Preparation of 4-Amino-3-hydrazino-1,2,4-triazol-5-thiol-Modified Graphene Oxide and Its Greatly Enhanced Selective Adsorption of Gallium in Aqueous Solution
Abstract
1. Introduction
2. Results and Discussion
2.1. Characterizations
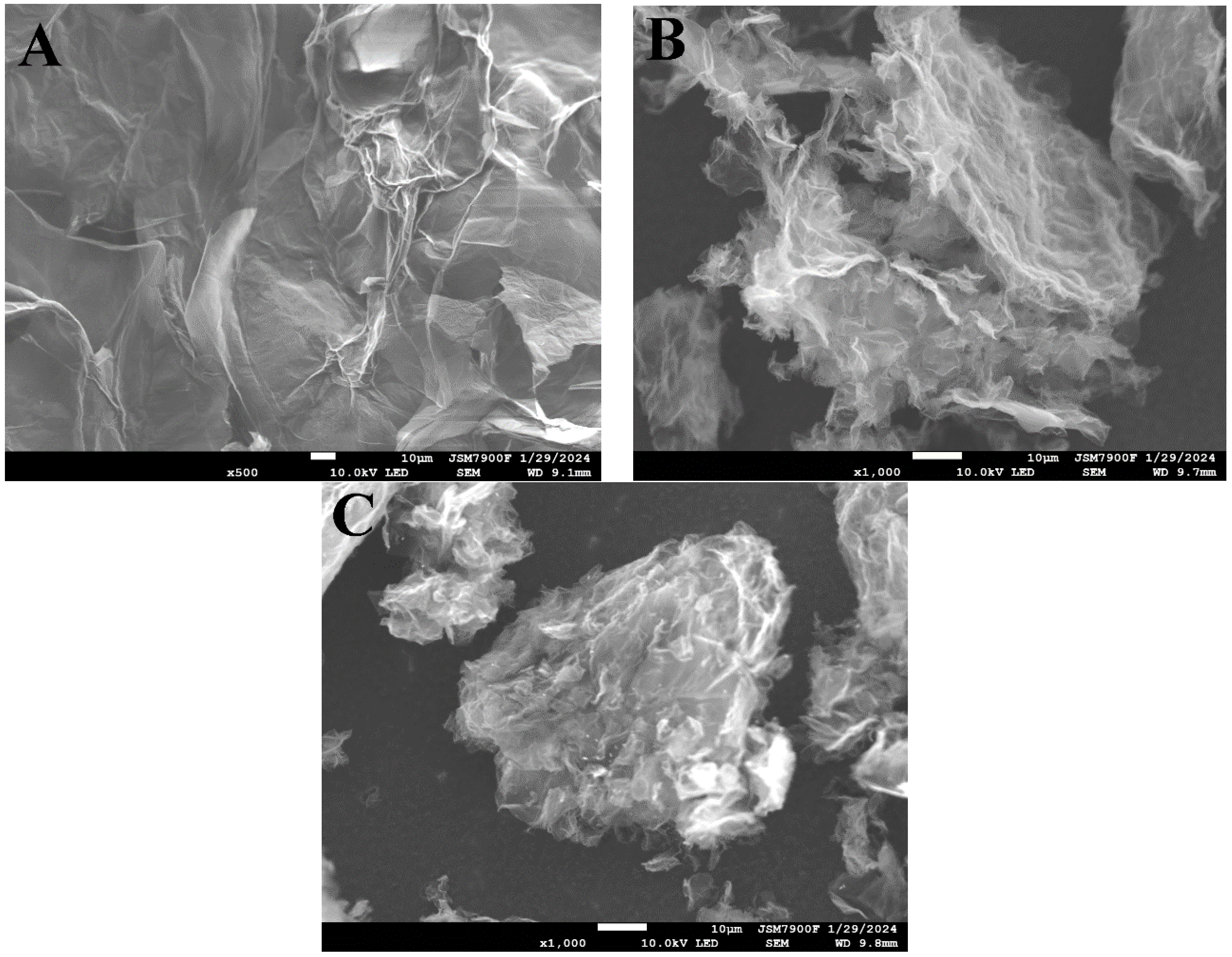
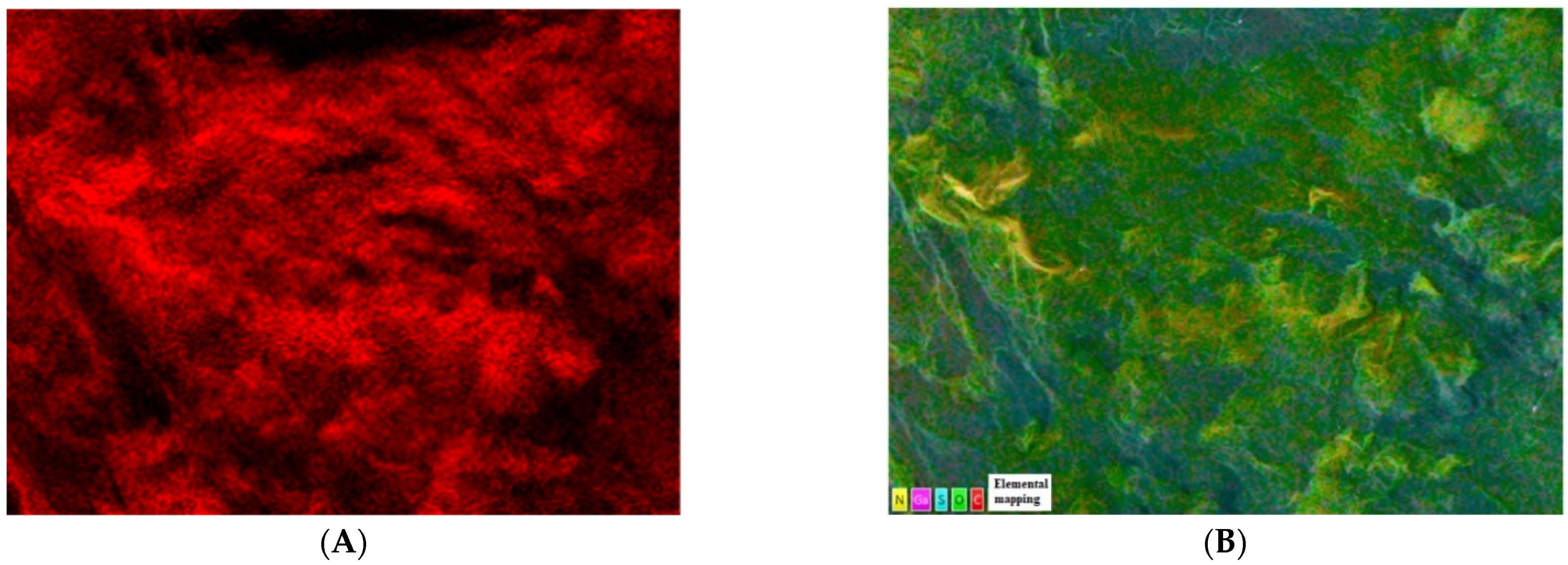
2.1.1. SEM, EDS, and Elemental Mapping Analyses
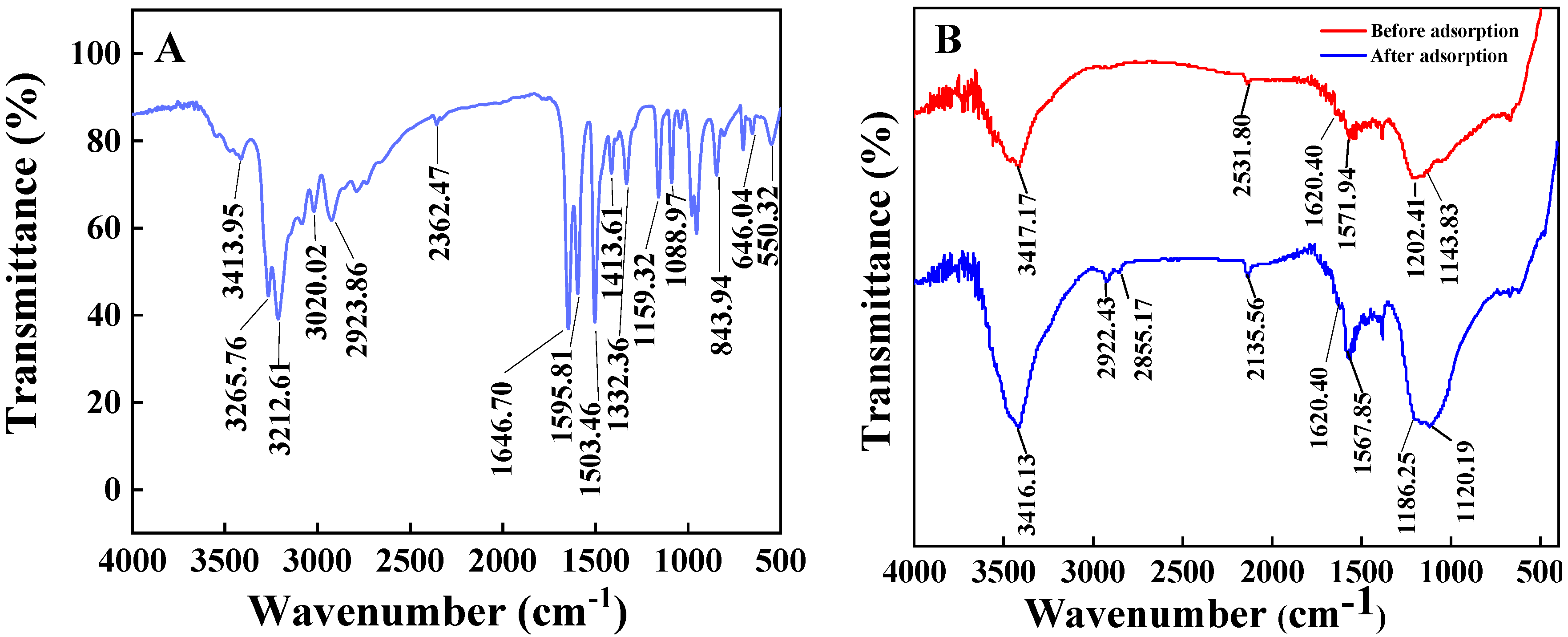
2.1.2. FT-IR Spectra
2.1.3. XPS
2.1.4. TGA
2.2. Adsorption Data
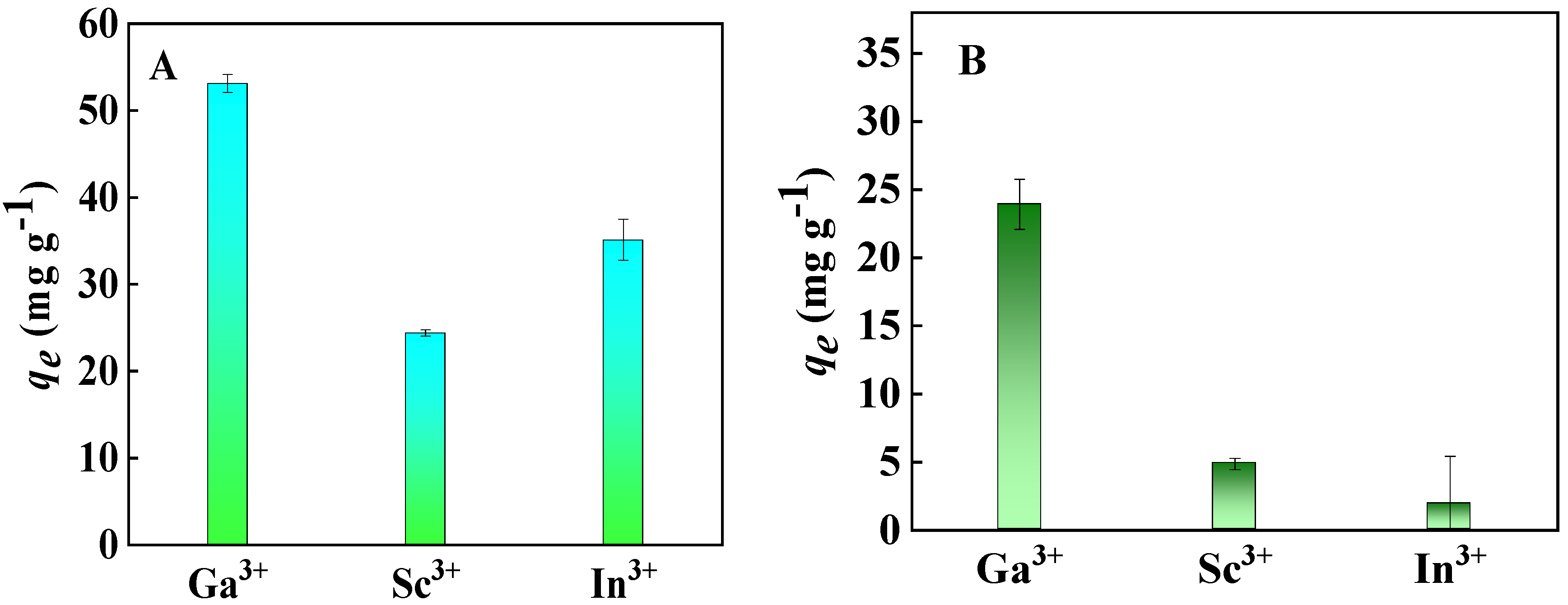
2.2.1. Adsorption Selectivity
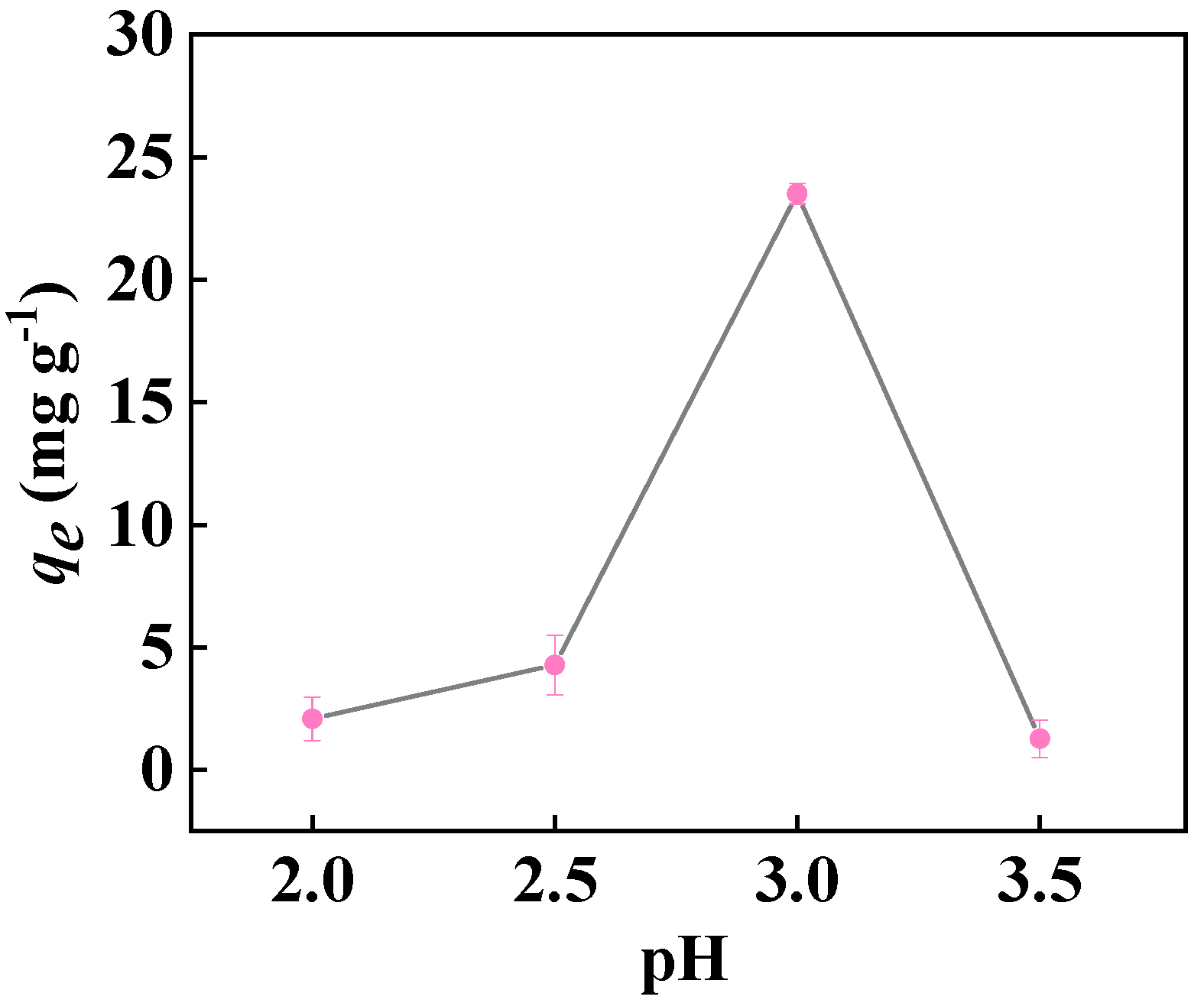
2.2.2. Effect of Solution pH
2.2.3. Effect of Time and Adsorption Kinetics
2.2.4. Effects of Initial Concentration and Contact Temperature, and Adsorption Isotherms
2.2.5. Adsorption Thermodynamics
2.2.6. Proposed Adsorption Mechanism
2.3. Desorption and Regeneration
2.4. Adsorption Performance in a Mixed Solution
3. Experimental Details
3.1. Reagents and Materials
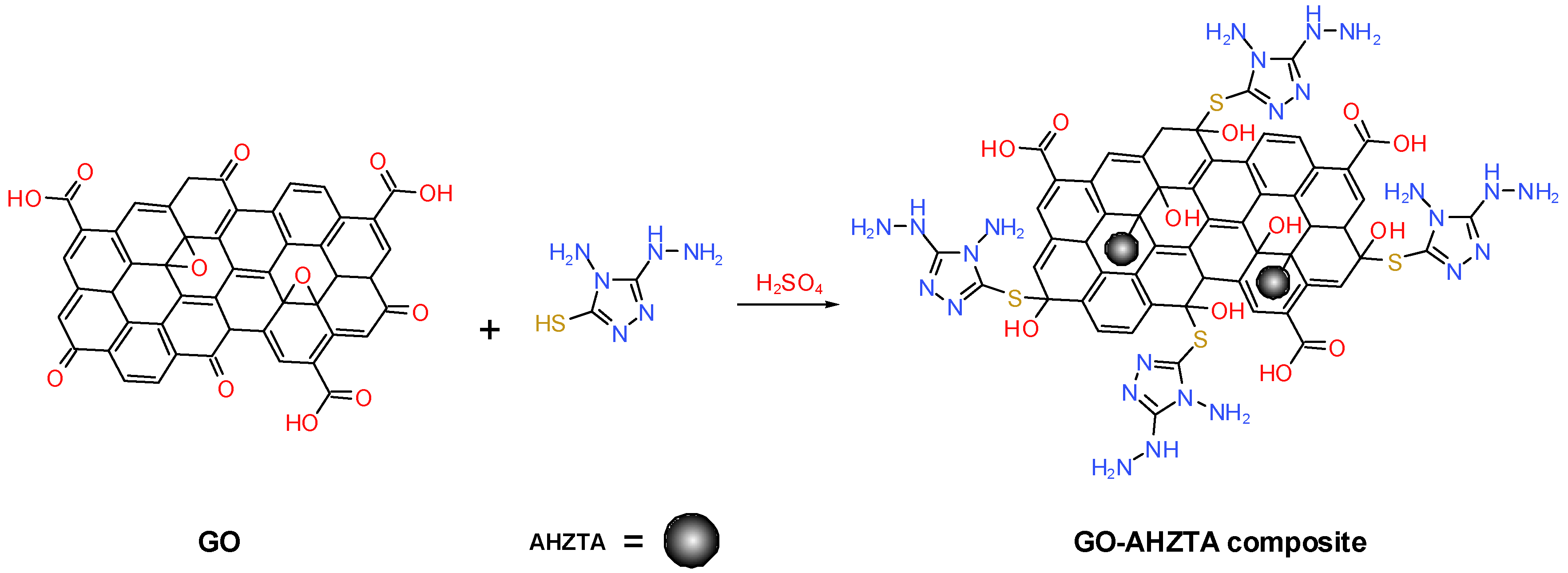
3.2. Preparation of the GO-AHTZT Composite
3.3. Characterization of the GO-AHZTA Composite
3.4. Adsorption Experiments
3.5. Batch Adsorption Experiments
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Syu, C.-H.; Chen, L.-Y.; Lee, D.-Y. The growth and uptake of gallium (Ga) and indium (In) of wheat seedlings in Ga- and In-contaminated soils. Sci. Total Environ. 2021, 759, 143943. [Google Scholar] [CrossRef] [PubMed]
- Wood, S.A.; Samson, I.M. The aqueous geochemistry of gallium, germanium, indium and scandium. Ore Geol. Rev. 2006, 28, 57–102. [Google Scholar] [CrossRef]
- Ergashev, S. Anomalously high diotovoltaic effect in thin films of gallium arsenide. Int. J. Adv. Sci. Res. 2023, 3, 143–149. [Google Scholar]
- Tarbi, A.; Chtouki, T.; Bouich, A.; Elkouari, Y.; Erguig, H.; Migalska-Zalas, A.; Aissat, A. InP/InGaAsP thin films based solar cells: Lattice mismatch impact on efficiency. Opt. Mater. 2022, 131, 112704. [Google Scholar] [CrossRef]
- Moskalyk, R.R. Gallium: The backbone of the electronics industry. Miner. Eng. 2003, 16, 921–929. [Google Scholar] [CrossRef]
