A Cost-Effective and Sensitive Method for the Determination of Lincomycin in Foods of Animal Origin Using High-Performance Liquid Chromatography
Abstract
1. Introduction
2. Results and Discussion
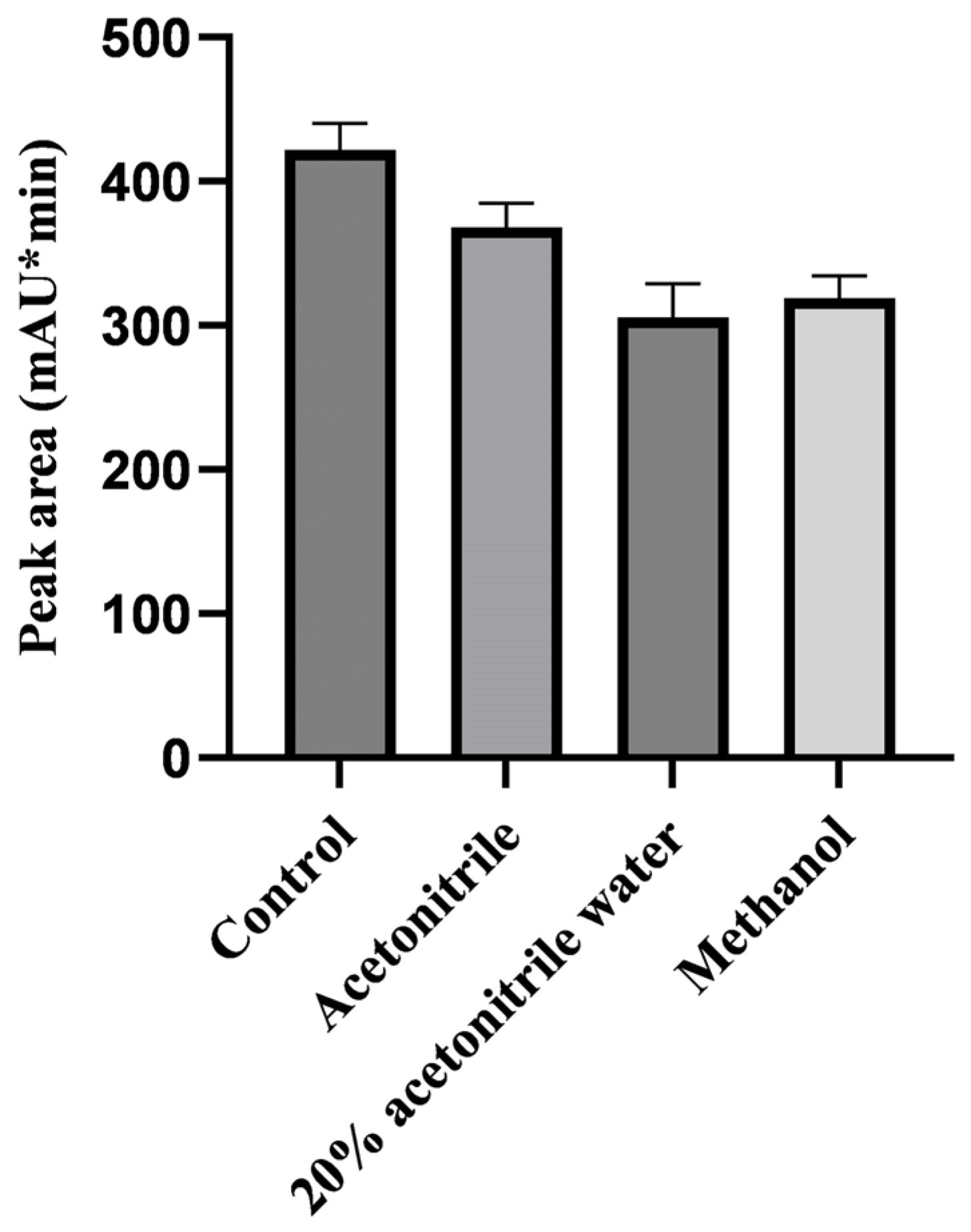
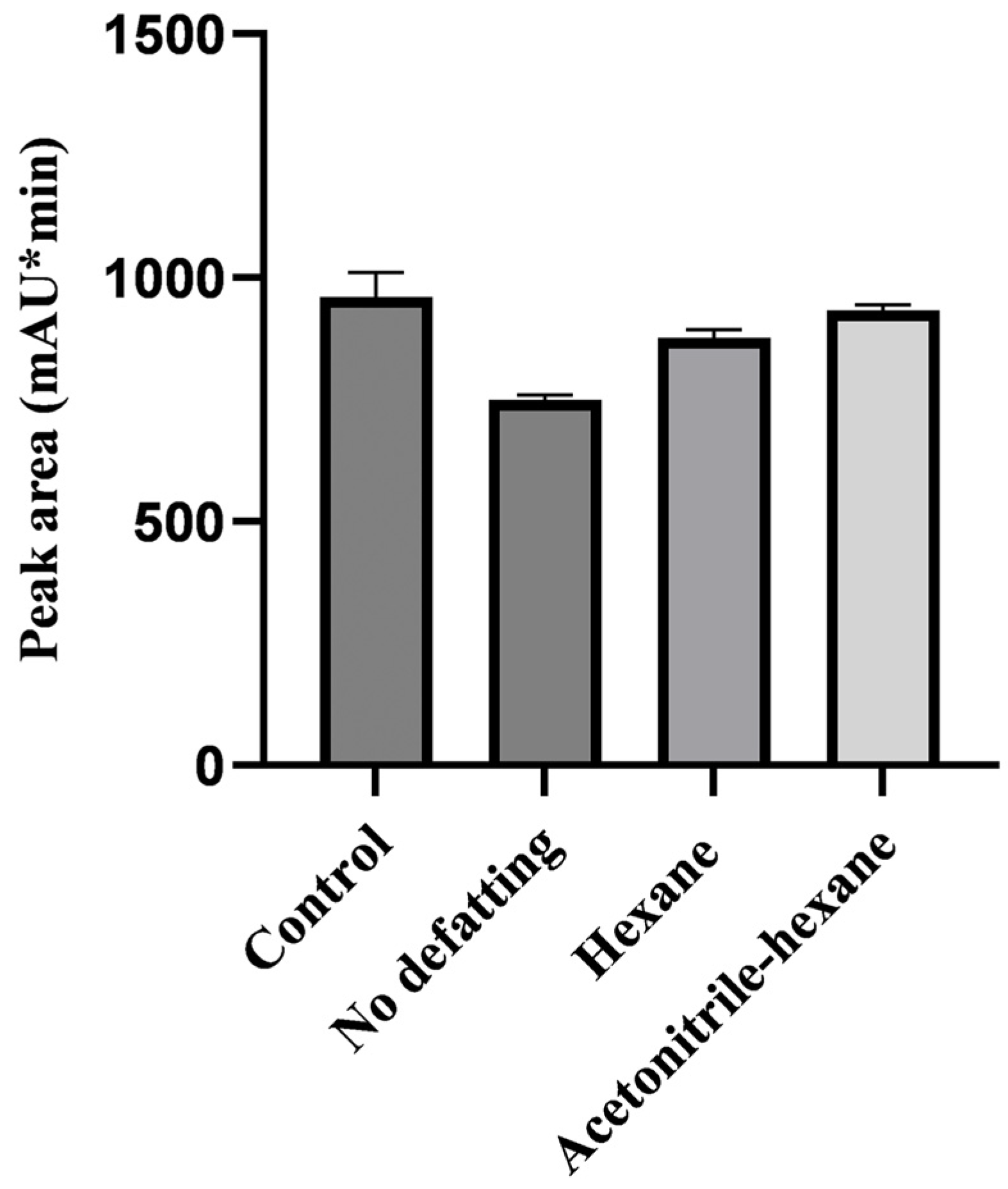
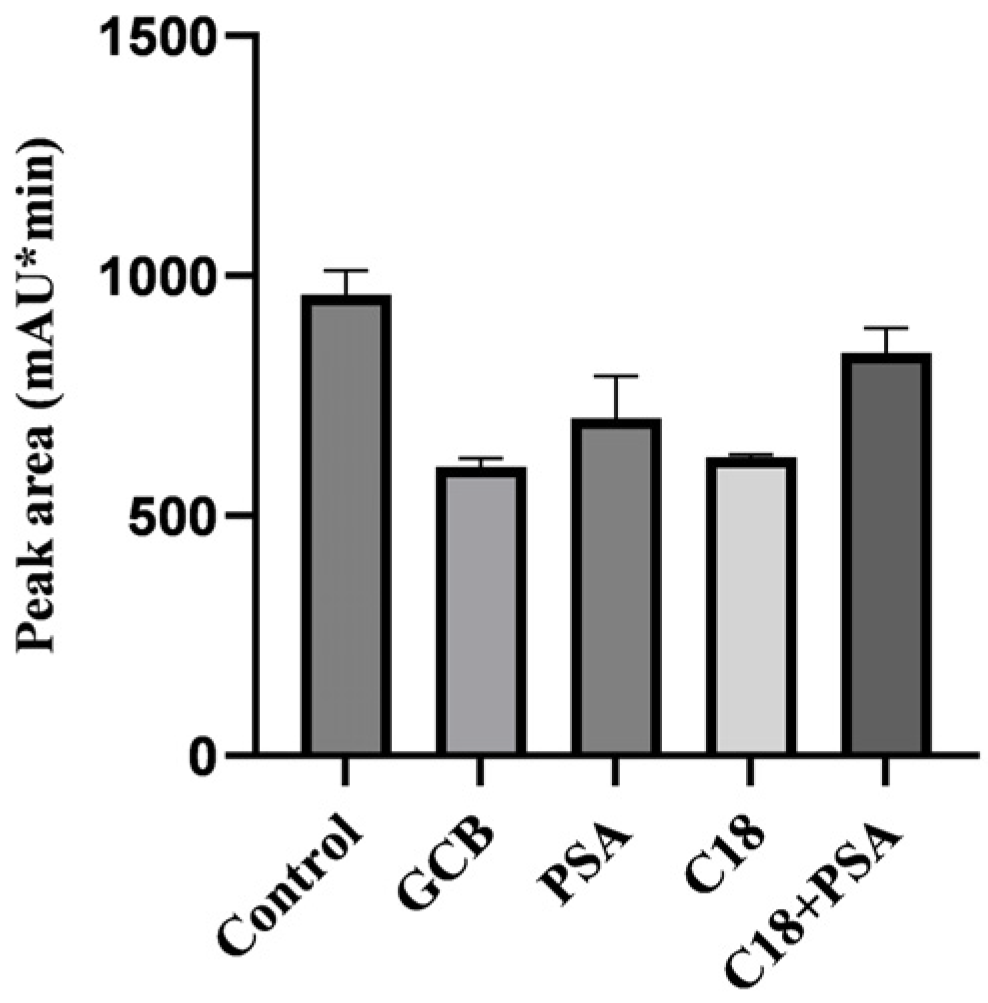
2.1. Optimization of the Sample Pretreatment
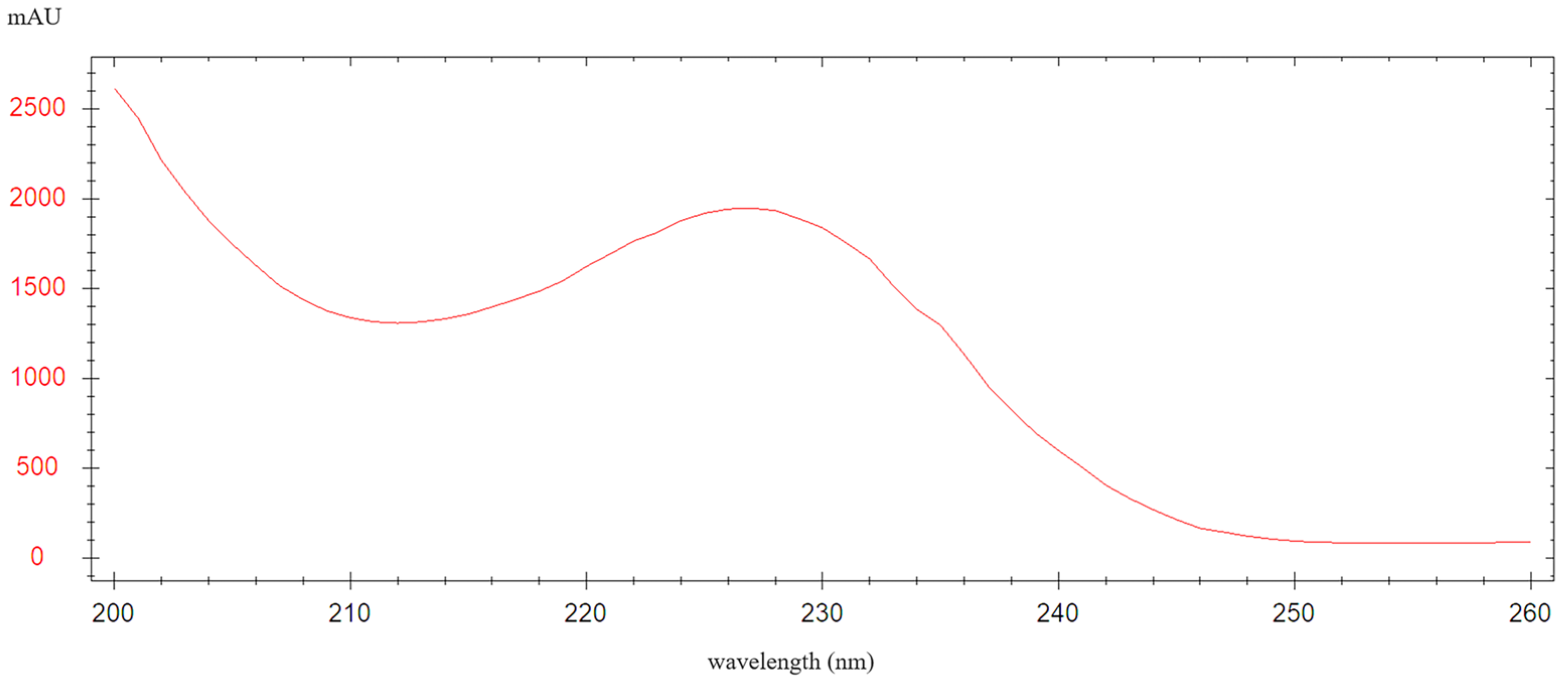
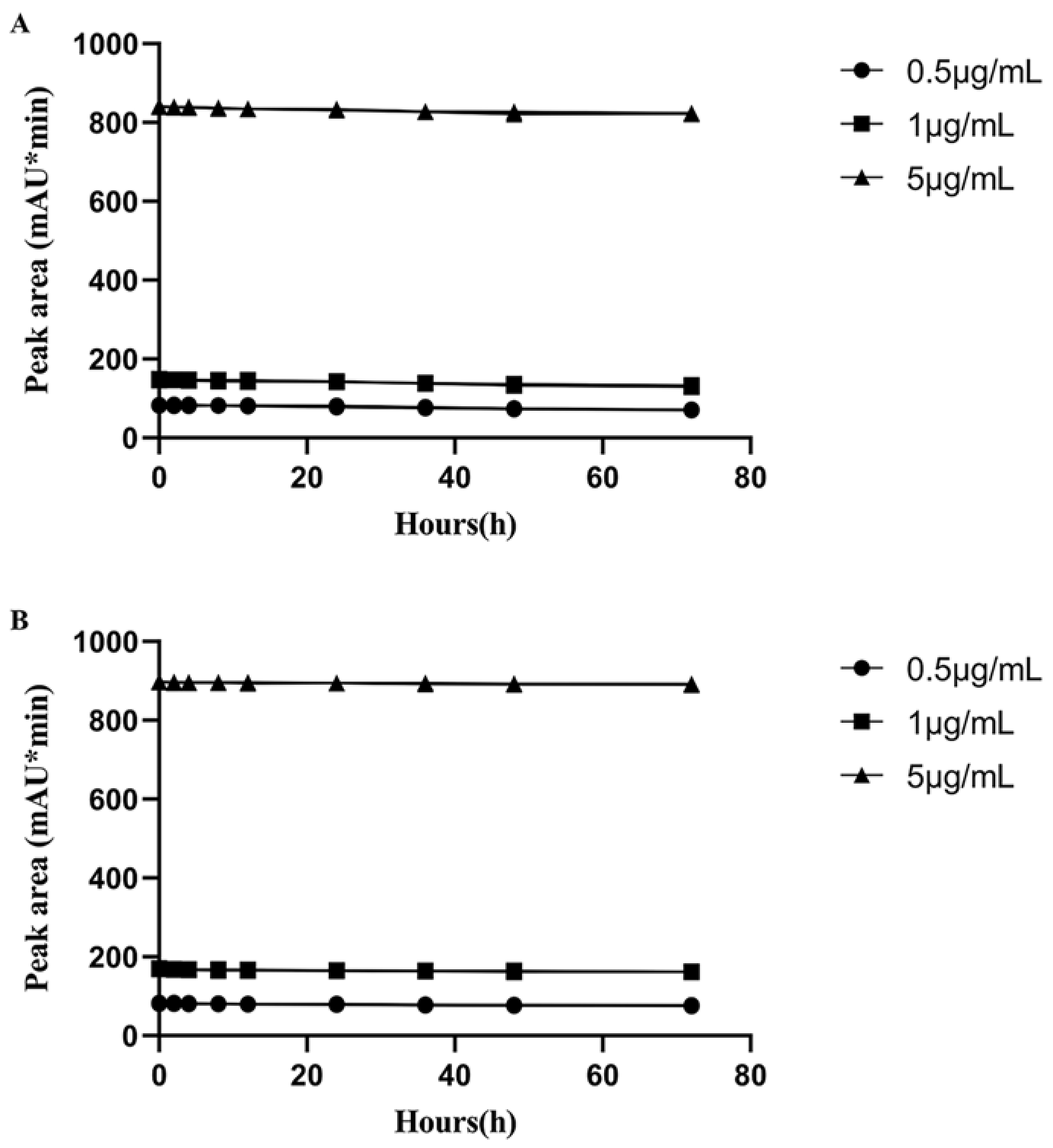
2.2. The Selection of Derivatization Reagent and the Stability of LIN Derivatives
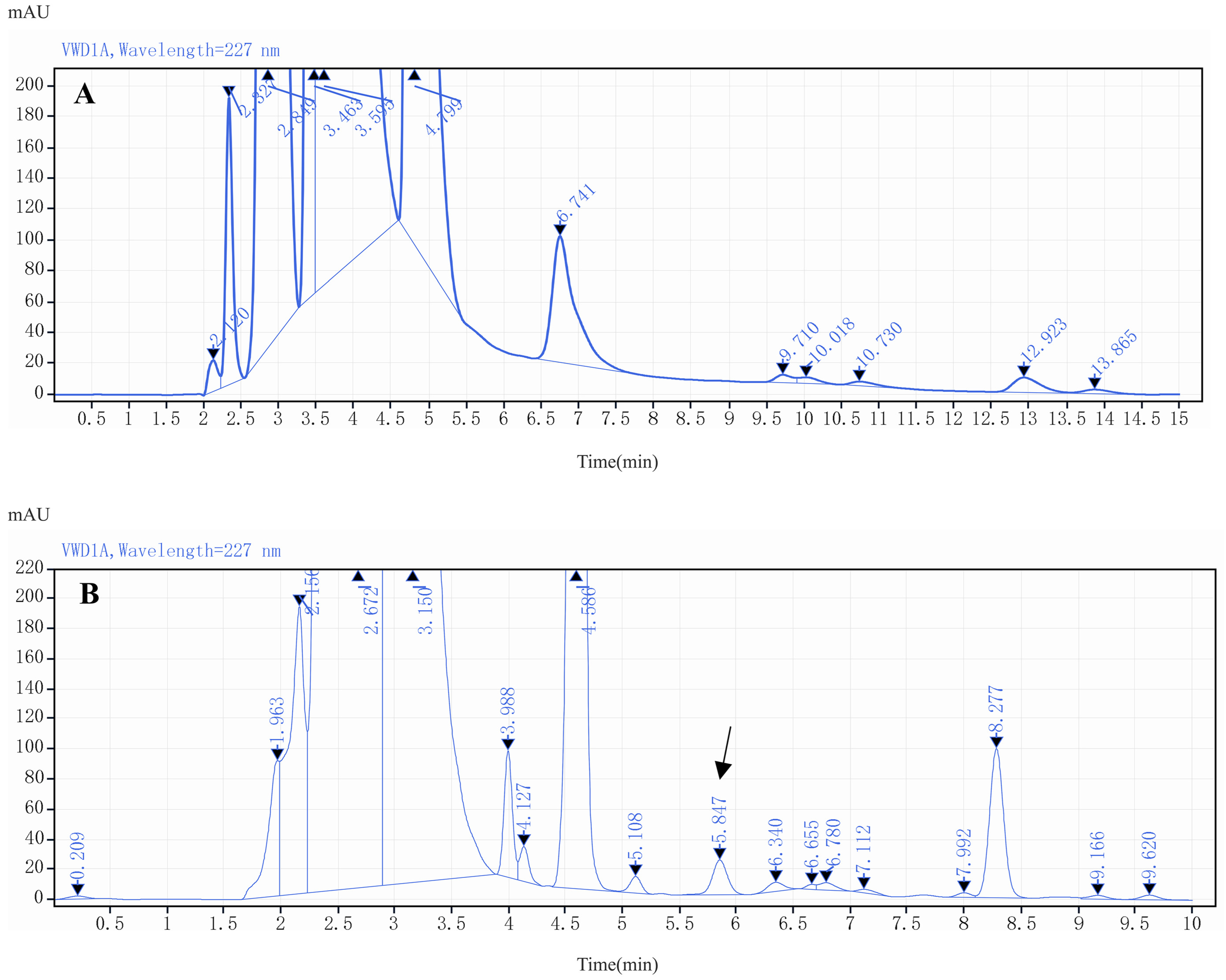
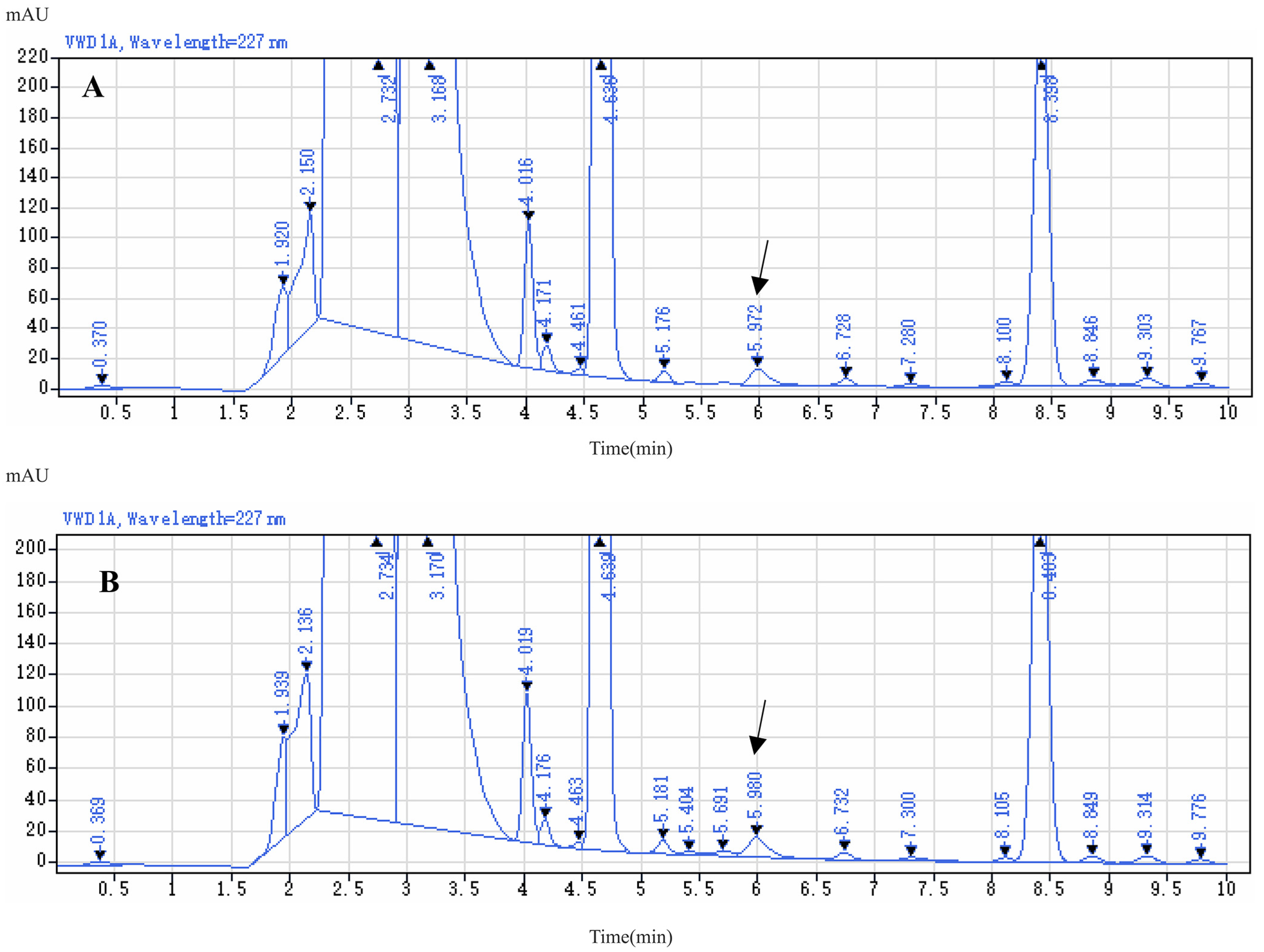
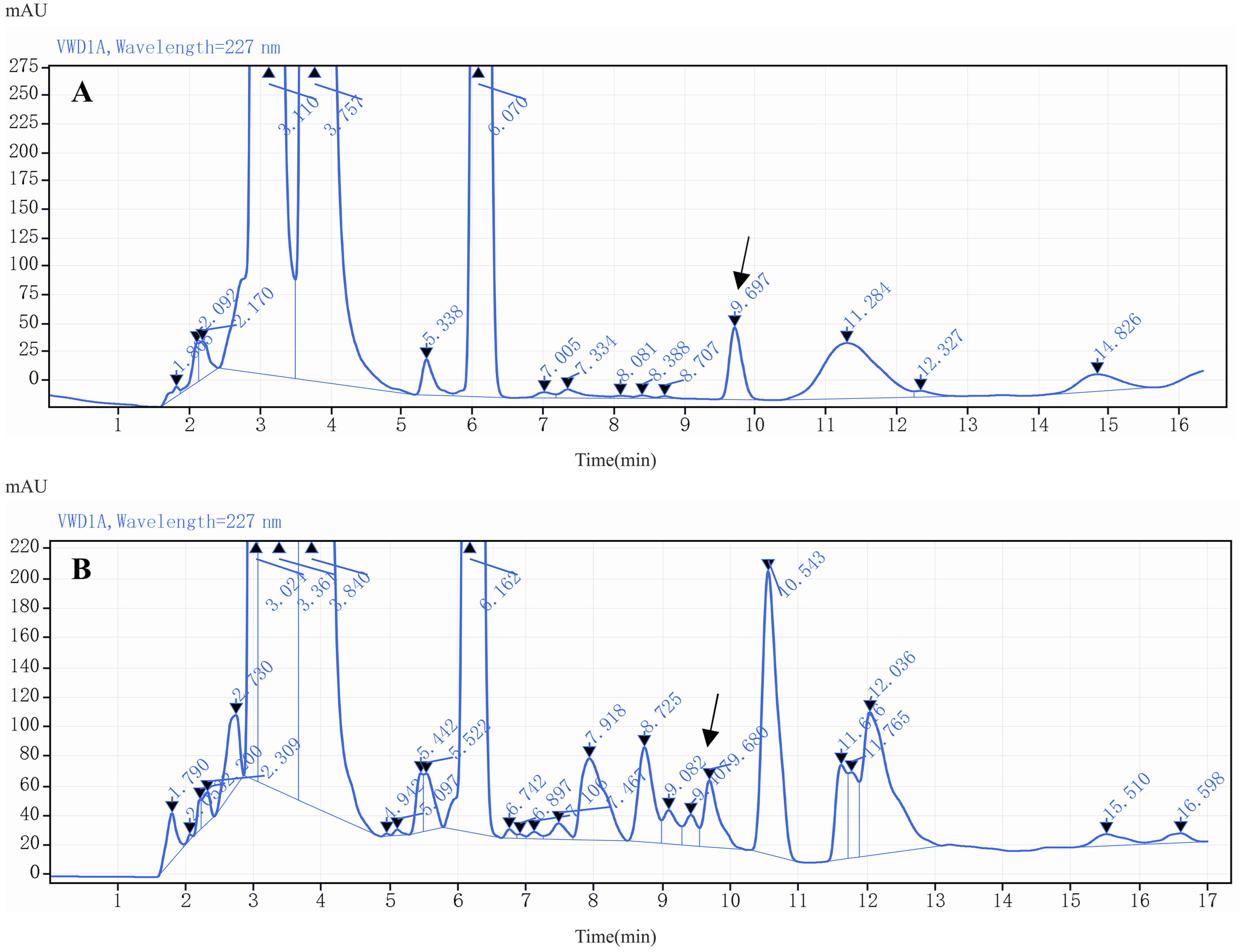
2.3. Optimization of HPLC Conditions
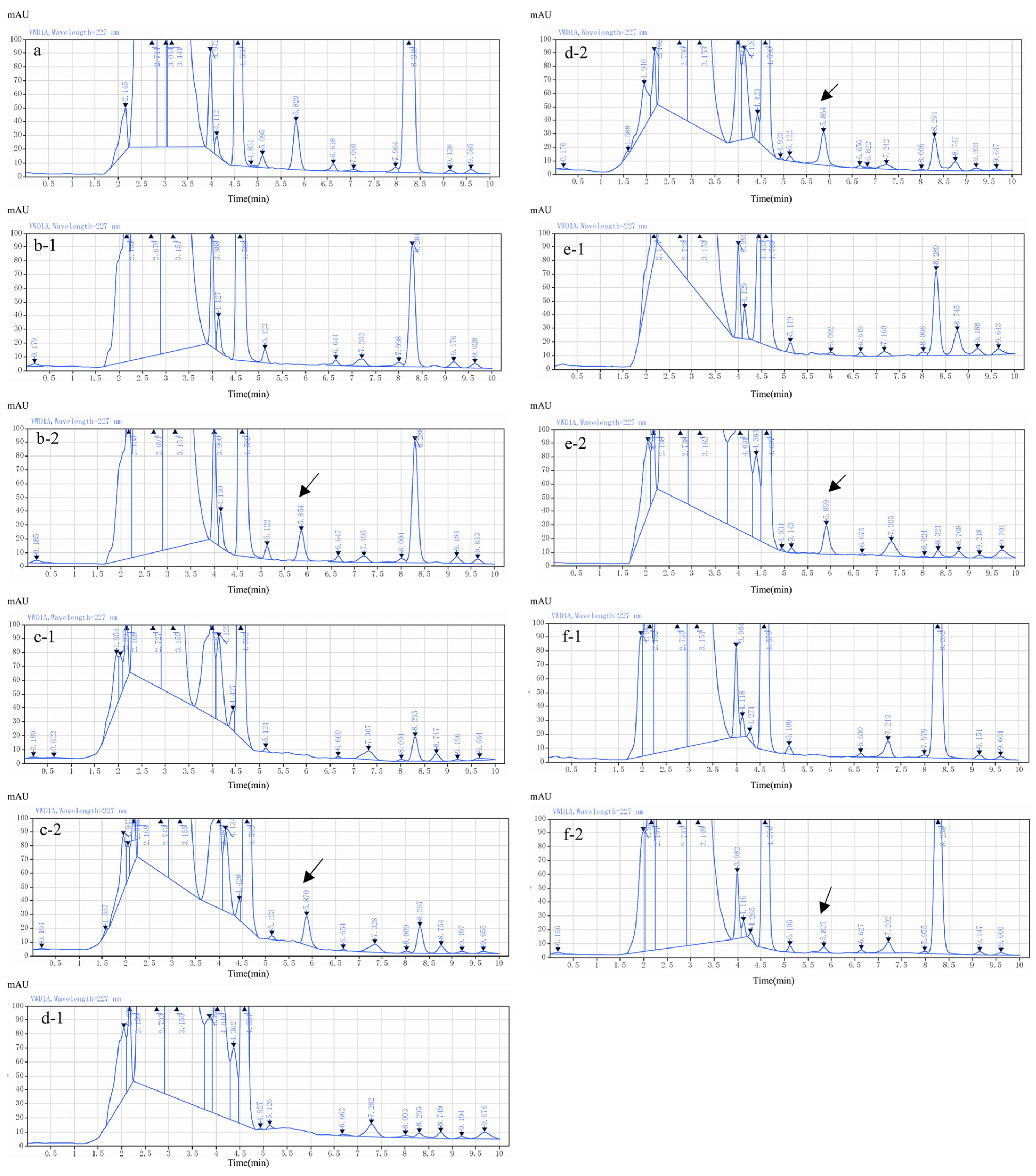
2.4. Method Validation
3. Materials and Methods
3.1. Solutions
3.2. Materials
3.3. Instrumentation and HPLC Conditions
3.4. Sample Pretreatment
3.5. Method Validation
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Lin, A.H.; Murray, R.W.; Vidmar, T.J.; Marotti, K.R. The oxazolidinone eperezolid binds to the 50S ribosomal subunit and competes with binding of chloramphenicol and lincomycin. Antimicrob. Agents Chemother. 1997, 41, 2127–2131. [Google Scholar] [CrossRef]
- Liu, C.-H.; Chuang, Y.-H.; Li, H.; Teppen, B.J.; Boyd, S.A.; Gonzalez, J.M.; Johnston, C.T.; Lehmann, J.; Zhang, W. Sorption of Lincomycin by Manure-Derived Biochars from Water. J. Environ. Qual. 2016, 45, 519–527. [Google Scholar] [CrossRef] [PubMed]
- Kuchta, S.L.; Cessna, A.J.; Elliott, J.A.; Peru, K.M.; Headley, J.V. Transport of lincomycin to surface and ground water from manure-amended cropland. J. Environ. Qual. 2009, 38, 1719–1727. [Google Scholar] [CrossRef]
- Mehrtens, A.; Licha, T.; Burke, V. Occurrence, effects and behaviour of the antibiotic lincomycin in the agricultural and aquatic environment—A review. Sci. Total. Environ. 2021, 778, 146306. [Google Scholar] [CrossRef]
