3-Hydroxytanshinone Inhibits the Activity of Hypoxia-Inducible Factor 1-α by Interfering with the Function of α-Enolase in the Glycolytic Pathway
Abstract
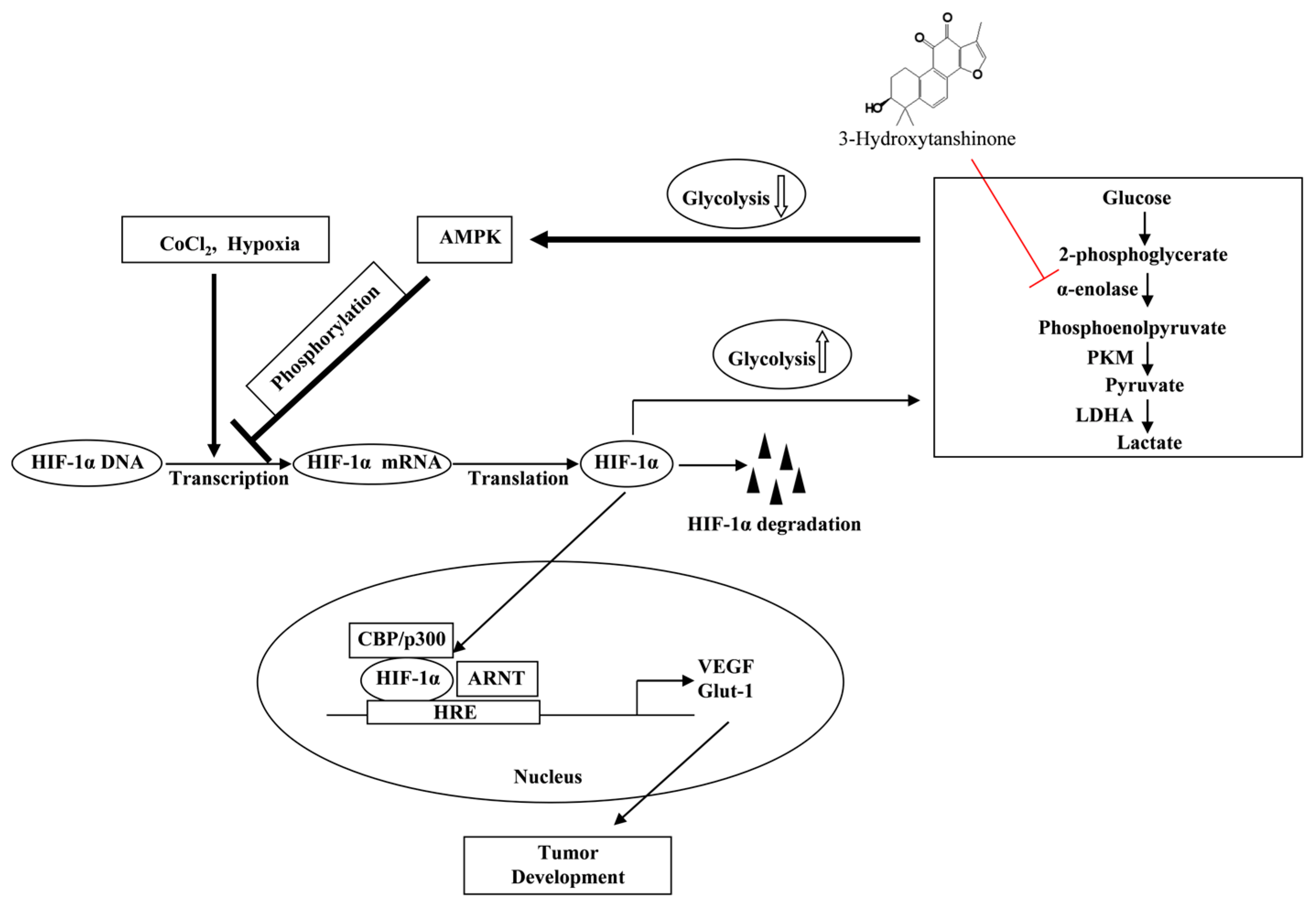
1. Introduction
2. Results
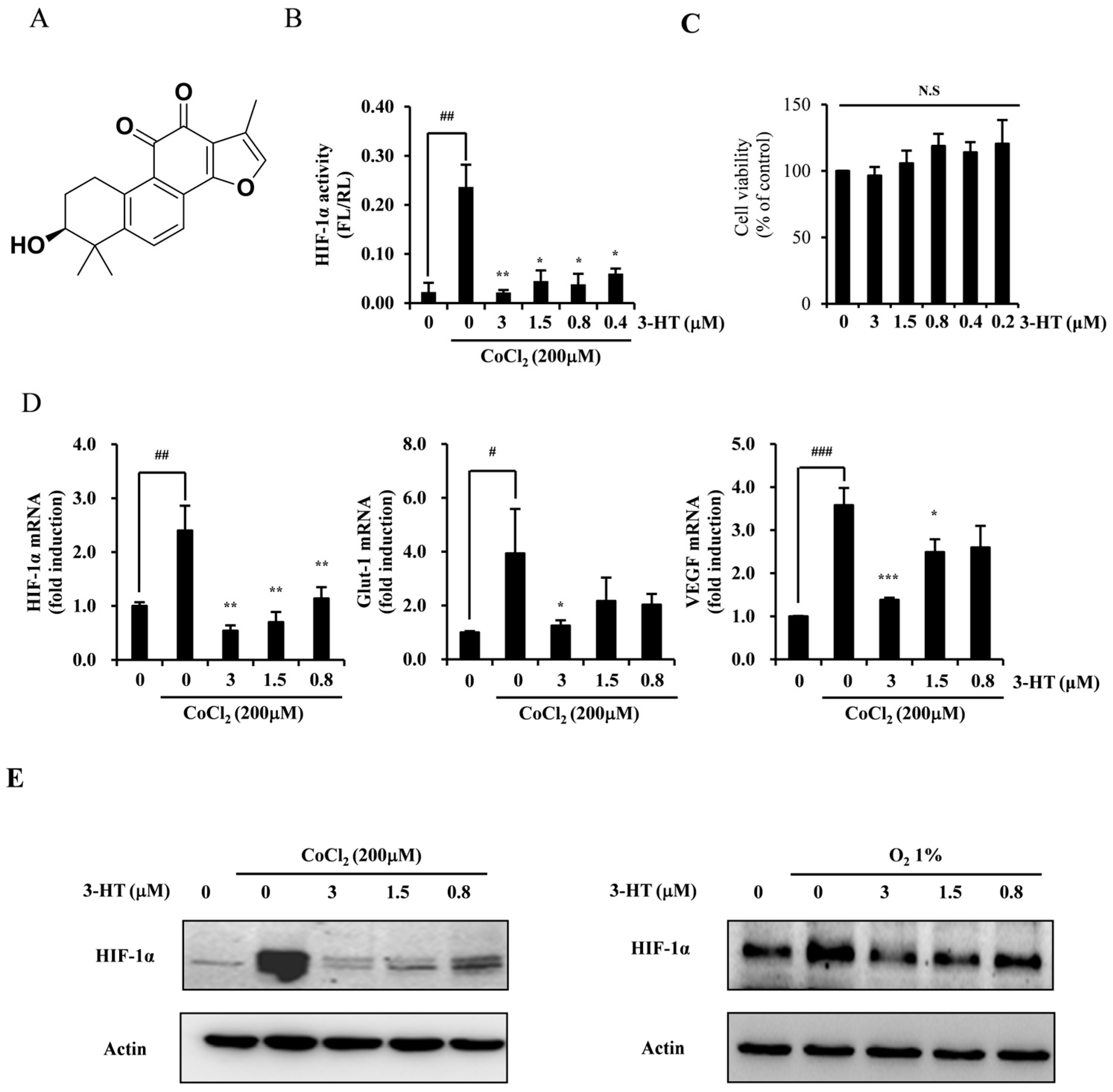
2.1. 3-HT Suppresses the Hypoxia-Induced Expression of HIF-1α
2.2. 3-HT Attenuates Hypoxia-Induced Angiogenesis in Both In Vitro and Ex Vivo
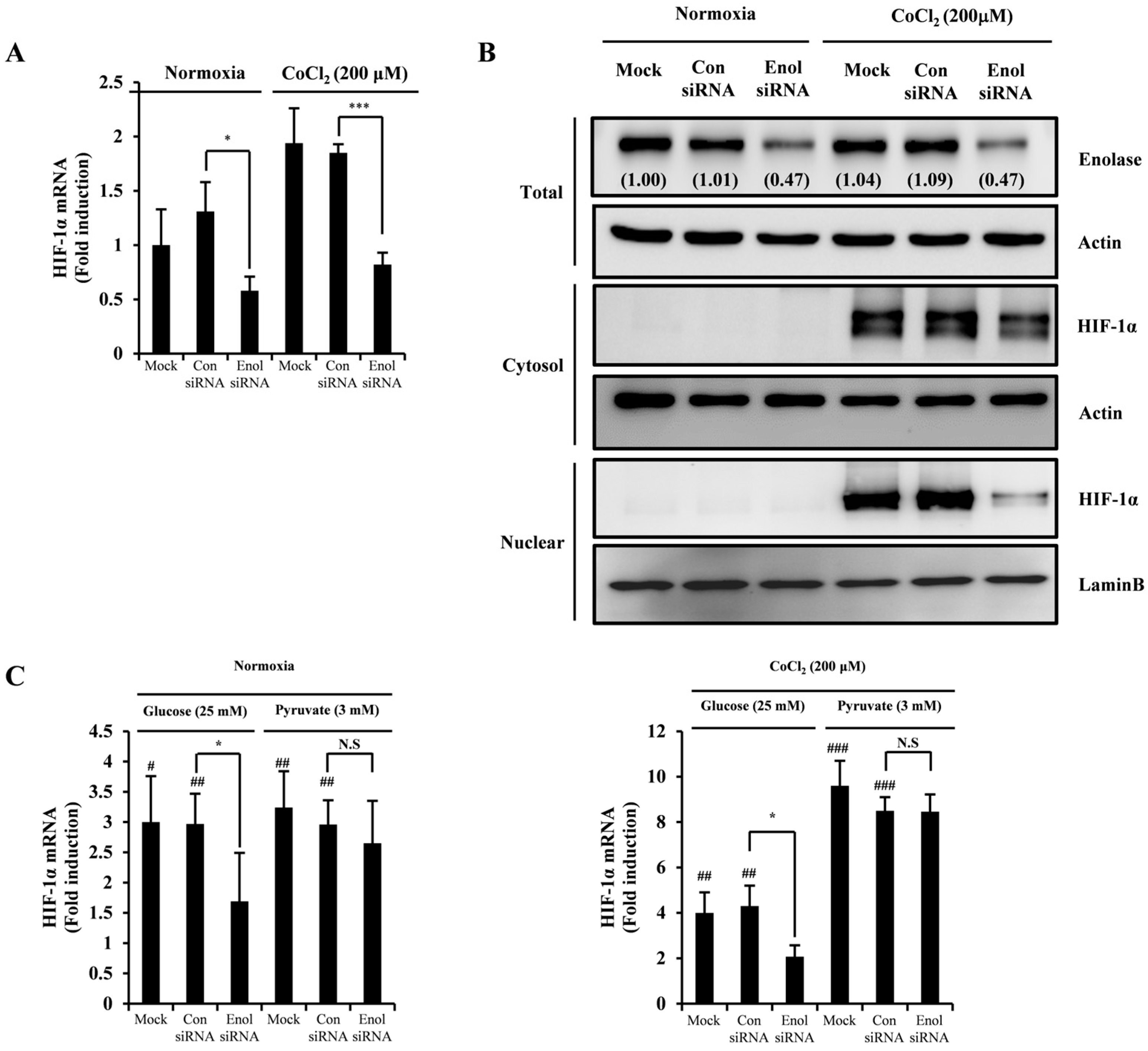
2.3. 3-HT Interacts with the α-Enolase Protein, Leading to the Inhibition of Its Enzymatic Activity
