Antibiotic Alternatives: Multifunctional Ultra-Small Metal Nanoclusters for Bacterial Infectious Therapy Application
Abstract
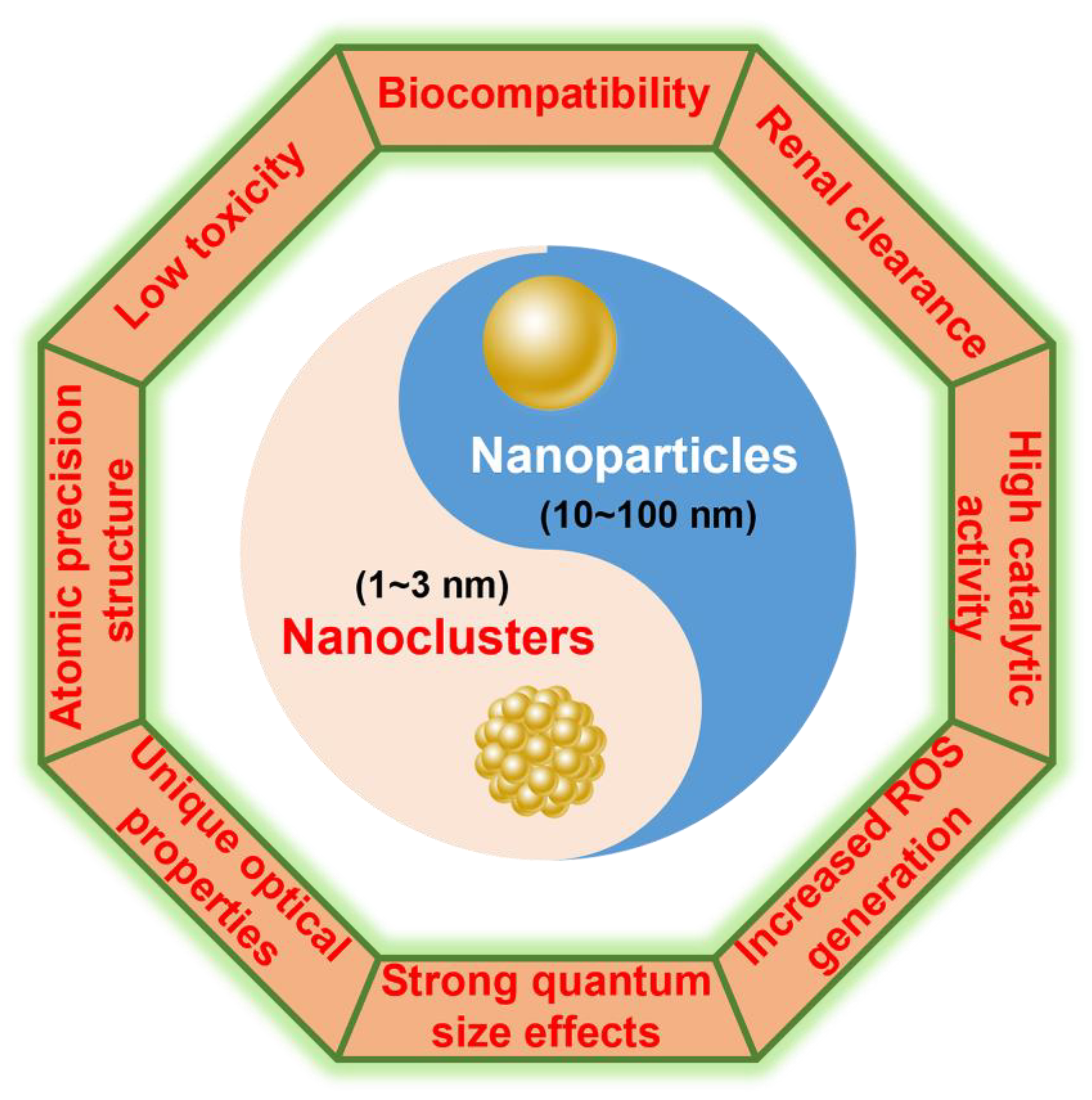
1. Introduction
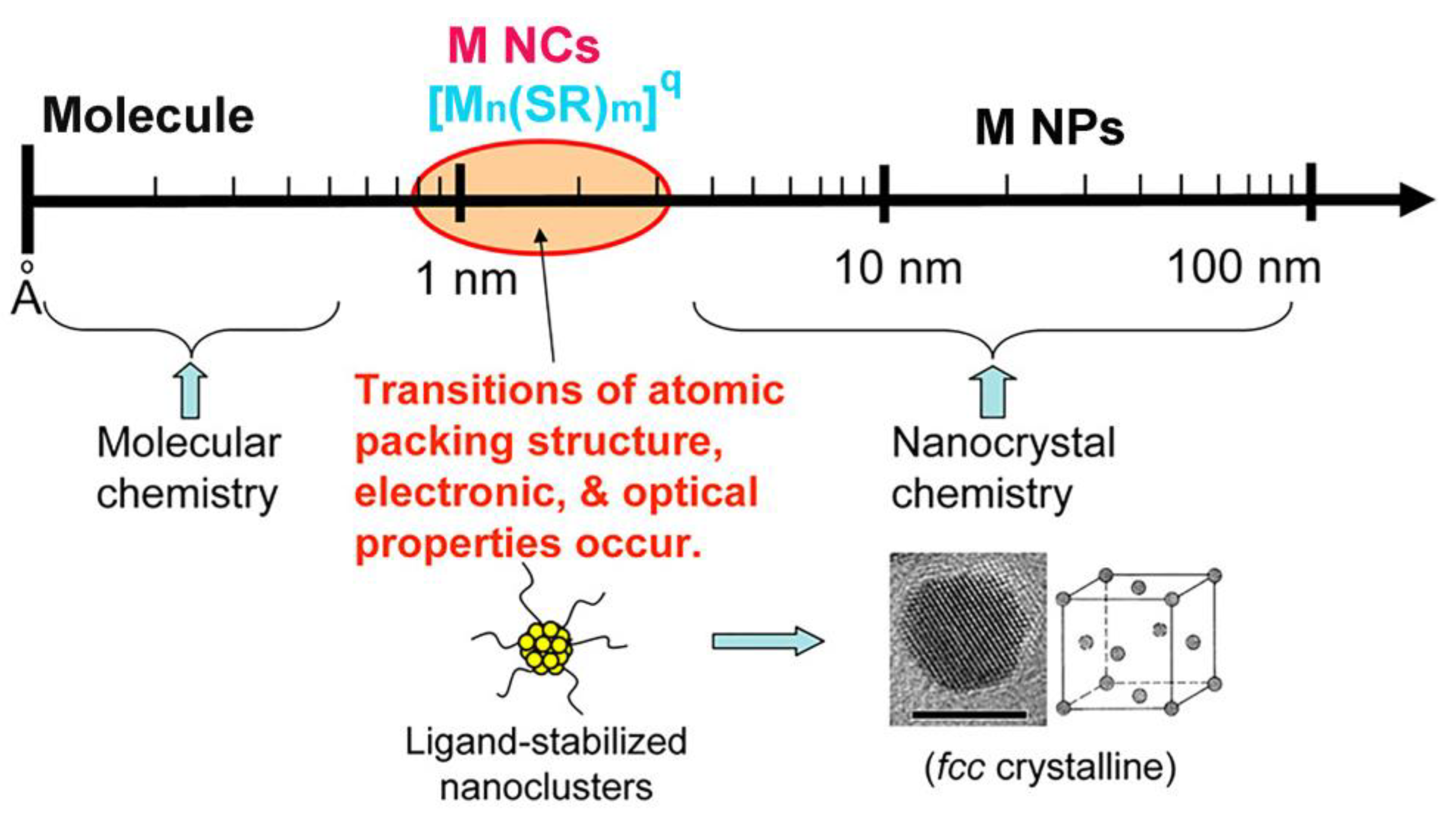
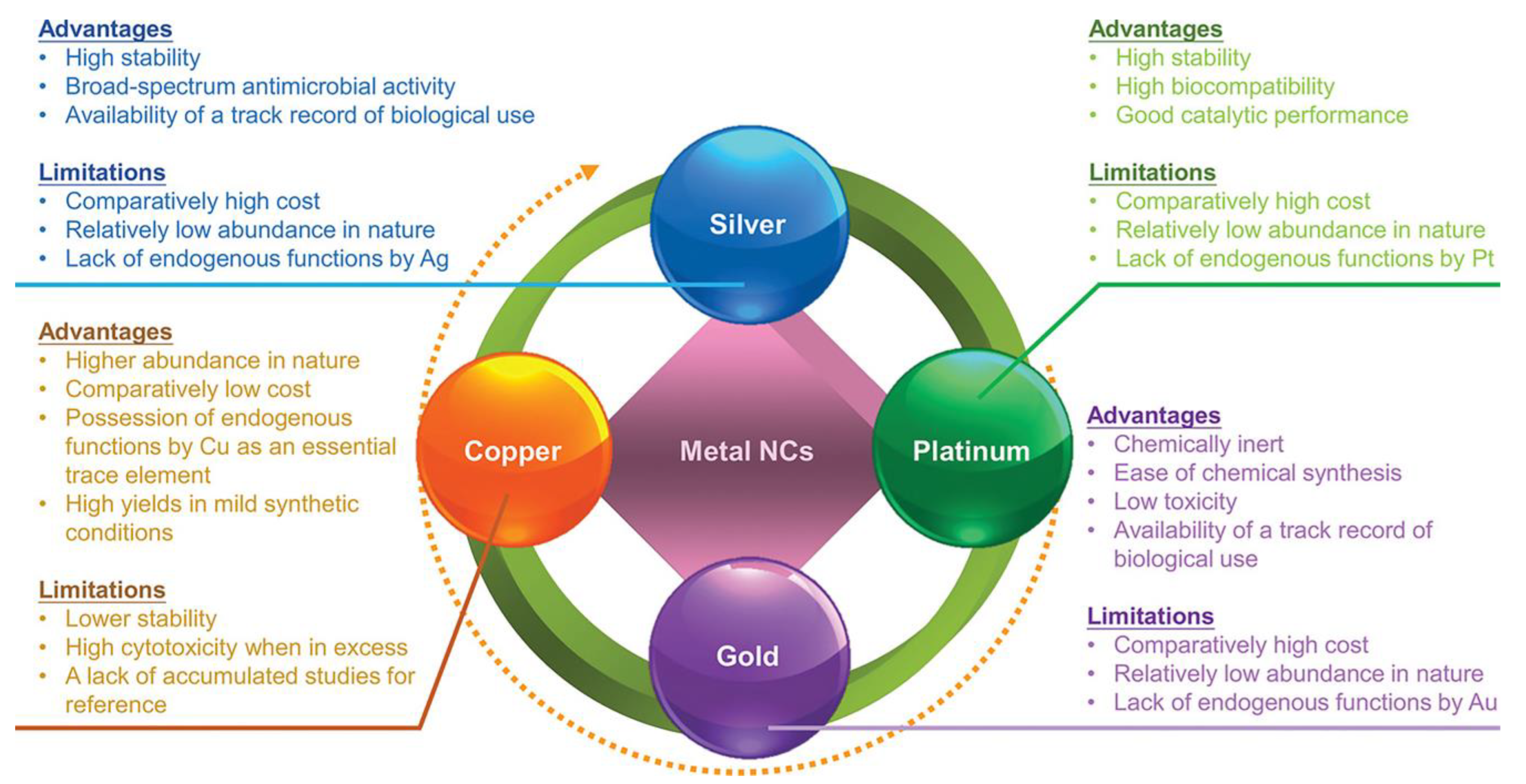
2. Synthesis and Properties of M NCs
3. Application of M NCs in Bacterial Infection
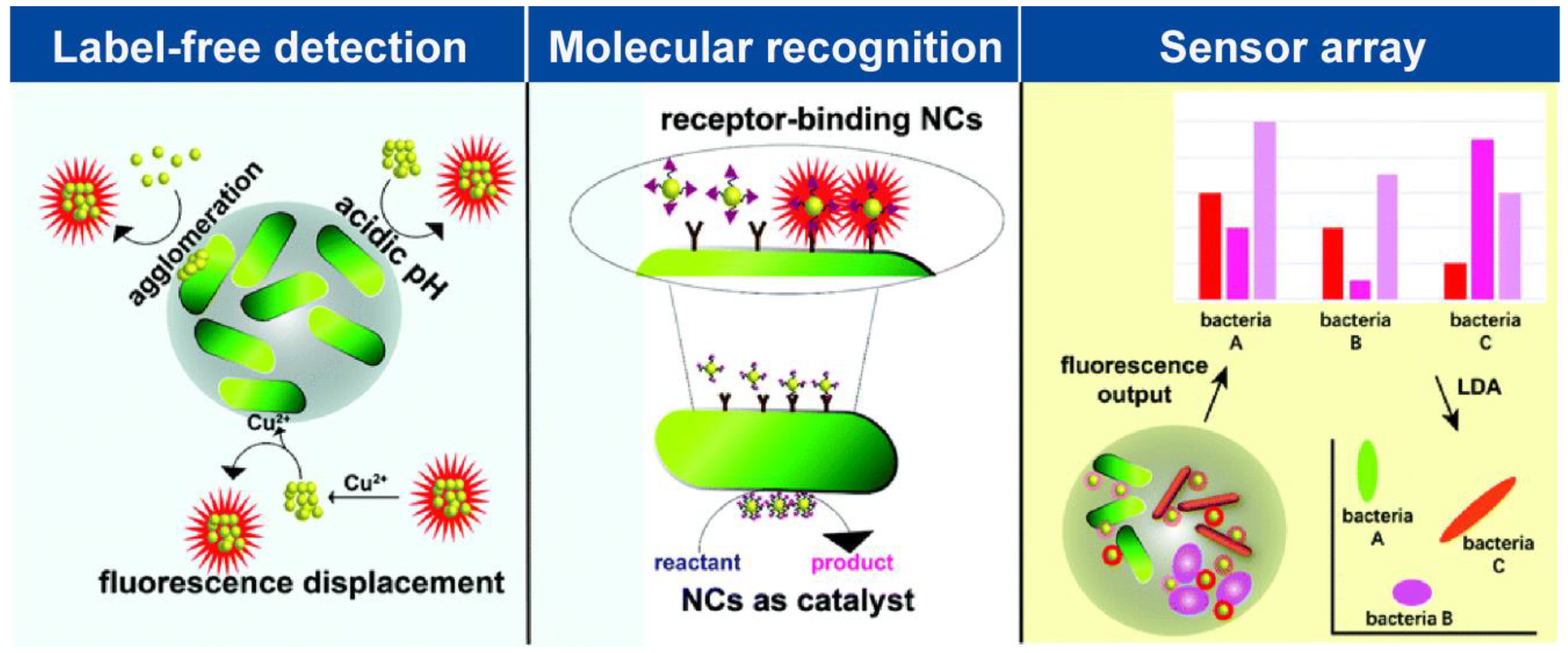
3.1. Detection of Pathogens by M NCs
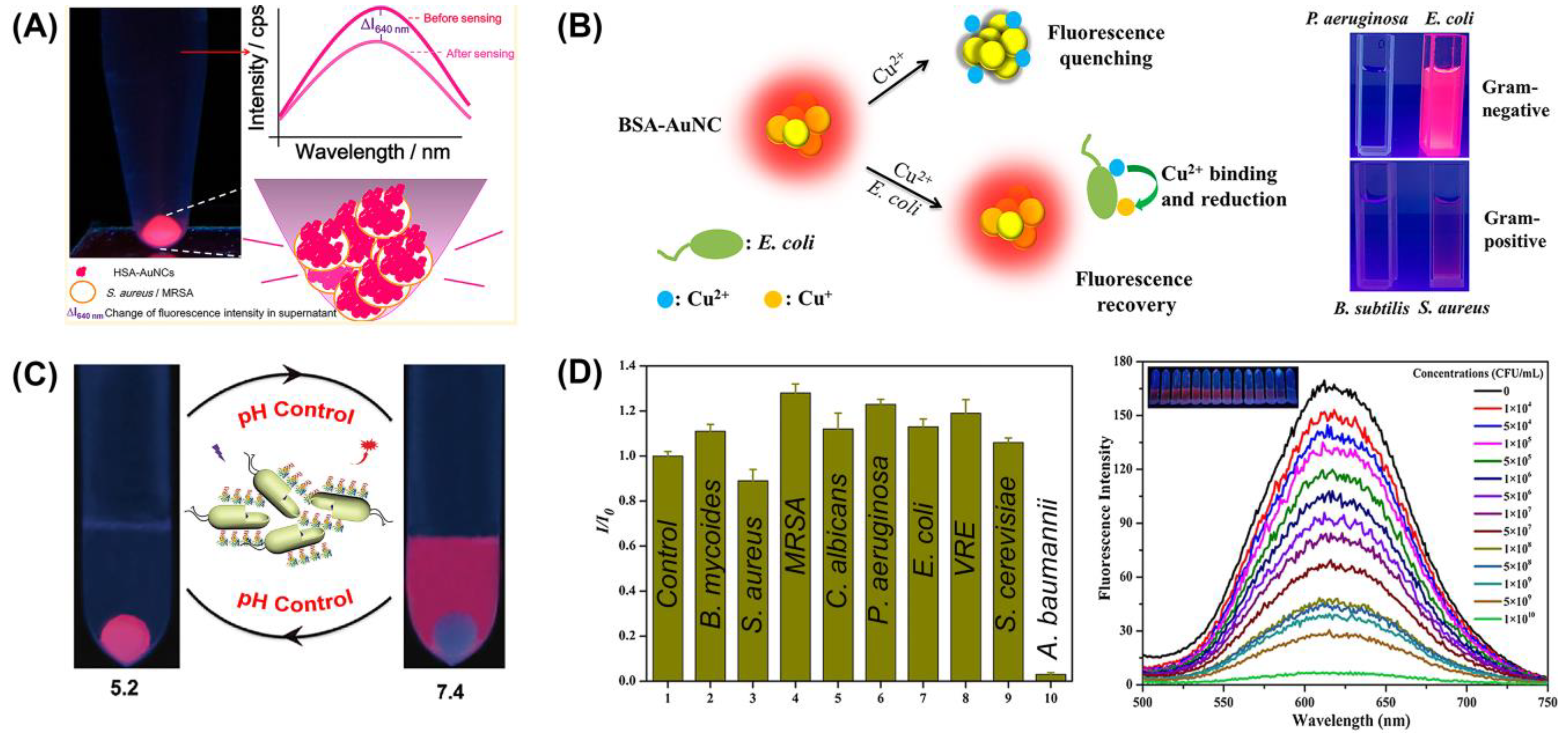
3.1.1. Label-Free Detection
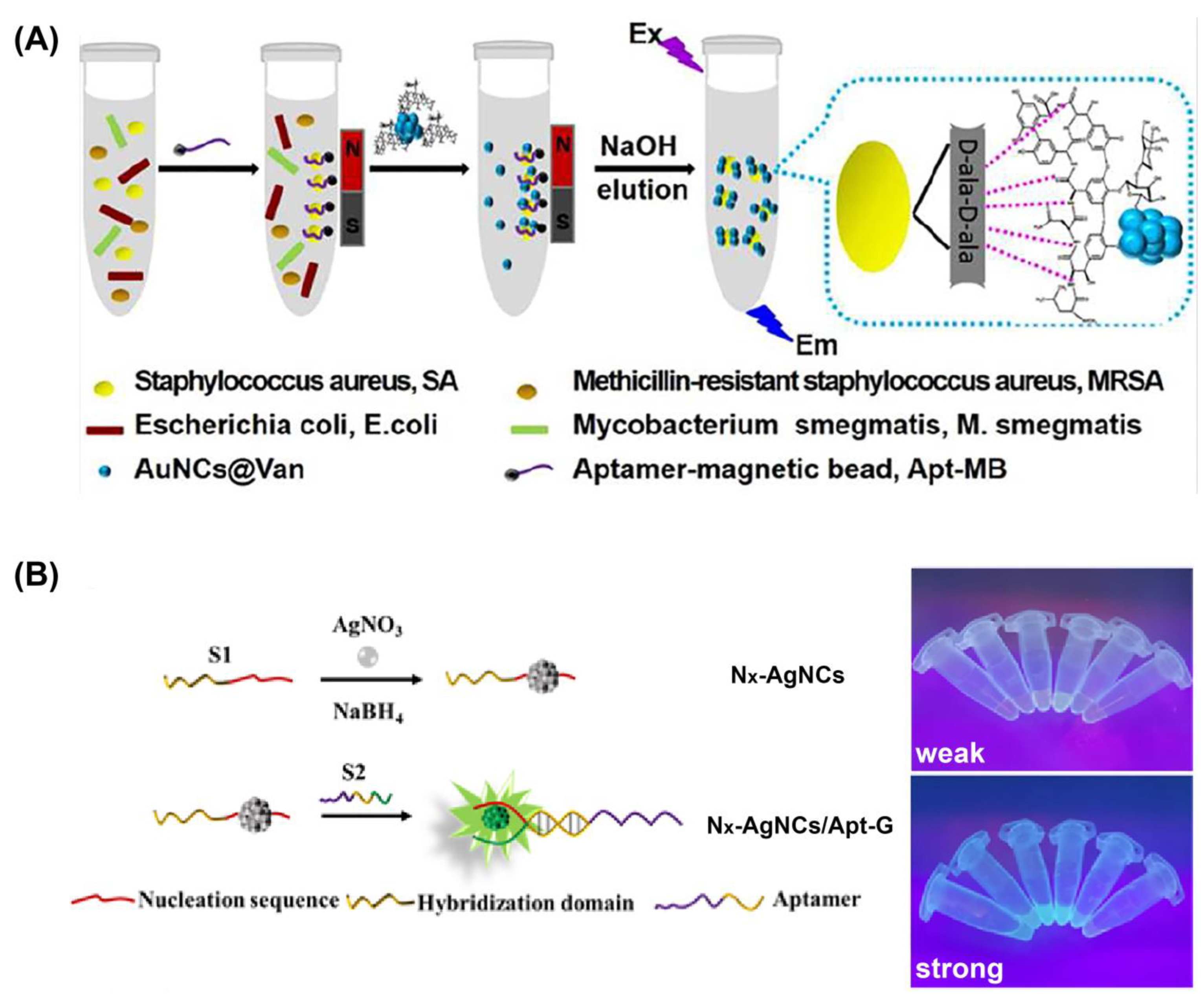
3.1.2. Molecular Recognition
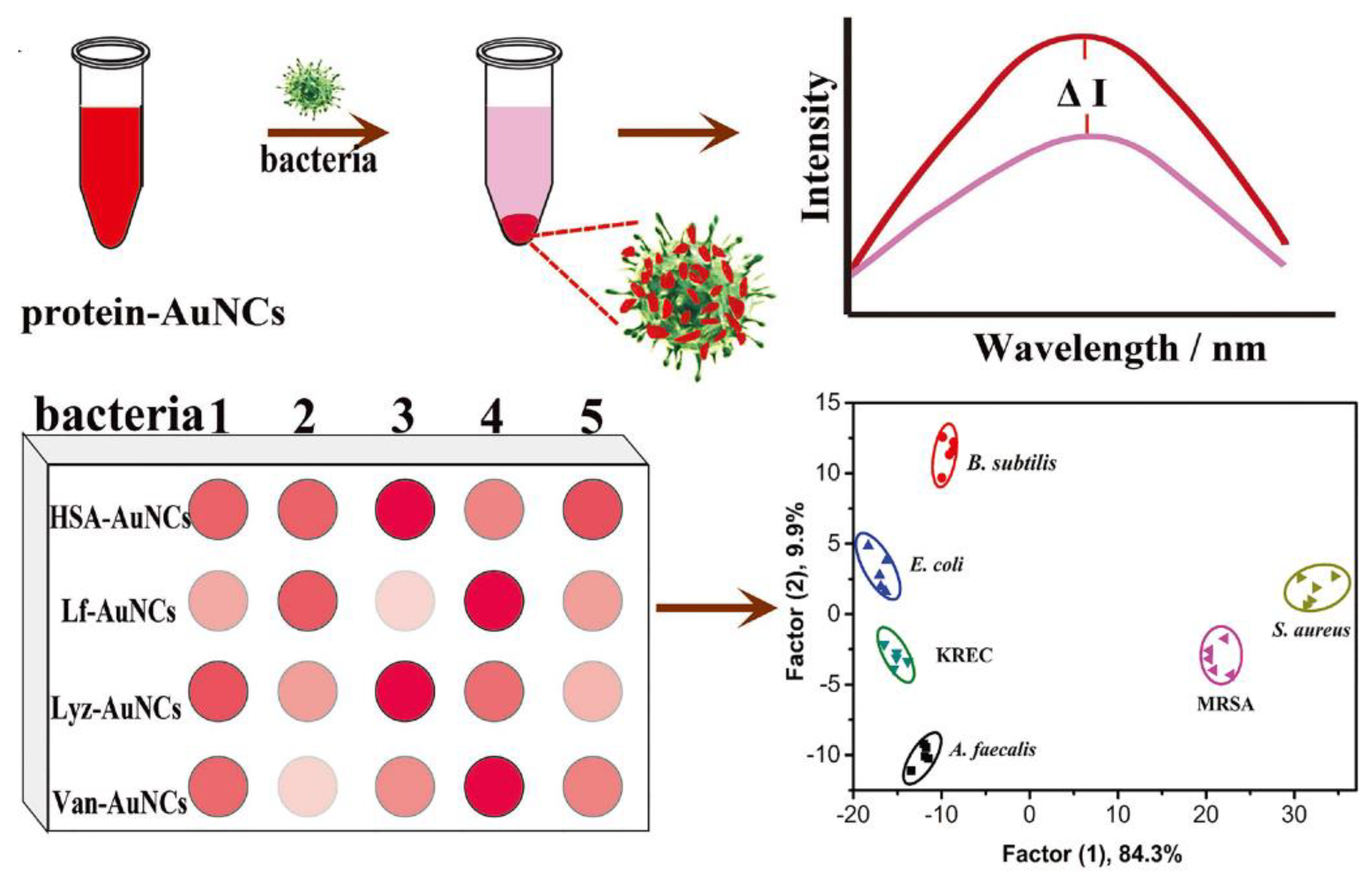
3.1.3. Sensor Array
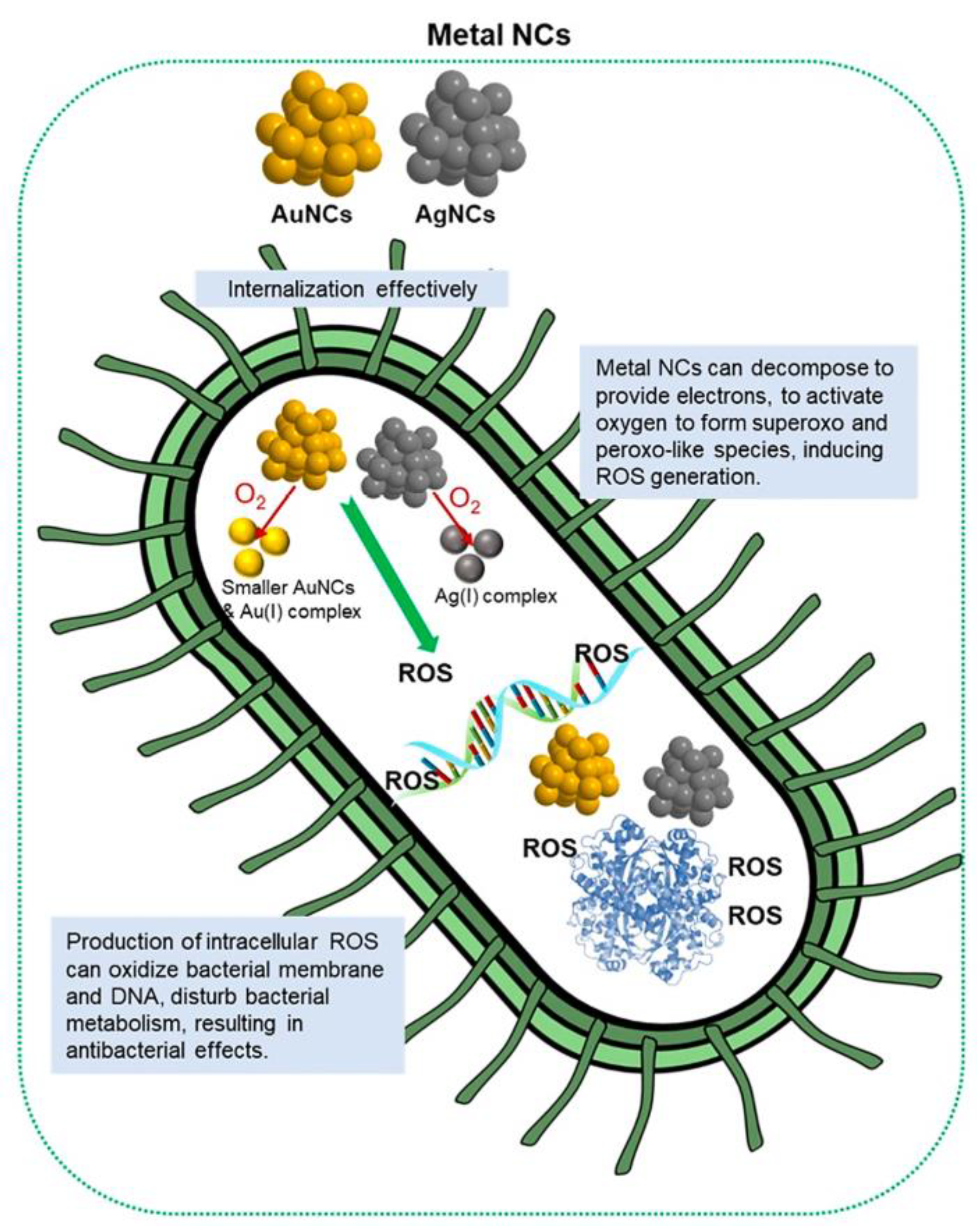
3.2. The Antibacterial Effect of M NCs
3.3. Antibacterial Infectious Therapy for M NCs
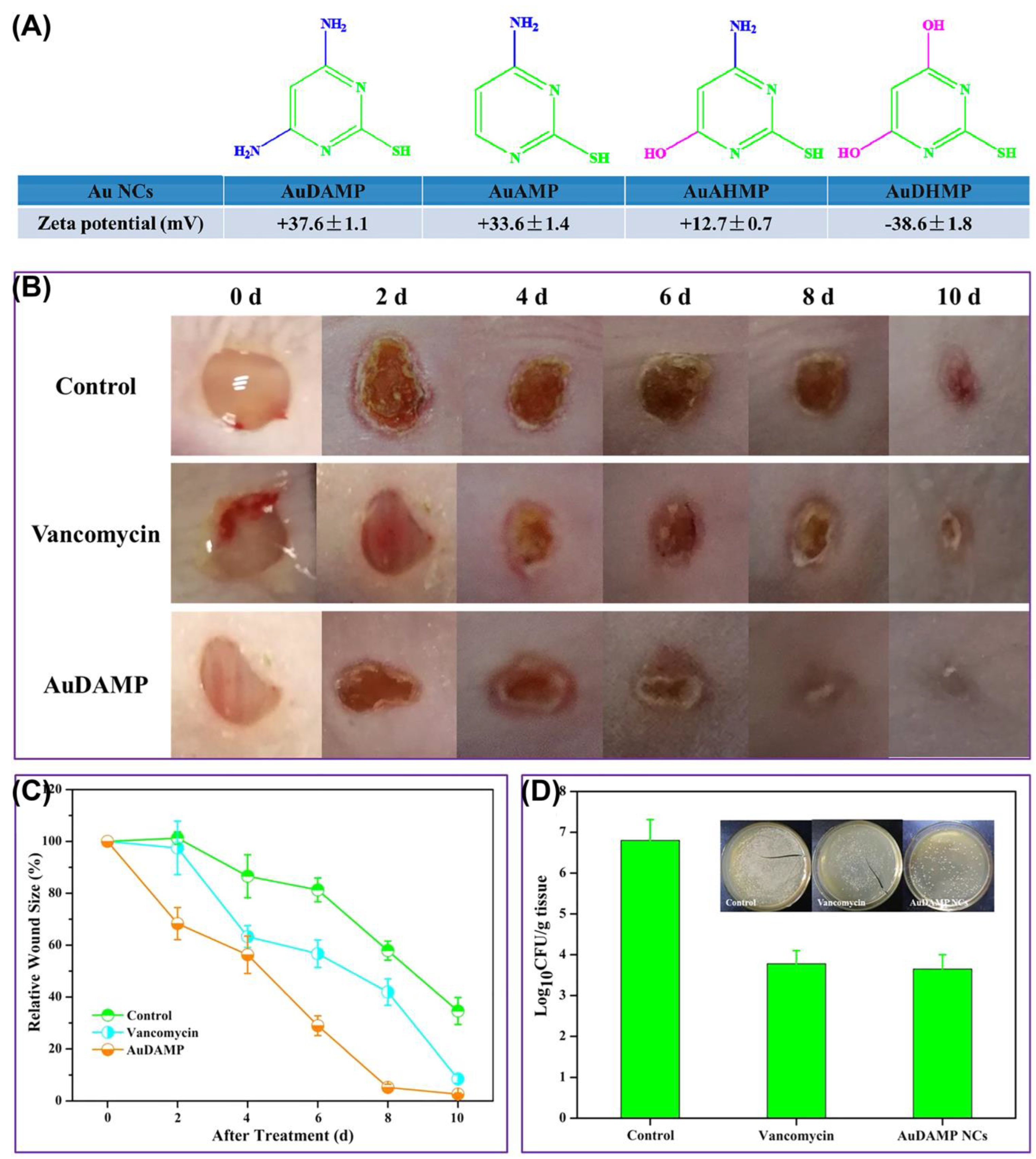
3.3.1. Promoting Wound Healing and Eliminating Inflammation
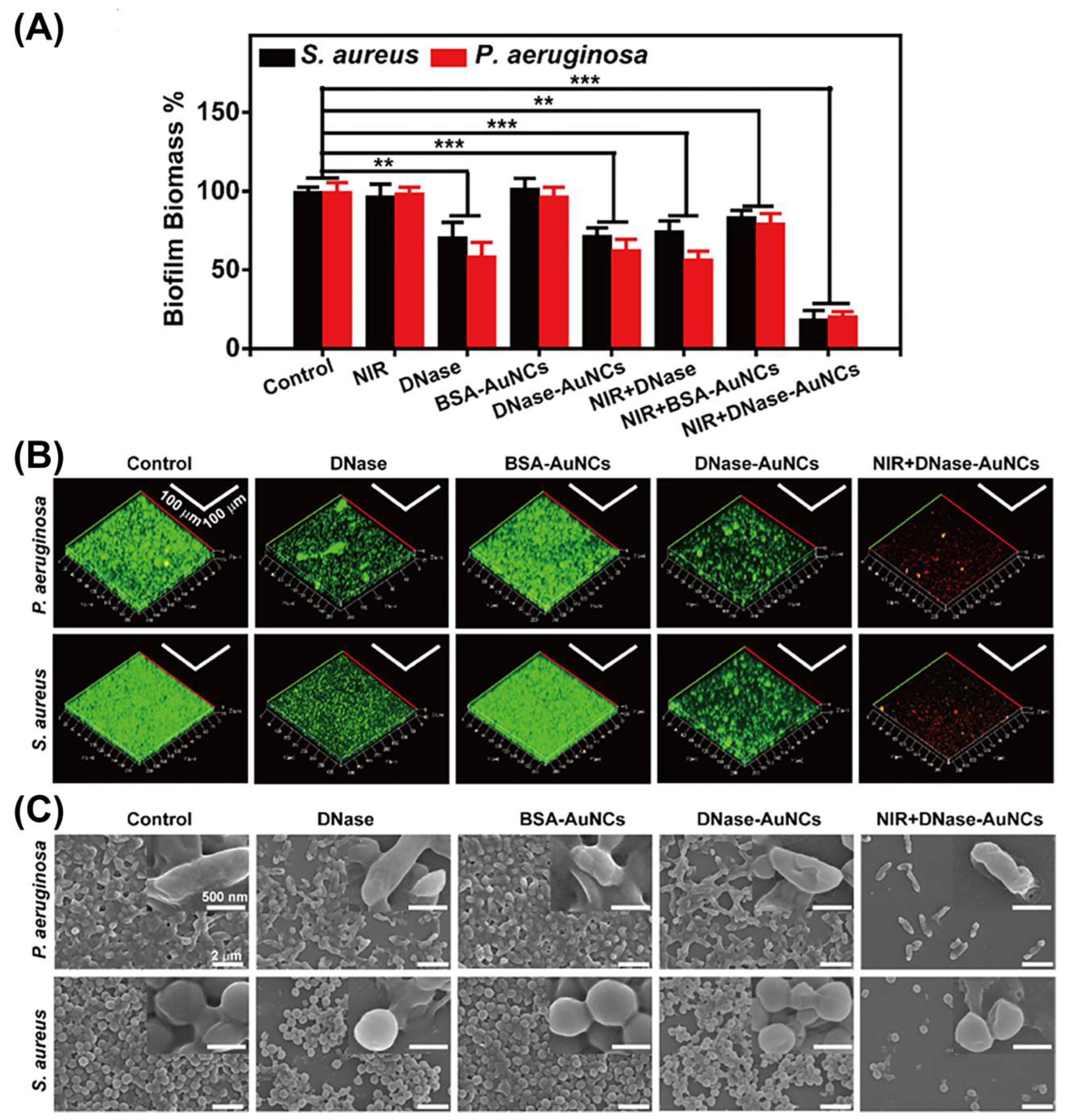
3.3.2. Clearing Biofilm and Preventing Its Formation
3.3.3. Treating Oral Bacterial Infections
4. Conclusions and Perspectives
Author Contributions
Funding
Conflicts of Interest
References
- Willyard, C. The drug-resistant bacteria that pose the greatest health threats. Nature 2017, 543, 15. [Google Scholar] [CrossRef]
- Shereen, M.A.; Khan, S.; Kazmi, A.; Bashir, N.; Siddique, R. COVID-19 infection: Emergence, transmission, and characteristics of human coronaviruses. J. Adv. Res. 2020, 24, 91–98. [Google Scholar] [CrossRef]
- Thompson, T. The staggering death toll of drug-resistant bacteria. Nature 2022. Available online: https://www.nature.com/articles/d41586-022-00228-x (accessed on 27 June 2024). [CrossRef]
- Cheng, G.; Dai, M.; Ahmed, S.; Hao, H.; Wang, X.; Yuan, Z. Antimicrobial drugs in fighting against antimicrobial resistance. Front. Microb. 2016, 7, 470. [Google Scholar] [CrossRef]
- Theuretzbacher, U.; Bush, K.; Harbarth, S.; Paul, M.; Rex, J.H.; Tacconelli, E.; Thwaites, G.E. Critical analysis of antibacterial agents in clinical development. Nat. Rev. Microbiol. 2020, 18, 286–298. [Google Scholar] [CrossRef]
- Zhao, N.; Yan, L.; Zhao, X.; Chen, X.; Li, A.; Zheng, D.; Zhou, X.; Dai, X.; Xu, F. Versatile types of organic/inorganic nanohybrids: From strategic design to biomedical applications. Chem. Rev. 2018, 119, 1666–1762. [Google Scholar] [CrossRef]
- Jian, W.; Hui, D.; Lau, D. Nanoengineering in biomedicine: Current development and future perspectives. Nanotechnol. Rev. 2020, 9, 700–715. [Google Scholar] [CrossRef]
- Makabenta, J.M.V.; Nabawy, A.; Li, C.H.; Schmidt-Malan, S.; Patel, R.; Rotello, V.R. Nanomaterial-based therapeutics for antibiotic-resistant bacterial infections. Nat. Rev. Microbiol. 2021, 19, 23–36. [Google Scholar] [CrossRef]
- Seifi, T.; Kamali, A.R. Anti-pathogenic activity of graphene nanomaterials: A review. Colloids Surf. B 2021, 199, 111509. [Google Scholar] [CrossRef]