- Zheng, K.; Benedetti, M.F.; van Hullebusch, E.D. Recovery technologies for indium, gallium, and germanium from end-of-life products (electronic waste)–A review. J. Environ. Manag. 2023, 347, 119043. [Google Scholar] [CrossRef] [PubMed]
- Du, J.; Zhang, M.; Dong, Z.; Yang, X.; Zhao, L. Facile fabrication of tannic acid functionalized microcrystalline cellulose for selective recovery of Ga (III) and In (III) from potential leaching solution. Sep. Purif. Technol. 2022, 286, 120442. [Google Scholar] [CrossRef]
- Hu, D.; Ma, B.; Li, X.; Lv, Y.; Zhang, W.; Chen, Y.; Wang, C. Efficient separation and recovery of gallium and indium in spent CIGS materials. Sep. Purif. Technol. 2022, 282, 120087. [Google Scholar] [CrossRef]
- Fabretti, A.C.; Franchini, G.C.; Peyronel, G. Cobalt(II), nickel(II), copper(II) and copper(I) complexes of 2-mercapto-5-methyl-1,3,4-thiadiazole and 2,5-bis(methylmercapto)-1,3,4-thiadiazole. Transit. Met. Chem. 1982, 7, 105–108. [Google Scholar] [CrossRef]
- Bharati, P.; Bharti, A.; Bharty, M.K.; Kashyap, S.; Singh, U.P.; Singh, N.K. Synthesis, spectral and structural characterization of Ni(II), Cu(II), Zn(II), Cd(II) and Hg(II) complexes with 2-mercapto-5-methyl-1,3,4-thiadiazole: A Zn(II) complex acting as a new sensitive and selective fluorescent probe for the detection of Hg2+ in H2O–MeOH medium. Polyhedron 2013, 63, 222–231. [Google Scholar]
- Raj, P.; Patel, M.; Karamalidis, A.K. Chemically modified polymeric resins with catechol derivatives for adsorption, separation and recovery of gallium from acidic solutions. J. Environ. Chem. Eng. 2023, 11, 110790. [Google Scholar] [CrossRef]
- Cui, J.; Cong, X.; Li, X.; Chen, X.; Wang, Y.; Gao, J.; Xiong, Y. Chitosan derived layered porous carbon and its performance on gallium adsorption. J. Chem. Technol. Biotechnol. 2023, 98, 1387–1394. [Google Scholar] [CrossRef]
- Li, S.; Fan, J.; Gao, L. Conductive biomass carbon aerogel with high adsorption performance for gallium in alkaline solution. Miner. Eng. 2023, 204, 108396. [Google Scholar] [CrossRef]
