Covalent Bonding of MXene/COF Heterojunction for Ultralong Cycling Li-Ion Battery Electrodes
Abstract
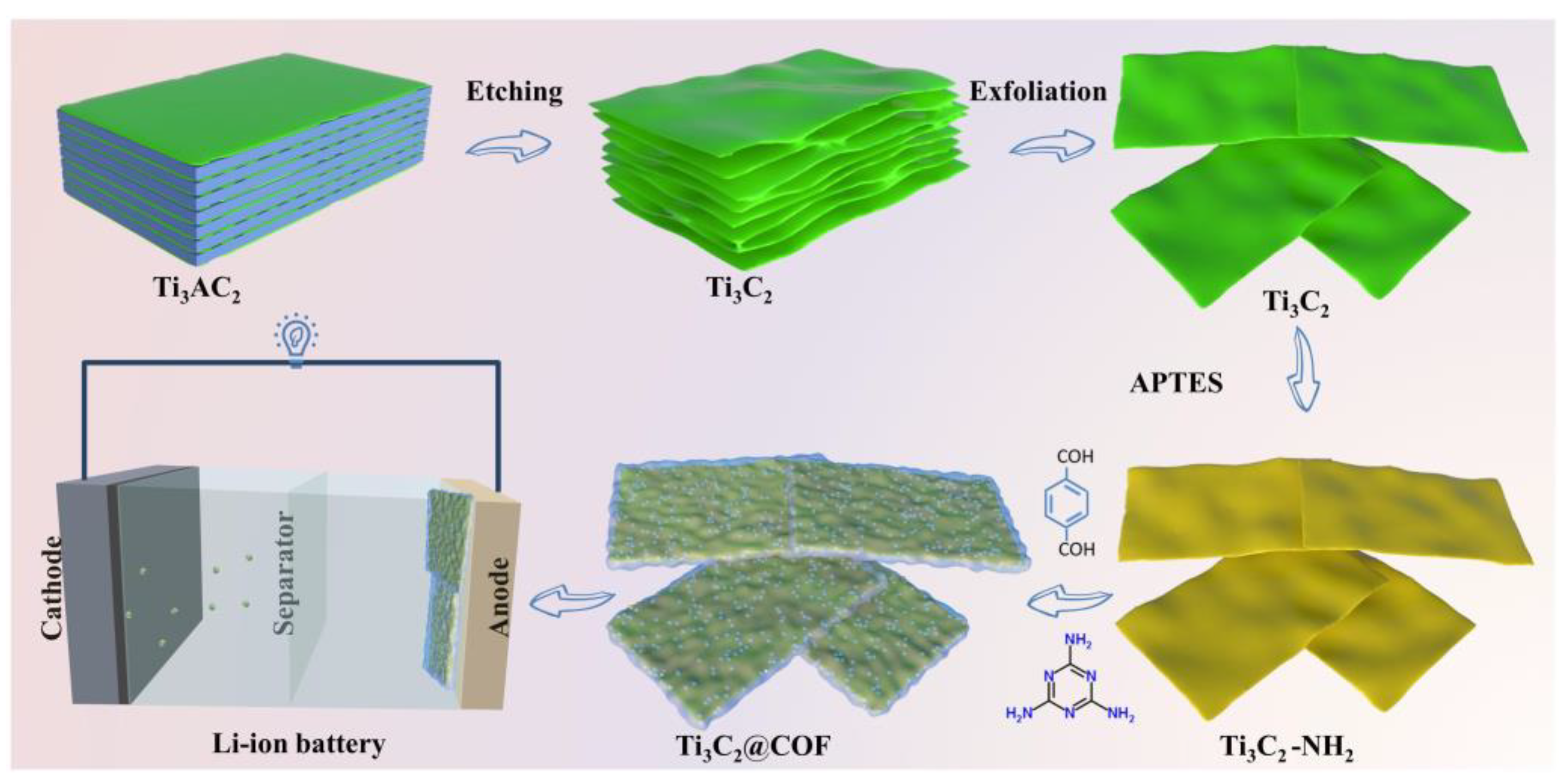
1. Introduction
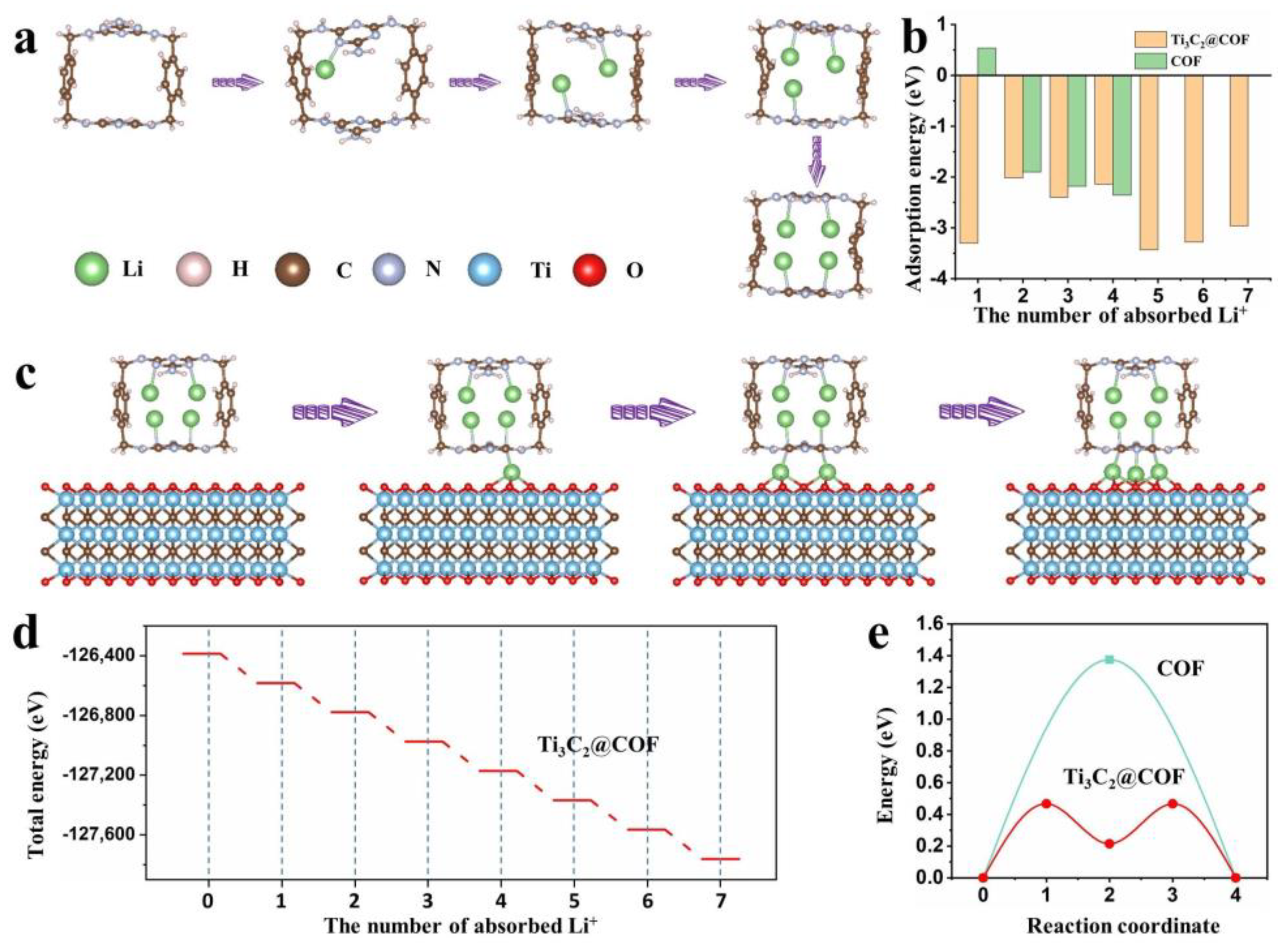
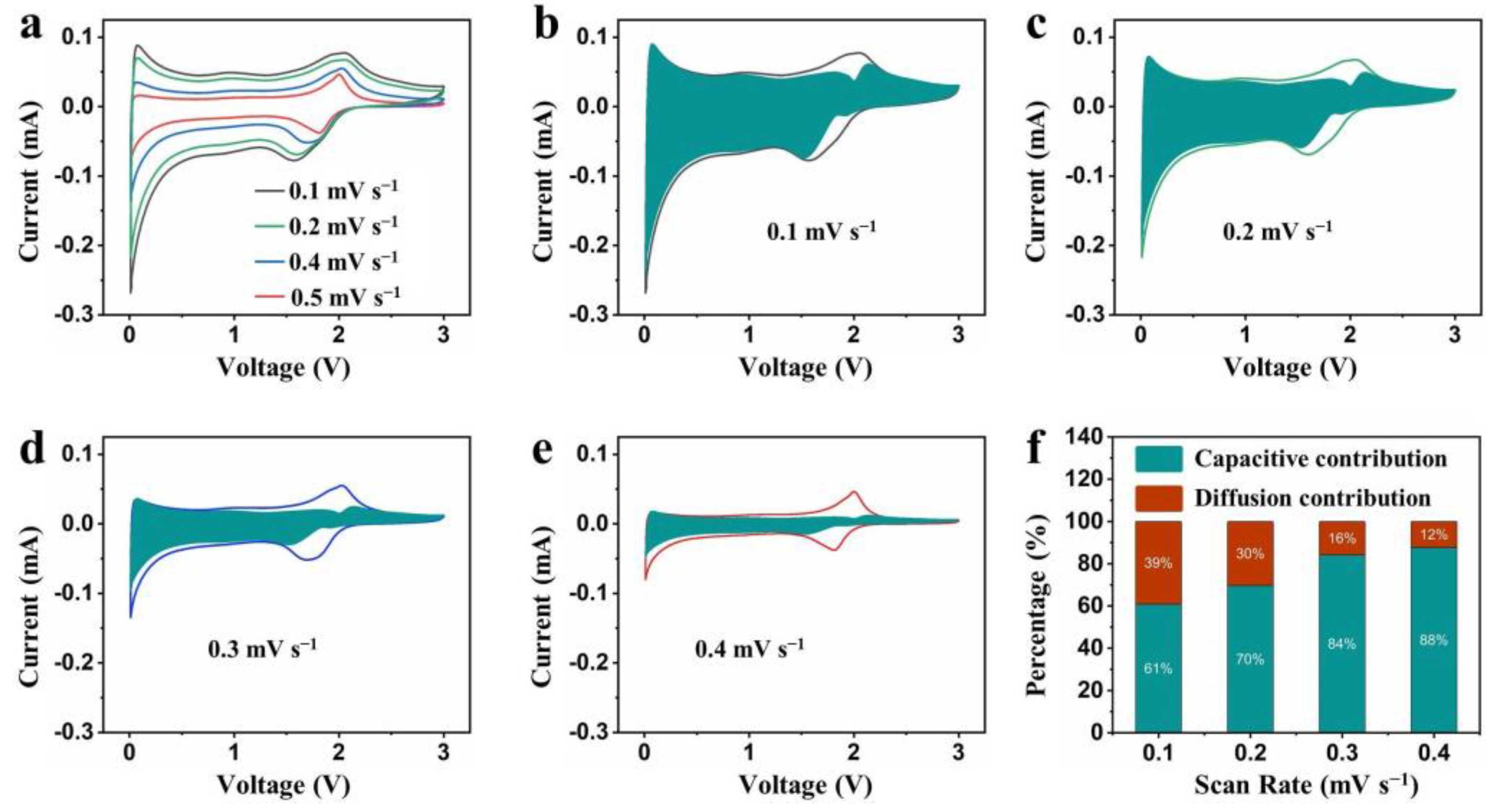
2. Results and Discussion
3. Materials and Methods
3.1. Preparation of Ti3C2 MXenes
3.2. Synthesis of COF and Ti3C2@COF Composite
3.3. Materials Characterization
3.4. Electrochemical Characterization
3.5. DFT Calculations
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Xia, H.; Tang, Y.; Malyi, O.I.; Zhu, Z.; Zhang, Y.; Zhang, W.; Ge, X.; Zeng, Y.; Chen, X. Deep Cycling for High-Capacity Li-Ion Batteries. Adv. Mater. 2021, 33, 2004998. [Google Scholar] [CrossRef]
- Ma, G.; Shao, H.; Xu, J.; Liu, Y.; Huang, Q.; Taberna, P.-L.; Simon, P.; Lin, Z. Li-ion storage properties of two-dimensional titanium-carbide synthesized via fast one-pot method in air atmosphere. Nat. Commun. 2021, 12, 5085. [Google Scholar] [CrossRef]
- Zhu, D.; Zhu, Y.; Chen, Y.; Yan, Q.; Wu, H.; Liu, C.-Y.; Wang, X.; Alemany, L.B.; Gao, G.; Senftle, T.P.; et al. Three-dimensional covalent organic frameworks with pto and mhq-z topologies based on Tri- and tetratopic linkers. Nat. Commun. 2023, 14, 2865. [Google Scholar] [CrossRef] [PubMed]
- Eshetu, G.G.; Zhang, H.; Judez, X.; Adenusi, H.; Armand, M.; Passerini, S.; Figgemeier, E. Production of high-energy Li-ion batteries comprising silicon-containing anodes and insertion-type cathodes. Nat. Commun. 2021, 12, 5459. [Google Scholar] [CrossRef]
- Zhao, G.; Sun, Y.; Yang, Y.; Zhang, C.; An, Q.; Guo, H. Molecular engineering regulation redox-dual-active-center covalent organic frameworks-based anode for high-performance Li storage. EcoMat 2022, 4, e12221. [Google Scholar] [CrossRef]
- Li, M.; Lu, J.; Chen, Z.; Amine, K. 30 Years of Lithium-Ion Batteries. Adv. Mater. 2018, 30, 1800561. [Google Scholar] [CrossRef]
