Study on Preparation of Calcium-Based Modified Coal Gangue and Its Adsorption Dye Characteristics
Abstract
1. Introduction
2. Results and Discussion
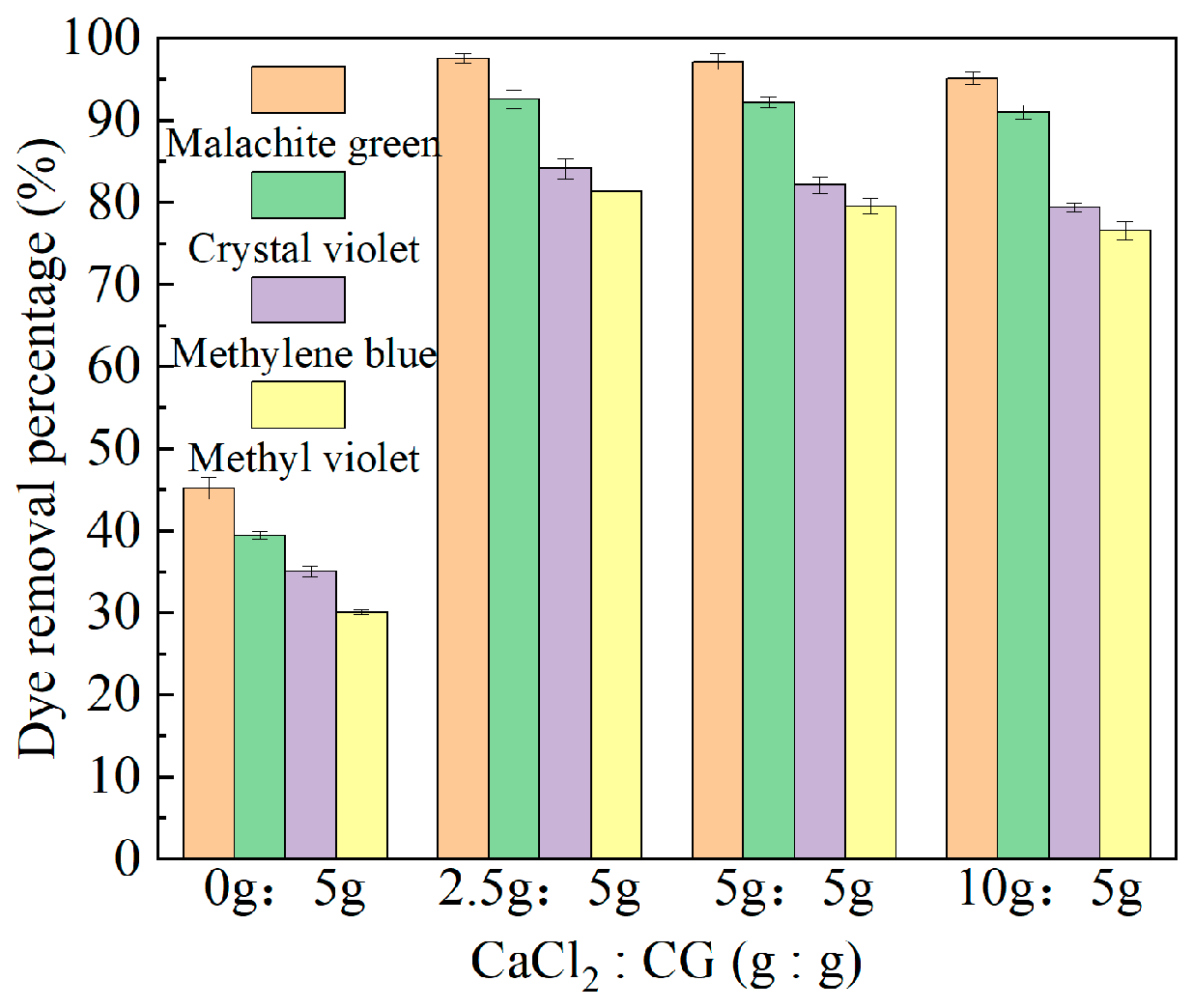
2.1. Experimental Results of Different Ca:CG Ratios of Ca-CG on Dye Removal Efficiency
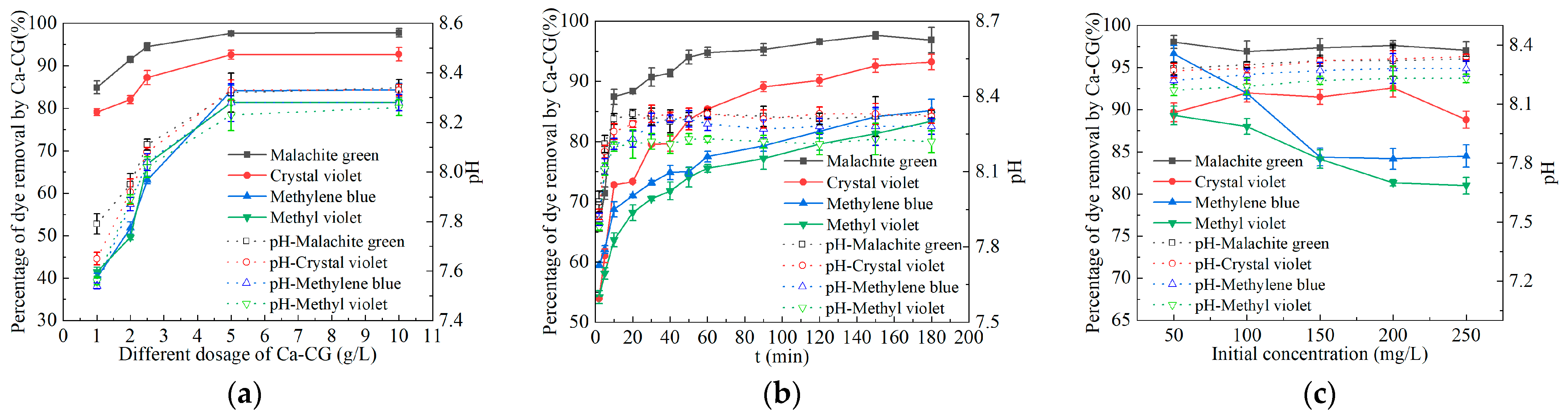
2.2. Batch Experiments
2.3. Adsorption Kinetics
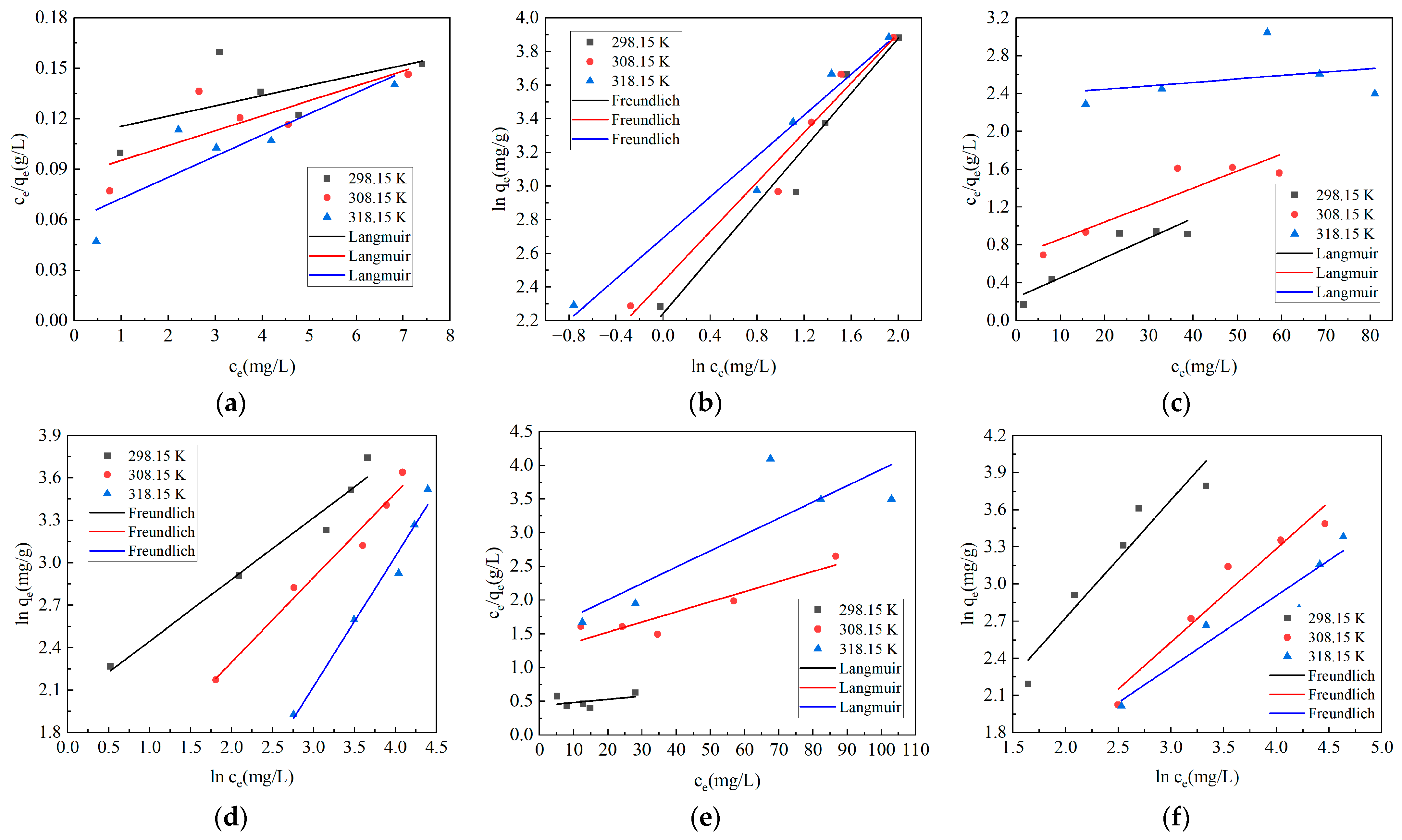
2.4. Adsorption Isotherm and Adsorption Thermodynamics
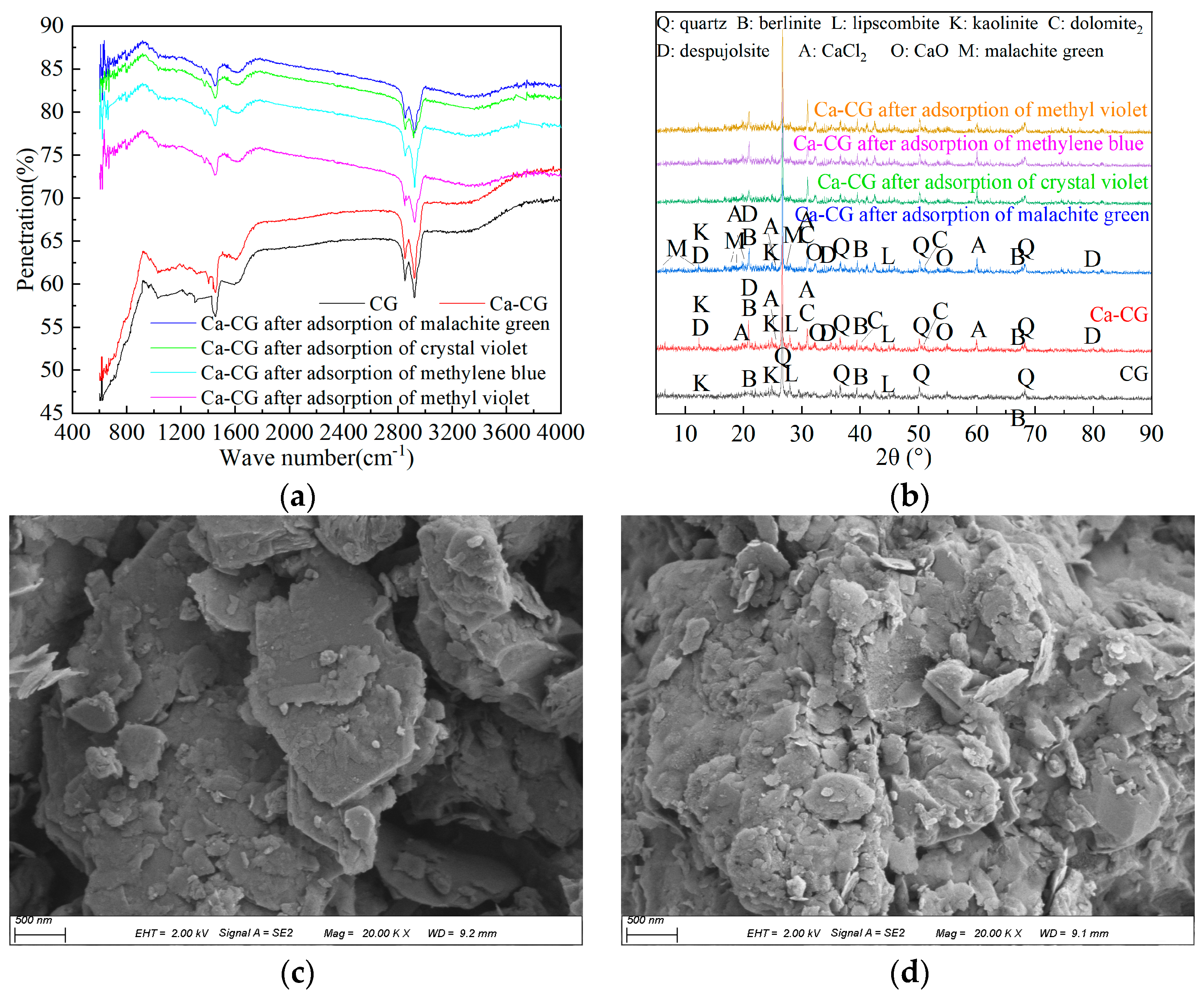
2.5. XRF, FTIR, XRD and SEM Analysis
3. Materials and Methods
3.1. Experimental Materials
3.2. Experimental Method
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Blackburn Richard, S. Natural polysaccharides and their interactions with dye molecules: Applications in effluent treatment. Environ. Sci. Technol. 2004, 38, 4905–4909. [Google Scholar] [CrossRef] [PubMed]
- Hussain, L.; Javed, F.; Tahir, M.W.; Munir, H.M.S.; Ikhlaq, A.; Wołowicz, A. Catalytic Ozonation of Reactive Black 5 in Aqueous Solution Using Iron-Loaded Dead Leaf Ash for Wastewater Remediation. Molecules 2024, 29, 836. [Google Scholar] [CrossRef] [PubMed]
- Abdi, J.; Vossoughi, M.; Mahmoodi, N.M.; Alemzadeh, I. Synthesis of metal-organic framework hybrid nanocomposites based on GO and CNT with high adsorption capacity for dye removal. Chem. Eng. J. 2017, 326, 1145–1158. [Google Scholar] [CrossRef]
- Zhou, Y.; Lu, J.; Zhou, Y.; Liu, Y. Recent advances for dyes removal using novel adsorbents: A review. Environ. Pollut. 2019, 252, 352–365. [Google Scholar] [CrossRef] [PubMed]
