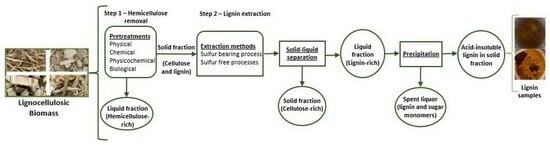
Lignin Extraction by Using Two-Step Fractionation: A Review
Abstract
1. Introduction
2. A Short Summary of General Pretreatment Methods
2.1. Physical Pretreatments
2.2. Chemical Pretreatments
2.2.1. Alkaline and Acid
2.2.2. Organic Solvent/Organosolv
2.2.3. Ionic Liquids
2.2.4. Deep-Eutectic Solvents
2.3. Physicochemical Pretreatments
2.3.1. Steam Pretreatment
2.3.2. Liquid Hot Water
2.3.3. Ammonia Fiber Explosion
2.4. Biological Pretreatments
3. Two-Step Fractionation for Lignin Extraction and Its Valorization
| Pretreatment | Feedstock | Conditions | Remarks of Studies | Lignin-Based Results | References |
|---|---|---|---|---|---|
| Two-step/pre-extraction and organosolv | Black spruce (Picea mariana) | Prior treatment with ethanol: water mixture in reflux reactor at 80 °C for 6 h; further treatment with EtOH: H2O ratios (50:50, 60:40, 70:30, and 80:20) for different temperatures (160, 180, and 200 °C) and residence times (60, 90, and 180 min) | Recovery yield reached 74% with pre-extraction from an average of 70% | 62% of the lignin precipitated | [86] |
| Two-step/Steam pretreatment and enzymatic mild acidolysis lignin extraction | Corncob residue (Zea mays) | Soaked with 0.5 wt.% H2SO4 for 12 h, steam pretreatment was at 180 °C for 10 min with 1.0 MPa, milling for 10 h, and cellulase concentration of 45FPU g−1 at 50 °C, 180 rpm for 72 h, and further rotary evaporated (45 °C, 90 bars) | Extracted lignin from CRSE EMAL contained a decent amount of β-O-4′ linkages, and it provides a good base for further lignin utilization | 99% purity of lignin, and the yield was 57.3% | [146] |
| Two-step/Milling and GVL-water fractionation | Eucalyptus globulus | Chips were ground to sawdust, and only those smaller than 125 microns were collected and further treated at 180 °C for 120 min | Precipitated lignin had a high phenolic content, relatively low polydispersity, and low molecular mass | 50–60% of the extracted lignin was precipitated | [147] |
| Multi-step/Steam pretreatment, enzymatic hydrolysis, and GVL-fractionation | Cornstalk (Zea mays L.) | Material treated with steam pretreatment @1.5 MPa for 5 h, then treated with 30 U/g cellulase loading at 50 °C for 48 h. Lastly, lignin was sequentially fractionated using GVL at water ratios of 60:40 (v/v), 40:60 (v/v), and 5:95 (v/v), respectively | The obtained three lignin fractions’ molecular weight had gradually decreased, and functional group contents increased with this phenomenon | Sequential lignin fractionation resulted in 41.10%, 29.13%, and 24.37%, respectively | [148] |
| Two-step/Alkaline extraction and black liquor precipitation | Sugarcane bagasse (Gramineae Saccharum officinarum L.) | Alkaline extraction was conducted using 6% w/w NaOH for 1 h at 90 °C with a liquid-solid ratio of 15. The obtained solid fraction was washed until the pH reached neutral, and then the obtained black liquor was precipitated using mineral and organic acids at 45 °C up to a final pH of 4 | The precipitation yields of black liquor ranged from 9 to 15%; lactic acid had the highest value | The solubilization and delignification yields were up to 53% and 81%, respectively | [128] |
| Two-step/Milling and oxy-organosolv | Wheat straw (Triticum aestivum) | First, the straw was pretreated by removing the peel and cutting it up. 1 to 5 mm and 100–500 μm sizes of straw were used for further The water-to-ethanol ratio was 3:7 (v/v) to 1:9 (v/v), and the straw-to-liquid ratio was 1:15 to 1:25 with a temperature range of 70 °C to 90 °C and a rate of 2 °C/min for 1 h @1000 rpm | Continuous oxygen flow was used to contain the inside pressure @ 0.8 MPa and lignin fractions were precipitated with and without oxygen assistance | The lignin yield was achieved at 46% with oxygen assistance @ 90 °C, and the range of lignin yield was between 19 and 46% | [149] |
