Hypervalent Iodine-Catalyzed Fluorination of Diene-Containing Compounds: A Computational Study
Abstract
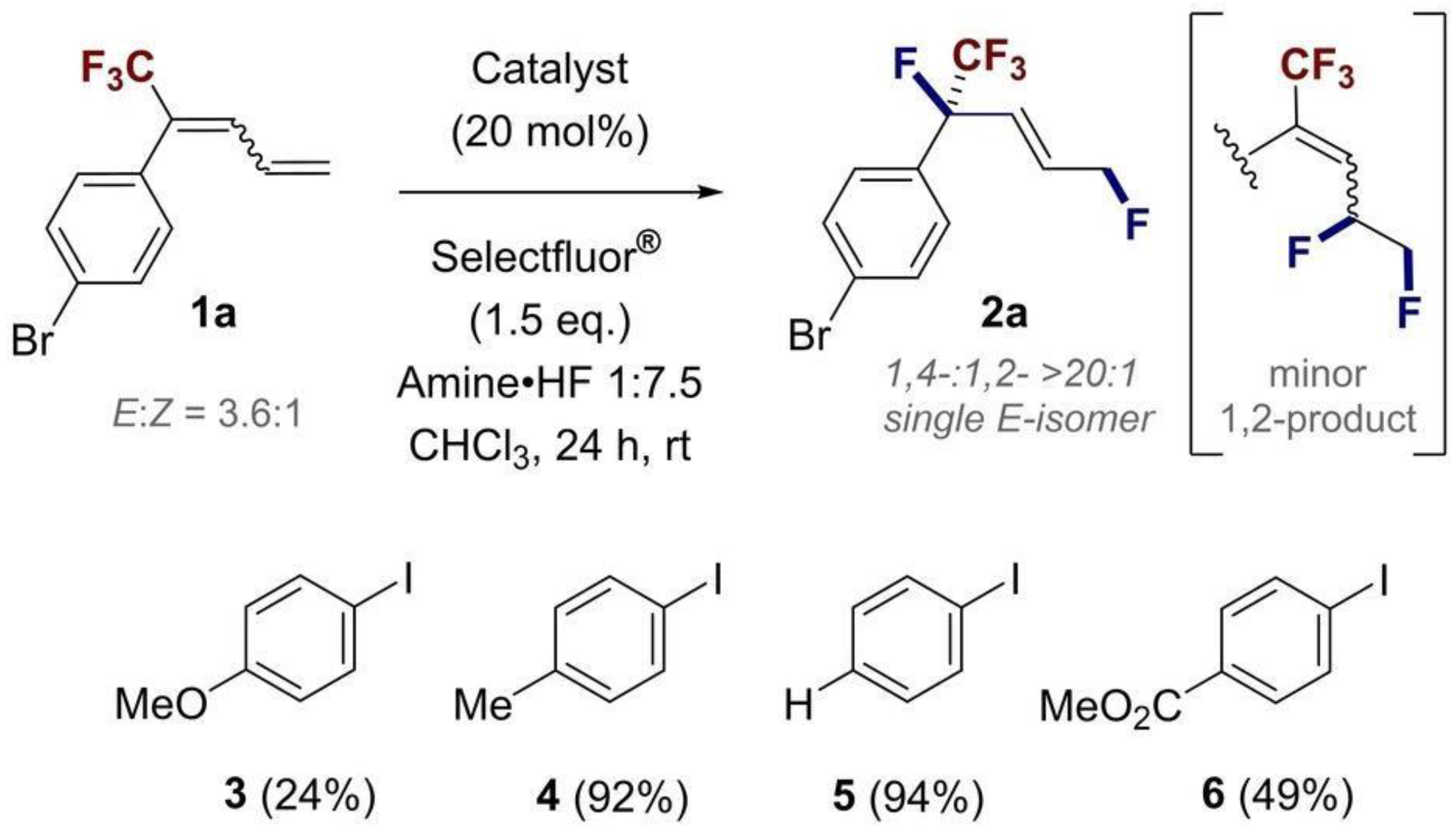
1. Introduction
2. Results
2.1. Electrostatic Potential Analysis
2.2. The Mechanism of Diene Difluoride
2.2.1. Difluorination of E-Configured Dienes Catalyzed by Hypervalent Iodine Reagents
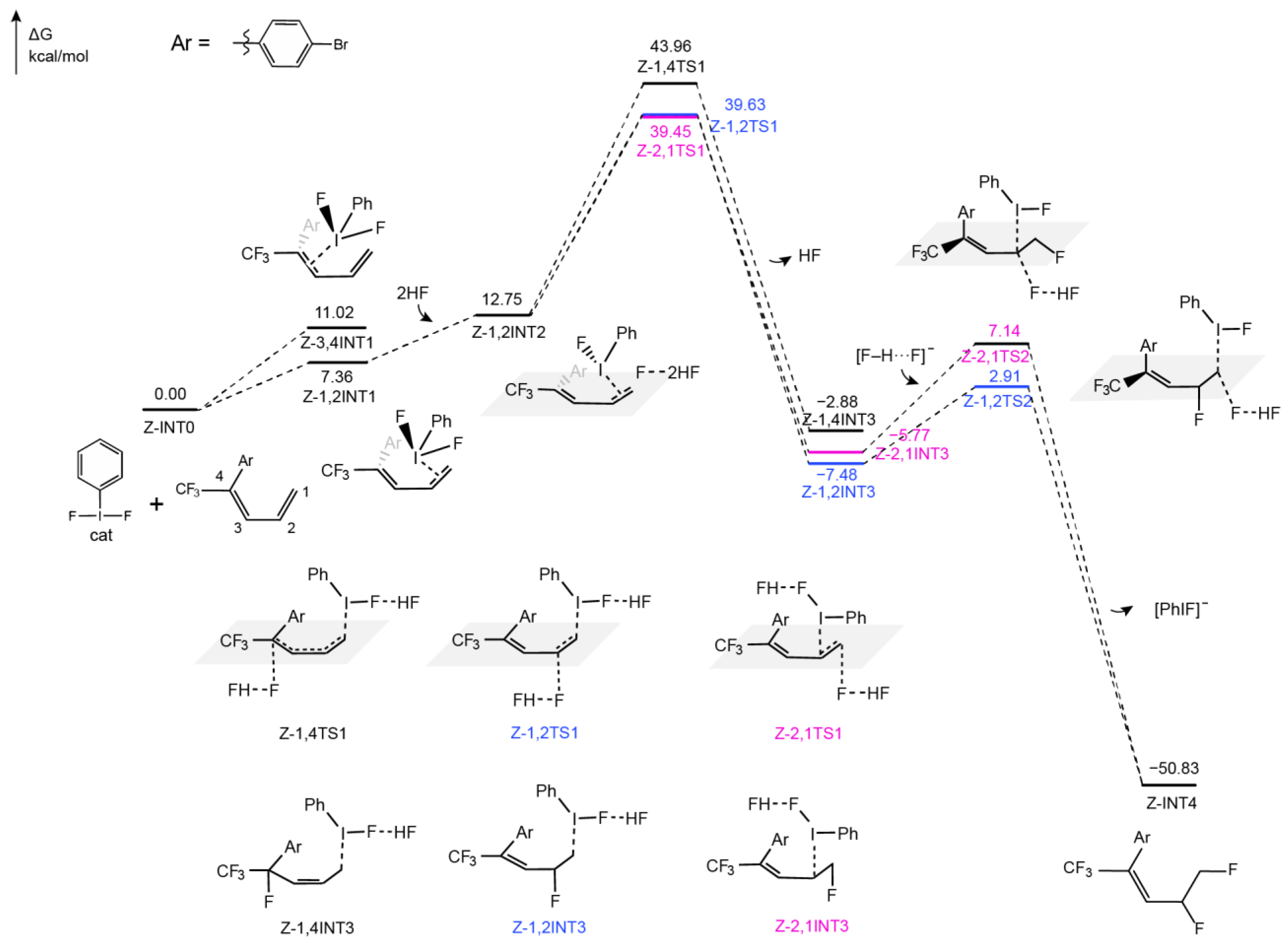
2.2.2. Difluorination of Z-Configured Dienes Catalyzed by Hypervalent Iodine Reagents
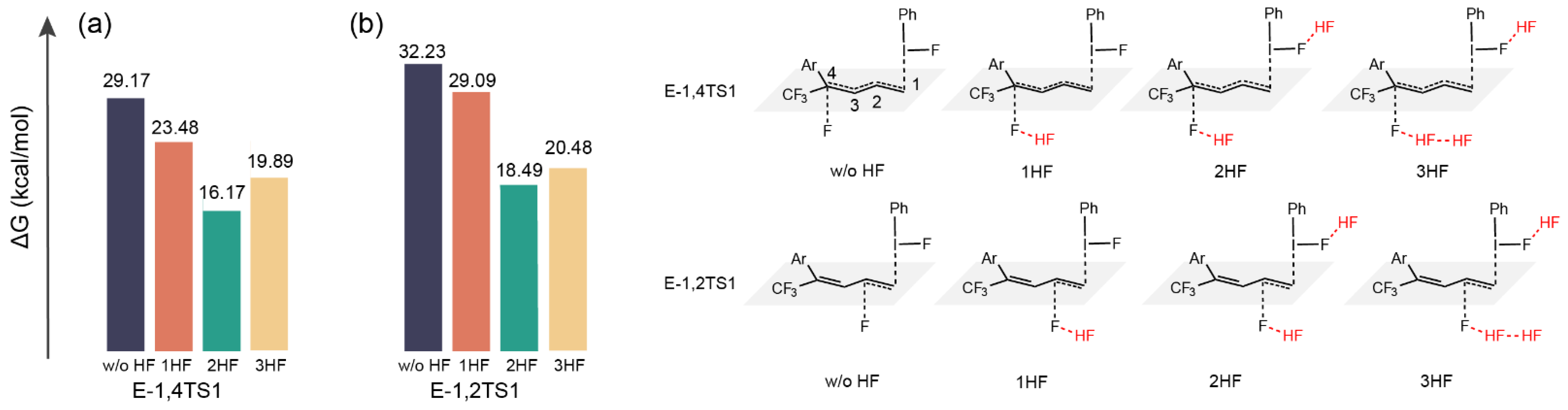
2.3. The Role of HF
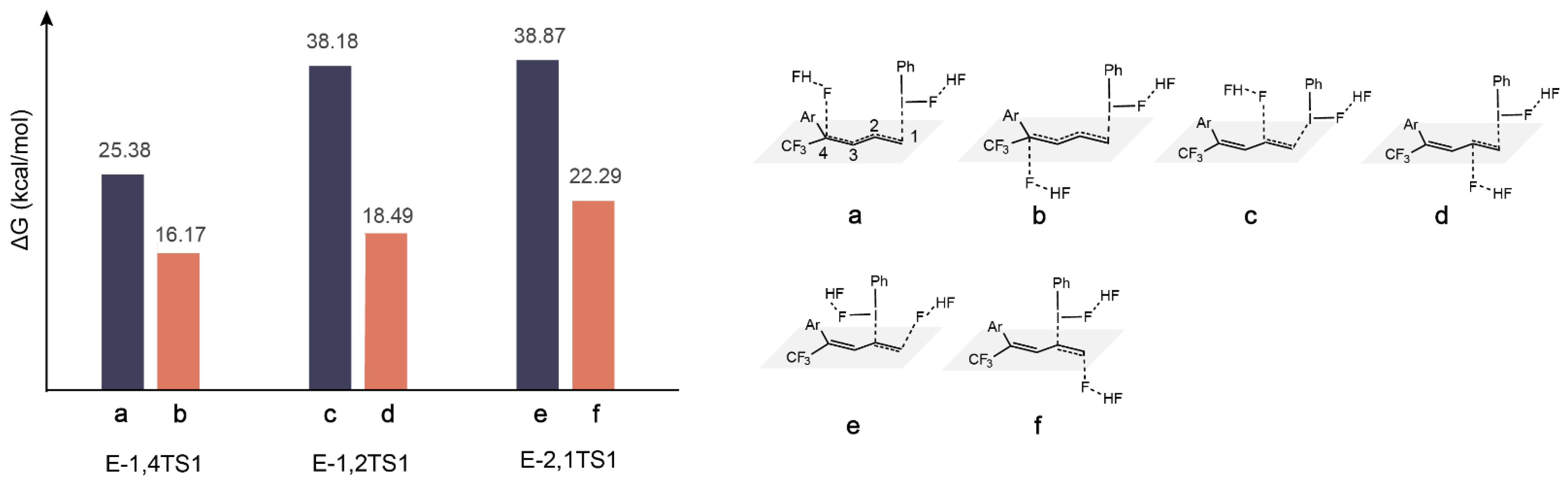
2.4. The Direction of Preference in F− Attacking C
3. Materials and Methods
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- O’Hagan, D. Understanding Organofluorine Chemistry. An Introduction to the C–F Bond. Chem. Soc. Rev. 2008, 37, 308–319. [Google Scholar] [CrossRef] [PubMed]
- Yan, G.; Qiu, K.; Guo, M. Recent Advance in the C–F Bond Functionalization of Trifluoromethyl-Containing Compounds. Org. Chem. Front. 2021, 8, 3915–3942. [Google Scholar] [CrossRef]
- Zhu, Y.; Han, J.; Wang, J.; Shibata, N.; Sodeoka, M.; Soloshonok, V.A.; Coelho, J.A.S.; Toste, F.D. Modern Approaches for Asymmetric Construction of Carbon–Fluorine Quaternary Stereogenic Centers: Synthetic Challenges and Pharmaceutical Needs. Chem. Rev. 2018, 118, 3887–3964. [Google Scholar] [CrossRef]
- Huang, Y.; Hayashi, T. Rhodium-Catalyzed Asymmetric Arylation/Defluorination of 1-(Trifluoromethyl)Alkenes Forming Enantioenriched 1,1-Difluoroalkenes. J. Am. Chem. Soc. 2016, 138, 12340–12343. [Google Scholar] [CrossRef] [PubMed]