- Peerakiatkhajohn, P.; Butburee, T.; Sul, J.-H.; Thaweesak, S.; Yun, J.-H. Efficient and Rapid Photocatalytic Degradation of Methyl Orange Dye Using Al/ZnO Nanoparticles. Nanomaterials 2021, 11, 1059. [Google Scholar] [CrossRef] [PubMed]
- Xu, R.; Chi, T.; Tian, J.; Chen, L. A new sustainable wastewater management: Simultaneous mineralization and dehalogenation treatment of high salinity dye wastewater. J. Clean. Prod. 2024, 445, 141046. [Google Scholar] [CrossRef]
- Vinayagam, V.; Palani, K.N.; Ganesh, S.; Rajesh, S.; Akula, V.V.; Avoodaiappan, R.; Kushwaha, O.S.; Pugazhendhi, A. Recent developments on advanced oxidation processes for degradation of pollutants from wastewater with focus on antibiotics and organic dyes. Environ. Res. 2024, 240, 117500. [Google Scholar] [CrossRef] [PubMed]
- Sartaj, S.; Ali, N.; Khan, A.; Malik, S.; Bilal, M.; Khan, M.; Ali, N.; Hussain, S.; Khan, H.; Khan, S. Performance evaluation of photolytic and electro-chemical oxidation processes for enhanced degradation of food dyes laden wastewater. Water Sci. Technol. J. Int. Assoc. Water Pollut. Res. 2020, 81, 971–984. [Google Scholar] [CrossRef] [PubMed]
- Georgianos, P.; Pournara, A.D.; Andreou, E.K.; Armatas, G.S.; Manos, M.J. Composite Materials Based on a Zr4+ MOF and Aluminosilicates for the Simultaneous Removal of Cationic and Anionic Dyes from Aqueous Media. Molecules 2023, 28, 815. [Google Scholar] [CrossRef] [PubMed]
