Unraveling the Impact of Adsorbed Molecules on Photocatalytic Processes: Advancements in Understanding Facet-Controlled Semiconductor Photocatalysts
Abstract
1. Introduction
2. Results and Discussion
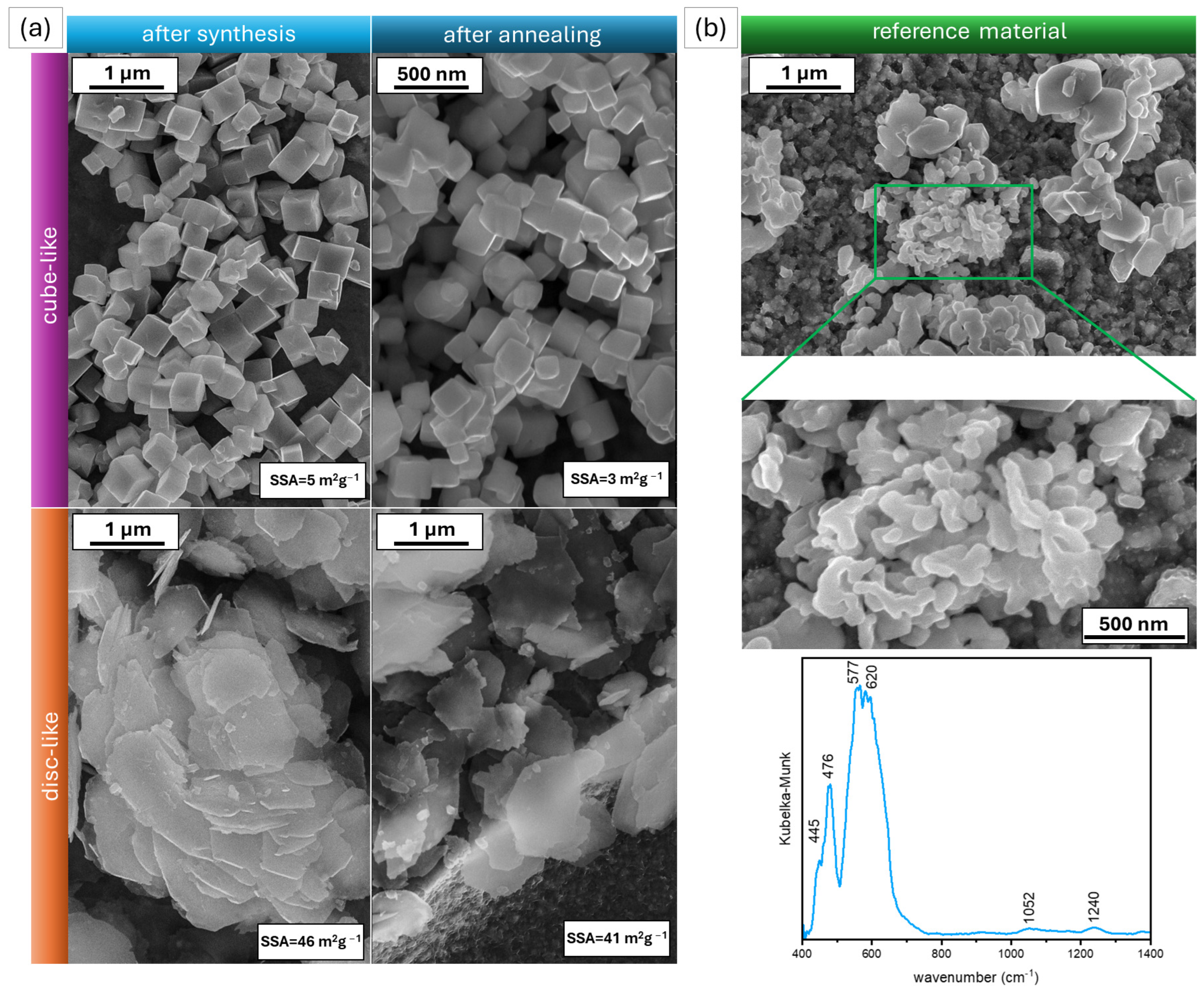
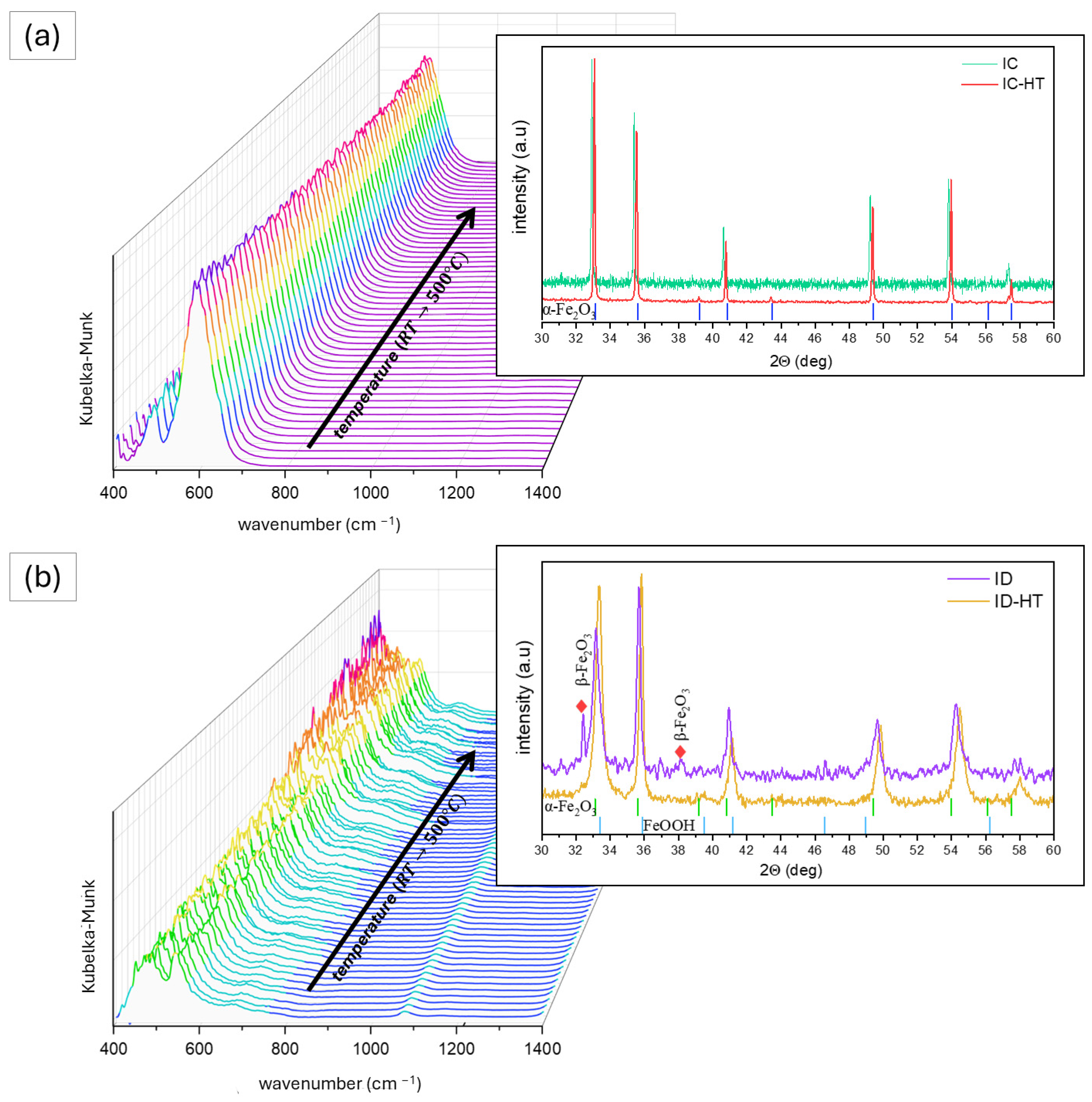
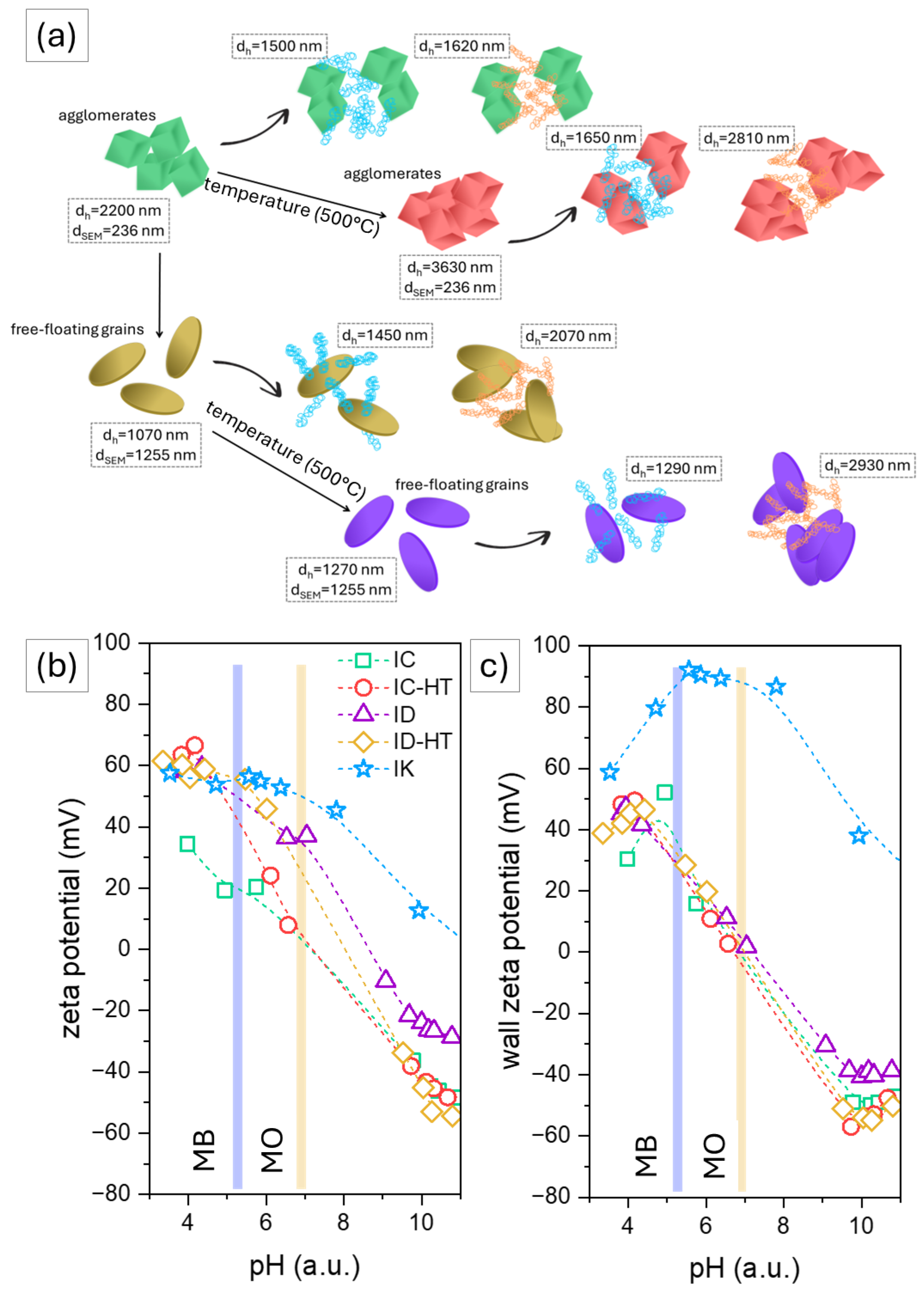
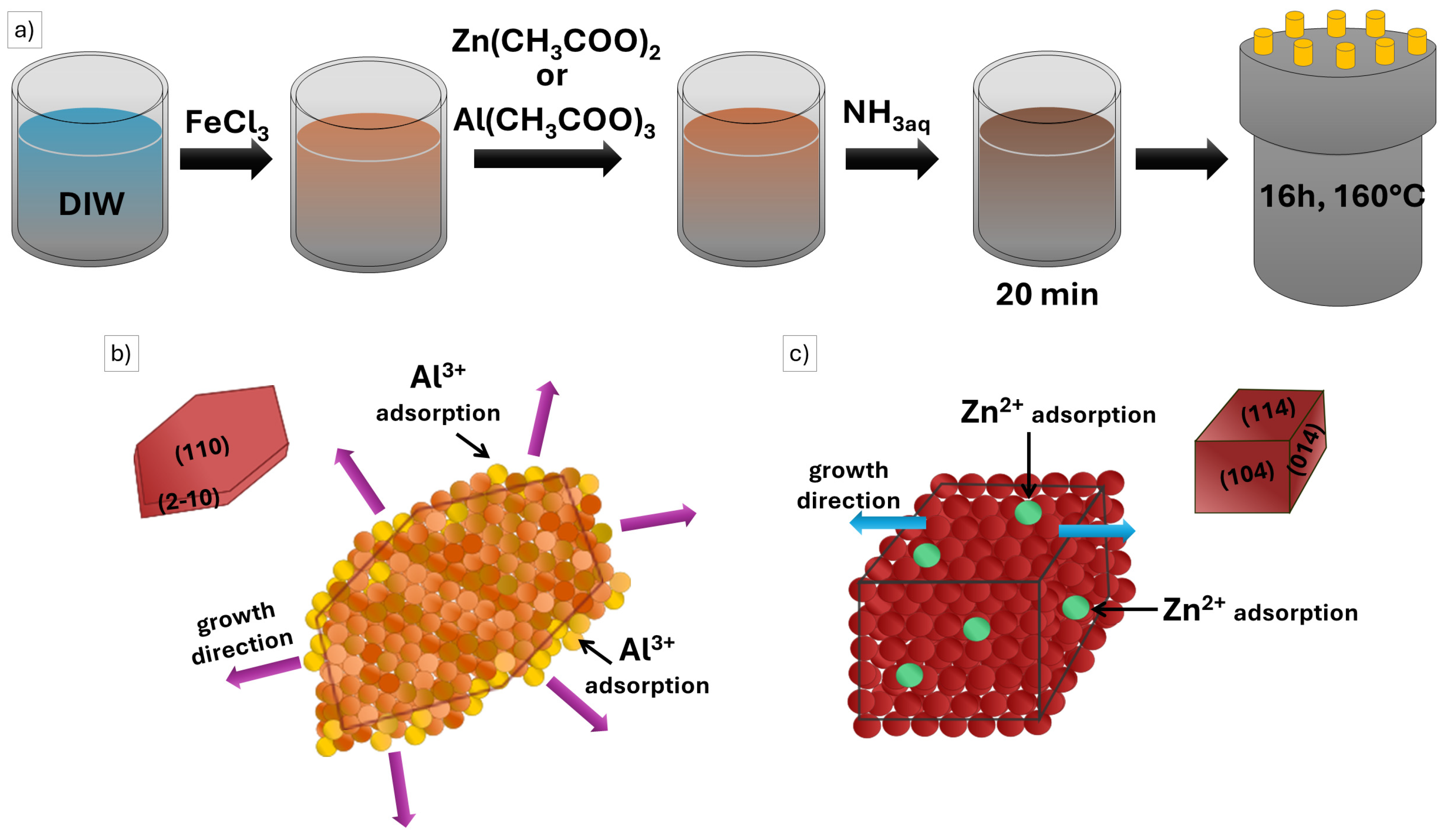
2.1. Photocatalysts Characterization
2.2. Photocatalytic Tests
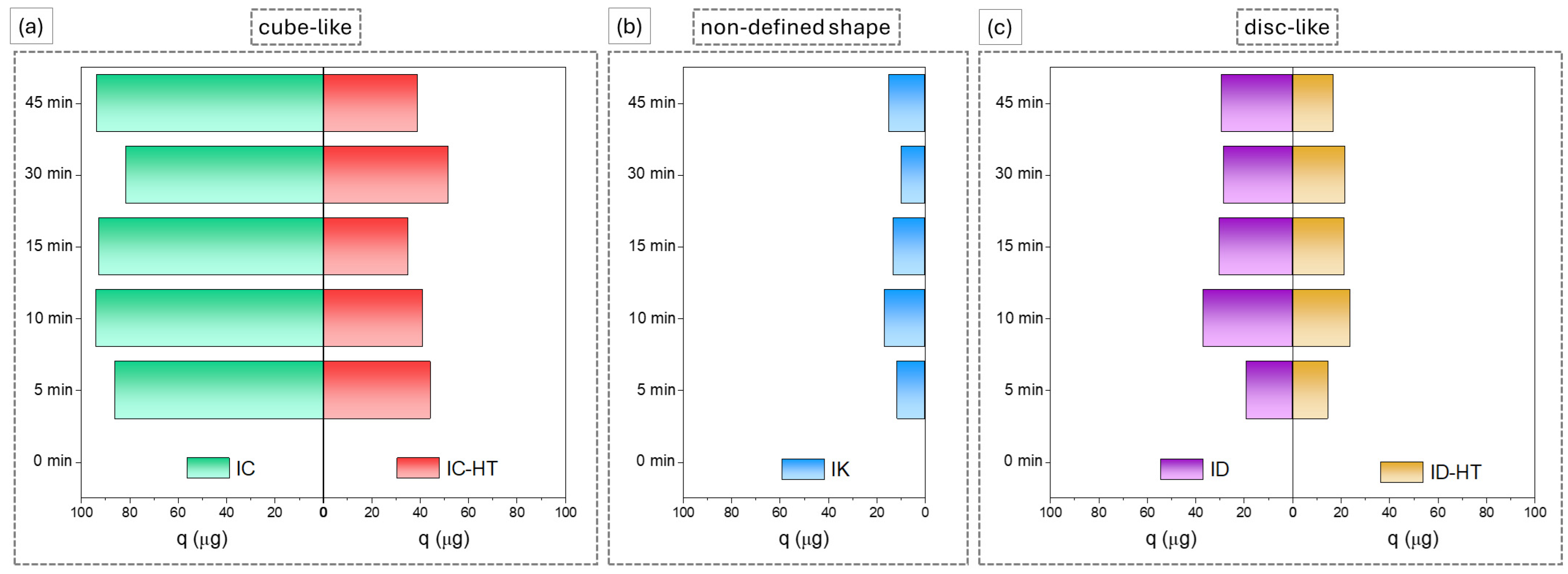
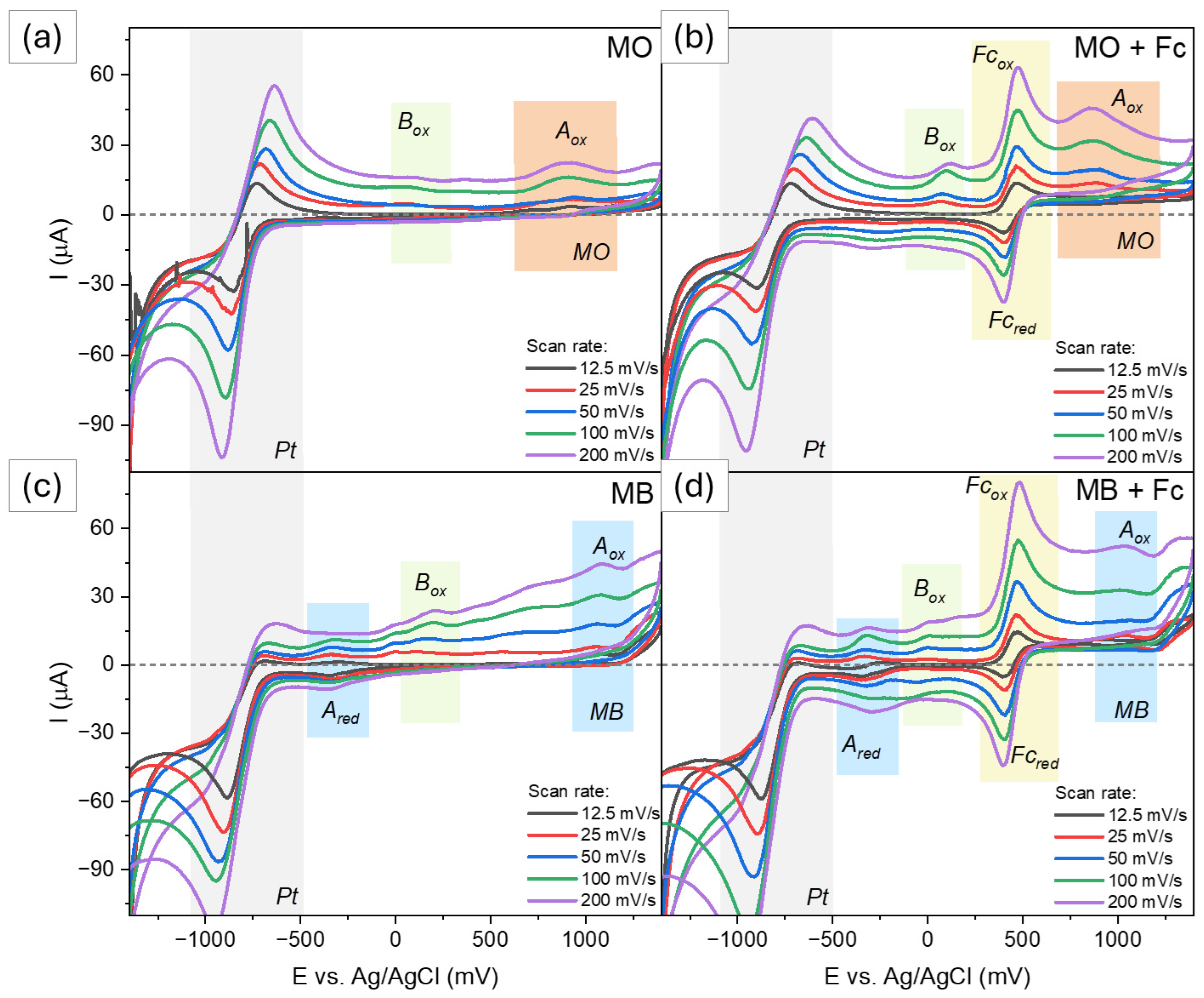
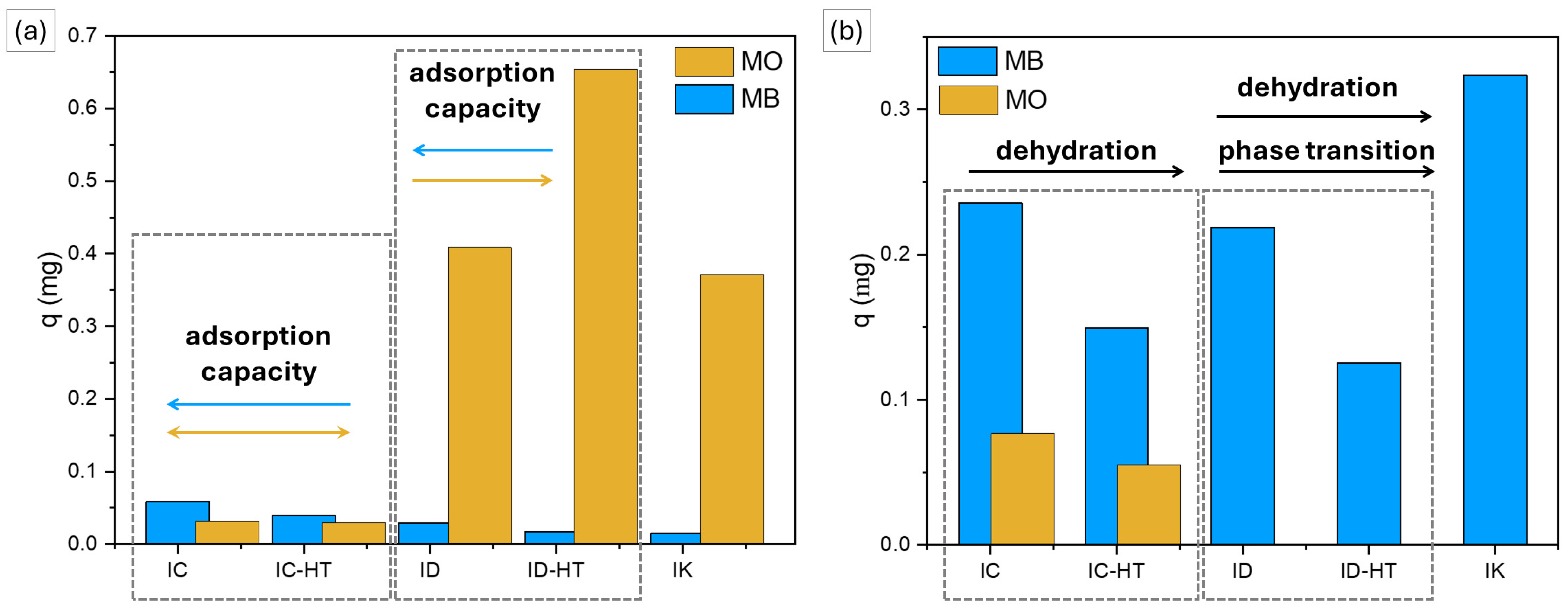
2.2.1. Cationic Dye
2.2.2. Anionic Dye
3. Experiment
3.1. Synthesis
3.2. Materials Characterization
3.3. Photocatalytic Activity
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Tong, H.; Ouyang, S.; Bi, Y.; Umezawa, N.; Oshikiri, M.; Ye, J. Nano-Photocatalytic Materials: Possibilities and Challenges. Adv. Mater. 2012, 24, 229–251. [Google Scholar] [CrossRef] [PubMed]
- Fresno, F.; Portela, R.; Suárez, S.; Coronado, J.M. Photocatalytic Materials: Recent Achievements and near Future Trends. J. Mater. Chem. A Mater. 2014, 2, 2863–2884. [Google Scholar] [CrossRef]
- Mills, A.; Le Hunte, S. An Overview of Semiconductor Photocatalysis. J. Photochem. Photobiol. A Chem. 1997, 108, 1–35. [Google Scholar] [CrossRef]
- Gusain, R.; Gupta, K.; Joshi, P.; Khatri, O.P. Adsorptive Removal and Photocatalytic Degradation of Organic Pollutants Using Metal Oxides and Their Composites: A Comprehensive Review. Adv. Colloid. Interface Sci. 2019, 272, 102009. [Google Scholar] [CrossRef] [PubMed]