- Jankovský, O.; Šimek, P.; Klímová, K.; Sedmidubský, D.; Pumera, M.; Sofer, Z. Highly selective removal of Ga3+ ions from Al3+/Ga3+ mixtures using graphite oxide. Carbon 2015, 89, 121–129. [Google Scholar] [CrossRef]
- Deshwal, N.; Singh, M.B.; Bahadur, I.; Kaushik, N.; Kaushik, N.K.; Singh, P.; Kumari, K. A review on recent advancements on removal of harmful metal/metal ions using graphene oxide: Experimental and theoretical approaches. Sci. Total Environ. 2023, 858, 159672. [Google Scholar] [PubMed]
- Li, X.; Zhang, J.; Pang, Z.; Zhu, Y.; Chen, X.; Sun, Q.; Li, Y. Photoelectrocatalytic decolorization of methylene blue using reduced graphene oxide modified TiO2 on filter paper. Water Sci. Technol. 2019, 80, 1673–1682. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Liu, X.; Wang, Y.; Lou, Z.; Shan, W.; Xiong, Y. Polyacrylic acid-functionalized graphene oxide for high-performance adsorption of gallium from aqueous solution. J. Colloid Interface Sci. 2019, 556, 102–110. [Google Scholar] [PubMed]
- Ji, Q.; Hu, C.; Liu, H.; Qu, J. Development of nitrogen-doped carbon for selective metal ion capture. Chem. Eng. J. 2018, 350, 608–615. [Google Scholar] [CrossRef]
- Xu, W.; Zhou, S.; Wang, B.; Zhang, P.; Tang, K. Efficient adsorption of Au (III) from acidic solution by a novel N, S-containing metal–organic framework. Sep. Purif. Technol. 2022, 288, 120646. [Google Scholar]
- Deng, J.; Liu, Y.; Liu, S.; Zeng, G.; Tan, X.; Huang, B.; Tang, X.; Wang, S.; Hua, Q.; Yan, Z. Competitive adsorption of Pb (II), Cd (II) and Cu (II) onto chitosan-pyromellitic dianhydride modified biochar. J. Colloid Interface Sci. 2017, 506, 355–364. [Google Scholar] [CrossRef]
- Yin, Z.; Hu, Y.; Sun, W.; Zhang, C.; He, J.; Xu, Z.; Zou, J.; Guan, C.; Zhang, C.; Guan, Q. Adsorption mechanism of 4-amino-5-mercapto-1, 2, 4-triazole as flotation reagent on chalcopyrite. Langmuir 2018, 34, 4071–4083. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Cheng, J.; Liu, N.; Zou, H.; Yan, H.; Lu, J.; Liu, H.; Li, Y.; Dou, J.; Wang, S. Two Co-based metal–organic framework isomers with similar metal-carboxylate sheets: Turn-on ratiometric luminescence sensing activities toward biomarker N-acetylneuraminic acid and discrimination of Ga3+ and In3+. Inorg. Chem. 2023, 62, 2083–2094. [Google Scholar] [CrossRef] [PubMed]
