Recent Advances in Photoswitchable Fluorescent and Colorimetric Probes
Abstract
1. Introduction
2. Types of Molecular Photoswitches and Construction Strategies for Photoswitchable Fluorescent and Colorimetric Probes
2.1. Spiropyrans
2.2. Diarylethenes
2.3. Azobenzenes
2.4. Donor–Acceptor Stenhouse Adducts (DASAs)
2.5. Hemithioindigos
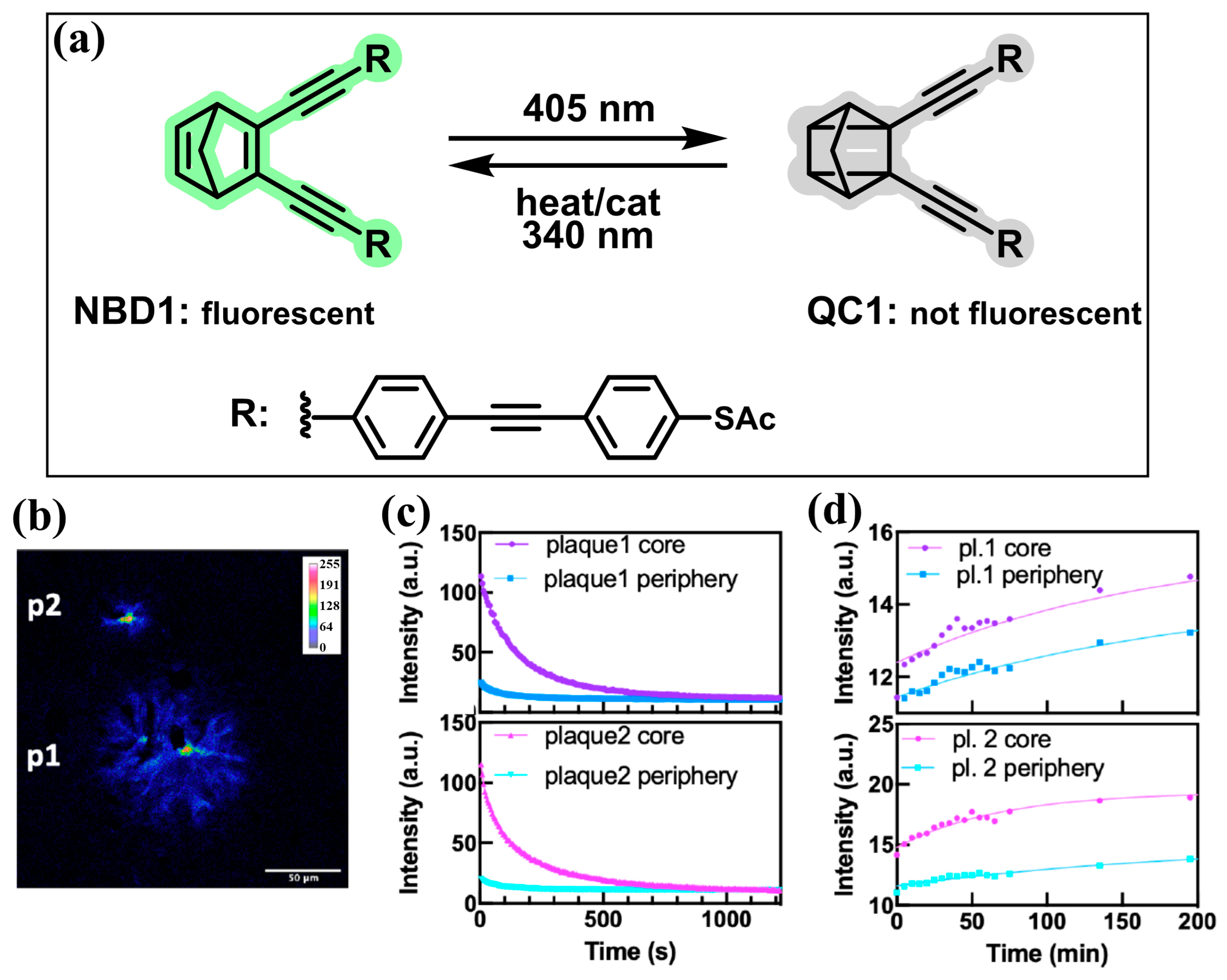
2.6. Norbornadienes
3. Applications of Photoswitchable Fluorescent and Colorimetric Probes
3.1. Cations
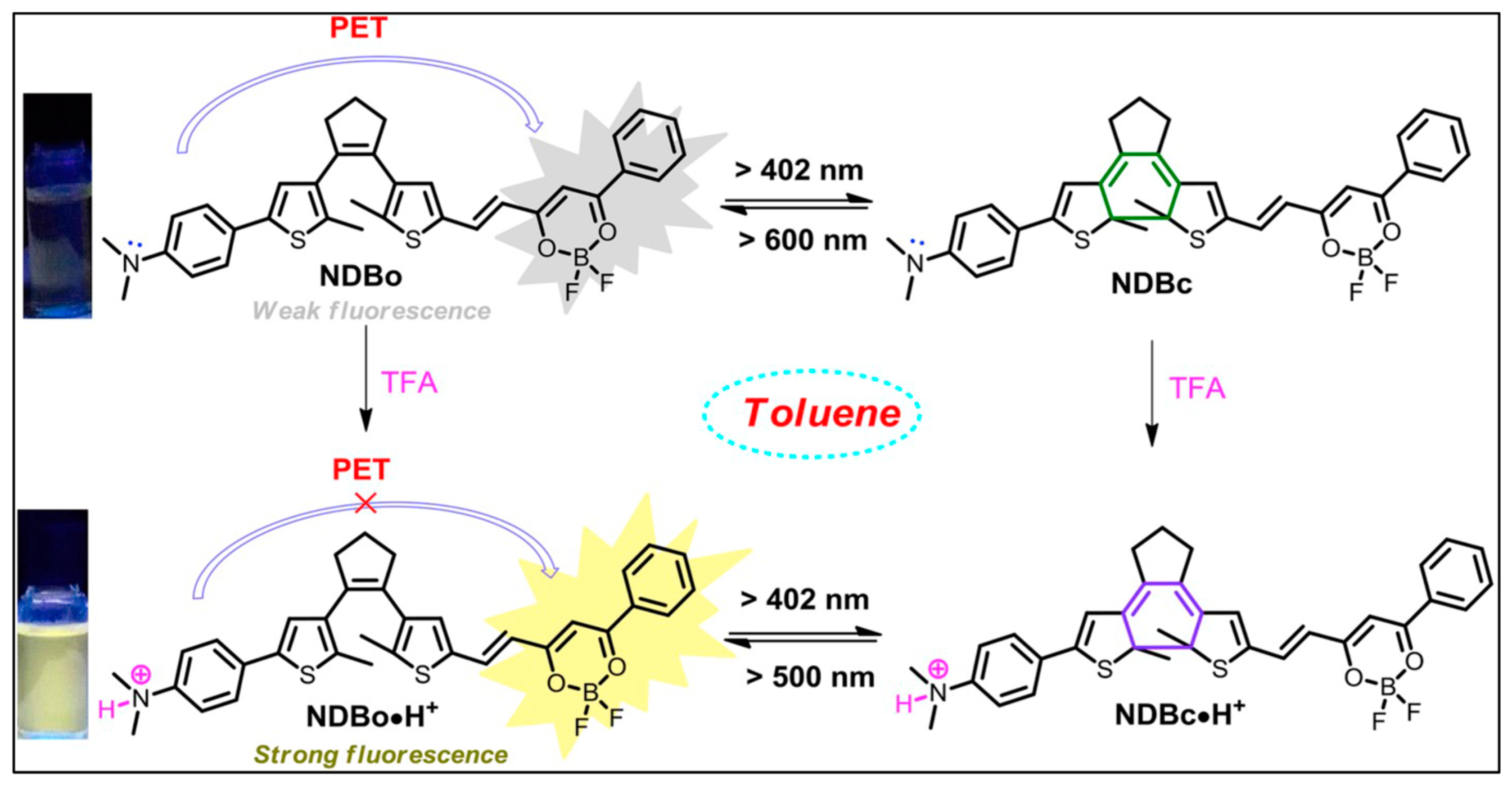
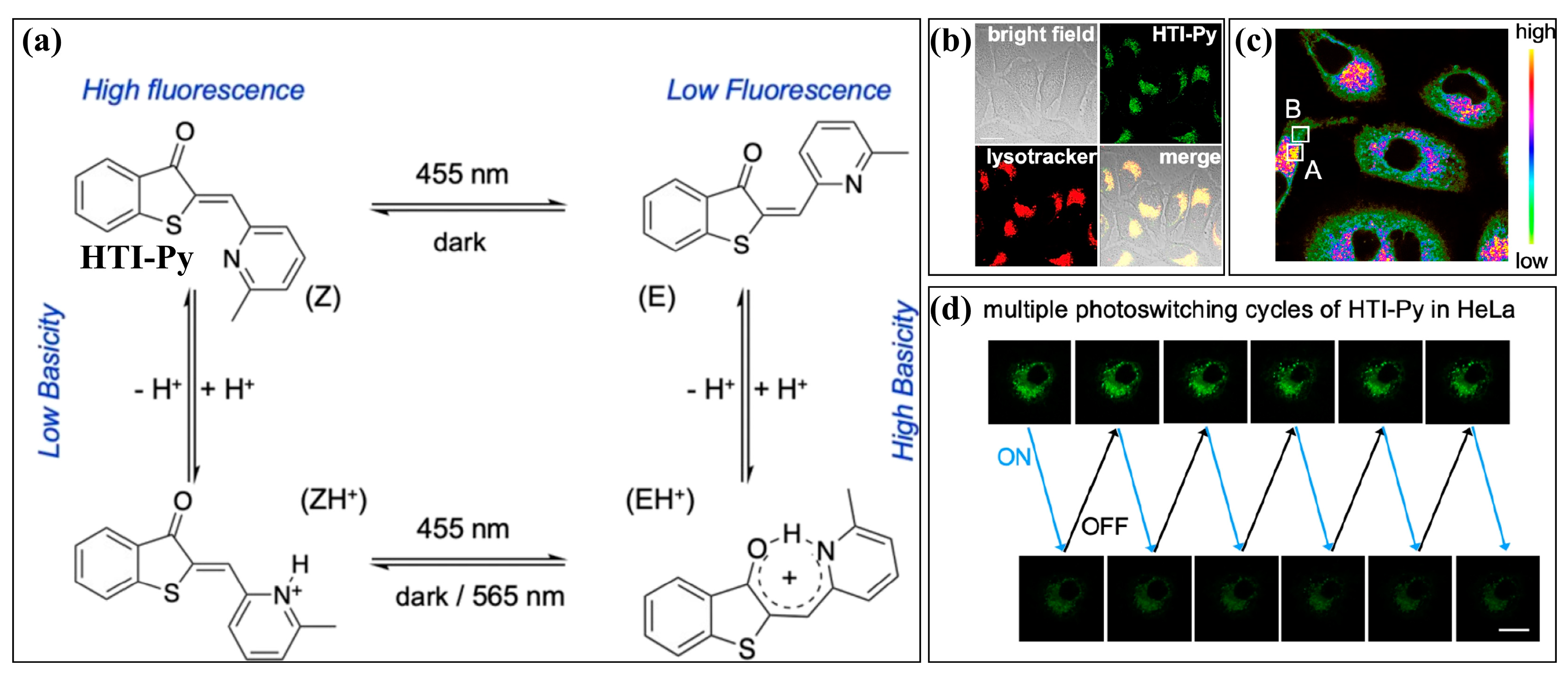
3.1.1. H+ (pH) or Acid
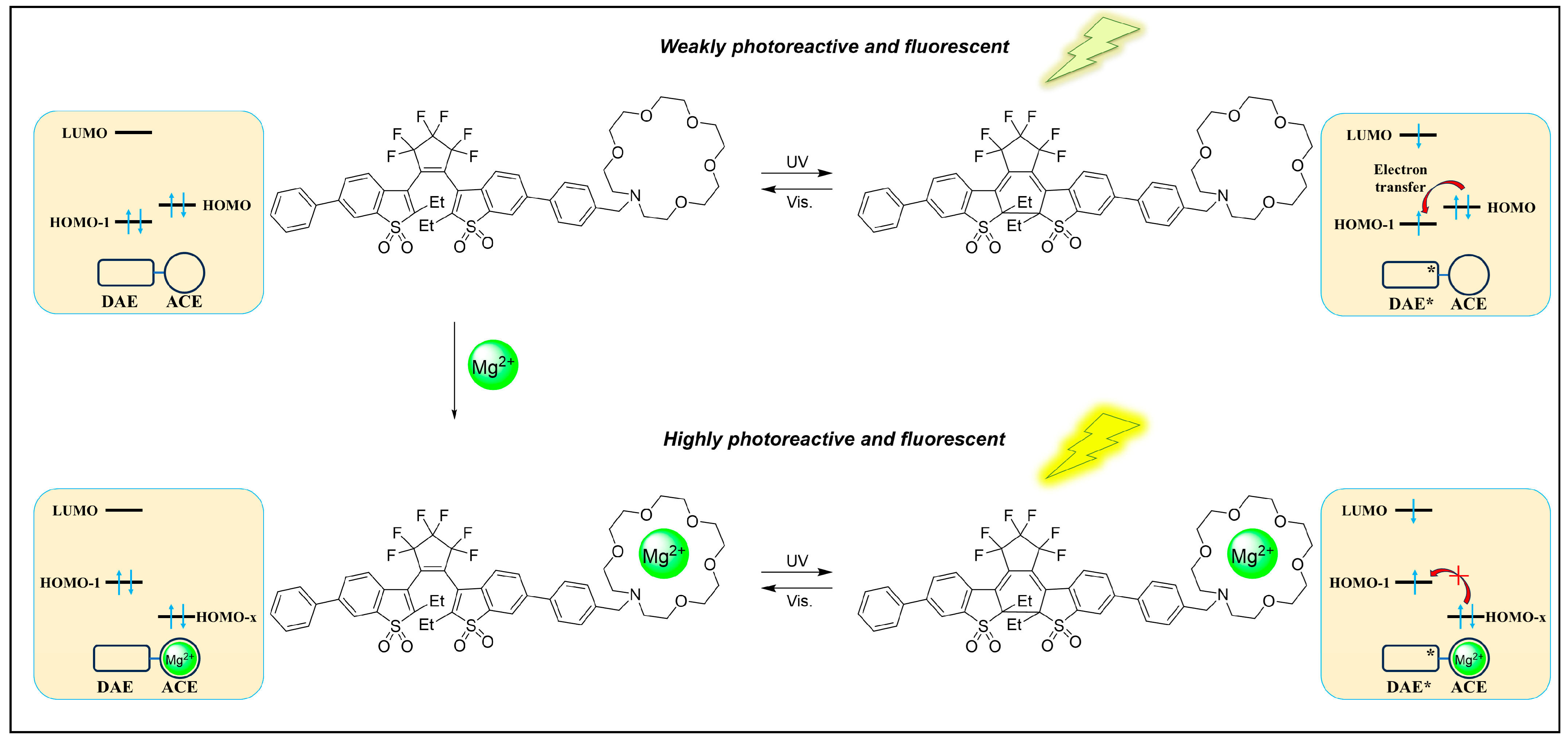
3.1.2. Mg2+ and Ca2+
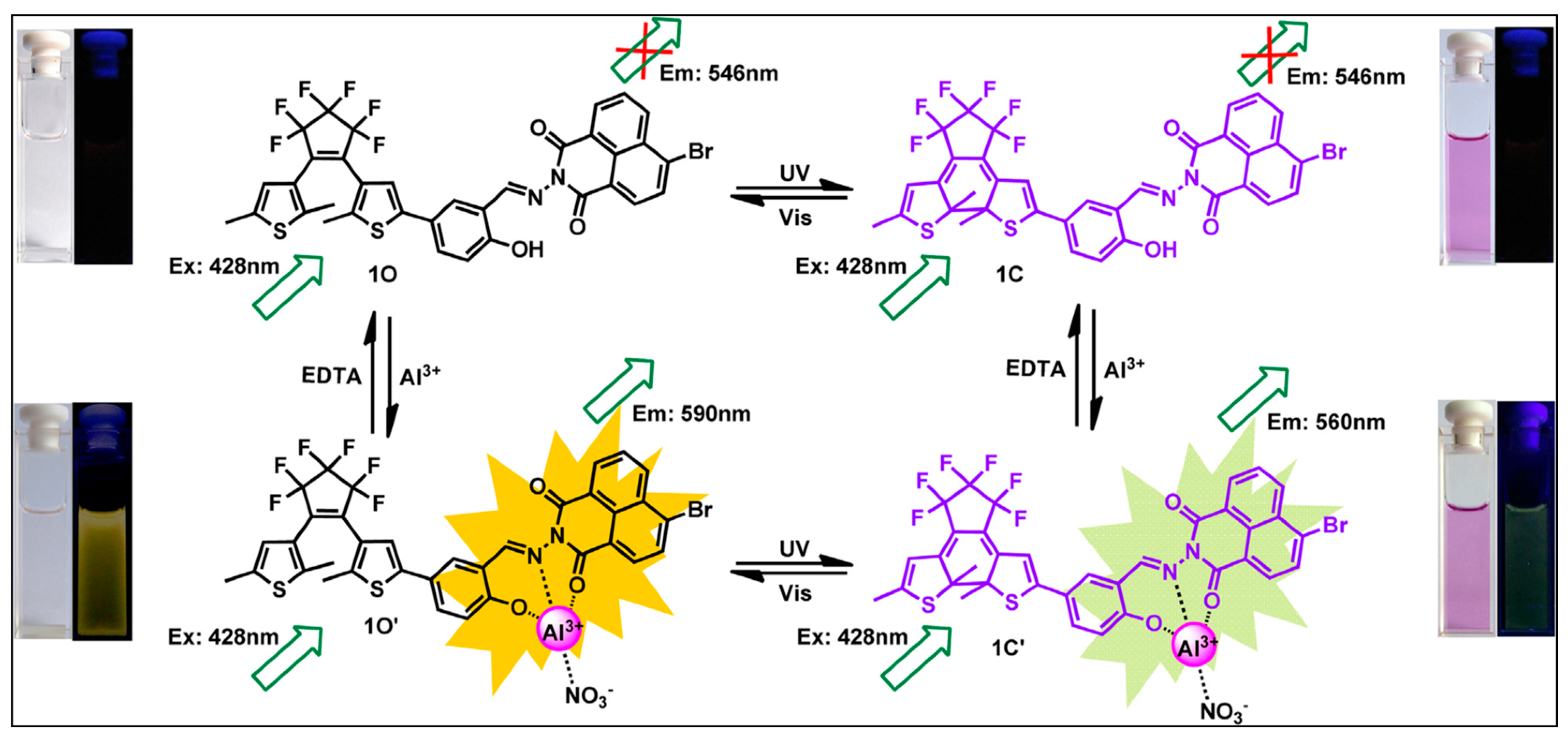
3.1.3. Al3+
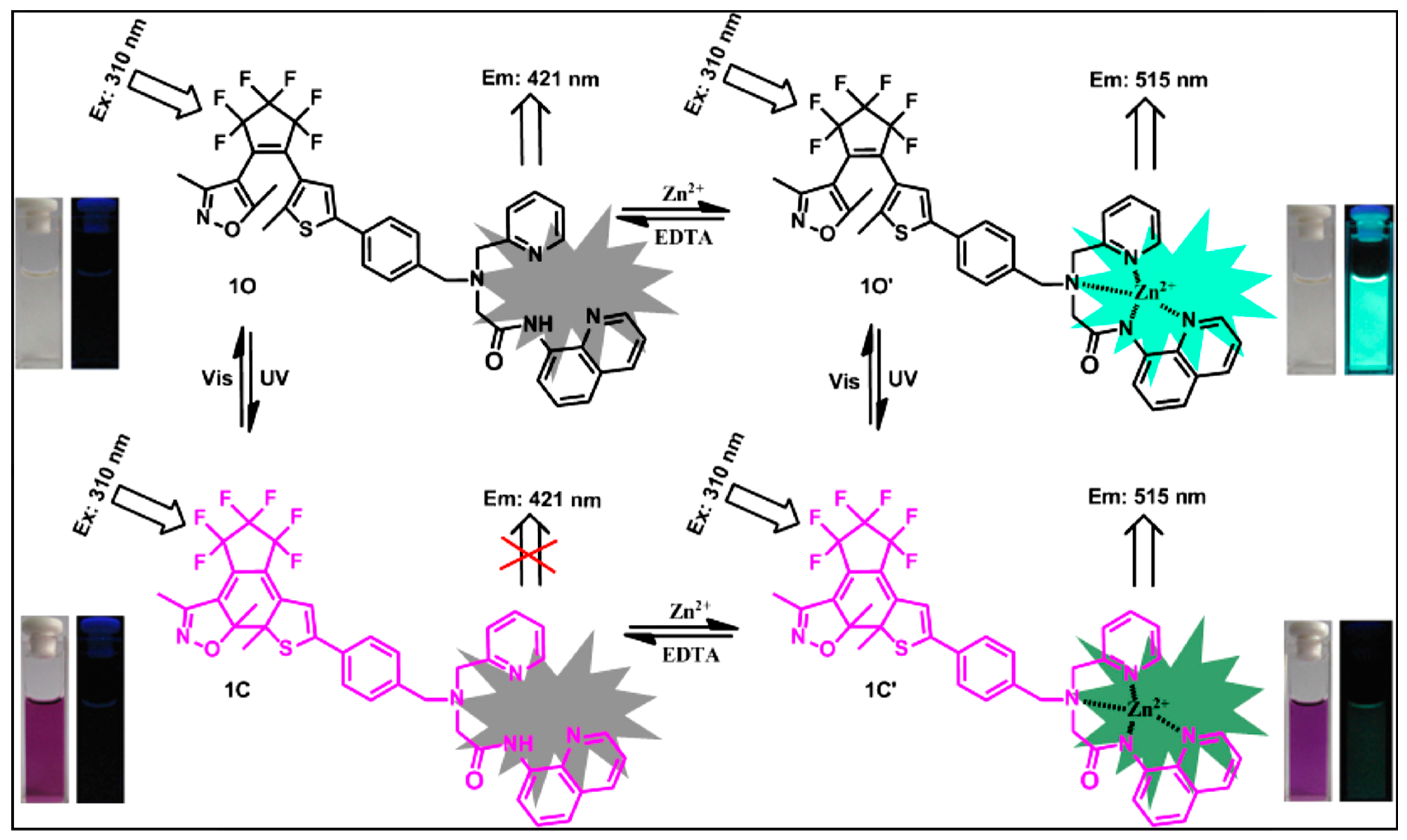
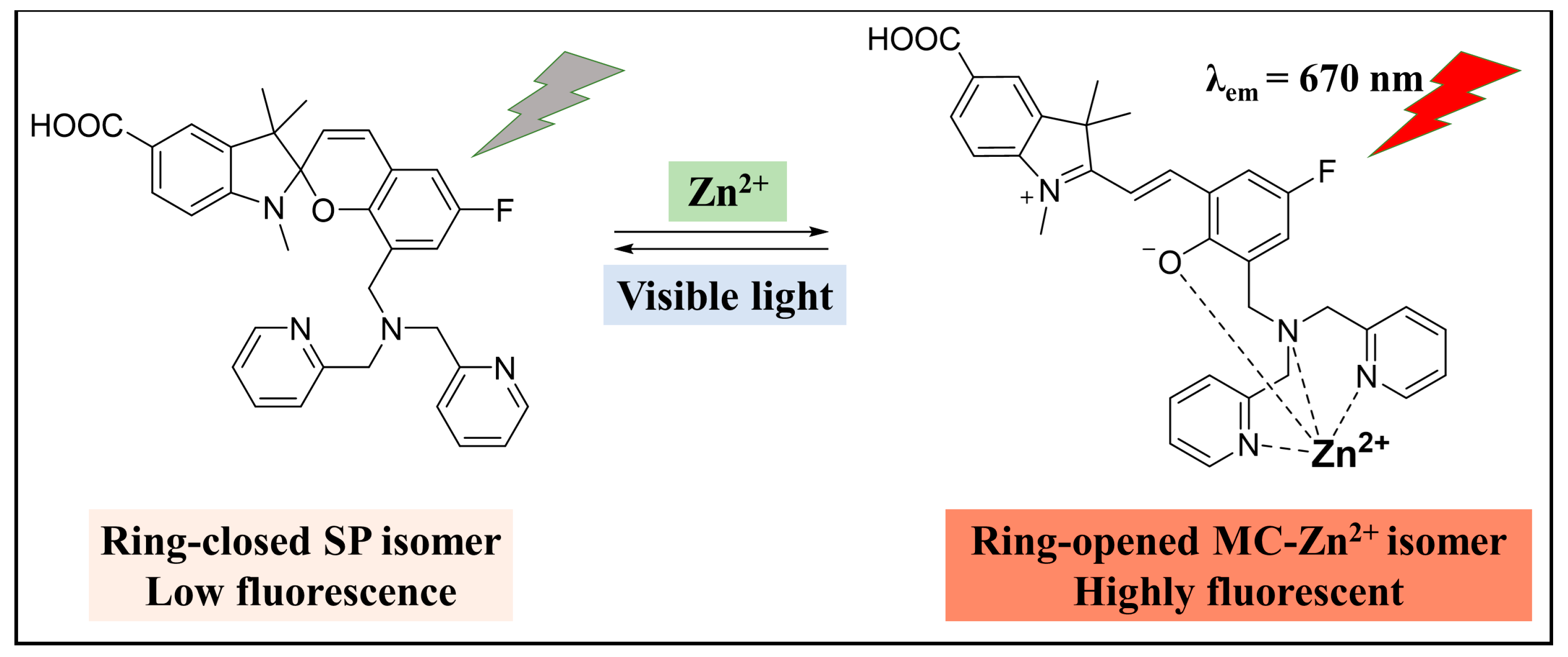
3.1.4. Zn2+
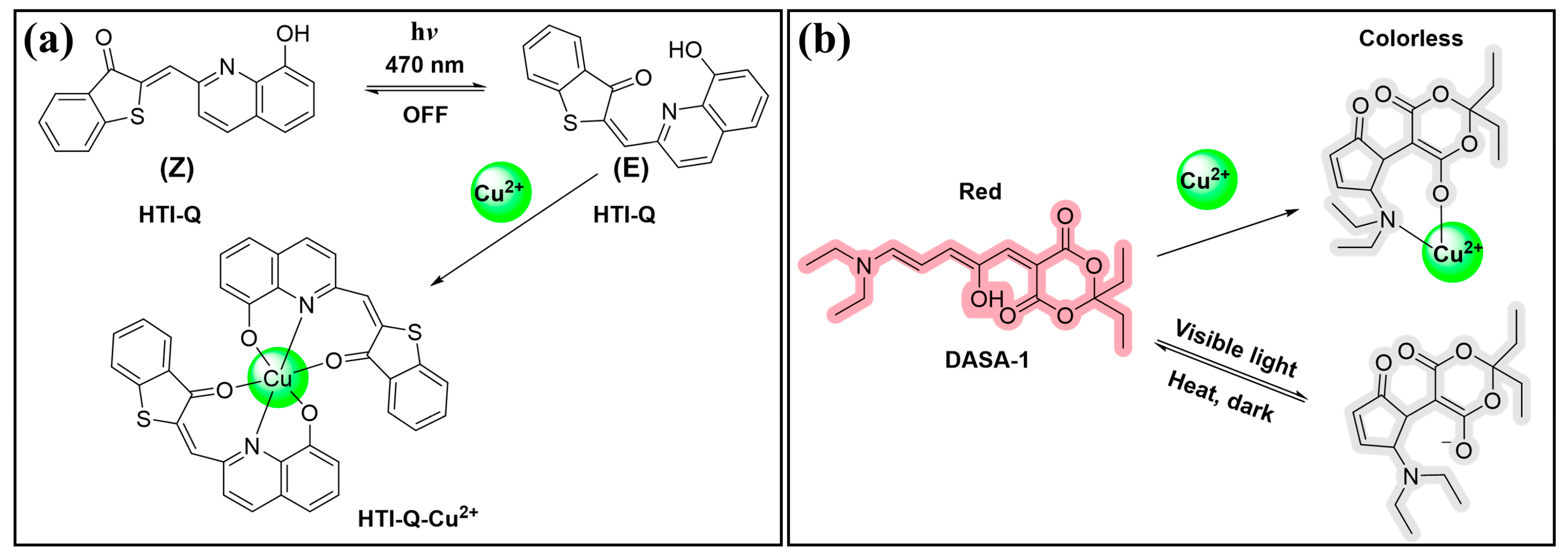
3.1.5. Cu2+
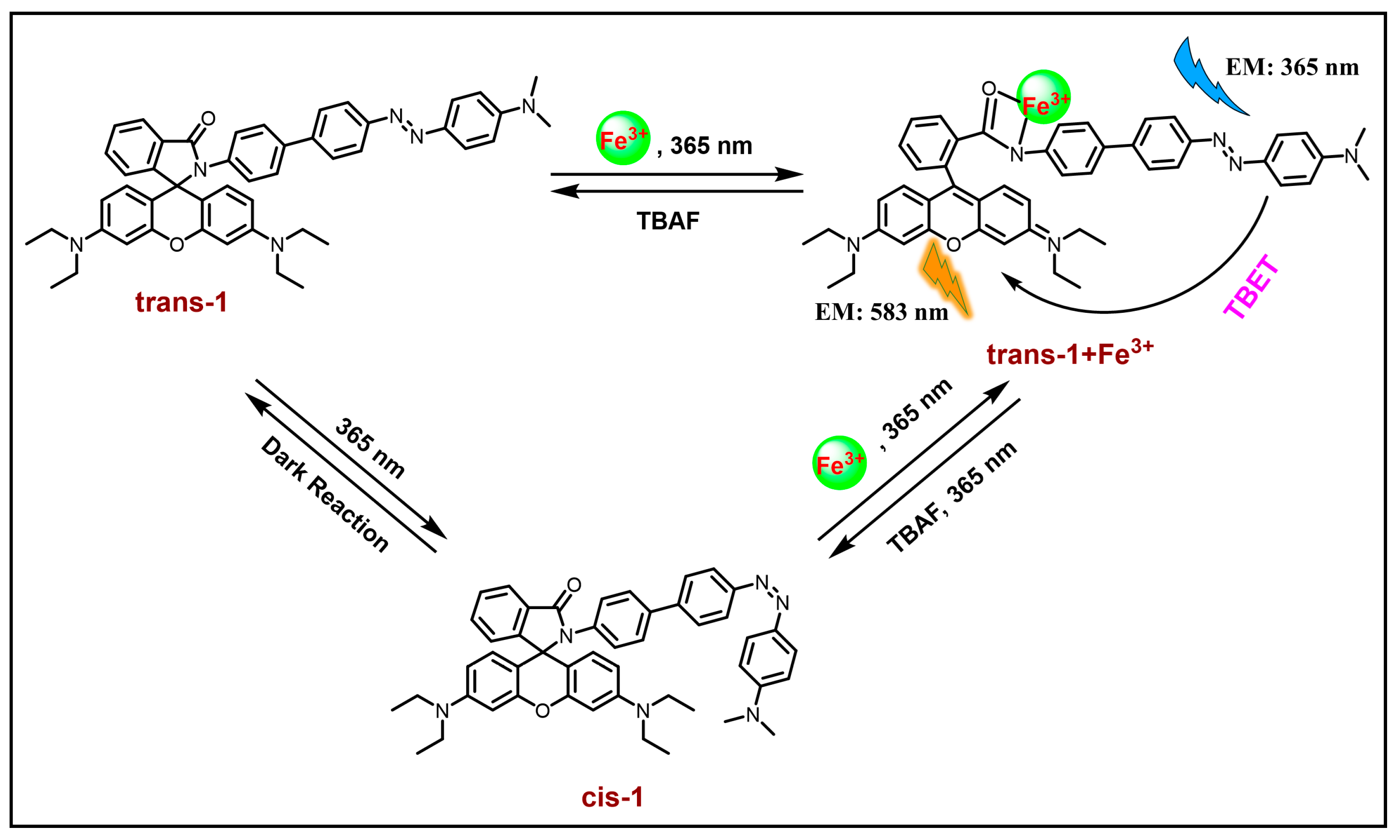
3.1.6. Other Metal Ions (Fe3+, Hg2+, Cd2+, Cr3+, Sn2+)
3.2. Anions
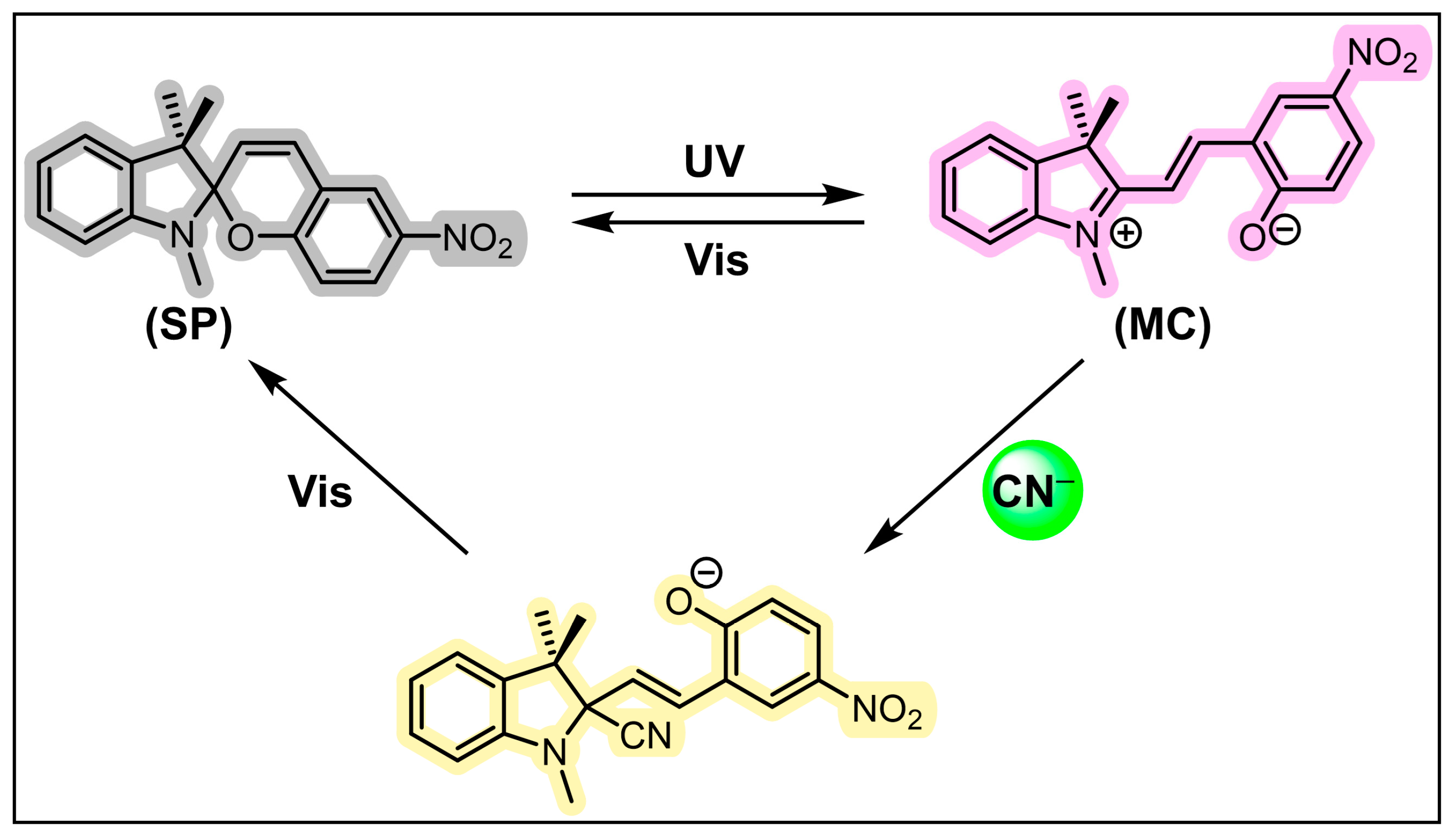
3.2.1. CN−
3.2.2. F−
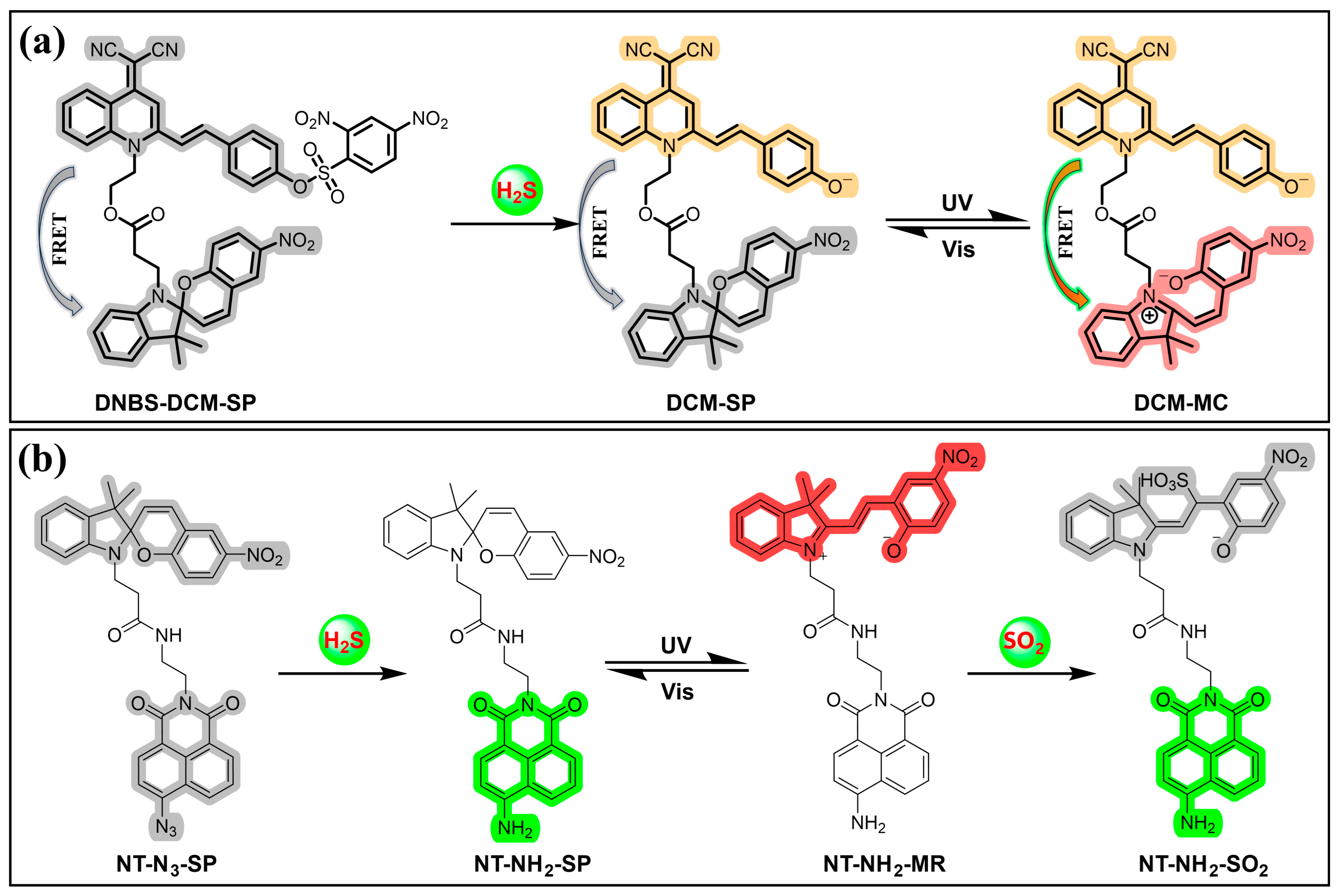
3.2.3. SO32− (HSO3−, SO2, H2SO3)
3.2.4. S2− (HS−, H2S)
3.3. Small Molecules
3.3.1. Amines
3.3.2. Thiols
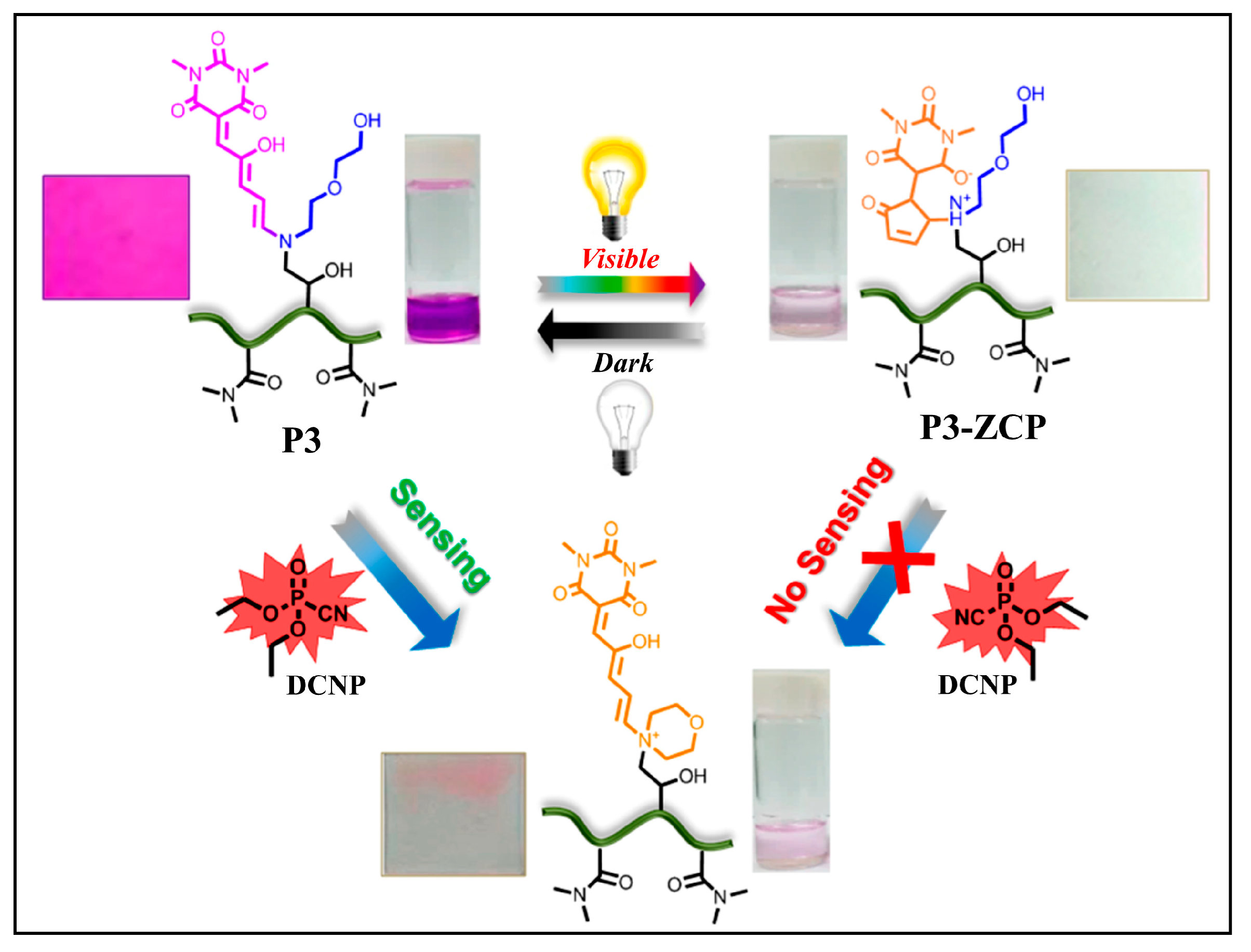
3.3.3. Diethyl Cyanophosphonate
3.4. Biomacromolecules
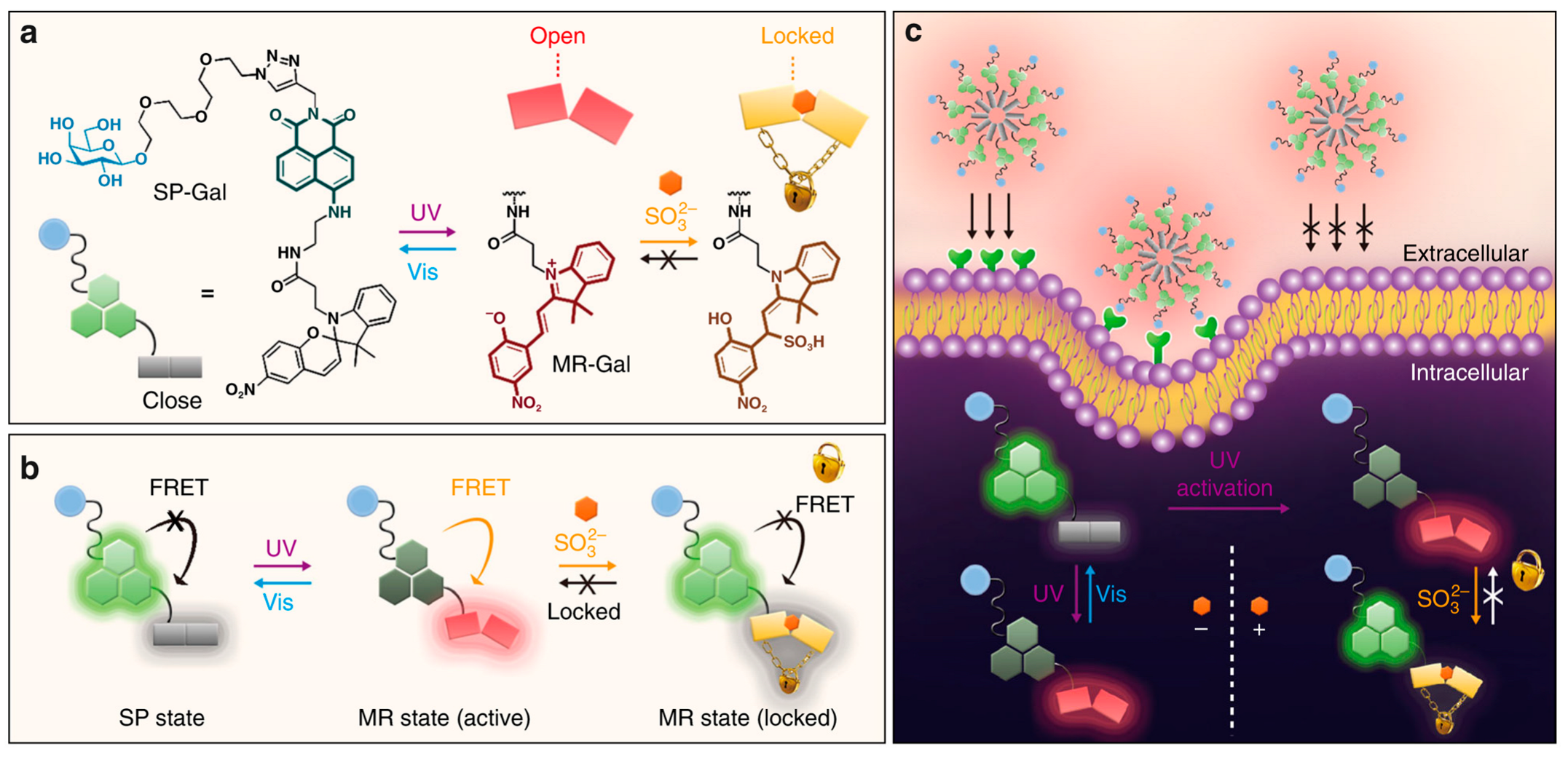
3.4.1. β-Galactosidase
3.4.2. β-Amyloid Protein
3.4.3. Nucleic Acids
4. Conclusions and Perspectives
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Abbreviations
| Aβ | Amyloid Beta |
| AD | Alzheimer’s disease |
| ANCA | Aminonaphthalenyl-2-cyano-acrylate |
| ATS | Amphetamine-Type Stimulants |
| BF2bdk | Difluoroboron β-diketonate |
| DAE | Diarylethene |
| DASA | Donor–Acceptor Stenhouse Adduct |
| DCNP | Diethyl cyanophosphonate |
| DMPA | Dimethylphenylamine |
| DNBS | Dinitrobenzenesulfonyl |
| FRET | Fluorescence resonance energy transfer |
| GMA | Glycidyl methacrylate |
| GSH | Glutathione |
| Hcy | Homocysteine |
| HEK 293 | Human Embryonic Kidney 293 Cells |
| HTI | Hemithioindigo |
| HSA | Human serum albumin |
| ICT | Internal charge transfer |
| LMCT | Ligand–metal charge transfer |
| MAHD-SP | Spiro[azahomoadamantane-pyran] |
| MC | Merocyanine |
| NBD | Nitrobenzofurazan |
| NIR | Near-infrared |
| NMR | Nuclear magnetic resonance |
| PET | Positron emission tomography |
| PFO | Polyfluorene |
| RAFT | Reversible addition–fragmentation chain transfer |
| SPECT | Single-photon emission computed tomography |
| SP | Spiropyran |
| STORM | Stochastic optical reconstruction microscopy |
| TBET | Through-bond energy transfer |
References
- Kikuchi, K.; Adair, L.D.; Lin, J.; New, E.J.; Kaur, A. Photochemical Mechanisms of Fluorophores Employed in Single-Molecule Localization Microscopy. Angew. Chem. Int. Ed. 2023, 62, e202204745. [Google Scholar] [CrossRef]
- Liu, X.; Chang, Y.T. Fluorescent probe strategy for live cell distinction. Chem. Soc. Rev. 2022, 51, 1573–1591. [Google Scholar] [CrossRef]
- Ma, J.; Sun, R.; Xia, K.; Xia, Q.; Liu, Y.; Zhang, X. Design and Application of Fluorescent Probes to Detect Cellular Physical Microenvironments. Chem. Rev. 2024, 124, 1738–1861. [Google Scholar] [CrossRef]
- Wu, L.L.; Huang, J.G.; Pu, K.Y.; James, T.D. Dual-locked spectroscopic probes for sensing and therapy. Nat. Rev. Chem. 2021, 5, 406–421. [Google Scholar] [CrossRef]
- Tian, M.; Wu, R.; Xiang, C.; Niu, G.; Guan, W. Recent Advances in Fluorescent Probes for Cancer Biomarker Detection. Molecules 2024, 29, 1168. [Google Scholar] [CrossRef]
- Liu, B.; Zhang, Y.; Hao, Y.; Zhu, X.; Zhang, Y.; Zhou, Y.; Tan, H.; Xu, M. All-in-One Luminescent Lanthanide Coordination Polymer Nanoprobe for Facile Detection of Protein Kinase Activity. Anal. Chem. 2022, 94, 10730–10736. [Google Scholar] [CrossRef]
- Lee, H.; Lee, S.; Han, M.S. Turn-On Fluorescent pH Probes for Monitoring Alkaline pHs Using Bis[2-(2′-hydroxyphenyl)benzazole] Derivatives. Sensors 2023, 23, 2044. [Google Scholar] [CrossRef]
- Munan, S.; Yadav, R.; Pareek, N.; Samanta, A. Ratiometric fluorescent probes for pH mapping in cellular organelles. Analyst 2023, 148, 4242–4262. [Google Scholar] [CrossRef]
- Wang, F.; Lan, Y.; Zuo, Y. Polysiloxane-Based Molecular Logic Gate for Dual-Channel Visualizing Mitochondrial pH and Sulphite Changes during Cuproptosis. Anal. Chem. 2023, 95, 14484–14493. [Google Scholar] [CrossRef]
- Xu, M.; Xing, J.; Yuan, B.; He, L.; Lu, L.; Chen, N.; Cai, P.; Wu, A.; Li, J. Organic small-molecule fluorescent probe-based detection for alkali and alkaline earth metal ions in biological systems. J. Mater. Chem. B 2023, 11, 3295–3306. [Google Scholar] [CrossRef]
- Park, S.H.; Kwon, N.; Lee, J.H.; Yoon, J.; Shin, I. Synthetic ratiometric fluorescent probes for detection of ions. Chem. Soc. Rev. 2020, 49, 143–179. [Google Scholar] [CrossRef]
- Hao, Y.Q.; Chen, S.; Zhou, Y.L.; Zhang, Y.T.; Xu, M.T. Recent Progress in Metal-Organic Framework (MOF) Based Luminescent Chemodosimeters. Nanomaterials 2019, 9, 974. [Google Scholar] [CrossRef]
- Reja, S.I.; Minoshima, M.; Hori, Y.; Kikuchi, K. Near-infrared fluorescent probes: A next-generation tool for protein-labeling applications. Chem. Sci. 2021, 12, 3437–3447. [Google Scholar] [CrossRef]
- Yu, X.K.; Li, C.M.; Wang, B.Z.; Ding, X.P.; Wang, N.; Xing, B.A.; Zhang, Z.J. Protein-mediated fluorescent probes for bioimaging and biosensing: From fundamentals to applications. TrAC Trends Anal. Chem. 2024, 170, 117462. [Google Scholar] [CrossRef]
- Wan, W.; Huang, Y.A.; Xia, Q.X.; Bai, Y.L.; Chen, Y.W.; Jin, W.H.; Wang, M.D.; Shen, D.; Lyu, H.C.; Tang, Y.Q.; et al. Covalent Probes for Aggregated Protein Imaging via Michael Addition. Angew. Chem. Int. Ed. 2021, 60, 11335–11343. [Google Scholar] [CrossRef]
- Yang, G.Q.; Liu, Z.J.; Zhang, R.L.; Tian, X.H.; Chen, J.; Han, G.M.; Liu, B.H.; Han, X.Y.; Fu, Y.; Hu, Z.J.; et al. A Multi-responsive Fluorescent Probe Reveals Mitochondrial Nucleoprotein Dynamics with Reactive Oxygen Species Regulation through Super-resolution Imaging. Angew. Chem. Int. Ed. 2020, 59, 16154–16160. [Google Scholar] [CrossRef] [PubMed]
- Zhi, X.; Qian, Y. A novel red-emission phenothiazine fluorescent protein chromophore based on oxygen-chlorine bond (O-Cl) formation for real-time detection of hypochlorous acid in cells. Talanta 2021, 222, 121503. [Google Scholar] [CrossRef] [PubMed]
- Hao, Y.; Zhang, Y.; Sun, Q.; Chen, S.; Tang, Z.; Zeng, R.; Xu, M. Phenothiazine-coumarin-pyridine hybrid as an efficient fluorescent probe for ratiometric sensing hypochlorous acid. Microchem. J. 2021, 171, 106851. [Google Scholar] [CrossRef]
- Jin, X.L.; Ma, X.H.; Zhong, W.; Cao, Y.X.; Zhao, H.Q.; Leng, X.; Yang, J.J.; Zhou, H.W.; She, M.Y. Fluorescent sensing film decorated with ratiometric probe for visual and recyclable monitoring of Cu2+. Spectrochim. Acta Part A 2021, 249, 119217. [Google Scholar] [CrossRef]
- Wang, Y.F.; Li, Z.; Jiang, M.; Yu, X.; Xu, L. “Two-in-one” sulfur and nitrogen co-doped fluorescent silicon nanoparticles: Simultaneous as the fluorescent probe and photocatalyst for in-situ real time visual monitoring and degradation of tetracycline antibiotics. Sci. Total Environ. 2022, 846, 157470. [Google Scholar] [CrossRef]
- Hao, Y.Q.; Yin, Q.Y.; Zhang, Y.T.; Xu, M.T.; Chen, S. Recent Progress in the Development of Fluorescent Probes for Thiophenol. Molecules 2019, 24, 3716. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Hao, Y. Selective Near-Infrared Fluorescent Probe for Monitoring Thiophenol in Real Water Samples and Living Cells. Anal. Lett. 2021, 54, 1366–1376. [Google Scholar] [CrossRef]