- Hernández-Alonso, M.D.; Fresno, F.; Suárez, S.; Coronado, J.M. Development of Alternative Photocatalysts to TiO2: Challenges and Opportunities. Energy Environ. Sci. 2009, 2, 1231–1257. [Google Scholar] [CrossRef]
- Li, J.; Wu, N. Semiconductor-Based Photocatalysts and Photoelectrochemical Cells for Solar Fuel Generation: A Review. Catal. Sci. Technol. 2015, 5, 1360–1384. [Google Scholar] [CrossRef]
- Jing, L.; Zhou, W.; Tian, G.; Fu, H. Surface Tuning for Oxide-Based Nanomaterials as Efficient Photocatalysts. Chem. Soc. Rev. 2013, 42, 9509–9549. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Yu, J.; Jaroniec, M. Hierarchical Photocatalysts. Chem. Soc. Rev. 2016, 45, 2603–2636. [Google Scholar] [CrossRef]
- Daghrir, R.; Drogui, P.; Robert, D. Modified TiO2 for Environmental Photocatalytic Applications: A Review. Ind. Eng. Chem. Res. 2013, 52, 3581–3599. [Google Scholar] [CrossRef]
- Kisch, H. Semiconductor Photocatalysis-Mechanistic and Synthetic Aspects. Angew. Chem.-Int. Ed. 2013, 52, 812–847. [Google Scholar] [CrossRef]
- Marschall, R. Semiconductor Composites: Strategies for Enhancing Charge Carrier Separation to Improve Photocatalytic Activity. Adv. Funct. Mater. 2014, 24, 2421–2440. [Google Scholar] [CrossRef]
- Sudha, D.; Sivakumar, P. Review on the Photocatalytic Activity of Various Composite Catalysts. Chem. Eng. Process. Process Intensif. 2015, 97, 112–133. [Google Scholar] [CrossRef]
