Covalent Organic Frameworks Based Electrocatalysts for Two-Electron Oxygen Reduction Reaction: Design Principles, Recent Advances, and Perspective
Abstract
1. Introduction
2. Introduction to Electrocatalytic ORR
3. Design Principles for COFs with High Electrocatalytic 2e− ORR Performance
4. COFs for Electrocatalytic H2O2 Production
4.1. Metal-Free Active Site Design
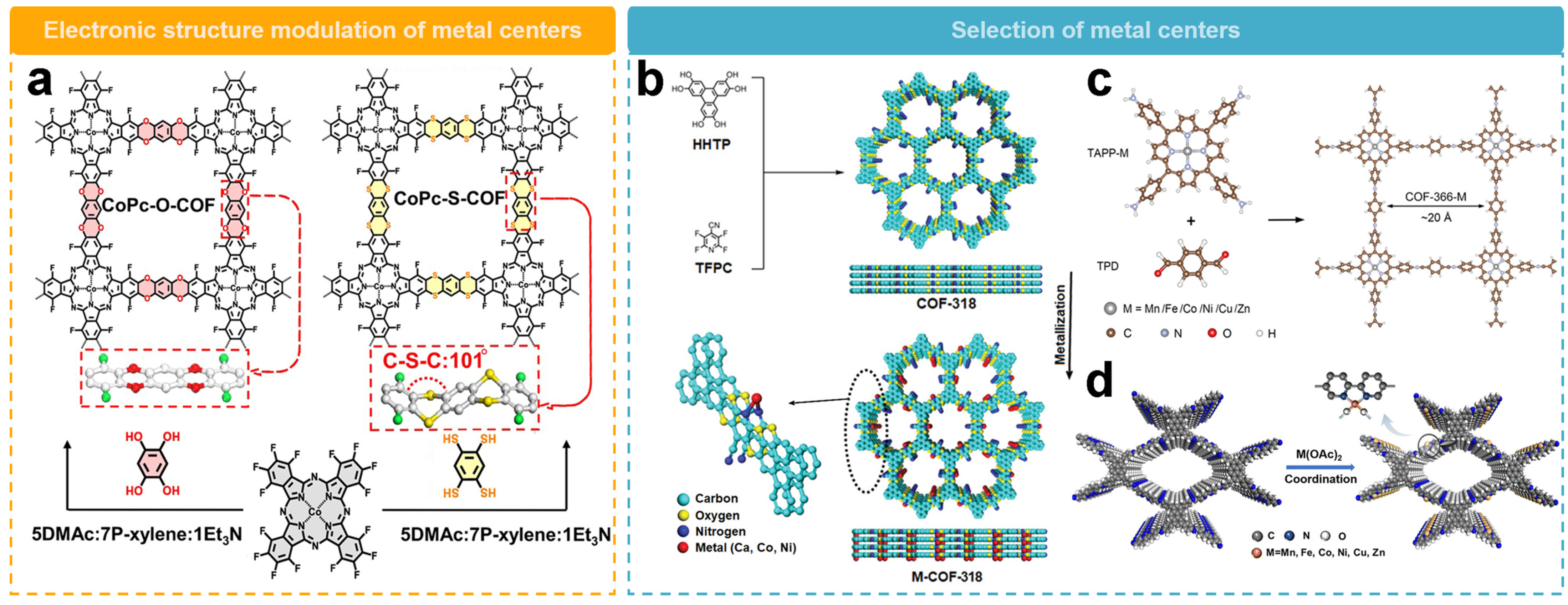
4.2. Metalated Active Sites Design
5. Conclusions and Perspective
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Li, S.; Ma, J.; Xu, F.; Wei, L.; He, D. Fundamental principles and environmental applications of electrochemical hydrogen peroxide production: A review. Chem. Eng. J. 2023, 452, 139371. [Google Scholar] [CrossRef]
- Zhang, X.; Xia, Y.; Xia, C.; Wang, H. Insights into practical-scale electrochemical H2O2 synthesis. Trends Chem. 2020, 2, 942–953. [Google Scholar] [CrossRef]
- Liu, C.; Yu, Z.; She, F.; Chen, J.; Liu, F.; Qu, J.; Cairney, J.M.; Wu, C.; Liu, K.; Yang, W. Heterogeneous molecular Co–N–C catalysts for efficient electrochemical H2O2 synthesis. Energy Environ. Sci. 2023, 16, 446–459. [Google Scholar] [CrossRef]
- Yang, H.; Lu, N.; Zhang, J.; Wang, R.; Tian, S.; Wang, M.; Wang, Z.; Tao, K.; Ma, F.; Peng, S. Ultra-low single-atom Pt on g-C3N4 for electrochemical hydrogen peroxide production. Carbon Energy 2023, 5, e337. [Google Scholar] [CrossRef]
- Iglesias, D.; Giuliani, A.; Melchionna, M.; Marchesan, S.; Criado, A.; Nasi, L.; Bevilacqua, M.; Tavagnacco, C.; Vizza, F.; Prato, M. N-doped graphitized carbon nanohorns as a forefront electrocatalyst in highly selective O2 reduction to H2O2. Chem 2018, 4, 106–123. [Google Scholar] [CrossRef]
- Chang, Q.; Zhang, P.; Mostaghimi, A.H.B.; Zhao, X.; Denny, S.R.; Lee, J.H.; Gao, H.; Zhang, Y.; Xin, H.L.; Siahrostami, S. Promoting H2O2 production via 2-electron oxygen reduction by coordinating partially oxidized Pd with defect carbon. Nat. Commun. 2020, 11, 2178. [Google Scholar] [CrossRef] [PubMed]
- Wang, N.; Ma, S.; Zuo, P.; Duan, J.; Hou, B. Recent progress of electrochemical production of hydrogen peroxide by two-electron oxygen reduction reaction. Sci. Adv. 2021, 8, 2100076. [Google Scholar] [CrossRef] [PubMed]
- Tian, Y.; Deng, D.; Xu, L.; Li, M.; Chen, H.; Wu, Z.; Zhang, S. Strategies for sustainable production of hydrogen peroxide via oxygen reduction reaction: From catalyst design to device setup. Nano-Micro Lett. 2023, 15, 122. [Google Scholar] [CrossRef] [PubMed]
- Espinoza-Montero, P.J.; Alulema-Pullupaxi, P.; Frontana-Uribe, B.A.; Barrera-Diaz, C.E. Electrochemical production of hydrogen peroxide on Boron-Doped diamond (BDD) electrode. Curr. Opin. Solid State Mater. Sci. 2022, 26, 100988. [Google Scholar] [CrossRef]