- Qiu, X.-Y.; Liu, S.-J.; Hao, Y.-Q.; Sun, J.-W.; Chen, S. Phenothiazine-based fluorescence probe for ratiometric imaging of hydrazine in living cells with remarkable Stokes shift. Spectrochim. Acta Part A 2020, 227, 117675. [Google Scholar] [CrossRef] [PubMed]
- Zhu, J.L.; Jia, P.P.; Li, N.; Tan, S.Y.; Huang, J.H.; Xu, L. Small-molecule fluorescent probes for the detection of carbon dioxide. Chin. Chem. Lett. 2018, 29, 1445–1450. [Google Scholar] [CrossRef]
- Shershov, V.E.; Miftakhov, R.A.; Kuznetsova, V.E.; Timofeev, E.N.; Grechishnikova, I.V.; Spitsyn, M.A.; Guseinov, T.O.; Lapa, S.A.; Zasedatelev, A.S.; Chudinov, A.V. Fluorescent Labeling of Oligonucleotide Probes for Double Indicator Microarray Hybridization Analysis. Russ. J. Bioorg. Chem. 2019, 45, 217–220. [Google Scholar] [CrossRef]
- Hao, Y.Q.; Zhang, Y.T.; Zhang, A.M.; Sun, Q.L.; Zhu, J.; Qu, P.; Chen, S.; Xu, M.T. A benzothiazole-based ratiometric fluorescent probe for detection of formaldehyde and its applications for bioimaging. Spectrochim. Acta Part A 2020, 229, 117988. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Su, W.; Zheng, H.; Yang, S.; Yang, T.; Han, T.; Dessie, W.; He, X.; Jiang, Y.; Hao, Y. Two phenanthroimidazole turn-on probes for the rapid detection of selenocysteine and its application in living cells imaging. Spectrochim. Acta Part A 2022, 267, 120585. [Google Scholar] [CrossRef]
- Wang, Z.; Yang, S.; Liu, X.; Yang, T.; Han, T.; He, X.; Jiang, Y.; Hao, Y. A near-infrared turn-on fluorescent probe for the rapid detection of selenocysteine and its application of imaging in living cells and mice. Microchem. J. 2021, 170, 106681. [Google Scholar] [CrossRef]
- You, J.B.; Lohse, D.; Zhang, X.H. Surface nanodroplet-based nanoextraction from sub-milliliter volumes of dense suspensions. Lab Chip 2021, 21, 2574–2585. [Google Scholar] [CrossRef]
- Shenashen, M.A.; Emran, M.Y.; El Sabagh, A.; Selim, M.M.; Elmarakbi, A.; El-Safty, S.A. Progress in sensory devices of pesticides, pathogens, coronavirus, and chemical additives and hazards in food assessment: Food safety concerns. Prog. Mater. Sci. 2022, 124, 100866. [Google Scholar] [CrossRef]
- Gao, M.; Yu, F.B.; Lv, C.J.; Choo, J.; Chen, L.X. Fluorescent chemical probes for accurate tumor diagnosis and targeting therapy. Chem. Soc. Rev. 2017, 46, 2237–2271. [Google Scholar] [CrossRef] [PubMed]
- Nerella, S.G.; Singh, P.; Thacker, P.S.; Arifuddin, M.; Supuran, C.T. PET radiotracers and fluorescent probes for imaging human carbonic anhydrase IX and XII in hypoxic tumors. Bioorg. Chem. 2023, 133, 106399. [Google Scholar] [CrossRef] [PubMed]
- Olesińska-Mönch, M.; Deo, C. Small-molecule photoswitches for fluorescence bioimaging: Engineering and applications. Chem. Commun. 2023, 59, 660–669. [Google Scholar] [CrossRef] [PubMed]
- Keyvan Rad, J.; Balzade, Z.; Mahdavian, A.R. Spiropyran-based advanced photoswitchable materials: A fascinating pathway to the future stimuli-responsive devices. J. Photochem. Photobiol. C 2022, 51, 100487. [Google Scholar] [CrossRef]
- Li, M.; Zhao, J.; Chu, H.; Mi, Y.; Zhou, Z.; Di, Z.; Zhao, M.; Li, L. Light-Activated Nanoprobes for Biosensing and Imaging. Adv. Mater. 2019, 31, 1804745. [Google Scholar] [CrossRef]
- Jia, S.; Fong, W.-K.; Graham, B.; Boyd, B.J. Photoswitchable Molecules in Long-Wavelength Light-Responsive Drug Delivery: From Molecular Design to Applications. Chem. Mater. 2018, 30, 2873–2887. [Google Scholar] [CrossRef]
- Huo, Z.; Li, G.; Xiao, X. Applications of Organic Photochromic Materials in Rapid Visual Detection. Prog. Chem. 2017, 29, 252–261. [Google Scholar] [CrossRef]
- Zhong, W.C.; Liang, K.Q.; Liu, W.F.; Shang, L. Ligand-protected nanocluster-mediated photoswitchable fluorescent nanoprobes towards dual-color cellular imaging. Chem. Sci. 2023, 14, 8823–8830. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Ye, Z.J.; Deng, Y.A.; Yuan, J.; Wei, L.; Xiao, L.H. Blinking fluorescent probes for single-molecule localization-based super-resolution imaging. TrAC Trends Anal. Chem. 2023, 169, 117359. [Google Scholar] [CrossRef]
- Zhang, Z.; Wang, W.; O’Hagan, M.; Dai, J.; Zhang, J.; Tian, H. Stepping Out of the Blue: From Visible to Near-IR Triggered Photoswitches. Angew. Chem. Int. Ed. 2022, 61, e202205758. [Google Scholar] [CrossRef]
- Jia, S.; Sletten, E.M. Spatiotemporal Control of Biology: Synthetic Photochemistry Toolbox with Far-Red and Near-Infrared Light. ACS Chem. Biol. 2022, 17, 3255–3269. [Google Scholar] [CrossRef] [PubMed]
- Dong, M.; Babalhavaeji, A.; Collins, C.V.; Jarrah, K.; Sadovski, O.; Dai, Q.; Woolley, G.A. Near-Infrared Photoswitching of Azobenzenes under Physiological Conditions. J. Am. Chem. Soc. 2017, 139, 13483–13486. [Google Scholar] [CrossRef] [PubMed]
- Chatterjee, S.; Liu, B.; Peng, H.-S. Chelation strategies in spiropyran-based chemosensors for colorimetric and fluorescent sensing of metal ions and anions. Coord. Chem. Rev. 2024, 508, 215779. [Google Scholar] [CrossRef]
- Kumar, A.; Kumar, S. Light-controlled receptors for environmentally and biologically relevant anions. Chem. Eng. J. 2023, 474, 145493. [Google Scholar] [CrossRef]
- Ali, A.A.; Kharbash, R.; Kim, Y. Chemo- and biosensing applications of spiropyran and its derivatives—A review. Anal. Chim. Acta 2020, 1110, 199–223. [Google Scholar] [CrossRef]
- Mandal, M.; Banik, D.; Karak, A.; Manna, S.K.; Mahapatra, A.K. Spiropyran–Merocyanine Based Photochromic Fluorescent Probes: Design, Synthesis, and Applications. ACS Omega 2022, 7, 36988–37007. [Google Scholar] [CrossRef]
- Xia, H.; Xie, K.; Zou, G. Advances in Spiropyrans/Spirooxazines and Applications Based on Fluorescence Resonance Energy Transfer (FRET) with Fluorescent Materials. Molecules 2017, 22, 2236. [Google Scholar] [CrossRef]
- Lee, J.; Choi, E.J.; Kim, I.; Lee, M.; Satheeshkumar, C.; Song, C. Tuning Sensory Properties of Triazole-Conjugated Spiropyrans: Metal-Ion Selectivity and Paper-Based Colorimetric Detection of Cyanide. Sensors 2017, 17, 1816. [Google Scholar] [CrossRef] [PubMed]
- Shi, C.; Zhu, R.; Guo, K.; Zhang, L.; Liu, Z.; Liu, X.; Ai, Q.; Hu, X. Recent Progress of Gated Diarylethenes: Strategies, Mechanism, and Applications. Adv. Opt. Mater. 2023, 11, 2301844. [Google Scholar] [CrossRef]
- Cheng, H.-B.; Zhang, S.; Bai, E.; Cao, X.; Wang, J.; Qi, J.; Liu, J.; Zhao, J.; Zhang, L.; Yoon, J. Future-Oriented Advanced Diarylethene Photoswitches: From Molecular Design to Spontaneous Assembly Systems. Adv. Mater. 2022, 34, 2108289. [Google Scholar] [CrossRef]
- Pu, S.-Z.; Sun, Q.; Fan, C.-B.; Wang, R.-J.; Liu, G. Recent advances in diarylethene-based multi-responsive molecular switches. J. Mater. Chem. C 2016, 4, 3075–3093. [Google Scholar] [CrossRef]
- Zhang, G.F.; Chen, T.; Li, C.; Gong, W.L.; Aldred, M.P.; Zhu, M.Q. Spiropyran-Based Molecular Photoswitches. Chin. J. Org. Chem. 2013, 33, 927–942. [Google Scholar] [CrossRef]
- Li, C.; Iscen, A.; Palmer, L.C.; Schatz, G.C.; Stupp, S.I. Light-Driven Expansion of Spiropyran Hydrogels. J. Am. Chem. Soc. 2020, 142, 8447–8453. [Google Scholar] [CrossRef] [PubMed]
- Klajn, R. Spiropyran-based dynamic materials. Chem. Soc. Rev. 2014, 43, 148–184. [Google Scholar] [CrossRef] [PubMed]