- Zhu, S.; Wang, D. Photocatalysis: Basic Principles, Diverse Forms of Implementations and Emerging Scientific Opportunities. Adv. Energy Mater. 2017, 7, 1700841. [Google Scholar] [CrossRef]
- Radecka, M.; Kusior, A.; Trenczek-Zajac, A.; Zakrzewska, K. Oxide Nanomaterials for Photoelectrochemical Hydrogen Energy Sources; Elsevier Ltd.: Amsterdam, The Netherlands, 2018; Volume 72, ISBN 9780128150771. [Google Scholar]
- Mishra, M.; Chun, D.M. α-Fe2O3 as a Photocatalytic Material: A Review. Appl. Catal. A Gen. 2015, 498, 126–141. [Google Scholar] [CrossRef]
- Henderson, M.A. A Surface Science Perspective on TiO2 Photocatalysis. Surf. Sci. Rep. 2011, 66, 185–297. [Google Scholar] [CrossRef]
- Friedmann, D.; Mendive, C.; Bahnemann, D. TiO2 for Water Treatment: Parameters Affecting the Kinetics and Mechanisms of Photocatalysis. Appl. Catal. B 2010, 99, 398–406. [Google Scholar] [CrossRef]
- Devi, L.G.; Kavitha, R. A Review on Non Metal Ion Doped Titania for the Photocatalytic Degradation of Organic Pollutants under UV/Solar Light: Role of Photogenerated Charge Carrier Dynamics in Enhancing the Activity. Appl. Catal. B 2013, 140–141, 559–587. [Google Scholar] [CrossRef]
- Yagub, M.T.M.T.; Sen, T.K.T.K.; Afroze, S.; Ang, H.M.M. Dye and Its Removal from Aqueous Solution by Adsorption: A Review. Adv. Colloid. Interface Sci. 2014, 209, 172–184. [Google Scholar] [CrossRef]
- Salleh, M.A.M.; Mahmoud, D.K.; Karim, W.A.W.A.; Idris, A. Cationic and Anionic Dye Adsorption by Agricultural Solid Wastes: A Comprehensive Review. Desalination 2011, 280, 1–13. [Google Scholar] [CrossRef]