- Siahrostami, S.; Verdaguer-Casadevall, A.; Karamad, M.; Deiana, D.; Malacrida, P.; Wickman, B.; Escudero-Escribano, M.; Paoli, E.A.; Frydendal, R.; Hansen, T.W. Enabling direct H2O2 production through rational electrocatalyst design. Nat. Mater. 2013, 12, 1137–1143. [Google Scholar] [CrossRef]
- Xue, Y.; Wang, Y.; Pan, Z.; Sayama, K. Electrochemical and photoelectrochemical water oxidation for hydrogen peroxide production. Angew. Chem. Int. Ed. 2021, 60, 10469–10480. [Google Scholar] [CrossRef]
- Liu, J.; Gong, Z.; Yan, M.; He, G.; Gong, H.; Ye, G.; Fei, H. Electronic Structure Regulation of Single-Atom Catalysts for Electrochemical Oxygen Reduction to H2O2. Small 2022, 18, 2103824. [Google Scholar] [CrossRef] [PubMed]
- Zhao, J.; Lian, J.; Zhao, Z.; Wang, X.; Zhang, J. A review of in-situ techniques for probing active sites and mechanisms of electrocatalytic oxygen reduction reactions. Nano-Micro Lett. 2023, 15, 19. [Google Scholar] [CrossRef] [PubMed]
- Dai, L.; Xue, Y.; Qu, L.; Choi, H.-J.; Baek, J.-B. Metal-free catalysts for oxygen reduction reaction. Chem. Rev. 2015, 115, 4823–4892. [Google Scholar] [CrossRef] [PubMed]
- Ren, X.; Dong, X.; Liu, L.; Hao, J.; Zhu, H.; Liu, A.; Wu, G. Research progress of electrocatalysts for the preparation of H2O2 by electrocatalytic oxygen reduction reaction. SusMat 2023, 3, 442–470. [Google Scholar] [CrossRef]
- von Weber, A.; Baxter, E.T.; White, H.S.; Anderson, S.L. Cluster size controls branching between water and hydrogen peroxide production in electrochemical oxygen reduction at Pt n/ITO. J. Phys. Chem. C 2015, 119, 11160–11170. [Google Scholar] [CrossRef]
- Choi, C.H.; Kim, M.; Kwon, H.C.; Cho, S.J.; Yun, S.; Kim, H.-T.; Mayrhofer, K.J.; Kim, H.; Choi, M. Tuning selectivity of electrochemical reactions by atomically dispersed platinum catalyst. Nat. Commun. 2016, 7, 10922. [Google Scholar] [CrossRef] [PubMed]
- Huang, S.; Zheng, J.; Qin, Z.; Li, Y.; Xu, F.; Duan, F.; Zhu, H.; Lu, S.; Du, M. Constructing abundant active interfaces in ultrafine Ru nanoparticles doped nickel–iron layered double hydroxide to promote electrocatalytic oxygen evolution. Electrochim. Acta 2022, 427, 140835. [Google Scholar] [CrossRef]
- Huang, S.; Lu, S.; Gong, S.; Zhang, Q.; Duan, F.; Zhu, H.; Gu, H.; Dong, W.; Du, M. Sublayer stable Fe dopant in porous Pd metallene boosts oxygen reduction reaction. ACS Nano 2021, 16, 522–532. [Google Scholar] [CrossRef]
- Anantharaj, S.; Pitchaimuthu, S.; Noda, S. A review on recent developments in electrochemical hydrogen peroxide synthesis with a critical assessment of perspectives and strategies. Adv. Colloid Interface Sci. 2021, 287, 102331. [Google Scholar] [CrossRef]
