Development of Potential Therapeutic Agents from Black Elderberries (the Fruits of Sambucus nigra L.)
Abstract
1. Introduction
2. Elderberries Show Potent Antioxidant Activity and Have Been Used as Functional Foods
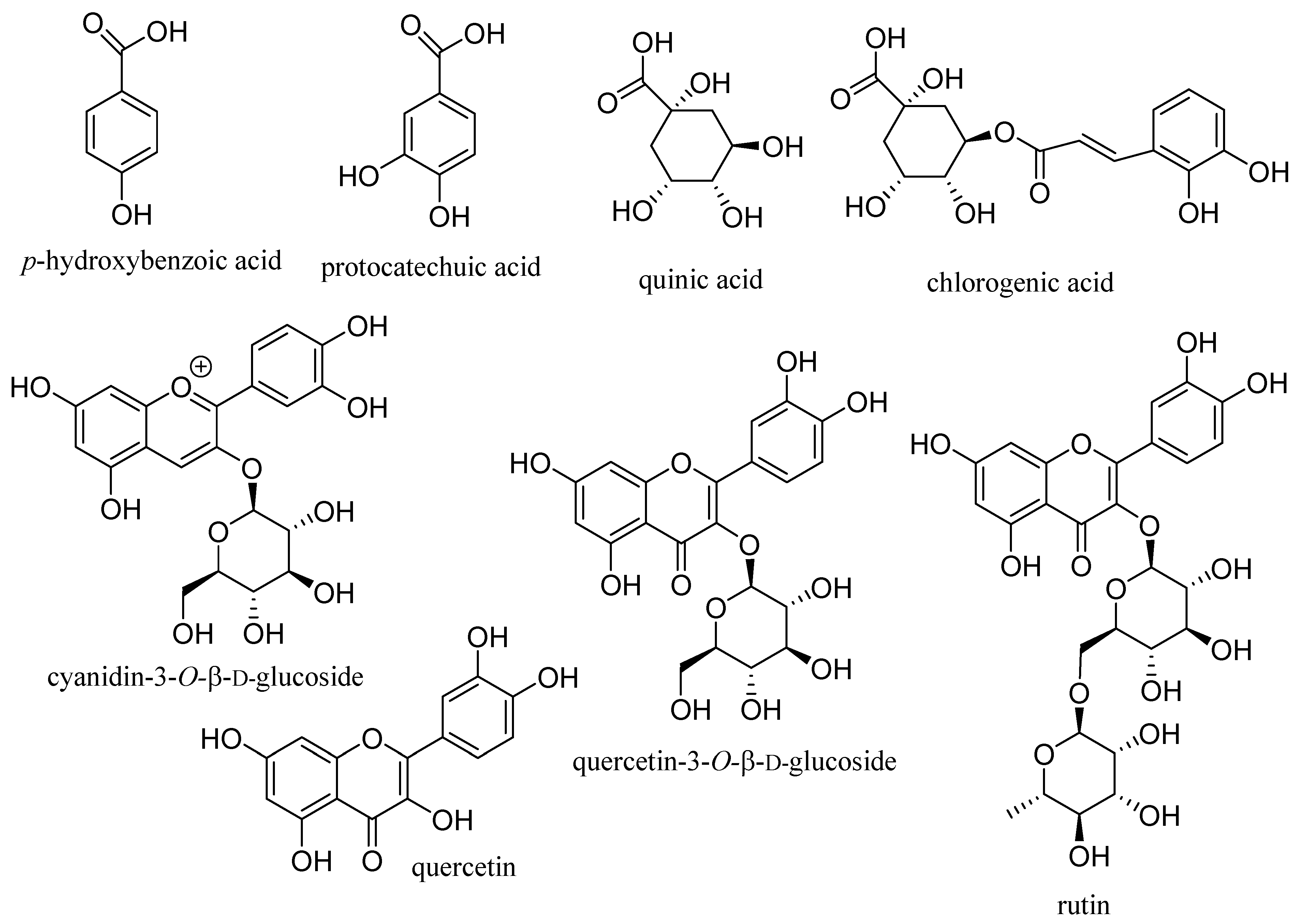
3. Development of Potential Therapeutic Agents from Elderberries
4. Development of Potential Therapeutic Agents from the Major Second Metabolites of Elderberries
5. Concluding Remarks
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| AChE | Acetylcholinesterase |
| AMPK | AMP-activated protein kinase |
| BChE | Butyrylcholinesterase |
| β-CD | β-cyclodextrin |
| CDDO-Me | Bardoxolone methyl |
| 6Cl-TGQ | 6-Chloro-6-deoxy-1,2,3,4-tetra-O-galloyl-α-d-glucopyranose |
| COX | Cyclooxygenase |
| IFN-γ | Interferon-γ |
| IL | Interleukin |
| JNK | c-Jun amino-terminal kinase |
| LPS | Lipopolysaccharide |
| MAPK | Mitogen-activated protein kinase |
| MVA | Modified vaccinia Ankara virus |
| NF-κB | Nuclear factor κB |
| Nrf2 | Nuclear factor erythroid 2-related factor 2 |
| PGG | Pentagalloylglucoside |
| ROS | Reactive oxygen species |
| SAR | Structure–activity relationship |
| SGLT-2 | Sodium–glucose co-transporter 2 |
| T1DM | Type 1 diabetes mellitus |
| T2DM | Type 2 diabetes mellitus |
| TME | Tumor microenvironment |
| TNF | Tumor necrosis factor |
References
- Tejero, J.; Jiménez, P.; Quinto, E.J.; Cordoba-Diaz, D.; Garrosa, M.; Cordoba-Diaz, M.; Gayoso, M.J.; Girbés, T. Elderberries: A source of ribosome-inactivating proteins with lectin activity. Molecules 2015, 20, 2364–2387. [Google Scholar] [CrossRef] [PubMed]
- Sala, G.; Pasta, S.; Maggio, A.; La Mantia, T. Sambucus nigra L. (fam. Viburnaceae) in Sicily: Distribution, ecology, traditional use and therapeutic properties. Plants 2023, 12, 3457. [Google Scholar] [CrossRef] [PubMed]
- Uhl, K.; Mitchell, A.E. Elderberry, an ancient remedy: A comprehensive study of the bioactive compounds in three Sambucus nigra L. subspecies. Annu. Rev. Food Sci. Technol. 2024, 15, 14.1–14.25. [Google Scholar] [CrossRef] [PubMed]
- Stabnikova, O.; Stabnikov, V.; Paredes-López, O. Fruits of wild-grown shrubs for health nutrition. Plant Foods Hum. Nutr. 2024, 79, 20–37. [Google Scholar] [CrossRef] [PubMed]
- Nawirska-Olszanska, A.; Oziemblowski, M.; Brandova, P.; Czaplicka, M. Comparison of the chemical composition of selected varieties of elderberry with wild-growing elderberry. Molecules 2022, 27, 5050. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.; Finn, C.E. Anthocyanins and other polyphenolics in American elderberry (Sambucus canadensis) and European elderberry (S. nigra) cultivars. J. Sci. Food Agric. 2007, 87, 2665–2675. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Gao, Y.G.; Giusti, M.M. Accumulation of anthocyanins and other phytochemicals in American elderberry cultivars during fruit ripening and its impact on color expression. Plants 2020, 9, 1721. [Google Scholar] [CrossRef] [PubMed]
- Corrado, G.; Basile, B.; Mataffo, A.; Yousefi, S.; Salami, S.A.; Perrone, A.; Martinelli, F. Cultivation, phytochemistry, health claims, and genetic diversity of Sambucus nigra, a versatile plant with many beneficial properties. Horticulturae 2023, 9, 488. [Google Scholar] [CrossRef]
- Papagrigoriou, T.; Iliadi, P.; Mitic, M.N.; Mrmošanin, J.M.; Papanastasi, K.; Karapatzak, E.; Maloupa, E.; Gkourogianni, A.V.; Badeka, A.V.; Krigas, N.; et al. Wild-growing and conventionally or organically cultivated Sambucus nigra germplasm: Fruit phytochemical profile, total phenolic content, antioxidant activity, and leaf elements. Plants 2023, 12, 1701. [Google Scholar] [CrossRef]
- Ferreira, S.S.; Silva, P.; Silva, A.M.; Nunes, F.M. Effect of harvesting year and elderberry cultivar on the chemical composition and potential bioactivity: A three-year study. Food Chem. 2020, 302, 125366. [Google Scholar] [CrossRef]