- Kortekaas, L.; Browne, W.R. The evolution of spiropyran: Fundamentals and progress of an extraordinarily versatile photochrome. Chem. Soc. Rev. 2019, 48, 3406–3424. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.J.; Zou, Q.; Tian, H. Photochromic Materials: More Than Meets The Eye. Adv. Mater. 2013, 25, 378–399. [Google Scholar] [CrossRef] [PubMed]
- Tang, Y.Y.; Zeng, Y.L.; Xiong, R.G. Contactless Manipulation of Write-Read-Erase Data Storage in Diarylethene Ferroelectric Crystals. J. Am. Chem. Soc. 2022, 144, 8633–8640. [Google Scholar] [CrossRef]
- Li, E.L.; He, W.X.; Yu, R.J.; He, L.H.; Wu, X.M.; Chen, Q.Z.; Liu, Y.; Chen, H.P.; Guo, T.L. High-Density Reconfigurable Synaptic Transistors Targeting a Minimalist Neural Network. ACS Appl. Mater. Interfaces 2021, 13, 28564–28573. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.; Aktalay, A.; Jensen, N.; Uno, K.; Bossi, M.L.; Belov, V.N.; Hell, S.W. Supramolecular Complex of Photochromic Diarylethene and Cucurbit[7]uril: Fluorescent Photoswitching System for Biolabeling and Imaging. J. Am. Chem. Soc. 2022, 144, 14235–14247. [Google Scholar] [CrossRef]
- Li, Z.Q.; Liu, X.; Wang, G.N.; Li, B.; Chen, H.Z.; Li, H.R.; Zhao, Y.L. Photoresponsive supramolecular coordination polyelectrolyte as smart anticounterfeiting inks. Nat. Commun. 2021, 12, 1363. [Google Scholar] [CrossRef]
- Lin, S.Y.; Gutierrez-Cuevas, K.G.; Zhang, X.F.; Guo, J.B.; Li, Q. Fluorescent Photochromic α-Cyanodiarylethene Molecular Switches: An Emerging and Promising Class of Functional Diarylethene. Adv. Funct. Mater. 2021, 31, 2007957. [Google Scholar] [CrossRef]
- Göstl, R.; Hecht, S. Photoreversible Prodrugs and Protags: Switching the Release of Maleimides by Using Light under Physiological Conditions. Chem.-Eur. J. 2015, 21, 4422–4427. [Google Scholar] [CrossRef] [PubMed]
- Yue, C.; Liao, C.L.; Yang, Z.W.; Hu, F. Recent Advances in Photoswitchable Cation Chemosensors. Curr. Org. Chem. 2018, 22, 1458–1467. [Google Scholar] [CrossRef]
- Cheng, H.B.; Zhang, S.C.; Qi, J.; Liang, X.J.; Yoon, J. Advances in Application of Azobenzene as a Trigger in Biomedicine: Molecular Design and Spontaneous Assembly. Adv. Mater. 2021, 33, 2007290. [Google Scholar] [CrossRef] [PubMed]
- Schultzke, S.; Puylaert, P.; Wang, H.; Schultzke, I.; Gerken, J.; Staubitz, A. Centennial Isomers: A Unique Fluorinated Azobenzene Macrocyclus with Dual Stability Over 120 Years. Adv. Funct. Mater. 2024, 2313268. [Google Scholar] [CrossRef]
- Fukaminato, T.; Kato, T.; Hashimoto, T.; Matsumoto, Y.; Kim, S.; Kurihara, S. Non-volatile optical memory based on cooperative orientation switching: Improvement of recording speed and contrast by utilizing out-of-plane orientation mode. Photochem. Photobiol. Sci. 2023, 22, 857–865. [Google Scholar] [CrossRef] [PubMed]
- Yu, S.H.; Hassan, S.Z.; Nam, G.H.; An, S.; Kang, B.; Chung, D.S. Consideration of Azobenzene-Based Self-Assembled Monolayer Deposition Conditions for Maximizing Optoelectronic Switching Performances. Chem. Mater. 2021, 33, 5991–6002. [Google Scholar] [CrossRef]
- Yao, J.B.; Wu, W.H.; Xiao, C.; Su, D.; Zhong, Z.H.; Mori, T.; Yang, C. Overtemperature-protection intelligent molecular chiroptical photoswitches. Nat. Commun. 2021, 12, 2600. [Google Scholar] [CrossRef] [PubMed]
- Kuang, Z.Y.; Deng, Y.; Hu, J.; Tao, L.; Wang, P.; Chen, J.; Xie, H.L. Responsive Smart Windows Enabled by the Azobenzene Copolymer Brush with Photothermal Effect. ACS Appl. Mater. Interfaces 2019, 11, 37026–37034. [Google Scholar] [CrossRef]
- Oh, S.W.; Kim, S.H.; Yoon, T.H. Self-shading by optical or thermal control of transmittance with liquid crystals doped with push-pull azobenzene. Sol. Energy Mater. Sol. Cells 2018, 183, 146–150. [Google Scholar] [CrossRef]
- Zhu, J.D.; Guo, T.; Wang, Z.; Zhao, Y.J. Triggered azobenzene-based prodrugs and drug delivery systems. J. Control. Release 2022, 345, 475–493. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.C.; Shang, Y.Y.; Liu, J.; Wang, J.X.; Ikeda, T.; Jiang, L. Janus Photochemical/Photothermal Azobenzene Inverse Opal Actuator with Shape Self-Recovery toward Sophisticated Motion. ACS Appl. Mater. Interfaces 2022, 14, 1727–1739. [Google Scholar] [CrossRef] [PubMed]
- Varghese, S.; Fredrich, S.; Vantomme, G.; Prabhu, S.R.; Teyssandier, J.; De Feyter, S.; Severn, J.; Bastiaansen, C.W.M.; Schenning, A. Epitaxial growth of light-responsive azobenzene molecular crystal actuators on oriented polyethylene films. J. Mater. Chem. C 2020, 8, 694–699. [Google Scholar] [CrossRef]
- Gomes, R.F.A.; Coelho, J.A.S.; Afonso, C.A.M. Synthesis and Applications of Stenhouse Salts and Derivatives. Chem.-Eur. J. 2018, 24, 9170–9186. [Google Scholar] [CrossRef] [PubMed]
- Duan, Y.; Song, M.; Sun, F.; Xu, Y.; Shi, F.; Wang, H.; Zheng, Y.; He, C.; Liu, X.; Wei, C.; et al. Controlling Isomerization of Photoswitches to Modulate 2D Logic-in-Memory Devices by Organic–Inorganic Interfacial Strategy. Adv. Sci. 2023, 10, 2207443. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Zheng, S.; Liang, B.; Wu, X.; Wang, D.; Dong, Y.; Huang, W.; Liu, Y.; Yu, X.; Shen, J.; et al. Realizing a Brain-Like Transistor Memory with Triple Data-Storage Modes by One Single Smart Molecular Dopant in the Dielectric Layer. Chem. Mater. 2023, 35, 2808–2819. [Google Scholar] [CrossRef]
- Stricker, F.; Sanchez, D.M.; Raucci, U.; Dolinski, N.D.; Zayas, M.S.; Meisner, J.; Hawker, C.J.; Martínez, T.J.; de Alaniz, J.R. A multi-stage single photochrome system for controlled photoswitching responses. Nat. Chem. 2022, 14, 942–948. [Google Scholar] [CrossRef] [PubMed]
- Clerc, M.; Sandlass, S.; Rifaie-Graham, O.; Peterson, J.A.; Bruns, N.; Read de Alaniz, J.; Boesel, L.F. Visible light-responsive materials: The (photo)chemistry and applications of donor–acceptor Stenhouse adducts in polymer science. Chem. Soc. Rev. 2023, 52, 8245–8294. [Google Scholar] [CrossRef]
- Kitzig, S.; Thilemann, M.; Cordes, T.; Rück-Braun, K. Light-Switchable Peptides with a Hemithioindigo Unit: Peptide Design, Photochromism, and Optical Spectroscopy. Chemphyschem 2016, 17, 1252–1263. [Google Scholar] [CrossRef]
- Kohl, F.; Gerwien, A.; Hampel, F.; Mayer, P.; Dube, H. Hemithioindigo-Based Trioxobicyclononadiene: 3D Multiswitching of Electronic and Geometric Properties. J. Am. Chem. Soc. 2022, 144, 2847–2852. [Google Scholar] [CrossRef]
- Köttner, L.; Schildhauer, M.; Wiedbrauk, S.; Mayer, P.; Dube, H. Oxidized Hemithioindigo Photoswitches-Influence of Oxidation State on (Photo)physical and Photochemical Properties. Chem.-Eur. J. 2020, 26, 10712–10718. [Google Scholar] [CrossRef] [PubMed]
- Sailer, A.; Meiring, J.C.M.; Heise, C.; Pettersson, L.N.; Akhmanova, A.; Thorn-Seshold, J.; Thorn-Seshold, O. Pyrrole Hemithioindigo Antimitotics with Near-Quantitative Bidirectional Photoswitching that Photocontrol Cellular Microtubule Dynamics with Single-Cell Precision. Angew. Chem. Int. Ed. 2021, 60, 23695–23704. [Google Scholar] [CrossRef]
- Leng, A.; Weiss, C.; Strassner, N.; Hirsch, A. Reversible Photoinduced Conversion of Unprecedented Norbornadiene-Based Photoswitches with Redox-Active Naphthalene Diimide Functionalities. Chem.-Eur. J. 2022, 28, e202201446. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.H.; Erhart, P.; Li, T.; Zhang, Z.Y.; Sampedro, D.; Hu, Z.Y.; Wegner, H.A.; Brummel, O.; Libuda, J.; Nielsen, M.B.; et al. Storing energy with molecular photoisomers. Joule 2021, 5, 3116–3136. [Google Scholar] [CrossRef]