- He, Y.; Zhang, H.-J.; Yang, Y.; Xue, Y. Progress of metal-free carbon alloy electrocatalysts for Hydrogen peroxide by Two-Electron oxygen reduction reaction. J. Electroanal. Chem. 2024, 955, 118050. [Google Scholar] [CrossRef]
- Quílez-Bermejo, J.; Morallón, E.; Cazorla-Amorós, D. Metal-free heteroatom-doped carbon-based catalysts for ORR: A critical assessment about the role of heteroatoms. Carbon 2020, 165, 434–454. [Google Scholar] [CrossRef]
- Huang, N.; Wang, P.; Jiang, D. Covalent organic frameworks: A materials platform for structural and functional designs. Nat. Rev. Mater. 2016, 1, 16068. [Google Scholar] [CrossRef]
- Diercks, C.S.; Yaghi, O.M. The atom, the molecule, and the covalent organic framework. Science 2017, 355, eaal1585. [Google Scholar] [CrossRef] [PubMed]
- Sahoo, R.; Mondal, S.; Pal, S.C.; Mukherjee, D.; Das, M.C. Covalent-organic frameworks (COFs) as proton conductors. Adv. Energy Mater. 2021, 11, 2102300. [Google Scholar] [CrossRef]
- Lyle, S.J.; Waller, P.J.; Yaghi, O.M. Covalent organic frameworks: Organic chemistry extended into two and three dimensions. Trends Chem. 2019, 1, 172–184. [Google Scholar] [CrossRef]
- Lin, C.Y.; Zhang, D.; Zhao, Z.; Xia, Z. Covalent organic framework electrocatalysts for clean energy conversion. Adv. Mater. 2018, 30, 1703646. [Google Scholar] [CrossRef] [PubMed]
- Xiao, L.; Qi, L.; Sun, J.; Husile, A.; Zhang, S.; Wang, Z.; Guan, J. Structural regulation of covalent organic frameworks for advanced electrocatalysis. Nano Energy 2023, 120, 109155. [Google Scholar] [CrossRef]
- Caballero, R.; Cohen, B.; Gutiérrez, M. Thiophene-based covalent organic frameworks: Synthesis, photophysics and light-driven applications. Molecules 2021, 26, 7666. [Google Scholar] [CrossRef]
- Yue, Y.; Cai, P.; Xu, K.; Li, H.; Chen, H.; Zhou, H.-C.; Huang, N. Stable bimetallic polyphthalocyanine covalent organic frameworks as superior electrocatalysts. J. Am. Chem. Soc. 2021, 143, 18052–18060. [Google Scholar] [CrossRef]
- Bhunia, S.; Peña-Duarte, A.; Li, H.; Li, H.; Sanad, M.F.; Saha, P.; Addicoat, M.A.; Sasaki, K.; Strom, T.A.; Yacamán, M.J.; et al. [2,1,3]-Benzothiadiazole-Spaced Co-Porphyrin-Based Covalent Organic Frameworks for O2 Reduction. ACS Nano 2023, 17, 3492–3505. [Google Scholar] [CrossRef] [PubMed]
- Zhu, H.-J.; Lu, M.; Wang, Y.-R.; Yao, S.-J.; Zhang, M.; Kan, Y.-H.; Liu, J.; Chen, Y.; Li, S.-L.; Lan, Y.-Q. Efficient electron transmission in covalent organic framework nanosheets for highly active electrocatalytic carbon dioxide reduction. Nat. Commun. 2020, 11, 497. [Google Scholar] [CrossRef] [PubMed]