- Johnson, M.C.; Thomas, A.L.; Greenlief, C.M. Impact of frozen storage on the anthocyanin and polyphenol contents of American elderberry fruit juice. J. Agric. Food Chem. 2015, 63, 5653–5659. [Google Scholar] [CrossRef]
- Neves, C.M.B.; Pinto, A.; Goncalves, F.; Wessel, D.F. Changes in elderberry (Sambucus nigra L.) juice concentrate polyphenols during storage. Appl. Sci. 2021, 11, 6941. [Google Scholar] [CrossRef]
- Terzić, M.; Majkić, T.; Beara, I.; Zengin, G.; Miljić, U.; Djurović, S.; Mollica, A.; Radojković, M. Elderberry (Sambucus nigra L.) wine as a novel potential functional food product. Food Biosci. 2022, 50, 102047. [Google Scholar] [CrossRef]
- Pascariu, O.-E.; Israel-Roming, F. Bioactive compounds from elderberry: Extraction, health benefits, and food applications. Processes 2022, 10, 2288. [Google Scholar] [CrossRef]
- Liu, D.; He, X.-Q.; Wu, D.-T.; Li, H.-B.; Feng, Y.-B.; Zou, L.; Gan, R.-Y. Elderberry (Sambucus nigra L.): Bioactive compounds, health functions, and applications. J. Agric. Food Chem. 2022, 70, 4202–4220. [Google Scholar] [CrossRef]
- Sidor, A.; Gramza-Michalowska, A. Advanced research on the antioxidant and health benefit of elderberry (Sambucus nigra) in food—A review. J. Funct. Foods 2015, 18, 941–958. [Google Scholar] [CrossRef]
- Radojković, M.; Vujanović, M.; Majkić, T.; Zengin, G.; Beara, I.; Catauro, M.; Montesano, D. Evaluation of Sambucus nigra L. biopotential as an unused natural resource. Appl. Sci. 2021, 11, 11207. [Google Scholar] [CrossRef]
- Mocanu, M.L.; Amariei, S. Elderberries—A source of bioactive compounds with antiviral action. Plants 2022, 11, 740. [Google Scholar] [CrossRef] [PubMed]
- Harnett, J.; Oakes, K.; Carè, J.; Leach, M.; Brown, D.; Cramer, H.; Pinder, T.-A.; Steel, A.; Anheyer, D. The effects of Sambucus nigra berry on acute respiratory viral infections: A rapid review of clinical studies. Adv. Integr. Med. 2020, 7, 240–246. [Google Scholar] [CrossRef] [PubMed]
- Wieland, S.L.; Piechotta, V.; Feinberg, T.; Ludeman, E.; Hutton, B.; Kanji, S.; Seely, D.; Garritty, C. Elderberry for prevention and treatment of viral respiratory illnesses: A systematic review. BMC Complement. Med. Ther. 2021, 21, 112. [Google Scholar] [CrossRef]
- Porter, R.S.; Bode, R.F. A review of the antiviral properties of black elder (Sambucus nigra L.) products. Phytother. Res. 2017, 31, 533–554. [Google Scholar] [CrossRef] [PubMed]
- Mahboubi, M. Sambucus nigra (black elder) as alternative treatment for cold and flu. Adv. Trad. Med. 2021, 21, 405–414. [Google Scholar] [CrossRef]
- Festa, J.; Singh, H.; Hussain, A.; Da Boit, M. Elderberries as a potential supplement to improve vascular function in a SARS-CoV-2 environment. J. Food Biochem. 2022, 46, e14091. [Google Scholar] [CrossRef] [PubMed]
- Asgary, S.; Pouramini, A. The pros and cons of using elderberry (Sambucus nigra) for prevention and treatment of COVID-19. Adv. Biomed. Res. 2022, 11, 96. [Google Scholar] [CrossRef] [PubMed]
- Dominguez, R.; Pateiro, M.; Munekata, P.E.S.; Santos López, E.M.; Rodriguez, J.A.; Barros, L.; Lorenzo, J.M. Potential use of elderberry (Sambucus nigra L.) as natural colorant and antioxidant in the food industry. A review. Foods 2021, 10, 2713. [Google Scholar] [CrossRef] [PubMed]
- Młynarczyk, K.; Walkowiak-Tomczak, D.; Lysiak, G. Bioactive properties of Sambucus nigra L. as a functional ingredient for food and pharmaceutical industry. J. Funct. Foods 2018, 40, 377–390. [Google Scholar] [CrossRef] [PubMed]
- Ferreira, S.S.; Silva, A.M.; Nunes, F.M. Sambucus nigra L. fruits and flowers: Chemical composition and related bioactivities. Food Rev. Int. 2022, 38, 1237–1265. [Google Scholar] [CrossRef]
