The Ninhydrin Reaction Revisited: Optimisation and Application for Quantification of Free Amino Acids
Abstract
1. Introduction
2. Results and Discussion
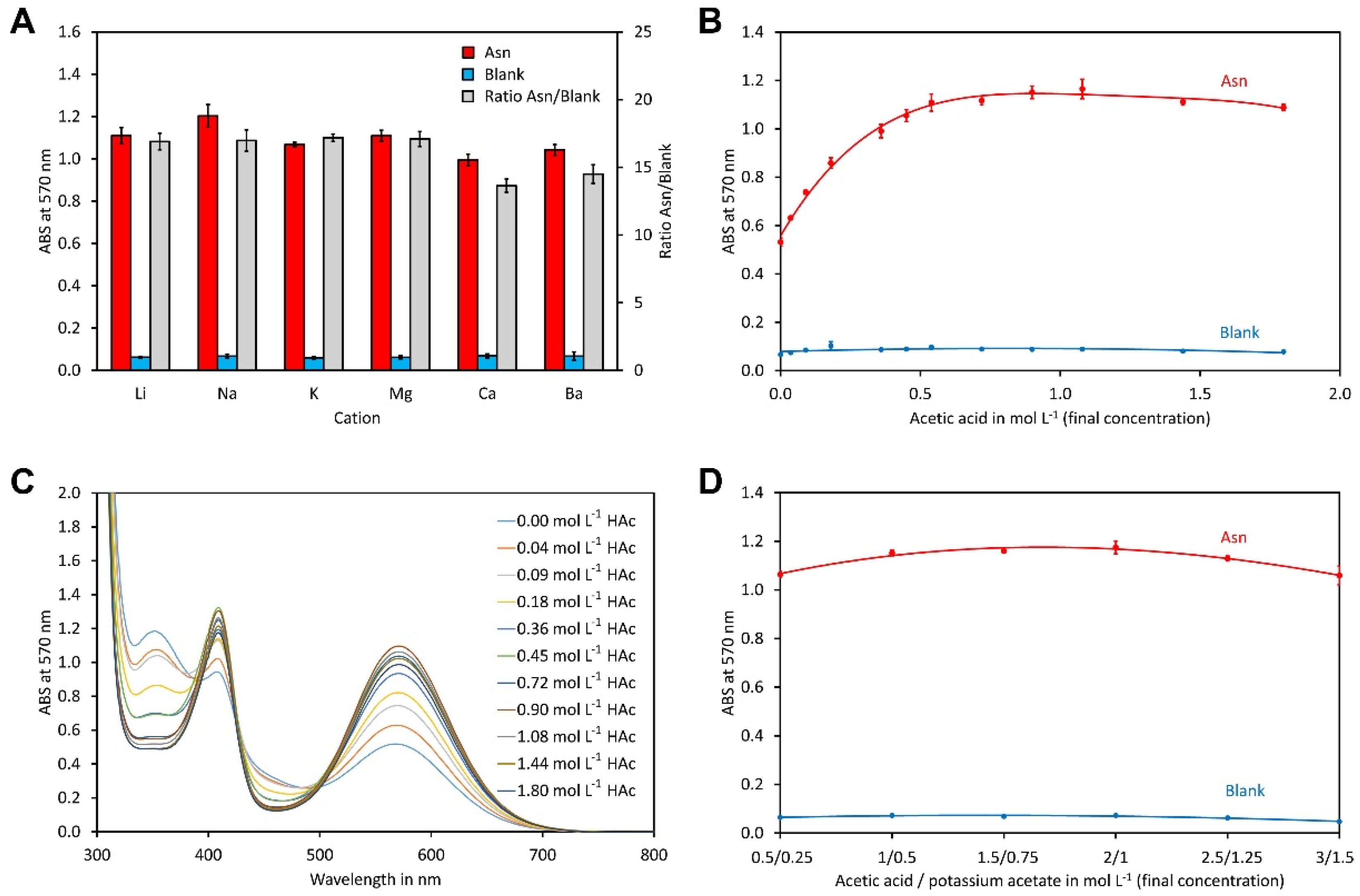
2.1. Buffer Composition
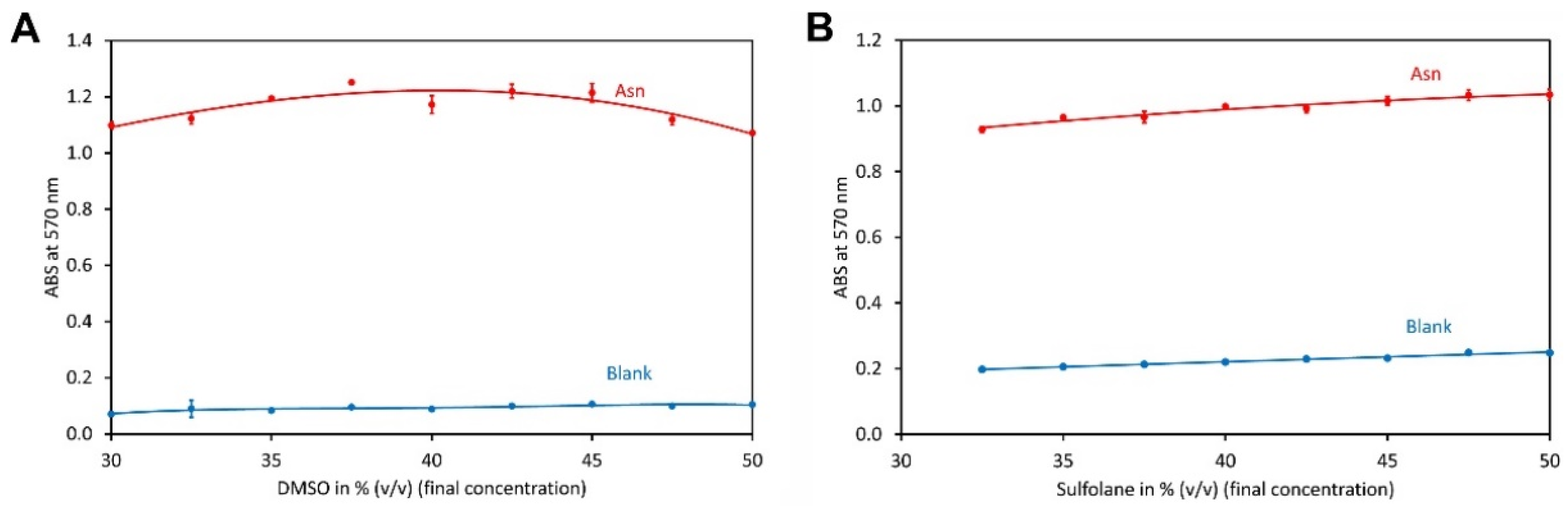
2.2. Type and Amount of Organic Solvent
2.3. Ninhydrin and Hydrindantin Concentration
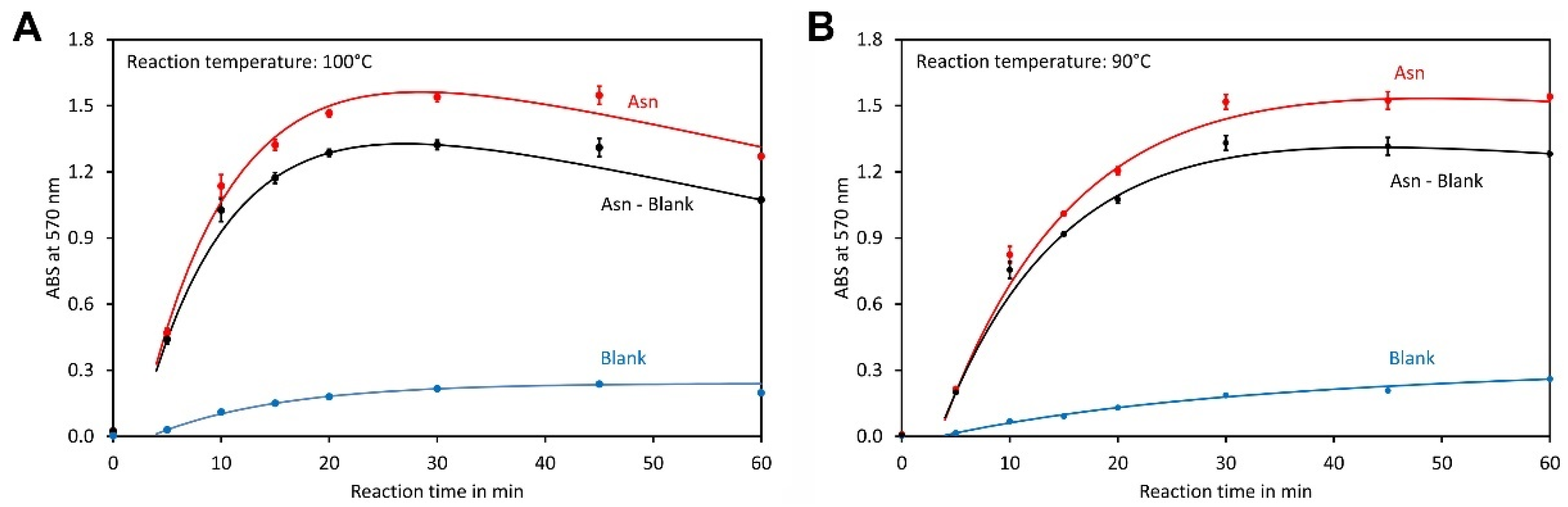
2.4. Reaction Conditions
2.5. Response of Different Amino Acids
2.6. Validation of the Method
3. Materials and Methods
3.1. Chemicals and Reagents
3.2. Analytical Procedure for Quantification of Total Amino Acids
3.3. Response of Different Amino Acids
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Sorrequieta, A.; Ferraro, G.; Boggio, S.B.; Valle, E.M. Free amino acid production during tomato fruit ripening: A focus on L-glutamate. Amino Acids 2010, 38, 1523–1532. [Google Scholar] [CrossRef]
- Sorrequieta, A.; Abriata, L.A.; Boggio, S.B.; Valle, E.M. Off-the-Vine Ripening of Tomato Fruit Causes Alteration in the Primary Metabolite Composition. Metabolites 2013, 3, 967–978. [Google Scholar] [CrossRef]
- Fuke, S.; Konosu, S. Taste-active components in some foods: A review of Japanese research. Physiol. Behav. 1991, 49, 863–868. [Google Scholar] [CrossRef]
- Kliewer, W.M. Changes in the Concentration of Free Amino Acids in Grape Berries During Maturation. Am. J. Enol. Vitic. 1968, 19, 166–174. [Google Scholar] [CrossRef]
- Wang, Y.; Wyllie, S.G.; Leach, D.N. Chemical Changes during the Development and Ripening of the Fruit of Cucumis melo (Cv. Makdimon). J. Agric. Food Chem. 1996, 44, 210–216. [Google Scholar] [CrossRef]
- Patel, P.R.; Gol, N.B.; Ramana Rao, T.V. Physiochemical changes in sunberry (Physalis minima L.) fruit during growth and ripening. Fruits 2011, 66, 37–46. [Google Scholar] [CrossRef]
- Song, J.; Bi, J.; Chen, Q.; Wu, X.; Lyu, Y.; Meng, X. Assessment of sugar content, fatty acids, free amino acids, and volatile profiles in jujube fruits at different ripening stages. Food Chem. 2019, 270, 344–352. [Google Scholar] [CrossRef]
