Abstract
A set of 2-aryl-9-H or methyl-6-morpholinopurine derivatives were synthesized and assayed through radioligand binding tests at human A1, A2A, A2B, and A3 adenosine receptor subtypes. Eleven purines showed potent antagonism at A1, A3, dual A1/A2A, A1/A2B, or A1/A3 adenosine receptors. Additionally, three compounds showed high affinity without selectivity for any specific adenosine receptor. The structure-activity relationships were made for this group of new compounds. The 9-methylpurine derivatives were generally less potent but more selective, and the 9H-purine derivatives were more potent but less selective. These compounds can be an important source of new biochemical tools and/or pharmacological drugs.
1. Introduction
Adenosine, a purine nucleoside composed of an adenine linked to a ribose via a β-N9-glycosidic bond, modulates many physiological conditions related to neurological, immunologic, and cardiovascular systems [1,2]. Adenosine receptors, namely A1, A2A, A2B, and A3, belong to the G protein-coupled receptor (GPCR) superfamily and are widely recognized as attractive targets for the design and development of new therapeutic agents against different clinical disorders [3,4,5]. The activation of the A1 and A3 receptors inhibits adenylyl cyclase activity through the action of Gi/o proteins and, as a result, intracellular cyclic AMP (cAMP) decreases, while the A2A and A2B receptors increase cAMP production through the action of Gs proteins [6]. So, it is of therapeutic importance to synthesize new drugs active on adenosine receptors A1, A3, and, additionally, dual A1/A3 to study the synergistic effect between these two receptor subtypes as they preferentially couple to the same G proteins [2]. Indeed, there are many patents for adenosine receptor ligands, and some of these ligands are already in clinical trials or waiting for FDA approval [4,5,7]. For example, A1 ligands are useful for glaucoma, heart failure, angina, seizures, ischemia, depression, obesity, asthma, renal protection, edema associated with congestive heart failure, atrial arrhythmias, type II diabetes, neuropathic pain, neuroprotection, atrial fibrillation, tachycardia, cardioprotection, and sleep regulation [1,8,9,10,11,12]. In addition, A3 ligands are useful for liver regeneration, hepatitis, psoriasis, rheumatoid arthritis, inflammation, dry eye syndrome, fibrotic diseases, neurodegeneration, ischaemia, asthma, chronic obstructive pulmonary disease, glaucoma, and cancer [13,14,15,16,17,18,19,20], with A3 antagonist PBF-677 (Palobiofarma SL), that has reached clinical trials for glaucoma, ulcerative colitis, and eosinophilic esophagitis [21,22]. Finally, dual A1/A3 ligands can be useful as potential therapeutics for treating glaucoma, kidney failure, pulmonary diseases, and Alzheimer’s disease [23,24]. Despite the fact that several adenosine receptor ligands are already in clinical trials, achieving selectivity is still a challenge. Additionally, a number of side effects were reported for A1 antagonists in the literature, such as an increase in the frequency of seizures, strokes [25,26], dizziness, nausea, transient hypertension, and transient hypotension [27] in treated patients, which justifies the search for new high-affinity and selective drugs.
In our previous work, we synthesized and identified a new scaffold for adenosine receptor antagonists based on the adenine nucleus (Figure 1) [28]. Highly potent and selective compounds were identified as having a piperidinyl group in C-6, a proton in N-9, and several selected aryl groups in C-2 of the purine nucleus. Indeed, the structure-activity relationship (SAR) study indicated that an aryl unit at C-2 is crucial for activity, and a proton at N-9 increased potency. Furthermore, a piperidinyl group in C-6 instead of a 4-N-methylpiperazinyl group led to purines with higher or similar affinity for the adenosine receptors A1 and A3 but with higher selectivity.
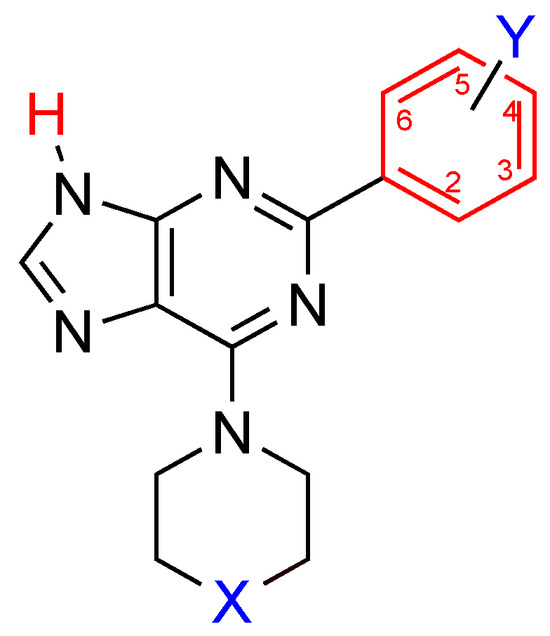
Figure 1.
The adenosine receptor antagonists scaffold identified in our previous work.
As an attempt to increase the affinity and selectivity of the compounds obtained in our previous work and to establish the importance of group X (Figure 1), herein, we synthesized new 6-morpholino purine derivatives substituted with a proton or a methyl group in N-9 and a selection of aryl substituents in the C-2 position of the purine nucleus. The results will allow us to complete the SAR study for the adenine-based scaffold identified in our previous publication.
2. Results and Discussion
2.1. Chemistry
The target compounds 3a–x were synthesized through the synthetic approach described in Scheme 1. The starting reagents 1 [29] and 2 [28,30] were obtained following the procedures described in previous work. Briefly, the commercially available diaminomaleonitrile was treated with triethyl orthoformate, under reflux. The solid obtained was reacted with the convenient amine followed by treatment with a base to generate imidazoles 1. The compounds 2 were obtained in excellent yield through reaction of 1 with the excess of morpholine, in acetonitrile, at room temperature.
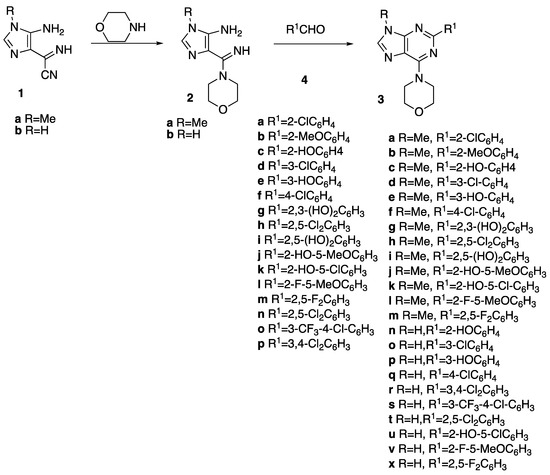
Scheme 1.
Synthetic approach to 2-aryl-adenine derivatives 3a–x.
The products 3 were obtained through reaction of compounds 2 with different aldehydes 4 following a previously described methodology [28,30]. The experimental conditions used in each reaction mainly depended on the aldehyde. Generally, when imidazoles 1 were reacted with non-phenolic aldehydes 4, the reactions were performed in basic medium. Considering that compounds 2 are temperature-sensitive, the reactions always started at low to moderate temperature. When the reactions were carried out under those conditions (Method A), they were slower, but the products 3 were precipitated pure from solution and isolated through simple filtration from the reaction medium. In order to accelerate the reactions, most of them started at moderate temperature, but, when the TLC showed the absence of imidazoles 2, the temperature was increased and the reactions were monitored by TLC until the spots assigned to the intermediates were absent. In these cases, the reactions led to black-greyish solids that required purification. The purification was achieved through filtration of a dichloromethane solution of the compounds through a silica gel column.
To obtain the phenolic derivatives, the reactions were performed in an acidic medium until complete consumption of the reagents (evidenced by TLC) and then continued in a basic medium at a temperature between 40 and 80 °C. In these reactions, degradation of the reaction mixture also occurred when higher temperatures were used. The pure products were obtained following the purification procedure described above for the other derivatives. The new compounds were fully characterized by usual techniques (1H and 13C NMR spectra are presented in the supporting information).
2.2. Pharmacology
All compounds synthesized in this work have a morpholine group as a substituent at C-6, an H atom or a methyl group at N-9, and different substituted aryl groups at C-2 of the purine nucleus. The compounds (3a–x) were assessed through radioligand binding assays at all human adenosine subtype receptors (A1, A2A, A2B, and A3) expressed in mammalian cell lines. The percentage of inhibition (%inhib) of radioligand binding was determined for all compounds at 10 µM concentration. Those compounds showing a %inhib higher than 80% were tested at different concentrations to determine their affinities (calculated as pKi) at the diverse receptors. Table 1 shows the binding affinities of all the synthesized compounds 3 at adenosine receptors A1, A2A, A2B, and A3.

Table 1.
Binding affinities of compounds 3 at all human adenosine receptors expressed as %inhib at 10 µM or pKi. Values are reported as mean ± SEM of three experiments with duplicate measurements.
Analysis of the data presented in the Table 1 shows that at 10 µM, several compounds presented a percentage inhibition higher than 80%. The pKi was determined, being in the range of 5.28 ≤ pKi ≤ 8.23 (3l, 3x). Both series, R = H and R = Me, gave ligands with high affinity to the four receptors subtypes; however, higher affinities are observed for derivatives with R = H (3d vs. 3h, 3c vs. 3n, 3e vs. 3p, 3k vs. 3u, 3l vs. 3v, and 3m vs. 3x). The higher potency of derivatives with a proton at N-9 agrees with the results published in our previous work [28], but the increase in potency of N-9-methyl derivatives having a morpholine unit at C-6 may indicate that the morpholine ring is interacting with the target through the oxygen atom. When we look for selectivity, we find selective ligands either having a methyl group at N-9 (3d, 3e, 3l and 3m) or a proton (3p, 3r, 3s, 3t and 3v). Additionally, the ligands with a methyl group at N-9 show high potency mainly for the A1 receptor (3c, 3d, 3e, 3i, 3j, 3k, and 3m), and ligands with a proton at N-9 presented high potency for both the A1 and A3 receptors (3n–3x). To evaluate the influence of the R1 group on potency and selectivity against the four receptors, we analyzed first the R1 = chloro-derivatives, compounds 3a, 3d, 3f, 3h, 3o, 3q, 3r, 3s, and 3t.