- Osman, A.G.; Avula, B.; Katragunta, K.; Ali, Z.; Chittiboyina, A.G.; Khan, I.A. Elderberry extracts: Characterization of the polyphenolic chemical composition, quality consistency, safety, adulteration, and attenuation of oxidative stress- and inflammation-induced health disorders. Molecules 2023, 28, 3148. [Google Scholar] [CrossRef] [PubMed]
- Stępień, A.E.; Trojniak, J.; Tabarkiewicz, J. Health-promoting properties: Anti-inflammatory and anticancer properties of Sambucus nigra L. flowers and fruits. Molecules 2023, 28, 6235. [Google Scholar] [CrossRef]
- Kolesarova, A.; Baldovska, S.; Kohut, L.; Sirotkin, A.V. Black elder and its constituents: Molecular mechanisms of action associated with female reproduction. Pharmaceuticals 2022, 15, 239. [Google Scholar] [CrossRef]
- Netzel, M.; Strass, G.; Herbst, M.; Dietrich, H.; Bitsch, R.; Bitsch, I.; Frank, T. The excretion and biological antioxidant activity of elderberry antioxidants in healthy humans. Food Res. Int. 2005, 38, 905–910. [Google Scholar] [CrossRef]
- Vujanović, M.; Majkić, T.; Zengin, G.; Beara, I.; Tomović, V.; Sojić, B.; Djurović, S.; Radojković, M. Elderberry (Sambucus nigra L.) juice as a novel functional product rich in health-promoting compounds. RSC Adv. 2020, 10, 44805–44814. [Google Scholar] [CrossRef]
- Mutavski, Z.; Nastić, N.; Živković, J.; Šavikin, K.; Veberič, R.; Medič, A.; Pastor, K.; Jokić, S.; Vidović, S. Black elderberry press cake as a source of bioactive ingredients using green-based extraction approaches. Biology 2022, 11, 1465. [Google Scholar] [CrossRef]
- Banach, M.; Khaidakov, B.; Korewo, D.; Wesierska, M.; Cyplik, W.; Kujawa, J.; Ahrné, L.M.; Kujawski, W. The chemical and cytotoxic properties of Sambucus nigra extracts—A natural food colorant. Sustainability 2021, 13, 12702. [Google Scholar] [CrossRef]
- Sibuyi, N.R.S.; Thipe, V.C.; Panjtan-Amiri, K.; Meyer, M.; Katti, K.V. Green synthesis of gold nanoparticles using Acai berry and Elderberry extracts and investigation of their effect on prostate and pancreatic cancer cells. Nanobiomedicine 2021, 8, 1849543521995310. [Google Scholar] [CrossRef]
- Filip, G.A.; Florea, A.; Olteanu, D.; Clichici, S.; David, L.; Moldovan, B.; Cenariu, M.; Scrobota, I.; Potara, M.; Baldea, I. Biosynthesis of silver nanoparticles using Sambucus nigra L. fruit extract for targeting cell death in oral dysplastic cells. Mater. Sci. Eng. C 2021, 123, 111974. [Google Scholar] [CrossRef] [PubMed]
- Wójciak, M.; Ziemlewska, A.; Zagórska-Dziok, M.; Nizioł-Łukaszewska, Z.; Szczepanek, D.; Oniszczuk, T.; Sowa, I. Anti-inflammatory and protective effects of water extract and bioferment from Sambucus nigra fruit in LPS-induced human skin fibroblasts. Int. J. Mol. Sci. 2023, 24, 10286. [Google Scholar] [CrossRef]
- Okihiro, M.S.A.; Rizvi, M.S.S.; Blumenthal, E.J. Elderberry extracts suppress melanoma growth in vitro. J. Anim.Vet. Adv. 2015, 14, 197–204. [Google Scholar]
- Baldovska, S.; Roychoudhury, S.; Bandik, M.; Mihal, M.; Mnahoncakova, E.; Arvay, J.; Pavlik, A.; Slama, P.; Kolesarova, A. Ovarian steroid hormone secretion by human granulosa cells after supplementation of Sambucus nigra L. extract. Physiol. Res. 2021, 70, 755–764. [Google Scholar] [CrossRef]
- Glensk, M.; Czapińska, E.; Woźniak, M.; Ceremuga, I.; Wlodarczyk, M.; Terlecki, G.; Ziólkowski, P.; Seweryn, E. Triterpenoid acids as important antiproliferative constituents of European elderberry fruits. Nutr. Cancer 2017, 69, 643–651. [Google Scholar] [CrossRef]
- Ren, Y.; Kinghorn, A.D. Natural product triterpenoids and their semi-synthetic derivatives with potential anticancer activity. Planta Med. 2019, 85, 802–814. [Google Scholar] [CrossRef]