- Nosaka, Y.; Nosaka, A.Y. Generation and Detection of Reactive Oxygen Species in Photocatalysis. Chem. Rev. 2017, 117, 11302–11336. [Google Scholar] [CrossRef]
- Banerjee, S.; Pillai, S.C.; Falaras, P.; O’shea, K.E.; Byrne, J.A.; Dionysiou, D.D. New Insights into the Mechanism of Visible Light Photocatalysis. J. Phys. Chem. Lett. 2014, 5, 2543–2554. [Google Scholar] [CrossRef] [PubMed]
- Kusior, A.; Michalec, K.; Jelen, P.; Radecka, M. Shaped Fe2O3 Nanoparticles–Synthesis and Enhanced Photocatalytic Degradation towards RhB. Appl. Surf. Sci. 2019, 476, 342–352. [Google Scholar] [CrossRef]
- Kusior, A.; Banas, J.; Trenczek-Zajac, A.; Zubrzycka, P.; Micek-Ilnicka, A.; Radecka, M. Structural Properties of TiO2 Nanomaterials. J. Mol. Struct. 2018, 1157, 327–336. [Google Scholar] [CrossRef]
- Cambier, P. Infrared Study of Goethites of Varying Crystallinity and Particle Size: I. Interpretation of OH and Lattice Vibration Frequencies. Clay Miner. 1986, 21, 191–200. [Google Scholar] [CrossRef]
- Cambier, P. Infrared Study of Goethites of Varying Crystallinity and Particle Size: II. Crystallographic and Morphological Changes in Series of Synthetic Goethites. Clay Miner. 1986, 21, 201–210. [Google Scholar] [CrossRef]
- Stanjek, H.; Schwertmann, U.D.O. The Inluence of Alulinum on Iron Oxides, Part XVI: Hydroxyl and Aluminum Substitution on Synthethic Hematites. Clays Clay Miner. 1992, 40, 347–354. [Google Scholar] [CrossRef]
