Abstract
Bletilla striata is the dried tuber of B. striata (Thund.) Reichb.f., which has antibacterial, anti-inflammatory, anti-tumor, antioxidant and wound healing effects. Traditionally, it has been used for hemostasis therapy, as well as to treat sores, swelling and chapped skin. In this study, we used the ultraviolet (UV) absorbance rate of B. striata extracts as the index, and the extraction was varied with respect to the solid–liquid ratio, ethanol concentration, ultrasonic time and temperature in order to optimize the extraction process for its sunscreen components. The main compounds in the sunscreen ingredients of Baiji (B. striata) were analyzed using ultra-high-performance liquid chromatography combined with quadrupole time-of-flight tandem mass spectrometry. The sunscreen properties were subsequently evaluated in vitro using the 3M tape method. The results show that the optimal extraction conditions for the sunscreen components of B. striata were a solid–liquid ratio of 1:40 (g/mL), an ethanol concentration of 50%, an ultrasonic time of 50 min and a temperature of 60 °C. A power of 100 W and an ultrasonic frequency of 40 Hz were used throughout the experiments. Under these optimized conditions, the UV absorption rate of the isolated sunscreen components in the UVB region reached 84.38%, and the RSD was 0.11%. Eighteen compounds were identified, including eleven 2-isobutyl malic acid glucose oxybenzyl esters, four phenanthrenes, two bibenzyl and one α-isobutylmalic acid. An evaluation of the sunscreen properties showed that the average UVB absorption values for the sunscreen samples from different batches of B. striata ranged from 0.727 to 1.201. The sunscreen ingredients of the extracts from B. striata had a good UV absorption capacity in the UVB area, and they were effective in their sunscreen effects under medium-intensity sunlight. Therefore, this study will be an experimental reference for the extraction of sunscreen ingredients from the B. striata plant, and it provides evidence for the future development of B. striata as a candidate cosmetic raw material with UVB protection properties.
1. Introduction
Studies suggest that excessive ultraviolet (UV) radiation can cause skin redness, tanning, sunburn and even the risk of skin cancer [1,2]. Sunscreen preparations can effectively prevent overexposure to UV radiation and significantly reduce the skin damage caused [3,4]. However, the properties of the sunscreens currently in use have certain shortcomings, including poor skin sensation with some their physical effects, as well as irritation and allergic reactions caused by the chemical components in the skin [5]. Compared with some of these physical and chemical sunscreen methods, natural plant sunscreen constituents have the advantages of high safety, and they can be more comfortable when applied to the skin. Therefore, in recent years, some substances for performing this task have been obtained from Chinese herbal medicines and have increased in popularity, and this is one of the major directions of research and development for sunscreen skincare products [6].
Bletilla striata (Thund., named ‘‘Bai Ji” in Chinese) is the dried tuber of B. striata (Thund.) Reichb.f., which is a relatively rare traditional Chinese medicine (TCM), with a history of over 1500 years [7]. The B. striata plant is mainly distributed in the humid river valleys and coastlands in the most southeastern three provinces of China. Outside China, its global distribution is limited, only growing in the Korean Peninsula, Japan and Myanmar [8]. Traditionally, it has been used for hemostasis therapy, as well as to treat sores, swelling and chapped skin [9]. Additionally, in ancient times, the dry tubers of B. striata were ground into a powder and then applied to the skin in order to achieve beautifying and skincare effects. Nowadays, some regions of China still retain this ancient method of skincare [10]. The main chemical components of B. striata consist of a variety of chemical substances, such as polysaccharides, benzenes, dihydrophenanthrolines and phenanthrenes, flavonoids, diphenylenes and diphenylene ethers and quinones [8,11]. Therefore, B. striata has a wide range of bio-pharmacological activities, such as bacteriostasis, anti-inflammatory [12], hemostasis [13], anti-tumor [14], cell-growth-promoting and wound healing properties [11,15]. Additionally, it has been shown to have a significant antioxidant effect in vitro [8,16,17]. Most of the chemical structures of B. striata compounds contain benzene ring skeletons and even multiple phenolic hydroxyl groups. It has been reported that polyphenols have good free radical scavenging and antioxidant properties [18,19], and these are capable of absorbing UV light and inhibiting the skin aging caused by this type of radiation [20]. As a TCM used for whitening the skin [21], it was speculated that B. striata contained components that had sunscreen properties. At present, the extraction methods for the total polyphenols in B. striata include the traditional heated reflux [22] and flash extraction methods [23], as well as the high-shear mixing emulsification technique [24]. These conventional methods have been in use for many decades, but they have major limitations. The flash extraction method and the high-shear mixing emulsification technique require specialized instrumentation which are not common in laboratories. Therefore, a practical and efficient extraction method is crucial for its wider use. In this context, the application of an ultrasound technique to the extraction of bioactive components is considered as a promising alternative, and it is emerging as a green technology due to the lower costs during operation. It has the advantages of shorter operation times and lower energy consumption when compared to conventional methods [25].
However, at present, there are few studies on the optimization process for the ultrasound-assisted extraction of polyphenols from B. striata. There are many studies on the antioxidant activity of polyphenols from B. striata but only a few reports on the sunscreen properties of extracts from B. striata. In order to explore the sunscreen components, the extraction process and the sunscreen effects of B. striata, we used an ultrasound-assisted method to extract its sunscreen compounds. The UV absorption rate was used as an index to optimize the conditions of the extraction process for the sunscreen ingredients obtained from this plant. Four parameters, including the solid–liquid ratio, ethanol concentration, ultrasonic time and temperature, were varied. The main compounds in the sunscreen ingredients of Baiji (B. striata) extracted under the optimal processing conditions were then identified using ultra-high-performance liquid chromatography combined with quadrupole time-of-flight tandem mass spectrometry (UPLC-Q-TOF-MS). The 3M tape method was subsequently used to preliminarily evaluate the sunscreen effects of the ingredients obtained with a view to providing a reference for the extraction procedure. This will aid in the future development and utilization of B. striata extracts as a functional ingredient in the skincare industry.