- Xin, Q.; Shah, H.; Nawaz, A.; Xie, W.; Akram, M.Z.; Batool, A.; Tian, L.; Jan, S.U.; Boddula, R.; Guo, B.; et al. Antibacterial carbon-based nanomaterials. Adv. Mater. 2019, 31, 1804838. [Google Scholar] [CrossRef]
- Su, C.; Huang, K.; Li, H.; Lu, Y.; Zheng, D. Antibacterial properties of functionalized gold nanoparticles and their application in oral biology. J. Nanomater. 2020, 2020, 1–13. [Google Scholar]
- Zheng, K.; Setyawati, M.I.; Leong, D.T.; Xie, J. Antimicrobial silver nanomaterials. Coord. Chem. Rev. 2018, 357, 1–17. [Google Scholar] [CrossRef]
- Woźniak-Budych, M.J.; Staszak, K.; Staszak, M. Copper and copper-based nanoparticles in medicine-perspectives and challenges. Molecules 2023, 28, 6687. [Google Scholar] [CrossRef]
- Vimbela, G.V.; Ngo, S.M.; Fraze, C.; Yang, L.; Stout, D.A. Antibacterial properties and toxicity from metallic nanomaterials. Int. J. Nanomed. 2017, 12, 3941–3965. [Google Scholar] [CrossRef]
- El-Meligy, M.A.; Abd El-Monaem, E.M.; Eltaweil, A.S.; Mohy-Eldin, M.S.; Ziora, Z.M.; Heydari, A.; Omer, A.M. Recent advancements in metallic Au- and Ag-based chitosan nanocomposite derivatives for enhanced anticancer drug delivery. Molecules 2024, 29, 2393. [Google Scholar] [CrossRef]
- Xie, M.; Gao, M.; Yun, Y.; Malmsten, M.; Rotello, V.M.; Zboril, R.; Akhavan, O.; Kraskouski, A.; Amalraj, J.; Cai, X.; et al. Antibacterial nanomaterials: Mechanisms, impacts on antimicrobial resistance and design principles. Angew. Chem. Int. Ed. 2023, 62, e202217345. [Google Scholar] [CrossRef]
- Huh, A.J.; Kwon, Y.J. “Nanoantibiotics”: A new paradigm for treating infectious diseases using nanomaterials in the antibiotics resistant era. J. Control. Release 2011, 156, 128–145. [Google Scholar] [CrossRef]
- Jin, R.; Zeng, C.; Zhou, M.; Chen, Y. Atomically precise colloidal metal nanoclusters and nanoparticles: Fundamentals and opportunities. Chem. Rev. 2016, 116, 10346–10413. [Google Scholar] [CrossRef]
- Borghei, Y.S.; Hosseinkhani, S.; Ganjali, M.R. Bridging from metallic nanoclusters to biomedical in understanding physicochemical interactions at the nano–bio interface. Part. Part. Syst. Charact. 2022, 39, 2100202. [Google Scholar] [CrossRef]
- Du, X.; Jin, R. Atomically precise metal nanoclusters for catalysis. ACS Nano 2019, 13, 7383–7387. [Google Scholar] [CrossRef]
- Xue, X.; Wang, Y.; Yang, H. Preparation and characterization of boron-doped titania nano-materials with antibacterial activity. Appl. Surf. Sci. 2013, 264, 94–99. [Google Scholar] [CrossRef]
- Niu, M.; Liu, X.; Dai, J.; Hou, W.; Wei, L.; Xu, B. Molecular structure and properties of wool fiber surface-grafted with nano-antibacterial materials. Spectrochim. Acta Part A 2012, 86, 289–293. [Google Scholar] [CrossRef]
- Loynachan, C.N.; Soleimany, A.P.; Dudani, J.S.; Lin, Y.; Najer, A.; Bekdemir, A.; Chen, Q.; Bhatia, S.N.; Stevens, M.M. Renal clearable catalytic gold nanoclusters for in vivo disease monitoring. Nat. Nanotechnol. 2019, 14, 883–890. [Google Scholar] [CrossRef]
