. [Google Scholar] [CrossRef]
- Butler, R.; Morris, A.D.; Belch, J.J.; Hill, A.; Struthers, A.D. Allopurinol Normalizes Endothelial Dysfunction in Type 2 Diabetics with Mild Hypertension. Hypertension 2000, 35, 746–751. [Google Scholar] [CrossRef]
- Desco, M.-C.; Asensi, M.; Márquez, R.; Martínez-Valls, J.; Vento, M.; Pallardó, F.V.; Sastre, J.; Viña, J. Xanthine Oxidase Is Involved in Free Radical Production in Type 1 Diabetes: Protection by Allopurinol. Diabetes 2002, 51, 1118–1124. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.-D.; Yang, Y.-Y. Ferroptosis as a Novel Therapeutic Target for Diabetes and Its Complications. Front. Endocrinol. 2022, 13, 853822. [Google Scholar] [CrossRef] [PubMed]
- Luo, E.-F.; Li, H.-X.; Qin, Y.-H.; Qiao, Y.; Yan, G.-L.; Yao, Y.-Y.; Li, L.-Q.; Hou, J.-T.; Tang, C.-C.; Wang, D. Role of Ferroptosis in the Process of Diabetes-Induced Endothelial Dysfunction. World J. Diabetes 2021, 12, 124–137. [Google Scholar] [CrossRef]
- Taguchi, K.; Okudaira, K.; Matsumoto, T.; Kobayashi, T. Ginkgolide B Caused the Activation of the Akt/eNOS Pathway through the Antioxidant Effect of SOD1 in the Diabetic Aorta. Pflugers Arch. 2023, 475, 453–463. [Google Scholar] [CrossRef]
- Juarez, J.C.; Manuia, M.; Burnett, M.E.; Betancourt, O.; Boivin, B.; Shaw, D.E.; Tonks, N.K.; Mazar, A.P.; Doñate, F. Superoxide Dismutase 1 (SOD1) Is Essential for H2O2-Mediated Oxidation and Inactivation of Phosphatases in Growth Factor Signaling. Proc. Natl. Acad. Sci. USA 2008, 105, 7147–7152. [Google Scholar] [CrossRef]
- Morikawa, K.; Shimokawa, H.; Matoba, T.; Kubota, H.; Akaike, T.; Talukder, M.A.H.; Hatanaka, M.; Fujiki, T.; Maeda, H.; Takahashi, S.; et al. Pivotal Role of Cu,Zn-Superoxide Dismutase in Endothelium-Dependent Hyperpolarization. J. Clin. Investig. 2003, 112, 1871–1879. [Google Scholar] [CrossRef] [PubMed]
- Connor, K.M.; Subbaram, S.; Regan, K.J.; Nelson, K.K.; Mazurkiewicz, J.E.; Bartholomew, P.J.; Aplin, A.E.; Tai, Y.-T.; Aguirre-Ghiso, J.; Flores, S.C.; et al. Mitochondrial H2O2 Regulates the Angiogenic Phenotype via PTEN Oxidation. J. Biol. Chem. 2005, 280, 16916–16924. [Google Scholar] [CrossRef] [PubMed]
- Hua, Y.-Y.; Zhang, Y.; Gong, W.-W.; Ding, Y.; Shen, J.-R.; Li, H.; Chen, Y.; Meng, G.-L. Dihydromyricetin Improves Endothelial Dysfunction in Diabetic Mice via Oxidative Stress Inhibition in a SIRT3-Dependent Manner. Int. J. Mol. Sci. 2020, 21, 6699. [Google Scholar] [CrossRef] [PubMed]
- Xi, J.; Rong, Y.; Zhao, Z.; Huang, Y.; Wang, P.; Luan, H.; Xing, Y.; Li, S.; Liao, J.; Dai, Y.; et al. Scutellarin Ameliorates High Glucose-Induced Vascular Endothelial Cells Injury by Activating PINK1/Parkin-Mediated Mitophagy. J. Ethnopharmacol. 2021, 271, 113855. [Google Scholar] [CrossRef] [PubMed]
- Leff, J.A.; Oppegard, M.A.; Terada, L.S.; McCarty, E.C.; Repine, J.E. Human Serum Catalase Decreases Endothelial Cell Injury from Hydrogen Peroxide. J. Appl. Physiol. 1991, 71, 1903–1906. [Google Scholar] [CrossRef] [PubMed]
- Margis, R.; Dunand, C.; Teixeira, F.K.; Margis-Pinheiro, M. Glutathione Peroxidase Family - an Evolutionary Overview. FEBS J. 2008, 275, 3959–3970. [Google Scholar] [CrossRef]
- Rezabakhsh, A.; Rahbarghazi, R.; Malekinejad, H.; Fathi, F.; Montaseri, A.; Garjani, A. Quercetin Alleviates High Glucose-Induced Damage on Human Umbilical Vein Endothelial Cells by Promoting Autophagy. Phytomedicine 2019, 56, 183–193. [Google Scholar] [CrossRef]
- Park, M.H.; Ju, J.-W.; Kim, M.; Han, J.-S. The Protective Effect of Daidzein on High Glucose-Induced Oxidative Stress in Human Umbilical Vein Endothelial Cells. Z. Naturforschung C J. Biosci. 2016, 71, 21–28. [Google Scholar] [CrossRef]
- Rezabakhsh, A.; Fathi, F.; Bagheri, H.S.; Malekinejad, H.; Montaseri, A.; Rahbarghazi, R.; Garjani, A. Silibinin Protects Human Endothelial Cells from High Glucose-Induced Injury by Enhancing Autophagic Response. J. Cell. Biochem. 2018, 119, 8084–8094. [Google Scholar] [CrossRef]
- Sharma, P.; Aggarwal, K.; Awasthi, R.; Kulkarni, G.T.; Sharma, B. Behavioral and Biochemical Investigations to Explore the Efficacy of Quercetin and Folacin in Experimental Diabetes Induced Vascular Endothelium Dysfunction and Associated Dementia in Rats. J. Basic Clin. Physiol. Pharmacol. 2021, 0, 000010151520200159. [Google Scholar] [CrossRef]
- El Eter, E.; Al Masri, A.; Habib, S.; Al Zamil, H.; Al Hersi, A.; Al Hussein, F.; Al Omran, M. Novel Links among Peroxiredoxins, Endothelial Dysfunction, and Severity of Atherosclerosis in Type 2 Diabetic Patients with Peripheral Atherosclerotic Disease. Cell Stress Chaperones 2014, 19, 173–181. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.R.; Zhu, H.; Danelisen, I. Role of Peroxiredoxins in Protecting Against Cardiovascular and Related Disorders. Cardiovasc. Toxicol. 2020, 20, 448–453. [Google Scholar] [CrossRef]
- Nakamura, T.; Nakamura, H.; Hoshino, T.; Ueda, S.; Wada, H.; Yodoi, J. Redox Regulation of Lung Inflammation by Thioredoxin. Antioxid. Redox Signal. 2005, 7, 60–71. [Google Scholar] [CrossRef] [PubMed]