- Brahma, G.S.; Mohanty, P. Kinetics and mechanism of complex formation between pentamminesuccinatocobalt (III) and gallium (III). Pol. J. Chem. 2003, 77, 1221–1228. [Google Scholar]
- Mateo, C.; Grazu, V.; Palomo, J.M.; Lopez-Gallego, F.; Fernandez-Lafuente, R.; Guisan, J.M. Immobilization of enzymes on heterofunctional epoxy supports. Nat. Protoc. 2007, 2, 1022–1033. [Google Scholar] [CrossRef] [PubMed]
- Grazú, V.; Abian, O.; Mateo, C.; Batista-Viera, F.; Fernández-Lafuente, R.; Guisán, J.M. Novel bifunctional epoxy/thiol-reactive support to immobilize thiol containing proteins by the epoxy chemistry. Biomacromolecules 2003, 4, 1495–1501. [Google Scholar] [CrossRef] [PubMed]
- Cote, L.J.; Kim, J.; Zhang, Z.; Sun, C.; Huang, J. Tunable assembly of graphene oxide surfactant sheets: Wrinkles, overlaps and impacts on thin film properties. Soft Matter 2010, 6, 6096–6101. [Google Scholar] [CrossRef]
- Ru, J.; Wang, X.; Wang, F.; Cui, X.; Du, X.; Lu, X. UiO series of metal-organic frameworks composites as advanced sorbents for the removal of heavy metal ions: Synthesis, applications and adsorption mechanism. Ecotoxicol. Environ. Saf. 2021, 208, 111577. [Google Scholar] [CrossRef] [PubMed]
- Jiang, H.; Yang, Y.; Lin, Z.; Zhao, B.; Wang, J.; Xie, J.; Zhang, A. Preparation of a novel bio-adsorbent of sodium alginate grafted polyacrylamide/graphene oxide hydrogel for the adsorption of heavy metal ion. Sci. Total Environ. 2020, 744, 140653. [Google Scholar] [CrossRef] [PubMed]
- Branca, C.; D’Angelo, G.; Crupi, C.; Khouzami, K.; Rifici, S.; Ruello, G.; Wanderlingh, U. Role of the OH and NH vibrational groups in polysaccharide-nanocomposite interactions: A FTIR-ATR study on chitosan and chitosan/clay films. Polymer 2016, 99, 614–622. [Google Scholar] [CrossRef]