| Two-step/Hydrothermal pretreatment and organosolv | Sweetgum (Liquidambar styraciflua) | First, the wood chips were pretreated at 180 °C for 40 min with a liquid/solid ratio of 4. Acetone, methanol, and acetone/methanol mixture (6:1, v/v) were used for extraction according to the solvent’s boiling point for 8 h | The extracted lignin had a low molecular weight, high phenolic hydroxyls, and low native lignin interunit linkages | Lignin yields ranged from 26.9% to 33.2% | [150] |
| Two-step/Alkaline and combined alkaline and acid pretreatment | Jerusalem artichoke stalks (Helianthus tuberosus L.) | Raw material was first treated with 2% (w/v) NaOH at 121 °C for 30 min. After, the spent filtrate was concentrated using H2SO4 (98.3%, w/v) at 60 °C and then kept at 70 °C for 1 h to precipitate lignin. | In the first step, 57–69% of the lignin was removed, and this study’s main aim was to increase enzyme accessibility. The results showed that the two-step approach was significantly better than the single-step approach | Lignin recovery yield was 36.78% | [151] |
| Two-step/Mannitol (MT) assisted p-toluenesulfonic acid/pentanol pretreatment | Poplar chip (Populus) | TsOH/pentanol with a solid-to-liquid ratio of 1:10 was used at 120 °C for 40 min. Simultaneously, different concentrations of MT were loaded into the pretreatment, and experiments were carried out under the same conditions. | The lignin obtained from the organic phase during pretreatment showcased β-O-4 bond characteristics akin to those found in native cellulosic enzyme lignin. | In the presence of 5% MT, the delignification rate reached 29%. | [152] |
| Two-step/Alkaline and deep eutectic solvent pretreatment | Bagasse (Gramineae Saccharum officinarum L.) | For alkaline pretreatment, 8 wt.% of NaOH was used at 90 °C for 2 h with a solid-to-liquid ratio of 1:30. After the alkaline step, La/ChCl was used as DES solution with alkaline extracted solid-to-DES (1:25) at 110 °C for 12 h. | The results of this study showed that separating lignin after DES recycling and reuse was possible. | The lignin removal rate was 86.7% | [43] |
| Two-step/Alkaline deep eutectic solvent and sequential acid precipitation | Wheat straw (Triticum aestivum) | The mixture of glycerol and K2CO3 with a molar ratio of 5:1 was used as alkaline DES. The material was transferred to K2CO3-Gly DES at 3 wt.% and stirred at 100 °C for 16 h. For acid-precipitated fractions, the spent liquor was concentrated to pH 2 using HCl. | Using sequential acid precipitation, three different lignin fractions were extracted from DES lignin, and the purity of each fraction was improved, respectively. | DES lignin was precipitated in a pH-6 condition at 59% wt. | [153] |
| Two-step/Ionic liquid and acid pretreatment | Kraft lignin | As a raw material, Kraft lignin was pretreated at 80–160 °C for 30–150 min and then concentrated to pH 2–3 using HCl. The solid fraction that was precipitated from spent liquor is called regenerated lignin. | The results showed that the effect of temperature was more essential than the residence time. FT-IR results also showed that there were no major differences between kraft lignin and regenerated lignin. | The lignin degradation rate was up to 27%, and it showed that the strong acidity of ionic liquids could destroy the lignin structure while increasing its degradation rate. | [154] |
| Two-step/organic solvent and solid organic acid combination | Hybrid poplar | Material treated with a mixture of p-TsOH (30 mL) and GVL-H2O (95:5, m/m) at different temperatures (60–100 °C) for 30, 45, 60, and 90 min. | The isolated lignin has a low molecular weight with a high phenolic hydroxyl group content | Lignin removal up to 86.14% under optimum conditions | [155] |