- Torabian, G.; Valtchev, P.; Adil, Q.; Dehghani, F. Anti-influenza activity of elderberry (Sambucus nigra). J. Funct. Foods 2019, 54, 353–360. [Google Scholar] [CrossRef]
- Ochnik, M.; Franz, D.; Sobczyński, M.; Naporowski, P.; Banach, M.; Orzechowska, B.; Sochocka, M. Inhibition of human respiratory influenza A virus and human betacoronavirus-1 by the blend of double-standardized extracts of Aronia melanocarpa (Michx.) Elliot and Sambucus nigra L. Pharmaceuticals 2022, 15, 619. [Google Scholar] [CrossRef] [PubMed]
- Schön, C.; Mödinger, Y.; Krüger, F.; Doebis, C.; Pischel, I.; Bonnländer, B. A new high-quality elderberry plant extract exerts antiviral and immunomodulatory effects in vitro and ex vivo. Food Agric. Immunol. 2021, 32, 650–662. [Google Scholar] [CrossRef]
- Kim, H.; Calderón, A.I. Rational and safe use of the top two botanical dietary supplements to enhance the immune system. Comb. Chem. High Throughput Screen. 2022, 25, 1129–1130. [Google Scholar] [CrossRef]
- Zakay-Rones, Z.; Varsano, N.; Zlotnik, M.; Manor, O.; Regev, L.; Schlesinger, M.; Mumcuoglu, M. Inhibition of several strains of influenza virus in vitro and reduction of symptoms by an elderberry extract (Sambucus nigra L.) during an outbreak of influenza B Panama. J. Altern. Complement. Med. 1995, 1, 361–369. [Google Scholar] [CrossRef] [PubMed]
- Barak, V.; Halperin, T.; Kalickman, I. The effect of Sambucol®, a black elderberry-based, natural product, on the production of human cytokines: I. Inflammatory cytokines. Eur. Cytokine Netw. 2001, 12, 290–296. [Google Scholar]
- Waknine-Grinberg, J.H.; El-On, J.; Barak, V.; Barenholz, Y.; Golenser, J. The immunomodulatory effect of Sambucol® on leishmanial and malarial infections. Planta Med. 2009, 75, 581–586. [Google Scholar] [CrossRef]
- Ribeiro, A.M.; Estevinho, B.N.; Rocha, F. Microencapsulation of polyphenols—The specific case of the microencapsulation of Sambucus nigra L. extracts—A review. Trends Food Sci. Technol. 2020, 105, 454–467. [Google Scholar] [CrossRef]
- del Olmo, A.; Calzada, J.; Nuñez, M. Benzoic acid and its derivatives as naturally occurring compounds in foods and as additives: Uses, exposure, and controversy. Crit. Rev. Food Sci. Nutr. 2017, 57, 3084–3103. [Google Scholar] [CrossRef]
- Mao, X.; Yang, Q.; Chen, D.; Yu, B.; He, J. Benzoic acid used as food and feed additives can regulate gut functions. BioMed Res. Int. 2019, 2019, 5721585. [Google Scholar] [CrossRef] [PubMed]
- Du, J.J.; Stanton, S.A.; Williams, P.A.; Ong, J.A.; Groundwater, P.W.; Overgaard, J.; Platts, J.A.; Hibbs, D.E. Using electron density to predict synthon formation in a 4-hydroxybenzoic acid: 4,4′-Bipyridine cocrystal. Cryst. Growth Des. 2018, 18, 1786–1798. [Google Scholar] [CrossRef]
- Farrugia, L.J. WinGX and ORTEP for Windows: An update. J. Appl. Cryst. 2012, 45, 849–854. [Google Scholar] [CrossRef]
- Manuja, R.; Sachdeva, S.; Jain, A.; Chaudhary, J. A comprehensive review on biological activities of p-hydroxy benzoic acid and its derivatives. Int. J. Pharm. Sci. Rev. Res. 2013, 22, 109–115. [Google Scholar]
- Pugazhendhi, D.; Pope, G.S.; Darbre, P.D. Oestrogenic activity of p-hydroxybenzoic acid (common metabolite of paraben esters) and methylparaben in human breast cancer cell lines. J. Appl. Toxicol. 2005, 25, 301–309. [Google Scholar] [CrossRef] [PubMed]
- Przybylska-Balcerek, A.; Szablewski, T.; Szwajkowska-Michalek, L.; Świerk, D.; Cegielska-Radziejewska, R.; Krejpcio, Z.; Suchowilska, E.; Tomczyk, L.; Stuper-Szablewska, K. Sambucus nigra extracts—Natural antioxidants and antimicrobial compounds. Molecules 2021, 26, 2910. [Google Scholar] [CrossRef] [PubMed]
- Sundaralingam, M.; Jensen, L.H. Refinement of the structure of salicylic acid. Acta Cryst. 1965, 18, 1053–1058. [Google Scholar] [CrossRef] [PubMed]