- Schwertmann, U.; Wolska, E. The Influence of Aluminum on Iron Oxides. XV. Al-for-Fe Substitution in Synthetic Lepidocrocite. Clays Clay Miner. 1990, 38, 209–212. [Google Scholar] [CrossRef]
- Russell, J.D.; Paterson, E.; Fraser, A.R.; Farmer, V.C. Adsorption of Carbon Dioxide on Goethite (α-FeOOH) Surfaces, and Its Implications for Anion Adsorption. J. Chem. Soc. Faraday Trans. 1 Phys. Chem. Condens. Phases 1975, 71, 1623–1630. [Google Scholar] [CrossRef]
- Amami, B.; Addou, M.; Monty, C. Selfdiffusion and Point Defects in Iron Oxides: FeO, Fe3O4, α-Fe2O3. Defect. Diffus. Forum 2001, 194–199, 1051–1056. [Google Scholar] [CrossRef]
- Reboiras, M.D.; Kaszuba, M.; Connah, M.T.; Jones, M.N. Measurement of Wall Zeta Potentials and Their Time-Dependent Changes Due to Adsorption Processes: Liposome Adsorption on Glass. Langmuir 2001, 17, 5314–5318. [Google Scholar] [CrossRef]
- Cornell, R.M.; Schwertmann, U. The Iron Oxides. Structure, Properties, Reactions, Occurrences and Uses, 2nd ed.; WILEY-VCH Verlag GmbH & Co. KGaA: Weinheim, Germany, 2003; ISBN 3527302743. [Google Scholar]
- Kusior, A.; Trenczek-Zajac, A.; Mazurków, J.; Michalec, K.; Synowiec, M.; Radecka, M. Interface Design, Surface-Related Properties, and Their Role in Interfacial Electron Transfer. Part I: Materials-Related Topics. In Advances in Inorganic Chemistry; Academic Press Inc.: Cambridge, MA, USA, 2022; Volume 79, pp. 373–409. ISBN 9780323999724. [Google Scholar]
- Lagergren, S. About the Theory of So-Called Adsorption of Soluble Substances. K. Sven. Vetenskapsakademiens Handl. 1898, 24, 1–39. [Google Scholar]