- Zheng, Y.; Liu, W.; Qin, Z.; Chen, Y.; Jiang, H.; Wang, X. Mercaptopyrimidine-conjugated gold nanoclusters as nanoantibiotics for combating multidrug-resistant superbugs. Bioconjugate Chem. 2018, 29, 3094–3103. [Google Scholar] [CrossRef]
- Zheng, K.; Xie, J. Composition-dependent antimicrobial ability of full-spectrum AuxAg25-x Alloy nanoclusters. ACS Nano 2020, 14, 11533–11541. [Google Scholar] [CrossRef]
- Zheng, K.; Xie, J. Engineering ultrasmall metal nanoclusters as promising theranostic agents. Trends Chem. 2020, 2, 665–679. [Google Scholar] [CrossRef]
- Zheng, K.; Setyawati, M.I.; Leong, D.T.; Xie, J. Overcoming bacterial physical defenses with molecule-like ultrasmall antimicrobial gold nanoclusters. Bioact. Mater. 2021, 6, 941–950. [Google Scholar] [CrossRef]
- Zheng, K.; Yuan, X.; Goswami, N.; Zhang, Q.; Xie, J. Recent advances in the synthesis, characterization, and biomedical applications of ultrasmall thiolated silver nanoclusters. RSC Adv. 2014, 4, 60581–60596. [Google Scholar] [CrossRef]
- Yao, Q.; Cao, Y.; Chen, T.; Xie, J. Total synthesis of thiolate-protected noble metal nanoclusters. In Atomically Precise Nanochemistry; WILEY Publishing: Hoboken, NJ, USA, 2023. [Google Scholar]
- Goswami, N.; Yao, Q.; Chen, T.; Xie, J. Mechanistic exploration and controlled synthesis of precise thiolate-gold nanoclusters. Coord. Chem. Rev. 2016, 329, 1–15. [Google Scholar] [CrossRef]
- Pandit, S.; Kundu, S. Methods of synthesis of metal nanoclusters. In Luminescent Metal. Nanoclusters; Woodhead Publishing: Sawston, UK, 2022; pp. 17–55. [Google Scholar]
- Yao, Q.; Wu, Z.; Liu, Z.; Lin, Y.; Yuan, X.; Xie, J. Molecular reactivity of thiolate-protected noble metal nanoclusters: Synthesis, self-assembly, and applications. Chem. Sci. 2021, 12, 99–127. [Google Scholar] [CrossRef]
- Cao, Y.; Fung, V.; Yao, Q.; Chen, T.; Zang, S.; Jiang, D.; Xie, J. Control of single-ligand chemistry on thiolated Au25 nanoclusters. Nat. Commun. 2020, 11, 5498. [Google Scholar] [CrossRef] [PubMed]
- Jana, A.; Jash, M.; Poonia, A.K.; Paramasivam, G.; Islam, M.R.; Chakraborty, P.; Antharjanam, S.; Machacek, J.; Ghos, S.; Adarsh, K.N.; et al. Light-activated intercluster conversion of an atomically precise silver nanocluster. ACS Nano 2021, 15, 15781–15793. [Google Scholar] [CrossRef]
- Rong, W.; Zou, H.; Zang, W.; Xi, S.; Wei, S.; Long, B.; Hu, J.; Ji, Y.; Duan, L. Size-dependent activity and selectivity of atomic-level copper nanoclusters during CO/CO2 electroreduction. Angew. Chem. Int. Ed. 2021, 60, 466–472. [Google Scholar] [CrossRef]
- Duan, X.; Cao, F.; Ding, R.; Li, Q.; Aisha, R.; Zhang, S.; Hua, K.; Rui, Z.; Wu, Y.; Li, J.; et al. Cobalt-doping stabilized active and durable sub-2 nm Pt nanoclusters for low-Pt-loading PEMFC cathode. Adv. Energy Mater. 2022, 12, 2103144. [Google Scholar] [CrossRef]