- Paluch, D.; Bazan-Wozniak, A.; Wolski, R.; Nosal-Wiercińska, A.; Pietrzak, R. Removal of Methyl Red from Aqueous Solution Using Biochar Derived from Fennel Seeds. Molecules 2023, 28, 7786. [Google Scholar] [CrossRef] [PubMed]
- Sultana, M.; Rownok, M.H.; Sabrin, M.; Rahaman, H.; Alam, S. A review on experimental chemically modified activated carbon to enhance dye and heavy metals adsorption. Clean. Eng. Technol. 2022, 6, 100382. [Google Scholar] [CrossRef]
- Rios, R.D.F.; Bueno, P.J.B.; Terra, J.C.S.; Moura, F.C.C. Influence of the surface modification of granularactivated carbon synthesized from macauba on heavy metal sorption. Environ. Sci. Pollut. Res. 2023, 30, 31881–31894. [Google Scholar] [CrossRef] [PubMed]
- Ye, T.; Min, X.; Jiang, X.; Sun, M.; Li, X. Adsorption and Desorption of Coal Gangue toward Available Phosphorus through Calcium-Modification with Different pH. Minerals 2022, 12, 801. [Google Scholar] [CrossRef]
- Shang, Z.; Zhang, L.; Zhao, X.; Liu, S.; Li, D. Removal of Pb(II), Cd(II) and Hg(II) from aqueous solution by mercaptomodified coal gangue. J. Environ. Manag. 2019, 231, 391–396. [Google Scholar] [CrossRef] [PubMed]
- Mohammadi, R.; Azadmehr, A.; Maghsoudi, A. Enhancing of competitive adsorptive removal of zinc and manganese from aqueous solution by iron oxide-combusted coal gangue composite. Sep. Sci. Technol. 2020, 55, 3343–3361. [Google Scholar] [CrossRef]
- Qiu, B.; Duan, F. Synthesis of industrial solid wastes/biochar composites and their use for adsorption of phosphate: From surface properties to sorption mechanism. Colloids Surf. A Physicochem. Eng. Asp. 2019, 571, 86–93. [Google Scholar] [CrossRef]