- Silva, A.L.R.; Gonçalves, J.M.; Morais, V.M.F.; Ribeiro da Silva, M.D.M.C. Thermodynamic properties of 2-mercapto-, 2,5-dimethyl- and 2-mercapto-5-methyl-1,3,4-thiadiazole. J. Chem. Thermodyn. 2022, 165, 106644. [Google Scholar] [CrossRef]
- Ren, F.; Li, Z.; Tan, W.Z.; Liu, X.H.; Sun, Z.F.; Ren, P.G.; Yan, D.X. Facile preparation of 3D regenerated cellulose/graphene oxide composite aerogel with high-efficiency adsorption towards methylene blue. J. Colloid Interf. Sci. 2018, 532, 58–67. [Google Scholar] [CrossRef] [PubMed]
- Miyata, O.; Shinada, T.; Ninomiya, I.; Naito, T.; Date, T.; Okamura, K.; Inagaki, S. Stereospecific nucleophilic addition reactions to olefins. Addition of thiols to. a,b-unsaturated carboxylic acid derivatives. J. Org. Chem. 1991, 56, 6556–6564. [Google Scholar] [CrossRef]
- Ramana, C.V.; Rubio, E.J.; Barraza, C.D.; Miranda Gallardo, A.; McPeak, S.; Kotru, S.; Grant, J.T. Chemical bonding, optical constants, and electrical resistivity of sputter-deposited gallium oxide thin films. J. Appl. Phys. 2014, 115, 043508. [Google Scholar] [CrossRef]
- Zhang, J.; Chen, L.; Yang, J.; Bian, C.; He, W. Thermal behaviors, thermal decomposition mechanism, kinetic model analysis and thermal hazard prediction of 3,6,7-triamino-7H-[1,2,4]triazolo [4,3-b][1,2,4]triazole (TATOT). Thermochim. Acta 2023, 724, 179515. [Google Scholar] [CrossRef]
- Chen, X.; Wang, X.; Fang, D. A review on C1s XPS-spectra for some kinds of carbon materials. Fuller. Nanotub. Carbon Nanostruct. 2020, 28, 1048–1058. [Google Scholar] [CrossRef]
- Briggs, D.; Beamson, G. XPS studies of the oxygen 1s and 2s levels in a wide range of functional polymers. Anal. Chem. 1993, 65, 1517–1523. [Google Scholar] [CrossRef]
- Poolwong, J.; Del Gobbo, S.; D’Elia, V. Transesterification of dimethyl carbonate with glycerol by perovskite-based mixed metal oxide nanoparticles for the atom-efficient production of glycerol carbonate. J. Ind. Eng. Chem. 2021, 104, 43–60. [Google Scholar] [CrossRef]