2. Results and Discussion
2.1. Screening of Sunscreen Components Extraction Conditions
2.1.1. The Effect of Ultrasonic Time on the Extraction Effect of Sunscreen Ingredients from B. striata
As shown in Figure 1, it can be seen that within 20~50 min, the UVB absorption rate showed a trend of first increasing and then decreasing with the extension of the extraction time, and the extraction rate was the highest at 50 min, which is statistically significant when compared to the average value. This may be due to the prolongation of the time in the early stage and the continuous penetration of the extraction solution, resulting in an increase in the extraction rate. However, as the extraction time increased, the oxidation of polyphenols in the extraction solution accelerated, resulting in decreased yields [23]. Therefore, the optimal range for the ultrasonic extraction time was determined to be 40~60 min.
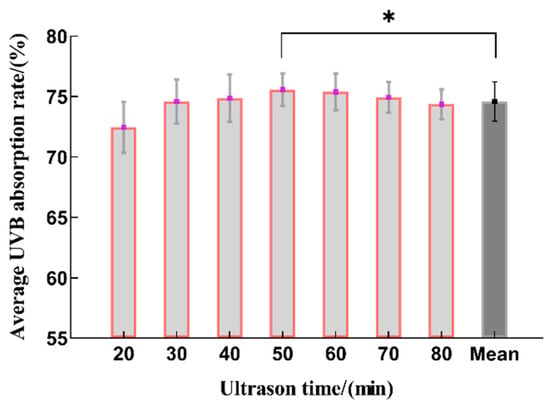
Figure 1.
The effect on the sunscreen activity for different time periods used for extraction (n = 3, * represents p < 0.05).
2.1.2. The Influence of Ethanol Concentration on the Extraction Effect of Sunscreen Ingredients from B. striata
The UVB absorption rate showed a trend of first increasing and then decreasing as the ethanol concentration increased, with the highest extraction rate seen at 60% ethanol, which is statistically significant when compared to the average value (Figure 2). It is possible that as the ethanol volume fraction increased, the dissolution of some alcohol-soluble impurities and highly lipophilic components increased. These components affected the dissolution of polyphenolic compounds from the raw material particles, resulting in a decrease in the total phenol yield of the extract [23]. Therefore, the optimal range for the ethanol concentration was determined to be 50~70% ethanol.
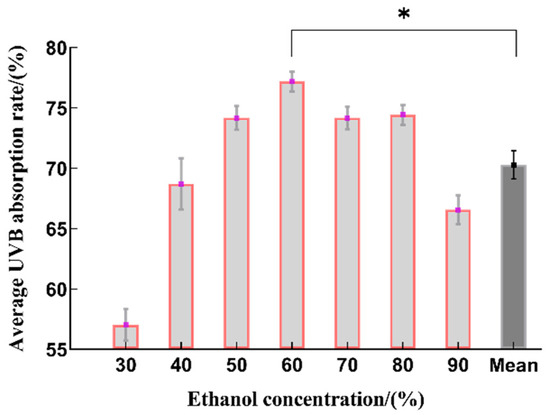
Figure 2.
The effect on the sunscreen activity obtained by using different concentrations of ethanol for extraction (n = 3, * represents p < 0.05).
2.1.3. The Effect of the Solid–Liquid Ratio on the Extraction of Sunscreen Ingredients from B. striata
With respect to solid–liquid ratios from 1:10 to 1:60 (g/mL), the extraction rate of the sunscreen components increased with an increase in the matter solid–liquid ratio, and this reached its maximum at 1:40 (g/mL), which is statistically significant when compared to the average value (Figure 3). However, as the dose of the solvent increased, there was a relatively small decrease in the UVB absorption rate. This is probably because a greater percentage of the sunscreen components was released as the solvents increased when the solid–liquid ratio was less than 1:40 (g/mL). However, excessive use of solvents was not conducive to the extraction of the sunscreen ingredients because the solute became saturated in the solvent, which adversely affected the mass transfer efficiency [26]. Therefore, the optimal range for the solid–liquid ratio was determined to be 1:30~1:50 (g/mL).
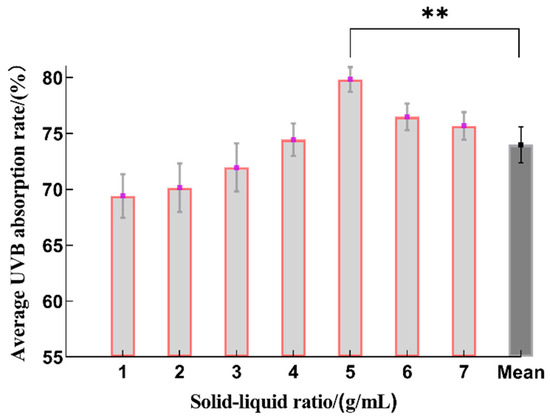
Figure 3.
The effect on the sunscreen activity of different solid–liquid ratios during the extraction process. Ratios of solid–liquid of 1:10, 1:15, 1:20, 1:30, 1:40, 1:50 and 1:60 (g/mL) correspond to 1, 2, 3, 4, 5, 6 and 7 along the x-axis (n = 3, ** represents p < 0.01).
2.1.4. The Effect of Ultrasonic Temperature on the Extraction of Sunscreen Ingredients from B. striata
From 20 to 50 °C, the UVB absorption rate increased as the ultrasonic temperature rose (Figure 4). A plausible reason is that an increase in temperature can improve the mass transfer efficiency and facilitate the extraction of sunscreen substances [8]. The sunscreen substances had a maximum extraction rate at 50 °C, which is statistically significant when compared to the average value. At higher temperatures, the UVB absorption rate fell as the ultrasonic temperature rose. This may have been due to the degradation of the sunscreen components at the higher temperatures, thereby reducing the extraction yield. Therefore, the optimal range for the extraction temperature was determined to be 40~60 °C.
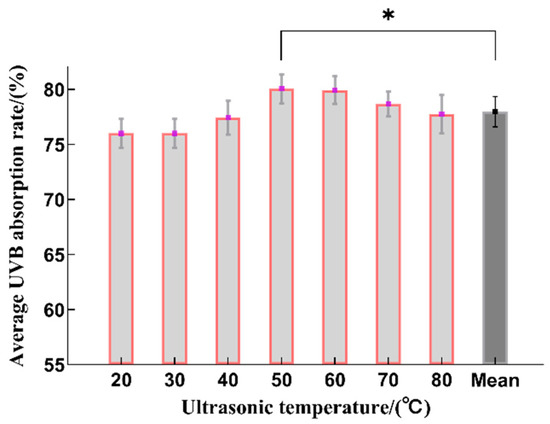
Figure 4.