- Zhou, L.; Zhou, H.; Hu, Y.; Yan, S.; Yang, J. Adsorption removal of cationic dyes from aqueous solutions using ceramic adsorbents prepared from industrial waste coal gangue. J. Environ. Manag. 2019, 234, 245–252. [Google Scholar] [CrossRef]
- Li, H.; Du, H.; Kang, L.; Zhang, Y.; Lu, T.; Zhang, Y.; Yang, L.; Song, S. Biomass Carbon Improves the Adsorption Performance of Gangue-Based Ceramsites: Adsorption Kinetics and Mechanism Analysis. J. Renew. Mater. 2023, 11, 4161–4174. [Google Scholar] [CrossRef]
- He, C.; Shi, L.; Lou, S.; Liu, B.; Zhang, W.; Zhang, L. Synthesis of spherical magnetic calcium modified chitosan microparticles with excellent adsorption performance for anionic-cationic dyes. Int. J. Biol. Macromol. 2019, 128, 593–602. [Google Scholar] [CrossRef] [PubMed]
- An, S.; Liu, X.; Yang, L.; Zhang, L. Enhancement removal of crystal violet dye using magnetic calcium ferrite nanoparticle: Study in single- and binary-solute systems. Chem. Eng. Res. Des. 2015, 94, 726–735. [Google Scholar] [CrossRef]
- Balci, B.; Al Dafiry, M.H.A.; Erkurt, F.E.; Basibuyuk, M.; Zaimoglu, Z.; Budak, F.; Yesiltas, H.K. Fe2O3-powder activated caron/CaO2 as an efficient hybrid process to remove a reactive dye from textile wastewater. Chem. Eng. Commun. 2023, 210, 1445–1464. [Google Scholar] [CrossRef]
- Cruz, E.D.; Missau, J.; Collinson, S.R.; Tanabe, E.H.; Bertuol, D.A. Efficient removal of congo red dye using activated lychee peel biochar supported Ca-Cr layered double hydroxide. Environ. Nanotechnol. Monit. Manag. 2023, 20, 100835. [Google Scholar] [CrossRef]