- Schulte, R.; Afflerbach, S.; Paululat, T.; Ihmels, H. Bis- and Tris-norbornadienes with High Energy Densities for Efficient Molecular Solar Thermal Energy Storage. Angew. Chem. Int. Ed. 2023, 62, e202309544. [Google Scholar] [CrossRef] [PubMed]
- Ren, T.B.; Wang, Z.Y.; Xiang, Z.; Lu, P.; Lai, H.H.; Yuan, L.; Zhang, X.B.; Tan, W.H. A General Strategy for Development of Activatable NIR-II Fluorescent Probes for In Vivo High-Contrast Bioimaging. Angew. Chem. Int. Ed. 2021, 60, 800–805. [Google Scholar] [CrossRef]
- Hou, J.T.; Ren, W.X.; Li, K.; Seo, J.; Sharma, A.; Yu, X.Q.; Kim, J.S. Fluorescent bioimaging of pH: From design to applications. Chem. Soc. Rev. 2017, 46, 2076–2090. [Google Scholar] [CrossRef]
- Wang, R.; Wang, N.; Pu, S.; Zhang, X.; Liu, G.; Dai, Y. A acid/base gated photochromic and fluorescent sensor based on a diarylethene with a 2-(1H-dithienobenzoimidazole)phenol unit. Dye. Pigment. 2017, 146, 445–454. [Google Scholar] [CrossRef]
- Li, Z.; Wang, Y.; Li, M.; Chen, H.; Xie, Y.; Li, P.; Guo, H.; Ya, H. Solvent-dependent and visible light-activated NIR photochromic dithienylethene modified by difluoroboron β-diketonates as fluorescent turn-on pH sensor. Dye. Pigment. 2019, 162, 339–347. [Google Scholar] [CrossRef]
- Keyvan Rad, J.; Ghomi, A.R.; Karimipour, K.; Mahdavian, A.R. Progressive Readout Platform Based on Photoswitchable Polyacrylic Nanofibers Containing Spiropyran in Photopatterning with Instant Responsivity to Acid–Base Vapors. Macromolecules 2020, 53, 1613–1622. [Google Scholar] [CrossRef]
- Zhang, W.; Cheng, Y.; Wu, M.; Xie, X. Photoswitchable chemical sensing based on the colorimetric pH response of ring-opened naphthopyrans. Sens. Actuators B 2024, 407, 135475. [Google Scholar] [CrossRef]
- Hou, I.C.-Y.; Berger, F.; Narita, A.; Müllen, K.; Hecht, S. Proton-Gated Ring-Closure of a Negative Photochromic Azulene-Based Diarylethene. Angew. Chem. Int. Ed. 2020, 59, 18532–18536. [Google Scholar] [CrossRef] [PubMed]
- Zhong, D.; Cao, Z.; Wu, B.; Zhang, Q.; Wang, G. Polymer dots of DASA-functionalized polyethyleneimine: Synthesis, visible light/pH responsiveness, and their applications as chemosensors. Sens. Actuators B 2018, 254, 385–392. [Google Scholar] [CrossRef]
- Li, J.; Ma, X.; Wang, Y.; Cheng, Y.; Qin, Y.; Zhai, J.; Xie, X. Proton-Coupled Photochromic Hemithioindigo: Toward Photoactivated Chemical Sensing and Imaging. Anal. Chem. 2023, 95, 11664–11671. [Google Scholar] [CrossRef]
- Hiruta, Y.; Shindo, Y.; Oka, K.; Citterio, D. Small Molecule-based Alkaline-earth Metal Ion Fluorescent Probes for Imaging Intracellular and Intercellular Multiple Signals. Chem. Lett. 2021, 50, 870–887. [Google Scholar] [CrossRef]
- Matsui, Y.; Kowada, T.; Ding, Y.; Sahoo, P.R.; Kikuchi, K.; Mizukami, S. Long-term imaging of intranuclear Mg2+ dynamics during mitosis using a localized fluorescent probe. Chem. Commun. 2023, 59, 7048–7051. [Google Scholar] [CrossRef] [PubMed]
- Zhou, X.Q.; Belavek, K.J.; Miller, E.W. Origins of Ca2+ Imaging with Fluorescent Indicators. Biochemistry 2021, 60, 3547–3554. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Cui, S.; Qiu, S.; Pu, S. A highly selective fluorescence “turn-on” sensor for Ca2+ based on diarylethene with a triazozoyl hydrazine unit. RSC Adv. 2018, 8, 29295–29300. [Google Scholar] [CrossRef] [PubMed]
- Takaku, S.; Nishimura, R.; Morimoto, M. A turn-on mode fluorescent diarylethene having an azacrown ether receptor: Metal-ion-gated enhancement of the photoreactivity and fluorescence. Dye. Pigment. 2023, 216, 111354. [Google Scholar] [CrossRef]
- Zhang, Y.; Li, H.; Jiang, D.; Pu, S. A highly selective fluorescent chemosensor for Mg2+ based on a diarylethene with a quinoxaline unit. J. Phys. Org. Chem. 2020, 33, e4030. [Google Scholar] [CrossRef]
- Yuwen, Z.; Mei, H.; Li, H.; Pu, S. A novel diarylethene probe with high selective recognition of CN− and Mg2+ and its application. J. Photochem. Photobiol. A 2020, 401, 112754. [Google Scholar] [CrossRef]
- Wang, Z.; Cui, S.; Qiu, S.; Pu, S. A novel diarylethene-based fluorescent “turn-on” sensor for the selective detection of Mg2+. RSC Adv. 2019, 9, 6021–6026. [Google Scholar] [CrossRef] [PubMed]
- Ducrot, A.; Tron, A.; Bofinger, R.; Sanz Beguer, I.; Pozzo, J.-L.; McClenaghan, N.D. Photoreversible stretching of a BAPTA chelator marshalling Ca2+-binding in aqueous media. Beilstein J. Org. Chem. 2019, 15, 2801–2811. [Google Scholar] [CrossRef] [PubMed]
- Kou, X.D.; Hu, C.T.; Pang, Z.; Zhang, X.Y.; Wang, H.Y.; Shen, R.; Yang, A.H. A coumarin-based multifunctional chemosensor for Cu2+/Al3+ as an AD theranostic agent: Synthesis, X-ray single crystal analysis and activity study. Anal. Chim. Acta 2023, 1279, 341818. [Google Scholar] [CrossRef] [PubMed]
- Li, W.T.; Zhang, L.M.; Jiang, N.J.; Chen, Y.Q.; Gao, J.; Zhang, J.H.; Yang, B.S.; Liu, J.L. Fabrication of Orange Fluorescent Boron-Doped Graphene Quantum Dots for Al3+ Ion Detection. Molecules 2022, 27, 6771. [Google Scholar] [CrossRef] [PubMed]
- Pu, S.; Zhang, C.; Fan, C.; Liu, G. Multi-controllable properties of an antipyrine-based diarylethene and its high selectivity for recognition of Al3+. Dye. Pigment. 2016, 129, 24–33. [Google Scholar] [CrossRef]
- Wang, Z.; Shu, L.; Luo, X.; Tu, Y.; Lv, J.; Liu, G.; Fan, C.; Pu, S. A New High Selective and Sensitive Fluorescent Probe for Al3+ based on Photochromic Salicylaldehyde Hydrazyl Diarylethene. J. Fluoresc. 2022, 32, 2213–2222. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Li, H.; Liu, G.; Pu, S. A novel fluorescent sensor for Al3+ based on a new diarylethene with a naphthalimide unit. J. Photochem. Photobiol. A 2017, 338, 192–200. [Google Scholar] [CrossRef]
- Wang, Z.; Cui, S.; Qiu, S.; Pu, S. A dual-functional fluorescent sensor based on diarylethene for Zn2+ and Al3+ in different solvents. J. Photochem. Photobiol. A 2019, 376, 185–195. [Google Scholar] [CrossRef]
- Lv, J.; Fu, Y.; Liu, G.; Fan, C.; Pu, S. A turn-on fluorescence sensor for the highly selective detection of Al3+ based on diarylethene and its application on test strips. RSC Adv. 2019, 9, 10395–10404. [Google Scholar] [CrossRef]
- Li, S.Y.; Zhang, D.B.; Wang, J.Y.; Lu, R.M.; Zheng, C.H.; Pu, S.Z. A novel diarylethene-hydrazinopyridine-based probe for fluorescent detection of aluminum ion and naked-eye detection of hydroxide ion. Sens. Actuators B 2017, 245, 263–272. [Google Scholar] [CrossRef]
- Zhang, X.; Wang, R.; Liu, G.; Fan, C.; Pu, S. A highly selective fluorescence probe for Al3+ based on a new diarylethene with a 6-(hydroxymethyl)picolinohydrazide unit. Tetrahedron 2016, 72, 8449–8455. [Google Scholar] [CrossRef]
- Lu, R.; Cui, S.; Li, S.; Pu, S. A highly sensitive and selective fluorescent sensor for Al3+ and Zn2+ based on diarylethene with an aminouracil unit. Tetrahedron 2017, 73, 915–922. [Google Scholar] [CrossRef]
- Liang, Y.; Diao, L.; Wang, R.; Wang, N.; Pu, S. A bifunctional probe for Al3+ and Zn2+ based on diarylethene with an ethylimidazo[2,1-b]thiazole-6-hydrazide unit. Tetrahedron Lett. 2019, 60, 106–112. [Google Scholar] [CrossRef]
- Lu, M.; Qiu, S.; Cui, S.; Pu, S. A double target fluorescent sensor based on diarylethene for detection of Al3+ and Zn2+. Tetrahedron Lett. 2020, 61, 152372. [Google Scholar] [CrossRef]
- Raman, A.; Augustine, G.; Ayyadurai, N.; Easwaramoorthi, S. Photoswitchable azobenzene–rhodamine tweezers for biosensing of Al3+ ions. New J. Chem. 2018, 42, 9300–9305. [Google Scholar] [CrossRef]