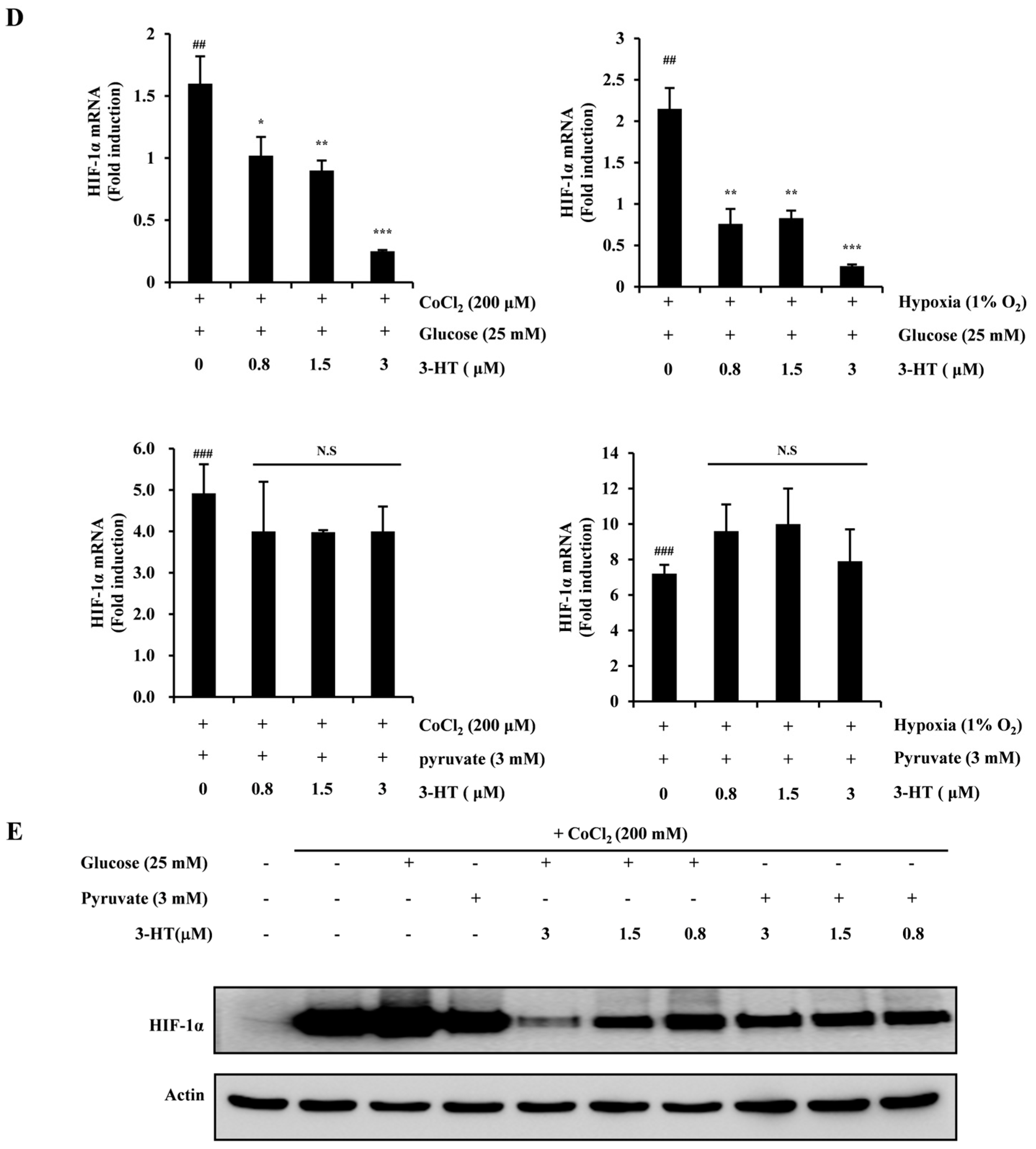
2.4. 3-HT Inhibits the Expression of HIF-1α by Directly Modulating the Activity of α-Enolase
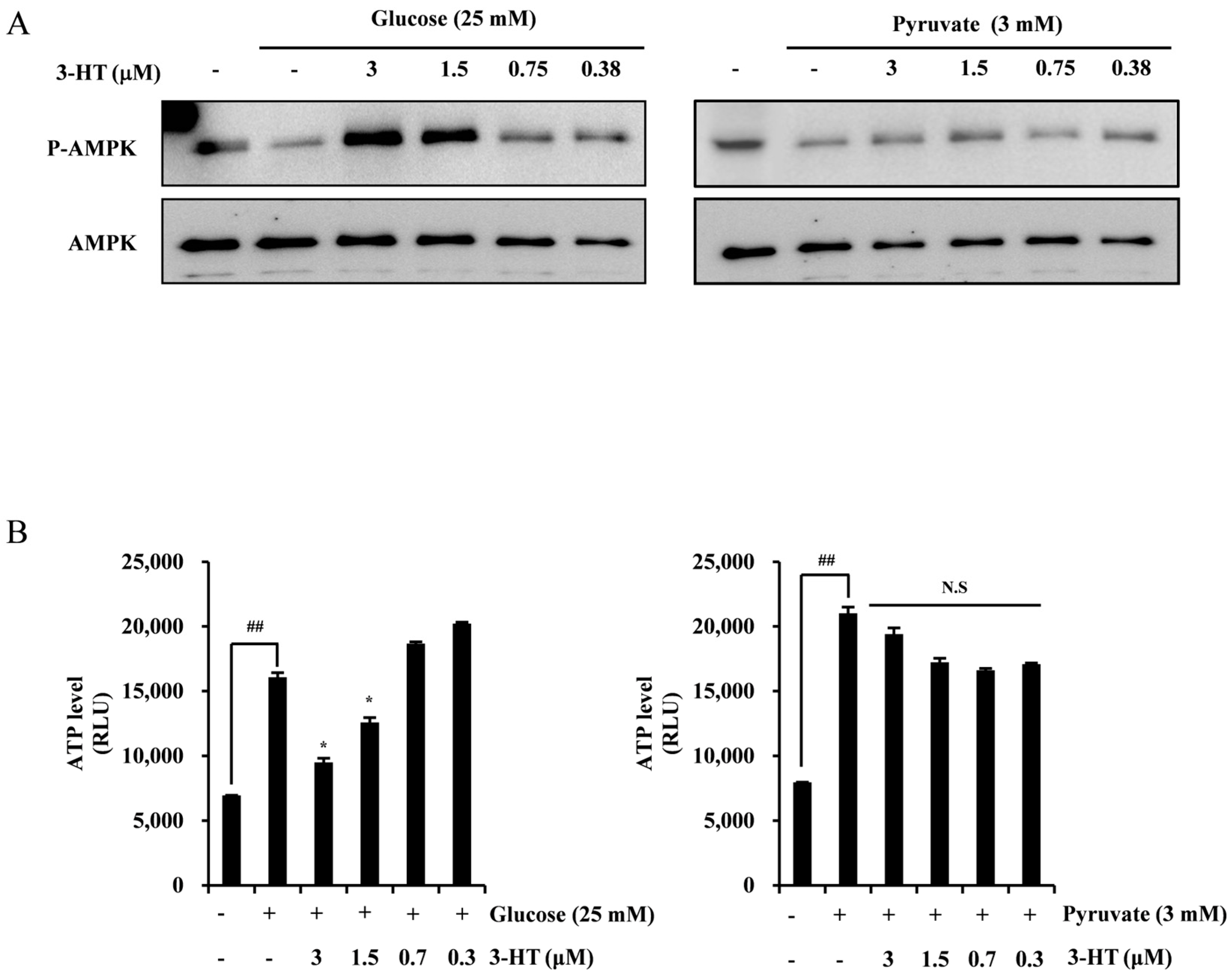
2.5. 3-HT Negatively Regulates HIF-1α through the Activation of AMPK
3. Discussion
4. Materials and Methods
4.1. Cell Culture
4.2. Reagents
4.3. Chemicals
4.4. Stable Cell Lines and Luciferase Assay
4.5. Cell Proliferation Assay
4.6. Real-Time PCR
4.7. Western Blot Analysis
4.8. In Vitro Capillary Tube Formation Assay