- Wang, P.; Chen, W.; Zhang, R.; Xing, Y. Enhanced Removal of Malachite Green Using Calcium-Functionalized Magnetic Biochar. Int. J. Environ. Res. Public Health 2022, 19, 3247. [Google Scholar] [CrossRef] [PubMed]
- Ziyat, H.; Bennani, M.N.; Dehmani, Y.; Houssaini, J.; Allaoui, S.; Kacimi, R.; Hajjaj, H. Adsorptive performance of a synthesized Mg-Al Hydrotalcite compound for removal of malachite green: Kinetic, isotherm, thermodynamic, and mechanism study. Int. J. Environ. Anal. Chem. 2024, 104, 1072–1091. [Google Scholar] [CrossRef]
- Amoh, P.O.; Samy, M.; Elkady, M.; Shokry, H.; Mensah, K. Surface modification of toner-based recyclable iron oxide self-doped graphite nanocomposite to enhance methylene blue and tetracycline adsorption. J. Environ. Manag. 2024, 357, 120786. [Google Scholar] [CrossRef]
- Sarkar, S.; Tiwari, N.; Behera, M.; Chakrabortty, S.; Jhingran, K.; Sanjay, K.; Banerjee, S.; Tripathy, S.K. Facile synthesis, characterization and application of magnetic Fe3O4-coir pith composites for the removal of methyl violet from aqueous solution: Kinetics, isotherm, thermodynamics and parametric optimization. J. Indian Chem. Soc. 2022, 99, 100447. [Google Scholar] [CrossRef]
- Daneshgar, H.; Sojdeh, S.; Salehi, G.; Edrisi, M.; Bagherzadeh, M.; Rabiee, N. Comparative study of synthesis methods and pH-dependent adsorption of methylene blue dye on UiO-66 and NH2-UiO-66. Chemosphere 2024, 353, 141543. [Google Scholar] [CrossRef] [PubMed]