- Albertus, P.; Babinec, S.; Litzelman, S.; Newman, A. Status and challenges in enabling the lithium metal electrode for high-energy and low-cost rechargeable batteries. Nat. Energy 2018, 3, 16. [Google Scholar] [CrossRef]
- Lu, J.; Chen, Z.; Pan, F.; Cui, Y.; Amine, K. High-Performance Anode Materials for Rechargeable Lithium-Ion Batteries. Electrochem. Energy Rev. 2018, 1, 35. [Google Scholar] [CrossRef]
- Ghidiu, M.; Lukatskaya, M.R.; Zhao, M.-Q.; Gogotsi, Y.; Barsoum, M.W. Conductive two-dimensional titanium carbide ‘clay’ with high volumetric capacitance. Nature 2014, 516, 78. [Google Scholar] [CrossRef]
- Wang, Y.; An, N.; Wen, L.; Wang, L.; Jiang, X.; Hou, F.; Yin, Y.; Liang, J. Recent progress on the recycling technology of Li-ion batteries. J. Energy Chem. 2021, 55, 391. [Google Scholar] [CrossRef]
- Lukatskaya, M.R.; Kota, S.; Lin, Z.; Zhao, M.-Q.; Shpigel, N.; Levi, M.D.; Halim, J.; Taberna, P.-L.; Barsoum, M.W.; Simon, P.; et al. Ultra-high-rate pseudocapacitive energy storage in two-dimensional transition metal carbides. Nat. Energy 2017, 2, 17105. [Google Scholar] [CrossRef]
- Li, Y.; Shao, H.; Lin, Z.; Lu, J.; Liu, L.; Duployer, B.; Persson, P.O.Å.; Eklund, P.; Hultman, L.; Li, M.; et al. A general Lewis acidic etching route for preparing MXenes with enhanced electrochemical performance in non-aqueous electrolyte. Nat. Mater. 2020, 19, 894. [Google Scholar] [CrossRef] [PubMed]
- Zheng, C.; Yao, Y.; Rui, X.; Feng, Y.; Yang, D.; Pan, H.; Yu, Y. Functional MXene-Based Materials for Next-Generation Rechargeable Batteries. Adv. Mater. 2022, 34, 2204988. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Q.; Zhu, Q.; Miao, J.; Zhang, P.; Wan, P.; He, L.; Xu, B. Flexible 3D Porous MXene Foam for High-Performance Lithium-Ion Batteries. Small 2019, 15, 1904293. [Google Scholar] [CrossRef] [PubMed]