In this set, the most potent and selective compound for the A1 receptor was 3d, with R1 = 3-ClC6H4 [pKi (A1) = 6.80 ± 0.07]. Compounds 3r (R1 = 3,4-Cl2C6H3) and 3s (R1 = 3-CF3-4-ClC6H3) were highly potent and selective for the A3 receptor. Compound 3q (R1 = 4-ClC6H4) showed dual selectivity for A1/A3 receptors. These results seem to indicate that the presence of an electron-withdrawing chlorine atom at the meta position together with a hydrophobic methyl group at N-9 interacts efficiently with the A1 receptor (3d). However, when the hydrophilic hydrogen was at N-9, a promiscuous ligand resulted (3o). The presence of a chlorine atom at the para position and a proton at N-9 interacts efficiently with receptors A1 and A3 (3q). Compound 3q is a potential dual ligand for A1/A3 receptors with therapeutic interest, as A1 and A3 receptors, principally coupled to Gi/o proteins, can simultaneously inhibit the adenylyl cyclase activity [6,31,32].
In addition, selectivity was achieved when a second chlorine or a CF3 group was simultaneously at the meta position (3r, 3s), suggesting volume constraints at the A1, A2A, and A2B pockets interacting with the ligands’ meta position. This hypothesis is also supported by the lower pKi observed for compound 3s (R1 = 3-CF3-4-Cl-C6H3; pKi = 6.02 ± 0.04) when compared to the pKi of compound 3r (R1 = 3,4-Cl2C6H3; pKi = 7.15 ± 0.08).
The sub-set of derivatives with hydroxyl groups at ortho or meta positions of the R1 group (3c, 3e, 3g, 3i, 3j, 3k, 3n, 3p, and 3u) will be analyzed. Generally, a hydroxyl group at ortho or meta positions of R1 led to very potent but not selective ligands (3c, 3i, 3j, 3k, 3n, and 3u), except compounds 3e and 3p, which have a hydroxyl group at the meta position of R1 and showed selectivity for the A1 receptor. Derivative 3d (R1 = 3-ClC6H4) also showed selectivity for the A1 receptor. These results suggest that an electron-withdrawing group produced through the inductive effect at the meta position of R1 interacts efficiently with a polar moiety at the A1 receptor. Comparing the affinity values between 3b (R1 = 2-MeOC6H4) and 3c (R1 = 2-HOC6H4), the increase of affinity of 3c suggests that a hydrogen-bond donor (HBD) group at the ortho position of R1 is important for the interaction with the adenosine receptors A1, A2A, and A2B. However, the possibility of a hydrogen-bond-acceptor (HBA) interaction of the ligand with the target should also be considered. Hydroxyl and methoxyl groups may interact that way with the targets; however, if the receptor pockets have volume constraints, this will explain the lack of activity of derivative 3b and support the hypothesis of HBD interaction. Furthermore, compound 3c has almost the same submicromolar affinity for the A1 and A2A receptors. This result indicates that 3c is a dual ligand for A1/A2A receptors. Dual affinity for the A1/A2A receptors was also observed for ligand 2k. These ligands could have medicinal interest, as compounds with dual affinity at A1/A2A receptors have shown therapeutic efficacy in animal models of Parkinson’s disease [33,34]. On the other hand, compound 3u showed a dual affinity for receptors A1/A2B. A comparison of affinities registered for 3c, 3d, and 3e with 3n, 3o, and 3p suggests more hydrophilic or small pockets at receptors to accommodate the group at N-9 of the ligands. Additionally, compound 3e (R1 = 3-HOC6H4) is a very potent and selective ligand for the A1 receptor with pKi = 6.16 ± 0.21, while compound 3g (R1 = 2,3-(HO)2C6H3) has a low affinity for all of the receptors. This may indicate that an intramolecular hydrogen bond between the hydroxyl groups of the ligand precludes the interaction with the target, leading to low activity.
Finally, the fluoro derivatives (3l, 3m, 3v, and 3x) presented high potency and selectivity for receptors A1 and A3, except compound 3x, which showed no selectivity. Compounds 3l and 3v with R1 = 2-F-5-MeOC6H3 were selective to the A3 receptor, with pKi = 5.28 ± 0.11 and pKi = 7.83 ± 0.16, respectively. Compound 3v also showed a high affinity for receptor A2B (pKi = 6.40 ± 0.16). These results suggest volume restrictions in the pockets of receptors A2B and A3 to lodge the methyl group at N-9 of the ligand, with the available space in receptor A3 bigger than in receptor A2B. Compound 3m showed high affinity and selectivity for the A1 receptor, while compound 3x showed higher potency for all receptors but no selectivity. These results also support the hypothesis that there is limited space in the receptors’ pockets to accommodate bulky groups at N-9. The difference in the selectivity of compounds 3l and 3m may indicate that receptor A3 has a hydrophobic pocket to accommodate the substituent MeO present at the meta position of the ligand, while receptor A1 does not. This hypothesis is also supported by the results obtained for ligands 3v and 3x. Figure 2 represents a SAR model that summarizes the results.
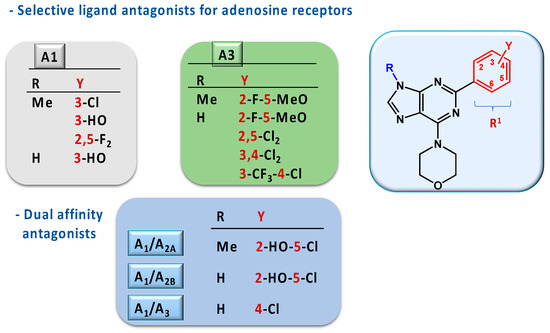
Figure 2.
Structure-activity relationship of 2-aryl-9-substituted-6-morpholino purine derivatives for the adenosine receptor antagonists.
The most potent compounds for A1 (3x; pKi = 8.23 ± 0.06; Figure 3a) and A3 (3v; pKi = 7.83 ± 0.16) receptors were selected and tested in intracellular cAMP tests to study their functional activity. The agonist/antagonist behavior of the two compounds selected was determined by assessing their effect on the modulation of cAMP levels by the receptor agonist NECA. Figure 3b shows the result of a representative test for the antagonist potency of compound 3x at A1 receptor. The results confirmed that the compounds 3v and 3x are antagonists of the A3 and A1 receptors, and their antagonist potencies, expressed as pKB values, were 8.24 ± 0.11 and 8.25 ± 0.16, respectively (Table 2). Based on their structural similarity, we can generalize that all compounds of this work are antagonists of adenosine receptors.
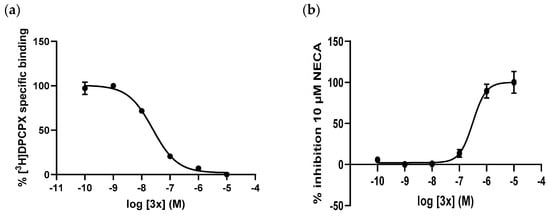
Figure 3.
(a) Competition binding curve for compound 3x at A1 receptors. (b) Concentration-response curve of compound 3x in the presence of 10 µM of NECA at human A1 receptor expressed in CHO cells. Points represent the mean ± standard deviation (vertical bars) of duplicate measurements.

Table 2.
Antagonist potency (pKB) of the most potent compounds at human A1 and A3 adenosine receptors in cAMP tests. Values are reported as mean ± SEM of three experiments with duplicate measurements.
In this work, we synthesized highly potent and selective hits based on the scaffold for adenosine receptor antagonists identified in our previous publication [28]. These results indicate that the purines substituted with a morpholine group at C-6, a hydrogen atom or a methyl group at N-9, and a specific aryl group at C-2 of purine nucleus, gave high affinity and selective antagonists for adenosine A1 and A3 receptors, as well as dual antagonists for receptors A1/A2A, A1/A2B, and A1/A3. In addition, four compounds (3i, 3j, 3n, and 3o) showed high affinity, although they were not selective for any specific adenosine receptor subtype.
2.3. Docking Studies
Compound 3x was computationally docked to the orthosteric site of the A1 receptor using a crystal structure representing an inactive conformation (PDB accession code: 5UEN) [35]. The ligand was predicted to form hydrogen bonds with Asn254 and π-π stacking with Phe171, which are interactions characteristic of adenosine receptor antagonists (Figure 4) [36]. The predicted binding mode of compound 3x in the A1 receptor also explained observed SARs. For example, replacing the fluorine at the 5-position of the phenyl ring with a methoxy group led to a substantial loss of affinity at the A1 receptor (pKi < 5, compound 3v). Consistent with this observation, increasing the size of this substituent in our model of the complex led to clashes with Asn184 and His251.