The effect on the sunscreen activity after using different ultrasonic temperatures during the extraction process (n = 3, * represents p < 0.05).
2.2. Statistical Analysis of the Orthogonal Test
According to the results of the single-parameter tests, the UV absorption of B. striata extracts in the UVA region was found to be relatively weak, with an average absorption rate of less than 30%. However, the UV absorption in the UVB region was relatively strong, with an average absorption rate of more than 55%. On the basis of the single-factor tests, the UVB absorption rate was used as the response value, with the solid–liquid ratio (A), the ultrasonic temperature (B), the ethanol concentration (C) and the ultrasonic time (D) as the self-variables. The above factors and levels were then transferred into an L9 (34) orthogonal table (Table 1). The last column in the table refers to the UVB absorption rate, which is the experimental detection index. Each row in the table represents a set of parameter combination experiments, and all four columns are balanced, orthogonal and independent. A total of nine groups were used to conduct the analysis with the different combinations of processing parameters.

Table 1.
An L9 (34) table of the sunscreen ingredient extraction parameters.
Range analysis can be used to analyze orthogonal experimental data, and it can demonstrate the advantages of factors, as well as the advantages and disadvantages of factors at specific levels. From the range analysis shown in Table 2, it can be seen that the R values (factor range value) obtained reflect a comparison of the results of the four parameters used for the extraction of sunscreen products from B. striata. The largest value is RC, followed by RB and then RA, and RD is the smallest. This indicates that the factors which affected the extraction of the sunscreen ingredients most, in order, were ethanol concentration, which had the greatest impact, followed by ultrasonic temperature, then the solid–liquid ratio and finally ultrasonic time had the least impact. When specifically combined with the optimal levels for each factor, it can be seen that the second level of factor 1 was the solid–liquid ratio, which was optimal at 1:40 (g/mL), and the third level of factor 2 was the ultrasonic temperature, which was optimal at 60 °C. In addition, the first level, which was the ethanol concentration of factor 3 (50%), and the second level of factor 4, which was the ultrasound time (50 min), were optimal. In summary, the optimal factor was found to be ethanol concentration. The optimal combination is A2B3C1D2. That is, a ratio of solid–liquid of 1:40 (g/mL), an ultrasonic temperature of 60 °C, an ethanol concentration of 50% and an ultrasonic time of 50 min.

Table 2.
The results obtained for the range analysis of orthogonal experiments.
2.3. Verification Test
Three parallel verification tests were conducted based on the obtained optimized extraction process conditions. In Table 3, using a solid–liquid ratio of 1:40 (g/mL), an ultrasonic temperature of 60 °C, an ethanol concentration of 50% and an ultrasonic time of 50 minutes as the extraction conditions, the average UVB absorption rate of the extracted products from B. striata reached 84.38%. The average UVB absorption rate under these conditions was equivalent to the test results of No. 6 in Table 1, with an RSD of 0.11%. The results of this experimental group showed that the optimal extraction process conditions obtained through orthogonal experiments had the best effect.

Table 3.
The results of the verification tests (n = 3).
2.4. Analysis Using UPLC-Q-TOF-MS
Using the abovementioned optimal extraction process conditions, the sunscreen substances in B. striata were extracted, and the compounds in the extracts were analyzed and identified using UPLC-Q-TOF-MS. Figure 5 represents a total ion chromatogram in the negative state obtained using optimized chromatographic and mass spectrometric conditions. Eighteen compounds were identified through analysis, and these included eleven 2-isobutyl malic acid glucose oxybenzyl esters, four phenanthrenes, two bibenzyl and one α-isobutylmalic acid (Table 4). The structures of these compounds contain benzene ring skeletons, and some also had phenolic hydroxyl groups. These groups give the compounds the ability to absorb UV radiation [27]. Therefore, analysis of the chemical components of the extracts showed that the extracts from B. striata had the effect of absorbing UV rays, and polyphenols are the components responsible for the sun-protective effects.
2.5. Evaluation of Sunscreen Effects in the UVB Region
In Table 5, the average absorbance values, Ai, of sunscreen Samples 1 and 2 in the UVB region as measured using the 3M tape method were 0.727 and 1.201, respectively. According to the sunscreen effect evaluation criteria shown in Table 6, if the absorbance was in the range of 0.5~1.0, this had a minimal UV protection effect, and the sample could be used in winter sunlight and summer morning and evening sunlight, as well as on cloudy days. If the absorbance was within the range of 1.1~1.5, this meant the sample had a medium UV protection effect, and it could be used in medium-intensity sunlight. It can be seen from the experimental results that the tested sunscreen samples had some sunscreening capacity. Therefore, Sample 1 could be used for basic sun protection in winter sun and on cloudy days, and Sample 2 could be used to protect against moderate sunlight.
The evaluation techniques to test the effectiveness of sunscreen products are divided into in vivo and in vitro methods. The in vivo method is based on the degree of the response of the human body to the production of erythema and pigmentation in the skin as caused by UV stimulation. Test results from the human body method are highly reliable, but there are also some obvious shortcomings. For example, testers need professional training, the test costs are high and large doses of UV rays can cause damage to the skin of the testers. Therefore, during the process of scientific research and product development, it is not practical to directly use in vivo methods to evaluate the sunscreen effects of samples. With the in vitro method, quartz sheets are often used to mimic biological tissues such as the cuticle and epidermis of the human body, UV-protective products are applied to these and the absorbance (transmittance) of the samples are measured using a UV spectrophotometer. The sun protection properties of a product can be evaluated based on the absorbance values obtained. Therefore, as in this study, it is more practical to use in vitro methods to evaluate the sun protection efficacy of UV-protective products during research and development [26].
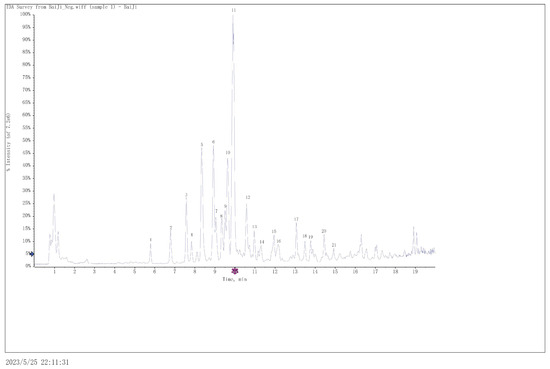


Figure 5.