| Two-step/pysical and chemical pretreatment | Poplar chip (Populus) | Wood chips were treated with steam in a twin-extruder for 5 min. After, treated wood chips were added to a p-TsOH (60% and 70% wt.) solution and heated at 70 and 80 °C for 1 h | The isolated lignin had a high hydroxyl content, higher β-O-4 aryl ether linkages, and narrow polydispersity | Lignin removal was between 65–85% | [156] |
| Two-step/Ball milling and GVL-assisted fractionation | Pinewood (Pinus sylvestris L.) | The material was subjected to milling for 20 h and then treated with 80% aqueous GVL at different temperatures of 140, 160, and 180 °C for 2 and 4 h | The highest lignin yield was obtained at 180 °C for 4 h with 50% solid recovery | Lignin yield ranged between 3–33% | [82] |
| Two-step/Hybrid steam pretreatment and organosolv | Spruce (Picea abies) | 200 g of spruce was mixed with 400 h of ethanol and manually fed into the hybrid reactor. After that, 52% v/v of ethanol was loaded into the reactor. The reactor heated up to 200 °C for 30 min | This study has comparable results for hardwood using the same hybrid pretreatment method for the production of phenolics and aromatics | Isolated lignin had 65% wt. of C content with a very low sulfur content | [157] |
| Two-step/combinatorial pretreatment of dilute acid, liquid hot water, sodium hydroxide, and ethanol and sequential fermentation step | Corn stover (Zea mays ssp. mays L.) | First step: the material was pretreated with dilute sulfuric acid or liquid hot water with a 10% (w/w) solid loading. Second step: the pretreated solid fraction loaded as in the first step and pretreated with NaOH or/and ethanol at different conditions. The liquid fraction, which was lignin-rich, was collected for lipid fermentation | The results showed that the combinatorial pretreatment, together with fermentation optimization, improved lipid production while using lignin as the carbon source | Alkaline fractioned lignin as a potential carbon source | [158] |
| Two-step/Alkaline pretreatment and acid precipitation | Bamboo chips (Bambusa vulgaris) | Wood chips were treated by different NaOH conditions (0.1–1.0%), various solid loadings (5–15%), and various residence times (60–240 min) at 120 °C. Acid precipitation was carried out to the pretreated material by adjusting the pH to 2 using 2 M HCl | The statistical model showed that the optimum pretreatment conditions were: 1.3% (w/v) NaOH concentration, 10% (w/v) solid loading, and 150 min of alkaline pretreatment | Soda lignin recovery 104.6 mg/g of biomass | [159] |
| Two-step/Aqueous ammonia and dilute acid pretreatment | Rice straw (Oryza sativa) | The first step was performed at 100–190 °C and 8 mL/min for 20 min using 15 wt.% aqueous ammonia, and the second step was performed at 130 °C and 8 mL/min for 20 min using sulfuric acid | The first stage was to remove the lignin selectively | The delignification rate for the two-step strategy varied between 69.2% and 83.6% | [160] |
| Two-step/Liquid hot water and imidazole treatment | Elephant grass (Pennisetum purpureum) | LHW pretreatments were performed at 160 °C, 180 °C, 200 °C, and 220 °C under non-isothermal conditions for 60 min. The second step, imidazole treatment, was carried out at 140 °C for 182.5 min | The results showed that the combination of these pretreatments promotes the use of less severe conditions during hydrothermal pretreatment | Resulted in an 83.8% delignification rate | [161] |
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- World Energy Outlook 2022. 2022. Available online: www.iea.org/t&c/ (accessed on 21 December 2023).
- Sustainable Bioenergy at the Heart of Global Net Zero Glasgow Declaration on Sustainable Bioenergy 1 © Glasgow Declaration on Sustainable Bioenergy 2021. 2021. Available online: www.sustainablebioenergy.org (accessed on 21 December 2023).