- Doǧan, M.; Özdemir, Y.; Alkan, M. Adsorption Kinetics and Mechanism of Cationic Methyl Violet and Methylene Blue Dyes onto Sepiolite. Dye. Pigment. 2007, 75, 701–713. [Google Scholar] [CrossRef]
- Yao, C.; Chen, T. A Film-Diffusion-Based Adsorption Kinetic Equation and Its Application. Chem. Eng. Res. Des. 2017, 119, 87–92. [Google Scholar] [CrossRef]
- Wang, S.; Li, H. Kinetic Modelling and Mechanism of Dye Adsorption on Unburned Carbon. Dye. Pigment. 2007, 72, 308–314. [Google Scholar] [CrossRef]
- Srivastava, V.C.; Swamy, M.M.; Mall, I.D.; Prasad, B.; Mishra, I.M. Adsorptive Removal of Phenol by Bagasse Fly Ash and Activated Carbon: Equilibrium, Kinetics and Thermodynamics. Colloids Surf. A Physicochem. Eng. Asp. 2006, 272, 89–104. [Google Scholar] [CrossRef]
- Fondell, M.; Jacobsson, T.J.; Boman, M.; Edvinsson, T. Optical Quantum Confinement in Low Dimensional Hematite. J. Mater. Chem. A Mater. 2014, 2, 3352–3363. [Google Scholar] [CrossRef]
- Butler, M.A.; Ginley, D.S. Prediction of Flatband Potentials at Semiconductor-Electrolyte Interfaces from Atomic. J. Electrochem. Soc. 1987, 125, 228–232. [Google Scholar] [CrossRef]
- Zhang, L.; Jaroniec, M. Toward Designing Semiconductor-Semiconductor Heterojunctions for Photocatalytic Applications. Appl. Surf. Sci. 2018, 430, 2–17. [Google Scholar] [CrossRef]
- Kissling, G.P.; Ruhstaller, B.; Pernstich, K.P. Measuring Frontier Orbital Energy Levels of OLED Materials Using Cyclic Voltammetry in Solution. Org. Electron. 2023, 122, 106888. [Google Scholar] [CrossRef]