- Tangyen, N.; Natongchai, W.; Del Gobbo, S.; D’Elia, V. Revisiting the potential of group Vi inorganic precatalysts for the ethenolysis of fatty acids through a mechanochemical approach. ACS Omega 2024, 9, 19712–19722. [Google Scholar] [CrossRef]
- Wang, R.; Huang, J.; Deng, Y.; Fan, K.; Xu, B.; Zhao, Z. Preparation and properties of protein-derived carbon-modified CoS2 applied to high-performance lithium-sulfur battery. Mater. Des. 2024, 238, 112696. [Google Scholar] [CrossRef]
- Farivar, F.; Yap, P.L.; Hassan, K.; Tung, T.T.; Tran, D.N.; Pollard, A.J.; Losic, D. Unlocking thermogravimetric analysis (TGA) in the fight against “Fake graphene” materials. Carbon 2021, 179, 505–513. [Google Scholar] [CrossRef]
- Li, W.; Zhou, C.; Li, C.; Zhu, W.; Shi, J.; Liu, G. Synthesis of UiO-66 series metal–organic framework composites and the adsorption effect on gallium. Chem. Eng. J. 2023, 455, 140881. [Google Scholar] [CrossRef]
- Alhamami, M.A.M.; Algethami, J.S.; Khan, S. A review on thiazole based colorimetric and fluorimetric chemosensors for the detection of heavy metal ions. Crit. Rev. Anal. Chem. 2023, 1–25. [Google Scholar] [CrossRef]
- Kubicek, V.; Havlickova, J.; Kotek, J.; Tircsó, G.; Hermann, P.; Tóth, É.; Lukes, I. Gallium (III) complexes of DOTA and DOTA−monoamide: Kinetic and thermodynamic studies. Inorg. Chem. 2010, 49, 10960–10969. [Google Scholar] [CrossRef] [PubMed]
- Simecek, J.; Schulz, M.; Notni, J.; Plutnar, J.; Kubicek, V.; Havlickova, J.; Hermann, P. Complexation of metal ions with TRAP (1, 4, 7-triazacyclononane phosphinic acid) ligands and 1,4,7-triazacyclononane-1,4,7-triacetic acid: Phosphinate-containing ligands as unique chelators for trivalent gallium. Inorg. Chem. 2012, 51, 577–590. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Guo, X. Adsorption kinetic models: Physical meanings, applications, and solving methods. J. Hazard. Mater. 2020, 390, 122156. [Google Scholar] [CrossRef] [PubMed]
- Nithya, K.; Sathish, A.; Senthil Kumar, P.; Ramachandran, T. Fast kinetics and high adsorption capacity of green extract capped superparamagnetic iron oxide nanoparticles for the adsorption of Ni(II) ions. J. Ind. Eng. Chem. 2018, 59, 230–241. [Google Scholar] [CrossRef]