- Liang, X.; Zhao, Z.; Shi, R.; Yang, L.; Zhao, B.; Qiao, H.; Zhai, L. Modulating the Oxygen Reduction Reaction Performance via Precisely Tuned Reactive Sites in Porphyrin-Based Covalent Organic Frameworks. Molecules 2023, 28, 4680. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Zhu, M.; Schmidt, O.G.; Chen, S.; Zhang, K. Covalent organic frameworks for efficient energy electrocatalysis: Rational design and progress. Adv. Energy Sustain. Res. 2021, 2, 2000090. [Google Scholar] [CrossRef]
- Martínez-Fernández, M.; Martínez-Periñán, E.; Martínez, J.I.; Gordo-Lozano, M.; Zamora, F.; Segura, J.L.; Lorenzo, E. Evaluation of the Oxygen Reduction Reaction Electrocatalytic Activity of Postsynthetically Modified Covalent Organic Frameworks. ACS Sustain. Chem. Eng. 2023, 11, 1763–1773. [Google Scholar] [CrossRef]
- Cui, X.; Gao, L.; Ma, R.; Wei, Z.; Lu, C.-H.; Li, Z.; Yang, Y. Pyrolysis-free covalent organic framework-based materials for efficient oxygen electrocatalysis. J. Mater. Chem. A 2021, 9, 20985–21004. [Google Scholar] [CrossRef]
- Tang, J.; Su, C.; Shao, Z. Covalent organic framework (COF)-Based hybrids for electrocatalysis: Recent advances and perspectives. Small Methods 2021, 5, 2100945. [Google Scholar] [CrossRef] [PubMed]
- Sprachmann, J.; Wachsmuth, T.; Bhosale, M.; Burmeister, D.; Smales, G.J.; Schmidt, M.; Kochovski, Z.; Grabicki, N.; Wessling, R.; List-Kratochvil, E.J. Antiaromatic covalent organic frameworks based on dibenzopentalenes. J. Am. Chem. Soc. 2023, 145, 2840–2851. [Google Scholar] [CrossRef]
- Li, M.-H.; Xu, C.; Yang, Y.-W. Macrocycle-embedded metal-covalent organic frameworks for catalysis: A bridge between covalent and non-covalent functional frameworks. Coord. Chem. Rev. 2024, 512, 215894. [Google Scholar] [CrossRef]
- Wang, Q.; Ren, L.; Zhang, J.; Chen, X.; Chen, C.; Zhang, F.; Wang, S.; Chen, J.; Wei, J. Recent Progress on the Catalysts and Device Designs for (Photo) Electrochemical On-Site H2O2 Production. Adv. Energy Mater. 2023, 13, 2301543. [Google Scholar] [CrossRef]
- Choi, S.; Do, H.W.; Jin, D.; Kim, S.; Lee, J.; Soon, A.; Moon, J.; Shim, W. Revisiting the Role of the Triple-Phase Boundary in Promoting the Oxygen Reduction Reaction in Aluminum–Air Batteries. Adv. Funct. Mater. 2021, 31, 2101720. [Google Scholar] [CrossRef]
- Liang, Y.; Han, Y.; Li, J.-s.; Wang, J.; Liu, D.; Fan, Q. Wettability control in electrocatalyst: A mini review. J. Energy Chem. 2022, 70, 643–655. [Google Scholar] [CrossRef]
- Yang, H.; An, N.; Kang, Z.; Menezes, P.W.; Chen, Z. Understanding Advanced Transition Metal-Based Two Electron Oxygen Reduction Electrocatalysts from the Perspective of Phase Engineering. Adv. Mater. 2024, 8, e2400140. [Google Scholar] [CrossRef] [PubMed]