- Chen, K.-H.; Goel, V.; Namkoong, M.J.; Wied, M.; Müller, S.; Wood, V.; Sakamoto, J.; Thornton, K.; Dasgupta, N.P. Enabling 6C Fast Charging of Li-Ion Batteries with Graphite/Hard Carbon Hybrid Anodes. Adv. Energy Mater. 2021, 11, 2003336. [Google Scholar] [CrossRef]
- Pomerantseva, E.; Bonaccorso, F.; Feng, X.; Cui, Y.; Gogotsi, Y. Energy storage: The future enabled by nanomaterials. Science 2019, 366, eaan8285. [Google Scholar] [CrossRef] [PubMed]
- Wang, T.; Yao, K.; Hua, Y.; Shankar, E.G.; Shanthappa, R.; Yu, J.S. Rational design of MXene-MoS2 heterostructure with rapid ion transport rate as an advanced anode for sodium-ion batteries. Chem. Eng. J. 2023, 457, 141363. [Google Scholar] [CrossRef]
- Li, X.; Huang, Z.; Shuck, C.E.; Liang, G.; Gogotsi, Y.; Zhi, C. MXene chemistry, electrochemistry and energy storage applications. Nat. Rev. Chem. 2022, 6, 389. [Google Scholar] [CrossRef]
- Li, J.; Han, L.; Li, Y.; Li, J.; Zhu, G.; Zhang, X.; Lu, T.; Pan, L. MXene-decorated SnS2/Sn3S4 hybrid as anode material for high-rate lithium-ion batteries. Chem. Eng. J. 2020, 380, 122590. [Google Scholar] [CrossRef]
- Wang, C.; Xie, H.; Chen, S.; Ge, B.; Liu, D.; Wu, C.; Xu, W.; Chu, W.; Babu, G.; Ajayan, P.M.; et al. Atomic Cobalt Covalently Engineered Interlayers for Superior Lithium-Ion Storage. Adv. Mater. 2018, 30, 1802525. [Google Scholar] [CrossRef]
- Zhao, G.; Zhang, Y.; Gao, Z.; Li, H.; Liu, S.; Cai, S.; Yang, X.; Guo, H.; Sun, X. Dual Active Site of the Azo and Carbonyl-Modified Covalent Organic Framework for High-Performance Li Storage. ACS Energy Lett. 2020, 5, 1022. [Google Scholar] [CrossRef]
- Zhu, D.; Xu, G.; Barnes, M.; Li, Y.; Tseng, C.-P.; Zhang, Z.; Zhang, J.-J.; Zhu, Y.; Khalil, S.; Rahman, M.M.; et al. Covalent Organic Frameworks for Batteries. Adv. Funct. Mater. 2021, 31, 2100505. [Google Scholar] [CrossRef]