- Sykes, E.M.E.; White, D.; McLaughlin, S.; Kumar, A. Salicylic acids and pathogenic bacteria: New perspectives on an old compound. Can. J. Microbiol. 2024, 70, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Elwood, P.; Protty, M.; Morgan, G.; Pickering, J.; Delon, C.; Watkins, J. Aspirin and cancer: Biological mechanisms and clinical outcomes. Open Biol. 2022, 12, 220124. [Google Scholar] [CrossRef]
- Tan, J.L.; Sidhu-Brar, S.; Woodman, R.; Chinnaratha, M.A. Regular aspirin use is associated with a reduced risk of hepatocellular carcinoma (HCC) in chronic liver disease: A systematic review and meta-analysis. J. Gastrointest. Cancer 2023, 54, 325–331. [Google Scholar] [CrossRef]
- Del Bianco-Rondeau, M.; Robert-Halabi, M.; Bloom, S.; Rabasa-Lhoret, R.; Tardif, J.-C.; Lordkipanidzé, M.; Marquis-Gravel, G. Aspirin for primary cardiovascular prevention in patients with diabetes: Uncertainties and opportunities. Thromb. Haemost. 2022, 122, 1443–1453. [Google Scholar] [CrossRef] [PubMed]
- Montgomery, S.; Miedema, M.D.; Dodson, J.A. Aspirin and statin therapy for primary prevention of cardiovascular disease in older adults. Heart 2022, 108, 1090–1097. [Google Scholar] [CrossRef] [PubMed]
- Aree, T. Understanding structures and thermodynamics of β-cyclodextrin encapsulation of chlorogenic, caffeic and quinic acids: Implications for enriching antioxidant capacity and masking bitterness in coffee. Food Chem. 2019, 293, 550–560. [Google Scholar] [CrossRef]
- Aree, T. Inclusion complex of β-cyclodextrin with coffee chlorogenic acid: New insights from a combined crystallographic and theoretical study. Acta Crystallogr. Sect. C Struct. Chem. 2019, 75, 15–21. [Google Scholar] [CrossRef] [PubMed]
- Benali, T.; Bakrim, S.; Ghchime, R.; Benkhaira, N.; El Omari, N.; Balahbib, A.; Taha, D.; Zengin, G.; Hasan, M.M.; Bibi, S.; et al. Pharmacological insights into the multifaceted biological properties of quinic acid. Biotechnol. Genet. Eng. Rev. 2022, 38, 1–30. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Pan, X.; Jiang, L.; Chu, Y.; Gao, S.; Jiang, X.; Zhang, Y.; Chen, Y.; Luo, S.; Peng, C. The biological activity mechanism of chlorogenic acid and its applications in food industry: A review. Front. Nutr. 2022, 9, 943911. [Google Scholar] [CrossRef] [PubMed]
- Huang, S.; Wang, L.-L.; Xue, N.-N.; Li, C.; Guo, H.-H.; Ren, T.-K.; Zhan, Y.; Li, W.-B.; Zhang, J.; Chen, X.-G.; et al. Chlorogenic acid effectively treats cancers through induction of cancer cell differentiation. Theranostics 2019, 9, 6745–6763. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Feng, Y.; Li, Y.; Hu, Y.; Zhang, Q.; Huang, Y.; Shi, K.; Ran, C.; Hou, J.; Zhou, G.; et al. Chlorogenic acid decreases malignant characteristics of hepatocellular carcinoma cells by inhibiting DNMT1 expression. Front. Pharmacol. 2020, 11, 867. [Google Scholar] [CrossRef] [PubMed]
- Salzillo, A.; Ragone, A.; Spina, A.; Naviglio, S.; Sapio, L. Chlorogenic acid enhances doxorubicin-mediated cytotoxic effect in osteosarcoma cells. Int. J. Mol. Sci. 2021, 22, 8586. [Google Scholar] [CrossRef]
- Ren, Y.; Frank, T.; Meyer, G.; Lei, J.; Grebenc, J.R.; Slaughter, R.; Gao, Y.G.; Kinghorn, A.D. Potential benefits of black chokeberry (Aronia melanocarpa) fruits and their constituents in improving human health. Molecules 2022, 27, 7823. [Google Scholar] [CrossRef]
- da Silva, R.F.R.; Barreira, J.C.M.; Heleno, S.A.; Barros, L.; Calhelha, R.C.; Ferreira, I.C.F.R. Anthocyanin profile of elderberry juice: A natural-based bioactive colouring ingredient with potential food application. Molecules 2019, 24, 2359. [Google Scholar] [CrossRef]
- Ueno, K.; Saito, N. Cyanidin bromide monohydrate (3,5,7,3′,4′-pentahydroxyflavylium bromide monohydrate). Acta Crystallogr. Sect. B Struct. Crystallogr. Cryst. Chem. 1977, 33, 114–116. [Google Scholar] [CrossRef]