- Cai, Y.-D.; Chen, T.-Y.; Chen, X.Q.; Bao, X. Multiresponsive Donor–Acceptor Stenhouse Adduct: Opportunities Arise from a Diamine Donor. Org. Lett. 2019, 21, 7445–7449. [Google Scholar] [CrossRef]
- Fu, P.; Yan, Q.; Wang, S.; Wu, H.; Cao, D. A visible-light-gated donor–acceptor Stenhouse adduct chemosensor: Synthesis, photochromism and naked-eye colorimetric/fluorometric sensing of Al3+ and Zn2+. New J. Chem. 2022, 46, 12600–12608. [Google Scholar] [CrossRef]
- Yu, J.H.; Yu, H.T.; Wang, S.S.; Qi, Y.Y. Progress in research of zinc ion fluorescent probes for biological imaging. J. Lumin. 2024, 266, 120318. [Google Scholar] [CrossRef]
- Liu, R.; Kowada, T.; Du, Y.Y.; Amagai, Y.; Matsui, T.; Inaba, K.; Mizukami, S. Organelle-Level Labile Zn2+ Mapping Based on TargetableFluorescent Sensors. ACS Sens. 2022, 7, 748–757. [Google Scholar] [CrossRef]
- Fu, Y.; Fan, C.; Liu, G.; Cui, S.; Pu, S. A highly selective and sensitive ratiometric fluorescent chemosensor for Zn2+ based on diarylethene with a benzyl-linked 8-aminoquinoline-2-aminomethylpyridine unit. Dye. Pigment. 2016, 126, 121–130. [Google Scholar] [CrossRef]
- Zhang, X.; Li, H.; Liu, G.; Pu, S. A novel diarylethene-based fluorescent switch with a carboxamidoquinoline unit for sensing of Zn(II) ion. J. Photochem. Photobiol. A 2016, 330, 22–29. [Google Scholar] [CrossRef]
- Wang, R.; Wang, N.; Tu, Y.; Liu, G.; Pu, S. A new fluorescence sensor based on diarylethene with a N′-(quinolin-8-ylmethylene)benzohydrazide group for Zn2+ detection. J. Photochem. Photobiol. A 2018, 364, 32–39. [Google Scholar] [CrossRef]
- Liu, F.; Fan, C.; Pu, S. A new “turn-on” fluorescent chemosensor for Zn2+ based on a diarylethene derivative and its practical applications. J. Photochem. Photobiol. A 2019, 371, 248–254. [Google Scholar] [CrossRef]
- Mao, L.; Ding, H.; Li, X.; Liu, G.; Pu, S. A diarylethene-based fluorescent chemosensor for highly selective recognition of Zn2+ and its application. J. Photochem. Photobiol. A 2022, 431, 114011. [Google Scholar] [CrossRef]
- Liang, R.; Luo, X.; Luo, Y.; Liu, F.; Hu, Z.; Xiong, Z.; Tu, Y.; Fan, C. A highly selective and sensitive fluorescent probe for Zn2+ based on diarylethene with a thienyl hydrazide unit. J. Photochem. Photobiol. A 2023, 445, 115107. [Google Scholar] [CrossRef]
- Tian, Z.; Cui, S.; Liu, G.; Wang, R.; Pu, S. A new fluorescent sensor for Zn2+ based on diarylethene with a 4-diethylamino-salicylaldehyde Schiff base unit. J. Phys. Org. Chem. 2016, 29, 421–429. [Google Scholar] [CrossRef]
- Lu, M.; Qiu, S.; Cui, S.; Pu, S. A novel diarylethene-based fluorescence sensor with a benzohydrazide unit for the detection of Zn2+. J. Phys. Org. Chem. 2020, 33, e4113. [Google Scholar] [CrossRef]
- Mao, L.; Li, X.; Ding, H.; Fan, C.; Liu, G.; Pu, S. A Highly Selective Hg2+ Fluorescent Chemosensor Based On Photochromic Diarylethene With Quinoline Unit. J. Fluoresc. 2022, 32, 2119–2128. [Google Scholar] [CrossRef] [PubMed]
- Feng, E.; Tu, Y.; Fan, C.; Liu, G.; Pu, S. A highly selective and sensitive fluorescent chemosensor for Zn2+ based on a diarylethene derivative. RSC Adv. 2017, 7, 50188–50194. [Google Scholar] [CrossRef]
- Tian, Z.; Cui, S.; Zheng, C.; Pu, S. A multi-state fluorescent switch based on a diarylethene with an acridine unit. Spectrochim. Acta Part A 2017, 173, 75–81. [Google Scholar] [CrossRef] [PubMed]
- Heng, S.; Reineck, P.; Vidanapathirana, A.K.; Pullen, B.J.; Drumm, D.W.; Ritter, L.J.; Schwarz, N.; Bonder, C.S.; Psaltis, P.J.; Thompson, J.G.; et al. Rationally Designed Probe for Reversible Sensing of Zinc and Application in Cells. ACS Omega 2017, 2, 6201–6210. [Google Scholar] [CrossRef] [PubMed]
- Sheline, C.T.; Choi, D.W. Cu2+ toxicity inhibition of mitochondrial dehydrogenases in vitro and in vivo. Ann. Neurol. 2004, 55, 645–653. [Google Scholar] [CrossRef] [PubMed]
- Falcone, E.; Okafor, M.; Vitale, N.; Raibaut, L.; Sour, A.; Faller, P. Extracellular Cu2+ pools and their detection: From current knowledge to next-generation probes. Coord. Chem. Rev. 2021, 433, 213727. [Google Scholar] [CrossRef]
- Yin, C.X.; Li, J.W.; Huo, F.J. Cu2+ Biological Imaging Probes Based on Different Sensing Mechanisms. Curr. Med. Chem. 2019, 26, 3958–4002. [Google Scholar] [CrossRef]
- Tang, L.J.; Xia, J.Y.; Zhong, K.L.; Tang, Y.W.; Gao, X.; Li, J.R. A simple AIE-active fluorogen for relay recognition of Cu2+ and pyrophosphate through aggregation-switching strategy. Dye. Pigment. 2020, 178, 108379. [Google Scholar] [CrossRef]
- Pu, S.; Ma, L.; Liu, G.; Ding, H.; Chen, B. A multiple switching diarylethene with a phenyl-linked rhodamine B unit and its application as chemosensor for Cu2+. Dye. Pigment. 2015, 113, 70–77. [Google Scholar] [CrossRef]
- Ma, L.; Liu, G.; Pu, S.; Ding, H.; Li, G. A highly selective fluorescent chemosensor for Cu2+ based on a new diarylethene with triazole-linked fluorescein. Tetrahedron 2016, 72, 985–991. [Google Scholar] [CrossRef]
- Guo, S.; Liu, G.; Fan, C.; Pu, S. A new diarylethene-derived probe for colorimetric sensing of Cu(II) and fluorometric sensing of Cu(II) and Zn(II): Photochromism and High Selectivity. Sens. Actuators B 2018, 266, 603–613. [Google Scholar] [CrossRef]
- Gao, W.; Li, H.; Pu, S. A highly selective fluorescent probe for Cu2+ based on a diarylethene with a benzo[1,2,5]oxadiazol-4-ylamine Schiff base unit. J. Photochem. Photobiol. A 2018, 364, 208–218. [Google Scholar] [CrossRef]
- Li, L.; Li, H.; Liu, G.; Pu, S. A colorimetric and fluorescent chemosensor for selective detection of Cu2+ based on a new diarylethene with a benzophenone hydrazone unit. Luminescence 2017, 32, 1473–1481. [Google Scholar] [CrossRef] [PubMed]
- Fu, Y.; Fan, C.; Liu, G.; Pu, S. A colorimetric and fluorescent sensor for Cu2+ and F− based on a diarylethene with a 1,8-naphthalimide Schiff base unit. Sens. Actuators B 2017, 239, 295–303. [Google Scholar] [CrossRef]
- Chen, Q.; Wang, Y.; Zhai, J.; Xie, X. Photoswitchable hemithioindigo inspired copper ion selective sensing with excellent selectivity and versatile operational modes. Sens. Actuators B 2023, 381, 133437. [Google Scholar] [CrossRef]
- Lin, Z.; Wang, S.; Yan, Q.; Yan, Q.; Cao, D. Visible light/Cu2+ dual triggered photochromic donor-acceptor stenhouse adducts: Synthesis, properties and applications. Dye. Pigment. 2021, 191, 109384. [Google Scholar] [CrossRef]
- Xu, H.; Ding, H.; Li, G.; Fan, C.; Liu, G.; Pu, S. A highly selective fluorescent chemosensor for Fe3+ based on a new diarylethene with a rhodamine 6G unit. RSC Adv. 2017, 7, 29827–29834. [Google Scholar] [CrossRef]
- Karmakar, M.; Pal, A.; Mondal, B.; Adarsh, N.N.; Thakur, A. Light-Triggered Metal Coordination Dynamics in Photoswitchable Dithienylethene–Ferrocene System. Inorg. Chem. 2021, 60, 6086–6098. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Zheng, C.; Xu, D.; Liao, G.; Pu, S. A fluorescent sensor for Zn2+ and Cd2+ based on a diarylethene derivative with an indole-2-methylhydrazone moiety. J. Photochem. Photobiol. A 2022, 424, 113634. [Google Scholar] [CrossRef]
- Wang, R.; Li, M.; Li, G.; Pu, S. High-contrast multicolour photoswitching based on dithienylethene Schiff base with a hydrazinylquinoline moiety. Spectrochim. Acta Part A 2024, 308, 123679. [Google Scholar] [CrossRef] [PubMed]
- Kang, H.; Xu, H.; Fan, C.; Liu, G.; Pu, S. A new sensitive symmetric fluorescein-linked diarylethene chemosensor for Hg2+ detection. J. Photochem. Photobiol. A 2018, 367, 465–470. [Google Scholar] [CrossRef]