- Yang, X.; Gong, L.; Liu, X.; Zhang, P.; Li, B.; Qi, D.; Wang, K.; He, F.; Jiang, J. Mesoporous Polyimide-Linked Covalent Organic Framework with Multiple Redox-Active Sites for High-Performance Cathodic Li Storage. Angew. Chem. Int. Ed. 2022, 61, e202207043. [Google Scholar] [CrossRef] [PubMed]
- Javed, M.S.; Mateen, A.; Hussain, I.; Ahmad, A.; Mubashir, M.; Khan, S.; Assiri, M.A.; Eldin, S.M.; Shah, S.S.A.; Han, W. Recent progress in the design of advanced MXene/metal oxides-hybrid materials for energy storage devices. Energy Storage Mater. 2022, 53, 827. [Google Scholar] [CrossRef]
- Xu, X.; Zhang, S.; Xu, K.; Chen, H.; Fan, X.; Huang, N. Janus Dione-Based Conjugated Covalent Organic Frameworks with High Conductivity as Superior Cathode Materials. J. Am. Chem. Soc. 2023, 145, 1022. [Google Scholar] [CrossRef] [PubMed]
- Zhai, L.; Li, G.; Yang, X.; Park, S.; Han, D.; Mi, L.; Wang, Y.; Li, Z.; Lee, S.-Y. 30 Li+-Accommodating Covalent Organic Frameworks as Ultralong Cyclable High-Capacity Li-Ion Battery Electrodes. Adv. Funct. Mater. 2022, 32, 2108798. [Google Scholar] [CrossRef]
- Wang, H.; Qian, C.; Liu, J.; Zeng, Y.; Wang, D.; Zhou, W.; Gu, L.; Wu, H.; Liu, G.; Zhao, Y. Integrating Suitable Linkage of Covalent Organic Frameworks into Covalently Bridged Inorganic/Organic Hybrids toward Efficient Photocatalysis. J. Am. Chem. Soc. 2020, 142, 4862. [Google Scholar] [CrossRef]
- Wu, X.; Hong, Y.-L.; Xu, B.; Nishiyama, Y.; Jiang, W.; Zhu, J.; Zhang, G.; Kitagawa, S.; Horike, S. Perfluoroalkyl-Functionalized Covalent Organic Frameworks with Superhydrophobicity for Anhydrous Proton Conduction. J. Am. Chem. Soc. 2020, 142, 14357. [Google Scholar] [CrossRef]
- Wei, S.; Wang, J.; Li, Y.; Fang, Z.; Wang, L.; Xu, Y. Recent progress in COF-based electrode materials for rechargeable metal-ion batteries. Nano Res. 2023, 16, 6753. [Google Scholar] [CrossRef]