- Chong, C.-R.; Chan, W.P.A.; Nguyen, T.H.; Liu, S.; Procter, N.E.K.; Ngo, D.T.; Sverdlov, A.L.; Chirkov, Y.Y.; Horowitz, J.D. Thioredoxin-Interacting Protein: Pathophysiology and Emerging Pharmacotherapeutics in Cardiovascular Disease and Diabetes. Cardiovasc. Drugs Ther. 2014, 28, 347–360. [Google Scholar] [CrossRef] [PubMed]
- Haendeler, J.; Popp, R.; Goy, C.; Tischler, V.; Zeiher, A.M.; Dimmeler, S. Cathepsin D and H2O2 Stimulate Degradation of Thioredoxin-1: Implication for Endothelial Cell Apoptosis. J. Biol. Chem. 2005, 280, 42945–42951. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Kover, K.L.; Heruth, D.P.; Watkins, D.J.; Guo, Y.; Moore, W.V.; He, L.G.; Zang, M.; Clements, M.A.; Yan, Y. Thioredoxin-Interacting Protein Promotes High-Glucose-Induced Macrovascular Endothelial Dysfunction. Biochem. Biophys. Res. Commun. 2017, 493, 291–297. [Google Scholar] [CrossRef] [PubMed]
- Dunn, L.L.; Simpson, P.J.L.; Prosser, H.C.; Lecce, L.; Yuen, G.S.C.; Buckle, A.; Sieveking, D.P.; Vanags, L.Z.; Lim, P.R.; Chow, R.W.Y.; et al. A Critical Role for Thioredoxin-Interacting Protein in Diabetes-Related Impairment of Angiogenesis. Diabetes 2014, 63, 675–687. [Google Scholar] [CrossRef] [PubMed]
- Lam, Y.T.; Tan, R.P.; Michael, P.; Yang, N.; Dunn, L.L.; Cooke, J.P.; Celermajer, D.S.; Wise, S.G.; Ng, M.K.C. Endothelial Thioredoxin Interacting Protein (TXNIP) Modulates Endothelium-Dependent Vasorelaxation in Hyperglycemia. Microvasc. Res. 2022, 143, 104396. [Google Scholar] [CrossRef] [PubMed]
- Idris-Khodja, N.; Ouerd, S.; Mian, M.O.R.; Gornitsky, J.; Barhoumi, T.; Paradis, P.; Schiffrin, E.L. Endothelin-1 Overexpression Exaggerates Diabetes-Induced Endothelial Dysfunction by Altering Oxidative Stress. Am. J. Hypertens. 2016, 29, 1245–1251. [Google Scholar] [CrossRef]
- Ouerd, S.; Idris-Khodja, N.; Trindade, M.; Ferreira, N.S.; Berillo, O.; Coelho, S.C.; Neves, M.F.; Jandeleit-Dahm, K.A.; Paradis, P.; Schiffrin, E.L. Endothelium-Restricted Endothelin-1 Overexpression in Type 1 Diabetes Worsens Atherosclerosis and Immune Cell Infiltration via NOX1. Cardiovasc. Res. 2021, 117, 1144–1153. [Google Scholar] [CrossRef]
- Binjawhar, D.N.; Alhazmi, A.T.; Bin Jawhar, W.N.; MohammedSaeed, W.; Safi, S.Z. Hyperglycemia-Induced Oxidative Stress and Epigenetic Regulation of ET-1 Gene in Endothelial Cells. Front. Genet. 2023, 14, 1167773. [Google Scholar] [CrossRef] [PubMed]
- Issa, E.; Moss, A.J.; Fischer, M.; Kang, M.; Ahmed, S.; Farah, H.; Bate, N.; Giakomidi, D.; Brindle, N.P. Development of an Orthogonal Tie2 Ligand Resistant to Inhibition by Ang2. Mol. Pharm. 2018, 15, 3962–3968. [Google Scholar] [CrossRef] [PubMed]
- Akwii, R.G.; Sajib, M.S.; Zahra, F.T.; Mikelis, C.M. Role of Angiopoietin-2 in Vascular Physiology and Pathophysiology. Cells 2019, 8, 471. [Google Scholar] [CrossRef] [PubMed]
- Puddu, A.; Sanguineti, R.; Maggi, D.; Nicolò, M.; Traverso, C.E.; Cordera, R.; Viviani, G.L. Advanced Glycation End-Products and Hyperglycemia Increase Angiopoietin-2 Production by Impairing Angiopoietin-1-Tie-2 System. J. Diabetes Res. 2019, 2019, 6198495. [Google Scholar] [CrossRef]
- Peng, X.; Wang, X.; Fan, M.; Zhao, J.; Lin, L.; Liu, J. Plasma Levels of von Willebrand Factor in Type 2 Diabetes Patients with and without Cardiovascular Diseases: A Meta-Analysis. Diabetes Metab. Res. Rev. 2020, 36, e3193. [Google Scholar] [CrossRef] [PubMed]
- Oggianu, L.; Lancellotti, S.; Pitocco, D.; Zaccardi, F.; Rizzo, P.; Martini, F.; Ghirlanda, G.; De Cristofaro, R. The Oxidative Modification of von Willebrand Factor Is Associated with Thrombotic Angiopathies in Diabetes Mellitus. PLoS ONE 2013, 8, e55396. [Google Scholar] [CrossRef]
- Kacso, I.M.; Potra, A.R.; Rusu, A.; Moldovan, D.; Rusu, C.C.; Kacso, G.; Hancu, N.D.; Muresan, A.; Bondor, C.I. Relationship of Endothelial Cell Selective Adhesion Molecule to Markers of Oxidative Stress in Type 2 Diabetes. Scand. J. Clin. Lab. Investig. 2014, 74, 170–176. [Google Scholar] [CrossRef]
- Inoue, M.; Ishida, T.; Yasuda, T.; Toh, R.; Hara, T.; Cangara, H.M.; Rikitake, Y.; Taira, K.; Sun, L.; Kundu, R.K.; et al. Endothelial Cell-Selective Adhesion Molecule Modulates Atherosclerosis through Plaque Angiogenesis and Monocyte-Endothelial Interaction. Microvasc. Res. 2010, 80, 179–187. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.; Ma, J.; Zhu, H.; Deng, S.; Gu, M.; Qu, S. Hydroxysafflor Yellow A Attenuates High Glucose-Induced Human Umbilical Vein Endothelial Cell Dysfunction. Hum. Exp. Toxicol. 2019, 38, 685–693. [Google Scholar] [CrossRef]
- Wu, W.; Xie, Z.; Zhang, Q.; Ma, Y.; Bi, X.; Yang, X.; Li, B.; Chen, J. Hyperoside Ameliorates Diabetic Retinopathy via Anti-Oxidation, Inhibiting Cell Damage and Apoptosis Induced by High Glucose. Front. Pharmacol. 2020, 11, 797. [Google Scholar] [CrossRef]
- Sun, S.; Liu, L.; Tian, X.; Guo, Y.; Cao, Y.; Mei, Y.; Wang, C. Icariin Attenuates High Glucose-Induced Apoptosis, Oxidative Stress, and Inflammation in Human Umbilical Venous Endothelial Cells. Planta Med. 2019, 85, 473–482. [Google Scholar] [CrossRef] [PubMed]