- Ackermann, J.; Fischer, M.; Amado, R. Changes in sugars, acids, and amino acids during ripening and storage of apples (cv. Glockenapfel). J. Agric. Food Chem. 1992, 40, 1131–1134. [Google Scholar] [CrossRef]
- Perez, A.G.; Rios, J.J.; Sanz, C.; Olias, J.M. Aroma components and free amino acids in strawberry variety Chandler during ripening. J. Agric. Food Chem. 1992, 40, 2232–2235. [Google Scholar] [CrossRef]
- Granvogl, M.; Wieser, H.; Koehler, P.; von Tucher, S.; Schieberle, P. Influence of sulfur fertilization on the amounts of free amino acids in wheat. correlation with baking properties as well as with 3-aminopropionamide and acrylamide generation during baking. J. Agric. Food Chem. 2007, 55, 4271–4277. [Google Scholar] [CrossRef]
- Hoffmann, C.M. Changes in N Composition of Sugar Beet Varieties in Response to Increasing N Supply. J. Agron. Crop Sci. 2005, 191, 138–145. [Google Scholar] [CrossRef]
- Hassan, I.; Mostafa, S. Influence of Sugar Beet Nitrogen Content on Quality and Efficiency of Sugar Extraction. J. Food Dairy. Sci. 2018, 9, 111–116. [Google Scholar] [CrossRef]
- Im, M.-H.; Choi, J.-D.; Chung, H.-C.; Lee, S.-H.; Lee, C.-W.; Choi, C.; Choi, K.-S. Improvement of Meju Preparation Method for the Production of Korean Traditional kanjang (Soy Sauce). Korean J. Food Sci. Technol. 1998, 30, 608–614. [Google Scholar]
- Izco, J.; Torre, P.; Barcina, Y. Ripening of Ossau-Iraty cheese: Determination of free amino acids by RP-HPLC and of total free amino acids by the TNBS method. Food Control 2000, 11, 7–11. [Google Scholar] [CrossRef]
- Niro, S.; Succi, M.; Tremonte, P.; Sorrentino, E.; Coppola, R.; Panfili, G.; Fratianni, A. Evolution of free amino acids during ripening of Caciocavallo cheeses made with different milks. J. Dairy. Sci. 2017, 100, 9521–9531. [Google Scholar] [CrossRef]
- Innocente, N. Free amino acids and water-soluble nitrogen as ripening indices in Montasio cheese. Le Lait 1997, 77, 359–369. [Google Scholar] [CrossRef]