- Long, Y.; Tao, Y.; Shang, T.; Yang, H.; Sun, Z.; Chen, W.; Yang, Q.-H. Roles of Metal Ions in MXene Synthesis, Processing and Applications: A Perspective. Adv. Sci. 2022, 9, 2200296. [Google Scholar] [CrossRef]
- Dong, Y.; Shi, H.; Wu, Z.-S. Recent Advances and Promise of MXene-Based Nanostructures for High-Performance Metal Ion Batteries. Adv. Funct. Mater. 2020, 30, 2000706. [Google Scholar] [CrossRef]
- Zhang, C.; Zhao, W.; Park, S.-H.; Guo, T.; Deng, S.; Seral-Ascaso, A.; Si, M.; Grissa, R.; Barwich, S.; Nicolosi, V. Interconnected Metallic Membrane Enabled by MXene Inks Toward High-Rate Anode and High-Voltage Cathode for Li-Ion Batteries. Adv. Funct. Mater. 2023, 33, 2213860. [Google Scholar] [CrossRef]
- Zhang, C.; Ma, Y.; Zhang, X.; Abdolhosseinzadeh, S.; Sheng, H.; Lan, W.; Pakdel, A.; Heier, J.; Nüesch, F. Two-Dimensional Transition Metal Carbides and Nitrides (MXenes): Synthesis, Properties, and Electrochemical Energy Storage Applications. Energy Environ. Mater. 2020, 3, 29. [Google Scholar] [CrossRef]
- VahidMohammadi, A.; Rosen, J.; Gogotsi, Y. The world of two-dimensional carbides and nitrides (MXenes). Science 2021, 372, eabf1581. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.; Zhou, C.; Filatov, A.S.; Cho, W.; Lagunas, F.; Wang, M.; Vaikuntanathan, S.; Liu, C.; Klie, R.F.; Talapin, D.V. Direct synthesis and chemical vapor deposition of 2D carbide and nitride MXenes. Science 2023, 379, 1242. [Google Scholar] [CrossRef] [PubMed]
- Xiong, D.; Shi, Y.; Yang, H.Y. Rational design of MXene-based films for energy storage: Progress, prospects. Mater. Today 2021, 46, 183. [Google Scholar] [CrossRef]
- Pang, J.; Mendes, R.G.; Bachmatiuk, A.; Zhao, L.; Ta, H.Q.; Gemming, T.; Liu, H.; Liu, Z.; Rummeli, M.H. Applications of 2D MXenes in energy conversion and storage systems. Chem. Soc. Rev. 2019, 48, 72. [Google Scholar] [CrossRef]