- Zhao, H.; Ding, H.; Kang, H.; Fan, C.; Liu, G.; Pu, S. A solvent-dependent chemosensor for fluorimetric detection of Hg2+ and colorimetric detection of Cu2+ based on a new diarylethene with a rhodamine B unit. RSC Adv. 2019, 9, 42155–42162. [Google Scholar] [CrossRef]
- Zhao, H.; Kang, H.; Fan, C.; Liu, G.; Pu, S. A new multi-functional fluorescent mercuric ion sensor based on diarylethene with triazole-linked rhodamine B unit. Tetrahedron 2020, 76, 131393. [Google Scholar] [CrossRef]
- Li, G.; Liu, G.; Zhang, D.-B.; Pu, S.-Z. A new fluorescence probe based on fluorescein-diarylethene fluorescence resonance energy transfer system for rapid detection of Cd2+. Tetrahedron 2016, 72, 6390–6396. [Google Scholar] [CrossRef]
- Xue, D.; Zheng, C.; Fan, C.; Liu, G.; Pu, S. A colorimetric fluorescent sensor for Cr3+ based on a novel diarylethene with a naphthalimide-rhodamine B group. J. Photochem. Photobiol. A 2015, 303–304, 59–66. [Google Scholar] [CrossRef]
- Guo, S.; Fan, C.; Liu, G.; Pu, S. A colorimetric and fluorescent chemosensor for Hg2+ based on a photochromic diarylethene with a quinoline unit. RSC Adv. 2018, 8, 39854–39864. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Wang, R.; Fan, C.; Liu, G.; Pu, S. A highly selective fluorescent sensor for Cd2+ based on a new diarylethene with a 1,8-naphthyridine unit. Dye. Pigment. 2017, 139, 208–217. [Google Scholar] [CrossRef]
- Duan, F.; Liu, G.; Liu, P.; Fan, C.; Pu, S. A fluorescent probe for Cd2+ based on a diarylethene with pyridinepiperazine-linked hydroxyquinoline group. Tetrahedron 2016, 72, 3213–3220. [Google Scholar] [CrossRef]
- Wang, Z.; Cui, S.; Qiu, S.; Pu, S. A new fluorescence probe based on diarylethene with a benzothiazine unit for selective detection of Cd2+. Tetrahedron 2018, 74, 7431–7437. [Google Scholar] [CrossRef]
- Heng, Z.; Shuli, G.; Huimin, K.; Congbin, F.; Gang, L.; Shouzhi, P. Multi-responsive molecular switch based on a novel photochromic diarylethene derivative bearing a benzocoumarin unit. Tetrahedron 2020, 76, 130955. [Google Scholar] [CrossRef]
- Pu, S.; Xue, Y.; Zheng, C.; Geng, W.; Cui, S.; Liu, G. A highly selective fluorescent probe for Sn2+ based on a new photochromic diarylethene with a phenol-containing Schiff base unit. Tetrahedron 2014, 70, 9070–9076. [Google Scholar] [CrossRef]
- Raman, A.; Augustine, G.; Ayyadurai, N.; Easwaramoorthi, S. Gated photochromism in azobenzene-appended rhodamine cassette: Through-bond energy transfer—A universal strategy towards “Lock and Unlock” system. J. Mater. Chem. C 2018, 6, 10497–10501. [Google Scholar] [CrossRef]
- Srivastava, A.; Grewal, S.; Singh, S.; Rajani; Venkataramani, S. Photoswitchable Rhodamine-Based Multianalyte Sensors for Metal Ion Detection. ChemPhotoChem 2023, 7, e202300029. [Google Scholar] [CrossRef]
- Leavesley, H.B.; Li, L.; Mukhopadhyay, S.; Borowitz, J.L.; Isom, G.E. Nitrite-Mediated Antagonism of Cyanide Inhibition of Cytochrome c Oxidase in Dopamine Neurons. Toxicol. Sci. 2010, 115, 569–576. [Google Scholar] [CrossRef] [PubMed]
- Nath, A.K.; Roberts, L.D.; Liu, Y.; Mahon, S.B.; Kim, S.; Ryu, J.H.; Werdich, A.; Januzzi, J.L.; Boss, G.R.; Rockwood, G.A.; et al. Chemical and metabolomic screens identify novel biomarkers and antidotes for cyanide exposure. FASEB J. 2013, 27, 1928–1938. [Google Scholar] [CrossRef] [PubMed]
- Udhayakumari, D. Chromogenic and fluorogenic chemosensors for lethal cyanide ion. A comprehensive review of the year 2016. Sens. Actuators B 2018, 259, 1022–1057. [Google Scholar] [CrossRef]
- Wang, F.; Wang, L.; Chen, X.Q.; Yoon, J.Y. Recent progress in the development of fluorometric and colorimetric chemosensors for detection of cyanide ions. Chem. Soc. Rev. 2014, 43, 4312–4324. [Google Scholar] [CrossRef] [PubMed]
- Shiraishi, Y.; Adachi, K.; Itoh, M.; Hirai, T. Spiropyran as a Selective, Sensitive, and Reproducible Cyanide Anion Receptor. Org. Lett. 2009, 11, 3482–3485. [Google Scholar] [CrossRef]
- Sumiya, S.; Doi, T.; Shiraishi, Y.; Hirai, T. Colorimetric sensing of cyanide anion in aqueous media with a fluorescein–spiropyran conjugate. Tetrahedron 2012, 68, 690–696. [Google Scholar] [CrossRef]
- Mahapatra, A.K.; Maiti, K.; Manna, S.K.; Maji, R.; Mukhopadhyay, C.D.; Pakhira, B.; Sarkar, S. Unique Fluorogenic Ratiometric Fluorescent Chemodosimeter for Rapid Sensing of CN− in Water. Chem. Asian J. 2014, 9, 3623–3632. [Google Scholar] [CrossRef]
- Hu, F.; Cao, M.; Ma, X.; Liu, S.H.; Yin, J. Visible-Light-Dependent Photocyclization: Design, Synthesis, and Properties of a Cyanine-Based Dithienylethene. J. Org. Chem. 2015, 80, 7830–7835. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Song, Y.; Dai, Y.; Pei, Y.; Lu, Z.; Guo, H. Dicyanovinyl-substituted D-A type dithienylethenes: Synthesis, photochromism and colorimetric sensing for cyanide anion. Opt. Mater. 2019, 95, 109235. [Google Scholar] [CrossRef]
- Zhuge, Y.; Xu, D.; Zheng, C.; Pu, S. An ionic liquid-modified diarylethene: Synthesis, properties and sensing cyanide ions. Anal. Chim. Acta 2019, 1079, 153–163. [Google Scholar] [CrossRef] [PubMed]
- Tang, X.G.; Liu, H.L.; Pu, S.Z. A diarylethene-derived probe for colorimetric detection of CN− and highly selective fluorescent recognition of I−. Photochem. Photobiol. Sci. 2016, 15, 1579–1585. [Google Scholar] [CrossRef] [PubMed]
- Feng, E.; Fan, C.; Wang, N.; Liu, G.; Pu, S. A highly selective diarylethene chemosensor for colorimetric detection of CN− and fluorescent relay-detection of Al3+/Cr3+. Dye. Pigment. 2018, 151, 22–27. [Google Scholar] [CrossRef]
- Frankenfield, K.; Marchany-Rivera, D.; Flanders, K.G.; Cruz-Balberdy, A.; Lopez-Garriga, J.; Cerda, J.F. Fluoride binding to characteristic heme-pocket centers: Insights into ligand stability. J. Inorg. Biochem. 2021, 224, 111578. [Google Scholar] [CrossRef] [PubMed]
- Wu, Z.S.; Tang, X.J. Visualizing Fluoride Ion in Mitochondria and Lysosome of Living Cells and in Living Mice with Positively Charged Ratiometric Probes. Anal. Chem. 2015, 87, 8613–8617. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Z.; Xiao, S.; Xu, J.; Liu, Z.; Shi, M.; Li, F.; Yi, T.; Huang, C. Modulation of the Photochromic Property in an Organoboron-Based Diarylethene by a Fluoride Ion. Org. Lett. 2006, 8, 3911–3914. [Google Scholar] [CrossRef]
- Li, Z.; Zhang, H.; Zhang, H.; Xie, Y.; Chen, H.; He, C.; Wang, Y.; Ya, H.; Guo, H. A Photoswitchable Colorimetric Sensor for Fluoride Based on a Dithienylethene Unit. J. Chem. Res. 2018, 42, 305–308. [Google Scholar] [CrossRef]
- Guan, X.; He, M.; Chang, J.; Wang, Z.; Chen, Z.; Fan, H. Photo-controllability of fluoride remediation by spiropyran-functionalized mesoporous silica powder. J. Environ. Chem. Eng. 2021, 9, 104655. [Google Scholar] [CrossRef]
- Zhang, W.; Lu, Y.; Cheng, Y.; Wang, Y.; Wu, Z.; Zhai, J.; Xie, X. Ion-selective response of visible light photoswitchable indole-hemithioindigo: Toward chemical sensing of fluoride and hydroxide. Chem. Commun. 2024, 60, 4202–4205. [Google Scholar] [CrossRef]
- Agarbati, A.; Canonico, L.; Comitini, F.; Ciani, M. Reduction of Sulfur Compounds through Genetic Improvement of Native Saccharomyces cerevisiae Useful for Organic and Sulfite-Free Wine. Foods 2020, 9, 658. [Google Scholar] [CrossRef]
