A Highly Sensitive and Group-Specific Enzyme-Linked Immunosorbent Assay (ELISA) for the Detection of AFB1 in Agriculture and Aquiculture Products
Abstract
1. Introduction
2. Results and Discussion
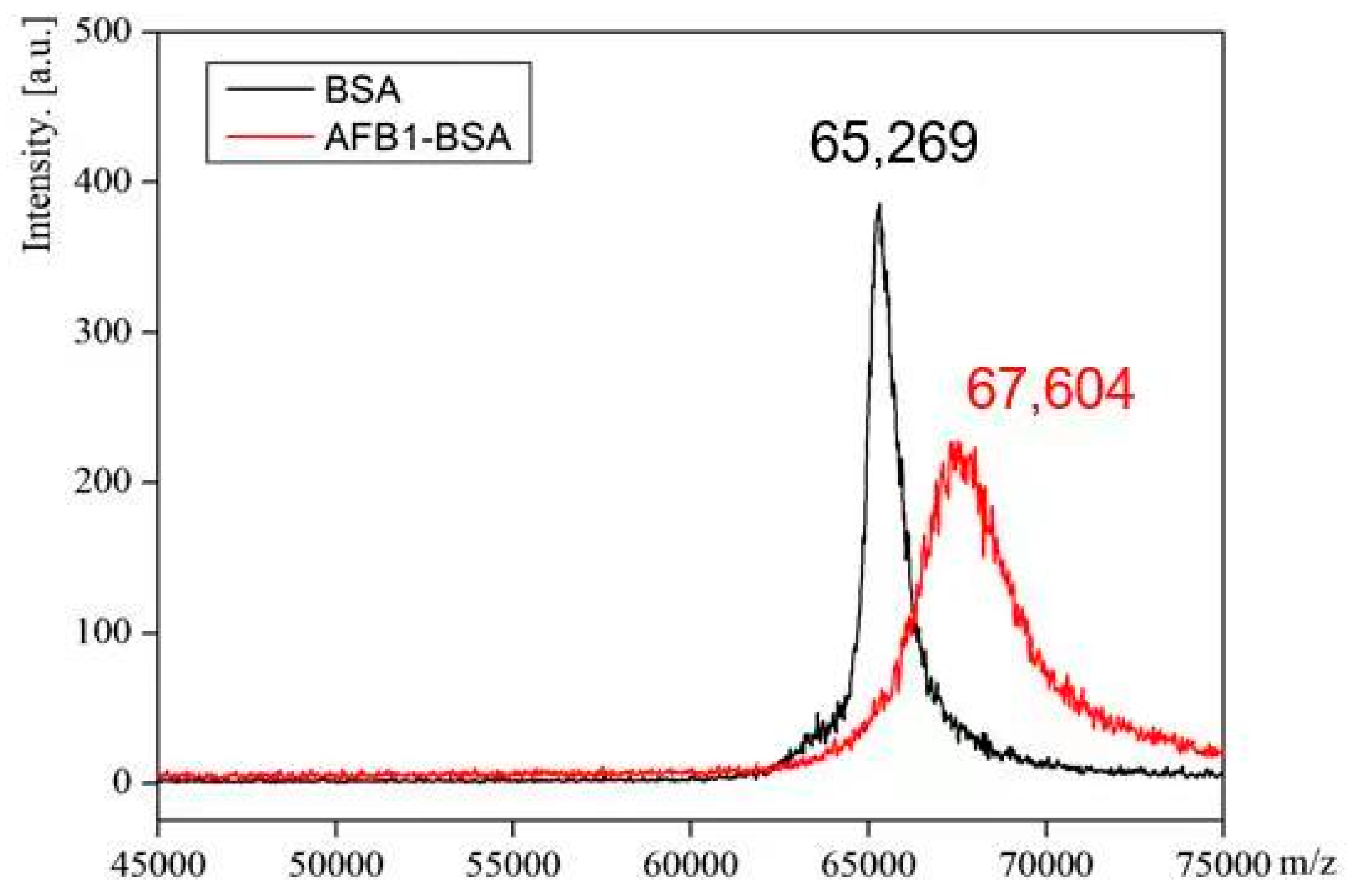
2.1. Synthesis of Immunogen and Coating Antigen
2.2. Preparation of mAb against AFB1
2.3. Optimization of ELISA Conditions
2.4. Sensitivity of ELISA
2.5. Specificity of the ELISA
2.6. Accuracy and Precision of the ELISA
2.7. Analysis of Wheat Flour Sample Spiked with Total AFs
3. Materials and Methods
3.1. Reagents, Materials and Apparatus
3.2. Buffers and Solutions
3.3. Synthesis of Immunogen and Coating Antigen
3.4. Production of mAb against AFB1
3.5. Procedures of ELISA
3.6. Cross-Reactivity Testing
3.7. Spiking Experiment
3.8. Analysis of Wheat Flour Sample Spiked with Total AFs
3.9. LC-MS/MS Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Pisoschi, A.M.; Iordache, F.; Stanca, L.; Petcu, A.I.; Purdoiu, L.; Geicu, O.I.; Bilteanu, O.I.; Serban, A.I. Comprehensive overview and critical perspective on the analytical techniques applied to aflatoxin determination–a review paper. Microchem. J. 2023, 191, 108770. [Google Scholar] [CrossRef]
- González-Curbelo, M.Á.; Kabak, B. Occurrence of mycotoxins in dried fruits worldwide, with a focus on aflatoxins and ochratoxin A: A review. Toxins 2023, 15, 576. [Google Scholar] [CrossRef]
- Abbas, H.K.; Accinelli, C.; Shier, W.T. Biological control of aflatoxin contamination in US crops and the use of bioplastic formulations of aspergillus flavus biocontrol strains to optimize application strategies. J. Agric. Food Chem. 2017, 65, 7081–7087. [Google Scholar] [CrossRef] [PubMed]