- Yang, S.-T. Bioprocessing-from Biotechnology to Biorefinery. In Bioprocessing for Value-Added Products from Renewable Resources; Elsevier: Amsterdam, The Netherlands, 2007; Chapter 1. [Google Scholar]
- World Bioenergy Association. 2021. Available online: www.worldbioenergy.org (accessed on 21 December 2023).
- Gavrilescu, D. Energy from biomass in pulp and paper mills. Environ. Eng. Manag. J. 2008, 7, 537–546. Available online: http://omicron.ch.tuiasi.ro/EEMJ/ (accessed on 21 December 2023). [CrossRef]
- Solarte-Toro, J.C.; Chacón-Pérez, Y.; Cardona-Alzate, C.A. Evaluation of biogas and syngas as energy vectors for heat and power generation using lignocellulosic biomass as raw material. Electron. J. Biotechnol. 2018, 33, 52–62. [Google Scholar] [CrossRef]
- Vohra, M.; Manwar, J.; Manmode, R.; Padgilwar, S.; Patil, S. Bioethanol production: Feedstock and current technologies. J. Environ. Chem. Eng. 2014, 2, 573–584. [Google Scholar] [CrossRef]
- Wagle, A.; Angove, M.J.; Mahara, A.; Wagle, A.; Mainali, B.; Martins, M.; Goldbeck, R.; Paudel, S.R. Multi-stage pre-treatment of lignocellulosic biomass for multi-product biorefinery: A review. Sustain. Energy Technol. Assess. 2021, 49, 101702. [Google Scholar] [CrossRef]
- Lobato-Peralta, D.R.; Duque-Brito, E.; Villafán-Vidales, H.I.; Longoria, A.; Sebastian, P.; Cuentas-Gallegos, A.K.; Arancibia-Bulnes, C.A.; Okoye, P.U. A review on trends in lignin extraction and valorization of lignocellulosic biomass for energy applications. J. Clean. Prod. 2021, 293, 126123. [Google Scholar] [CrossRef]
- Fitzpatrick, M.; Champagne, P.; Cunningham, M.F.; Whitney, R.A. A biorefinery processing perspective: Treatment of lignocellulosic materials for the production of value-added products. Bioresour. Technol. 2010, 101, 8915–8922. [Google Scholar] [CrossRef] [PubMed]
- Wyman, C.E.; Dale, B.E.; Elander, R.T.; Holtzapple, M.; Ladisch, M.R.; Lee, Y. Coordinated Development of Leading Biomass Pretreatment Technologies. Bioresour. Technol. 2005, 96, 1959–1966. [Google Scholar] [CrossRef] [PubMed]
- Abdel-Hamid, A.M.; Solbiati, J.O.; Cann, I.K.O. Insights into Lignin Degradation and its Potential Industrial Applications. In Advances in Applied Microbiology; Academic Press Inc.: Cambridge, MA, USA, 2013; Volume 82, pp. 1–28. [Google Scholar] [CrossRef]
- Chen, H. Biotechnology of lignocellulose. In Theory and Practice; Chemical Industry Press and Springer: Nanjing, China, 2014. [Google Scholar]
- Wool, R.P.; Sun, X.S. Lignin polymers and composites. In Bio-Based Polymers and Composites, 1st ed.; Wool, R.P., Sun, X.S., Eds.; Elsevier: Amsterdam, The Netherlands, 2011; pp. 551–598. [Google Scholar]
- Le Floch, A.; Jourdes, M.; Teissedre, P.-L. Polysaccharides and lignin from oak wood used in cooperage: Composition, interest, assays: A review. Carbohydr. Res. 2015, 417, 94–102. [Google Scholar] [CrossRef]
- Harmsen, P.; Huijgen, W.; Bermudez, L.; Bakker, R. Literature Review of Physical and Chemical Pretreatment Processes for Lignocellulosic Biomass; Wageningen UR, Food & Biobased Research: Wageningen, The Netherlands, 2010. [Google Scholar]