- Charuaud, L.; Jarde, E.; Jaffrezic, A.; Thomas, M.-F.; Le Bot, B. Veterinary pharmaceutical residues from natural water to tap water: Sales, occurrence and fate. J. Hazard. Mater. 2019, 361, 169–186. [Google Scholar] [CrossRef] [PubMed]
- Sarmah, A.K.; Meyer, M.T.; Boxall, A.B.A. A global perspective on the use, sales, exposure pathways, occurrence, fate and effects of veterinary antibiotics (VAs) in the environment. Chemosphere 2006, 65, 725–759. [Google Scholar] [CrossRef]
- Hornish, R.E.; Ron, S.; Nappier, J.M.; Gosline, E. Comparative Metabolism of Lincomycin in the Swine Chicken, and Cat. Drug Metab. Rev. 1987, 18, 177–214. [Google Scholar] [CrossRef] [PubMed]
- Carman, R.J.; Simon, M.A.; Fernández, H.; Miller, M.A.; Bartholomew, M.J. Ciprofloxacin at low levels disrupts colonization resistance of human fecal microflora growing in chemostats. Regul. Toxicol. Pharmacol. 2004, 40, 319–326. [Google Scholar] [CrossRef]
- Wang, B.; Wang, Y.; Xie, X.; Diao, Z.; Xie, K.; Zhang, G.; Zhang, T.; Dai, G. Quantitative Analysis of Spectinomycin and Lincomycin in Poultry Eggs by Accelerated Solvent Extraction Coupled with Gas Chromatography Tandem Mass Spectrometry. Foods 2020, 9, 651. [Google Scholar] [CrossRef]
- Gorbach, S.L.; Spanknebel, G.; Weinstein, L.; Plaut, A.G.; Nahas, L.; Levitan, R. Studies of intestinal microflora. 8. Effect of lincomycin on the microbial population of the human intestine. J. Infect. Dis. 1969, 120, 298–304. [Google Scholar] [CrossRef]
- Medina, M.B. Development of a Fluorescent Latex Immunoassay for detection of a spectinomycin antibiotic. J. Agric. Food Chem. 2004, 52, 3231–3236. [Google Scholar] [CrossRef]
- Kowalski, P.; Konieczna, L.; Olędzka, I.; Plenis, A.; Bączek, T. Development and Validation of Electromigration Technique for the Determination of Lincomycin and Clindamycin Residues in Poultry Tissues. Food Anal. Methods 2014, 7, 276–282. [Google Scholar] [CrossRef]