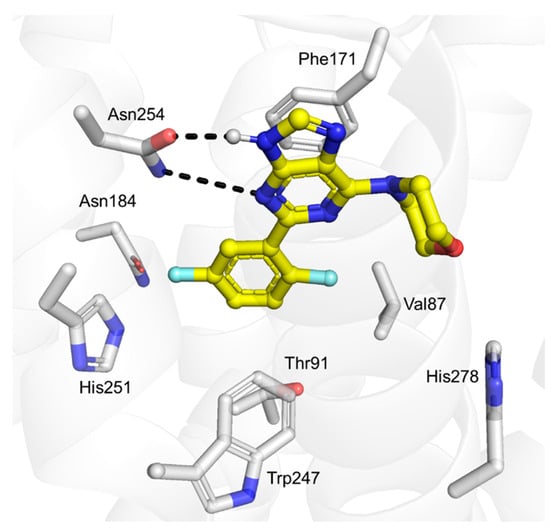
Figure 4.
Predicted binding mode of compound 3x in the orthosteric site of the A1 receptor. The receptor is depicted as a grey cartoon, with the ligand and key side chains represented as sticks. Hydrogen bonds are shown as black dashed lines.
3. Experimental Protocols
3.1. Chemistry
The imidazoles 1 used in this work were synthesized according to previously described procedures [29], and compounds 2 were synthesized according to the procedure described in [28,30]. Solvents and other commercially available chemicals were used as shipped. The melting points (m.p.) were determined with a Gallenkamp melting point apparatus, and they are uncorrected. The reactions were followed by thin layer chromatography (TLC) using Silica Gel 60 F254 (Merck, Darmstadt, Germany) plates with detection by UV light. The NMR spectra were obtained on a Varian Unity Plus (1H: 300 MHz, 13C: 75 MHz) or on a Bruker Avance III NMR spectrometer (1H: 400 MHz, 13C: 100 MHz) including the 1H and 13C bidimensional correlation spectra (HMQC and HMBC). For solutions, [D6]-DMSO residual [D6]-DMSO (δH = 2.49 ppm) or [D6]-DMSO (δC = 39.5 ppm) was used as the internal standard at 298 K. Chemical shifts (δ) were reported in parts per million (ppm) and the coupling constants, J, were presented in hertz (Hz). The purity of all tested compounds was higher than 95% according to elemental analysis, which was reported to be within 0.4% of the calculated values. The IR spectra were recorded with a FT-IR Bomem MB 104 using nujol mulls and NaCl cells. Elemental analyses were performed with a LECO CHNS-932 instrument.
3.2. General Procedure for the Synthesis of 3a–x
Method A: To a suspension of imidazole 2 in ethanol, an ethanol/acetonitrile mixture, or DMSO, the aldehyde 4 (1.1–1.5 equivalents) was added, followed by triethylamine (10 equivalents). The reaction was kept at room to moderate temperature and followed by TLC. Otherwise, when the TLC indicated the absence of the starting reagent but the presence of intermediates, the reaction continued at a higher temperature. When the TLC showed the absence of intermediate spots, the solvent was removed in the rotary evaporator, and an off-white solid was isolated after the addition of a small amount of ethanol to the residue. When the residue showed a dark color, it was dissolved in DCM. The solution was filtered through a column of silica gel (0.5 cm high), and the column was washed with an extra 30 mL of DCM. The resulting solution was concentrated until dry, diethyl ether was added to the oil, and an off-white solid was precipitated. The solid was filtered and washed with cold diethyl ether.
Method B: To a suspension of imidazole 2 in ethanol, the aldehyde 4 (1.1–1.5 equivalents) was added, followed by trifluoroacetic acid (1.3–2.0 equivalents). The reaction was kept at 22 °C under a magnetic stirrer until the TLC showed consumption of starting reagent 2. The solvent was eliminated in the rotary evaporator, dimethyl sulfoxide and/or ethanol was added, followed by triethylamine (10 equivalents), and the reaction continued at 40–80 °C. When the TLC indicated the end of the reaction, the solvents were removed in the rotary evaporator, and the product was precipitated through the addition of water. The isolated solid was dissolved in a mixture of DCM/THF/EtOH and purified through dry flash chromatography using DCM as the elution solvent.
3.2.1. 4-(2-(2-Chlorophenyl)-9-methyl-9H-purin-6-yl)morpholine 3a
Method A: Compound 2a (0.18 g, 0.89 mmol) in EtOH, aldehyde 4a (1.1 eq.), and Et3N (10 eq.) was kept at 22 °C for 20 days. Product 3a was isolated as an off-white solid (0.12 g, 0.28 mmol, 31%); m.p. = 99–101 °C; Found: C, 58.28; H, 4.86; N, 21.25. C16H16ClN5O requires C, 58.27; H, 4.89; N, 21.24; IR (Nujol mull) νmax: 1584 cm−1; 1H (300 MHz, DMSO-d6) δ: 8.21 (s, 1H), 7.75–7.72 (m, 1H), 7.53–7.50 (m, 1H), 7.45–7.38 (m, 2H), 4.23 (br s, 4H), 3.75 (s, 3H), 3.71 (t, J = 4.5 Hz, 4H); 13C (75 MHz, DMSO-d6) δ: 157.6, 152.7, 151.6, 141.4, 138.7, 131.6, 131.5, 130.1, 129.9, 126.8, 117.7, 66.2, 45.2, 29.6.
3.2.2. 4-(2-(2-Methoxyphenyl)-9-methyl-9H-purin-6-yl)morpholine 3b
Method A: Compound 2a (0.13 g, 0.64 mmol) in DMSO, aldehyde 4b (1.1 eq.), and Et3N (10 eq.) was kept at 60 °C for 6 days. Product 3b was isolated as an off-white solid (0.05 g, 0.23 mmol, 36%); m.p. = 114–116 °C; Found: C, 62.10; H, 5.92; N, 21.15. C17H19N5O2.0.2H2O requires C, 62.08; H, 5.90; N, 21.30; IR (Nujol mull) νmax: 1698, 1587, 1577 cm−1; 1H (300 MHz, DMSO-d6) δ: 8.15 (s, 1H), 7.50 (dd, J = 7.7 Hz, J = 1.8 Hz, 1H), 7.37 (td, J = 7.8 Hz, J = 1.8 Hz, 1H), 7.09 (d, J = 7.8 Hz, 1H), 6.99 (t, J = 7.8 Hz, 1H), 4.20 (br s, 4H), 3.80–3.50 (m, 10H). 13C (75 MHz, DMSO-d6) δ: 158.4, 157.2, 152.8, 151.7, 149.9, 130.6, 130.1, 129.7, 120.0, 112.5, 66.2, 55.9, 45.2, 29.5.
3.2.3. 2-(9-Methyl-6-morpholino-9H-purin-2-yl)phenol 3c
Method A: Compound 2a (0.08 g, 0.38 mmol) in a mixture of EtOH/CH3CN (1/1) and aldehyde 4c (1.5 eq.) was kept at 11 °C for 17 days. Product 3c was isolated as an off-white solid (0.08 g, 0.25 mmol, 66%); m.p. = 210–212 °C (Found: C, 61.17; H, 5.52; N, 21.00. C16H17N5O2.0.1H2O requires C, 61.45; H, 5.49; N, 22.40); IR (Nujol mull) νmax: 3097, 1591, 1530, 1519 cm−1; 1H (300 MHz, DMSO-d6) δ: 13.52 (s, 1H, OH), 8.38 (dd, J = 8.4 Hz, J = 1.8 Hz, 1H), 8.21 (s, 1H), 7.33 (td, J = 7.8 Hz, J = 1.8 Hz, 1H), 6.91 (t, J = 7.8 Hz, 1H), 6.89 (d, J = 7.8 Hz, 1H), 4.25 (br s, 4H), 3.78 (s, 4H), 3.77 (s, 3H); 13C (75 MHz, DMSO-d6) δ: 159.4, 157.5, 152.4, 149.7, 141.4, 132.0, 129.0, 119.3, 118.6, 117.8, 117.2, 66.1, 45.4, 29.6.
3.2.4. 4-(2-(3-Chlorophenyl)-9-methyl-9H-purin-6-yl)morpholine 3d
Method A: Compound 2a (0.31 g, 1.46 mmol) in EtOH, aldehyde 4d (1.1 eq.), and Et3N (10 eq.) was kept at 20 °C for 12 days and then at 70 °C for 21 h. The product 3d was isolated as an off-white solid (0.35 g, 1.06 mmol, 73%); m.p. = 160–162 °C (Found: C, 58.04; H, 4.76; N, 21.18. C16H16ClN5O requires C, 58.27; H, 4.89; N, 21.24); IR (Nujol mull) νmax: 1690, 1575 cm−1; 1H (300 MHz, DMSO-d6) δ: 8.31 (m, 2H), 8.17 (s, 1H), 7.48–7.50 (m, 2H), 4.26 (br s, 4H), 3.76 (br s, 4H), 3.73 (s, 3H); 13C (75 MHz, DMSO-d6) δ: 155.3, 153.0, 151.9, 141.5, 140.4, 133.2, 130.2, 129.6, 127.1, 126.3, 118.4, 66.2, 45.3, 29.5.
3.2.5. 3-(9-Methyl-6-morpholino-9H-purin-2-yl)phenol 3e
Method B: Compound 2b (0.22 g, 1.10 mmol) in EtOH, aldehyde 4e (1.1 eq.), and TFA (2 eq.) was kept at 22 °C for 3 days. Then, the reaction continued in DMSO and Et3N (10 eq.) at 60 °C for 2 days. The product 3e was isolated as an off-white solid (0.23 g, 0.75 mmol, 67%); m.p. = 230–232 °C (Found: C, 61.67; H, 5.52; N, 22.64. C16H17N5O2 requires C, 61.72; H, 5.50; N, 22.49); IR (Nujol mull) νmax: 3281, 1578, 1505 cm−1; 1H (300 MHz, DMSO-d6) δ: 9.45 (s, 1H, OH), 8.16 (s, 1H), 7.84 (br.s, 2H), 7.24 (t, J = 8.4 Hz, 1H), 6.82 (d, J = 7.8 Hz, 1H), 4.28 (br s, 4H), 3.77 (s, 3H), 3.75 (m, 4H); 13C (75 MHz, DMSO-d6) δ: 157.3, 156.8, 152.9, 152.0, 141.1, 139.7, 129.1, 118.6, 118.1, 116.7, 114.5, 66.2, 45.1, 29.4.