- Zhou, F.; Xin, H.; Liu, T.; Li, G.-Y.; Gao, Z.-Z.; Liu, J.; Li, W.-R.; Cui, W.-S.; Bai, G.-Y.; Park, N.C.; et al. Effects of Icariside II on Improving Erectile Function in Rats with Streptozotocin-Induced Diabetes. J. Androl. 2012, 33, 832–844. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Xu, Y.; Li, H.; Lei, H.; Guan, R.; Gao, Z.; Xin, Z. Antioxidant Icariside II Combined with Insulin Restores Erectile Function in Streptozotocin-Induced Type 1 Diabetic Rats. J. Cell. Mol. Med. 2015, 19, 960–969. [Google Scholar] [CrossRef] [PubMed]
- Aminzadeh, A.; Bashiri, H. Myricetin Ameliorates High Glucose-Induced Endothelial Dysfunction in Human Umbilical Vein Endothelial Cells. Cell Biochem. Funct. 2020, 38, 12–20. [Google Scholar] [CrossRef]
- Wang, W.; Wu, Q.-H.; Sui, Y.; Wang, Y.; Qiu, X. Rutin Protects Endothelial Dysfunction by Disturbing Nox4 and ROS-Sensitive NLRP3 Inflammasome. Biomed. Pharmacother. Biomedecine Pharmacother. 2017, 86, 32–40. [Google Scholar] [CrossRef] [PubMed]
- Kwak, S.; Ku, S.-K.; Bae, J.-S. Fisetin Inhibits High-Glucose-Induced Vascular Inflammation in Vitro and in Vivo. Inflamm. Res. 2014, 63, 779–787. [Google Scholar] [CrossRef] [PubMed]
- Patel, R.; Varghese, J.F.; Singh, R.P.; Yadav, U.C.S. Induction of Endothelial Dysfunction by Oxidized Low-Density Lipoproteins via Downregulation of Erk-5/Mef2c/KLF2 Signaling: Amelioration by Fisetin. Biochimie 2019, 163, 152–162. [Google Scholar] [CrossRef] [PubMed]
- Ch, L.; Jl, H.; Ol, W. 3’,4’-Dihydroxyflavonol Reduces Superoxide and Improves Nitric Oxide Function in Diabetic Rat Mesenteric Arteries. PLoS ONE 2011, 6, e20813. [Google Scholar] [CrossRef]
- Lian, D.; Yuan, H.; Yin, X.; Wu, Y.; He, R.; Huang, Y.; Chen, Y. Puerarin Inhibits Hyperglycemia-Induced Inter-Endothelial Junction through Suppressing Endothelial Nlrp3 Inflammasome Activation via ROS-Dependent Oxidative Pathway. Phytomedicine 2019, 55, 310–319. [Google Scholar] [CrossRef]
- Xu, Y.; Feng, L.; Wang, S.; Zhu, Q.; Lin, J.; Lou, C.; Xiang, P.; He, B.; Zheng, Z.; Tang, D.; et al. Phytoestrogen Calycosin-7-O-β-D-Glucopyranoside Ameliorates Advanced Glycation End Products-Induced HUVEC Damage. J. Cell. Biochem. 2011, 112, 2953–2965. [Google Scholar] [CrossRef]
- Zhang, D.; Gao, X.; Wang, Q.; Qin, M.; Liu, K.; Huang, F.; Liu, B. Kakkalide Ameliorates Endothelial Insulin Resistance by Suppressing Reactive Oxygen Species-Associated Inflammation. J. Diabetes 2013, 5, 13–24. [Google Scholar] [CrossRef]
- Xu, Y.; Zhang, Y.; Liang, H.; Liu, X. Coumestrol Mitigates Retinal Cell Inflammation, Apoptosis, and Oxidative Stress in a Rat Model of Diabetic Retinopathy via Activation of SIRT1. Aging 2021, 13, 5342–5357. [Google Scholar] [CrossRef]
- Ramírez-Sánchez, I.; Rodríguez, A.; Moreno-Ulloa, A.; Ceballos, G.; Villarreal, F. (−)-Epicatechin-Induced Recovery of Mitochondria from Simulated Diabetes: Potential Role of Endothelial Nitric Oxide Synthase. Diab. Vasc. Dis. Res. 2016, 13, 201–210. [Google Scholar] [CrossRef]
- Roghani, M.; Baluchnejadmojarad, T. Chronic Epigallocatechin-Gallate Improves Aortic Reactivity of Diabetic Rats: Underlying Mechanisms. Vascul. Pharmacol. 2009, 51, 84–89. [Google Scholar] [CrossRef]
- Bhardwaj, P.; Khanna, D.; Balakumar, P. Catechin Averts Experimental Diabetes Mellitus-Induced Vascular Endothelial Structural and Functional Abnormalities. Cardiovasc. Toxicol. 2014, 14, 41–51. [Google Scholar] [CrossRef]
- Fan, J.; Liu, H.; Wang, J.; Zeng, J.; Tan, Y.; Wang, Y.; Yu, X.; Li, W.; Wang, P.; Yang, Z.; et al. Procyanidin B2 Improves Endothelial Progenitor Cell Function and Promotes Wound Healing in Diabetic Mice via Activating Nrf2. J. Cell. Mol. Med. 2021, 25, 652–665. [Google Scholar] [CrossRef]
- Li, B.; Li, X.; Gao, H.; Zhang, J.; Cai, Q.; Cheng, M.; Lu, M. Grape Seed Procyanidin B2 Inhibits Advanced Glycation End Product-Induced Endothelial Cell Apoptosis through Regulating GSK3β Phosphorylation. Cell Biol. Int. 2011, 35, 663–669. [Google Scholar] [CrossRef]
- Wang, C.; Sun, Y.; Liu, W.; Liu, Y.; Afzal, S.; Grover, J.; Chang, D.; Münch, G.; Li, C.G.; Lin, S.; et al. Protective Effect of the Curcumin-Baicalein Combination against Macrovascular Changes in Diabetic Angiopathy. Front. Endocrinol. 2022, 13, 953305. [Google Scholar] [CrossRef]
- Wang, G.; Liang, J.; Gao, L.-R.; Si, Z.-P.; Zhang, X.-T.; Liang, G.; Yan, Y.; Li, K.; Cheng, X.; Bao, Y.; et al. Baicalin Administration Attenuates Hyperglycemia-Induced Malformation of Cardiovascular System. Cell Death Dis. 2018, 9, 234. [Google Scholar] [CrossRef]
- Shao, X.; Yu, J.; Ni, W. Wogonoside alleviates high glucose-induced dysfunction of retinal microvascular endothelial cells and diabetic retinopathy in rats by up-regulating SIRT1. Nan Fang Yi Ke Da Xue Xue Bao 2022, 42, 463–472. [Google Scholar] [CrossRef]
- Wang, D.; Wang, L.; Gu, J.; Yang, H.; Liu, N.; Lin, Y.; Li, X.; Shao, C. Scutellarin Inhibits High Glucose-Induced and Hypoxia-Mimetic Agent-Induced Angiogenic Effects in Human Retinal Endothelial Cells through Reactive Oxygen Species/Hypoxia-Inducible Factor-1α/Vascular Endothelial Growth Factor Pathway. J. Cardiovasc. Pharmacol. 2014, 64, 218–227. [Google Scholar] [CrossRef]