- Cao, S.; Song, S.; Liu, L.; Kong, N.; Kuang, H.; Xu, C. Comparison of an Enzyme-Linked Immunosorbent Assay with an Immunochromatographic Assay for Detection of Lincomycin in Milk and Honey. Immunol. Investig. 2015, 44, 438–450. [Google Scholar] [CrossRef] [PubMed]
- Haagsna, N.; Scherpenisse, P.; Simmonds, R.; Wood, S.; Rees, S. High-performance liquid chromatographic determination of spectinomycin in swine, calf and chicken plasma using post-column derivatization. J. Chromatogr. B Biomed. Sci. Appl. 1995, 672, 165–171. [Google Scholar] [CrossRef] [PubMed]
- Negarian, M.; Mohammadinejad, A.; Mohajeri, S.A. Preparation, evaluation and application of core–shell molecularly imprinted particles as the sorbent in solid-phase extraction and analysis of lincomycin residue in pasteurized milk. Food Chem. 2019, 288, 29–38. [Google Scholar] [CrossRef] [PubMed]
- Hamamoto, K.; Mizuno, Y.; Koike, R.; Yamaoka, R.; Takahashi, T.; Takahashi, Y. Residue analysis of spectinomycin in tissues of chicken and swine by HPLC. J. Food Hyg. Soc. Jpn. 2003, 44, 114–118. [Google Scholar] [CrossRef]
- Hou, L.; Jiang, Z.; Ye, M.; Sun, X.; Liu, K.; Zhu, Y.; Wang, X.; Chen, L.; Gu, R.; Fang, B. Determination of lincomycin residues of animal derived food by pre-column derivatization with HPLC -UVD. Arab. J. Chem. 2024, 17, 105439. [Google Scholar] [CrossRef]
- Zhou, J.; Zhang, L.; Wang, Y.; Yan, C. HPLC-ELSD analysis of spectinomycin dihydrochloride and its impurities. J. Sep. Sci. 2011, 34, 1811–1819. [Google Scholar] [CrossRef] [PubMed]
- Peru, K.M.; Kuchta, S.L.; Headley, J.V.; Cessna, A.J. Development of a hydrophilic interaction chromatography–mass spectrometry assay for spectinomycin and lincomycin in liquid hog manure supernatant and run-off from cropland. J. Chromatogr. A 2006, 1107, 152–158. [Google Scholar] [CrossRef]
- Maddaleno, A.; Pokrant, E.; Yanten, F.; Martin, B.S.; Cornejo, J. Implementation and Validation of an Analytical Method for Lincomycin Determination in Feathers and Edible Tissues of Broiler Chickens by Liquid Chromatography Tandem Mass Spectrometry. J. Anal. Methods Chem. 2019, 2019, 4569707. [Google Scholar] [CrossRef]