4.9. Chorioallantoic Membrane Assay
4.10. Target Identification
4.11. Dotting Assay
4.12. Surface Plasmon Resonance Assay
4.13. α-Enolase Enzymatic Activity Assay
4.14. ATP Assay
4.15. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| 3-HT | 3-Hydroxytanshinone |
| AMPK | AMP-activated protein kinase |
| ATP | Adenosine triphosphate |
| CAM | Chick embryo chorioallantoic membrane |
| CoCl2 | Cobalt chloride |
| DMSO | Dimethyl sulfoxide |
| FPLC | Fast Protein Liquid Chromatography |
| GLUT-1 | Glucose transporter 1 |
| HIF-1α | Hypoxia Inducible Factor-1α |
| HIST3H2A | Histone H2A type 3 |
| HUVECs | Human umbilical vein endothelial cells |
| LC-MS/MS | Liquid chromatography-tandem mass spectrometry |
| MUC1 | Mucin1 |
| RA | Retinoic acid |
| SPR | Surface plasmon resonance |
| VEGF | Vascular endothelial growth factor |
References
- Global Cancer Burden Growing, Amidst Mounting Need for Services. Available online: https://www.who.int/news/item/01-02-2024-global-cancer-burden-growing--amidst-mounting-need-for-services (accessed on 18 March 2024).