- Lin, S.Y.; Dence, C.W. Methods in Lignin Chemistry; Springer Science & Business Media: Berlin/Heidelberg, Germany, 1992. [Google Scholar]
- Serrano-Ruiz, J.C.; West, R.M.; Dumesic, J.A. Catalytic Conversion of Renewable Biomass Resources to Fuels and Chemicals. Annu. Rev. Chem. Biomol. Eng. 2010, 1, 79–100. [Google Scholar] [CrossRef]
- Kaparaju, P.; Serrano, M.; Thomsen, A.B.; Kongjan, P.; Angelidaki, I. Bioethanol, biohydrogen and biogas production from wheat straw in a biorefinery concept. Bioresour. Technol. 2009, 100, 2562–2568. [Google Scholar] [CrossRef]
- Singh, N.; Singhania, R.R.; Nigam, P.S.; Dong, C.-D.; Patel, A.K.; Puri, M. Global status of lignocellulosic biorefinery: Challenges and perspectives. Bioresour. Technol. 2021, 344, 126415. [Google Scholar] [CrossRef] [PubMed]
- Yoo, C.G.; Meng, X.; Pu, Y.; Ragauskas, A.J. The critical role of lignin in lignocellulosic biomass conversion and recent pretreatment strategies: A comprehensive review. Bioresour. Technol. 2020, 301, 122784. [Google Scholar] [CrossRef] [PubMed]
- Banu, J.R.; Preethi; Kavitha, S.; Tyagi, V.K.; Gunasekaran, M.; Karthikeyan, O.P.; Kumar, G. Lignocellulosic biomass based biorefinery: A successful platform towards circular bioeconomy. Fuel 2021, 302, 121086. [Google Scholar] [CrossRef]
- De Wild, P.J.; Huijgen, W.J.J.; Gosselink, R.J. Lignin pyrolysis for profitable lignocellulosic biorefineries. Biofuels Bioprod. Biorefin. 2014, 8, 645–657. [Google Scholar] [CrossRef]
- Tarasov, D.; Leitch, M.; Fatehi, P. Lignin–carbohydrate complexes: Properties, applications, analyses, and methods of extraction: A review. Biotechnol. Biofuels 2018, 11, 269. [Google Scholar] [CrossRef] [PubMed]
- Al-Rudainy, B. Galactoglucomannan Recovery from Softwood Spent Sulfite Liquor Challenges, Process Design and Techno-economic Evaluations. Ph.D. Thesis, Lund University, Lund, Sweden, 2020. [Google Scholar]
- Huang, Y.; Liu, H.; Yuan, H.; Zhuang, X.; Yuan, S.; Yin, X.; Wu, C. Association of chemical structure and thermal degradation of lignins from crop straw and softwood. J. Anal. Appl. Pyrolysis 2018, 134, 25–34. [Google Scholar] [CrossRef]
- Lupoi, J.S.; Singh, S.; Parthasarathi, R.; Simmons, B.A.; Henry, R.J. Recent innovations in analytical methods for the qualitative and quantitative assessment of lignin. Renew. Sustain. Energy Rev. 2015, 49, 871–906. [Google Scholar] [CrossRef]
- Smith, R.L., Jr.; Fang, Z. (Eds.) Biofuels and Biorefineries 6 Production of Biofuels and Chemicals from Lignin; Springer: Berlin/Heidelberg, Germany, 2016; Volume 6. [Google Scholar] [CrossRef]
- Rishikesh, M.S.; Harish, S.; Prasanth, S.M.; Prakash, D.G. A comprehensive review on lignin obtained from agro-residues: Potential source of useful chemicals. Biomass-Convers. Biorefin. 2021, 13, 5533–5556. [Google Scholar] [CrossRef]
- Gellerstedt, G.L.F.; Henriksson, E.G. Lignins: Major Sources, Structure and Properties. Monomers Polym. Compos. Renew. Resour. 2008, Chapter 9, 201–224. [Google Scholar] [CrossRef]