- Shim, Y.-S.; Yoon, W.-J.; Ha, J.; Seo, D.; Lee, K.-W.; Lee, W.-Y.; Kwon, K.-I.; Kang, T.-S.; Lee, J.-H.; Kim, H.-J.; et al. Method validation of 16 types of structural amino acids using an automated amino acid analyzer. Food Sci. Biotechnol. 2013, 22, 1567–1571. [Google Scholar] [CrossRef]
- Zhang, H.; Wang, Z.-Y.; Yang, X.; Zhao, H.-T.; Zhang, Y.-C.; Dong, A.-J.; Jing, J.; Wang, J. Determination of free amino acids and 18 elements in freeze-dried strawberry and blueberry fruit using an Amino Acid Analyzer and ICP-MS with micro-wave digestion. Food Chem. 2014, 147, 189–194. [Google Scholar] [CrossRef]
- Verni, M. Determination of the Content of Free Amino Acids and Their Profiling. In Basic Methods and Protocols on Sourdough; Gobbetti, M., Rizzello, C.G., Eds.; Springer: New York, NY, USA, 2024; pp. 71–79. ISBN 978-1-0716-3705-0. [Google Scholar]
- McDermott, A.; Visentin, G.; de Marchi, M.; Berry, D.P.; Fenelon, M.A.; O′Connor, P.M.; Kenny, O.A.; McParland, S. Prediction of individual milk proteins including free amino acids in bovine milk using mid-infrared spectroscopy and their correlations with milk processing characteristics. J. Dairy Sci. 2016, 99, 3171–3182. [Google Scholar] [CrossRef]
- Bidlingmeyer, B.A.; Cohen, S.A.; Tarvin, T.L. Rapid analysis of amino acids using pre-column derivatization. J. Chromatogr. 1984, 336, 93–104. [Google Scholar] [CrossRef]
- Cohen, S.A.; Bidlingmeyer, B.A.; Tarvin, T.L. PITC derivatives in amino acid analysis. Nature 1986, 320, 769–770. [Google Scholar] [CrossRef]
- Klikarová, J.; Česlová, L.; Fischer, J. Rapid analysis of phenyl isothiocyanate derivatives of amino acids present in Czech meads. J. Chromatogr. A 2021, 1644, 462134. [Google Scholar] [CrossRef]
- Zhang, P.; Liu, W.; Li, W.; Wang, Q.; Zhang, G.; Zhao, L.; Li, J.; Ji, W.; Wang, C.; Wang, J. A Rapid and Sensitive Method for Free Amino Acids in Nasal Feeding Nutrition by Liquid Chromatography with Liquid Extraction-Derivatization. Chromatographia 2020, 83, 293–297. [Google Scholar] [CrossRef]