The total ion chromatogram obtained in the negative mode.

Table 4.
The individual components isolated from the ethanol extract samples of Bletilla striata after analysis by UPLC-Q-TOF-MS.
Table 4.
The individual components isolated from the ethanol extract samples of Bletilla striata after analysis by UPLC-Q-TOF-MS.
| No. | tR (min) | Extracting ions | Calculated (m/z) | Observed (m/z) | Error (ppm) | Area (%) | Formula | Main Secondary Fragment Ions | Identity | Category | References |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 5.787 | [M − H]− | 351.1297 | 351.1308 | 3.2 | 0.792 | C14H24O10 | 171.0666, 127.0771 | Dactylorhin C | 2-isobutyl malic acid glucose oxybenzyl esters | [28,29] |
| 2 | 6.788 | [M − H]− | 189.0768 | 189.0777 | 4.5 | 1.763 | C8H14O5 | 171.0648, 129.0556 | α-isobutylmalic acid | α-isobutylmalic acid | [28] |
| 3 | 7.57 | [M − H]− | 619.2244 | 619.2259 | 2.5 | 3.261 | C27H40O16 | 439.1605, 171.0661, 153.0554 | Dactylorhin E | 2-isobutyl malic acid glucose oxybenzyl esters | [28,29] |
| 4 | 7.833 | [M − H]− | 593.1876 | 593.1881 | 0.9 | 1.039 | C28H34O14 | 431.1346, 325.0930, 269.0802, 163.0399 | 2,4-dimethoxyphenanthrene-3,7-O-β-D-diglucopyranoside | Phenanthrenes | [28] |
| 5 | 8.345 | [M − H]− | 887.319 | 887.3216 | 2.9 | 7.538 | C40H56O22 | 619.2248, 439.1607, 179.0564, 171.0658, 153.0555 | Dactylorhin A | 2-isobutyl malic acid glucose oxybenzyl esters | [28,29] |
| 6 | 8.925 | [M − H]− | 457.1715 | 457.1721 | 1.2 | 6.611 | C21H30O11 | 171.0660, 161.0450, 153.0555, 129.0562, 127.0768, 123.0456 | Gymnoside II | 2-isobutyl malic acid glucose oxybenzyl esters | [29,30] |
| 7 | 9.044 | [M − H]− | 723.5053 | 723.5049 | −0.5 | 2.332 | C41H72O10 | 661.2368, 481.1685, 439.1621, 153.0562 | Unknown | ||
| 8 | 9.341 | [M − H]− | 661.2349 | 661.2369 | 3 | 2.593 | C29H42O17 | 439.1610, 171.0663, 153.0557 | Isomers | 2-isobutyl malic acid glucose oxybenzyl esters | [30] |
| 9 | 9.51 | [M − H]− | 853.5776 | 853.579 | 1.6 | 2.51 | C58H78O5 | 661.2358, 481.1727, 439.1611, 171.0667, 153.0553 | Unknown | ||
| 10 | 9.637 | [M − H]− | 929.3296 | 929.3323 | 2.9 | 6.621 | C42H58O23 | 661.2353, 439.1610, 221.0662, 171.0666, 161.0443, 153.0555 | Gymnoside Ⅲ | 2-isobutyl malic acid glucose oxybenzyl esters | [28,29] |
| 11 | 9.908 | [M − H]− | 725.2662 | 725.2686 | 3.3 | 19.932 | C34H46O17 | 457.1726, 285.0984, 171.0662, 153.0555, 129.0561, 127.0769 | Militarine | 2-isobutyl malic acid glucose oxybenzyl esters | [28] |
| 12 | 10.574 | [M − H]− | 971.3402 | 971.3432 | 3.1 | 3.396 | C44H60O24 | 703.2466, 481.1735, 439.1619, 153.0554 | Gymnoside VIII | 2-isobutyl malic acid glucose oxybenzyl esters | [28,29,30] |
| 13 | 10.963 | [M − H]− | 971.3402 | 971.3429 | 2.8 | 1.369 | C44H60O24 | 749.2674, 703.2490, 661.2341, 481.1725, 439.1619, 153.0550 | Habenarioside | 2-isobutyl malic acid glucose oxybenzyl esters | [28,29] |
| 14 | 11.287 | [M − H]− | 753.2611 | 753.2624 | 1.7 | 1.007 | C35H46O18 | 439.1585, 171.0661, 153.0537 | Unknown | ||
| 15 | 11.938 | [M − H]− | 241.087 | 241.087 | 0.8 | 2.088 | C15H14O3 | 226.0640, 225.0566, 198.0686, 197.0600, 181.0678 | Coelonin | Phenanthrenes | [31] |
| 16 | 12.148 | [M − H]− | 1017.3609 | 1017.3621 | 1.9 | 1.67 | C49H62O23 | 897.3407, 767.2793, 499.1825, 457.1680, 439.1532, 285.0985, 153.0559 | Gymnoside IV | 2-isobutyl malic acid glucose oxybenzyl esters | [28,29] |
| 17 | 13.064 | [M − H]− | 1059.3715 | 1059.3738 | 2.2 | 2.24 | C51H64O24 | 837.3036, 791.2795, 439.1623, 569.2024, 661.2389, 153.0557 | Gymnoside X | 2-isobutyl malic acid glucose oxybenzyl esters | [28,29] |
| 18 | 13.49 | [M − H]− | 243.1027 | 243.1034 | 3.8 | 1.017 | C15H16O3 | 243.1044, 227.0722, 183.0819, 136.0539, 106.0424 | Batatasin Ⅲ | Bibenzyl | [31] |
| 19 | 13.775 | [M − H]− | 347.1289 | 347.1296 | 2.1 | 0.912 | C22H20O4 | 332.1050, 331.0973, 304.1091, 239.0707, 238.0632, 237.0562, 225.0550 | 1-(p-hydroxybenzyl)-4-methoxy-9,10-dihydroph -enanthrene-2,7-diol. | Phenanthrenes | [32] |
| 20 | 14.446 | [M − H]− | 481.1657 | 481.1660 | 0.7 | 1.755 | C30H26O6 | 466.1434, 465.1347, 451.1219, 435.1243, 225.0568 | Blestrianol A or Gymconopin C or Blesriarene A | Phenanthrenes | [32] |
| 21 | 14.921 | [M − H]− | 455.1816 | 455.1866 | 0.4 | 0.601 | C29H28O5 | 361.1440, 346.1183, 331.0959, 255.1010 | 3,3’-dihydroxy-2’,6’-bis(p-hydroxybenzyl)-5-methoxybibenzyl | Bibenzyl | [33] |
There are certain limitations to this study. Firstly, the extract from B. strata is a dark and unattractive product due to the presence of pigment. For its use as a cosmetic raw material, the pigment must be removed, which will improve its appearance and presence. Therefore, decolorization treatments will need to be considered in the future. Secondly, the extract of B. strata has to be evaluated for its toxicity in order to be used on human skin. Therefore, the extract requires local toxicological safety evaluation, such as multiple skin irritation, acute eye irritation, skin allergy and further tests [34]. Thirdly, as of yet, there is a lack of availability of pure compounds for use as internal standards, and this will have to be remedied in future experiments. In future studies, these limitations must be addressed prior to its use as a product in the cosmetic industry.