- van Holthoon, F.L.; Essers, M.L.; Mulder, P.J.; Stead, S.L.; Caldow, M.; Ashwin, H.M.; Sharman, M. A generic method for the quantitative analysis of aminoglycosides (and spectinomycin) in animal tissue using methylated internal standards and liquid chromatography tandem mass spectrometry. Anal. Chim. Acta 2009, 637, 135–143. [Google Scholar] [CrossRef] [PubMed]
- Sin, D.W.-M.; Wong, Y.-C.; Ip, A.C.-B. Quantitative analysis of lincomycin in animal tissues and bovine milk by liquid chromatography electrospray ionization tandem mass spectrometry. J. Pharmaceut. Biomed. 2004, 34, 651–659. [Google Scholar] [CrossRef] [PubMed]
- Tao, Y.; Chen, D.; Yu, G.; Yu, H.; Pan, Y.; Wang, Y.; Huang, L.; Yuan, Z. Simultaneous determination of lincomycin and spectinomycin residues in animal tissues by gas chromatography-nitrogen phosphorus detection and gas chromatography-mass spectrometry with accelerated solvent extraction. J. Sci. Food Agr. 2014, 94, 301–307. [Google Scholar] [CrossRef] [PubMed]
- Luo, W.; Yin, B.; Ang, C.Y.; Rushing, L.; Thompson, H.C. Determination of lincomycin residues in salmon tissues by gas chromatography with nitrogen-phosphorus detection. J. Chromatogr. B Biomed. Sci. Appl. 1996, 687, 405–411. [Google Scholar] [CrossRef]
- Zhou, D.; Li, Y.; Huang, L.; Qian, M.; Li, D.; Sun, G.; Yang, B. A reliable and cost-efficient TLC-HPLC method for determining total florfenicol residues in porcine edible tissues. Food Chem. 2020, 303, 125399. [Google Scholar] [CrossRef] [PubMed]
- Xie, K.; Jia, L.; Yao, Y.; Xu, D.; Chen, S.; Xie, X.; Pei, Y.; Bao, W.; Dai, G.; Wang, J.; et al. Simultaneous determination of thiamphenicol, florfenicol and florfenicol amine in eggs by reversed-phase high-performance liquid chromatography with fluorescence detection. J. Chromatogr. B 2011, 879, 2351–2354. [Google Scholar] [CrossRef] [PubMed]