- Guo, X.; Lin, S.; Gu, J.; Zhang, S.; Chen, Z.; Huang, S. Simultaneously achieving high activity and selectivity toward two-electron O2 electroreduction: The power of single-atom catalysts. Acs Catal. 2019, 9, 11042–11054. [Google Scholar] [CrossRef]
- Stamenkovic, V.R.; Fowler, B.; Mun, B.S.; Wang, G.; Ross, P.N.; Lucas, C.A.; Markovic, N.M. Improved oxygen reduction activity on Pt3Ni (111) via increased surface site availability. Science 2007, 315, 493–497. [Google Scholar] [CrossRef] [PubMed]
- Jiao, J.; Pan, Y.; Wang, B.; Yang, W.; Liu, S.; Zhang, C. Melamine-assisted pyrolytic synthesis of bifunctional cobalt-based core–shell electrocatalysts for rechargeable zinc–air batteries. J. Energy Chem. 2021, 53, 364–371. [Google Scholar] [CrossRef]
- Yan, L.; Wang, C.; Wang, Y.; Wang, Y.; Wang, Z.; Zheng, L.; Lu, Y.; Wang, R.; Chen, G. Optimizing the binding of the *OOH intermediate via axially coordinated Co-N5 motif for efficient electrocatalytic H2O2 production. Appl. Catal. B 2023, 338, 123078. [Google Scholar] [CrossRef]
- Tong, Y.; Liu, J.; Su, B.J.; Juang, J.Y.; Hou, F.; Yin, L.; Dou, S.X.; Liang, J. High-rate electrochemical H2O2 production over multimetallic atom catalysts under acidic–neutral conditions. Carbon Energy 2024, 6, e378. [Google Scholar] [CrossRef]
- Zhong, L.; Li, S. Unconventional oxygen reduction reaction mechanism and scaling relation on single-atom catalysts. ACS Catal. 2020, 10, 4313–4318. [Google Scholar] [CrossRef]
- Liang, Z.; Wang, H.-Y.; Zheng, H.; Zhang, W.; Cao, R. Porphyrin-based frameworks for oxygen electrocatalysis and catalytic reduction of carbon dioxide. Chem. Soc. Rev. 2021, 50, 2540–2581. [Google Scholar] [CrossRef]
- Dong, J.; Han, X.; Liu, Y.; Li, H.; Cui, Y. Metal–covalent organic frameworks (MCOFs): A bridge between metal–organic frameworks and covalent organic frameworks. Angew. Chem. Int. Ed. 2020, 59, 13722–13733. [Google Scholar] [CrossRef]
- Zhi, Q.; Jiang, R.; Yang, X.; Jin, Y.; Qi, D.; Wang, K.; Liu, Y.; Jiang, J. Dithiine-linked metalphthalocyanine framework with undulated layers for highly efficient and stable H2O2 electroproduction. Nat. Commun. 2024, 15, 678. [Google Scholar] [CrossRef]
- Guo, Y.; Xu, Q.; Yang, S.; Jiang, Z.; Yu, C.; Zeng, G. Precise design of covalent organic frameworks for electrocatalytic hydrogen peroxide production. Chem. Asian J. 2021, 16, 498–502. [Google Scholar] [CrossRef]
- Liu, C.; Li, H.; Liu, F.; Chen, J.; Yu, Z.; Yuan, Z.; Wang, C.; Zheng, H.; Henkelman, G.; Wei, L. Intrinsic activity of metal centers in metal–nitrogen–carbon single-atom catalysts for hydrogen peroxide synthesis. J. Am. Chem. Soc. 2020, 142, 21861–21871. [Google Scholar] [CrossRef]