Table 5.
The UVB absorption values obtained for the sunscreen components of different fractions.
Table 5.
The UVB absorption values obtained for the sunscreen components of different fractions.
| No. | Wavelength (nm) | |||||
|---|---|---|---|---|---|---|
| A280 | A290 | A300 | A310 | A320 | Ai | |
| Control | 0.537 | 0.570 | 0.468 | 0.336 | 0.203 | 0.409 |
| 1 | 0.948 | 0.873 | 0.790 | 0.622 | 0.403 | 0.727 |
| 2 | 1.569 | 1.350 | 1.221 | 1.054 | 0.811 | 1.201 |

Table 6.
Evaluation of the sunscreen effects of B. striata.
Table 6.
Evaluation of the sunscreen effects of B. striata.
| Absorbance (A) | Sunscreen Effect | Conditions of Use |
|---|---|---|
| 0.5~1.0 | Minimum UV protection effect | Winter sunshine, summer morning and evening sunshine and overcast days |
| 1.1~1.5 | Moderate UV protection | Moderate sunlight |
| 1.6~2.0 | High-efficiency protection against ultraviolet radiation | Outdoor work, strong sunlight in summer |
| 2.1 | Fully protected against UV radiation | Outdoor work, strong sunlight in summer |
3. Materials and Methods
3.1. Materials and Instruments
3.1.1. Materials and Chemical Regents
B. striata (batch number: 20181101) was purchased from Guangdong Huiqun Herbal Medicine Co., Ltd. (Shantou, China). HPLC-grade acetonitrile and formic acid were from Merck (Merck Group, Darmstadt, Germany). Stearic acid, octadecyl alcohol, Tween 80, Span 60, liquid paraffin, vitamin E, carbomer, EDTA disodium, polydimethylsiloxane and glycerin were from Shanghai Yuanye Biotechnology Co., Ltd. (Shanghai, China). Sodium sorbate was from Henan Heisenlin Biotechnology Co., Ltd. (shangqiu, China), and medical tape was purchased from 3M Company (St. Paul, MN, USA). All the other reagents used were analytical-grade.
3.1.2. Instruments
The UPLC-Q-TOF-MS analyses were performed on an AB Sciex Triple 6600+ mass spectrometer (AB Sciex Pte. Ltd., Atlanta, GA, USA). A WJX-A250 high-speed multi-function crusher (Shanghai Yuanwo Industry and Trade Co., Ltd., Shanghai, China) and a KQ-500DE CNC ultrasonic cleaner (Kunshan Ultrasonic Instrument Co., Ltd., Kunshan, China) were used throughout this study. In addition, a UV-mini 1240 UV spectrophotometer (Shimadzu Corporation, Kyoto, Japan), A TD5A-WS medical centrifuge (Xiangyi Centrifuge Instrument Co., Ltd., Changsha, China) and an XS205DU One Hundred Thousand Electronic Balance (Mettler Toledo Co., Ltd., Zurich, Switzerland) were also used.
3.2. Methods
3.2.1. Extraction of the Sunscreen Ingredients from B. striata
The herbs of B. striata were first crushed in a grinder and sieved through a 100-mesh sieve. Then, 1.0 g of B. striata powder was placed in a 100 mL triangular flask with a stopper and subjected to ultrasonic extraction at 100 W and at an ultrasonic frequency of 40 Hz. Different ultrasonic times, ethanol concentrations, solid–liquid ratios and ultrasonic temperatures were used. The extracted products were kept at room temperature, centrifuged and filtered, the filtrates were transferred into a 100 mL volumetric flask and water was added to obtain a standardized volume.
3.2.2. The Determination of the Absorbance UV
UV can generally be divided into three wave bands, namely UVA (320~400 nm), UVB (280~320 nm) and UVC (200~280 nm) in the long-, medium- and short-wave zones, respectively [35]. The transmission of UVC in the short-wave zone can only reach the cuticle, and most of it is blocked by ozone before reaching the ground. It is generally believed that UVC in the short-wave zone will not cause damage to the skin [36].
A total of 25.0 mL of the test solution obtained in Section 3.2.1 was transferred into a 50 mL volumetric flask, and this was used as the blank control to measure the UV absorbance values of the extracts of B. striata in the UVB and UVA regions, respectively. This was then used to evaluate the UV absorption (sun protection) capacity of the extracts. The values obtained were converted into the UV absorbance values. These were used to calculate the UV absorbance according to the formula [37]:
UV absorbance/% = 100% − average transmittance T
3.2.3. Single-Factor Tests on the Extraction Process of Sun Protection Ingredients from B. striata
Briefly, 7 × 1.0 g of powder of B. striata was weighed, and the ultrasonic temperature used was 50 °C, the ratio of material to liquid was 1:20 (g/mL) and the ethanol concentration was 50%. These were extracted by ultrasonication for 20, 30, 40, 50, 60, 70 and 80 minutes, respectively. The extracts were brought to room temperature and filtered, and the filtrates were made up to 100 mL in volumetric flasks. Similar extractions were carried out to determine the concentration of ethanol by using 30, 40, 50, 60, 70, 80 and 90%, respectively. The solid–liquid ratio used in the extraction process was determined using ratios of 1:10, 1:15, 1:20, 1:30, 1:40, 1:50 and 1:60 (g/mL), and for the ultrasonic temperature determination, we used 20, 30, 40, 50, 60, 70 and 80 °C, respectively.