- Mozaffari Majd, M.; Kordzadeh-Kermani, V.; Ghalandari, V.; Askari, A.; Sillanpää, M. Adsorption isotherm models: A comprehensive and systematic review (2010−2020). Sci. Total Environ. 2022, 812, 151334. [Google Scholar] [CrossRef] [PubMed]
- Yi, Q.; Hao, X.; Li, X.; Dong, H.; Sun, L. Effect of TiO2 nanoparticles on the mass transfer process of absorption of toluene: Experimental investigation and molecular dynamics simulation. J. Environ. Chem. Eng. 2023, 11, 109474. [Google Scholar] [CrossRef]
- Mujtaba, G.; Ullah, A.; Khattak, D.; Shah, M.U.H.; Daud, M.; Ahmad, S.; Hai, A.; Ahmed, F.; Alshahrani, T.; Banat, F. Simultaneous adsorption of methylene blue and amoxicillin by starch-impregnated MgAl layered double hydroxide: Parametric optimization, isothermal studies and thermo-kinetic analysis. Environ. Res. 2023, 235, 116610. [Google Scholar] [CrossRef]
- Chen, H.; Zhao, J.; Wu, J.; Dai, G. Isotherm, thermodynamic, kinetics and adsorption mechanism studies of methyl orange by surfactant modified silkworm exuviae. J. Hazard. Mater. 2011, 192, 246–254. [Google Scholar] [CrossRef]
- Fang, Y.; Liu, Q.; Song, Y.; Jia, F.; Yang, Y.; Li, H. Rational design of anti-interference Fe/Co MOF-coupled PMS process for As (III) removal in DOM-rich groundwater: 1O2-dominated As (III) oxidation and chemisorption of As (V). Chem. Eng. J. 2023, 470, 144386. [Google Scholar] [CrossRef]
- Hong, S.; Wen, C.; He, J.; Gan, F.X.; Ho, Y.S. Adsorption thermodynamics of Methylene Blue onto bentonite. J. Hazard. Mater. 2009, 167, 630–633. [Google Scholar] [CrossRef] [PubMed]
- Olsson, E.; Chai, G.; Dove, M.; Cai, Q. Adsorption and migration of alkali metals (Li, Na, and K) on pristine and defective graphene surfaces. Nanoscale 2019, 11, 5274–5284. [Google Scholar] [CrossRef] [PubMed]
- Chen, D.; Feng, H.; Li, J. Graphene oxide: Preparation, functionalization, and electrochemical applications. Chem. Rev. 2012, 112, 6027–6053. [Google Scholar] [CrossRef]