- Xu, J.; Li, C.; Dai, L.; Xu, C.; Zhong, Y.; Yu, F.; Si, C. Biomass Fractionation and Lignin Fractionation towards Lignin Valorization. ChemSusChem 2020, 13, 4284–4295. [Google Scholar] [CrossRef]
- Lancefield, C.S.; Rashid, G.M.M.; Bouxin, F.; Wasak, A.; Tu, W.-C.; Hallett, J.; Zein, S.; Rodríguez, J.; Jackson, S.D.; Westwood, N.J.; et al. Investigation of the Chemocatalytic and Biocatalytic Valorization of a Range of Different Lignin Preparations: The Importance of β-O-4 Content. ACS Sustain. Chem. Eng. 2016, 4, 6921–6930. [Google Scholar] [CrossRef]
- Peretti, S.W.; Barton, R.; Mendonca, R.T. Lignin as feedstock for fibers and chemicals. In RSC Green Chemistry; Royal Society of Chemistry: London, UK, 2016; pp. 132–165. [Google Scholar] [CrossRef]
- Laurichesse, S.; Avérous, L. Chemical modification of lignins: Towards biobased polymers. Prog. Polym. Sci. 2014, 39, 1266–1290. [Google Scholar] [CrossRef]
- Ralph, J.; Lapierre, C.; Boerjan, W. Lignin structure and its engineering. Curr. Opin. Biotechnol. 2019, 56, 240–249. [Google Scholar] [CrossRef] [PubMed]
- John, M.J.; Lefatle, M.C.; Sithole, B. Lignin fractionation and conversion to bio-based functional products. Sustain. Chem. Pharm. 2022, 25, 100594. [Google Scholar] [CrossRef]
- Yang, Z.; Xu, Z.; Feng, M.; Cort, J.R.; Gieleciak, R.; Heyne, J.; Yang, B. Lignin-based jet fuel and its blending effect with conventional jet fuel. Fuel 2022, 321, 124040. [Google Scholar] [CrossRef]
- Ruan, H.; Qin, Y.; Heyne, J.; Gieleciak, R.; Feng, M.; Yang, B. Chemical compositions and properties of lignin-based jet fuel range hydrocarbons. Fuel 2019, 256, 115947. [Google Scholar] [CrossRef]
- Zhang, K.; Pei, Z.; Wang, D. Organic solvent pretreatment of lignocellulosic biomass for biofuels and biochemicals: A review. Bioresour. Technol. 2016, 199, 21–33. [Google Scholar] [CrossRef]
- Geng, X.; Henderson, W.A. Pretreatment of corn stover by combining ionic liquid dissolution with alkali extraction. Biotechnol. Bioeng. 2011, 109, 84–91. [Google Scholar] [CrossRef]
- De la Torre, M.J.; Moral, A.; Hernández, M.D.; Cabeza, E.; Tijero, A. Organosolv lignin for biofuel. Ind. Crop. Prod. 2013, 45, 58–63. [Google Scholar] [CrossRef]
- Fu, D.; Mazza, G.; Tamaki, Y. Lignin Extraction from Straw by Ionic Liquids and Enzymatic Hydrolysis of the Cellulosic Residues. J. Agric. Food Chem. 2010, 58, 2915–2922. [Google Scholar] [CrossRef]
- Xu, B.; Wang, N.; Wang, X.; Lang, J.; Zhang, H. Experimental study on the separation of bagasse lignin and cellulose by using deep eutectic solvent based on alkaline pretreatment. Biomass-Convers. Biorefin. 2022, 1–11. [Google Scholar] [CrossRef]
- Attard, T.M.; Clark, J.H.; McElroy, C.R. Recent developments in key biorefinery areas. Curr. Opin. Green Sustain. Chem. 2019, 21, 64–74. [Google Scholar] [CrossRef]
- Toledano, A.; Serrano, L.; Garcia, A.; Mondragon, I.; Labidi, J. Comparative study of lignin fractionation by ultrafiltration and selective precipitation. Chem. Eng. J. 2010, 157, 93–99. [Google Scholar] [CrossRef]
- Abu-Omar, M.M.; Barta, K.; Beckham, G.T.; Luterbacher, J.S.; Ralph, J.; Rinaldi, R.; Román-Leshkov, Y.; Samec, J.S.M.; Sels, B.F.; Wang, F. Guidelines for performing lignin-first biorefining. Energy Environ. Sci. 2020, 14, 262–292. [Google Scholar] [CrossRef]