- Su, Y.; Xue, T.; Liu, Y.; Qi, J.; Jin, R.; Lin, Z. Luminescent metal nanoclusters for biomedical applications. Nano Res. 2019, 12, 1251–1265. [Google Scholar] [CrossRef]
- Lai, W.F.; Wong, W.T.; Rogach, A.L. Development of copper nanoclusters for in vitro and in vivo theranostic applications. Adv. Mater. 2020, 32, 1906872. [Google Scholar] [CrossRef]
- Tang, M.; Zhang, J.; Yang, C.; Zheng, H.; Jiang, H. Gold nanoclusters for bacterial detection and infection therapy. Front. Chem. 2020, 8, 181. [Google Scholar] [CrossRef]
- Yang, L.; Hou, P.; Wei, J.; Li, B.; Gao, A.; Yuan, Z. Recent advances in gold nanocluster-based biosensing and therapy: A review. Molecules 2024, 29, 1574. [Google Scholar] [CrossRef] [PubMed]
- Tao, Y.; Li, M.; Ren, J.; Qu, X. Metal nanoclusters: Novel probes for diagnostic and therapeutic applications. Chem. Soc. Rev. 2015, 44, 8636–8663. [Google Scholar] [CrossRef]
- Li, D.; Kumari, B.; Makabenta, J.M.; Gupta, A.; Rotello, V. Effective detection of bacteria using metal nanoclusters. Nanoscale 2019, 11, 22172–22181. [Google Scholar] [CrossRef]
- Qian, S.; Wang, Z.; Zuo, Z.; Wang, X.; Wang, Q.; Yuan, X. Engineering luminescent metal nanoclusters for sensing applications. Coord. Chem. Rev. 2022, 451, 214268. [Google Scholar] [CrossRef]
- Xiao, Y.; Wu, Z.; Yao, Q.; Xie, J. Luminescent metal nanoclusters: Biosensing strategies and bioimaging applications. Aggregate 2021, 2, 114–132. [Google Scholar] [CrossRef]
- Chan, P.H.; Chen, Y.C. Human serum albumin stabilized gold nanoclusters as selective luminescent probes for Staphylococcus aureus and methicillin-resistant Staphylococcus aureus. Anal. Chem. 2012, 84, 8952–8956. [Google Scholar] [CrossRef] [PubMed]
- Yan, R.; Shou, Z.; Chen, J.; Wu, H.; Zhao, Y.; Qiu, L.; Jiang, P.; Mou, X.; Wang, J.; Li, Y. On-off-on gold nanocluster-based fluorescent probe for rapid Escherichia coli differentiation, detection and bactericide screening. ACS Sustain. Chem. Eng. 2018, 6, 4504–4509. [Google Scholar] [CrossRef]
- Liu, P.; Wang, H.; Hiltunen, J.K.; Chen, Z.; Shen, J. Cross-linked proteins with gold nanoclusters: A dual-purpose pH-responsive material for controllable cell Imaging and antibiotic delivery. Part. Part. Syst. Charact. 2015, 32, 749–755. [Google Scholar] [CrossRef]
- Zheng, Y.; Wang, X.; Jiang, H. Label-free detection of Acinetobacter baumannii through the induced fluorescence quenching of thiolated AuAg nanoclusters. Sens. Actuators B 2018, 277, 388–393. [Google Scholar] [CrossRef]
- Kailasa, S.K.; Borse, S.; Koduru, J.R.; Murthy, Z.V.P. Biomolecules as promising ligands in the synthesis of metal nanoclusters: Sensing, bioimaging and catalytic applications. Trends Environ. Anal. Chem. 2021, 32, e00140. [Google Scholar] [CrossRef]
- Cheng, D.; Yu, M.; Fu, F.; Han, W.; Li, G.; Xie, J.; Song, Y.; Swihart, M.T.; Song, E. Dual recognition strategy for specific and sensitive detection of bacteria using aptamer-coated magnetic beads and antibiotic-capped gold nanoclusters. Anal. Chem. 2016, 88, 820–825. [Google Scholar] [CrossRef]