- Osawa, T.; Tsuchida, R.; Muramatsu, M.; Shimamura, T.; Wang, F.; Suehiro, J.; Kanki, Y.; Wada, Y.; Yuasa, Y.; Aburatani, H.; et al. Inhibition of histone demethylase JMJD1A improves anti-angiogenic therapy and reduces tumor-associated macrophages. Cancer Res. 2013, 73, 3019–3028. [Google Scholar] [CrossRef]
- Infantino, V.; Santarsiero, A.; Convertini, P.; Todisco, S.; Iacobazzi, V. Cancer Cell Metabolism in Hypoxia: Role of HIF-1 as Key Regulator and Therapeutic Target. Int. J. Mol. Sci. 2021, 22, 5703. [Google Scholar] [CrossRef]
- Krock, B.L.; Skuli, N.; Simon, M.C. Hypoxia-induced angiogenesis: Good and evil. Genes. Cancer 2011, 2, 1117–1133. [Google Scholar] [CrossRef] [PubMed]
- Kierans, S.J.; Taylor, C.T. Regulation of glycolysis by the hypoxia-inducible factor (HIF): Implications for cellular physiology. J. Physiol. 2021, 599, 23–37. [Google Scholar] [CrossRef] [PubMed]
- Melkonian, E.A.; Schury, M.P. Biochemistry, Anaerobic Glycolysis. In StatPearls; Statpearls Publishing, LLC.: St. Petersburg, FL, USA, 2024. [Google Scholar]
- Courtnay, R.; Ngo, D.C.; Malik, N.; Ververis, K.; Tortorella, S.M.; Karagiannis, T.C. Cancer metabolism and the Warburg effect: The role of HIF-1 and PI3K. Mol. Biol. Rep. 2015, 42, 841–851. [Google Scholar] [CrossRef]
- Huang, C.K.; Sun, Y.; Lv, L.; Ping, Y. ENO1 and Cancer. Mol. Ther. Oncolytics 2022, 24, 288–298. [Google Scholar] [CrossRef]
- Lin, S.C.; Hardie, D.G. AMPK: Sensing Glucose as well as Cellular Energy Status. Cell Metab. 2018, 27, 299–313. [Google Scholar] [CrossRef] [PubMed]
- Kishton, R.J.; Barnes, C.E.; Nichols, A.G.; Cohen, S.; Gerriets, V.A.; Siska, P.J.; Macintyre, A.N.; Goraksha-Hicks, P.; de Cubas, A.A.; Liu, T.; et al. AMPK Is Essential to Balance Glycolysis and Mitochondrial Metabolism to Control T-ALL Cell Stress and Survival. Cell Metab. 2016, 23, 649–662. [Google Scholar] [CrossRef]
- Atanasov, A.G.; Zotchev, S.B.; Dirsch, V.M.; International Natural Product Sciences, T.; Supuran, C.T. Natural products in drug discovery: Advances and opportunities. Nat. Rev. Drug Discov. 2021, 20, 200–216. [Google Scholar] [CrossRef] [PubMed]
- Banwo, K.; Olojede, A.O.; Adesulu-Dahunsi, A.T.; Verma, D.K.; Thakur, M.; Tripathy, S.; Singh, S.; Patel, A.R.; Gupta, A.K.; Aguilar, C.N.; et al. Functional importance of bioactive compounds of foods with Potential Health Benefits: A review on recent trends. Food Biosci. 2021, 43, 101320. [Google Scholar] [CrossRef]
- Li, W.; Huang, T.; Xu, S.; Che, B.; Yu, Y.; Zhang, W.; Tang, K. Molecular Mechanism of Tanshinone against Prostate Cancer. Molecules 2022, 27, 5594. [Google Scholar] [CrossRef] [PubMed]
- Gao, S.; Liu, Z.; Li, H.; Little, P.J.; Liu, P.; Xu, S. Cardiovascular actions and therapeutic potential of tanshinone IIA. Atherosclerosis 2012, 220, 3–10. [Google Scholar] [CrossRef] [PubMed]
- Subedi, L.; Gaire, B.P. Tanshinone IIA: A phytochemical as a promising drug candidate for neurodegenerative diseases. Pharmacol. Res. 2021, 169, 105661. [Google Scholar] [CrossRef]
- Fang, Z.Y.; Zhang, M.; Liu, J.N.; Zhao, X.; Zhang, Y.Q.; Fang, L. Tanshinone IIA: A Review of its Anticancer Effects. Front. Pharmacol. 2020, 11, 611087. [Google Scholar] [CrossRef]
- Wang, Y.; Liu, L.; Qu, Z.; Wang, D.; Huang, W.; Kong, L.; Yan, L. Tanshinone Ameliorates Glucocorticoid-Induced Bone Loss via Activation of AKT1 Signaling Pathway. Front. Cell Dev. Biol. 2022, 10, 878433. [Google Scholar] [CrossRef]