- Xi, Y.; Hartwig, J.F. Mechanistic Studies of Copper-Catalyzed Asymmetric Hydroboration of Alkenes. J. Am. Chem. Soc. 2017, 139, 12758–12772. [Google Scholar] [CrossRef] [PubMed]
- Harsanyi, A.; Sandford, G. Organofluorine Chemistry: Applications, Sources and Sustainability. Green Chem. 2015, 17, 2081–2086. [Google Scholar] [CrossRef]
- Müller, K.; Faeh, C.; Diederich, F. Fluorine in Pharmaceuticals: Looking Beyond Intuition. Science 2007, 317, 1881–1886. [Google Scholar] [CrossRef] [PubMed]
- Inoue, M.; Sumii, Y.; Shibata, N. Contribution of Organofluorine Compounds to Pharmaceuticals. ACS Omega 2020, 5, 10633–10640. [Google Scholar] [CrossRef]
- Benedetto Tiz, D.; Bagnoli, L.; Rosati, O.; Marini, F.; Sancineto, L.; Santi, C. New Halogen-Containing Drugs Approved by FDA in 2021: An Overview on Their Syntheses and Pharmaceutical Use. Molecules 2022, 27, 1643. [Google Scholar] [CrossRef]
- Fujiwara, T.; O’Hagan, D. Successful Fluorine-Containing Herbicide Agrochemicals. J. Fluor. Chem. 2014, 167, 16–29. [Google Scholar] [CrossRef]
- Jeschke, P. Recent Developments in Fluorine-containing Pesticides. Pest Manag. Sci. 2024, 80, 3065–3087. [Google Scholar] [CrossRef]
- Wang, Y.; Wu, Z.; Azad, F.M.; Zhu, Y.; Wang, L.; Hawker, C.J.; Whittaker, A.K.; Forsyth, M.; Zhang, C. Fluorination in Advanced Battery Design. Nat. Rev. Mater. 2023, 9, 119–133. [Google Scholar] [CrossRef]
- Berger, R.; Resnati, G.; Metrangolo, P.; Weber, E.; Hulliger, J. Organic Fluorine Compounds: A Great Opportunity for Enhanced Materials Properties. Chem. Soc. Rev. 2011, 40, 3496. [Google Scholar] [CrossRef] [PubMed]