- Shen, J.; Xia, X.; Jiang, H.; Li, C.; Li, J.; Li, X.; Ding, S. Determination of chloramphenicol, thiamphenicol, florfenicol, and florfenicol amine in poultry and porcine muscle and liver by gas chromatography-negative chemical ionization mass spectrometry. J. Chromatogr. B 2009, 877, 1523–1529. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Liu, Z.; Guo, X.; Cheng, L.; Wang, Z.; Shen, J. Simultaneous determination and confirmation of chloramphenicol, thiamphenicol, florfenicol and florfenicol amine in chicken muscle by liquid chromatography–tandem mass spectrometry. J. Chromatogr. B 2008, 875, 399–404. [Google Scholar] [CrossRef]
- Li, J.; Ding, S.; Zhang, S.; Li, C.; Li, X.; Liu, Z.; Liu, J.; Shen, J. Residue Depletion of Florfenicol and Its Metabolite Florfenicol Amine in Swine Tissues after Intramuscular Administration. J. Agric. Food Chem. 2006, 54, 9614–9619. [Google Scholar] [CrossRef]
- van de Riet, J.M.; Potter, R.A.; Christie-Fougere, M.; Burns, B.G. Simultaneous Determination of Residues of Chloramphenicol, Thiamphenicol, Florfenicol, and Florfenicol Amine in Farmed Aquatic Species by Liquid Chromatography/Mass Spectrometry. J. AOAC Int. 2003, 86, 510–514. [Google Scholar] [CrossRef]
- Tao, X.; Yu, X.; Zhang, D.; Shi, W.; Jiang, H.; Wang, X.; Wang, Z.; Niu, L.; Wu, X.; Xia, X.; et al. Development of a rapid chemiluminescent ciELISA for simultaneous determination of florfenicol and its metabolite florfenicol amine in animal meat products. J. Sci. Food Agric. 2014, 94, 301–307. [Google Scholar] [CrossRef]
- Luo, P.; Chen, X.; Liang, C.; Kuang, H.; Lu, L.; Jiang, Z.; Wang, Z.; Li, C.; Zhang, S.; Shen, J. Simultaneous determination of thiamphenicol, florfenicol and florfenicol amine in swine muscle by liquid chromatography–tandem mass spectrometry with immunoaffinity chromatography clean-up. J. Chromatogr. B 2010, 878, 207–212. [Google Scholar] [CrossRef] [PubMed]