- Tang, K.; Zhang, J.; Wang, Q. Effect of pH on adsorption capacity of activated carbon. Chin. Leather 2007, 36, 7–10. [Google Scholar] [CrossRef]
- Fu, S.; Di, J.; Guo, X.; Dong, Y.; Bao, S.; Li, H. Preparation of lignite-loaded nano-FeS and its performance for treating acid Cr(VI)-containing wastewater. Environ. Sci. Pollut. Res. 2023, 30, 3351–3366. [Google Scholar] [CrossRef] [PubMed]
- Abebe, B.; Taddesse, A.M.; Kebede, T.; Teju, E.; Diaz, I. Fe-Al-Mn ternary oxide nanosorbent: Synthesis, characterization and phosphate sorption property. J. Environ. Chem. Eng. 2017, 5, 1330–1340. [Google Scholar] [CrossRef]
- Liu, Y.; Wang, S.; Huo, J.; Zhang, X.; Wen, H.; Zhang, D.; Zhao, Y.; Kang, D.; Guo, W.; Ngo, H.H. Adsorption recovery of phosphorus in contaminated water by calcium modified biochar derived from spent coffee grounds. Sci. Total Environ. 2023, 909, 168426. [Google Scholar] [CrossRef] [PubMed]
- Yadav, S.; Yadav, A.; Sharma, N.; Sharma, A.K.; Kumar, S. Exploring the Potential of Pennisetum glaucum Composite for the Removal of Cationic and Anionic Dyes at Natural pH for Sustainable Waste Water Remediation. Water Air Soil Pollut. 2024, 235, 220. [Google Scholar] [CrossRef]
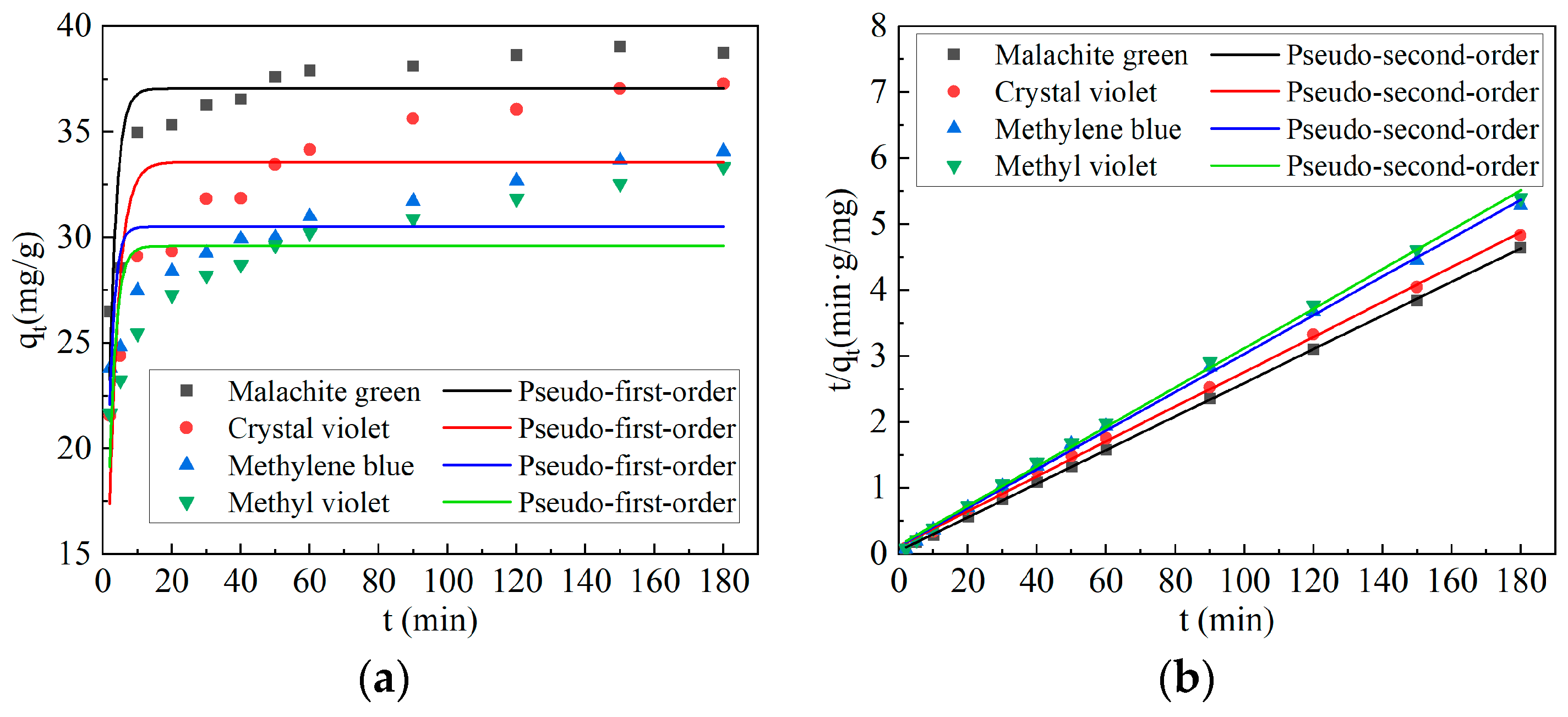

| Adsorption Kinetics Model | Parameter | Dye | |||
|---|---|---|---|---|---|
| Malachite Green | Methylene Blue | Crystal Violet | Methyl Violet | ||
| Pseudo-first-order | qe (mg/g) | 37.05617 | 30.521 | 33.57172 | 29.60306 |
| k1 (min−1) | 0.49497 | 0.64269 | 0.36501 | 0.52046 | |
| R2 | 0.68002 | 0.41459 | 0.61455 | 0.471 | |