- Kumar, V.V. Aflatoxins: Properties, toxicity and detoxification. Nutr. Food Sci. Int. J. 2018, 6, 555696. [Google Scholar]
- Benkerroum, N. Aflatoxins: Producing-molds, structure, health issues and incidence in southeast asian and sub-saharan african countries. Int. J. Environ. Res. Public Health 2020, 17, 1215. [Google Scholar] [CrossRef] [PubMed]
- Zitomer, N.; Rybak, M.E.; Li, Z.; Walters, M.J.; Holman, M.R. Determination of aflatoxin B1 in smokeless tobacco products by use of UHPLC-MS/MS. J. Agric. Food Chem. 2015, 63, 9131–9138. [Google Scholar] [CrossRef] [PubMed]
- Sun, L.L.; Zhao, Q. Direct fluorescence anisotropy approach for aflatoxin B1 detection and affinity binding study by using single tetramethylrhodamine labeled aptamer. Talanta 2018, 189, 442–450. [Google Scholar] [CrossRef]
- Ventura, M.; Gómez, A.; Anaya, I.; Díaz, J.; Broto, F.; Agut, M.; Comellas, L. Determination of aflatoxins B1, G1, B2 and G2 in medicinal herbs by liquid chromatography-tandem mass spectrometry. J. Chromatogr. A 2004, 1048, 25–29. [Google Scholar]
- European Commission. Commission Regulation (EC) No. 1881/2006 of 19 December 2006 setting maximum levels for certain contaminants in foodstuffs. Off. J. Eur. Union 2006, L364, 20–23. [Google Scholar]
- GB2761-2017; Chinese National Food Safety Standard, Maximum Residue Limits for Mycotoxins in Food. National Medical Products Administration: Beijing, China, 2017.