- Li, D.; Wang, P.; Luo, Y.; Zhao, M.; Chen, F. Health benefits of anthocyanins and molecular mechanisms: Update from recent decade. Crit. Rev. Food Sci. Nutr. 2017, 57, 1729–1741. [Google Scholar] [CrossRef] [PubMed]
- Strugała, P.; Loi, S.; Bażanów, B.; Kuropka, P.; Kucharska, A.Z.; Włoch, A.; Gabrielska, J. A comprehensive study on the biological activity of elderberry extract and cyanidin 3-O-glucoside and their interactions with membranes and human serum albumin. Molecules 2018, 23, 2566. [Google Scholar] [CrossRef]
- Klitou, P.; Parisi, E.; Bordignon, S.; Bravetti, F.; Rosbottom, I.; Dell’Aera, M.; Cuocci, C.; Chierotti, M.R.; Altomare, A.; Simone, E. Navigating the complex solid form landscape of the quercetin flavonoid molecule. Cryst. Growth Des. 2023, 23, 6034–6045. [Google Scholar] [CrossRef]
- Lai, W.-F.; Wong, W.-T. Design and optimization of quercetin-based functional foods. Crit. Rev. Food Sci. Nutr. 2022, 62, 7319–7335. [Google Scholar] [CrossRef]
- Asgharian, P.; Tazekand, A.P.; Hosseini, K.; Forouhandeh, H.; Ghasemnejad, T.; Ranjbar, M.; Hasan, M.; Kumar, M.; Beirami, S.M.; Tarhriz, V.; et al. Potential mechanisms of quercetin in cancer prevention: Focus on cellular and molecular targets. Cancer Cell Int. 2022, 22, 257. [Google Scholar] [CrossRef]
- Lotfi, N.; Yousefi, Z.; Golabi, M.; Khalilian, P.; Ghezelbash, B.; Montazeri, M.; Shams, M.H.; Baghbadorani, P.Z.; Eskandari, N. The potential anti-cancer effects of quercetin on blood, prostate and lung cancers: An update. Front. Immunol. 2023, 14, 1077531. [Google Scholar] [CrossRef] [PubMed]
- McKay, T.B.; Emmitte, K.A.; German, C.; Karamichos, D. Quercetin and related analogs as therapeutics to promote tissue repair. Bioengineering 2023, 10, 1127. [Google Scholar] [CrossRef]
- Bhat, I.U.H.; Bhat, R. Quercetin: A bioactive compound imparting cardiovascular and neuroprotective benefits: Scope for exploring fresh produce, their wastes, and by-products. Biology 2021, 10, 586. [Google Scholar] [CrossRef]
- Islam, S.M.; Quispe, C.; Hossain, R.; Islam, M.T.; Al-Harrasi, A.; Al-Rawahi, A.; Martorell, M.; Mamurova, A.; Seilkhan, A.; Altybaeva, N.; et al. Neuropharmacological effects of quercetin: A literature-based review. Front. Pharmacol. 2021, 12, 665031. [Google Scholar] [CrossRef] [PubMed]
- Arabi, S.M.; Jazinaki, M.S.; Chambari, M.; Bahrami, L.S.; Maleki, M.; Sukhorukov, V.N.; Sahebkar, A. The effects of quercetin supplementation on cardiometabolic outcomes: An umbrella review of meta-analyses of randomized controlled trials. Phytother. Res. 2023, 37, 5080–5091. [Google Scholar] [CrossRef] [PubMed]
- Prakash, C.; Tyagi, J.; Rabidas, S.S.; Kumar, V.; Sharma, D. Therapeutic potential of quercetin and its derivatives in epilepsy: Evidence from preclinical studies. NeuroMol. Med. 2023, 25, 163–178. [Google Scholar] [CrossRef] [PubMed]
- Terzić, M.; Majkić, T.; Zengin, G.; Beara, I.; Cespedes-Acuña, C.L.; Čavić, D.; Radojković, M. Could elderberry fruits processed by modern and conventional drying and extraction technology be considered a valuable source of health-promoting compounds? Food Chem. 2023, 405, 134766. [Google Scholar] [CrossRef] [PubMed]
- Maugeri, A.; Lombardo, G.E.; Cirmi, S.; Süntar, I.; Barreca, D.; Laganà, G.; Navarra, M. Pharmacology and toxicology of tannins. Arch. Toxicol. 2022, 96, 1257–1277. [Google Scholar] [CrossRef] [PubMed]
- de Melo, L.F.M.; Aquino-Martins, V.G.Q.; da Silva, A.P.; Rocha, H.A.O.; Scortecci, K.C. Biological and pharmacological aspects of tannins and potential biotechnological applications. Food Chem. 2023, 414, 135645. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Kim, J.; Li, J.; Liu, F.; Liu, X.; Himmeldirk, K.; Ren, Y.; Wagner, T.E.; Chen, X. Natural anti-diabetic compound 1,2,3,4,6-penta-O-galloyl-D-glucopyranose binds to insulin receptor and activates insulin-mediated glucose transport signaling pathway. Biochem. Biophys. Res. Commun. 2005, 336, 430–437. [Google Scholar] [CrossRef] [PubMed]