3.2.4. The Orthogonal Optimization Test
An orthogonal test can be used analyze experimental results using fewer experimental groups to obtain better process parameters, and this method was adapted for use to determine the effects of various extraction parameters on the extraction of B. striata sunscreen components. Therefore, based on the results of the single-factor experiments, an orthogonal experiment was designed to optimize the extraction process. When designing the orthogonal test, the UVB absorption rate was taken as the evaluation index, and the factors for the four parameters of the solid–liquid ratio (A), ultrasonic temperature (B), ethanol concentration (C) and ultrasonic time (D) were taken as the independent variables. A 4-factor, 3-level L9 (34) standard orthogonal table was selected to optimize the conditions for the extraction process of the sunscreen ingredients from B. striata in the UVB region. The individual test factors and levels used in this study are given in Table 7.

Table 7.
The factors and levels of extraction parameters used for the orthogonal tests.
3.2.5. UPLC-Q-TOF-MS Analysis
Sample Preparation for UPLC-Q-TOF-MS
The ethanol-extracted samples obtained under the optimized processing conditions from B. striata were filtered using 0.22 μm filter membranes.
UPLC-Q-TOF-MS Analysis Conditions
The chromatographic separation was performed using a Luna Omega Polar C18 column with 3 μm, 2.1 mm × 100 mm. The composition of the two mobile phases was 0.1% (v/v) formic acid in water (A) and acetonitrile (B): 0~6 min, 3%~20% B; 6~13 min, 20~45% B; 13~16 min, 45~75% B; 16~18 min, 75~95% B; 18~20 min, 95% B. The separations were performed with a constant flow rate of 300 μL/min. The column temperatures were set to 45 °C. Then, 5 μL of the samples were injected in full loop injection mode. We then performed electrospray MS detection in the negative detection mode with the ion source voltage set to −4.5 kV. Nitrogen was used as the sheath gas. The mass spectrum scanning range was set to 100~1500 Da. An 80 V declustering potential, −40 V collision energy and 10 V collision energy spread were used in the mass detection.
3.2.6. Evaluation of the Sunscreen Effects of the Extracts from B. striata in the UVB Region
Preparation formulae for the sunscreen samples: Component A (glycerin 3.5% and Tween 80 0.72%, residual purified water); component B (stearic acid 3%, octadecyl alcohol 1%, liquid paraffin 11%, Span 60 5%, polydimethylsiloxane 2%, vitamin E 0.6% and sodium sorbate 0.3%); component C (carbomer 0.35%, EDTA disodium 0.05% and the appropriate amount of purified water); component D (triethanolamine 0.7%); Component E (B. striata extract 5~10%, appropriate amount of essence).
Preparation process: The fully swollen component C consisting of the water-soluble polymers at room temperature was heated to 75 °C and mixed. Components A and B were dissolved uniformly at 75~80 °C, and components B and C were slowly added to A while stirring them at 80 °C for about 15 minutes to obtain a uniform mixture. This was cooled to 50 °C, components D and E were added and the mixture was stirred. The temperature was reduced to 45 °C, and this yielded the sunscreen products. A control group sample (without B. striata extract), sunscreen Sample 1 (with 5% B. striata extract) and sunscreen Sample 2 (with 10% B. striata extract) were also prepared using the process described above.
The control and sunscreen Samples 1 and 2 prepared as described above were evaluated for their sunscreen effects using the national standard QB/T 2410-1998 [26]. This employed the 3M tape method, which is based on the principle that the UV-shielding agent and the UV absorbance in sunscreen cosmetics can block and absorb the UV rays from the sun. The samples were directly and evenly smeared onto the cuticle of the skin and other biological materials (such as a quartz pool and medical breathable tapes). This simulated the process of a person applying the cosmetic products onto the surface of their skin. A total of 8 mg of the products was pasted evenly onto a 1 × 4 cm piece of 3M medical tape on the transparent side of the quartz absorption cell and left for 30 min. A UV spectrophotometer was then used to measure the UV absorbance A value of the sample in the UVB region (280~320 nm), and the results of the sunscreen effect evaluation are shown in Table 6.
3.2.7. Statistical Analysis
Statistical analysis was performed by using the SPSSAU website (https://spssau.com/index.html, accessed on 19 May 2024). The t test was used for the single-factor analysis, and the range analysis method was used to analyze the orthogonal experiment results. Statistically significant differences were labeled as * and ** for p < 0.05 and <0.01, respectively. GraphPad 8.0.2 was used to depict the graphs. PeakView 2.0 was used to load the results of the published compounds into the Analyst@TF 1.6 Software (PeakView 2.0) function block for analysis.
4. Conclusions
In this study, the UV absorption rate of the extracted chemical components of the orchid plant, B. striata, was determined. The orthogonal method was used to optimize the extraction process. The optimal extraction process was found to be when the ratio of solid-liquid was 1:40 (g/mL), the ultrasonic temperature was 60 °C, the ethanol concentration was 50% and the ultrasonic time was 50 min. Under these conditions, the extracts obtained from B. striata had a good absorption effect with respect to UVB, with an average UVB absorption rate of 84.38% and an RSD of 0.11%. This indicated that the process was stable and feasible. We subsequently used UPLC-Q-TOF-MS to identify eighteen compounds with sunscreen properties in ethanol extracts of B. striata, including eleven 2-isobutyl malic acid glucose oxybenzyl esters, four phenanthrenes, two bibenzyl and one α-isobutylmalic acid. These compounds are the basis for its sunscreen properties. The 3M tape method showed that the average Ai values of the sunscreen samples containing high and low concentrations of B. striata extracts in the UVB region were 1.201 and 0.727, respectively. This verified that the extracts from B. striata have a good UV absorption ability in the UVB region and they can achieve a sun protection effect in moderate sunlight. Therefore, the B. striata plant can be used as one of the candidates for cosmetic sunscreen purposes, and this study provides an experimental basis for the further development and utilization of B. striata in skincare products.