- Altmann, F. Determination of amino sugars and amino acids in glycoconjugates using precolumn derivatization with o-phthalaldehyde. Anal. Biochem. 1992, 204, 215–219. [Google Scholar] [CrossRef]
- Lindroth, P.; Mopper, K. High performance liquid chromatographic determination of subpicomole amounts of amino acids by precolumn fluorescence derivatization with o-phthaldialdehyde. Anal. Chem. 1979, 51, 1667–1674. [Google Scholar] [CrossRef]
- Radjai, M.K.; Hatch, R.T. Fast determination of free amino acids by ion-pair high-performance liquid chromatography using on-line post-column derivatization. J. Chromatogr. A 1980, 196, 319–322. [Google Scholar] [CrossRef]
- de Sousa Fontes, V.M.; Colombo Pimentel, T.; Da Martins Silva, A.B.; Suely Madruga, M.; Magnani, M.; Dos Santos Lima, M. An improved method for determining free amino acids by RP-HPLC/DAD with o-phthalaldehyde derivatization: Method evaluation in beers and wines. Food Chem. 2024, 435, 137591. [Google Scholar] [CrossRef]
- Liyanaarachchi, G.V.V.; Mahanama, K.R.R.; Somasiri, H.P.P.S.; Punyasiri, P.A.N. Validation of a reversed-phase high-performance liquid chromatographic method for the determination of free amino acids in rice using l-theanine as the internal standard. Food Chem. 2018, 240, 196–203. [Google Scholar] [CrossRef]
- Molnár-Perl, I. Advancement in the derivatizations of the amino groups with the o-phthaldehyde-thiol and with the 9-fluorenylmethyloxycarbonyl chloride reagents. J. Chromatogr. B Analyt. Technol. Biomed. Life Sci. 2011, 879, 1241–1269. [Google Scholar] [CrossRef]
- Cohen, S.A.; Michaud, D.P. Synthesis of a fluorescent derivatizing reagent, 6-aminoquinolyl-N-hydroxysuccinimidyl carbamate, and its application for the analysis of hydrolysate amino acids via high-performance liquid chromatography. Anal. Biochem. 1993, 211, 279–287. [Google Scholar] [CrossRef]
- Zeng, F.; Ou, J.; Huang, Y.; Li, Q.; Xu, G.; Liu, Z.; Yang, S. Determination of 21 Free Amino Acids in Fruit Juices by HPLC Using a Modification of the 6-Aminoquinolyl-N-hydroxysuccinimidyl Carbamate (AQC) Method. Food Anal. Methods 2015, 8, 428–437. [Google Scholar] [CrossRef]