- Rezende, D.; Filho, N.F.; Rocha, G. Simultaneous determination of chloramphenicol and florfenicol in liquid milk, milk powder and bovine muscle by LC–MS/MS. Food Addit. Contam. Part A 2012, 29, 559–570. [Google Scholar] [CrossRef] [PubMed]
- Martel, A.; Zeggane, S. HPLC Determination of Sulfathiazole in French Honeys. J. Liq. Chromatogr. Relat. Technol. 2003, 26, 953–961. [Google Scholar] [CrossRef]
- Masiá, A.; Suarez-Varela, M.M.; Llopis-Gonzalez, A.; Picó, Y. Determination of pesticides and veterinary drug residues in food by liquid chromatography-mass spectrometry: A review. Anal. Chim. Acta 2016, 936, 40–61. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Zhang, H.Y.; Wang, L.; Duan, Z.J.; Kennedy, I. Analysis of sulphonamide residues in edible animal products: A review. Food Addit. Contam. 2006, 23, 362–384. [Google Scholar] [CrossRef] [PubMed]
- Casado, N.; Morante-Zarcero, S.; Sierra, I. Application of the QuEChERS Strategy as a Useful Sample Preparation Tool for the Multiresidue Determination of Pyrrolizidine Alkaloids in Food and Feed Samples: A Critical Overview. Appl. Sci. 2022, 12, 4325. [Google Scholar] [CrossRef]
- Gil-Agustí, M.; Capella-Peiró, M.E.; Monferrer-Pons, L.; García-Alvarez-Coque, M.C.; Esteve-Romero, J. Chromatographic analysis of phenethylamine-antihistamine combinations using C8, C18 or cyano columns and micellar sodium dodecyl sulfate-pentanol mixtures. Analyst 2001, 126, 457–464. [Google Scholar] [CrossRef]
- Taghvaei, M.; Smith, B. Development and Optimization of a Reversed-Phase HPLC Method to Separate Pulse Proteins. Food Anal. Methods 2020, 13, 1482–1491. [Google Scholar] [CrossRef]
- GB 29685-2013; National Standard for Food Safety-Determination of Lincomycin, Clindamycin and Macroscopycin Residues in Animal Foods by Gas Chromatography-Mass Spectrometry. Ministry of Agriculture of the People’s Republic of China: Beijing, China; National Health and Family Planning Commission, People’s Republic of China: Beijing, China, 2013.