- Huang, Y.; Duan, Y.; Qiu, S.; Wang, M.; Ju, C.; Cao, H.; Fang, Y.; Tan, T. Lignin-first biorefinery: A reusable catalyst for lignin depolymerization and application of lignin oil to jet fuel aromatics and polyurethane feedstock. Sustain. Energy Fuels 2017, 2, 637–647. [Google Scholar] [CrossRef]
- Monção, M.; Hrůzová, K.; Rova, U.; Matsakas, L.; Christakopoulos, P. Organosolv Fractionation of Birch Sawdust: Establishing a Lignin-First Biorefinery. Molecules 2021, 26, 6754. [Google Scholar] [CrossRef] [PubMed]
- Matsakas, L.; Raghavendran, V.; Yakimenko, O.; Persson, G.; Olsson, E.; Rova, U.; Olsson, L.; Christakopoulos, P. Lignin-first biomass fractionation using a hybrid organosolv—Steam explosion pretreatment technology improves the saccharification and fermentability of spruce biomass. Bioresour. Technol. 2018, 273, 521–528. [Google Scholar] [CrossRef] [PubMed]
- Tian, D.; Chandra, R.P.; Lee, J.-S.; Lu, C.; Saddler, J.N. A comparison of various lignin-extraction methods to enhance the accessibility and ease of enzymatic hydrolysis of the cellulosic component of steam-pretreated poplar. Biotechnol. Biofuels 2017, 10, 157. [Google Scholar] [CrossRef]
- Lyu, H.; Zhou, J.; Geng, Z.; Lyu, C.; Li, Y. Two-stage processing of liquid hot water pretreatment for recovering C5 and C6 sugars from cassava straw. Process Biochem. 2018, 75, 202–211. [Google Scholar] [CrossRef]
- Zhao, Y.; Shakeel, U.; Rehman, M.S.U.; Li, H.; Xu, X.; Xu, J. Lignin-carbohydrate complexes (LCCs) and its role in biorefinery. J. Clean. Prod. 2020, 253, 120076. [Google Scholar] [CrossRef]
- Sarangi, P.K.; Nanda, S.; Mohanty, P. Recent Advancements in Biofuels and Bioenergy Utilization; Springer: Singapore, 2018. [Google Scholar]
- Chen, H.; Liu, J.; Chang, X.; Chen, D.; Xue, Y.; Liu, P.; Lin, H.; Han, S. A review on the pretreatment of lignocellulose for high-value chemicals. Fuel Process Technol. 2017, 160, 196–206. [Google Scholar] [CrossRef]
- Ewanick, S.; Bura, R. Hydrothermal pretreatment of lignocellulosic biomass. In Bioalcohol Production: Biochemical Conversion of Lignocellulosic Biomass; Woodhead Publishing: Cambridge, UK, 2010; pp. 3–23. [Google Scholar] [CrossRef]
- Agbor, V.B.; Cicek, N.; Sparling, R.; Berlin, A.; Levin, D.B. Biomass pretreatment: Fundamentals toward application. Biotechnol. Adv. 2011, 29, 675–685. [Google Scholar] [CrossRef] [PubMed]
- Tu, W.-C.; Hallett, J.P. Recent advances in the pretreatment of lignocellulosic biomass. Curr. Opin. Green Sustain. Chem. 2019, 20, 11–17. [Google Scholar] [CrossRef]
- Mankar, A.R.; Pandey, A.; Modak, A.; Pant, K. Pretreatment of lignocellulosic biomass: A review on recent advances. Bioresour. Technol. 2021, 334, 125235. [Google Scholar] [CrossRef] [PubMed]
- Galbe, M.; Wallberg, O. Pretreatment for biorefineries: A review of common methods for efficient utilisation of lignocellulosic materials. Biotechnol. Biofuels 2019, 12, 294. [Google Scholar] [CrossRef]