- Chiu, Y.H.; Chang, T.F.M.; Chen, C.Y.; Sone, M.; Hsu, Y.J. Mechanistic Insights into Photodegradation of Organic Dyes Using Heterostructure Photocatalysts. Catalysts 2019, 9, 430. [Google Scholar] [CrossRef]
- Yang, S.J.; Lin, Y.K.; Pu, Y.C.; Hsu, Y.J. Crystal Facet Dependent Energy Band Structures of Polyhedral Cu2O Nanocrystals and Their Application in Solar Fuel Production. J. Phys. Chem. Lett. 2022, 13, 6298–6305. [Google Scholar] [CrossRef] [PubMed]
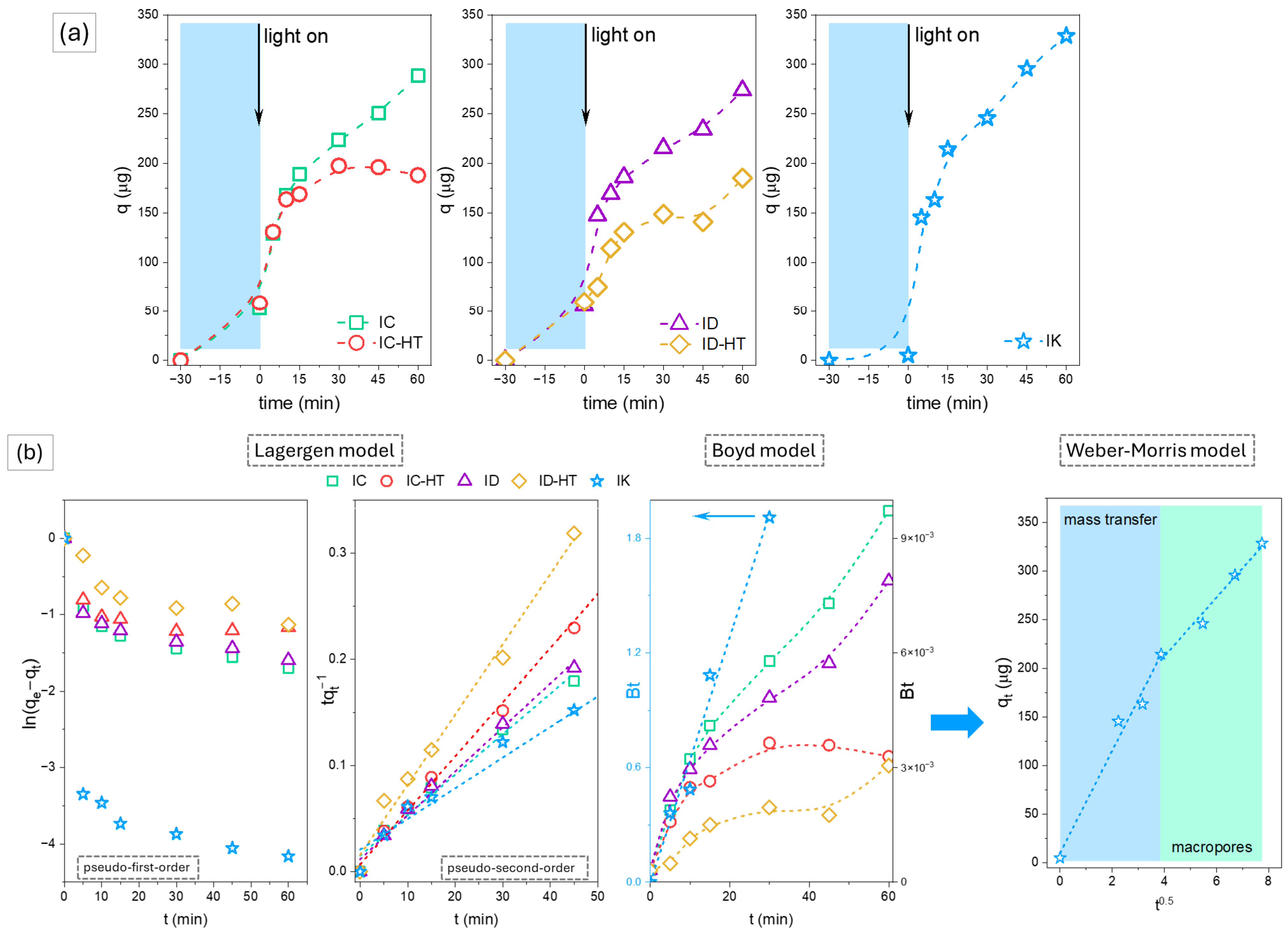
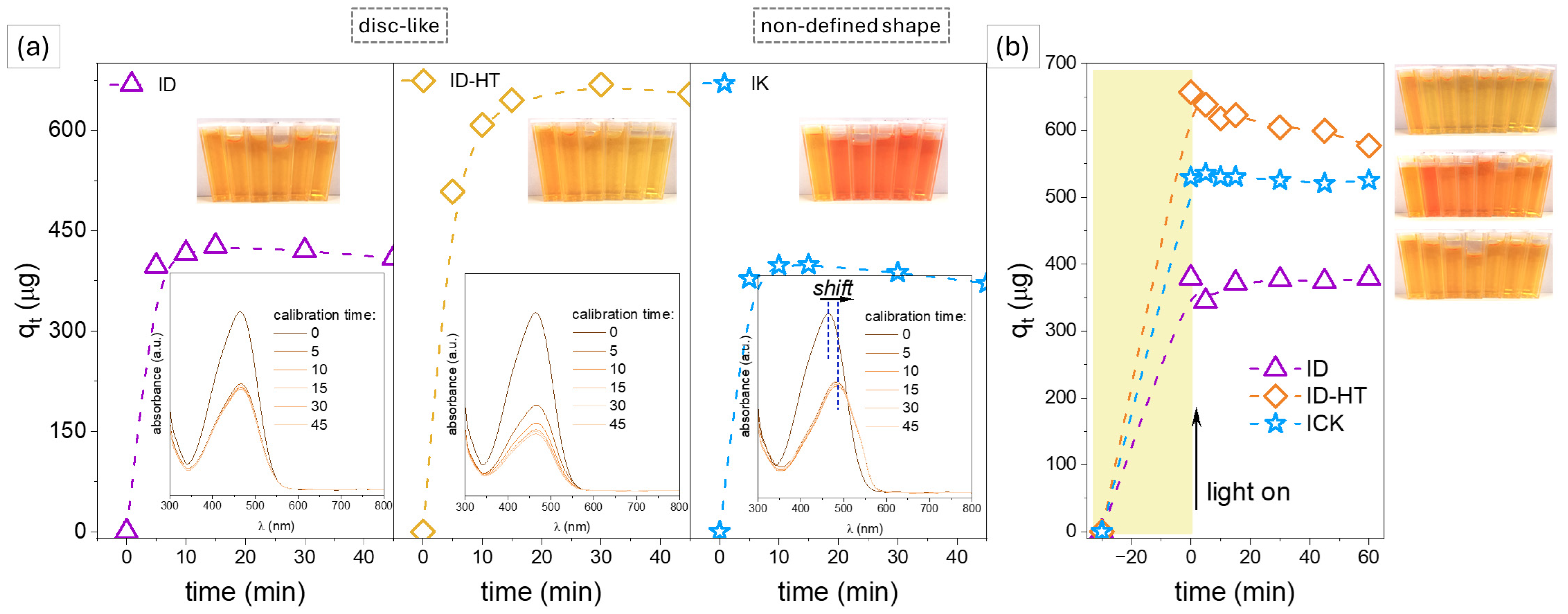
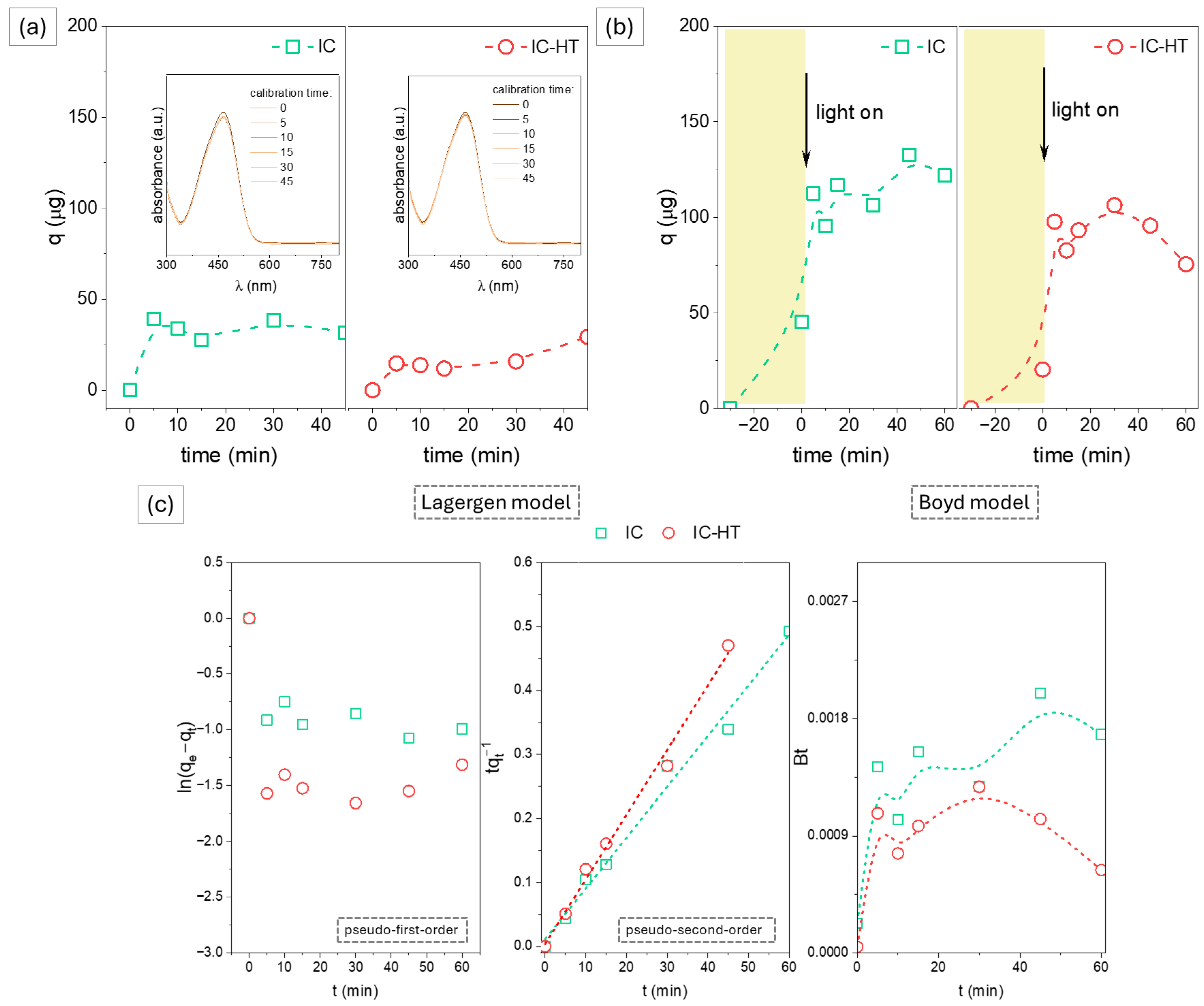
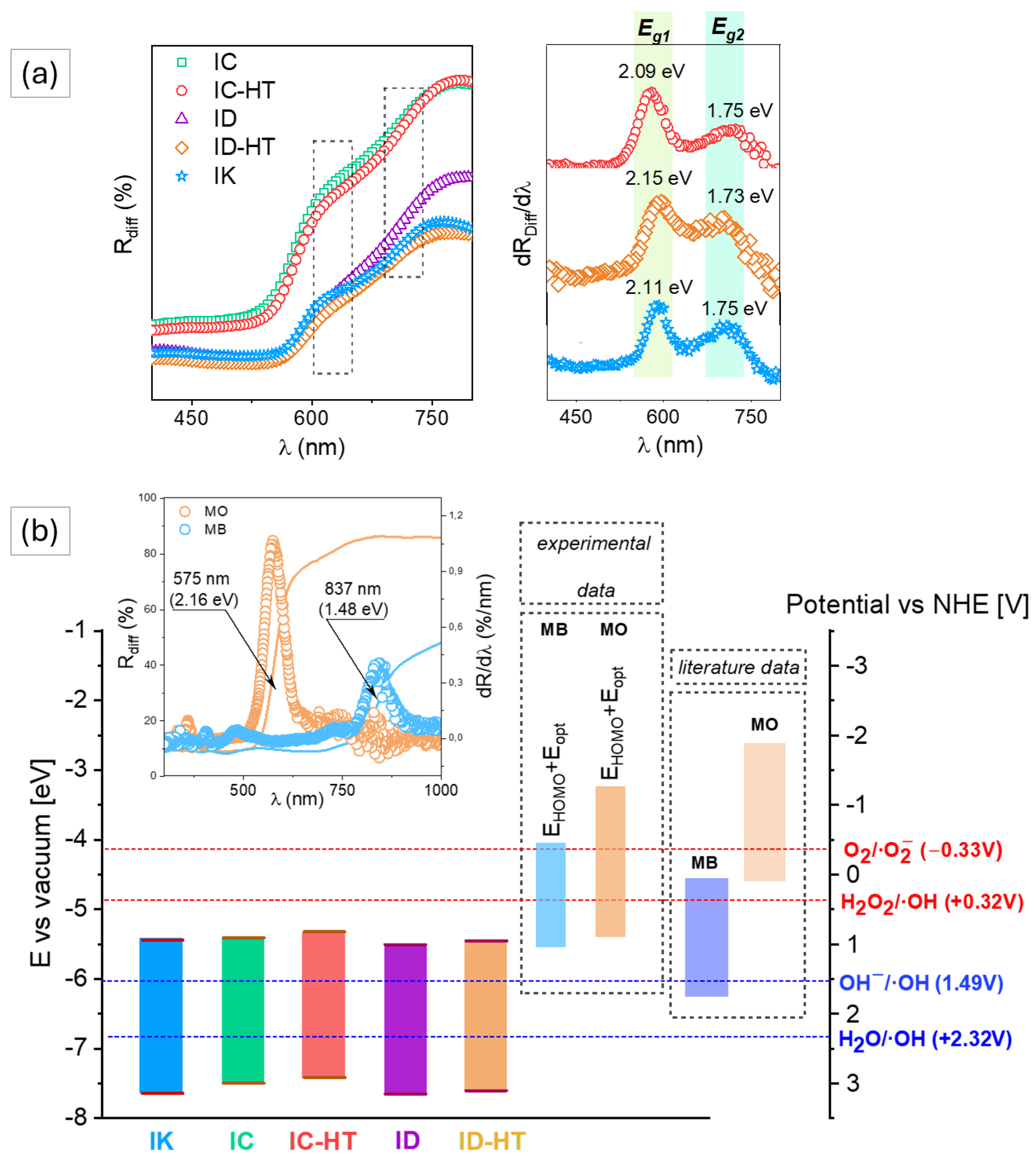
| Parameter | IC | IC-HT | ID | ID-HT | IK |
|---|---|---|---|---|---|
| k2 (gmin−1) | 0.17 | 0.29 | 0.21 | 0.10 | 0.17 |
| qe (g) | 0.0026 | 0.0020 | 0.0024 | 0.0015 | 0.0035 |
| Scan Rate (mVs−1) | MO | MB | ||||||
|---|---|---|---|---|---|---|---|---|
| Eredox (V) | E(HOMO) (V) | Eopt (eV) | E(LUMO)1 (V) | Eredox (V) | E(HOMO) (V) | Eopt (eV) | E(LUMO)1 (V) | |
| 12.5 | n/a | n/a | 2.16 | −3.24 | n/a | n/a | 1.48 | −4.07 |
| 25 | 0.44 | −5.43 | 0.57 | −5.56 | ||||
| 50 | 0.44 | −5.43 | 0.56 | −5.55 | ||||
| 100 | 0.38 | −5.37 | 0.55 | −5.54 | ||||
| 200 | 0.37 | −5.36 | 0.56 | −5.55 | ||||
| average | 0.41 | −5.40 | 0.56 | −5.55 | ||||
| Synthesis Parameters | Cube-like | Disc-like | Reference | ||
|---|---|---|---|---|---|
| IC | IC-HT | ID | ID-HT | IK | |
| metal precursor | 16 mmol FeCl3·9H2O | commercial powder Chempur, >96%, Fe2O3 | |||