- Liu, M.; Yang, S.; Liu, S.; Miao, Q.; Yang, X.; Li, X.; Xu, Q.; Zeng, G. Construction of Atomic Metal-N2 Sites by Interlayers of Covalent Organic Frameworks for Electrochemical H2O2 Synthesis. Small 2022, 18, 2204757. [Google Scholar] [CrossRef]
- Huang, S.; Zhang, B.; Sun, H.; Hu, H.; Wang, J.; Duan, F.; Zhu, H.; Du, M.; Lu, S. Constructing single atom sites on bipyridine covalent organic frameworks for selective electrochemical production of H2O2. Chem. Commun. 2023, 59, 10424–10427. [Google Scholar] [CrossRef]
- Peng, L.-Z.; Liu, P.; Cheng, Q.-Q.; Hu, W.-J.; Liu, Y.A.; Li, J.-S.; Jiang, B.; Jia, X.-S.; Yang, H.; Wen, K. Highly effective electrosynthesis of hydrogen peroxide from oxygen on a redox-active cationic covalent triazine network. Chem. Commun. 2018, 54, 4433–4436. [Google Scholar] [CrossRef]
- Martínez-Fernández, M.; Martínez-Periñán, E.; de la Peña Ruigómez, A.; Cabrera-Trujillo, J.J.; Navarro, J.A.; Aguilar-Galindo, F.; Rodríguez-San-Miguel, D.; Ramos, M.; Vismara, R.; Zamora, F.; et al. Scalable synthesis and electrocatalytic performance of highly fluorinated covalent organic frameworks for oxygen reduction. Angew. Chem. Int. Ed. 2023, 135, e202313940. [Google Scholar] [CrossRef]
- Yang, S.; Lu, L.; Li, J.; Cheng, Q.; Mei, B.; Li, X.; Mao, J.; Qiao, P.; Sun, F.; Ma, J. Boosting hydrogen peroxide production via establishment and reconstruction of single-metal sites in covalent organic frameworks. SusMat 2023, 3, 379–389. [Google Scholar] [CrossRef]
- Zhang, Y.; Qiao, Z.; Zhang, R.; Wang, Z.; Wang, H.J.; Zhao, J.; Cao, D.; Wang, S. Multicomponent Synthesis of Imidazole-Linked Fully Conjugated 3D Covalent Organic Framework for Efficient Electrochemical Hydrogen Peroxide Production. Angew. Chem. Int. Ed. 2023, 135, e202314539. [Google Scholar] [CrossRef]
- An, S.; Li, X.; Shang, S.; Xu, T.; Yang, S.; Cui, C.X.; Peng, C.; Liu, H.; Xu, Q.; Jiang, Z.; et al. One-Dimensional Covalent Organic Frameworks for the 2e− Oxygen Reduction Reaction. Angew. Chem. Int. Ed. 2023, 62, e202218742. [Google Scholar] [CrossRef] [PubMed]
- Huang, S.; Zhang, B.; Wu, D.; Xu, Y.; Hu, H.; Duan, F.; Zhu, H.; Du, M.; Lu, S. Linkage engineering in covalent organic frameworks as metal-free oxygen reduction electrocatalysts for hydrogen peroxide production. Appl. Catal. B 2024, 340, 123216. [Google Scholar] [CrossRef]
- Huang, S.; Lu, S.; Hu, Y.; Cao, Y.; Li, Y.; Duan, F.; Zhu, H.; Jin, Y.; Du, M.; Zhang, W. Covalent Organic Frameworks with Molecular Electronic Modulation as Metal-Free Electrocatalysts for Efficient Hydrogen Peroxide Production. Small Struct. 2023, 4, 2200387. [Google Scholar] [CrossRef]