- Lu, M.; Lan, X.; Wu, X.; Fang, X.; Zhang, Y.; Luo, H.; Gao, W.; Wu, D. Salvia miltiorrhiza in cancer: Potential role in regulating MicroRNAs and epigenetic enzymes. Front. Pharmacol. 2022, 13, 1008222. [Google Scholar] [CrossRef]
- Su, C.Y.; Ming, Q.L.; Rahman, K.; Han, T.; Qin, L.P. Salvia miltiorrhiza: Traditional medicinal uses, chemistry, and pharmacology. Chin. J. Nat. Med. 2015, 13, 163–182. [Google Scholar] [CrossRef]
- Jiang, Z.; Gao, W.; Huang, L. Tanshinones, Critical Pharmacological Components in Salvia miltiorrhiza. Front. Pharmacol. 2019, 10, 202. [Google Scholar] [CrossRef]
- Hu, K.B.; Lu, X.M.; Wang, H.Y.; Liu, H.L.; Wu, Q.Y.; Liao, P.; Li, S.; Long, Z.Y.; Wang, Y.T. Effects and mechanisms of tanshinone IIA on PTSD-like symptoms. Phytomedicine 2023, 120, 155032. [Google Scholar] [CrossRef] [PubMed]
- Bi, H.C.; Zuo, Z.; Chen, X.; Xu, C.S.; Wen, Y.Y.; Sun, H.Y.; Zhao, L.Z.; Pan, Y.; Deng, Y.; Liu, P.Q.; et al. Preclinical factors affecting the pharmacokinetic behaviour of tanshinone IIA, an investigational new drug isolated from Salvia miltiorrhiza for the treatment of ischaemic heart diseases. Xenobiotica 2008, 38, 185–222. [Google Scholar] [CrossRef]
- Zhang, F.; Zheng, W.; Pi, R.; Mei, Z.; Bao, Y.; Gao, J.; Tang, W.; Chen, S.; Liu, P. Cryptotanshinone protects primary rat cortical neurons from glutamate-induced neurotoxicity via the activation of the phosphatidylinositol 3-kinase/Akt signaling pathway. Exp. Brain Res. 2009, 193, 109–118. [Google Scholar] [CrossRef]
- Park, O.K.; Choi, J.H.; Park, J.H.; Kim, I.H.; Yan, B.C.; Ahn, J.H.; Kwon, S.H.; Lee, J.C.; Kim, Y.S.; Kim, M.; et al. Comparison of neuroprotective effects of five major lipophilic diterpenoids from Danshen extract against experimentally induced transient cerebral ischemic damage. Fitoterapia 2012, 83, 1666–1674. [Google Scholar] [CrossRef] [PubMed]
- Jing, X.; Wei, X.; Ren, M.; Wang, L.; Zhang, X.; Lou, H. Neuroprotective Effects of Tanshinone I Against 6-OHDA-Induced Oxidative Stress in Cellular and Mouse Model of Parkinson’s Disease Through Upregulating Nrf2. Neurochem. Res. 2016, 41, 779–786. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Jing, H.; Yang, H.; Liu, Z.; Guo, H.; Chai, L.; Hu, L. Tanshinone I selectively suppresses pro-inflammatory genes expression in activated microglia and prevents nigrostriatal dopaminergic neurodegeneration in a mouse model of Parkinson’s disease. J. Ethnopharmacol. 2015, 164, 247–255. [Google Scholar] [CrossRef]
- Chen, Z.; Han, F.; Du, Y.; Shi, H.; Zhou, W. Hypoxic microenvironment in cancer: Molecular mechanisms and therapeutic interventions. Signal Transduct. Target. Ther. 2023, 8, 70. [Google Scholar] [CrossRef]
- Liu, W.; Shen, S.M.; Zhao, X.Y.; Chen, G.Q. Targeted genes and interacting proteins of hypoxia inducible factor-1. Int. J. Biochem. Mol. Biol. 2012, 3, 165–178. [Google Scholar] [PubMed]
- Gleadle, J.M.; Ratcliffe, P.J. Induction of hypoxia-inducible factor-1, erythropoietin, vascular endothelial growth factor, and glucose transporter-1 by hypoxia: Evidence against a regulatory role for Src kinase. Blood 1997, 89, 503–509. [Google Scholar] [CrossRef]
- Tang, N.; Wang, L.; Esko, J.; Giordano, F.J.; Huang, Y.; Gerber, H.P.; Ferrara, N.; Johnson, R.S. Loss of HIF-1alpha in endothelial cells disrupts a hypoxia-driven VEGF autocrine loop necessary for tumorigenesis. Cancer Cell 2004, 6, 485–495. [Google Scholar] [CrossRef]