- Shen, J.; Hu, Y.; Shi, M.; Lu, X.; Qin, C.; Li, C.; Ye, M. Fast and facile preparation of graphene oxide and reduced graphene oxide nanoplatelets. Chem. Mater. 2009, 21, 3514–3520. [Google Scholar] [CrossRef]



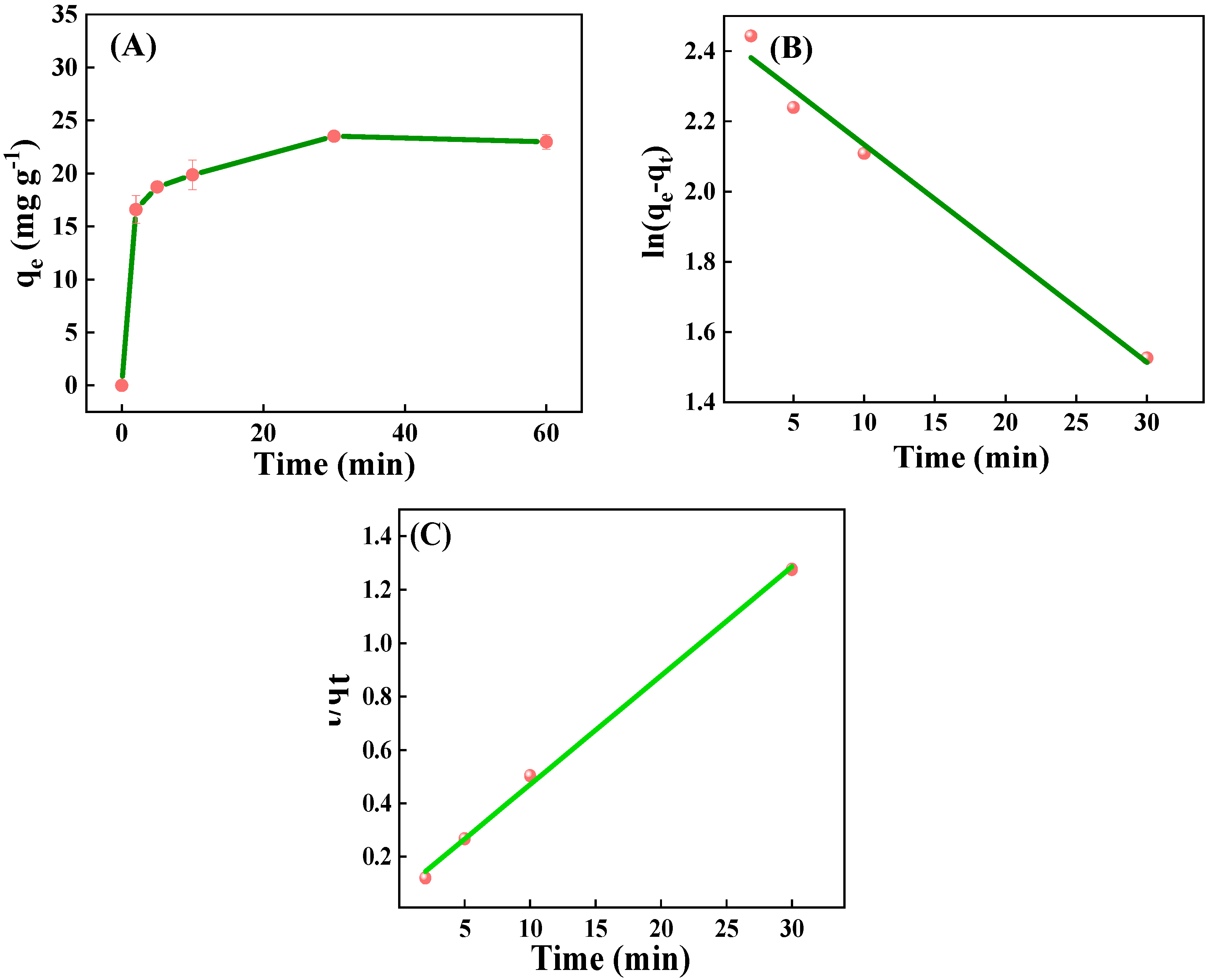


| Adsorption Kinetic Model | R2 | k1 (min−1) | k2 (min−1) | qe (mg g−1) |
|---|---|---|---|---|
| Linear pseudo-first-order | 0.985 | 0.031 | - | 6.641 |
| Linear pseudo-second order | 0.997 | - | 0.0264 | 24.534 |
| T (K) | Linear Langmuir Model | Linear Freundlich Model | ||||
|---|---|---|---|---|---|---|
| R2 | kL | qm (mg g−1) | R2 | kF | n | |
| 288 | 0.416 | 0.026 | 56.850 | 0.708 | 1.869 | 1.295 |
| 293 | 0.898 | 0.064 | 41.356 | 0.809 | 3.684 | 1.709 |
| 298 | 0.964 | 0.225 | 33.211 | 0.863 | 7.530 | 6.411 |
| 303 | 0.961 | 0.256 | 33.818 | 0.650 | 8.141 | 10.778 |
| 308 | 0.980 | 0.304 | 34.722 | 0.752 | 8.208 | 9.320 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhu, X.; Guo, Y.; Zheng, B. Preparation of 4-Amino-3-hydrazino-1,2,4-triazol-5-thiol-Modified Graphene Oxide and Its Greatly Enhanced Selective Adsorption of Gallium in Aqueous Solution. Molecules 2024, 29, 2778. https://doi.org/10.3390/molecules29122778
Zhu X, Guo Y, Zheng B. Preparation of 4-Amino-3-hydrazino-1,2,4-triazol-5-thiol-Modified Graphene Oxide and Its Greatly Enhanced Selective Adsorption of Gallium in Aqueous Solution. Molecules. 2024; 29(12):2778. https://doi.org/10.3390/molecules29122778
Chicago/Turabian StyleZhu, Xi, Yong Guo, and Baozhan Zheng. 2024. "Preparation of 4-Amino-3-hydrazino-1,2,4-triazol-5-thiol-Modified Graphene Oxide and Its Greatly Enhanced Selective Adsorption of Gallium in Aqueous Solution" Molecules 29, no. 12: 2778. https://doi.org/10.3390/molecules29122778
APA StyleZhu, X., Guo, Y., & Zheng, B. (2024). Preparation of 4-Amino-3-hydrazino-1,2,4-triazol-5-thiol-Modified Graphene Oxide and Its Greatly Enhanced Selective Adsorption of Gallium in Aqueous Solution. Molecules, 29(12), 2778. https://doi.org/10.3390/molecules29122778