- Fu, L.; Huang, D.; Peng, J.; Li, N.; Liu, Z.; Shen, Y.; Zhao, X.; Gu, Y.; Xiang, Y. Charge redistribution in covalent organic frameworks via linkage conversion enables enhanced selective reduction of oxygen to H2O2. J. Mater. Chem. A 2023, 11, 18945–18952. [Google Scholar] [CrossRef]
- Xu, X.; Gao, Y.; Yang, Q.; Liang, T.; Luo, B.; Kong, D.; Li, X.; Zhi, L.; Wang, B. Regulating the activity of intrinsic sites in covalent organic frameworks by introducing electro-withdrawing groups towards highly selective H2O2 electrosynthesis. Nano Today 2023, 49, 101792. [Google Scholar] [CrossRef]
- Li, X.; Fu, Y.; An, Q.; Yang, S.; Yang, X.; Xu, Q.; Zeng, G.; Jiang, Z. Micro-modulation of linkers of covalent organic frameworks as catalysts for 2e− oxygen reduction reaction. Appl. Catal. B 2024, 344, 123611. [Google Scholar] [CrossRef]


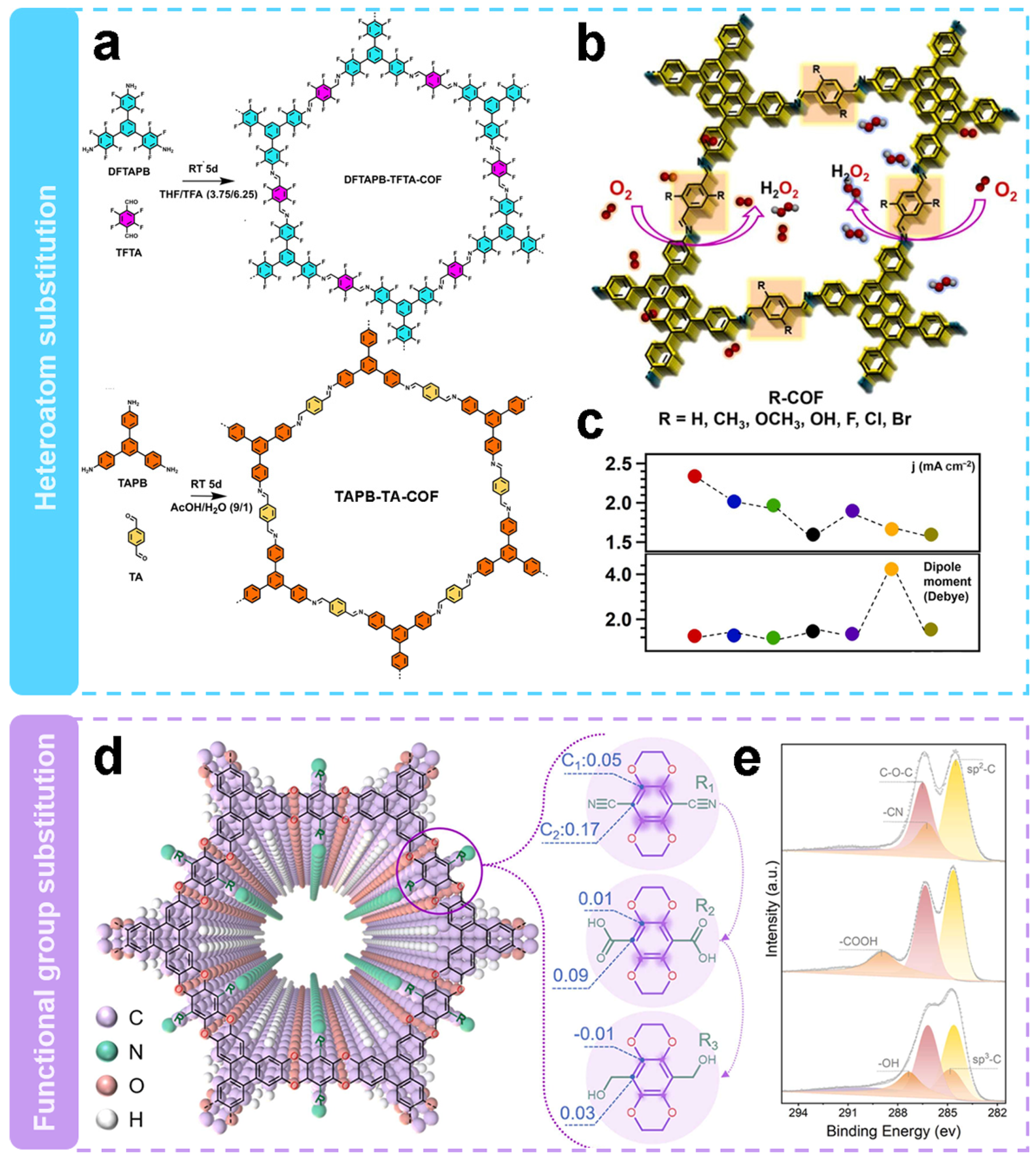
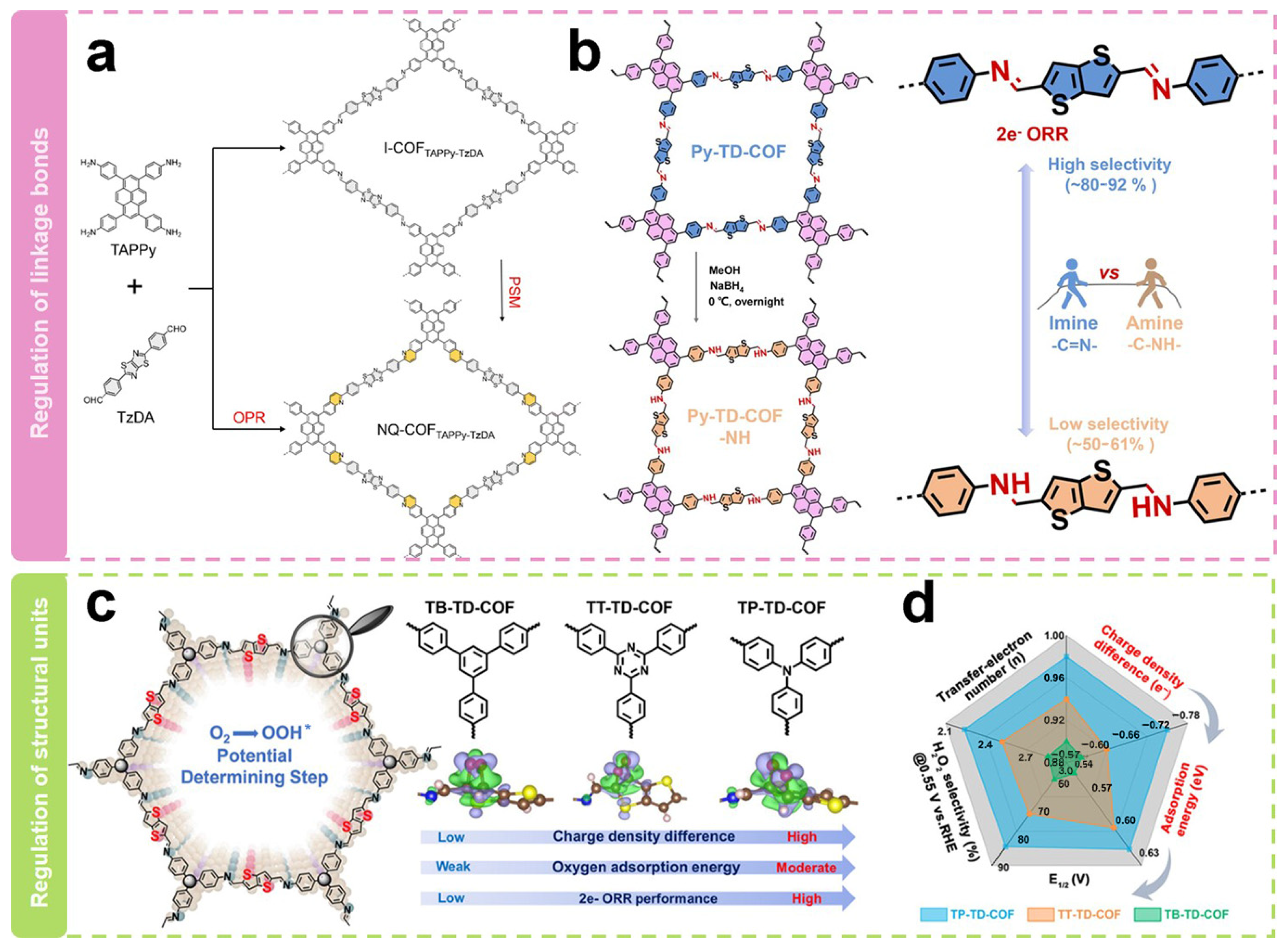
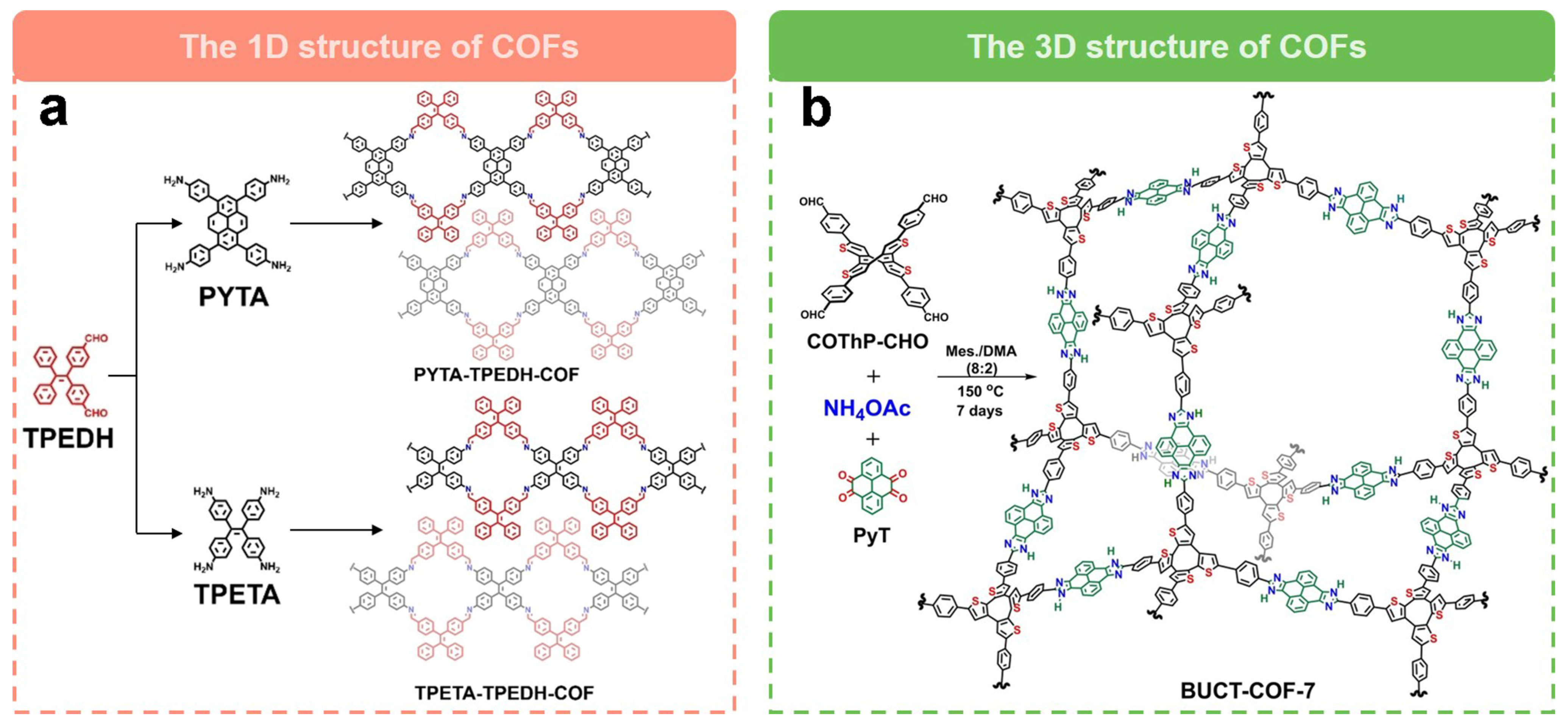
| Electrocatalysts | Electrolyte | n | H2O2 Selectivity (%) | FE (%) | E0/V (vs. RHE) | E1/2/V (vs. RHE) | Jlim/(mA cm−2) | Ref. |
|---|---|---|---|---|---|---|---|---|
| cCTN:Cl− | 0.1 M KOH | 2.2 | 85.3 | N/A | 0.75 | ~0.60 | N/A | [57] |
| DFTAPB-TFTA-COF | 0.1 M NaOH | 2.1 | 96.25 | 71.1 | 0.698 | ~0.60 | 1.70 | [58] |
| TP-TTA-COF | 0.1 M KOH | 2.58–2.68 | 66.0–70.9 | N/A | 0.622 | ~0.57 | 1.32 | [59] |
| BUCT-COF-7/CNT | 0.1 M KOH | 2.41 | 83.4 | ~80 | 0.82 | ~0.71 | N/A | [60] |
| PYTA-TPEDH-COF | 0.1 M KOH | 2.28–2.36 | 82–85.8 | ~80 | 0.69 | ~0.60 | 2.14 | [61] |
| TPETA-TPEDH-COF | 2.40–2.50 | 75.2–79.8 | N/A | 0.69 | ~0.60 | 1.90 | ||
| PYTA-TPETH-COF | 2.54–2.62 | 69–72.9 | N/A | 0.69 | ~0.60 | 2.13 | ||
| Py-TD-COF | 0.1 M KOH | 2.2–2.5 | 80–92 | N/A | 0.834 | 0.698 | 2.898 | [62] |
| Py-TD-COF-NH | 2.8–3.0 | 50–61 | N/A | 0.829 | 0.693 | 2.891 | ||
| TB-TD-COF | 0.1 M KOH | 2.86 | 50.9–67.4 | N/A | 0.71 | 0.55 | N/A | [63] |
| TP-TD-COF | 2.24 | 81.9–86.2 | N/A | 0.76 | 0.62 | N/A | ||
| TT-TD-COF | 2.52 | 68.9–81.8 | N/A | 0.74 | 0.60 | N/A | ||
| NQ-COFTAPPy-TzDA-OPR | 0.1 M Na2SO4 | 2.61–2.77 | 61–69 | 66.7 | ~0.12 | ~−0.2 | N/A | [64] |
| I-COFTAPPy-TzDA | ~3.2 | 34–41 | 39.5 | ~0.02 | ~−0.3 | N/A | ||
| NQ-COFTAPPy-TzDA-PSM | ~2.8 | 48–57 | 61.3 | ~−0.02 | ~−0.25 | N/A | ||
| COF-CN | 0.1 M KOH | 2.06 | 97.2 | N/A | 0.72 | ~0.64 | N/A | [65] |
| COF-CH2OH | ~2.3 | ~82% | N/A | 0.68 | ~0.60 | N/A | ||
| COF-COOH | ~2.2 | ~89 | N/A | ~0.70 | ~0.62 | N/A | ||
| Br-COF | 0.1 M KOH | 2.26–2.29 | 85.4–85.5 | 80.6 | 0.70 | 0.61 | 2.34 | [66] |
| Cl-COF | ~2.31 | 82.4–84.4 | N/A | 0.67 | 0.60 | 1.99 | ||
| OCH3-COF | ~2.33 | 80.1–81.9 | N/A | 0.66 | 0.59 | 1.67 | ||
| COF-366 | 0.1 M KOH | 2.4 | 78 | 64 | ~0.60 | ~0.51 | N/A | [54] |