- An, H.; Lee, S.; Lee, J.M.; Jo, D.H.; Kim, J.; Jeong, Y.S.; Heo, M.J.; Cho, C.S.; Choi, H.; Seo, J.H.; et al. Novel Hypoxia-Inducible Factor 1alpha (HIF-1alpha) Inhibitors for Angiogenesis-Related Ocular Diseases: Discovery of a Novel Scaffold via Ring-Truncation Strategy. J. Med. Chem. 2018, 61, 9266–9286. [Google Scholar] [CrossRef]
- Lee, H.P.; Liu, Y.C.; Chen, P.C.; Tai, H.C.; Li, T.M.; Fong, Y.C.; Chang, C.S.; Wu, M.H.; Chiu, L.P.; Wang, C.J.; et al. Tanshinone IIA inhibits angiogenesis in human endothelial progenitor cells in vitro and in vivo. Oncotarget 2017, 8, 109217–109227. [Google Scholar] [CrossRef]
- Xing, Y.; Tu, J.; Zheng, L.; Guo, L.; Xi, T. Anti-angiogenic effect of tanshinone IIA involves inhibition of the VEGF/VEGFR2 pathway in vascular endothelial cells. Oncol. Rep. 2015, 33, 163–170. [Google Scholar] [CrossRef] [PubMed]
- Ganapathy-Kanniappan, S.; Geschwind, J.F. Tumor glycolysis as a target for cancer therapy: Progress and prospects. Mol. Cancer 2013, 12, 152. [Google Scholar] [CrossRef] [PubMed]
- Liberti, M.V.; Locasale, J.W. The Warburg Effect: How Does it Benefit Cancer Cells? Trends Biochem. Sci. 2016, 41, 211–218. [Google Scholar] [CrossRef]
- Lee, S.H.; Golinska, M.; Griffiths, J.R. HIF-1-Independent Mechanisms Regulating Metabolic Adaptation in Hypoxic Cancer Cells. Cells 2021, 10, 2371. [Google Scholar] [CrossRef] [PubMed]
- Qi, H.; Chen, Z.; Qin, Y.; Wang, X.; Zhang, Z.; Li, Y. Tanshinone IIA inhibits cell growth by suppressing SIX1-induced aerobic glycolysis in non-small cell lung cancer cells. Oncol. Lett. 2022, 23, 184. [Google Scholar] [CrossRef]
- Qiao, G.; Wu, A.; Chen, X.; Tian, Y.; Lin, X. Enolase 1, a Moonlighting Protein, as a Potential Target for Cancer Treatment. Int. J. Biol. Sci. 2021, 17, 3981–3992. [Google Scholar] [CrossRef] [PubMed]
- Chung, I.C.; Huang, W.C.; Huang, Y.T.; Chen, M.L.; Tsai, A.W.; Wu, P.Y.; Yuan, T.T. Unrevealed roles of extracellular enolase-1 (ENO1) in promoting glycolysis and pro-cancer activities in multiple myeloma via hypoxia-inducible factor 1alpha. Oncol. Rep. 2023, 50, 205. [Google Scholar] [CrossRef] [PubMed]
- Zheng, F.; Jang, W.C.; Fung, F.K.; Lo, A.C.; Wong, I.Y. Up-Regulation of ENO1 by HIF-1alpha in Retinal Pigment Epithelial Cells after Hypoxic Challenge Is Not Involved in the Regulation of VEGF Secretion. PLoS ONE 2016, 11, e0147961. [Google Scholar] [CrossRef] [PubMed]
- Semenza, G.L.; Jiang, B.H.; Leung, S.W.; Passantino, R.; Concordet, J.P.; Maire, P.; Giallongo, A. Hypoxia response elements in the aldolase A, enolase 1, and lactate dehydrogenase A gene promoters contain essential binding sites for hypoxia-inducible factor 1. J. Biol. Chem. 1996, 271, 32529–32537. [Google Scholar] [CrossRef]
- Liu, Y.; Liao, L.; An, C.; Wang, X.; Li, Z.; Xu, Z.; Liu, J.; Liu, S. alpha-Enolase Lies Downstream of mTOR/HIF1alpha and Promotes Thyroid Carcinoma Progression by Regulating CST1. Front. Cell Dev. Biol. 2021, 9, 670019. [Google Scholar] [CrossRef]
- Zhan, P.; Zhao, S.; Yan, H.; Yin, C.; Xiao, Y.; Wang, Y.; Ni, R.; Chen, W.; Wei, G.; Zhang, P. alpha-enolase promotes tumorigenesis and metastasis via regulating AMPK/mTOR pathway in colorectal cancer. Mol. Carcinog. 2017, 56, 1427–1437. [Google Scholar] [CrossRef] [PubMed]
- Qi, D.; Young, L.H. AMPK: Energy sensor and survival mechanism in the ischemic heart. Trends Endocrinol. Metab. 2015, 26, 422–429. [Google Scholar] [CrossRef] [PubMed]