- Sun, Y.-X.; Zhao, H.-Y.; Liu, Y.-J.; Dai, Z.-Q.; Fang, B.-H. Toxicokinetics of T-2 Toxin, HT-2 Toxin and T-2 Triol after Intravenously Administrated T-2 Toxin in Swine. J. Anim. Vet. Adv. 2012, 11, 1977–1981. [Google Scholar] [CrossRef][Green Version]
- She, Y.-X.; Liu, J.; Wang, J.; Liu, Y.; Wang, R.; Cao, W. Determination of sulfonamides in bovine milk by ultra performance liquid chromatography combined with quadrupole mass spectrometry. Anal. Lett. 2010, 43, 2246–2256. [Google Scholar] [CrossRef]
- Zaidon, S.Z.; Bin Ho, Y.; Hamsan, H.; Hashim, Z.; Saari, N.; Praveena, S.M. Improved QuEChERS and solid phase extraction for multi-residue analysis of pesticides in paddy soil and water using ultra-high performance liquid chromatography tandem mass spectrometry. Microchem. J. 2019, 145, 614–621. [Google Scholar] [CrossRef]
- Liu, L.; Rao, L.; Li, W.; Zhou, W.; Li, B.; Tang, L. Detection of Glyamifop residues in rice and its environment by the QuEChERS method combined with HPLC–MS. Microchem. J. 2020, 158, 105157. [Google Scholar] [CrossRef]
- Kwon, C.H.; Lee, Y.D.; Im, M.H. Simultaneous Determination of Orysastrobin and Its Isomers in Rice Using HPLC-UV and LC-MS/MS. J. Agric. Food Chem. 2011, 59, 10826–10830. [Google Scholar] [CrossRef]
- Wahed, P.; Razzaq, M.; Dharmapuri, S.; Corrales, M. Determination of formaldehyde in food and feed by an in-house validated HPLC method. Food Chem. 2016, 202, 476–483. [Google Scholar] [CrossRef]


| Animal | Type | Batch | Linear Range (μg/kg) | Calibration Equations | R2 |
|---|---|---|---|---|---|
| Pig | Liver | 1 | 60~1200 | y = 0.731x + 10.36 | 0.9948 |
| 2 | 60~1200 | y = 0.899x + 0.400 | 0.9993 | ||
| 3 | 60~1200 | y = 1.050x + 9.441 | 0.9978 | ||
| Muscle | 1 | 60~1200 | y = 1.176x + 8.105 | 0.9995 | |
| 2 | 60~1200 | y = 1.009x + 24.099 | 0.9986 | ||
| 3 | 60~1200 | y = 1.092x + 20.015 | 0.9989 | ||
| Chicken | Kidney | 1 | 60~3000 | y = 0.970x + 3.134 | 0.9971 |
| 2 | 60~3000 | y = 0.915x + 26.74 | 0.9929 | ||
| 3 | 60~3000 | y = 0.720x + 13.94 | 0.9938 | ||
| Liver | 1 | 60~1200 | y = 1.086x − 11.53 | 0.9962 | |
| 2 | 60~1200 | y = 0.994x + 17.58 | 0.9945 | ||
| 3 | 60~1200 | y = 1.076x + 20.76 | 0.9960 | ||
| Eggs | 1 | 30~200 | y = 0.969x + 3.134 | 0.9971 | |
| 2 | 30~200 | y = 0.915x + 26.743 | 0.9929 | ||
| 3 | 30~200 | y = 0.720x + 13.937 | 0.9938 | ||
| Cow | Fat | 1 | 30~200 | y = 1.079x − 0.670 | 0.9993 |
| 2 | 30~200 | y = 1.205x − 1.801 | 0.9958 | ||
| 3 | 30~200 | y = 1.341x + 10.84 | 0.9906 | ||
| Liver | 1 | 60~1200 | y = 1.160x + 17.07 | 0.9946 | |
| 2 | 60~1200 | y = 0.837x − 0.631 | 0.9983 | ||
| 3 | 60~1200 | y = 0.728x + 9.432 | 0.9948 | ||
| Milk | 1 | 60~1200 | y = 0.911x − 1.3203 | 0.9980 | |
| 2 | 60~1200 | y = 0.971x + 15.73 | 0.9969 | ||
| 3 | 60~1200 | y = 1.007x + 8.696 | 0.9917 | ||
| Goat | Muscle | 1 | 60~1200 | y = 1.107x + 0.5321 | 0.9982 |
| 2 | 60~1200 | y = 1.033x − 4.560 | 0.9986 | ||
| 3 | 60~1200 | y = 1.071x + 26.805 | 0.9986 | ||
| Liver | 1 | 60~1200 | y = 0.788x − 5.920 | 0.9989 | |
| 2 | 60~1200 | y = 1.038x + 26.80 | 0.9994 | ||
| 3 | 60~1200 | y = 0.874x − 11.96 | 0.9917 | ||
| Milk | 1 | 60~1200 | y = 1.018x + 6.593 | 0.9979 | |
| 2 | 60~1200 | y = 1.125x + 4.823 | 0.9995 | ||
| 3 | 60~1200 | y = 1.045x + 4.727 | 0.9996 |
| Matrix | Spiked Concentration (μg/kg) | Recovery (X ± SD, %, n = 6) | Intra-Day RSD (%, n = 6) | Inter-Day RSD (%, n = 18) | ||||
|---|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 1 | 2 | 3 | |||
| Pig muscle | 60 | 86.9 ± 6.3 | 95.0 ± 4.1 | 81.8 ± 5.3 | 7.3 | 4.3 | 6.4 | 8.5 |
| 200 | 97.5 ± 2.1 | 84.9 ± 2.0 | 81.0 ± 10.2 | 2.1 | 2.4 | 12.6 | 10.5 | |
| 400 | 96.6 ± 2.3 | 97.1 ± 1.4 | 97.2 ± 1.9 | 2.3 | 1.5 | 2.0 | 1.9 | |
| Pig liver | 60 | 90.0 ± 6.3 | 89.1 ± 9.2 | 94.1 ± 5.0 | 7.0 | 10.4 | 5.0 | 7.6 |