| 0.1 M PBS | 2.8 | 58 | 41 | ~0.50 | ~0.31 | N/A | ||
| 0.1 M ABS | 2.9 | 53 | 36 | ~0.25 | ~0.13 | N/A | ||
| Py-Bpy-COF | 0.1 M KOH | 2.69 | 63.5 | N/A | ~0.67 | ~0.60 | N/A | [56] |
| H2P-DHTA-COF | 0.1 M KOH | 2.45–2.49 | 76.1 | N/A | 0.67 | 0.57 | 1.4 | [53] |
| Electrocatalysts | Electrolyte | n | H2O2 Selectivity (%) | FE (%) | E0/V (vs. RHE) | E1/2/V (vs. RHE) | Jlim/(mA cm−2) | Ref. |
|---|---|---|---|---|---|---|---|---|
| MgP-DHTA-COF | 0.1 M KOH | 2.11–2.15 | 96 | 90.6 | 0.68 | 0.60 | 2.0 | [53] |
| PtCl-COF | 0.1 M KOH | 2.26–2.37 | 81.6–87.2 | N/A | 0.675 | ~0.58 | 1.83 | [59] |
| CoPc-S-COF | 0.1 M KOH | 2.0–2.2 | ~94 | ~95 | 0.81 | ~0.72 | N/A | [52] |
| CoPc-O-COF | ~2.3 | ~88 | ~90 | 0.78 | ~0.68 | N/A | ||
| Py-Bpy-COF-Zn | 0.1 M KOH | 2.06 | 99.1 | N/A | ~0.75 | ~0.65 | N/A | [56] |
| Py-Bpy-COF-Fe | 2.61 | 45.1 | N/A | ~0.79 | ~0.66 | N/A | ||
| Py-Bpy-COF-Ni | 2.29 | 79.8 | N/A | ~0.75 | ~0.66 | N/A | ||
| Ca-COF-318 | 0.1 M KOH 0.1 M KOH 0.1 M KOH | 2.1 | 94–95 | 91 | 0.75 | 0.61 | 2.80 | [55] |
| COF-366-Co | 0.1 M KOH | 2.2 | 91 | 84 | ~0.67 | ~0.58 | N/A | [54] |
| COF-366-Ni | 2.3 | 86 | 75 | ~0.68 | ~0.60 | N/A | ||
| COF-366-Cu | 2.5 | 76 | 61 | ~0.64 | ~0.55 | N/A | ||
| COF-366-Co | 0.1 M PBS | 2.3 | 86 | 75 | ~0.60 | ~0.20 | N/A | |
| COF-366-Ni | 2.4 | 80 | 67 | ~0.60 | ~0.20 | N/A | ||
| COF-366-Cu | 2.5 | 75 | 60 | ~0.50 | ~0.20 | N/A | ||
| COF-366-Co | 0.1 M ABS | 2.3 | 87 | 77 | ~0.43 | ~0.19 | N/A | |
| COF-366-Ni | 2.4 | 79 | 65 | ~0.42 | ~0.19 | N/A | ||
| COF-366-Cu | 2.7 | 65 | 48 | ~0.25 | ~0.13 | N/A |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Qiao, R.; Wang, J.; Hu, H.; Lu, S. Covalent Organic Frameworks Based Electrocatalysts for Two-Electron Oxygen Reduction Reaction: Design Principles, Recent Advances, and Perspective. Molecules 2024, 29, 2563. https://doi.org/10.3390/molecules29112563
Qiao R, Wang J, Hu H, Lu S. Covalent Organic Frameworks Based Electrocatalysts for Two-Electron Oxygen Reduction Reaction: Design Principles, Recent Advances, and Perspective. Molecules. 2024; 29(11):2563. https://doi.org/10.3390/molecules29112563
Chicago/Turabian StyleQiao, Rui, Jinyan Wang, Hongyin Hu, and Shuanglong Lu. 2024. "Covalent Organic Frameworks Based Electrocatalysts for Two-Electron Oxygen Reduction Reaction: Design Principles, Recent Advances, and Perspective" Molecules 29, no. 11: 2563. https://doi.org/10.3390/molecules29112563
APA StyleQiao, R., Wang, J., Hu, H., & Lu, S. (2024). Covalent Organic Frameworks Based Electrocatalysts for Two-Electron Oxygen Reduction Reaction: Design Principles, Recent Advances, and Perspective. Molecules, 29(11), 2563. https://doi.org/10.3390/molecules29112563