- Ren, Y.; Himmeldirk, K.; Chen, X. Synthesis and structure-activity relationship study of antidiabetic penta-O-galloyl-D-glucopyranose and its analogs. J. Med. Chem. 2006, 49, 2829–2837. [Google Scholar] [CrossRef] [PubMed]
- Cao, Y.; Li, Y.; Kim, J.; Ren, Y.; Himmeldirk, K.; Liu, Y.; Qian, Y.; Liu, F.; Chen, X. Orally efficacious novel small molecule 6-chloro-6-deoxy-1,2,3,4-tetra-O-galloyl-α-D-glucopyranose selectively and potently stimulates insulin receptor and alleviates diabetes. J. Mol. Endocrinol. 2013, 51, 15–26. [Google Scholar] [CrossRef][Green Version]
- Xie, T.; Zhao, L.-J. Synthetic approaches and clinical application of small-molecule inhibitors of sodium-dependent glucose transporters 2 for the treatment of type 2 diabetes mellitus. Eur. J. Med. Chem. 2024, 269, 116343. [Google Scholar] [CrossRef]
- Lam-Chung, C.E. Comprehensive review of SGLT2 inhibitors’ efficacy through their diuretic mode of action in diabetic patients. Front. Endocrinol. 2023, 14, 1174692. [Google Scholar] [CrossRef] [PubMed]
- Maxson, R.; Starr, J.; Sewell, J.; Lyas, C. SGLT2 inhibitors to slow chronic kidney disease progression: A review. Clin. Ther. 2024, 46, e23–e28. [Google Scholar] [CrossRef] [PubMed]
- Kern, J.S.; Sprecher, E.; Fernandez, M.F.; Schauer, F.; Bodemer, C.; Cunningham, T.; Löwe, S.; Davis, C.; Sumeray, M.; Bruckner, A.L.; et al. Efficacy and safety of oleogel-S10 (birch triterpenes) for epidermolysis bullosa: Results from the phase III randomized double-blind phase of the EASE study. Br. J. Dermatol. 2023, 188, 12–21. [Google Scholar] [CrossRef] [PubMed]
- Laszczyk, M.; Jäger, S.; Simon-Haarhaus, B.; Scheffler, A.; Schempp, C.M. Physical, chemical and pharmacological characterization of a new oleogel-forming triterpene extract from the outer bark of birch (Betulae Cortex). Planta Med. 2006, 72, 1389–1395. [Google Scholar] [CrossRef] [PubMed]
- Ren, Y.; Anaya-Eugenio, G.D.; Czarnecki, A.A.; Ninh, T.N.; Yuan, C.; Chai, H.-B.; Soejarto, D.D.; Burdette, J.E.; Carcache de Blanco, E.J.; Kinghorn, A.D. Cytotoxic and NF-κB and mitochondrial transmembrane potential inhibitory pentacyclic triterpenoids from Syzygium corticosum and their semi-synthetic derivatives. Bioorg. Med. Chem. 2018, 26, 4452–4460. [Google Scholar] [CrossRef] [PubMed]
- Ren, Y.; Yu, J.; Kinghorn, A.D. Development of anticancer agents from plant-derived sesquiterpene lactones. Curr. Med. Chem. 2016, 23, 2397–2420. [Google Scholar] [CrossRef]
- Ren, Y.; Kinghorn, A.D. Development of potential antitumor agents from the scaffolds of plant-derived terpenoid lactones. J. Med. Chem. 2020, 63, 15410–15448. [Google Scholar] [CrossRef] [PubMed]
- Amirova, K.M.; Dimitrova, P.A.; Leseva, M.N.; Koycheva, I.K.; Dinkova-Kostova, A.T.; Georgiev, M.I. The triterpenoid Nrf2 activator, CDDO-Me, decreases neutrophil senescence in a murine model of joint damage. Int. J. Mol. Sci. 2023, 24, 8775. [Google Scholar] [CrossRef] [PubMed]
- Ihunnah, C.A.; Ghosh, S.; Hahn, S.; Straub, A.C.; Ofori-Acquah, S.F. Nrf2 activation with CDDO-methyl promotes beneficial and deleterious clinical effects in transgenic mice with sickle cell anemia. Front. Pharmacol. 2022, 13, 880834. [Google Scholar] [CrossRef]
- Bondi, C.D.; Hartman, H.L.; Tan, R.J. NRF2 in kidney physiology and disease. Physiol. Rep. 2024, 12, e15961. [Google Scholar] [CrossRef]
- Chertow, G.M.; Appel, G.B.; Andreoli, S.; Bangalore, S.; Block, G.A.; Chapman, A.B.; Chin, M.P.; Gibson, K.L.; Goldsberry, A.; Iijima, K.; et al. Study design and baseline characteristics of the CARDINAL trial: A phase 3 study of bardoxolone methyl in patients with Alport syndrome. Am. J. Nephrol. 2021, 52, 180–189. [Google Scholar] [CrossRef] [PubMed]