| 500 | 88.7 ± 4.4 | 81.2 ± 6.0 | 89.6 ± 5.4 | 4.9 | 7.4 | 6.1 | 7.3 | |
| 1000 | 85.1 ± 4.4 | 75.1 ± 2.7 | 74.1 ± 2.9 | 5.2 | 3.6 | 3.9 | 7.7 | |
| Chicken liver | 60 | 88.1 ± 4.9 | 91.2 ± 5.8 | 88.3 ± 5.5 | 5.6 | 6.4 | 6.2 | 5.9 |
| 500 | 77.4 ± 3.7 | 73.7 ± 1.9 | 81.3 ± 5.8 | 4.8 | 2.6 | 7.1 | 6.5 | |
| 1000 | 86.0 ± 3.2 | 91.6 ± 4.5 | 83.7 ± 3.6 | 3.8 | 4.9 | 4.3 | 5.7 | |
| Chicken kidney | 60 | 88.0 ± 6.7 | 76.9 ± 3.2 | 89.6 ± 7.9 | 7.6 | 4.2 | 8.8 | 9.7 |
| 500 | 80.5 ± 10.1 | 74.8 ± 3.9 | 89.9 ± 5.9 | 12.2 | 5.2 | 6.5 | 11.1 | |
| 1000 | 75.4 ± 3.3 | 73.5 ± 1.9 | 85.8 ± 5.2 | 4.3 | 2.6 | 6.0 | 8.4 | |
| Chicken egg | 30 | 82.0 ± 5.1 | 80.1 ± 8.2 | 76.4 ± 6.7 | 6.2 | 10.3 | 8.8 | 9.3 |
| 50 | 81.5 ± 8.5 | 80.6 ± 7.7 | 89.1 ± 7.9 | 10.4 | 9.5 | 8.8 | 10.8 | |
| 100 | 79.2 ± 3.5 | 80.8 ± 11.3 | 90.6 ± 7.2 | 4.4 | 13.9 | 7.9 | 12.1 | |
| Cow fat | 30 | 88.0 ± 9.8 | 84.7 ± 8.4 | 84.0 ± 5.3 | 11.1 | 10.0 | 6.3 | 9.1 |
| 50 | 92.0 ± 4.2 | 91.2 ± 6.4 | 79.2 ± 8.0 | 4.5 | 7.0 | 11.2 | 10.0 | |
| 100 | 95.1 ± 1.9 | 89.3 ± 3.3 | 81.5 ± 5.0 | 2.0 | 3.7 | 6.1 | 7.5 | |
| Cow liver | 60 | 87.3 ± 8.5 | 89.2 ± 5.8 | 88.6 ± 6.3 | 9.7 | 6.5 | 7.1 | 7.5 |
| 500 | 87.5 ± 8.5 | 82.4 ± 5.8 | 92.2 ± 3.9 | 9.7 | 7.0 | 4.3 | 8.3 | |
| 1000 | 73.9 ± 6.2 | 78.6 ± 7.7 | 86.8 ± 5.3 | 8.5 | 9.8 | 6.1 | 10.3 | |
| Cow milk | 60 | 89.0 ± 9.0 | 91.0 ± 3.0 | 84.7 ± 5.6 | 10.1 | 3.3 | 6.6 | 7.4 |
| 150 | 93.5 ± 5.3 | 80.5 ± 2.3 | 87.3 ± 7.1 | 5.6 | 2.9 | 8.1 | 8.4 | |
| 300 | 77.6 ± 8.6 | 76.9 ± 5.3 | 75.1 ± 7.8 | 11.0 | 7.7 | 10.5 | 10.7 | |
| Goat muscle | 60 | 89.6 ± 4.9 | 89.0 ± 2.4 | 88.9 ± 7.1 | 8.0 | 2.7 | 9.1 | 5.4 |
| 100 | 90.3 ± 7.2 | 95.3 ± 2.5 | 83.6 ± 10.2 | 8.0 | 2.6 | 12.2 | 9.4 | |
| 200 | 87.8 ± 9.1 | 90.4 ± 5.0 | 79.3 ± 5.5 | 10.4 | 5.6 | 6.9 | 9.4 | |
| Goat liver | 60 | 75.6 ± 5.1 | 79.1 ± 4.9 | 93.9 ± 6.6 | 6.8 | 6.2 | 7.0 | 11.7 |
| 500 | 80.6 ± 8.0 | 84.2 ± 5.1 | 81.6 ± 3.2 | 9.9 | 6.1 | 3.9 | 6.9 | |
| 1000 | 82.2 ± 6.3 | 79.8 ± 6.1 | 75.6 ± 4.3 | 7.6 | 7.6 | 5.7 | 7.5 | |
| Goat milk | 60 | 82.4 ± 11.0 | 93.4 ± 4.9 | 84.7 ± 5.6 | 13.4 | 5.2 | 5.1 | 10.2 |
| 150 | 89.5 ± 6.5 | 95.8 ± 3.0 | 87.3 ± 7.1 | 7.3 | 3.1 | 11.4 | 8.6 | |
| 300 | 88.1 ± 5.5 | 94.3 ± 6.1 | 75.1 ± 7.8 | 6.2 | 6.4 | 5.0 | 6.4 | |
| Animal | Type | MRL (μg/kg) | LOD (μg/kg) | LOQ (μg/kg) |
|---|---|---|---|---|
| Pig | Liver | 500 | 40 | 60 |
| Muscle | 200 | 30 | 60 | |
| Chicken | Liver | 500 | 40 | 60 |
| Kidney | 500 | 40 | 60 | |
| Eggs | 50 | 25 | 40 | |
| Cow | Fat | 50 | 25 | 40 |
| Liver | 500 | 40 | 60 | |
| Milk | 150 | 30 | 60 | |
| Goat | Muscle | 200 | 30 | 60 |
| Liver | 500 | 40 | 60 | |
| Milk | 200 | 30 | 60 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ye, M.; Hou, L.; Jiang, Z.; Sun, X.; Chen, L.; Fang, B. A Cost-Effective and Sensitive Method for the Determination of Lincomycin in Foods of Animal Origin Using High-Performance Liquid Chromatography. Molecules 2024, 29, 3054. https://doi.org/10.3390/molecules29133054
Ye M, Hou L, Jiang Z, Sun X, Chen L, Fang B. A Cost-Effective and Sensitive Method for the Determination of Lincomycin in Foods of Animal Origin Using High-Performance Liquid Chromatography. Molecules. 2024; 29(13):3054. https://doi.org/10.3390/molecules29133054
Chicago/Turabian StyleYe, Minqi, Limin Hou, Zongpei Jiang, Xueyan Sun, Liangzhu Chen, and Binghu Fang. 2024. "A Cost-Effective and Sensitive Method for the Determination of Lincomycin in Foods of Animal Origin Using High-Performance Liquid Chromatography" Molecules 29, no. 13: 3054. https://doi.org/10.3390/molecules29133054
APA StyleYe, M., Hou, L., Jiang, Z., Sun, X., Chen, L., & Fang, B. (2024). A Cost-Effective and Sensitive Method for the Determination of Lincomycin in Foods of Animal Origin Using High-Performance Liquid Chromatography. Molecules, 29(13), 3054. https://doi.org/10.3390/molecules29133054