- Simmaco, M.; de Biase, D.; Barra, D.; Bossa, F. Automated amino acid analysis using precolumn derivatization with dansylchloride reversed-phase high-performance liquid chromatography. J. Chromatogr. A 1990, 504, 129–138. [Google Scholar] [CrossRef]
- Rozhon, W.; Wang, W.; Berthiller, F.; Mayerhofer, J.; Chen, T.; Petutschnig, E.; Sieberer, T.; Poppenberger, B.; Jonak, C. Bikinin-like inhibitors targeting GSK3/Shaggy-like kinases: Characterisation of novel compounds and elucidation of their catabolism in planta. BMC Plant Biol. 2014, 14, 172. [Google Scholar] [CrossRef]
- Kırkan, E.; Aydoğan, C. Free Amino Acid Analysis in Honey Samples by Hydrophilic Interaction Liquid Chromatography with UV Detection Using Precolumn Derivatization with Dansyl Chloride. Chromatographia 2021, 84, 127–133. [Google Scholar] [CrossRef]
- Zhang, X.; Zhao, T.; Cheng, T.; Liu, X.; Zhang, H. Rapid resolution liquid chromatography (RRLC) analysis of amino acids using pre-column derivatization. J. Chromatogr. B Analyt. Technol. Biomed. Life Sci. 2012, 906, 91–95. [Google Scholar] [CrossRef]
- Agius, C.; von Tucher, S.; Rozhon, W. The Effect of Salinity on Fruit Quality and Yield of Cherry Tomatoes. Horticulturae 2022, 8, 59. [Google Scholar] [CrossRef]
- Fisher, G.H.; Arias, I.; Quesada, I.; D′Aniello, S.; Errico, F.; Di Fiore, M.M.; D′Aniello, A. A fast and sensitive method for measuring picomole levels of total free amino acids in very small amounts of biological tissues. Amino Acids 2001, 20, 163–173. [Google Scholar] [CrossRef]
- Satake, K.; Take, T.; Matsuo, A.; Tazaki, K.; Hiraga, Y. Amino acid analyzer using 2,4,6-trinitrobenzenesulfonic acid. J. Biochem. 1966, 60, 12–16. [Google Scholar] [CrossRef]
- Palmer, D.W.; Peters, T. Automated determination of free amino groups in serum and plasma using 2,4,6-trinitrobenzene sulfonate. Clin. Chem. 1969, 15, 891–901. [Google Scholar] [CrossRef]