- Nishikawa, D.; Nakajima, T.; Ohzawa, Y.; Koh, M.; Yamauchi, A.; Kagawa, M.; Aoyama, H. Thermal and Oxidation Stability of Organo-Fluorine Compound-Mixed Electrolyte Solutions for Lithium Ion Batteries. J. Power Sources 2013, 243, 573–580. [Google Scholar] [CrossRef]
- Guo, Z.; Yu, Q.; Chen, Y.; Liu, J.; Li, T.; Peng, Y.; Yi, W. Fluorine-Containing Functional Group-Based Energetic Materials. Chem. Rec. 2023, 23, e202300108. [Google Scholar] [CrossRef]
- Li, R.; Gao, T.; Wang, Y.; Chen, Y.; Luo, W.; Wu, Y.; Xie, Y.; Wang, Y.; Zhang, Y. Engineering of Bimetallic Au–Pd Alloyed Particles on Nitrogen Defects Riched g-C3N4 for Efficient Photocatalytic Hydrogen Production. Int. J. Hydrogen Energy 2024, 63, 1116–1127. [Google Scholar] [CrossRef]
- Grabowski, S.J. Halogen Bonds between Diiodotetrafluorobenzenes and Halide Anions: Theoretical Analysis. Cryst. Growth Des. 2023, 23, 489–500. [Google Scholar] [CrossRef]
- Yoshimura, A.; Zhdankin, V.V. Advances in Synthetic Applications of Hypervalent Iodine Compounds. Chem. Rev. 2016, 116, 3328–3435. [Google Scholar] [CrossRef] [PubMed]
- Arndt, T.; Raina, A.; Breugst, M. Iodine-Catalyzed Claisen-Rearrangements of Allyl Aryl Ethers and Subsequent Iodocyclizations. Chem. Asian J. 2023, 18, e202201279. [Google Scholar] [CrossRef] [PubMed]
- Fujita, M. Mechanistic Aspects of Alkene Oxidation Using Chiral Hypervalent Iodine Reagents. Tetrahedron Lett. 2017, 58, 4409–4419. [Google Scholar] [CrossRef]
- Soni, R.; Sihag, M.; Rani, N.; Kinger, M.; Aneja, D.K. Aqueous Mediated Reactions Involving Hypervalent Iodine Reagents. Asian J. Org. Chem. 2022, 11, e202200125. [Google Scholar] [CrossRef]