3.2.6. 4-(2-(4-Chlorophenyl)-9-methyl-9H-purin-6-yl)morpholine 3f
Method A: Compound 2a (0.34 g, 1.57 mmol) in EtOH, aldehyde 4f (1.1 eq.), and Et3N (10 eq.) was kept at 21 °C for 8 days and then at 70 °C for 6 h. The product 3f was isolated as an off-white solid (0.25 g, 0.75 mmol, 48%); m.p. = 226–228 °C (Found: C, 57.99; H, 4.86; N, 21.13. C16H16ClN5O requires: C, 58.27; H, 4.89; N, 21.24); IR (Nujol mull) νmax: 1706, 1587, 1571 cm−1; 1H (300 MHz, DMSO-d6) δ: 8.40 (d, J = 8.4 Hz, 2H), 8.17 (s, 1H), 7.52 (d, J = 8.4 Hz, 2H), 4.28 (br s, 4H), 3.79 (s, 3H), 3.74 (m, 4H); 13C (75 MHz, DMSO-d6) δ: 155.7, 152.9, 151.0, 141.4, 137.1, 134.5, 129.4, 128.3, 118.2, 66.2, 45.1, 29.5.
3.2.7. 3-(9-Methyl-6-morpholino-9H-purin-2-yl)benzene-1,2-diol 3g
Method B: Compound 2a (0.15 g, 0.69 mmol) in EtOH, aldehyde 4g (1.1 eq.), and TFA (2 eq.) was kept at 22 °C for 3 days. Then, the reaction continued in a mixture of DMSO/EtOH (0.1/1) and Et3N (10 eq.) at 80 °C for 6 h. The product 3g was isolated as an off-white solid (0.14 g, 0.43 mmol, 62%); m.p. = 254–256 °C (Found: C, 58.87; H, 5.55; N, 21.02. C16H17N5O3 C16H17N5O3 requires C, 58.71; H, 5.23; N, 21.39); IR (Nujol mull) νmax: 3415, 1588, 1569 cm−1; 1H (300 MHz, DMSO-d6) δ: 13.73 (s, 1H, OH), 8.83 (br s, 1H, OH), 8.19 (s, 1H), 7.83 (dd, J = 8.0 Hz, J = 1.5 Hz, 1H), 6.84 (dd, J = 7.5 Hz, J = 1.5 Hz, 1H), 6.70 (t, J = 7.8 Hz, 1H), 4.23 (br s, 4H), 3.77 t, J = 5.4 Hz, 7H); 13C (75 MHz, DMSO-d6) δ: 157.9, 152.3, 149.8, 148.3, 146.1, 141.3, 119.5, 119.1, 117.9, 117.6, 117.3, 66.0, 45.4.
3.2.8. 4-(2-(2,5-Dichlorophenyl)-9-methyl-9H-purin-6-yl)morpholine 3h
Method A: Compound 2a (0.25 g, 1.22 mmol) in EtOH, aldehyde 4h (1.1 eq.), and Et3N (10 eq.) was kept at 40 °C for 4 days and then at 50 °C for 10 days. The product 3h was isolated as an off-white solid (0.18 g, 0.50 mmol, 41%); m.p. = 141–142 °C (Found: C, 52.64; H, 4.11; N, 19.45. C16H15Cl2N5O requires C, 52.76; H, 4.15; N, 19.23); IR (Nujol mull) νmax: 3048, 1585 cm−1; 1H (300 MHz, DMSO-d6) δ: 8.22 (s, 1H), 7.78(d, J = 2.7 Hz, 1H), 7.56 (d, J = 8.4 Hz, 1H), 7.50 (dd, J = 8.4 Hz, J = 2.4 Hz, 1H), 4.23 (br s, 4H), 3.76 (s, 3H), 3.72 (t, J = 4.5 Hz, 4H); 13C (75 MHz, DMSO-d6) δ: 156.3, 152.7, 151.5, 141.6, 140.0, 131.9, 131.4, 130.9, 130.5, 129.7, 117.9, 66.2, 45.2, 29.6.
3.2.9. 2-(9-Methyl-6-morpholino-9H-purin-2-yl)benzene-1,4-diol 3i
Method B: Compound 2a (0.20 g, 0.96 mmol) in EtOH, aldehyde 4i (1.1 eq.), and TFA (2 eq.) was kept at 22 °C for 5 h. Then, the reaction continued in a mixture of DMSO/EtOH (0.1/1) and Et3N (10 eq.) at 60 °C for 3 days. The product 3i was isolated as an off-white solid (0.20 g, 0.61 mmol, 64%); m.p. = 288–290 °C (Found: C, 57.21; H, 5.09; N, 20.61. C16H17N5O3.0.5H2O requires C, 57.14; H, 5.36; N, 20.83); IR (Nujol mull) νmax: 3353, 1574, 1500 cm−1; 1H (300 MHz, DMSO-d6) δ: 12.81 (s, 1H, OH), 8.88 (s, 1H, OH), 8.20 (s, 1H), 7.80 (d, J = 2.7 Hz, 1H), 6.77 (dd, J = 8.6 Hz, J = 2.7 Hz, 1H), 6.72 (d, J = 8.7 Hz, 1H), 4.25 (br s, 4H), 3.77 (t, J = 3.6 Hz, 7H); 13C (75 MHz, DMSO-d6) δ: 157.6, 152.4, 152.2, 149.9, 149.2, 141.4, 119.8, 119.3, 117.7 (2C), 114.1, 66.1, 45.4, 29.7.
3.2.10. 4-Methoxy-2-(9-methyl-6-morpholino-9H-purin-2-yl)phenol 3j
Method B: Compound 2a (0.15 g, 0.70 mmol) in EtOH, aldehyde 4j (1.2 eq.), and TFA (2 eq.) was kept at 22 °C for 2 days. Then, the reaction continued in EtOH and Et3N (10 eq.) at 25 °C for 5 days. The product 3j was isolated as an off-white solid (0.14 g, 0.43 mmol, 62%); m.p. = 146–148 °C (Found: C, 59.75; H, 5.67; N, 20.42. C17H19N5O3 requires C, 59.81; H, 5.61; N, 20.52); IR (Nujol mull) νmax: 3011, 1594, 1578 cm−1; 1H (300 MHz, DMSO-d6) δ: 12.99 (s, 1H, OH), 8.20 (s, 1H), 7.89 (d, J = 3.2 Hz, 1H), 6.97 (dd, J = 8.8 Hz, J = 3.2 Hz, 1H), 6.83 (d, J = 8.8 Hz, 1H), 4.23 (s, 4H), 377 (m, 7H); 13C (75 MHz, DMSO-d6) δ: 157.2, 153.4, 152.4, 151.5, 149.8, 141.4, 119.3, 118.7, 117.9, 117.8, 112.6, 66.0, 55.4, 45.4, 29.6.
3.2.11. 4-Chloro-2-(9-methyl-6-morpholino-9H-purin-2-yl)phenol 3k
Method B: Compound 2a (0.13 g, 0.64 mmol) in EtOH, aldehyde 4k (1.1 eq.), and TFA (2 eq.) was kept at 22 °C for 2 days. Then, the reaction continued in a mixture of DMSO/EtOH (0.2/1) and Et3N (10 eq.) at 60 °C for 6 days. The product 3k was isolated as an off-white solid (0.104 g, 0.41 mmol, 64%); m.p. = 179–180 °C (Found: C, 55.65; H, 4.50; N, 20.00. C16H16ClN5O2 requires C, 55.58; H, 4.66; N, 20.25); IR (Nujol mull) νmax: 3016, 1589, 1528 cm−1; 1H (300 MHz, DMSO-d6) δ: 13.59 (s, 1H, OH), 8.24 (d, J = 2.8 Hz, 1H), 8.20 (s, 1H), 7.32 (dd, J = 8.8 Hz, J = 2.8 Hz, 1H), 6.90 (d, J = 8.8 Hz, 1H), 4.20 (s, 4H), 3.77 (t, J = 4.8 Hz, 7H); 13C (75 MHz, DMSO-d6) δ: 158.0, 156.0, 152.2, 149.5, 141.5, 131.4, 127.7, 122.2, 120.5, 119.1, 118.0, 66.0, 45.3, 29.6.
3.2.12. 4-(2-(2-Fluoro-5-methoxyphenyl)-9-methyl-9H-purin-6-yl)morpholine 3l
Method A: Compound 2a (0.16 g, 0.77 mmol) in EtOH, aldehyde 4l (1.3 eq.), and Et3N (10 eq.) was kept at 22 °C for 1 day and then at 60 °C for 4 days. The product 3l was isolated as an off-white solid (0.15 g, 0.44mmol, 57%); m.p. = 102–104 °C (Found: C, 58.76; H, 4.93; N, 19.99. C17H18FN5O2·0.2H2O requires C, 58.86; H, 5.31; N, 20.19); IR (Nujol mull) νmax: 3122, 1598, 1579 cm−1; 1H (300 MHz, DMSO-d6) δ: 8.19 (s, 1H), 7.52 (dd, J = 3.2 Hz, J = 6.4 Hz, 1H), 7.18 (dd, J = 8.0 Hz, J = 6.9 Hz, 1H), 7.01 (dt, J = 6.9 Hz, J = 2.4 Hz, 1H), 4.23 (br s, 4H), 3.80–3.70 (m, 10H). 13C (75 MHz, DMSO-d6) δ: 155.2 (d, J = 4 Hz), 154.9 (d, J = 2 Hz), 154.7 (d, J = 244 Hz), 153.4, 151.7, 141.4, 127.7 (d, J = 11 Hz), 117.8, 117.3 (d, J = 24 Hz), 115.9 (d, J = 2 Hz), 115.8 (d, J = 8 Hz), 66.2, 55.6, 45.2, 29.5.