- Cayot, P.; Tainturier, G. The quantification of protein amino groups by the trinitrobenzenesulfonic acid method: A reexamination. Anal. Biochem. 1997, 249, 184–200. [Google Scholar] [CrossRef]
- Chen, L.; Chen, Q.; Zhang, Z.; Wan, X. A novel colorimetric determination of free amino acids content in tea infusions with 2,4-dinitrofluorobenzene. J. Food Compos. Anal. 2009, 22, 137–141. [Google Scholar] [CrossRef]
- Agius, C.; von Tucher, S.; Poppenberger, B.; Rozhon, W. Quantification of Glutamate and Aspartate by Ultra-High Performance Liquid Chromatography. Molecules 2018, 23, 1389. [Google Scholar] [CrossRef]
- Moore, S. Amino Acid Analysis: Aqueous Dimethyl Sulfoxide As Solvent for the Ninhydrin Reaction. J. Biol. Chem. 1968, 243, 6281–6293. [Google Scholar] [CrossRef]
- Sun, S.-W.; Lin, Y.-C.; Weng, Y.-M.; Chen, M.-J. Efficiency improvements on ninhydrin method for amino acid quantification. J. Food Compos. Anal. 2006, 19, 112–117. [Google Scholar] [CrossRef]
- Rosen, H. A Modified Ninhydrin Colorimetric Analysis for Amino Acids. Arch. Biochem. Biophys. 1957, 67, 10–15. [Google Scholar] [CrossRef]
- Fisher, L.J.; Bunting, S.L.; Rosenberg, L.E. A Modified Ninhydrin Colorimetric Method for the Determination of Plasma Alpha-Amino Nitrogen. Clin. Chem. 1963, 9, 573–581. [Google Scholar] [CrossRef]
- Standara, S.; Drdák, M.; Veselá, M. Amino acid analysis: Reduction of ninhydrin by sodium borohydride. Nahrung 1999, 43, 410–413. [Google Scholar] [CrossRef]
- Yokoyama, S.; Hiramatsu, J.-I. A modified ninhydrin reagent using ascorbic acid instead of potassium cyanide. J. Biosci. Bioeng. 2003, 95, 204–205. [Google Scholar] [CrossRef]
- Waraksa, E.; Kowalski, K.; Rola, R.; Kłodzińska, E.; Bieńkowski, T.; Namieśnik, J. Determination of free tyrosine in equestrian supplements by LC–MS/MS and comparison of its quantity with total free amino acids content in view of doping control. Microchem. J. 2019, 146, 56–65. [Google Scholar] [CrossRef]
- Ye, H.; Cai, Y.; Zhang, L.; Yu, W.; Shi, Y.; Tian, L. Determination of total free amino acids in Sipunculus nudus by UV spectrophotometry. E3S Web Conf. 2020, 189, 02013. [Google Scholar] [CrossRef]