| Pseudo-second-order | qe (mg/g) | 39.23107 | 34.27005 | 37.87879 | 33.46720 |
| k2 (mg/(g·min)) | 0.01304 | 0.00691 | 0.00550 | 0.00656 | |
| R2 | 0.99987 | 0.99846 | 0.99892 | 0.99849 | |
| Type of Dyes | T (K) | Langmuir Model | Freundlich Model | ||||
|---|---|---|---|---|---|---|---|
| qm (mg/g) | KL (L/mg) | R2 | KF (mg(1−1/n)·L1/n·g−1) | n | R2 | ||
| Malachite green | 298.15 | 165.01650 | 0.05531 | 0.35049 | 9.44089 | 1.22424 | 0.95946 |
| 308.15 | 112.86682 | 0.10250 | 0.61541 | 11.41799 | 1.35811 | 0.96814 | |
| 318.15 | 79.68127 | 0.20847 | 0.76412 | 14.78318 | 1.64493 | 0.96388 | |
| Methylene blue | 298.15 | 47.59638 | 0.08607 | 0.86264 | 7.46937 | 2.29216 | 0.96673 |
| 308.15 | 55.71031 | 0.02622 | 0.83852 | 3.01413 | 1.67193 | 0.97531 | |
| 318.15 | 274.72527 | 0.00153 | 0.10702 | 0.52860 | 1.08516 | 0.97551 | |
| Crystal violet | 298.15 | 207.90021 | 0.01106 | 0.18798 | 2.28022 | 1.05048 | 0.91143 |
| 308.15 | 66.71114 | 0.01224 | 0.86068 | 1.30412 | 1.32366 | 0.94166 | |
| 318.15 | 41.28819 | 0.01592 | 0.72858 | 1.83543 | 1.73986 | 0.92135 | |
| Methyl violet | 298.15 | 67.88866 | 0.02738 | 0.95627 | 3.08892 | 1.50707 | 0.99065 |
| 308.15 | 49.60317 | 0.02770 | 0.91203 | 2.78250 | 1.68277 | 0.91439 | |
| 318.15 | 41.91115 | 0.01661 | 0.91301 | 2.06630 | 1.81018 | 0.97279 | |
| Type of Dyes | ΔG (kJ/mol) | ΔH (kJ/mol) | ΔS (kJ/(mol·K)) | ||
|---|---|---|---|---|---|
| 298.15 K | 308.15 K | 318.15 K | |||
| Malachite green | −4.19610 | −4.33684 | −4.47758 | −2.94918 × 10−9 | 0.01407 |
| Methylene blue | −0.60784 | −0.62823 | −0.64861 | −1.05108 × 10−8 | 0.00204 |