- Ferreira, D.; Barbosa, A.C.C.; Oliveira, G.P.; Catarino, T.; Venceslau, S.S.; Pereira, I.A.C. The DsrD functional marker protein is an allosteric activator of the DsrAB dissimilatory sulfite reductase. Proc. Natl. Acad. Sci. USA 2022, 119, e2118880119. [Google Scholar] [CrossRef]
- Oshanova, D.; Kurmanbayeva, A.; Bekturova, A.; Soltabayeva, A.; Nurbekova, Z.; Standing, D.; Dubey, A.K.; Sagi, M. Level of Sulfite Oxidase Activity Affects Sulfur and Carbon Metabolism in Arabidopsis. Front. Plant Sci. 2021, 12, 690830. [Google Scholar] [CrossRef] [PubMed]
- Li, K.; Li, L.L.; Zhou, Q.; Yu, K.K.; Kim, J.S.; Yu, X.Q. Reaction-based fluorescent probes for SO2 derivatives and their biological applications. Coord. Chem. Rev. 2019, 388, 310–333. [Google Scholar] [CrossRef]
- Li, Y.F.; Wang, Y.; Niu, H.W.; Chen, X.J.; Li, Z.Z.; Wang, Y.G. Research Progress of Sulfur Dioxide Fluorescent Probe Targeting Mitochondria. Chin. J. Org. Chem. 2023, 43, 1952–1962. [Google Scholar] [CrossRef]
- Zhang, Y.; Hao, Y.; Peng, Y.; Wang, X.; Zhou, Y.; Chen, S.; Xu, M. Responsive Photoactive Probe Based Photoelectrochemical Sensor for Sulfur Dioxide. Electroanalysis 2024, e202400041. [Google Scholar] [CrossRef]
- Liu, F.T.; Han, W.W.; Ren, H.; Wang, R.N.; Yang, W.J.; Miao, J.Y.; Zhao, B.X.; Lin, Z.M. A dicyanoisophorone-quinolinium-based near-infrared-emission fluorescent probe for ratiometric sensing of bisulfite/sulfite in living cells. Sens. Actuators B 2023, 380, 133305. [Google Scholar] [CrossRef]
- Hu, W.; Zeng, L.Y.; Zhai, S.Y.; Li, C.C.; Feng, W.Q.; Feng, Y.; Liu, Z.H. Developing a ratiometric two-photon probe with baseline resolved emissions by through band energy transfer strategy: Tracking mitochondrial SO2 during neuroinflammation. Biomaterials 2020, 241, 119910. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Fu, Y.; Han, H.-H.; Zang, Y.; Li, J.; He, X.-P.; Feringa, B.L.; Tian, H. Remote light-controlled intracellular target recognition by photochromic fluorescent glycoprobes. Nat. Commun. 2017, 8, 987. [Google Scholar] [CrossRef] [PubMed]
- Fu, Y.; Han, H.-H.; Zhang, J.; He, X.-P.; Feringa, B.L.; Tian, H. Photocontrolled Fluorescence “Double-Check” Bioimaging Enabled by a Glycoprobe–Protein Hybrid. J. Am. Chem. Soc. 2018, 140, 8671–8674. [Google Scholar] [CrossRef]
- Tang, J.; Yang, X.; Li, J.; Zhang, D.; Wang, Y.; Ye, Y. Photo-controlled fluorescence “Double-Check” for human serum albumin and its applications. Sens. Actuators B 2022, 350, 130814. [Google Scholar] [CrossRef]
- Fu, Y.; Zhang, X.; Liu, J.; Qian, G.; Xu, Z.P.; Zhang, R. Fluorescence detection and imaging of intracellular sulphite using a remote light activatable photochromic nanoprobe. J. Mater. Chem. B 2022, 10, 3366–3374. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Wang, H.; Chen, J.; Tian, Y.; Xiang, C.; Liu, W.; Zhou, Z.; Cui, J.; Chen, X. Visible-Light-Driven Photoswitchable Fluorescent Polymers for Photorewritable Pattern, Anti-Counterfeiting, and Information Encryption. Adv. Funct. Mater. 2023, 33, 2303765. [Google Scholar] [CrossRef]
- Li, X.; Lin, Z.; Tian, Y.; Zhang, C.; Zhang, P.; Zeng, R.; Qiao, L.; Chen, J. Rational design of a negative photochromic spiropyran-containing fluorescent polymeric nanoprobe for sulfur dioxide derivative ratiometric detection and cell imaging. Anal. Bioanal. Chem. 2023, 415, 715–724. [Google Scholar] [CrossRef] [PubMed]
- Feng, W.; Xiao, Q.C.; Wang, L.; Yang, Y.Y. A New Fluorescent Probe for Hydrogen Sulfide Detection in Solution and Living Cells. Molecules 2023, 28, 6195. [Google Scholar] [CrossRef] [PubMed]
- Lin, V.S.; Chen, W.; Xian, M.; Chang, C.J. Chemical probes for molecular imaging and detection of hydrogen sulfide and reactive sulfur species in biological systems. Chem. Soc. Rev. 2015, 44, 4596–4618. [Google Scholar] [CrossRef]
- He, W.M.; Zhou, Z.; Han, Z.; Li, S.; Zhou, Z.; Ma, L.F.; Zang, S.Q. Ultrafast Size Expansion and Turn-On Luminescence of Atomically Precise Silver Clusters by Hydrogen Sulfide. Angew. Chem. Int. Ed. 2021, 60, 8505–8509. [Google Scholar] [CrossRef]
- Hong, Y.; Zhang, P.; Wang, H.; Yu, M.; Gao, Y.; Chen, J. Photoswitchable AIE nanoprobe for lysosomal hydrogen sulfide detection and reversible dual-color imaging. Sens. Actuators B 2018, 272, 340–347. [Google Scholar] [CrossRef]
- Zhang, W.; Huo, F.; Yin, C. Photocontrolled Single-/Dual-Site Alternative Fluorescence Probes Distinguishing Detection of H2S/SO2 in Vivo. Org. Lett. 2019, 21, 5277–5280. [Google Scholar] [CrossRef] [PubMed]
- Vilanakis, E.; Papakonstantinou, E.; Paramera, E.; Argyri, I.; Drakou, E.; Kokkinou, E.; Zouvelou, V.; Outsika, C.; Pons, R. Cerebrospinal Fluid Concentrations of Neurotransmitters in a Greek Pediatric Reference Population. Neuropediatrics 2023, 54, 126–133. [Google Scholar] [CrossRef]
- Chiellini, G.; Bellusci, L.; Sabatini, M.; Zucchi, R. Thyronamines and Analogues—The Route from Rediscovery to Translational Research on Thyronergic Amines. Mol. Cell. Endocrinol. 2017, 458, 149–155. [Google Scholar] [CrossRef]
- Cao, Q.J.W.; Wang, Y.J.; Chen, B.M.; Ma, F.F.; Hao, L.; Li, G.Y.; Ouyang, C.Z.; Li, L.J. Visualization and Identification of Neurotransmitters in Crustacean Brain via Multifaceted Mass Spectrometric Approaches. ACS Chem. Neurosci. 2019, 10, 1222–1229. [Google Scholar] [CrossRef] [PubMed]
- Teknikel, E.; Unaleroglu, C. Recent Advances in Chemodosimeters Designed for Amines. Curr. Org. Synth. 2023, 20, 4–19. [Google Scholar] [CrossRef] [PubMed]
- Fredrich, S.; Bonasera, A.; Valderrey, V.; Hecht, S. Sensitive Assays by Nucleophile-Induced Rearrangement of Photoactivated Diarylethenes. J. Am. Chem. Soc. 2018, 140, 6432–6440. [Google Scholar] [CrossRef] [PubMed]
- Diaz, Y.J.; Page, Z.A.; Knight, A.S.; Treat, N.J.; Hemmer, J.R.; Hawker, C.J.; Read de Alaniz, J. A Versatile and Highly Selective Colorimetric Sensor for the Detection of Amines. Chem. Eur. J. 2017, 23, 3562–3566. [Google Scholar] [CrossRef] [PubMed]
- Sabahi-Agabager, L.; Eskandari, H.; Nasiri, F.; Shamkhali, A.N.; Baghi Sefidan, S. Properties of a furan ring-opening reaction in aqueous micellar solutions for selective sensing of mesalazine. Spectrochim. Acta Part A 2021, 258, 119846. [Google Scholar] [CrossRef] [PubMed]
- Cho, S.; Kim, Y. Donor–acceptor Stenhouse adduct formation for the simple and rapid colorimetric detection of amphetamine-type stimulants. Sens. Actuators B 2022, 355, 131274. [Google Scholar] [CrossRef]
- Xie, W.Z.; Jiang, J.Y.; Shu, D.J.; Zhang, Y.J.; Yang, S.; Zhang, K. Recent Progress in the Rational Design of Biothiol-Responsive Fluorescent Probes. Molecules 2023, 28, 4252. [Google Scholar] [CrossRef] [PubMed]
- Niu, L.Y.; Chen, Y.Z.; Zheng, H.R.; Wu, L.Z.; Tung, C.H.; Yang, Q.Z. Design strategies of fluorescent probes for selective detection among biothiols. Chem. Soc. Rev. 2015, 44, 6143–6160. [Google Scholar] [CrossRef]
- Huang, H.J.; Ji, X.R.; Jiang, Y.Q.; Zhang, C.Y.; Kang, X.Y.; Zhu, J.Q.; Sun, L.; Yi, L. NBD-based fluorescent probes for separate detection of cysteine and biothiols via different reactivities. Org. Biomol. Chem. 2020, 18, 4004–4008. [Google Scholar] [CrossRef]
- Li, T.; Hao, Y.; Dong, H.; Li, C.; Liu, J.; Zhang, Y.; Tang, Z.; Zeng, R.; Xu, M.; Chen, S. Target-Induced In Situ Formation of Organic Photosensitizer: A New Strategy for Photoelectrochemical Sensing. ACS Sens. 2022, 7, 415–422. [Google Scholar] [CrossRef]
- Hao, Y.-Q.; Zhang, Y.-T.; Zhu, D.-D.; Luo, L.-J.; Chen, L.; Tang, Z.-L.; Zeng, R.-J.; Xu, M.-T.; Chen, S. Dual-emission fluorescent probe for discriminative sensing of biothiols. Chin. J. Anal. Chem. 2022, 50, 100153. [Google Scholar] [CrossRef]