- Hyatt, I.F.D.; Dave, L.; David, N.; Kaur, K.; Medard, M.; Mowdawalla, C. Hypervalent Iodine Reactions Utilized in Carbon–Carbon Bond Formations. Org. Biomol. Chem. 2019, 17, 7822–7848. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Studer, A. Iodine(III) Reagents in Radical Chemistry. Acc. Chem. Res. 2017, 50, 1712–1724. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Chen, P.; Liu, G. Recent Advances in Hypervalent Iodine(III)-Catalyzed Functionalization of Alkenes. Beilstein J. Org. Chem. 2018, 14, 1813–1825. [Google Scholar] [CrossRef] [PubMed]
- He, J.; Du, F.-H.; Zhang, C.; Du, Y. Chemoselective Cycloisomerization of O-Alkenylbenzamides via Concomitant 1,2-Aryl Migration/Elimination Mediated by Hypervalent Iodine Reagents. Commun. Chem. 2023, 6, 126. [Google Scholar] [CrossRef] [PubMed]
- Banik, S.M.; Medley, J.W.; Jacobsen, E.N. Catalytic, Diastereoselective 1,2-Difluorination of Alkenes. J. Am. Chem. Soc. 2016, 138, 5000–5003. [Google Scholar] [CrossRef] [PubMed]
- Meyer, S.; Häfliger, J.; Schäfer, M.; Molloy, J.J.; Daniliuc, C.G.; Gilmour, R. Eine chirale pentafluorierte Isopropylgruppe durch Iod(I)/(III)-Katalyse. Angew. Chem. 2021, 133, 6501–6506. [Google Scholar] [CrossRef]
- Kohlhepp, S.V.; Gulder, T. Hypervalent Iodine(iii) Fluorinations of Alkenes and Diazo Compounds: New Opportunities in Fluorination Chemistry. Chem. Soc. Rev. 2016, 45, 6270–6288. [Google Scholar] [CrossRef] [PubMed]
- Yu, Y.; Schäfer, M.; Daniliuc, C.G.; Gilmour, R. Catalytic, Regioselective 1,4-Fluorodifunctionalization of Dienes. Angew. Chem. Int. Ed. 2023, 62, e202214906. [Google Scholar] [CrossRef]