- Liu, S.Y.; Qiu, F.; Kong, W.J.; Wei, J.; Xiao, X.H.; Yang, M.H. Development andvalidation of an accurate and rapid LC-ESI-MS/MS method for the simultaneous quantification of aflatoxin B1, B2, G1 and G2 in lotus seeds. Food Control 2013, 29, 156–161. [Google Scholar] [CrossRef]
- Miró-Abella, E.; Herrero, P.; Canela, N.; Arola, L.; Borrull, F.; Ras, R.; Fontanals, N. Determination of mycotoxins in plant-based beverages using QuEChERS and liquid chromatography–tandem mass spectrometry. Food Chem. 2017, 229, 366–372. [Google Scholar] [CrossRef]
- Khayoon, W.S.; Saad, B.; Lee, T.P.; Salleh, B. High performance liquid chromatographic determination of aflatoxins in chilli, peanut and rice using silica based monolithic column. Food Chem. 2012, 133, 489–496. [Google Scholar] [CrossRef] [PubMed]
- Cao, B.Y.; He, G.Z.; Yang, H.; Chang, H.F.; Li, S.Q.; Deng, A.P. Development of a highly sensitive and specific enzyme-linked immunosorbent assay (ELISA) for the detection of phenylethanolamine A in tissue and feed samples and confirmed by liquid chromatography tandem mass spectrometry (LC–MS/MS). Talanta 2013, 115, 624–630. [Google Scholar] [CrossRef]
- Pu, C.; Wu, Y.F.; Yang, H.; Deng, A.P. Trace analysis of contraceptive drug levonorgestrel in wastewater samples by a newly developed indirect competitive enzyme-linked immunosorbent assay (ELISA) coupled with solid phase extraction. Anal. Chim. Acta 2008, 628, 73–79. [Google Scholar] [CrossRef]
- Li, J.; Zhao, X.; Chen, L.J.; Qian, H.L.; Wang, W.L.; Yang, C.; Yan, X.P. p-bromophenol-enhanced bienzymatic chemiluminescence competitive immunoassay for ultrasensitive determination of aflatoxin B1. Anal. Chem. 2019, 91, 13191–13197. [Google Scholar] [CrossRef]
- Wang, X.; Lu, D.S.; Huang, Q.F.; Yang, J.Y. Microfluidics-based time-resolved fluorescence immunoassay for the on-site detection of aflatoxins B1 zearalenone and deoxynivalenol in cereals. Foods 2022, 11, 1319. [Google Scholar] [CrossRef]
- Liu, X.; Wen, Y.P.; Wang, W.J.; Zhao, Z.T.; Han, Y.; Tang, K.J.; Wang, D. Nanobody-based electrochemical competitive immunosensor for the detection of AFB1 through AFB1-HCR as signal amplifier. Microchim. Acta 2020, 187, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Mao, X.Y.; Yu, B.G.; Li, Z.J.; Li, Z.P.; Shi, G.Q. Comparison of lateral flow immunoassays based on oriented and nonoriented immobilization of antibodies for the detection of aflatoxin B1. Anal. Chim. Acta 2022, 1221, 340135. [Google Scholar] [CrossRef]
- Kolosova, A.Y.; Shim, W.B.; Yang, Z.Y.; Eremin, S.A.; Chung, D.H. Direct competitive ELISA based on a monoclonal antibody for detection of aflatoxin B1. Stabilization of ELISA kit components and application to grain samples. Anal. Bioanal. Chem. 2006, 384, 286–294. [Google Scholar] [CrossRef]
- Burkin, M.A.; Yakovleva, I.V.; Sviridov, V.V.; Burkin, A.A.; Kononenko, G.P.; Soboleva, N.A. Comparative immunochemical characterization of polyclonal and monoclonal antibodies to aflatoxin B1. Appl. Biochem. Microbiol. 2000, 36, 496–501. [Google Scholar] [CrossRef]
- Burkin, A.A.; Kononenko, G.P.; Soboleva, N.A.; Zotova, E.V. An enzyme immunoassay system for aflatoxin B1. Appl. Biochem. Microbiol. 2000, 36, 80–84. [Google Scholar] [CrossRef]