- Zhang, Y.; Hao, Y.; Ma, X.; Chen, S.; Xu, M. A dicyanoisophorone-based highly sensitive and selective near-infrared fluorescent probe for sensing thiophenol in water samples and living cells. Environ. Pollut. 2020, 265, 114958. [Google Scholar] [CrossRef] [PubMed]
- Hao, Y.; Zhang, Y.; Luo, L.; Zhu, D.; Xu, M.; Zeng, R.; Chen, S. Deep-Red Emissive Fluorescent Probe for Sensitive Detection of Cysteine in Milk and Living Cells. Food Anal. Methods 2022, 15, 2145–2154. [Google Scholar] [CrossRef]
- Hao, Y.; Li, T.; Luo, L.; Fan, S.; Chen, S.; Zhang, Y.; Tang, Z.; Xu, M.; Zeng, R.; Chen, S. A reaction based dual-modal probe for fluorescent and photoelectrochemical determination of thiophenol. Sens. Actuators B 2022, 369, 132405. [Google Scholar] [CrossRef]
- Wu, J.J.; Su, D.D.; Qin, C.Q.; Li, W.; Rodrigues, J.; Sheng, R.L.; Zeng, L.T. A fast responsive chromogenic and near-infrared fluorescence lighting-up probe for visual detection of toxic thiophenol in environmental water and living cells. Talanta 2019, 201, 111–118. [Google Scholar] [CrossRef]
- Zheng, C.; Pu, S.; Liu, G.; Chen, B.; Dai, Y. A highly selective colorimetric sensor for cysteine and homocysteine based on a new photochromic diarylethene. Dye. Pigment. 2013, 98, 280–285. [Google Scholar] [CrossRef]
- Li, G.; Ma, L.; Liu, G.; Fan, C.; Pu, S. A diarylethene-based “on–off–on” fluorescence sensor for the sequential recognition of mercury and cysteine. RSC Adv. 2017, 7, 20591–20596. [Google Scholar] [CrossRef]
- Zhai, L.; Tu, Y.; Shi, Z.; Pu, S. A colorimetric and fluorescent chemosensor based on diarylethene for simultaneous detection and discrimination of biothiols. Spectrochim. Acta Part A 2019, 218, 171–177. [Google Scholar] [CrossRef] [PubMed]
- Fu, Y.; Zhang, X.; Cao, F.; Wang, W.; Qian, G.; Zhang, J. Target-activated and ratiometric photochromic probe for “double-check” detection of toxic thiols in live cells. Sci. China Chem. 2019, 62, 1204–1212. [Google Scholar] [CrossRef]
- Guo, Y.; Zhang, D.; Wang, J.; Lu, H.; Pu, S. Luminescent probe based on photochromic cyclometalated iridium(III) complex for high selectivity detection of thiophenol. Dye. Pigment. 2020, 175, 108191. [Google Scholar] [CrossRef]
- Balamurugan, A.; Lee, H.-I. A Visible Light Responsive On–Off Polymeric Photoswitch for the Colorimetric Detection of Nerve Agent Mimics in Solution and in the Vapor Phase. Macromolecules 2016, 49, 2568–2574. [Google Scholar] [CrossRef]
- Li, L.L.; Jia, F.F.; Li, Y.X.; Peng, Y. Design strategies and biological applications of β-galactosidase fluorescent sensor in ovarian cancer research and beyond. RSC Adv. 2024, 14, 3010–3023. [Google Scholar] [CrossRef]
- Yao, Y.K.; Zhang, Y.T.; Yan, C.X.; Zhu, W.H.; Guo, Z.Q. Enzyme-activatable fluorescent probes for β-galactosidase: From design to biological applications. Chem. Sci. 2021, 12, 9885–9894. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.W.; Chen, L.L.; Xu, C.Y.; Li, Z.; Zhang, H.Y.; Zhang, X.B.; Tan, W.H. Recent progresses in small-molecule enzymatic fluorescent probes for cancer imaging. Chem. Soc. Rev. 2018, 47, 7140–7180. [Google Scholar] [CrossRef] [PubMed]
- Chai, X.; Han, H.-H.; Sedgwick, A.C.; Li, N.; Zang, Y.; James, T.D.; Zhang, J.; Hu, X.-L.; Yu, Y.; Li, Y.; et al. Photochromic Fluorescent Probe Strategy for the Super-resolution Imaging of Biologically Important Biomarkers. J. Am. Chem. Soc. 2020, 142, 18005–18013. [Google Scholar] [CrossRef]
- Hampel, H.; Hardy, J.; Blennow, K.; Chen, C.; Perry, G.; Kim, S.H.; Villemagne, V.L.; Aisen, P.; Vendruscolo, M.; Iwatsubo, T.; et al. The Amyloid-β pathway in Alzheimer’s disease. Mol. Psychiatry 2021, 26, 5481–5503. [Google Scholar] [CrossRef]
- Depp, C.; Sun, T.; Sasmita, A.O.; Spieth, L.; Berghoff, S.A.; Nazarenko, T.; Overhoff, K.; Steixner-Kumar, A.A.; Subramanian, S.; Arinrad, S.; et al. Myelin dysfunction drives amyloid-β deposition in models of Alzheimer’s disease. Nature 2023, 618, 349–357. [Google Scholar] [CrossRef]
- Aliyan, A.; Cook, N.P.; Martí, A.A. Interrogating Amyloid Aggregates using Fluorescent Probes. Chem. Rev. 2019, 119, 11819–11856. [Google Scholar] [CrossRef] [PubMed]
- Xu, L.; Gao, H.; Zhan, W.; Deng, Y.; Liu, X.; Jiang, Q.; Sun, X.; Xu, J.-J.; Liang, G. Dual Aggregations of a Near-Infrared Aggregation-Induced Emission Luminogen for Enhanced Imaging of Alzheimer’s Disease. J. Am. Chem. Soc. 2023, 145, 27748–27756. [Google Scholar] [CrossRef]
- Lv, G.; Cui, B.; Lan, H.; Wen, Y.; Sun, A.; Yi, T. Diarylethene based fluorescent switchable probes for the detection of amyloid-β pathology in Alzheimer’s disease. Chem. Commun. 2015, 51, 125–128. [Google Scholar] [CrossRef]
- Dreos, A.; Ge, J.; Najera, F.; Tebikachew, B.E.; Perez-Inestrosa, E.; Moth-Poulsen, K.; Blennow, K.; Zetterberg, H.; Hanrieder, J. Investigating New Applications of a Photoswitchable Fluorescent Norbornadiene as a Multifunctional Probe for Delineation of Amyloid Plaque Polymorphism. ACS Sens. 2023, 8, 1500–1509. [Google Scholar] [CrossRef] [PubMed]
- Bargstedt, J.; Reinschmidt, M.; Tydecks, L.; Kolmar, T.; Hendrich, C.M.; Jäschke, A. Photochromic Nucleosides and Oligonucleotides. Angew. Chem. Int. Ed. 2024, 63, e202310797. [Google Scholar] [CrossRef] [PubMed]
- Lubbe, A.S.; Szymanski, W.; Feringa, B.L. Recent developments in reversible photoregulation of oligonucleotide structure and function. Chem. Soc. Rev. 2017, 46, 1052–1079. [Google Scholar] [CrossRef] [PubMed]
- Wu, Z.; Zhang, L. Photoregulation between small DNAs and reversible photochromic molecules. Biomater. Sci. 2019, 7, 4944–4962. [Google Scholar] [CrossRef]
- Berdnikova, D.V.; Carloni, P.; Krauß, S.; Rossetti, G. Role and Perspective of Molecular Simulation-Based Investigation of RNA–Ligand Interaction: From Small Molecules and Peptides to Photoswitchable RNA Binding. Molecules 2021, 26, 3384. [Google Scholar] [CrossRef] [PubMed]
- Cui, Y.; Hu, D.; Markillie, L.M.; Chrisler, W.B.; Gaffrey, M.J.; Ansong, C.; Sussel, L.; Orr, G. Fluctuation localization imaging-based fluorescence in situ hybridization (fliFISH) for accurate detection and counting of RNA copies in single cells. Nucleic Acids Res. 2017, 46, e7. [Google Scholar] [CrossRef]
- Guo, C.; Zhai, J.; Wang, Y.; Yang, W.; Xie, X. Wash-Free Detection of Nucleic Acids with Photoswitch-Mediated Fluorescence Resonance Energy Transfer against Optical Background Interference. Anal. Chem. 2021, 93, 8128–8133. [Google Scholar] [CrossRef]








Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, H.; Tang, Z.; Yang, Y.; Hao, Y.; Chen, W. Recent Advances in Photoswitchable Fluorescent and Colorimetric Probes. Molecules 2024, 29, 2521. https://doi.org/10.3390/molecules29112521
Chen H, Tang Z, Yang Y, Hao Y, Chen W. Recent Advances in Photoswitchable Fluorescent and Colorimetric Probes. Molecules. 2024; 29(11):2521. https://doi.org/10.3390/molecules29112521
Chicago/Turabian StyleChen, Hongjuan, Zilong Tang, Yewen Yang, Yuanqiang Hao, and Wansong Chen. 2024. "Recent Advances in Photoswitchable Fluorescent and Colorimetric Probes" Molecules 29, no. 11: 2521. https://doi.org/10.3390/molecules29112521
APA StyleChen, H., Tang, Z., Yang, Y., Hao, Y., & Chen, W. (2024). Recent Advances in Photoswitchable Fluorescent and Colorimetric Probes. Molecules, 29(11), 2521. https://doi.org/10.3390/molecules29112521