- Yang, M.; Chen, X.; Zhu, L.; Lin, S.; Li, C.; Li, X.; Huang, K.; Xu, W. Aptamer-functionalized DNA–silver nanocluster nanofilm for visual detection and elimination of bacteria. ACS Appl. Mater. Interfaces 2021, 13, 38647–38655. [Google Scholar] [CrossRef]
- Hossein-Nejad-Ariani, H.; Kim, T.; Kaur, K. Peptide-based biosensor utilizing fluorescent gold nanoclusters for detection of Listeria monocytogenes. ACS Appl. Nano Mater. 2018, 1, 3389–3397. [Google Scholar] [CrossRef]
- Chahande, A.M.; Lathigara, D.; Prabhune, A.A.; Devi, R.N. Red fluorescent ultra-small gold nanoclusters functionalized with signal molecules to probe specificity in quorum sensing receptors in gram-negative bacteria. Arch. Microbiol. 2021, 203, 4293–4301. [Google Scholar] [CrossRef] [PubMed]
- Chang, X.; Gao, P.; Li, Q.; Liu, H.; Hou, H.; Wu, S.; Chen, J.; Gan, L.; Zhao, M.; Zhang, D.; et al. Fluorescent papain-encapsulated platinum nanoclusters for sensing lysozyme in biofluid and gram-positive bacterial identification. Sens. Actuators B 2021, 345, 130363. [Google Scholar] [CrossRef]
- Ji, H.; Wu, L.; Pu, F.; Ren, J.; Qu, X. Point-of-care identification of bacteria using protein-encapsulated gold nanoclusters. Adv. Healthc. Mater. 2018, 7, 1701370. [Google Scholar] [CrossRef] [PubMed]
- Gold, K.; Slay, B.; Knackstedt, M.; Gaharwar, A.K. Antimicrobial activity of metal and metal-oxide based nanoparticles. Adv. Ther. 2018, 1, 1700033. [Google Scholar] [CrossRef]
- Li, D.; Kumari, B.; Makabenta, J.M.; Tao, B.; Qian, K.; Mei, X.; Rotello, V.M. Development of coinage metal nanoclusters as antimicrobials to combat bacterial infections. J. Mater. Chem. B 2020, 8, 9466–9480. [Google Scholar] [CrossRef] [PubMed]
- Zheng, K.; Xie, J. Cluster materials as traceable antibacterial agents. Acc. Mater. Res. 2021, 2, 1104–1116. [Google Scholar] [CrossRef]
- Chang, T.K.; Cheng, T.M.; Chu, H.L.; Tan, S.H.; Kuo, J.C.; Hsu, P.H.; Su, C.Y.; Chen, H.M.; Lee, C.M.; Kuo, T.R. Metabolic mechanism investigation of antibacterial active cysteine-conjugated gold nanoclusters in Escherichia coli. ACS Sustain. Chem. Eng. 2019, 7, 15479–15486. [Google Scholar] [CrossRef]
- Huang, H.; Hwang, G.B.; Wu, G.; Karu, K.; Toit, H.D.; Wu, H.; Callison, J.; Parkin, I.P.; Gavriilidis, A. Rapid synthesis of [Au25 (Cys)18] nanoclusters via carbon monoxide in microfluidic liquid-liquid segmented flow system and their antimicrobial performance. Chem. Eng. J. 2020, 383, 123176. [Google Scholar] [CrossRef]
- Chen, Y.; Ren, L.; Sun, L.; Bai, X.; Zhuang, G.; Cao, B.; Hu, G.; Zheng, N.; Liu, S. Amphiphilic silver nanoclusters show active nano-bio interaction with compelling antibacterial activity against multidrug-resistant bacteria. NPG Asia Mater. 2020, 12, 1–15. [Google Scholar] [CrossRef]