- Newman, D.J.; Cragg, G.M. Natural products as sources of new drugs over the nearly four decades from 01/1981 to 09/2019. J. Nat. Prod. 2020, 83, 770–803. [Google Scholar] [CrossRef]
- Aldrich, L.N.; Burdette, J.E.; Carcache de Blanco, E.J.; Coss, C.C.; Eustaquio, A.S.; Fuchs, J.R.; Kinghorn, A.D.; MacFarlane, A.; Mize, B.K.; Oberlies, N.H.; et al. Discovery of anticancer agents of diverse natural origin. J. Nat. Prod. 2022, 85, 702–719. [Google Scholar] [CrossRef] [PubMed]
- Boedtkjer, E.; Pedersen, S.F. The acidic tumor microenvironment as a driver of cancer. Annu. Rev. Physiol. 2020, 82, 103–126. [Google Scholar] [CrossRef] [PubMed]
- Anari, F.; Ramamurthy, C.; Zibelman, M. Impact of tumor microenvironment composition on therapeutic responses and clinical outcomes in cancer. Future Oncol. 2018, 14, 1409–1421. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, S.M.U.; Luo, L.; Namani, A.; Wang, X.J.; Tang, X. Nrf2 signaling pathway: Pivotal roles in inflammation. Biochim. Biophys. Acta 2017, 1863, 585–597. [Google Scholar] [CrossRef] [PubMed]
- Iqbal, M.J.; Kabeer, A.; Abbas, Z.; Siddiqui, H.A.; Calina, D.; Sharifi-Rad, J.; Cho, W.C. Interplay of oxidative stress, cellular communication and signaling pathways in cancer. Cell Commun. Signal. 2024, 22, 7. [Google Scholar] [CrossRef]
- Feng, J.; Read, O.J.; Dinkova-Kostova, A.T. Nrf2 in TIME: The emerging role of nuclear factor erythroid 2-related factor 2 in the tumor immune microenvironment. Mol. Cells 2023, 46, 142–152. [Google Scholar] [CrossRef]




| Year(s) | Clinical ID | Investigation Title |
|---|---|---|
| 25 May 2023–26 November 2023 | NCT05994586 | The efficacy of an AP029 mix in patients with impaired carbohydrate metabolism |
| 7 July 2021–2 September 2022 | NCT05723497 | Elderberries and obesity |
| 1 September 2016–1 December 2019 | NCT02414607 | Effect of elderberry juice on cognition and inflammation in patients with mild cognitive impairment |
| 29 January 2018–25 June 2019 | NCT03410862 | Evaluating the safety and clinical efficacy of elderberry extract in patients with influenza |
| 10 October 2022–present | NCT05435144 | Elderberry for immune support |
| 1 September 2006–1 September 2009 | NCT00375115 | Efficacy of Sambucol® in the treatment of influenza |
| 5 January 2021–16 May 2022 | NCT05489770 | BERRY—a study of Sambucol® in the treatment and reduction of symptoms in participants with COVID-19 (BERRY) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ren, Y.; Meyer, G.; Anderson, A.T.; Lauber, K.M.; Gallucci, J.C.; Gao, G.; Kinghorn, A.D. Development of Potential Therapeutic Agents from Black Elderberries (the Fruits of Sambucus nigra L.). Molecules 2024, 29, 2971. https://doi.org/10.3390/molecules29132971
Ren Y, Meyer G, Anderson AT, Lauber KM, Gallucci JC, Gao G, Kinghorn AD. Development of Potential Therapeutic Agents from Black Elderberries (the Fruits of Sambucus nigra L.). Molecules. 2024; 29(13):2971. https://doi.org/10.3390/molecules29132971
Chicago/Turabian StyleRen, Yulin, Gunnar Meyer, Andrew T. Anderson, Kaitlyn M. Lauber, Judith C. Gallucci, Gary Gao, and Alan Douglas Kinghorn. 2024. "Development of Potential Therapeutic Agents from Black Elderberries (the Fruits of Sambucus nigra L.)" Molecules 29, no. 13: 2971. https://doi.org/10.3390/molecules29132971
APA StyleRen, Y., Meyer, G., Anderson, A. T., Lauber, K. M., Gallucci, J. C., Gao, G., & Kinghorn, A. D. (2024). Development of Potential Therapeutic Agents from Black Elderberries (the Fruits of Sambucus nigra L.). Molecules, 29(13), 2971. https://doi.org/10.3390/molecules29132971