| Crystal violet | −2.62222 | −2.71017 | −2.79812 | −4.545 × 10−9 | 0.00879 |
| Methyl violet | −0.01334 | −0.01379 | −0.01424 | 1.2415 × 10−8 | 4.47441 × 10−5 |
| Components | SiO2 | Al2O3 | Fe2O3 | MgO | K2O | CaO | Na2O | TiO2 | MnO | P2O5 | SO3 | CO2 | Other |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Coal gangue | 60.81 | 18.47 | 4.98 | 2.95 | 2.19 | 1.87 | 1.54 | 0.81 | 0.08 | 2.35 | 0.28 | 2.56 | 1.11 |
| Ca-CG | 59.29 | 17.71 | 4.95 | 3.04 | 2.18 | 3.84 | 1.58 | 0.81 | 0.08 | 2.37 | 0.33 | 2.94 | 0.88 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, Y.; Dong, Y.; Shao, J.; Zhao, Z.; Zhai, H. Study on Preparation of Calcium-Based Modified Coal Gangue and Its Adsorption Dye Characteristics. Molecules 2024, 29, 2183. https://doi.org/10.3390/molecules29102183
Wang Y, Dong Y, Shao J, Zhao Z, Zhai H. Study on Preparation of Calcium-Based Modified Coal Gangue and Its Adsorption Dye Characteristics. Molecules. 2024; 29(10):2183. https://doi.org/10.3390/molecules29102183
Chicago/Turabian StyleWang, Yihan, Yanrong Dong, Junli Shao, Zilong Zhao, and Hongyu Zhai. 2024. "Study on Preparation of Calcium-Based Modified Coal Gangue and Its Adsorption Dye Characteristics" Molecules 29, no. 10: 2183. https://doi.org/10.3390/molecules29102183
APA StyleWang, Y., Dong, Y., Shao, J., Zhao, Z., & Zhai, H. (2024). Study on Preparation of Calcium-Based Modified Coal Gangue and Its Adsorption Dye Characteristics. Molecules, 29(10), 2183. https://doi.org/10.3390/molecules29102183