- Jin, J.; Wu, X.; Xu, J.; Wang, B.; Jiang, F.; Liu, Y. Ultrasmall silver nanoclusters: Highly efficient antibacterial activity and their mechanisms. Biomater. Sci. 2017, 5, 247–257. [Google Scholar] [CrossRef]
- Li, Y.; Zhen, J.; Tian, Q.; Shen, C.; Zhang, L.; Yang, K.; Shang, L. One step synthesis of positively charged gold nanoclusters as effective antimicrobial nanoagents against multidrug-resistant bacteria and biofilms. J. Colloid Interface Sci. 2020, 569, 235–243. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Y.; Liu, W.; Chen, Y.; Li, C.; Jiang, H.; Wang, X. Conjugating gold nanoclusters and antimicrobial peptides: From aggregation-induced emission to antibacterial synergy. J. Colloid Interface Sci. 2019, 546, 1–10. [Google Scholar] [CrossRef]
- Wang, S.; Wang, Y.; Peng, Y.; Yang, X. Exploring the antibacteria performance of multicolor Ag, Au, and Cu nanoclusters. ACS Appl. Mater. Interfaces 2019, 11, 8461–8469. [Google Scholar] [CrossRef]
- Zheng, K.; Setyawati, M.I.; Lim, T.P.; Leong, D.T.; Xie, J. Antimicrobial cluster bombs: Silver nanoclusters packed with daptomycin. ACS Nano 2016, 10, 7934–7942. [Google Scholar] [CrossRef] [PubMed]
- Ye, Z.; Zhu, H.; Zhang, S.; Li, J.; Wang, J.; Wang, E. Highly efficient nanomedicine from cationic antimicrobial peptide-protected Ag nanoclusters. J. Mater. Chem. B 2021, 9, 307–313. [Google Scholar] [CrossRef] [PubMed]
- Xie, Y.; Liu, Y.; Yang, J.; Liu, Y.; Hu, F.; Zhu, K.; Jiang, X. Gold nanoclusters for targeting methicillin-resistant staphylococcus aureus in vivo. Angew. Chem. Int. Ed. 2018, 57, 3958–3962. [Google Scholar] [CrossRef] [PubMed]
- Xu, D.; Wang, Q.; Yang, T.; Cao, J.; Lin, Q.; Yuan, Z.; Li, L. Polyethyleneimine capped silver nanoclusters as efficient antibacterial agents. Int. J. Environ. Res. Public Health 2016, 13, 334. [Google Scholar] [CrossRef]
- Yang, L.; Yao, C.; Li, F.; Dong, Y.; Zhang, Z.; Yang, D. Synthesis of branched DNA scaffolded super-nanoclusters with enhanced antibacterial performance. Small 2018, 14, 1800185. [Google Scholar] [CrossRef] [PubMed]
- Zhu, H.; Li, J.; Wang, J.; Wang, E. Lighting up the gold nanoclusters via host–guest recognition for high-efficiency antibacterial performance and imaging. ACS Appl. Mater. Interfaces 2019, 11, 36831–36838. [Google Scholar] [CrossRef]
- Xie, Y.; Zheng, W.; Jiang, X. Near-infrared light-activated phototherapy by gold nanoclusters for dispersing biofilms. ACS Appl. Mater. Interfaces 2020, 12, 9041–9049. [Google Scholar] [CrossRef]
- Okamoto, I.; Miyaji, H.; Miyata, S.; Shitomi, K.; Sugaya, T.; Ushijima, N.; Akasaka, T.; Enya, S.; Saita, S.; Kawasaki, H. Antibacterial and antibiofilm photodynamic activities of lysozyme-Au nanoclusters/rose bengal conjugates. ACS Omega 2021, 6, 9279–9290. [Google Scholar] [CrossRef] [PubMed]
- Ravindran, G.A.; Balasubramanian, S.; Bright, R.; Cowin, A.J.; Goswami, N.; Vasilev, K. Ultrasmall gold nanocluster based antibacterial nanoaggregates for infectious wound healing. ChemNanoMat 2019, 5, 1176–1181. [Google Scholar] [CrossRef]