3.2.13. 4-(2-(2,5-Difluorophenyl)-9-methyl-9H-purin-6-yl)morpholine 3m
Method A: Compound 2a (0.31 g, 1.48 mmol) in EtOH, aldehyde 4m (1.1 eq.), and Et3N (10 eq.) was kept at 40 °C for 4 days and then at 50 °C for 6 days. The product 3m was isolated as an off-white solid (0.30 g, 0.91 mmol, 61%); m.p. = 143–145 °C (Found: C, 57.90; H, 4.77; N, 20.97. C16H15F2N5O requires C, 58.00; H, 4.56; N, 21.14); IR (Nujol mull) νmax: 3083, 1616, 1578 cm−1; 1H (400 MHz, DMSO-d6) δ: 8.20 (s, 1H), 7.80–7.79 (m, 1H), 7.34–7.30 (m, 1H), 4.24 (br s, 4H), 3.77 (s, 3H), 3.73 (t, J = 4.5 Hz, 4H); 13C (100 MHz, DMSO-d6) δ: 157.7 (dd, J = 230 Hz, J = 6 Hz), 156.5 (dd, J = 246 Hz, J = 4 Hz), 154.2 (dd, J = 5 Hz, J = 2 Hz), 152.9, 151.6, 141.6, 128.5 (dd, J = 11 Hz, J = 4 Hz), 118.4 (dd, J = 25 Hz, J = 9 Hz), 118.0, 117.5 (dd, J = 24 Hz, J = 9 Hz), 128.3 (dd, J = 25 Hz, J = 2 Hz), 66.2, 45.2, 29.5.
3.2.14. 2-(6-Morpholino-9H-purin-2-yl)phenol 3n
Method B: Compound 2b (0.30 g, 1.55 mmol) in EtOH, aldehyde 4c (1.1 eq.), and TFA (1.5 eq.) was kept at 22 °C for 4.5 h. Then, the reaction continued in DMSO and Et3N (10 eq.) at 40 °C for 10 days. The product 3n was isolated as an off-white solid (0.15 g, 0.52 mmol, 34%); (Found: C, 60.43; H, 5.12; N, 23.71. C15H15N5O2 requires C, 60.60; H, 5.09; N, 23.56); 1H (400 MHz, DMSO-d6) δ: 13.64 (s, 1H), 13.23 (br s, 1H), 8.35 (dd, J = 8.4 Hz, J = 2.0 Hz, 1H), 8.20 (s, 1H), 7.31 (dt, J = 8.4 Hz, J = 2.0 Hz, 1H), 6.90 (m, 2H), 4.28 (br s, 4H), 3.78 (m, 4H); 13C (100 MHz, DMSO-d6) δ: 159.4, 157.5, 152.5, 149.9, 139.0, 131.8, 128.9, 119.4, 118.6, 117.6, 117.2, 66.1, 45.5.
3.2.15. 4-(2-(3-Chlorophenyl)-9H-purin-6-yl)morpholine 3o
Method A: Compound 2b (0.14 g, 0.72 mmol) in EtOH, aldehyde 4d (1.1 eq.), and Et3N (10 eq.) was kept at 40 °C for 10 days. The product 3o was isolated as an off-white solid (0.10 g, 0.32 mmol, 44%); m.p. = 265–267 °C (Found: C, 56.21; H, 4.26; N, 21.66. C15H14ClN5O.0.3H2O requires C, 56.10; H, 4.55; N, 21.81); IR (Nujol mull) νmax: 3103, 1587, 1571 cm−1; 1H (300 MHz, DMSO-d6) δ: 13.16 (s, 1H, NH), 8.18 (s, 1H), 8.55–7.48 (m, 2H), 8.32–8.28 (m, 2H), 4.29 (br s, 4H), 3.76 (t, J = 4.8 Hz, 4H); 13C (75 MHz, DMSO-d6) δ: 155.2, 152.9, 152.7, 140.7, 139.4, 133.1, 130.2, 129.3, 127.0, 126.1, 118.3, 66.2, 45.1.
3.2.16. 3-(6-Morpholino-9H-purin-2-yl)phenol 3p
Method B: Compound 2a (0.31 g, 1.58 mmol) in EtOH, aldehyde 4e (1.1 eq.), and TFA (1.5 eq.) was kept at 22 °C for 7 days. Then, the reaction continued in DMSO and Et3N (10 eq.) at 50 °C for 1 month. The product 3p was isolated as an off-white solid (0.24 g, 0.81 mmol, 51%); (Found: C, 60.53; H, 5.02; N, 23.52. C15H15N5O2 requires C, 60.60; H, 5.09; N, 23.56); 1H (300 MHz, DMSO-d6) δ: 13.07 (br s, 1H), 9.45 (br s, 1H), 8.13 (s, 1H), 7.77–7.79 (m, 2H), 7.23 (t, J = 7.8 Hz, 1H), 6.82 (ddd, J = 7.8 Hz, J = 3.3 Hz, J = 0.9 Hz, 1H), 4.28 (br s, 4H), 3.76 (m, 4H); 13C (75 MHz, DMSO-d6 δ: 157.3, 156.9, 152.9, 152.6, 139.9, 138.8, 129.2, 118.5, 117.9, 116.6, 114.4, 66.3, 45.1.
3.2.17. 4-(2-(4-Chlorophenyl)-9H-purin-6-yl)morpholine 3q
Method A: Compound 2b (0.31 g, 1.58 mmol) in EtOH, aldehyde 4f (1.1 eq.), and Et3N (10 eq.) was kept at 20 °C for 13 days and then at 50 °C for 21 h. The product 3q was isolated as an off-white solid (0.15 g, 0.47 mmol, 30%); m.p. = 284 °C (dec.) (Found: C, 57.00; H, 4.51; N, 22.05. C15H14ClN5O requires C, 57.06; H, 4.47; N, 22.18); IR (Nujol mull) νmax: 1583 cm−1; 1H (300 MHz, DMSO-d6) δ: 13.13 (s, 1H), 8.34 (s, 1H), 8.16 (d, J = 9.0 Hz, 2H), 7.50 (d, J = 9.0 Hz, 2H), 4.28 (br s, 4H), 3.75 (m, 4H); 13C (75 MHz, DMSO-d6) δ: 155.8, 153.0, 152.5, 139.0, 137.3, 134.4, 129.3, 128.3, 118.1, 66.2, 45.3.
3.2.18. 4-(2-(3,4-Dichlorophenyl)-9H-purin-6-yl)morpholine 3r
Method A: Compound 2b (0.31 g, 1.57 mmol) in EtOH, aldehyde 4p (1.1 eq.), and Et3N (10 eq.) was kept at 40 °C for 2 days and then at 50 °C for 6 days. The product 3t was isolated as an off-white solid (0.18 g, 0.51 mmol, 33%); m.p. > 300 °C (Found: C, 51.28; H, 3.91; N, 19.93. C15H13Cl2N5O requires C, 51.45; H, 3.74; N, 20.00); IR (Nujol mull) νmax: 3010, 1606, 1584, 1561 cm−1; 1H (300 MHz, DMSO-d6) δ: 13.05 (br s, 1H), 8.45 (d, J = 2.1 Hz, 1H), 8.29 (dd, J = 8.7 Hz, J = 2.1 Hz, 1H), 8.20 (s, 1H), 6.90 (d, J = 8.7 Hz, 1H), 4.29 (br s, 4H), 3.76 (t, J = 4.8 Hz, 4H); 13C (75 MHz, DMSO-d6) δ: 154.5, 152.9, 139.3, 139.1, 132.2, 131.2, 130.6, 128.9, 127.5, 118.2, 66.2, 45.2.
3.2.19. 4-(2-(4-Chloro-3-(trifluoromethyl)phenyl)-9H-purin-6-yl)morpholine 3s
Method A: Compound 2b (0.27 g, 1.38 mmol) in EtOH, aldehyde 4o (1.1 eq.), and Et3N (10 eq.) was kept at 22 °C for 7 days and then at 40 °C for 5 days. The product 3u was isolated as an off-white solid (0.28 g, 0.76 mmol, 55%); m.p. = 248–250 °C (Found: C, 50.00; H, 3.53; N, 18.28. C16H13ClF3N5O requires C, 50.00; H, 3.39; N, 18.25); IR (Nujol mull) νmax: 3191, 1587, 1575 cm−1; 1H (300 MHz, DMSO-d6) δ: 13.24 (s, 1H, NH), 8.72 (d, J = 2.1 Hz, 1H), 8.60 (dd, J = 8.4 Hz, J = 2.1 Hz, 1H), 8.21 (s, 1H), 7.82 (d, J = 8.4 Hz, 1H), 4.30 (br s, 4H), 3.76 (t, J = 4.5 Hz, 4H); 13C (75 MHz, DMSO-d6) δ: 156.3, 152.9, 152.4, 139.3, 137.9, 132.6, 131.8, 131.6 (q, J = 1Hz), 126.6 (q, J = 1Hz), 126.6 (q, J = 31 Hz), 126.0 (q, J = 6 Hz), 122.9 (q, J = 271 Hz), 118.3, 66.2, 45.1.