Author Contributions
Y.L. conceived and designed the main ideas of this work, and contributed to the initial draft. Z.T. and H.Z. were involved in the experiments performed. S.T. was responsible for drawing charts. S.R.S. revised and polished the manuscript. J.X. led the supervision and administration of the project, and he was responsible for reviewing and guiding the manuscript. All authors have read and agreed to the published version of the manuscript.
Funding
This project was funded by the NMPA Key Laboratory for Quality Monitoring and Evaluation of Traditional Chinese Medicine, Chinese Materia Medica (KFKT2022-6), Open Project of Guangxi Key Laboratory of Regenerative Medicine, Guangxi Medical University (GuiZaiZhongKai 202201) and the Collaborative Innovation Centre of Regenerative Medicine and Medical Bioresource Development and Application Co-constructed by the Province and Ministry (CICRMMBR-SR-2023001).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The authors declare that all relevant data supporting the results of this study are available within the article.
Acknowledgments
Thanks to Qingquan Huang from Guangxi Institute of Drug Control for providing the photos of B. striata in the graphic abstract.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Greinert, R.; de Vries, E.; Erdmann, F.; Espina, C.; Auvinen, A.; Kesminiene, A.; Schüz, J. European Code against Cancer 4th Edition: Ultraviolet radiation and cancer. Cancer Epidemiol. 2015, 39, S75–S83. [Google Scholar] [CrossRef] [PubMed]
- Romanhole, R.C.; Ataide, J.A.; Moriel, P.; Mazzola, P.G. Update on ultraviolet A and B radiation generated by the sun and artificial lamps and their effects on skin. Int. J. Cosmet. Sci. 2015, 37, 366–370. [Google Scholar] [CrossRef] [PubMed]
- Seité, S.; Colige, A.; Piquemal-Vivenot, P.; Montastier, C.; Fourtanier, A.; Lapière, C.; Nusgens, B. A full-UV spectrum absorbing daily use cream protects human skin against biological changes occurring in photoaging. Photodermatol. Photoimmunol. Photomed. 2002, 16, 147–155. [Google Scholar] [CrossRef] [PubMed]
- Popiół, J.; Gunia-Krzyżak, A.; Słoczyńska, K.; Koczurkiewicz-Adamczyk, P.; Piska, K.; Wójcik-Pszczoła, K.; Żelaszczyk, D.; Krupa, A.; Żmudzki, P.; Marona, H.; et al. The Involvement of Xanthone and (E)-Cinnamoyl Chromophores for the Design and Synthesis of Novel Sunscreening Agents. Int. J. Mol. Sci. 2020, 22, 34. [Google Scholar] [CrossRef] [PubMed]
- Saewan, N.; Jimtaisong, A. Natural products as photoprotection. J. Cosmet. Dermatol. 2015, 14, 47–63. [Google Scholar] [CrossRef] [PubMed]
- Nunes, A.R.; Vieira, Í.G.P.; Queiroz, D.B.; Leal, A.L.A.B.; Maia Morais, S.; Muniz, D.F.; Calixto-Junior, J.T.; Coutinho, H.D.M. Use of Flavonoids and Cinnamates, the Main Photoprotectors with Natural Origin. Adv. Pharmacol. Sci. 2018, 5341487. [Google Scholar] [CrossRef] [PubMed]
- Gou, K.; Li, Y.; Qu, Y.; Li, H.; Zeng, R. Advances and prospects of Bletilla striata polysaccharide as promising multifunctional biomedical materials. Mater. Des. 2022, 223, 111198. [Google Scholar] [CrossRef]
- Luo, L.; Fan, W.; Qin, J.; Guo, S.; Xiao, H.; Tang, Z. Study on Process Optimization and Antioxidant Activity of Polysaccharide from Bletilla striata Extracted via Deep Eutectic Solvents. Molecules 2023, 28, 5538. [Google Scholar] [CrossRef]
- Commission, N.P. Pharmacopoeia of the People′s Republic of China: Part One; China Medical Science Press: Beijing, China, 2020. [Google Scholar]
- Fan, Y.X.; Long, C.L. Traditional skin-care knowledge of Bletilla plants. Chin. Det. Cosmet. 2020, 43, 41–48. [Google Scholar] [CrossRef]
- He, X.; Wang, X.; Fang, J.; Zhao, Z.; Huang, L.; Guo, H.; Zheng, X. Bletilla striata: Medicinal uses, phytochemistry and pharmacological activities. J. Ethnopharmacol. 2017, 195, 20–38. [Google Scholar] [CrossRef]
- Jiang, F.; Li, M.; Wang, H.; Ding, B.; Zhang, C.; Ding, Z.; Yu, X.; Lv, G. Coelonin, an Anti-Inflammation Active Component of Bletilla striata and Its Potential Mechanism. Int. J. Mol. Sci. 2019, 20, 4422. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Qi, C.; Wang, H.; Xiao, X.; Zhuang, Y.; Gu, S.; Zhou, Y.; Wang, L.; Yang, H.; Xu, W. Biocompatible and degradable Bletilla striata polysaccharide hemostasis sponges constructed from natural medicinal herb Bletilla striata. Carbohydr. Polym. 2019, 226, 115304. [Google Scholar] [CrossRef] [PubMed]
- Zhao, L.; Sun, D.; Lu, H.; Han, B.; Zhang, G.; Guan, Q. In vitro characterization of pH-sensitive Bletilla Striata polysaccharide copolymer micelles and enhanced tumour suppression in vivo. J. Pharm. Pharmacol. 2018, 70, 797–807. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; He, Y.; Chen, Z.; Shi, J.; Qu, Y.; Zhang, J. Effect of Polysaccharides from Bletilla striata on the Healing of Dermal Wounds in Mice. Evid.-Based Complement. Altern. Med. 2019, 9212314. [Google Scholar] [CrossRef] [PubMed]
- Qu, Y.; Li, C.; Zhang, C.; Zeng, R.; Fu, C. Optimization of infrared-assisted extraction of Bletilla striata polysaccharides based on response surface methodology and their antioxidant activities. Carbohydr. Polym. 2016, 148, 345–353. [Google Scholar] [CrossRef] [PubMed]