- Chen, M.B.; Zhang, Y.; Wei, M.X.; Shen, W.; Wu, X.Y.; Yao, C.; Lu, P.H. Activation of AMP-activated protein kinase (AMPK) mediates plumbagin-induced apoptosis and growth inhibition in cultured human colon cancer cells. Cell. Signal. 2013, 25, 1993–2002. [Google Scholar] [CrossRef] [PubMed]
- Treins, C.; Murdaca, J.; Van Obberghen, E.; Giorgetti-Peraldi, S. AMPK activation inhibits the expression of HIF-1alpha induced by insulin and IGF-1. Biochem. Biophys. Res. Commun. 2006, 342, 1197–1202. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.S.; Han, S.Y.; Kim, M.S.; Yu, C.M.; Kim, M.H.; Kim, S.H.; Min, Y.K.; Kim, B.T. Synthesis of novel chemical probes for the study of tanshinone binding proteins. Bioorg Med. Chem. Lett. 2006, 16, 4733–4737. [Google Scholar] [CrossRef]
- Choi, S.W.; Lee, K.S.; Lee, J.H.; Kang, H.J.; Lee, M.J.; Kim, H.Y.; Park, K.I.; Kim, S.L.; Shin, H.K.; Seo, W.D. Suppression of Akt-HIF-1alpha signaling axis by diacetyl atractylodiol inhibits hypoxia-induced angiogenesis. BMB Rep. 2016, 49, 508–513. [Google Scholar] [CrossRef]
- Rozen, S.; Skaletsky, H. Primer3 on the WWW for general users and for biologist programmers. Methods Mol. Biol. 2000, 132, 365–386. [Google Scholar] [CrossRef] [PubMed]
- Kim, K.H.; Jung, H.J.; Kwon, H.J. A new anti-angiogenic small molecule, G0811, inhibits angiogenesis via targeting hypoxia inducible factor (HIF)-1alpha signal transduction. Biochem. Biophys. Res. Commun. 2013, 441, 399–404. [Google Scholar] [CrossRef]
- Anderson, G.P.; Legler, P.M.; Zabetakis, D.; Goldman, E.R. Comparison of immunoreactivity of staphylococcal enterotoxin B mutants for use as toxin surrogates. Anal. Chem. 2012, 84, 5198–5203. [Google Scholar] [CrossRef]


| Target Gene | Forward Primer (5′–3′) | Reverse Primer (5′–3′) |
|---|---|---|
| Glut-1 | TGGATGTCCTATCTGAGCATCG | CTCCTCGGGTGTCTTGTCAC |
| VEGF | AACTTTCTGCTGTCTTGG | TTTGGTCTGCATTCACAT |
| HIF-1α | ACTTAAGAAGGAACCTGATG | TGGAGACATTGCCAAATTTA |
| HIST3H2A | CTTGACTCGGAAATGTCCGGTCG | AGTCAAGTACTCGAGCACCGCG |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Son, T.H.; Kim, S.-H.; Shin, H.-L.; Kim, D.; Kim, H.G.; Choi, Y.; Choi, S.-W. 3-Hydroxytanshinone Inhibits the Activity of Hypoxia-Inducible Factor 1-α by Interfering with the Function of α-Enolase in the Glycolytic Pathway. Molecules 2024, 29, 2218. https://doi.org/10.3390/molecules29102218
Son TH, Kim S-H, Shin H-L, Kim D, Kim HG, Choi Y, Choi S-W. 3-Hydroxytanshinone Inhibits the Activity of Hypoxia-Inducible Factor 1-α by Interfering with the Function of α-Enolase in the Glycolytic Pathway. Molecules. 2024; 29(10):2218. https://doi.org/10.3390/molecules29102218
Chicago/Turabian StyleSon, Tae Hyun, Shin-Hye Kim, Hye-Lim Shin, Dongsoo Kim, Hwan Gyu Kim, Yongseok Choi, and Sik-Won Choi. 2024. "3-Hydroxytanshinone Inhibits the Activity of Hypoxia-Inducible Factor 1-α by Interfering with the Function of α-Enolase in the Glycolytic Pathway" Molecules 29, no. 10: 2218. https://doi.org/10.3390/molecules29102218
APA StyleSon, T. H., Kim, S.-H., Shin, H.-L., Kim, D., Kim, H. G., Choi, Y., & Choi, S.-W. (2024). 3-Hydroxytanshinone Inhibits the Activity of Hypoxia-Inducible Factor 1-α by Interfering with the Function of α-Enolase in the Glycolytic Pathway. Molecules, 29(10), 2218. https://doi.org/10.3390/molecules29102218