3.2.20. 4-(2-(2,5-Dichlorophenyl)-9H-purin-6-yl)morpholine 3t
Method A: Compound 2b (0.28 g, 1.45 mmol) in EtOH, aldehyde 4h (1.1 eq.), and Et3N (10 eq.) was kept at 22 °C for 6 days and then at 40 °C for 6 days. The product 3r was isolated as an off-white solid (0.21 g, 0.60 mmol, 41%); m.p. = 259–261 °C (Found: C, 51.26; H, 3.69; N, 19.98. C15H13Cl2N5O requires C, 51.45; H, 3.74; N, 20.00); IR (Nujol mull) νmax: 3090, 1602, 1586, 1561 cm−1; 1H (300 MHz, DMSO-d6) δ: 13.23 (br s, 1H, NH), 8.22 (s, 1H), 7.76 (d, J = 2.1 Hz, 1H), 7.57 (d, J = 8.7 Hz, 1H), 7.50 (dd, J = 8.7 Hz, J = 2.1 Hz, 1H), 4.24 (br s, 4H), 3.72 (t, J = 4.5 Hz, 4H); 13C (75 MHz, DMSO-d6) δ: 156.3, 152.7, 152.0, 140.2, 139.3, 131.9, 131.4, 131.0, 130.4, 129.6, 117.7, 66.2, 45.2.
3.2.21. 5-Chloro-2-(6-morpholino-9H-purin-2-yl)phenol 3u
Method B: Compound 2b (0.18 g, 0.91 mmol) in EtOH, aldehyde 4k (1.1 eq.), and TFA (1.0 eq.) was kept at 22 °C for 2 days. Then, the reaction continued in EtOH and Et3N (10 eq.) at 40 °C for 24 days. The product 3s was isolated as an off-white solid (0.08 g, 0.24 mmol, 26%); m.p. > 300 °C. (Found: C, 54.33; H, 4.23; N, 21.31. C15H14ClN5O2 requires C, 54.31; H, 4.25; N, 21.11); 1H (400 MHz, DMSO-d6) δ: 13.76 (s, 1H), 13.32 (br s, 1H), 8.27 (d, J = 2.8 Hz, 1H), 8.23 (s, 1H), 7.34 (dd, J = 8.8 Hz, J = 2.8 Hz, 1H), 6.94 (d, J = 8.8 Hz, 1H), 4.27 (br s, 4H), 3.78 (t, J = 4.8 Hz, 4H); 13C (100 MHz, DMSO-d6) δ: 158.2, 156.2, 152.5, 149.6, 139.3, 131.4, 127.7, 122.2, 120.7, 119.2, 117.9, 66.1, 45.4.
3.2.22. 4-(2-(2-Fluoro-5-methoxyphenyl)-9H-purin-6-yl)morpholine 3v
Method A: Compound 2b (0.35 g, 1.80 mmol) in EtOH, aldehyde 4l (1.1 eq.), and Et3N (10 eq.) was kept at 20 °C for 19 days and then at 40 °C for 7 days. The product 3v was isolated as an off-white solid (0.17 g, 0.52 mmol, 29%); m.p. = 261–263 °C (Found: C, 57.99; H, 4.89; N, 20.98. C16H16FN5O2 requires C, 58.35; H, 4.90; N, 21.27); IR (Nujol mull) νmax: 1579, 1500 cm−1; 1H (300 MHz, DMSO-d6) δ: 13.17 (s, 1H), 8.18 (s, 1H), 7.50 (dd, J = 6.6 Hz, J = 3.6 Hz, 1H), 7.17 (dd, J = 10.5 Hz, J = 9.0 Hz, 1H), 7.02 (dt, J = 3.6 Hz, J = 3.3 Hz, 1H), 4.24 (br s, 4H), 3.77 (s, 3H), 3.72 (m, 4H); 13C (75 MHz, DMSO-d6) δ: 154.7 (d, J = 244 Hz), 155.2 (d, J = 5 Hz), 155.0 (d, J = 2 Hz), 152.9, 152.3, 139.2, 127.8 (d, J = 12 Hz), 117.7, 117.4 (d, J = 25 Hz), 116.0 (d, J = 8 Hz), 115.7 (d, J = 2 Hz), 66.2, 55.6, 45.1.
3.2.23. 4-(2-(2,5-Difluorophenyl)-9H-purin-6-yl)morpholine 3x
Method A: Compound 2b (0.31 g, 1.59 mmol) in EtOH, aldehyde 4m (1.1 eq.), and Et3N (10 eq.) was kept at 35 °C for 1 month. The product 3x was isolated as an off-white solid (0.28 g, 0.88 mmol, 56%); m.p. = 260–262 °C (Found: C, 56.78; H, 4.00; N, 21.89. C15H13F2N5O requires C, 56.78; H, 4.13; N, 22.07); IR (Nujol mull) νmax: 1581, 1500 cm−1; 1H (300 MHz, DMSO-d6) δ: 13.22 (s, 1H), 8.19 (s, 1H), 7.75–7.80 (m, 1H), 7.29–7.33 (m, 2H), 4.24 (br s, 4H), 3.73 (br s, 4H); 13C (75 MHz, DMSO-d6) δ: 157.8 (dd, J = 240 Hz, J = 4 Hz), 156.6 (dd, J = 250 Hz, J = 4 Hz), 154.2 (d, J = 3 Hz), 152.9, 152.2, 139.3, 128.7 (dd, J = 11 Hz, J = 8 Hz), 118.4 (dd, J = 25 Hz, J = 9 Hz), 117.9, 117.4 (dd, J = 23 Hz, J = 11 Hz), 117.3 (dd, J = 23 Hz, J = 5 Hz), 66.2, 45.2.
3.3. Radioligand Binding Assays
The %inhib of specific radioligand binding at the receptors by the compounds was tested at the concentration of 10 µM at all adenosine receptors following the conditions described below. Competition binding curves at several receptors were made by testing six different concentrations (ranging from 10 nM to 100 µM) for all of the compounds showing an %inhib ≥ 80%.
3.3.1. Human A1 Receptor
Competition binding experiments of adenosine A1 receptor were made with membranes from CHO-A1 cells (Euroscreen, Brussels, Belgium). Membranes were defrosted and suspended in an incubation buffer with 10 mM of MgCl2, 100 mM of NaCl, 20 mM of Hepes, 2 UI/mL of adenosine deaminase, and pH = 7.4. Each well of a GF/C multiscreen plate (Millipore, Madrid, Spain), prepared in duplicate, contained 15 μg of protein, 2 nM of [3H]DPCPX, and the tested compound. Non-specific binding was calculated with 10 μM of (R)-PIA. The reaction mixture was incubated at room temperature (25 °C) for 60 min, after which samples were filtered and measured in a microplate beta scintillation counter (Microbeta Trilux, Perkin Elmer, Madrid, Spain).
3.3.2. Human A2A Receptor
Competition binding experiments of adenosine A2A receptor were made in membranes from HeLa-A2A cells, which were provided by Dr. Mengod (Departament de Neuroquimica, Institut d’Investi-gacions Biomediques de Barcelona, CSIC-IDIBAPS, Barcelona, Spain). Membranes were defrosted and suspended in an incubation buffer with 1 mM of EDTA, 50 mM of Tris-HCl, 10 mM of MgCl2, and 2 UI/mL of adenosine deaminase, with pH = 7.4. Each well of a GF/C multiscreen plate (Millipore, Madrid, Spain), prepared in duplicate, contained 3 nM of [3H]ZM241385, 10 µg of protein, and the tested compound. Nonspecific binding was calculated with 50 μM of NECA. The reaction mixture was incubated at room temperature (25 °C) for 30 min, after which samples were filtered and measured in a microplate beta scintillation counter (Microbeta Trilux, Perkin Elmer, Madrid, Spain).
3.3.3. Human A2B Receptor
Competition binding experiments of adenosine A2B receptor were made in membranes from HEK-293-A2B cells (Euroscreen, Brussels, Belgium). Membranes were defrosted and suspended in incubation buffer with 1 mM of EDTA, 10 mM of MgCl2, 0.1 mM of benzamidine, 50 mM of Tris-HCl, 10 µg/mL of bacitracine, and 2 UI/mL of adenosine deaminase, with pH = 6.5. Each reaction well, prepared in duplicate, contained 35 nM of [3H]DPCPX, 18 µg of protein, and the tested compound. Non-specific binding was calculated with 400 µM of NECA. The reaction mixture was incubated at room temperature (25 °C) for 30 min, after which samples were filtered through a multiscreen GF/C microplate and measured in a microplate beta scintillation counter (Microbeta Trilux, Perkin Elmer, Madrid, Spain).
3.3.4. Human A3 Receptor
Competition binding experiments of adenosine A3 receptor were made in membranes from HeLa-A3 cells, which were provided by Dr. Mengod (Departament de Neuroquimica, Institut d’Investi-gacions Biomediques de Barcelona, CSIC-IDIBAPS, Barcelona, Spain). Membranes were defrosted and suspended in incubation buffer with 1 mM of EDTA, 5 mM of MgCl2, 50 mM of Tris-HCl, and 2 UI/mL of adenosine deaminase, with pH = 7.4. Each reaction well of a GF/B multiscreen plate (Millipore, Madrid, Spain), prepared in triplicate, contained 90 µg of protein, 30 nM of [3H]NECA, and the tested compound. Non-specific binding was determined with 100 µM of (R)-PIA. The reaction mixture was incubated at room temperature (25 °C) for 180 min, after which samples were filtered and measured in a microplate beta scintillation counter (Microbeta Trilux, Perkin Elmer, Madrid, Spain).