- Lu, J.D.; Wang, Z.; Qin, L.H. Drying methods affect bioactive compound contents and antioxidant capacity of Bletilla striata (Thunb.) Reichb.f. flower. Ind. Crop. Prod. 2021, 164, 113388. [Google Scholar] [CrossRef]
- Zillich, O.V.; Schweiggert-Weisz, U.; Eisner, P.; Kerscher, M. Polyphenols as active ingredients for cosmetic products. Int. J. Cosmet. Sci. 2015, 37, 455–464. [Google Scholar] [CrossRef] [PubMed]
- Lv, Q.Z.; Long, J.T.; Gong, Z.F.; Nong, K.Y.; Liang, X.M.; Qin, T.; Huang, W.; Yang, L. Current State of Knowledge on the Antioxidant Effects and Mechanisms of Action of Polyphenolic Compounds. Nat. Prod. Commun. 2021, 16, 1934578X211027745. [Google Scholar] [CrossRef]
- Roh, E.; Kim, J.-E.; Kwon, J.Y.; Park, J.S.; Bode, A.M.; Dong, Z.; Lee, K.W. Molecular mechanisms of green tea polyphenols with protective effects against skin photoaging. Crit. Rev. Food Sci. Nutr. 2015, 57, 1631–1637. [Google Scholar] [CrossRef]
- Jiang, J.; Chen, H.X.; Tang, X.L.; Zhou, Y.F. Consideration on development of Bletillae striata based on whitening theory in traditional Chinese medicine. Chin. Tradit. Herb. Drugs 2017, 48, 2313–2320. [Google Scholar] [CrossRef]
- Zhou, Y.K.; Li, W.P.; Tian, S.S.; Ma, D.D.; Jiang, F.S.; Ding, Z.S. Determination of Total Polyphenol Content in Different Parts of Rhizoma Bletillae Striatae. Chin. J. Exp. Tradit. Med. Form. 2012, 18, 161–164. [Google Scholar] [CrossRef]
- Peng, Y.; Tang, K.H. Study on Flash Extraction Process of Total Phenol and Determination of Its Content from Bletilla Striata. J. Anhui Agric. Sci. 2018, 46, 157–160. [Google Scholar]
- Chen, X.Y.; Song, W.; Tan, X.F.; Wang, T.T.; Geng, Y.C.; Wang, W.D.; Kang, Q.J.; Wu, Y.X. Optimization of extraction process of polyphenols from fibrous roots of Bletilla striata and study on its antioxidant and tyrosinase inhibitory activities. Nat. Prod. Res. Dev. 2020, 32, 1134–1142. [Google Scholar] [CrossRef]
- Mehta, N.; Kumar, P.; Verma, A.K.; Umaraw, P.; Khatkar, S.K.; Khatkar, A.B.; Pathak, D.; Kaka, U.; Sazili, A.Q. Ultrasound-Assisted Extraction and the Encapsulation of Bioactive Components for Food Applications. Foods 2022, 11, 2973. [Google Scholar] [CrossRef] [PubMed]
- Zhang, R. Preparation of Rabbit Hair Keratin and Its Application in Ultraviolet Protective Health Products. Master’s Thesis, Tianjin Polytechnic University, Tianjin, China, 2018. [Google Scholar]
- Ma, X.Y.; Zhu, Y. Application of plant polyphenols in cosmetics. Chin. Det. Cosmet. 2018, 41, 40–44. [Google Scholar]
- Liu, J.M.; Liu, G.; Liu, Y.C.; Sun, Q.W.; Zhang, Y.P. Analysis of Chemical Constituents in Baiji(Bletilla Striata) by UPLC-Q-TOF/MS. Chin. Arch. Trad. Chin. Med. 2022, 40, 72–76. [Google Scholar] [CrossRef]
- Li, M.; Guo, S.X.; Wang, C.L.; Yang, J.S.; Xiao, P.G. Distribution characteristics and pharmacological activities of 2-isobutyl malic acid glucose oxybenzyl ester compounds in orchid plants. Chin. Pharm. J. 2010, 45, 724–726. [Google Scholar]
- Li, L.M. Study on the Metabolite and Pharmacokinetics of Militarine from Bletilla Striata in Rats. Ph.D. Thesis, Shanghai University of Traditional Chinese Medicine, Shanghai, China, 2019. [Google Scholar]
- Qin, Y.D.; Wang, R.B.; Tang, H.; Li, L.H. Relationship between HPLC Fingerprint Chromatogram and Antibacterial Activity of Medicinal Herb of Bletilla Rchb. F. Tradit. Chin. Drug. Res. Clin. Pharmacol. 2020, 31, 832–837. [Google Scholar] [CrossRef]
- Gang, Y.Q. Studies on Chemical Constituents from Bletilla striata and Their Biological Activities. Master’s Thesis, South-Central University for Nationalities, Wuhan, China, 2020. [Google Scholar]
- Jiang, S.; Wan, K.; Lou, H.Y.; Yi, P.; Zhang, N.; Zhou, M.; Song, Z.Q.; Wang, W.; Wu, M.-K.; Pan, W.D. Antibacterial bibenzyl derivatives from the tubers of Bletilla striata. Phytochemistry 2019, 162, 216–223. [Google Scholar] [CrossRef]
- Tie, H.; Liao, F.; Wang, C.; Yan, Y.; Xie, X.; Su, N. The active ingredients and safety analysis of colla corii asini as a cosmetic raw material. China Surfactant Deterg. Cosmet. 2020, 50, 38–43+70. [Google Scholar] [CrossRef]
- Wang, L.; Zhang, Z.; Ge, R.; Zhang, J.; Liu, W.; Mou, K.; Lv, S.; Mu, X. Gossypetin Inhibits Solar-UV Induced Cutaneous Basal Cell Carcinoma Through Direct Inhibiting PBK/TOPK Protein Kinase. Anti-Cancer Agents Med. Chem. 2019, 19, 1029–1036. [Google Scholar] [CrossRef]
- Teixeira, M.A.C.; Piccirillo, C.; Tobaldi, D.M.; Pullar, R.C.; Labrincha, J.A.; Ferreira, M.O.; Castro, P.M.L.; Pintado, M.M.E. Effect of preparation and processing conditions on UV absorbing properties of hydroxyapatite-Fe2O3 sunscreen. Mater. Sci. Eng. C 2017, 71, 141–149. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.L. Study on Sunscreen Performance of the Hemp Plant Ingredients and Its Application in Military Cosmetics. Master’s Thesis, Guangdong Ocean University, Zhanjiang, China, 2013. [Google Scholar]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).