- Zhang, T.; Chang, L.; Zhang, X.; Wan, H.; Liu, N.; Zhou, L.; Xiao, X. Simultaneously tuning interlayer spacing and termination of MXenes by Lewis-basic halides. Nat. Commun. 2022, 13, 6731. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Wang, S.; Zhang, Z.; Lu, Q.; Zhang, Y.; Wang, L.; Fang, H.; Song, Y. Regulating interlayer spacing and surface terminations of N-doped 3D MXenes with high-rate Li-ion storage capability. Mater. Lett. 2023, 349, 134832. [Google Scholar] [CrossRef]
- Sun, L.; Wang, H.; Zhai, S.; Sun, J.; Fang, X.; Yang, H.; Zhai, D.; Liu, C.; Deng, W.-Q.; Wu, H. Dual-conductive metal-organic framework@MXene heterogeneity stabilizes lithium-ion storage. J. Energy Chem. 2023, 76, 368. [Google Scholar] [CrossRef]
- Zhang, R.; Xue, Z.; Qin, J.; Sawangphruk, M.; Zhang, X.; Liu, R. NiCo-LDH/Ti3C2 MXene hybrid materials for lithium ion battery with high-rate capability and long cycle life. J. Energy Chem. 2020, 50, 143. [Google Scholar] [CrossRef]
- Ramezanitaghartapeh, M.; Achazi, A.J.; Soltani, A.; Miró, P.; Mahon, P.J.; Hollenkamp, A.F.; Musameh, M. Sustainable cyanide-C60 fullerene cathode to suppress the lithium polysulfides in a lithium-sulfur battery. Sustain. Mater. Technol. 2022, 32, e00403. [Google Scholar] [CrossRef]
- Ramezanitaghartapeh, M.; Hollenkamp, A.F.; Musameh, M.; Mahon, P.J. High capacity polycarbazole-sulfur cathode for use in lithium-sulfur batteries. Electrochim. Acta 2021, 391, 138898. [Google Scholar] [CrossRef]
- Lup, A.N.K.; Soni, V.; Keenan, B.; Son, J.; Taghartapeh, M.R.; Morato, M.M.; Poya, Y.; Montañés, R.M. Sustainable energy technologies for the Global South: Challenges and solutions toward achieving SDG 7. Environ. Sci. Adv. 2023, 2, 570. [Google Scholar] [CrossRef]
- Ramezanitaghartapeh, M.; Musameh, M.; Hollenkamp, A.F.; Mahon, P.J. Conjugated Microporous Polycarbazole-Sulfur Cathode Used in a Lithium-Sulfur Battery. J. Electrochem. Soc. 2021, 168, 110542. [Google Scholar] [CrossRef]