- Qian, L.-B.; Wang, H.-P.; Chen, Y.; Chen, F.-X.; Ma, Y.-Y.; Bruce, I.C.; Xia, Q. Luteolin Reduces High Glucose-Mediated Impairment of Endothelium-Dependent Relaxation in Rat Aorta by Reducing Oxidative Stress. Pharmacol. Res. 2010, 61, 281–287. [Google Scholar] [CrossRef]
- Xu, F.; Liu, Y.; Zhu, X.; Li, S.; Shi, X.; Li, Z.; Ai, M.; Sun, J.; Hou, B.; Cai, W.; et al. Protective Effects and Mechanisms of Vaccarin on Vascular Endothelial Dysfunction in Diabetic Angiopathy. Int. J. Mol. Sci. 2019, 20, 4587. [Google Scholar] [CrossRef]
- Zhu, X.; Lei, Y.; Tan, F.; Gong, L.; Gong, H.; Yang, W.; Chen, T.; Zhang, Z.; Cai, W.; Hou, B.; et al. Vaccarin Protects Human Microvascular Endothelial Cells from Apoptosis via Attenuation of HDAC1 and Oxidative Stress. Eur. J. Pharmacol. 2018, 818, 371–380. [Google Scholar] [CrossRef]
- Ren, B.; Qin, W.; Wu, F.; Wang, S.; Pan, C.; Wang, L.; Zeng, B.; Ma, S.; Liang, J. Apigenin and Naringenin Regulate Glucose and Lipid Metabolism, and Ameliorate Vascular Dysfunction in Type 2 Diabetic Rats. Eur. J. Pharmacol. 2016, 773, 13–23. [Google Scholar] [CrossRef]
- Zhang, S.; Jin, S.; Zhang, S.; Li, Y.-Y.; Wang, H.; Chen, Y.; Lu, H. Vitexin Protects against High Glucose-Induced Endothelial Cell Apoptosis and Oxidative Stress via Wnt/β-Catenin and Nrf2 Signalling Pathway. Arch. Physiol. Biochem. 2022, 130, 275–284. [Google Scholar] [CrossRef]
- Fratantonio, D.; Speciale, A.; Ferrari, D.; Cristani, M.; Saija, A.; Cimino, F. Palmitate-Induced Endothelial Dysfunction Is Attenuated by Cyanidin-3-O-Glucoside through Modulation of Nrf2/Bach1 and NF-κB Pathways. Toxicol. Lett. 2015, 239, 152–160. [Google Scholar] [CrossRef]
- Qin, W.; Ren, B.; Wang, S.; Liang, S.; He, B.; Shi, X.; Wang, L.; Liang, J.; Wu, F. Apigenin and Naringenin Ameliorate PKCβII-Associated Endothelial Dysfunction via Regulating ROS/Caspase-3 and NO Pathway in Endothelial Cells Exposed to High Glucose. Vascul. Pharmacol. 2016, 85, 39–49. [Google Scholar] [CrossRef]
- Li, G.; Xu, Y.; Sheng, X.; Liu, H.; Guo, J.; Wang, J.; Zhong, Q.; Jiang, H.; Zheng, C.; Tan, M.; et al. Naringin Protects Against High Glucose-Induced Human Endothelial Cell Injury Via Antioxidation and CX3CL1 Downregulation. Cell. Physiol. Biochem. 2017, 42, 2540–2551. [Google Scholar] [CrossRef]
- Guzmán, L.; Balada, C.; Flores, G.; Álvarez, R.; Knox, M.; Vinet, R.; Martínez, J.L. T-Resveratrol Protects against Acute High Glucose Damage in Endothelial Cells. Plant Foods Hum. Nutr. 2018, 73, 235–240. [Google Scholar] [CrossRef]
- Shukla, K.; Sonowal, H.; Saxena, A.; Ramana, K.V. Didymin Prevents Hyperglycemia-Induced Human Umbilical Endothelial Cells Dysfunction and Death. Biochem. Pharmacol. 2018, 152, 1–10. [Google Scholar] [CrossRef]
- Zhang, X.; Song, Y.; Han, X.; Feng, L.; Wang, R.; Zhang, M.; Zhu, M.; Jia, X.; Hu, S. Liquiritin Attenuates Advanced Glycation End Products-Induced Endothelial Dysfunction via RAGE/NF-κB Pathway in Human Umbilical Vein Endothelial Cells. Mol. Cell. Biochem. 2013, 374, 191–201. [Google Scholar] [CrossRef]
- Zhao, K.; Li, Y.; Qiu, Y.; Huang, R.; Lin, M.; Chen, L.; Liu, Y. Norkurarinone and Isoxanthohumol Inhibit High Glucose and Hypoxia-Induced Angiogenesis via Improving Oxidative Stress and Regulating Autophagy in Human Retinal Microvascular Endothelial Cells. Biochem. Biophys. Res. Commun. 2022, 634, 20–29. [Google Scholar] [CrossRef]
- Kozłowska, A.; Szostak-Węgierek, D. Targeting Cardiovascular Diseases by Flavonols: An Update. Nutrients 2022, 14, 1439. [Google Scholar] [CrossRef]
- Anand David, A.; Arulmoli, R.; Parasuraman, S. Overviews of Biological Importance of Quercetin: A Bioactive Flavonoid. Pharmacogn. Rev. 2016, 10, 84. [Google Scholar] [CrossRef]
- Boesten, D.M.P.H.J.; von Ungern-Sternberg, S.N.I.; den Hartog, G.J.M.; Bast, A. Protective Pleiotropic Effect of Flavonoids on NAD+ Levels in Endothelial Cells Exposed to High Glucose. Oxid. Med. Cell. Longev. 2015, 2015, 894597. [Google Scholar] [CrossRef]
- Tian, R.; Jin, Z.; Zhou, L.; Zeng, X.-P.; Lu, N. Quercetin Attenuated Myeloperoxidase-Dependent HOCl Generation and Endothelial Dysfunction in Diabetic Vasculature. J. Agric. Food Chem. 2021, 69, 404–413. [Google Scholar] [CrossRef]
- Zhao, L.-R.; Du, Y.-J.; Chen, L.; Liu, Z.-G.; Pan, Y.-H.; Liu, J.-F.; Liu, B. Quercetin Protects against High Glucose-Induced Damage in Bone Marrow-Derived Endothelial Progenitor Cells. Int. J. Mol. Med. 2014, 34, 1025–1031. [Google Scholar] [CrossRef]
- Wang, X.; Yao, W.; Pan, Z.; Dong, J.; Liu, S.; Ding, X. Protective effects and mechanism of icariin against vascular function in diabetic mice. China Pharm. Univ. 2022, 53, 215–221. [Google Scholar] [CrossRef]
- Tang, Y.; Jacobi, A.; Vater, C.; Zou, L.; Zou, X.; Stiehler, M. Icariin Promotes Angiogenic Differentiation and Prevents Oxidative Stress-Induced Autophagy in Endothelial Progenitor Cells. Stem Cells Dayt. Ohio 2015, 33, 1863–1877. [Google Scholar] [CrossRef]
- Li, H.; Xu, Y.; Guan, R.; Matheu, M.; Lei, H.; Tian, W.; Gao, Z.; Lin, G.; Guo, Y.; Xin, Z.; et al. Icariside II Prevents High-Glucose-Induced Injury on Human Cavernous Endothelial Cells through Akt-eNOS Signaling Pathway. Andrology 2015, 3, 408–416. [Google Scholar] [CrossRef]