- Gadre, S.R.; Bhadane, P.K. Electrostatics in Chemistry: 3. Molecular Electrostatic Potential: Visualization and Topography. Resonance 1999, 4, 14–23. [Google Scholar] [CrossRef]
- Politzer, P.; Laurence, P.R. Molecular Electrostatic Potentials: An Effective Tool for the Elucidation of Biochemical Phenomena. Environ. Health Perspect. 1985, 61, 191–202. [Google Scholar] [CrossRef] [PubMed]
- Frisch, M.J.; Trucks, G.W.; Schlegel, H.B.; Scuseria, G.E.; Robb, M.A.; Cheeseman, J.R.; Scalmani, G.; Barone, V.; Petersson, G.A.; Nakatsuji, H.; et al. Gaussian~16 Revision A.03; Gaussian Inc.: Wallingford, CT, USA, 2016. [Google Scholar]
- Zhao, Y.; Truhlar, D.G. The M06 Suite of Density Functionals for Main Group Thermochemistry, Thermochemical Kinetics, Noncovalent Interactions, Excited States, and Transition Elements: Two New Functionals and Systematic Testing of Four M06-Class Functionals and 12 Other Functionals. Theor. Chem. Acc. 2008, 120, 215–241. [Google Scholar] [CrossRef]
- Hay, P.J.; Wadt, W.R. Ab Initio Effective Core Potentials for Molecular Calculations. Potentials for K to Au Including the Outermost Core Orbitals. J. Chem. Phys. 1985, 82, 299–310. [Google Scholar] [CrossRef]
- Zhou, B.; Haj, M.K.; Jacobsen, E.N.; Houk, K.N.; Xue, X.-S. Mechanism and Origins of Chemo- and Stereoselectivities of Aryl Iodide-Catalyzed Asymmetric Difluorinations of β-Substituted Styrenes. J. Am. Chem. Soc. 2018, 140, 15206–15218. [Google Scholar] [CrossRef] [PubMed]
- Shu, S.; Li, Y.; Jiang, J.; Ke, Z.; Liu, Y. Mechanism of Hypervalent Iodine Promoted Fluorocyclization of Unsaturated Alcohols: Metathesis via Double Acids Activation. J. Org. Chem. 2019, 84, 458–462. [Google Scholar] [CrossRef] [PubMed]