- Kowalska, S.; Szłyk, E.; Jastrzębska, A. Simple extraction procedure for free amino acids determination in selected gluten-free flour samples. Eur. Food Res. Technol. 2022, 248, 507–517. [Google Scholar] [CrossRef]
- Nayuni, N.K.; Cloutman-Green, E.; Hollis, M.; Hartley, J.; Martin, S.; Perrett, D. Critical evaluation of ninhydrin for monitoring surgical instrument decontamination. J. Hosp. Infect. 2013, 84, 97–102. [Google Scholar] [CrossRef]
- Pickering, M.V. Ninhydrin Reagent for Use in Amine and Amino Acid Analyses. U.S. Patent 4,274,833, 18 October 1979. [Google Scholar]
- Stigliano, P.L.; Pianta, N.; Bonizzoni, S.; Mauri, M.; Simonutti, R.; Lorenzi, R.; Vigani, B.; Berbenni, V.; Rossi, S.; Mustarelli, P.; et al. A physico-chemical investigation of highly concentrated potassium acetate solutions towards applications in electrochemistry. Phys. Chem. Chem. Phys. 2021, 23, 1139–1145. [Google Scholar] [CrossRef]
- Matthews, D.M.; Muir, G.G.; Baron, D.N. Estimation of alpha-amino nitrogen in plasma and urine by the colorimetric ninhydrin reaction. J. Clin. Pathol. 1964, 17, 150–153. [Google Scholar] [CrossRef]
- Friedman, M.; Pang, J.; Smith, G.A. Ninhydrin-Reactive Lysine in Food Proteins. J. Food Sci. 1984, 49, 10–13. [Google Scholar] [CrossRef]
- Moore, S.; Stein, W.H. Photometric ninhydrin method for use in the chromatography of amino acids. J. Biol. Chem. 1948, 176, 367–388. [Google Scholar] [CrossRef]
- Whitaker, J.R. Ninhydrin assay in the presence of thiol compounds. Nature 1961, 189, 662–663. [Google Scholar] [CrossRef]
- Prota, G.; Ponsiglione, E. On the reaction of ninhydrin with cysteine and its analogues. Tetrahedron 1973, 29, 4271–4274. [Google Scholar] [CrossRef]
- European Medicines Agency. ICH Q2(R2) Validation of Analytical Procedures—Scientific Guideline. Available online: https://www.ema.europa.eu/en/ich-q2r2-validation-analytical-procedures-scientific-guideline (accessed on 8 August 2023).