- Lee, N.A.; Rachmawati, S. A rapid ELISA for screening aflatoxin B1 in animal feed and feed ingredients in Indonesia. Food Agr. Immunol. 2006, 17, 91–104. [Google Scholar] [CrossRef]
- Chen, F.S.; Zhou, Q. Comparative study on the characterization of antisera of anti-aflatoxin B1 from rabbit and laying hen. Food/Nahrung. 2002, 46, 432–436. [Google Scholar] [CrossRef]
- Reddy, S.V.; Kiran Mayi, D.; Uma Reddy, M.; Thirumala-Devi, K.; Reddy, D.V.R. Aflatoxins B1 in different grades of chillies (Capsicum annum L.) in India as determined by indirect competitive-ELISA. Food Addit. Contam. 2001, 18, 553–558. [Google Scholar] [CrossRef]
- Jiang, W.X.; Wang, Z.H.; Nölke, G.; Zhang, J.; Niu, L.L.; Shen, J.Z. Simultaneous determination of aflatoxin B1 and aflatoxin M1 in food matrices by enzyme-linked immunosorbent assay. Food Anal. Meth. 2013, 6, 767–774. [Google Scholar] [CrossRef]
- Liu, B.H.; Hsu, Y.T.; Lu, C.C.; Yu, F.Y. Detecting aflatoxin B1 in foods and feeds by using sensitive rapid enzyme-linked immunosorbent assay and gold nanoparticle immunochromatographic strip. Food Control 2013, 30, 184–189. [Google Scholar] [CrossRef]
- Anfossi, L.; Di Nardo, F.; Giovannoli, C.; Passini, C.; Baggiani, C. Enzyme immunoassay for monitoring aflatoxins in eggs. Food Control 2015, 57, 115–121. [Google Scholar] [CrossRef]
- Liu, J.W.; Lu, C.C.; Liu, B.H.; Yu, F.Y. Development of novel monoclonal antibodies-based ultrasensitive enzyme-linked immunosorbent assay and rapid immunochromatographic strip for aflatoxin B1 detection. Food Control 2016, 59, 700–707. [Google Scholar] [CrossRef]
- Yamasaki, T.; Miyake, S.; Sato, N.; Hirakawa, Y.; Iwasa, S.; Narita, H.; Watanabe, T. Development of enzyme-linked immunosorbent assay for analysis of total aflatoxins based on monoclonal antibody reactive with aflatoxins B1, B2, G1 and G2. Food Hyg. Saf. Sci. 2018, 59, 200–205. [Google Scholar] [CrossRef]
- Zhang, X.; Feng, M.; Liu, L.Q.; Xing, C.R.; Kuang, H.; Peng, C.F.; Wang, L.B.; Xu, C.L. Detection of aflatoxins in tea samples based on a class-specific monoclonal antibody. Int. J. Food Sci. Technol. 2013, 48, 1269–1274. [Google Scholar] [CrossRef]
- Lee, N.A.; Wang, S.; Allan, R.D.; Kennedy, I.R. A Rapid Aflatoxin B1 ELISA: Development and validation with reduced matrix effects for peanuts, corn, pistachio, and soybeans. J. Agric. Food Chem. 2004, 52, 2746–2755. [Google Scholar] [CrossRef] [PubMed]
- Ye, Y.; Liu, A.P.; Wang, X.H.; Chen, F.S. Spectra analysis of coating antigen: A possible explanation for difference in anti-AFB1 polyclonal antibody sensitivity. J. Mol. Struct. 2016, 1121, 74–79. [Google Scholar] [CrossRef]
- Thilini Madurangika Jayasinghe, G.D.; Domínguez-Gonzalez, R.; Bermejo-Barrera, P.; Moreda-Pineiro, A. Room temperature phosphorescent determination of aflatoxins in fish feed based on molecularly imprinted polymer-Mn-doped ZnS quantum dots. Anal. Chim. Acta 2020, 1103, 183–191. [Google Scholar] [CrossRef] [PubMed]

| ELISA | Antibody | Sample | IC50 (ng mL−1) | LOD (ng mL−1) | Ref. |
|---|---|---|---|---|---|
| dc-ELISA | mAb | Rice | 0.62 | 0.05 | [20] |
| ic-ELISA | mAb | Food | 0.33 | / | [21] |