- Tian, W.; Lei, H.; Guan, R.; Xu, Y.; Li, H.; Wang, L.; Yang, B.; Gao, Z.; Xin, Z. Icariside II Ameliorates Diabetic Nephropathy in Streptozotocin-Induced Diabetic Rats. Drug Des. Devel. Ther. 2015, 9, 5147–5157. [Google Scholar] [CrossRef]
- Wang, X.; Zhao, X.; Feng, T.; Jin, G.; Li, Z. Rutin Prevents High Glucose-Induced Renal Glomerular Endothelial Hyperpermeability by Inhibiting the ROS/Rhoa/ROCK Signaling Pathway. Planta Med. 2016, 82, 1252–1257. [Google Scholar] [CrossRef]
- Woodman, O.L.; Malakul, W. 3’,4’-Dihydroxyflavonol Prevents Diabetes-Induced Endothelial Dysfunction in Rat Aorta. Life Sci. 2009, 85, 54–59. [Google Scholar] [CrossRef]
- Leo, C.-H.; Hart, J.L.; Woodman, O.L. 3’,4’-Dihydroxyflavonol Restores Endothelium-Dependent Relaxation in Small Mesenteric Artery from Rats with Type 1 and Type 2 Diabetes. Eur. J. Pharmacol. 2011, 659, 193–198. [Google Scholar] [CrossRef]
- Roghani, M.; Vaez Mahdavi, M.-R.; Jalali-Nadoushan, M.-R.; Baluchnejadmojarad, T.; Naderi, G.; Roghani-Dehkordi, F.; Taghi Joghataei, M.; Kord, M. Chronic Administration of Daidzein, a Soybean Isoflavone, Improves Endothelial Dysfunction and Attenuates Oxidative Stress in Streptozotocin-Induced Diabetic Rats. Phytother. Res. PTR 2013, 27, 112–117. [Google Scholar] [CrossRef]
- Xu, S.-Z.; Zhong, W.; Ghavideldarestani, M.; Saurabh, R.; Lindow, S.W.; Atkin, S.L. Multiple Mechanisms of Soy Isoflavones against Oxidative Stress-Induced Endothelium Injury. Free Radic. Biol. Med. 2009, 47, 167–175. [Google Scholar] [CrossRef]
- Luo, Y.; Jian, Y.; Liu, Y.; Jiang, S.; Muhammad, D.; Wang, W. Flavanols from Nature: A Phytochemistry and Biological Activity Review. Molecules 2022, 27, 719. [Google Scholar] [CrossRef]
- Ihm, S.-H.; Lee, J.-O.; Kim, S.-J.; Seung, K.-B.; Schini-Kerth, V.B.; Chang, K.; Oak, M.-H. Catechin Prevents Endothelial Dysfunction in the Prediabetic Stage of OLETF Rats by Reducing Vascular NADPH Oxidase Activity and Expression. Atherosclerosis 2009, 206, 47–53. [Google Scholar] [CrossRef]
- Pinna, C.; Morazzoni, P.; Sala, A. Proanthocyanidins from Vitis Vinifera Inhibit Oxidative Stress-Induced Vascular Impairment in Pulmonary Arteries from Diabetic Rats. Phytomedicine 2017, 25, 39–44. [Google Scholar] [CrossRef]
- Okudan, N.; Barışkaner, H.; Gökbel, H.; Sahin, A.S.; Belviranlı, M.; Baysal, H. The Effect of Supplementation of Grape Seed Proanthocyanidin Extract on Vascular Dysfunction in Experimental Diabetes. J. Med. Food 2011, 14, 1298–1302. [Google Scholar] [CrossRef]
- Zhang, F.-L.; Gao, H.-Q.; Wu, J.-M.; Ma, Y.-B.; You, B.-A.; Li, B.-Y.; Xuan, J.-H. Selective Inhibition by Grape Seed Proanthocyanidin Extracts of Cell Adhesion Molecule Expression Induced by Advanced Glycation End Products in Endothelial Cells. J. Cardiovasc. Pharmacol. 2006, 48, 47–53. [Google Scholar] [CrossRef]
- Zhang, F.-L.; Gao, H.-Q.; Shen, L. Inhibitory Effect of GSPE on RAGE Expression Induced by Advanced Glycation End Products in Endothelial Cells. J. Cardiovasc. Pharmacol. 2007, 50, 434–440. [Google Scholar] [CrossRef] [PubMed]
- Cerbaro, A.F.; Rodrigues, V.S.B.; Rigotti, M.; Branco, C.S.; Rech, G.; de Oliveira, D.L.; Salvador, M. Grape Seed Proanthocyanidins Improves Mitochondrial Function and Reduces Oxidative Stress through an Increase in Sirtuin 3 Expression in EA. Hy926 Cells in High Glucose Condition. Mol. Biol. Rep. 2020, 47, 3319–3330. [Google Scholar] [CrossRef]
- Zou, W.; Lu, Q.; Zhu, X.; Pan, Y.; Xu, Q.; Wang, K. Procyanidin B2 Protects TR-iBRB2 Cells Against Hyperglycemia Stress by Attenuating Oxidative Stress and Inflammasome Activation via Regulation of Redoxosomes/NF-kB Signaling. Curr. Mol. Med. 2022, 23, 1095–1103. [Google Scholar] [CrossRef]
- Panche, A.N.; Diwan, A.D.; Chandra, S.R. Flavonoids: An Overview. J. Nutr. Sci. 2016, 5, e47. [Google Scholar] [CrossRef]
- Chen, Y.; Zhou, B.; Yu, Z.; Yuan, P.; Sun, T.; Gong, J.; Zhang, Y.; Wang, T.; Wang, S.; Liu, K.; et al. Baicalein Alleviates Erectile Dysfunction Associated With Streptozotocin-Induced Type I Diabetes by Ameliorating Endothelial Nitric Oxide Synthase Dysfunction, Inhibiting Oxidative Stress and Fibrosis. J. Sex. Med. 2020, 17, 1434–1447. [Google Scholar] [CrossRef] [PubMed]
- Othman, A.; Ahmad, S.; Megyerdi, S.; Mussell, R.; Choksi, K.; Maddipati, K.R.; Elmarakby, A.; Rizk, N.; Al-Shabrawey, M. 12/15-Lipoxygenase-Derived Lipid Metabolites Induce Retinal Endothelial Cell Barrier Dysfunction: Contribution of NADPH Oxidase. PLoS ONE 2013, 8, e57254. [Google Scholar] [CrossRef] [PubMed]
- Queiroz, M.; Leandro, A.; Azul, L.; Figueirinha, A.; Seiça, R.; Sena, C.M. Luteolin Improves Perivascular Adipose Tissue Profile and Vascular Dysfunction in Goto-Kakizaki Rats. Int. J. Mol. Sci. 2021, 22, 13671. [Google Scholar] [CrossRef]
- Zhou, Q.; Cheng, K.-W.; Gong, J.; Li, E.T.S.; Wang, M. Apigenin and Its Methylglyoxal-Adduct Inhibit Advanced Glycation End Products-Induced Oxidative Stress and Inflammation in Endothelial Cells. Biochem. Pharmacol. 2019, 166, 231–241. [Google Scholar] [CrossRef]