| electrolyte | 80 mL distilled water | ||||
| modifier | 8 mmol (CH3COO)2Zn·2H2O | 8 mmol (CH3COO)3Al | |||
| precipitating agent | 80 mL NH3aq, 25% | ||||
| reaction | 160 °C, 16 h | ||||
| post-treatment | - | 500 °C, 180 min, air | - | 500 °C, 180 min, air | - |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kusior, A.; Michalec, K.; Micek-Ilnicka, A.; Radecka, M. Unraveling the Impact of Adsorbed Molecules on Photocatalytic Processes: Advancements in Understanding Facet-Controlled Semiconductor Photocatalysts. Molecules 2024, 29, 2290. https://doi.org/10.3390/molecules29102290
Kusior A, Michalec K, Micek-Ilnicka A, Radecka M. Unraveling the Impact of Adsorbed Molecules on Photocatalytic Processes: Advancements in Understanding Facet-Controlled Semiconductor Photocatalysts. Molecules. 2024; 29(10):2290. https://doi.org/10.3390/molecules29102290
Chicago/Turabian StyleKusior, Anna, Kinga Michalec, Anna Micek-Ilnicka, and Marta Radecka. 2024. "Unraveling the Impact of Adsorbed Molecules on Photocatalytic Processes: Advancements in Understanding Facet-Controlled Semiconductor Photocatalysts" Molecules 29, no. 10: 2290. https://doi.org/10.3390/molecules29102290
APA StyleKusior, A., Michalec, K., Micek-Ilnicka, A., & Radecka, M. (2024). Unraveling the Impact of Adsorbed Molecules on Photocatalytic Processes: Advancements in Understanding Facet-Controlled Semiconductor Photocatalysts. Molecules, 29(10), 2290. https://doi.org/10.3390/molecules29102290