3.4. Functional Studies
3.4.1. Human A1 Receptor
The agonist/antagonist behavior of tested compounds at A1 receptor was determined in CHO-A1 cells by measuring reversion induced by the tested compound of 10 µM of NECA-mediated inhibition of forkolin-stimulated cAMP production. Cells grown in 96-well plates with growth medium containing dialyzed fetal bovine serum were washed 2 times with F-12 nutrient mixture medium containing 25 mM of HEPES and 20 µM of the phosphodiesterase inhibitor rolipram, with pH = 7.4. After this time, the tested compounds were preincubated for 15 min in test medium at different concentrations (ranging from 0.1 nM to 10 µM). After this time, 10 µM of NECA and 3 µM of forskolin were added to each well. The incubation was continued for 15 min, and the reaction was stopped with a lysis buffer from the cAMP enzyme immunoassay kit (GE Healthcare, Chicago, IL, USA). Cell lysates were put in a plate with anti-IgG and anti-cAMP in the presence of cAMP–peroxidase during 60 min. Then, the wells were washed 4 times with the wash buffer from the kit, and TMB was added to each well and incubated for 60 min. The peroxidase reaction was stopped with 1 M of sulphuric acid, and the cAMP amount was determined from the optical density at 450 nm (Tecan M100 reader).
3.4.2. Human A3 Receptor
The agonist/antagonist behavior of tested compounds at A3 receptor was determined in CHO–A3 cells, which were in-house generated at BioFarma Research Group (Department of Pharma-cology, University of Santiago de Compostela, Spain), by measuring reversion induced by the tested compound of 10 µM of NECA inhibition of forskolin-stimulated cAMP production. Cells grown in 96-well plates with growth medium containing dialyzed fetal bovine serum were washed 2 times with DMEM F-12 nutrient medium containing 25 mM of HEPES, 30 μM of rolipram, with pH = 7.4. After this time, the tested compounds were preincubated for 15 min in test medium at different concentrations (ranging from 0.1 nM to 10 µM). Finally, 10 μM of NECA and 10 μM of forskolin were added to each well. The incubation lasted 15 min, and cAMP was determined through an enzyme immunoassay (Perkin Elmer) for Human A1 receptor.
3.5. Data Analysis
Concentration response curves were fitted with Prism 10.1.2 (Graph Pad, San Diego, CA, USA). Radioligand binding pKi values were extrapolated from the equation
where IC50 is the concentration that displaced specific radioligand binding at 50%, extrapolated from non-linear fitting, [L] is the concentration of radioligand employed, and KD is the dissociation constant of the radioligand. In cAMP studies, KB values were derived by employing the derivation of the Cheng-Prusoff equation, reported by Leffand Dougall in 1993 [37].
where A is the concentration of the agonist employed to stimulate the receptor, EC50 is the potency of the agonist for stimulating the receptor, and n is the Hill slope of the antagonist’s concentration-response curve.
pKi = −log(IC50/(1 + ([L]/KD)))
KB = IC50/(2 + (A/(EC50)n)1/n − 1);
3.6. Docking Studies
The molecular docking calculations were carried out using the Molecular Operating Environment (MOE) software (version 2022.02, https://www.chemcomp.com, accessed on 25 April 2024) and the crystal structure of the A1 receptor in complex with the ligand DU1 (PDB accession code: 5UEN) [35]. The receptor was prepared by removing non-protein atoms and adding missing side chains. Engineered mutations were reverted back to the wild-type sequence based on the side chain rotamers determined through MOE. The protonation states of ionizable residues were set to the most probable at physiological pH. The binding site was defined based on the co-crystallized ligand, and default parameters in MOE were used in the docking calculations.
4. Conclusions
In summary, several 6-morpholino-purine derivatives with different substituents in C-2 and a methyl or a hydrogen at N-9 of the purine ring were synthesized and pharmacologically tested at human A1, A2A, A2B, and A3 adenosine receptor subtypes. Four of the twenty-three synthesized purines showed high affinity and selectivity for A1 receptors, and five showed high affinity and selectivity for A3 receptors. Three compounds (3k, 3q, and 3u) showed a dual affinity for A1/A2A, A1/A3, and A1/A2B receptors, respectively. In addition, three compounds showed high affinity without selectivity for any specific adenosine receptor subtype (3n, 3o, and 3x). SAR studies showed that the substituent groups in C-2, C-6, and N-9 positions affect the potency and selectivity of the compounds for adenosine receptors. To generate highly potent antagonist ligands for adenosine receptors, a morpholine group and a hydrogen atom must be present in C-6 and N-9, respectively. Indeed, 9H-purines bind some 5–358 times more strongly than their corresponding 9-CH3-purines for the adenosine receptors. The substituted aryl group in C-2 is very important for governing the selectivity towards the adenosine receptor subtypes. Additionally, our results suggested that the A3 receptor subtype presents a larger hydrophobic pocket than that of the A1 receptor subtype, as A3 accommodates bigger substituent groups Y present at the meta position of substituent R1. In conclusion, several purine-based compounds are described herein, some with high affinity and antagonist selectivity for A1, A3, dual A1/A2A, A1/A2B, and A1/A3 receptors.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/molecules29112543/s1, 1H and 13C NMR spectra of representative synthesized compounds.
Author Contributions
Conceptualization, F.A., M.F.P. and M.A.C.; formal analysis, M.C., J.B., J.C. and M.A.C.; funding acquisition, J.C., M.I.L., M.F.P. and M.A.C.; investigation, F.A., C.C., A.R., S.T., M.C., J.B. and H.H.; methodology, M.C. and M.A.C.; project administration, M.I.L., M.F.P. and M.A.C.; supervision, J.C., M.F.P. and M.A.C.; writing—original draft, F.A.; writing—review and editing, M.A.C. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the Portuguese Fundação para a Ciência e Tecnologia/Ministério da Educação e Ciência (MEC) through funds in the framework of the Strategic Funding of CQUM (UID/QUI/00686/2020), (UID/QUI/00686/2018), FEDER funds through the Operational Programme for Competitiveness Factors (COMPETE 2020), Programa Operacional de Competitividade e Internacionalização (POCI) (POCI-01-0145-FEDER-031354) Rede Nacional de RMN (PINFRA/22161/2016), and PhD grants to Carla Correia and Ashly Rocha (SFRH/BD/22270/2005; SFRH/BD/85937/2012), Xunta de Galicia (ED431C 2022/20), and the European Regional Development Fund (ERDF). Filipe Areias gratefully acknowledges the Post-PhD grant from the Portuguese FCT (SFRH/BPD/26106/2005). J.C. received funding from the Olle Engkvist Foundation (219-0154) and the Swedish Research Council (2021-4186). The LECO instrument for elemental analysis TruSpec Micro CHN was purchased within the PT-OPENSCREEN project (NORTE-01—0145-FEDER-085468), financed by CCDR-N.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Data are contained within the article and Supplementary Materials.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Batra, R.; Jain, V.; Sharma, P. Adenosine: A partially discovered medicinal agent. Future J. Pharm. Sci. 2021, 7, 214. [Google Scholar] [CrossRef] [PubMed]
- Borea, P.A.; Gessi, S.; Merighi, S.; Vincenzi, F.; Varani, K. Pharmacology of adenosine receptors: The state of the art. Physiol. Rev. 2018, 98, 1591–1625. [Google Scholar] [CrossRef] [PubMed]
- Jamwal, S.; Mittal, A.; Kumar, P.; Alhayani, D.M.; Al-Aboudi, A. Therapeutic potential of agonists and antagonists of A1, A2a, A2b and A3 adenosine receptors. Curr. Pharm. Des. 2019, 25, 2892–2905. [Google Scholar] [CrossRef] [PubMed]
- Effendi, W.I.; Nagano, T.; Kobayashi, K.; Nishimura, Y. Focusing on adenosine receptors as a potential targeted therapy in human diseases. Cells 2020, 9, 785. [Google Scholar] [CrossRef] [PubMed]
- Ijzerman, A.P.; Jacobson, K.A.; Müller, C.E.; Cronstein, B.N.; Cunha, R.A. International union of basic and clinical pharmacology. CXII: Adenosine receptors: A further updates. Pharmacol. Rev. 2022, 74, 340–372. [Google Scholar] [CrossRef] [PubMed]
- Müller, C.E.; Jacobson, K.A. Recent developments in adenosine receptor ligands and their potential as novel drugs. Biochim. Biophys. Acta Biomembr. 2011, 1808, 1290–1308. [Google Scholar] [CrossRef] [PubMed]