- Putta, S.; Yarla, N.S.; Kumar K, E.; Lakkappa, D.B.; Kamal, M.A.; Scotti, L.; Scotti, M.T.; Ashraf, G.M.; Rao, B.S.B.; D, S.K.; et al. Preventive and Therapeutic Potentials of Anthocyanins in Diabetes and Associated Complications. Curr. Med. Chem. 2018, 25, 5347–5371. [Google Scholar] [CrossRef] [PubMed]
- Markovics, A.; Biró, A.; Kun-Nemes, A.; Fazekas, M.É.; Rácz, A.A.; Paholcsek, M.; Lukács, J.; Stündl, L.; Remenyik, J. Effect of Anthocyanin-Rich Extract of Sour Cherry for Hyperglycemia-Induced Inflammatory Response and Impaired Endothelium-Dependent Vasodilation. Nutrients 2020, 12, 3373. [Google Scholar] [CrossRef] [PubMed]
- Huang, W.; Hutabarat, R.P.; Chai, Z.; Zheng, T.; Zhang, W.; Li, D. Antioxidant Blueberry Anthocyanins Induce Vasodilation via PI3K/Akt Signaling Pathway in High-Glucose-Induced Human Umbilical Vein Endothelial Cells. Int. J. Mol. Sci. 2020, 21, 1575. [Google Scholar] [CrossRef] [PubMed]
- Huang, W.; Yan, Z.; Li, D.; Ma, Y.; Zhou, J.; Sui, Z. Antioxidant and Anti-Inflammatory Effects of Blueberry Anthocyanins on High Glucose-Induced Human Retinal Capillary Endothelial Cells. Oxid. Med. Cell. Longev. 2018, 2018, 1862462. [Google Scholar] [CrossRef] [PubMed]
- Hasanein, P.; Fazeli, F. Role of Naringenin in Protection against Diabetic Hyperalgesia and Tactile Allodynia in Male Wistar Rats. J. Physiol. Biochem. 2014, 70, 997–1006. [Google Scholar] [CrossRef] [PubMed]
- Xue, B.; Wang, Y. Naringenin Upregulates GTPCH1/eNOS to Ameliorate High Glucose-Induced Retinal Endothelial Cell Injury. Exp. Ther. Med. 2022, 23, 428. [Google Scholar] [CrossRef] [PubMed]
- Elkanzi, N.A.A.; Hrichi, H.; Alolayan, R.A.; Derafa, W.; Zahou, F.M.; Bakr, R.B. Synthesis of Chalcones Derivatives and Their Biological Activities: A Review. ACS Omega 2022, 7, 27769–27786. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Wang, T.; Liu, P.; Yang, F.; Wang, X.; Zheng, W.; Sun, W. Hesperetin Ameliorates Hepatic Oxidative Stress and Inflammation via the PI3K/AKT-Nrf2-ARE Pathway in Oleic Acid-Induced HepG2 Cells and a Rat Model of High-Fat Diet-Induced NAFLD. Food Funct. 2021, 12, 3898–3918. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Z.; Li, H.; Bai, S.; Xu, Z.; Jiao, Y. Loss of Serine/Threonine Protein Kinase 25 in Retinal Ganglion Cells Ameliorates High Glucose-Elicited Damage through Regulation of the AKT-GSK-3β/Nrf2 Pathway. Biochem. Biophys. Res. Commun. 2022, 600, 87–93. [Google Scholar] [CrossRef]
- Long, L.H.; Hoi, A.; Halliwell, B. Instability of, and Generation of Hydrogen Peroxide by, Phenolic Compounds in Cell Culture Media. Arch. Biochem. Biophys. 2010, 501, 162–169. [Google Scholar] [CrossRef]
- Dirimanov, S.; Högger, P. Screening of Inhibitory Effects of Polyphenols on Akt-Phosphorylation in Endothelial Cells and Determination of Structure-Activity Features. Biomolecules 2019, 9, 219. [Google Scholar] [CrossRef] [PubMed]
- Reis, A.; Rocha, S.; Dias, I.H.; Costa, R.; Soares, R.; Sánchez-Quesada, J.L.; Perez, A.; de Freitas, V. Type 2 Diabetes Mellitus Alters the Cargo of (Poly)Phenol Metabolome and the Oxidative Status in Circulating Lipoproteins. Redox Biol. 2023, 59, 102572. [Google Scholar] [CrossRef] [PubMed]
- Annunziata, G.; Jiménez-García, M.; Capó, X.; Moranta, D.; Arnone, A.; Tenore, G.C.; Sureda, A.; Tejada, S. Microencapsulation as a Tool to Counteract the Typical Low Bioavailability of Polyphenols in the Management of Diabetes. Food Chem. Toxicol. 2020, 139, 111248. [Google Scholar] [CrossRef] [PubMed]
- Lakshmanan, R.; Maulik, N. Graphene-Based Drug Delivery Systems in Tissue Engineering and Nanomedicine. Can. J. Physiol. Pharmacol. 2018, 96, 869–878. [Google Scholar] [CrossRef] [PubMed]
- Xue, B.; Wang, Y.; Tian, J.; Zhang, W.; Zang, Z.; Cui, H.; Zhang, Y.; Jiang, Q.; Li, B.; Hai Liu, R. Effects of Chitooligosaccharide-Functionalized Graphene Oxide on Stability, Simulated Digestion, and Antioxidant Activity of Blueberry Anthocyanins. Food Chem. 2022, 368, 130684. [Google Scholar] [CrossRef] [PubMed]
- Ostovar, S.; Pourmadadi, M.; Zaker, M.A. Co-Biopolymer of Chitosan/Carboxymethyl Cellulose Hydrogel Improved by Zinc Oxide and Graphene Quantum Dots Nanoparticles as pH-Sensitive Nanocomposite for Quercetin Delivery to Brain Cancer Treatment. Int. J. Biol. Macromol. 2023, 253, 127091. [Google Scholar] [CrossRef] [PubMed]
- Nielsen, I.L.F.; Dragsted, L.O.; Ravn-Haren, G.; Freese, R.; Rasmussen, S.E. Absorption and Excretion of Black Currant Anthocyanins in Humans and Watanabe Heritable Hyperlipidemic Rabbits. J. Agric. Food Chem. 2003, 51, 2813–2820. [Google Scholar] [CrossRef] [PubMed]
- Skibola, C.F.; Smith, M.T. Potential Health Impacts of Excessive Flavonoid Intake. Free Radic. Biol. Med. 2000, 29, 375–383. [Google Scholar] [CrossRef] [PubMed]
- Sahu, S.C.; Gray, G.C. Interactions of Flavonoids, Trace Metals, and Oxygen: Nuclear DNA Damage and Lipid Peroxidation Induced by Myricetin. Cancer Lett. 1993, 70, 73–79. [Google Scholar] [CrossRef]