| Amino Acid | Response in % Based on Asn Average ± SD |
|---|---|
| Asn (Reference) | 100 ± 2 |
| Ala | 96 ± 1 |
| Arg | 95 ± 2 |
| Asp | 111 ± 3 |
| Cys | 16 ± 2 |
| Glu | 117 ± 4 |
| Gln | 102 ± 2 |
| Gly | 81 ± 3 |
| His | 115 ± 1 |
| Ile | 100 ± 1 |
| Leu | 103 ± 1 |
| Lys | 212 ± 2 |
| Met | 95 ± 2 |
| Phe | 98 ± 1 |
| Pro | 6 ± 1 |
| Ser | 113 ± 1 |
| Thr | 101 ± 1 |
| Trp | 88 ± 1 |
| Tyr | 102 ± 2 |
| Val | 101 ± 1 |
| Ammonia (NH4+) | 117 ± 1 |
| Experiment | Repeats | Amino Acids | ||
|---|---|---|---|---|
| Average mmol L−1 | SD mmol L−1 | RSD % | ||
| Day 1 | 4 | 32.9 | 0.5 | 1.6 |
| Day 2 | 4 | 32.0 | 0.5 | 1.7 |
| Day 3 | 4 | 32.7 | 0.2 | 0.5 |
| Day 4 | 4 | 31.5 | 0.4 | 1.2 |
| Inter-day | 16 | 32.3 | 0.7 | 2.1 |
| Experiment | Repeats | Amino Acids | ||
|---|---|---|---|---|
| Average mmol L−1 | SD mmol L−1 | RSD % | ||
| Day 1 | 4 | 54.30 | 1.28 | 2.4 |
| Day 2 | 4 | 52.84 | 1.05 | 2.0 |
| Day 3 | 4 | 53.83 | 0.85 | 1.6 |
| Day 4 | 4 | 54.14 | 0.76 | 1.4 |
| Inter-day | 16 | 53.78 | 1.07 | 2.0 |
| Experiment | Repeats | Amino Acids | ||
|---|---|---|---|---|
| Average mmol L−1 | SD mmol L−1 | RSD % | ||
| Day 1 | 4 | 558 | 4 | 0.7 |
| Day 2 | 4 | 546 | 10 | 1.9 |
| Day 3 | 4 | 560 | 4 | 0.7 |
| Day 4 | 4 | 547 | 8 | 1.5 |
| Inter-day | 16 | 553 | 9 | 1.7 |
| Experiment | Equation | Pearson Correlation Coefficient |
|---|---|---|
| Day 1 | 0.6676x + 0.2021 | 0.9994 |
| Day 2 | 0.6686x + 0.2175 | 0.9996 |
| Day 3 | 0.6690x + 0.2018 | 0.9994 |
| Day 4 | 0.6821x + 0.2027 | 0.9998 |
| Sample | Spiking Level | Found | Recovery | |||
|---|---|---|---|---|---|---|
| mmol L−1 | Average mmol L−1 | SD mmol L−1 | RSD % | mmol L−1 | % | |
| Tomato juice | - | 29.8 | 0.4 | 1.2 | - | - |
| 10 | 40.1 | 0.4 | 0.9 | 10.3 | 103 | |
| 20 | 50.8 | 0.5 | 1.0 | 21.0 | 105 | |
| Soy sauce | - | 573 | 31 | 5.5 | - | - |
| 500 | 1077 | 18 | 1.7 | 504 | 101 | |
| 1000 | 1568 | 28 | 1.8 | 996 | 100 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Stauß, A.C.; Fuchs, C.; Jansen, P.; Repert, S.; Alcock, K.; Ludewig, S.; Rozhon, W. The Ninhydrin Reaction Revisited: Optimisation and Application for Quantification of Free Amino Acids. Molecules 2024, 29, 3262. https://doi.org/10.3390/molecules29143262
Stauß AC, Fuchs C, Jansen P, Repert S, Alcock K, Ludewig S, Rozhon W. The Ninhydrin Reaction Revisited: Optimisation and Application for Quantification of Free Amino Acids. Molecules. 2024; 29(14):3262. https://doi.org/10.3390/molecules29143262
Chicago/Turabian StyleStauß, Amelie Charlotte, Carolin Fuchs, Paulina Jansen, Sarah Repert, Kimberley Alcock, Sandra Ludewig, and Wilfried Rozhon. 2024. "The Ninhydrin Reaction Revisited: Optimisation and Application for Quantification of Free Amino Acids" Molecules 29, no. 14: 3262. https://doi.org/10.3390/molecules29143262
APA StyleStauß, A. C., Fuchs, C., Jansen, P., Repert, S., Alcock, K., Ludewig, S., & Rozhon, W. (2024). The Ninhydrin Reaction Revisited: Optimisation and Application for Quantification of Free Amino Acids. Molecules, 29(14), 3262. https://doi.org/10.3390/molecules29143262