- Zheng, K.; Li, K.; Chang, T.H.; Xie, J.; Chen, P. Synergistic antimicrobial capability of magnetically oriented graphene oxide conjugated with gold nanoclusters. Adv. Funct. Mater. 2019, 29, 1904603. [Google Scholar] [CrossRef]
- Chu, G.; Zhang, C.; Liu, Y.; Cao, Z.; Wang, L.; Chen, Y.; Zhou, W.; Gao, G.; Wang, K.; Cui, D. A gold nanocluster constructed mixed-metal metal-organic network film for combating iImplant-associated infections. ACS Nano 2020, 14, 15633–15645. [Google Scholar] [CrossRef] [PubMed]
- Li, K.; Li, D.; Li, C.H.; Zhuang, P.; Dai, C.; Hu, X.; Wang, D.; Liu, Y.; Mei, X.; Rotello, V.M. Efficient in vivo wound healing using noble metal nanoclusters. Nanoscale 2021, 13, 6531–6537. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Shen, B.; Zhang, Z.; Chen, Y.; Zhu, L.; Zhang, Y.; Huang, H.; Jiang, L. Multifunctional fluorescent gold nanoclusters with enhanced aggregation-induced emissions (AIEs) and excellent antibacterial effect for bacterial imaging and wound healing. Biomater. Adv. 2022, 137, 212841. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Li, C.; Shen, B.; Zhu, L.; Zhang, Y.; Jiang, L. Ultra-small Au/Pt NCs@GOX clusterzyme for enhancing cascade catalytic antibiofilm effect against F. nucleatum-induced periodontitis. Chem. Eng. J. 2023, 466, 143292. [Google Scholar] [CrossRef]
- Wu, Z.; Zhao, J.; Xu, R. Recent advances in oral nano-antibiotics for bacterial infection therapy. Int. J. Nanomed. 2020, 15, 9587–9610. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.; Chu, Z.; Jiang, Y.; Xu, L.; Qian, H.; Wang, Y.; Wang, W. Recent advances on nanomaterials for antibacterial treatment of oral diseases. Mater. Today Bio 2023, 20, 100635. [Google Scholar] [CrossRef]
- Xie, Y.; Zhang, M.; Zhang, W.; Liu, X.; Zheng, W.; Jiang, X. Gold nanoclusters-coated orthodontic devices can inhibit the formation of Streptococcus mutans biofilm. ACS Biomater. Sci. Eng. 2020, 6, 1239–1246. [Google Scholar] [CrossRef]
- Wu, T.; Sun, J.; Lei, J.; Fan, Q.; Tang, X.; Zhu, G.; Yan, Q.; Feng, X.; Shi, B. An efficient treatment of biofilm-induced periodontitis using Pt nanocluster catalysis. Nanoscale 2021, 13, 17912–17919. [Google Scholar] [CrossRef] [PubMed]

| System | Ligand | Formulation | Pathogens | Antibacterial Mechanism | Ref |
|---|---|---|---|---|---|
| M NCs modified with small molecules | Cyscein | Cys-Au NCs | E. coli | Intracellular ROS | [59] |
| Au25Cys18 | S. aureus | Photocatalytic generation of ROS | [60] | ||
| p-Mercaptobenzoic acid | Au25 NCs; Au102 NCs; Au144 NCs | S. aureus | ROS; membrane damage; metabolic inactivation | [27] | |
| Mercaptopyrimidine | AuDAMP | E. coli, MRSA | ROS; membrane damage; DNA disruption | [24] | |
| Mercaptosuccinic acid | Ag NCs | P. aeruginosa, A. baumannii, E. coli | Enzyme-like catalysis; ROS; Ag+ release | [61] | |