- Liu, F.; Nie, J.; Deng, M.-G.; Yang, H.; Feng, Q.; Yang, Y.; Li, X.; Li, X.; Yang, X.; Li, W.; et al. Dietary Flavonoid Intake Is Associated with a Lower Risk of Diabetic Nephropathy in US Adults: Data from NHANES 2007-2008, 2009-2010, and 2017-2018. Food Funct. 2023, 14, 4183–4190. [Google Scholar] [CrossRef]
- Mahoney, S.E.; Loprinzi, P.D. Influence of Flavonoid-Rich Fruit and Vegetable Intake on Diabetic Retinopathy and Diabetes-Related Biomarkers. J. Diabetes Complicat. 2014, 28, 767–771. [Google Scholar] [CrossRef] [PubMed]
- Yuan, S.; Xu, F.; Li, X.; Chen, J.; Zheng, J.; Mantzoros, C.S.; Larsson, S.C. Plasma Proteins and Onset of Type 2 Diabetes and Diabetic Complications: Proteome-Wide Mendelian Randomization and Colocalization Analyses. Cell Rep. Med. 2023, 4, 101174. [Google Scholar] [CrossRef]
- Zheng, D.; Li, N.; Hou, R.; Zhang, X.; Wu, L.; Sundquist, J.; Sundquist, K.; Ji, J. Glucagon-like Peptide-1 Receptor Agonists and Diabetic Retinopathy: Nationwide Cohort and Mendelian Randomization Studies. BMC Med. 2023, 21, 40. [Google Scholar] [CrossRef] [PubMed]
- David, L.; Danciu, V.; Moldovan, B.; Filip, A. Effects of In Vitro Gastrointestinal Digestion on the Antioxidant Capacity and Anthocyanin Content of Cornelian Cherry Fruit Extract. Antioxidants 2019, 8, 114. [Google Scholar] [CrossRef] [PubMed]
- McDougall, G.J.; Fyffe, S.; Dobson, P.; Stewart, D. Anthocyanins from Red Wine--Their Stability under Simulated Gastrointestinal Digestion. Phytochemistry 2005, 66, 2540–2548. [Google Scholar] [CrossRef]
- Carrera-Quintanar, L.; López Roa, R.I.; Quintero-Fabián, S.; Sánchez-Sánchez, M.A.; Vizmanos, B.; Ortuño-Sahagún, D. Phytochemicals That Influence Gut Microbiota as Prophylactics and for the Treatment of Obesity and Inflammatory Diseases. Mediators Inflamm. 2018, 2018, 9734845. [Google Scholar] [CrossRef]
| Flavonoid Subclass | Flavonoid Name | Molecular Formula | Mechanism | Model Used | References | |
|---|---|---|---|---|---|---|
| In Vivo | In Vitro | |||||
| Flavonol | Quercetin | 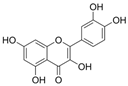 | p-eNOS↑ p-AMPK↑ p-Akt↑ | aorta of diabetic mice 10−6 M for 30 min | [41] | |
| MDA↓ ROS↓ p62↓ Beclin-1↑ LC3Ⅱ/Ⅰ↑ NO↑ | HG-HUVECs 20 μM for 24/48/72 h | [58] | ||||
| SOD↑ CAT↑ MDA↓ protein carbonyls groups↓ iNOS↓ | aorta of diabetic rats 30 mg/kg/day for 4 weeks gastric lavage | [39] | ||||
| Hyperoside | 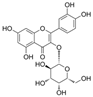 | caspase-3↓ Bax↓ caspase-9↓ CytC↓ Bcl-2↑ SOD↑ MDA↓ ROS↓ | retina of diabetic rats 20/50/100 mg/kg/day for 8 weeks gastric lavage | HG-RVECs 10 mg/mL for 72 h | [81] | |
| Icariin | 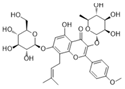 | SOD↑ MDA↓ ROS↓ NOX4↓ p-p47phox↓ lactate dehydrogenase↓ caspase-3↓ Bcl-2/Bax↑ p65↓ NF-κB↓ IL-6↓ p-ERK/ERK↓ ICAM-1↓ VCAM-1↓ E-selectin↓ | HG-HUVECs 50 μM for 72 h | [82] | ||
| Icariside II |  | α-smooth muscle actin↑ collagen I/collagen III↑ TGFβ1↓ CTGF↓Smad2↓ nNOS↑ VEGF↑ eNOS↑ SOD↑ MDA↓ RAGE↓ | penis of diabetic rats 1/5/10 mg/kg/day for 12 weeks, 5 mg/kg/day for 6 weeks gastric lavage | [83,84] | ||
| Myricetin | 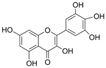 | LPO↓ TAC↑ total thiol molecules↑ Bax/Bcl-2↓ cleaved caspase-3↓ | HG-HUVECs 0.5/1 μM for 24 h | [85] | ||
| Rutin | 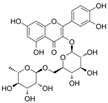 | NOX4↓ ROS↓ TXNIP↓ NLRP3↓ IL-1β↓ caspase-1↓ NO↑ | aorta of diabetic rats 35/70 mg/kg/day for 12 weeks | HG-HUVECs 10/30/100 μM for 0.5 h | [86] | |
| Fisetin | 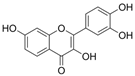 | CAMs↓ MCP-1↓IL-8↓p65↓ H2O2↓ | peritoneal exudates of mice 5.7/28.5 μg intravenous administration after 6h | HG-HUVECs 0.1/1/10/50μM for 6 h | [87] | |
| vWf↑ ICAM-1↓ eNOS↑ Erk-5↑ | oxidized LDL-HUVECs 0.5 μM for 48 h | [88] | ||||
| Morin | 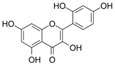 | NO↑ p-eNOS↑ p-Akt↑ | aorta of diabetic mice 10−6 M for 30 min | [41] | ||
| DiOHF |  | ROS↓ NOX2↓ eNOS↑ | aorta of diabetic rats 1mg/kg/day for 7 days subcutaneous injection | [89] | ||
| Isoflavone | Daidzein | 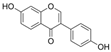 | LPO↓ ROS↓ COX-2↓ CAT↑ SOD↑ GPx↑ iNOS↓ NF-κB↓ | HG-HUVECs 0.02/0.04/0.1 μM for 20 h | [59] | |
| Puerarin | 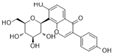 | GSH↑ CAT↑ MDA↓ ROS↓ TXNIP↓ HMGB1↓ NLRP3↓ cleaved caspase-1↓ | HG-mouse vascular endothelial Cells 1/10/25/50 μM for 24 h | [90] | ||
| C7G | 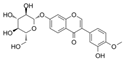 | Bad↓ Bcl-2↑ Bax↓ MDA↓ SOD↑ IL-6↓ ICAM-1↓ MCP-1↓ TGFβ1↓ RAGE↓ | AGEs-HUVECs 10−7/10−8/10−9 M for 24 h | [91] | ||
| Kakkalide | 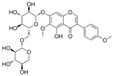 | IL-6↓ TNF-α↓ ROS↓ p-JNK↓ p-p65↓ IκB kinase β↓ p-Akt↑ p-eNOS↑ | palmitate–HUVECs palmitate–aorta of rats 0.1/1/10 μmol/L for 30 min | [92] | ||
| Coumestrol | 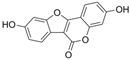 | SIRT1↑IL-6↓ TNF-α↓ CytC in cytoplasm↓ CytC in mitochondria↑ SOD↑ ROS↓MDA↓ iNOS↓ NO↓ VEGF↓ cleaved caspase-3↓ C-reactive protein↓ | retina of diabetic rats 10/50/100 mg/kg/day for 8 weeks subcutaneous injection | HG-HRECs | [93] | |
| Flavanols | Dihydromyricetin |  | TAC↑ SIRT3↑ SOD2↑ GSH/GSSG↑ MDA↓ ROS↓ | aorta of diabetic mice 250 mg/kg/day for 12 weeks gastric lavage | [54] | |
| Silibinin | 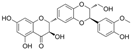 | MDA↓ ROS↓ SOD↑ GSH↑ Beclin-1↑ p62↓ LC3-Ⅱ/LC3-Ⅰ↑ NO↓ | HG-HUVECs 10 μM for 24/48/72 h | [60] | ||