| ic-ELISA | pAb | Food | 10 | / | [22] |
| dc-ELISA | pAb | Feed | 0.85 | 0.18 | [23] |
| dc-ELISA | pAb | Corn, wheat, peanut | 1.1 | / | [24] |
| ic-ELISA | pAb | Chilli | 1.2 | / | [25] |
| ic-ELISA | mAb | Peanuts, corn, rice, etc. | 0.13 | / | [26] |
| dc-ELISA | pAb | Food, Feed | 0.15 | / | [27] |
| dc-ELISA | pAb | Egg | 0.24 | / | [28] |
| dc-ELISA | mAb | rice, pet food, peanut, etc. | 0.051 | / | [29] |
| dc-ELISA | mAb | Peanuts | 0.09 | / | [30] |
| ic-ELISA | mAb | Tea | 0.057 | / | [31] |
| dc-ELISA | pAb | Peanuts, Corn, etc. | 0.8 | / | [32] |
| ic-ELISA | pAb | Food | 1.4 | / | [33] |
| ic-ELISA | mAb | Wheat, corn, Crab roe | 0.09 | 0.018 | This work |
| Compound | Molecular Structure | IC50 (ng mL−1) | CR (%) |
|---|---|---|---|
| AFB1 | 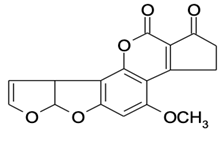 | 0.090 | 100 |
| AFB2 |  | 0.381 | 23.6 |
| AFG1 |  | 0.212 | 42.5 |
| AFG2 |  | 4.756 | 1.9 |
| AFM1 |  | 0.203 | 44.3 |
| AFM2 |  | 2.190 | 4.1 |
| PAT |  | >1000 | <0.01 |
| FB1 |  | >1000 | <0.01 |
| ZEN |  | >1000 | <0.01 |
| DON |  | >1000 | <0.01 |
| OTA |  | >1000 | <0.01 |
| Sample | Con. Spiked (ng g−1) | Con. Measured ±SD (ng g−1) | Recovery (%) | RSD (%, n = 3) |
|---|---|---|---|---|
| Wheat flour | 0 | 0.90 ± 0.05 | / | 5.56 |
| 2 | 3.21 ± 0.08 | 115.50 | 2.49 | |
| 5 | 6.73 ± 0.10 | 116.60 | 1.49 | |
| 10 | 11.27 ± 0.93 | 103.70 | 8.25 | |
| Corn flour | 0 | 0.21 ± 0.01 | / | 4.76 |
| 10 | 10.44 ± 0.75 | 102.30 | 7.18 | |
| 20 | 21.51 ± 0.32 | 106.50 | 1.49 | |
| 40 | 38.45 ± 0.62 | 95.60 | 1.61 | |
| Crab roe | 0 | 0.37 ± 0.10 | / | 27.03 |
| 10 | 8.20 ± 0.40 | 78.30 | 4.88 | |
| 20 | 20.67 ± 2.73 | 101.50 | 13.21 | |
| 40 | 37.67 ± 3.07 | 93.23 | 8.15 |
| Conc. of Total AFs Spiked (ng g−1) | Conc. of Total AFs Measured by LC-MS/MS (ng g−1) | Conc. Measured by ELISA (ng g−1) |
|---|---|---|
| 0 | 2.46 | 0.25 ± 0.03 |
| 12.5 | 12.16 | 5.67 ± 0.33 |
| 25 | 23.21 | 12.73 ± 1.13 |
| 50 | 47.25 | 24.00 ± 2.80 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cao, J.; Wang, T.; Wu, K.; Zhou, F.; Feng, Y.; Li, J.; Deng, A. A Highly Sensitive and Group-Specific Enzyme-Linked Immunosorbent Assay (ELISA) for the Detection of AFB1 in Agriculture and Aquiculture Products. Molecules 2024, 29, 2280. https://doi.org/10.3390/molecules29102280
Cao J, Wang T, Wu K, Zhou F, Feng Y, Li J, Deng A. A Highly Sensitive and Group-Specific Enzyme-Linked Immunosorbent Assay (ELISA) for the Detection of AFB1 in Agriculture and Aquiculture Products. Molecules. 2024; 29(10):2280. https://doi.org/10.3390/molecules29102280
Chicago/Turabian StyleCao, Junlin, Ting Wang, Kang Wu, Fengjie Zhou, Yuze Feng, Jianguo Li, and Anping Deng. 2024. "A Highly Sensitive and Group-Specific Enzyme-Linked Immunosorbent Assay (ELISA) for the Detection of AFB1 in Agriculture and Aquiculture Products" Molecules 29, no. 10: 2280. https://doi.org/10.3390/molecules29102280
APA StyleCao, J., Wang, T., Wu, K., Zhou, F., Feng, Y., Li, J., & Deng, A. (2024). A Highly Sensitive and Group-Specific Enzyme-Linked Immunosorbent Assay (ELISA) for the Detection of AFB1 in Agriculture and Aquiculture Products. Molecules, 29(10), 2280. https://doi.org/10.3390/molecules29102280