- Borah, P.; Deka, S.; Mailavaram, R.P.; Deb, P.K. P1 receptor agonists/antagonists in clinical trials-potential drug candidates of the future. Curr. Pharm. Des. 2019, 25, 2792–2807. [Google Scholar] [CrossRef] [PubMed]
- Gao, Z.-G.; Tosh, D.K.; Jain, S.; Yu, J.; Suresh, R.R.; Jacobson, K.A. A1 adenosine receptor agonists, antagonists, and allosteric modulators. In The Adenosine Receptors; Borea, P., Varani, K., Gessi, S., Merighi, S., Vincenzi, F., Eds.; Springer International Publishing: Cham, Germany, 2018; pp. 59–89. [Google Scholar]
- Stockwell, J.; Jakova, E.; Cayabyab, F. Adenosine A1 and A2A Receptors in the brain: Current research and their role in neurodegeneration. Molecules 2017, 22, 676. [Google Scholar] [CrossRef] [PubMed]
- Pak, E.S.; Cha, J.J.; Cha, D.R.; Kanasaki, K.; Ha, H. Adenosine receptors as emerging therapeutic targets for diabetic kidney disease. Kidney Res. Clin. Pract. 2022, 41, S74–S88. [Google Scholar] [CrossRef] [PubMed]
- Ramos-Barbon, D.; Brienza, N.S.; Bigorra Rodríguez, T.; Mateus Medina, É.F.; Gich Saladich, I.; Puntes Rodríguez, M.; Antonijoan Arbos, R.M.; Quirce Gancedo, S.; Castro Palomino Laria, N.; Castro Palomino Laria, J. PBF-680, an oral A1 adenosine receptor antagonist, inhibits the late allergic response (LAR) in mild-to-moderate atopic asthmatics: A Phase-IIa trial. Eur. Respir. J. 2020, 56, 4784. [Google Scholar] [CrossRef]
- Nguyen, A.T.N.; Tran, Q.L.; Baltos, J.-A.; McNeill, S.M.; Nguyen, D.T.N.; May, L.T. Small molecule allosteric modulation of the adenosine A1 receptor. Front. Endocrinol. 2023, 14, 1184360. [Google Scholar] [CrossRef] [PubMed]
- Sousa, J.B.; Fresco, P.; Diniz, C.; Goncalves, J. Adenosine receptor ligands on cancer therapy: A review of patent literature. Recent Pat. Anticancer. Drug Discov. 2018, 13, 40–69. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.; Kim, D.M.; Son, W.C.; Moon, B.G. A selective adenosine A3 receptor antagonist, HL3501, has therapeutic potential in preclinical liver and renal fibrosis models. Vivo 2022, 36, 2186–2193. [Google Scholar] [CrossRef] [PubMed]
- Baraldi, P.G.; Romagnoli, R.; Saponaro, G.; Baraldi, S.; Tabrizi, M.A.; Preti, D. A3 adenosine receptor antagonists: History and future perspectives. In A3 Adenosine Receptors from Cell Biology to Pharmacology and Therapeutics; Borea, P.A., Ed.; Springer Science+Business Media B.V.: Dordrecht, The Netherlands, 2010; pp. 121–147. [Google Scholar]
- Spinaci, A.; Buccioni, M.; Dal Ben, D.; Maggi, F.; Marucci, G.; Francucci, B.; Santoni, G.; Lambertucci, C.; Volpini, R. A3 adenosine receptor antagonists with nucleoside structures and their anticancer activity. Pharmaceuticals 2022, 15, 164. [Google Scholar] [CrossRef] [PubMed]
- Jacobson, K.A.; Merighi, S.; Varani, K.; Borea, P.A.; Baraldi, S.; Aghazadeh Tabrizi, M.; Romagnoli, R.; Baraldi, P.G.; Ciancetta, A.; Tosh, D.K.; et al. A3 adenosine receptors as modulators of inflammation: From medicinal chemistry to therapy. Med. Res. Rev. 2018, 38, 1031–1072. [Google Scholar] [CrossRef]
- Spinozzi, E.; Baldassarri, C.; Acquaticci, L.; Del Bello, F.; Grifantini, M.; Cappellacci, L.; Riccardo, P. Adenosine receptors as promising targets for the management of ocular diseases. Med. Chem. Res. 2021, 30, 353–370. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.; Yang, J.; Kim, J.Y.; Lee, J.M.; Son, W.C.; Moon, B.G. HL3501, a novel selective A3 adenosine receptor antagonist, lowers intraocular pressure (IOP) in animal glaucoma models. Transl. Vis. Sci. Technol. 2022, 11, 30. [Google Scholar] [CrossRef] [PubMed]
- Barkan, K.; Lagarias, P.; Stampelou, M.; Stamatis, D.; Hoare, S.; Safitri, D.; Klotz, K.N.; Vrontaki, E.; Kolocouris, A.; Ladds, G. Pharmacological characterisation of novel adenosine A3 receptor antagonists. Sci. Rep. 2020, 10, 20781. [Google Scholar] [CrossRef] [PubMed]
- Guglielmi, P.; Carradori, S.; Campestre, C.; Poce, G. Novel therapies for glaucoma: A patent review (2013–2019). Expert Opin. Ther. Pat. 2019, 29, 769–780. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.; Kim, S.; Kim, D.; Ahn, K.H.; Lee, G.B.; Kim, D.; Hwang, H.S. Compounds antagonizing A3 adenosine receptor, method for preparing them, and medical-use thereof. WO2017123058A1, 20 July 2017. [Google Scholar]
- Petrelli, R.; Torquati, I.; Kachler, S.; Luongo, L.; Maione, S.; Franchetti, P.; Grifantini, M.; Novellino, E.; Lavecchia, A.; Klotz, K.N.; et al. 5′-C-ethyl-tetrazolyl-N 6-substituted adenosine and 2-chloro-adenosine derivatives as highly potent dual acting A1 adenosine receptor agonists and A3 adenosine receptor antagonists. J. Med. Chem. 2015, 58, 2560–2566. [Google Scholar] [CrossRef]
- Abdelrahman, A.; Yerande, S.G.; Namasivayam, V.; Klapschinski, T.A.; Alnouri, M.W.; El-Tayeb, A.; Müller, C.E. Substituted 4-phenylthiazoles: Development of potent and selective A1, A3 and dual A1/A3 adenosine receptor antagonists. Eur. J. Med. Chem. 2020, 186, 111879. [Google Scholar] [CrossRef]
- Massie, B.M.; O’Connor, C.M.; Metra, M.; Ponikowski, P.; Teerlink, J.R.; Cotter, G.; Weatherley, B.D.; Cleland, J.G.F.; Givertz, M.M.; Voors, A.; et al. Rolofylline, an adenosine A1-receptor antagonist, in acute heart failure. N. Engl. J. Med. 2010, 363, 1419–1428. [Google Scholar] [CrossRef]
- Teerlink, J.R.; Iragui, V.J.; Mohr, J.P.; Carson, P.E.; Hauptman, P.J.; Lovett, D.H.; Miller, A.B.; Piña, I.L.; Thomson, S.; Varosy, P.D.; et al. The safety of an adenosine A1-receptor antagonist, rolofylline, in patients with acute heart failure and renal impairment. Drug Saf. 2012, 35, 233–244. [Google Scholar] [CrossRef]
- Mitrovic, V.; Seferovic, P.; Dodic, S.; Krotin, M.; Neskovic, A.; Dickstein, K.; de Voogd, H.; Bocker, C.; Ziegler, D.; Godes, M.; et al. Cardio-renal effects of the A1 adenosine receptor antagonist SLV320 in patients with heart failure. Circ. Hear. Fail. 2009, 2, 523–531. [Google Scholar] [CrossRef]
- Areias, F.; Correia, C.; Rocha, A.; Brea, J.; Castro, M.; Loza, M.I.; Proença, M.F.; Carvalho, M.A. 2-Aryladenine derivatives as a potent scaffold for A1, A3 and dual A1/A3 adenosine receptor antagonists: Synthesis and structure-activity relationships. Bioorganic Med. Chem. 2019, 27, 3551–3558. [Google Scholar] [CrossRef]
- Alves, M.J.; Booth, B.L.; Proença, M.F.J.R.P. Synthesis of 5-amino-4-(cyanoformimidoyl)-1H-imidazole: A reactive intermediate for the synthesis of 6-carbamoyl-1,2-dihydropurines and 6-carbamoylpurines. J. Chem. Soc. Perkin Trans. 1 1990, 1, 1705–1712. [Google Scholar] [CrossRef]
- Correia, C.; Carvalho, M.A.; Proença, M.F. Synthesis and in vitro activity of 6-amino-2,9-diarylpurines for Mycobacterium tuberculosis. Tetrahedron 2009, 65, 6903–6911. [Google Scholar] [CrossRef]
- Jacobson, K.A. Introduction to adenosine receptors as therapeutic targets. In Adenosine Receptors in Health and Disease; Wilson, C.N., Mustafa, S.J., Eds.; Springer: Berlin/Heidelberg, Germany, 2009; pp. 1–24. [Google Scholar]
- Giorgi, I.; Nieri, P. Adenosine A 1 modulators: A patent update (2008 to present). Expert Opin. Ther. Pat. 2013, 23, 1109–1121. [Google Scholar] [CrossRef]
- Shook, B.C.; Rassnick, S.; Osborne, M.C.; Davis, S.; Westover, L.; Boulet, J.; Hall, D.; Rupert, K.C.; Heintzelman, G.R.; Hansen, K.; et al. In vivo characterization of a dual adenosine A2A/A1 receptor antagonist in animal models of Parkinson’s disease. J. Med. Chem. 2010, 53, 8104–8115. [Google Scholar] [CrossRef]
- Jung, J.; Lee, Y.; Moon, A.-N.; Ann, J.; Jeong, J.J.; Do, N.; Lee, J. Discovery of Novel Dual Adenosine A2A and A1 receptor antagonists with 1H-Pyrazolo[3,4-d]pyrimidin-6-amine core scaffold as anti-Parkinson’s disease agents. Pharmaceuticals 2022, 15, 922. [Google Scholar] [CrossRef]
- Glukhova, A.; Thal, D.M.; Nguyen, A.T.; Vecchio, E.A.; Jörg, M.; Scammells, P.J.; May, L.T.; Sexton, P.M.; Christopoulos, A. Structure of the adenosine A1 receptor reveals the basis for subtype selectivity. Cell 2017, 168, 867–877. [Google Scholar] [CrossRef]
- Jacobson, K.A.; Gao, Z.; Matricon, P.; Eddy, M.T.; Carlsson, J. Adenosine A 2A receptor antagonists: From caffeine to selective non-xanthines. Br. J. Pharmacol. 2022, 179, 3496–3511. [Google Scholar] [CrossRef]
- Leff, P.; Dougall, I.G. Further concerns over Cheng-Prusoff analysis. Trends Pharmacol. Sci. 1993, 14, 110–112. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).