- Hafner, J. Ab-initio simulations of materials using VASP: Density-functional theory and beyond. J. Comput. Chem. 2008, 29, 2044. [Google Scholar] [CrossRef] [PubMed]
- Perdew, J.P.; Burke, K.; Ernzerhof, M. Generalized Gradient Approximation Made Simple. Phys. Rev. Lett. 1996, 77, 3865. [Google Scholar] [CrossRef] [PubMed]
- Blöchl, P.E. Projector augmented-wave method. Phys. Rev. B 1994, 50, 17953. [Google Scholar] [CrossRef] [PubMed]
- Grimme, S.; Antony, J.; Ehrlich, S.; Krieg, H. A consistent and accurate ab initio parametrization of density functional dispersion correction (DFT-D) for the 94 elements H-Pu. J. Chem. Phys. 2010, 132, 154104. [Google Scholar] [CrossRef]
- Henkelman, G.; Uberuaga, B.P.; Jónsson, H. A climbing image nudged elastic band method for finding saddle points and minimum energy paths. J. Chem. Phys. 2000, 113, 9901. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, Y.; Song, Y.; Lu, Q.; Zhang, L.; Du, L.; Yu, S.; Zhang, Y. Covalent Bonding of MXene/COF Heterojunction for Ultralong Cycling Li-Ion Battery Electrodes. Molecules 2024, 29, 2899. https://doi.org/10.3390/molecules29122899
Liu Y, Song Y, Lu Q, Zhang L, Du L, Yu S, Zhang Y. Covalent Bonding of MXene/COF Heterojunction for Ultralong Cycling Li-Ion Battery Electrodes. Molecules. 2024; 29(12):2899. https://doi.org/10.3390/molecules29122899
Chicago/Turabian StyleLiu, Yongbiao, Yang Song, Quanbing Lu, Linsen Zhang, Lulu Du, Shiying Yu, and Yongshang Zhang. 2024. "Covalent Bonding of MXene/COF Heterojunction for Ultralong Cycling Li-Ion Battery Electrodes" Molecules 29, no. 12: 2899. https://doi.org/10.3390/molecules29122899
APA StyleLiu, Y., Song, Y., Lu, Q., Zhang, L., Du, L., Yu, S., & Zhang, Y. (2024). Covalent Bonding of MXene/COF Heterojunction for Ultralong Cycling Li-Ion Battery Electrodes. Molecules, 29(12), 2899. https://doi.org/10.3390/molecules29122899