| Dihydrolipoic acid | DHLA-Ag NCs | E. coli | Destruction of the cell membrane and fluidity; ROS; destruction the cytoplasmic membrane respiratory chain and DNA | [62] | |
| MUTAB | MUTAB-Au NCs | B. Subtilis, S. pneumonia, E.coli | Membrane damage; DNA leakage; ROS | [63] | |
| M NCs modified with antibacterial substances | Daptomycin | Dap-AUDAMP NCs | MRSA | ROS; destruction of the cell membrane and DNA | [64] |
| Bacitracin | AuNCs@Bacitracin; AgNCs@Bacitracin; CuNCs@Bacitracin | S. aureus | ROS; destruction of the cell membrane | [65] | |
| Daptomycin | Ag NCs | S. aureus | ROS; destruction of the cell membrane and DNA | [66] | |
| CCLLLLRRRRRR (Dpep) | Dpep-Ag NCs | E. coli, S.aureus | ROS; Ag+ release | [67] | |
| Quaternary ammonium (QA) | QA-Au NCs | MRSA | ROS; destruction of the cell membrane; membrane depolarization | [68] | |
| Polyethyleneimine (PEI) | PEI-Ag NCs | E. coli | PEI penetration and sterilization; Ag+ release | [69] | |
| M NCs modified with biomacromolecules | DNA | DNA/Ag NC | E. coli | Ag+ | [70] |
| Protamine (Prot) | Prot/MTU-Au NCs | E. coli, MRSA | ROS; destruction the cell membrane | [71] | |
| DNase | DNase-Au NCs | E. coli, S. aureus | Photothermal and photodynamic effect | [72] | |
| Nanocluster hybrid system | Lysozyme (Lys) | Lys-Au NCs/RB | S. mutans, E. coli, A. naeslundii, P. gingivalis, P. intermedia | ROS; destruction of the cell membrane | [73] |
| Mercaptosuccinic acid (MSA) | Au NCs/CS | E. coli, S. aureus | ROS; destruction of the cell membrane | [74] | |
| 6-Mercaptohexanoic acid, cysteamine | Au NCs/Ho-GO | E. coli, S. aureus | Piercing of bacterial membranes; ROS; metabolic inactivation | [75] | |
| p-Mercaptobenzoic acid | GNCs-based mixed-metal metal−organic network (MM-MON) | E. coli, S. aureus | Destruction of the cell membrane | [76] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, Y.; Gu, M.; Cheng, J.; Wan, Y.; Zhu, L.; Gao, Z.; Jiang, L. Antibiotic Alternatives: Multifunctional Ultra-Small Metal Nanoclusters for Bacterial Infectious Therapy Application. Molecules 2024, 29, 3117. https://doi.org/10.3390/molecules29133117
Wang Y, Gu M, Cheng J, Wan Y, Zhu L, Gao Z, Jiang L. Antibiotic Alternatives: Multifunctional Ultra-Small Metal Nanoclusters for Bacterial Infectious Therapy Application. Molecules. 2024; 29(13):3117. https://doi.org/10.3390/molecules29133117
Chicago/Turabian StyleWang, Yuxian, Meng Gu, Jiangyang Cheng, Yusong Wan, Liying Zhu, Zhen Gao, and Ling Jiang. 2024. "Antibiotic Alternatives: Multifunctional Ultra-Small Metal Nanoclusters for Bacterial Infectious Therapy Application" Molecules 29, no. 13: 3117. https://doi.org/10.3390/molecules29133117
APA StyleWang, Y., Gu, M., Cheng, J., Wan, Y., Zhu, L., Gao, Z., & Jiang, L. (2024). Antibiotic Alternatives: Multifunctional Ultra-Small Metal Nanoclusters for Bacterial Infectious Therapy Application. Molecules, 29(13), 3117. https://doi.org/10.3390/molecules29133117