- Marenich, A.V.; Cramer, C.J.; Truhlar, D.G. Universal Solvation Model Based on Solute Electron Density and on a Continuum Model of the Solvent Defined by the Bulk Dielectric Constant and Atomic Surface Tensions. J. Phys. Chem. B 2009, 113, 6378–6396. [Google Scholar] [CrossRef]
- Andrae, D. Energy-Adjusted Ab Initio Pseudopotentials for the Second and Third Row Transition Elements. Theoret. Chim. Acta 1990, 77, 123–141. [Google Scholar] [CrossRef]
- Lu, T.; Chen, F. Multiwfn: A Multifunctional Wavefunction Analyzer. J. Comput. Chem. 2012, 33, 580–592. [Google Scholar] [CrossRef]
- Dennington, R.; Keith, T.A.; Millam, J.M. GaussView, Version 6; Semichem Inc.: Shawnee Mission, KS, USA, 2016. Available online: https://gaussian.com/gaussview6/ (accessed on 24 June 2024).



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, T.; Li, H.-B. Hypervalent Iodine-Catalyzed Fluorination of Diene-Containing Compounds: A Computational Study. Molecules 2024, 29, 3104. https://doi.org/10.3390/molecules29133104
Liu T, Li H-B. Hypervalent Iodine-Catalyzed Fluorination of Diene-Containing Compounds: A Computational Study. Molecules. 2024; 29(13):3104. https://doi.org/10.3390/molecules29133104
Chicago/Turabian StyleLiu, Tianci, and Hai-Bei Li. 2024. "Hypervalent Iodine-Catalyzed Fluorination of Diene-Containing Compounds: A Computational Study" Molecules 29, no. 13: 3104. https://doi.org/10.3390/molecules29133104
APA StyleLiu, T., & Li, H.-B. (2024). Hypervalent Iodine-Catalyzed Fluorination of Diene-Containing Compounds: A Computational Study. Molecules, 29(13), 3104. https://doi.org/10.3390/molecules29133104