| (–)-Epicatechin | 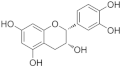 | superoxide in mitochondrial↓ ETC complex V↓ | HG-HUVECs 0.1/1 μM for 1 h | [17] | ||
| eNOS O-GlcNAc↓ SIRT1↑ NO↑ p-eNOS↑ | HG-HCACEs 100 μM for 48 h | [94] | ||||
| EGCG | 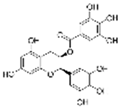 | SOD↑ MDA↓ | aorta of diabetic rats 25 mg/kg/day for 12 weeks gastric lavage | [95] | ||
| Catechin | 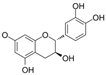 | MDA↓ ROS↓ | aorta of diabetic rats 50 mg/kg/day for 3 weeks gastric lavage | [96] | ||
| PCB2 | 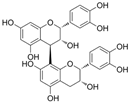 | MDA↓ROS↓Nrf2↑CAT↑ NADPH dehydrogenase quinone 1↑ | HG-EPCs 0.1/0.5/2.5 μmol/L for 6h | [97] | ||
| cleaved caspase-3↓ lactadherin↓ ROS↓ | AGE-HUVECs 2.5/5/10 μmol/l for 1 h | [98] | ||||
| Flavones | Baicalein | 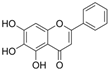 | TNF-α↓p-p38↓ p-JNK↓ caspase-3↓ ROS↓ Nrf2↑ HO-1↑ SOD↑ eNOS↑ NAD+/NADH↑ MPO↓ | aorta of HFD-Goto–Kakizaki rats 150 mg/kg/day for 4 weeks gastric lavage | H2O2-HUVECs 7.5/15 μg/mL for 1 h | [99] |
| Baicalin | 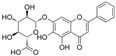 | VEGF-receptor 2↑ MDA↓ cleaved caspase-3↓ p62↓ | HG-induced chick embryos 6 μM for 48 h injection | [100] | ||
| Wogonoside | 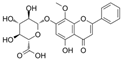 | VEGF↓ HIF-1α↓ SIRT1↑ IL-1β↓ IL-6↓ | retina of diabetic rats 30 mg/kg/day for 6 weeks | HG-HRECs 10/20/30/40 μmol/L | [101] | |
| Scutellarin | 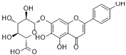 | proliferating cell nuclear antigen↓ VEGF↓ HIF-1α↓ ROS↓ MDA↓ NOX↓ | HG-HUVECs 1/10−1/ 10−2/10−3/ 10−4 μM for 48 h | [102] | ||
| Luteolin | 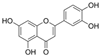 | ROS↓ OH·−↓ SOD↑ NO↑ NOS↑ | aorta of diabetic rats 10/50/100 mg/kg/day for 8 weeks | [103] | ||
| Vaccarin | 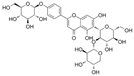 | NO↑ p-eNOS↑ p-AMPK↑ ROS↓ | aorta of diabetic mice 1 mg/kg/day for 4 weeks intraperitoneal injection | HG-HMEC-1 5 μM for 12 h | [104] | |
| Histone deacetylase 1↓ cleaved caspase-3↓ Bax/Bcl-2↓ SOD↑ CAT↑ GPx↑ | [105] | |||||
| Apigenin | 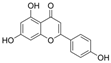 | SOD↑ MDA↓ ICAM-1↓ NO↑ p-p65↓ | artery of diabetic rats 50/100 mg/kg/day for 6 weeks gastric lavage | [106] | ||
| Vitexin | 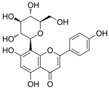 | Bcl-2/Bax↑ Wnt/b-catenin↑ SOD↑ Nrf2↑ ROS↓ MDA↓ | HG-HUVECs 15/30 μmol/L for 24 h | [107] | ||
| C3G | 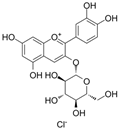 | O2·−↓ NADH dehydrogenase↑ succinate cytochrome c reductase↑ CytC↑ NOX4↓ CytB↑ NADH dehydrogenase 1↑ cleaved caspase-3↓ Bcl-2↑ | glyLDL-PAECs 30 μM for 12 h | [18] | ||
| E-selectin↓ VCAM-1↓ leukocyte adhesion↓ ROS↓ Nrf2↑ NF-κB↓ HO-1↑ GSH↑ BTB and CNC homology 1↓ NADH quinone oxidoreductase 1↑ | palmitic acid–HUVECs 20/40 μM for 24 h | [108] | ||||
| Flavanones | Naringenin | 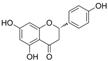 | PKCβII↓ ROS↓ p-p65↓ p-Akt↑ Bcl-2↑ Bax↓ caspase-3↓ NO↑ p-eNOS↑ | HG-HUVECs and HAECs 3/30 μM for 48 h | [109] | |
| Naringin | 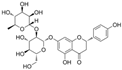 | CX3XL1↓ p-Akt↑ ROS↓ NO↑ | HG-HUVECs 50 μM for 48 h | [110] | ||
| t-RV | 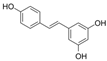 | ROS↓ | HG-HUVECs/rat aorta 0.1/1/10/ 100 μM for 3 h | [111] | ||
| Didymin | 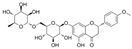 | MDA↓ ROS↓ iNOS/eNOS↓ Bcl-2↑ Bax↓ caspase-3↓ ICAM-1↓ VCAM-1↓ NF-κB ↓ EGF↓ FGF2↓ CX3CL1↓ TNF-α↓ Interferon-α2↓ Interferon-γ↓ MCP-1↓ IL-1β↓ IL-1α↓ IL-2↓ IL-5↓ IL-6↓ IL-8↓ IL-15↓ | HG-HUVECs 20 μM for 24 h | [112] | ||
| Liquiritin |  | TGFβ1↓ RAGE↓ NF-κB↓ ROS↓ MDA↓ SOD ↑ | AGEs-HUVECs 10−6/10−7/10−8 M for 48 h | [113] | ||
| IXM | 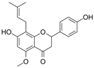 | HIF-1α↓MDA↓ SOD↑ LC3-Ⅱ/LC3-Ⅰ↓ Beclin-1↓ p62↑ autophagy-related gene 5↓ p-PI3K↑ p-Akt↑ p-mTOR↑ VEGF↓ | HG and hypoxia-HRECs high/ medium/ low dose for 24 h | [114] | ||
| NKR |  | |||||
| Chalcones | HSYA | 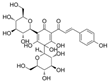 | NOX4↓ ROS↓ H2O2↓ E-selectin↓ VCAM-1↓ ICAM-1↓ VEGF↓ Fibroblast growth factor↓ | HG-HUVECs 50 μM for 24 h | [80] | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dou, B.; Zhu, Y.; Sun, M.; Wang, L.; Tang, Y.; Tian, S.; Wang, F. Mechanisms of Flavonoids and Their Derivatives in Endothelial Dysfunction Induced by Oxidative Stress in Diabetes. Molecules 2024, 29, 3265. https://doi.org/10.3390/molecules29143265
Dou B, Zhu Y, Sun M, Wang L, Tang Y, Tian S, Wang F. Mechanisms of Flavonoids and Their Derivatives in Endothelial Dysfunction Induced by Oxidative Stress in Diabetes. Molecules. 2024; 29(14):3265. https://doi.org/10.3390/molecules29143265
Chicago/Turabian StyleDou, Baolei, Yingying Zhu, Mengwei Sun, Lina Wang, Yu Tang, Shuo Tian, and Furong Wang. 2024. "Mechanisms of Flavonoids and Their Derivatives in Endothelial Dysfunction Induced by Oxidative Stress in Diabetes" Molecules 29, no. 14: 3265. https://doi.org/10.3390/molecules29143265
APA StyleDou, B., Zhu, Y., Sun, M., Wang, L., Tang, Y., Tian, S., & Wang, F. (2024). Mechanisms of Flavonoids and Their Derivatives in Endothelial Dysfunction Induced by Oxidative Stress in Diabetes. Molecules, 29(14), 3265. https://doi.org/10.3390/molecules29143265