- Bhatia, S.K.; Jagtap, S.S.; Bedekar, A.A.; Bhatia, R.K.; Patel, A.K.; Pant, D.; Banu, J.R.; Rao, C.V.; Kim, Y.-G.; Yang, Y.-H. Recent developments in pretreatment technologies on lignocellulosic biomass: Effect of key parameters, technological improvements, and challenges. Bioresour. Technol. 2020, 300, 122724. [Google Scholar] [CrossRef] [PubMed]
- De Jong, W. Physical Pretreatment of Biomass. In Biomass as a Sustainable Energy Source for the Future: Fundamentals of Conversion Processes; John Wiley & Son: Hoboken, NJ, USA, 2014; Volume 9781118304914, pp. 231–267. [Google Scholar] [CrossRef]
- Chiaramonti, D.; Prussi, M.; Ferrero, S.; Oriani, L.; Ottonello, P.; Torre, P.; Cherchi, F. Review of pretreatment processes for lignocellulosic ethanol production, and development of an innovative method. Biomass Bioenergy 2012, 46, 25–35. [Google Scholar] [CrossRef]
- Gallego-García, M.; Moreno, A.D.; Manzanares, P.; Negro, M.J.; Duque, A. Recent advances on physical technologies for the pretreatment of food waste and lignocellulosic residues. Bioresour. Technol. 2023, 369, 128397. [Google Scholar] [CrossRef]
- Karimi, K.; Shafiei, M.; Kumar, R. Progress in physical and chemical pretreatment of lignocellulosic biomass. In Biofuel Tech-nologies: Recent Developments; Springer: Berlin/Heidelberg, Germany, 2013; Volume 9783642345197, pp. 53–96. [Google Scholar] [CrossRef]
- Yang, M.; Xu, M.; Nan, Y.; Kuittinen, S.; Hassan, K.; Vepsäläinen, J.; Xin, D.; Zhang, J.; Pappinen, A. Influence of size reduction treatments on sugar recovery from Norway spruce for butanol production. Bioresour. Technol. 2018, 257, 113–120. [Google Scholar] [CrossRef]
- DeMartini, J.D.; Foston, M.; Meng, X.; Jung, S.; Kumar, R.; Ragauskas, A.J.; Wyman, C.E. How chip size impacts steam pretreatment effectiveness for biological conversion of poplar wood into fermentable sugars. Biotechnol. Biofuels 2015, 8, 209. [Google Scholar] [CrossRef]
- Jȩdrzejczyk, M.; Soszka, E.; Czapnik, M.; Ruppert, A.M.; Grams, J. Physical and Chemical Pretreatment of Lignocellulosic Biomass. In Second and Third Generation of Feedstocks: The Evolution of Biofuels; Elsevier: Amsterdam, The Netherlands, 2019; pp. 143–196. [Google Scholar] [CrossRef]
- Muley, P.D.; Mobley, J.K.; Tong, X.; Novak, B.; Stevens, J.; Moldovan, D.; Shi, J.; Boldor, D. Rapid microwave-assisted biomass delignification and lignin depolymerization in deep eutectic solvents. Energy Convers. Manag. 2019, 196, 1080–1088. [Google Scholar] [CrossRef]
- Monteil-Rivera, F.; Huang, G.H.; Paquet, L.; Deschamps, S.; Beaulieu, C.; Hawari, J. Microwave-assisted extraction of lignin from triticale straw: Optimization and microwave effects. Bioresour. Technol. 2012, 104, 775–782. [Google Scholar] [CrossRef] [PubMed]
- Du, X.; Gellerstedt, G.; Li, J. Universal fractionation of lignin–carbohydrate complexes (LCCs) from lignocellulosic biomass: An example using spruce wood. Plant J. 2013, 74, 328–338. [Google Scholar] [CrossRef] [PubMed]
- Jose, D.; Kitiborwornkul, N.; Sriariyanun, M.; Keerthi, K. A Review on Chemical Pretreatment Methods of Lignocellulosic Biomass: Recent Advances and Progress. Appl. Sci. Eng. Prog. 2022, 15, 6210. [Google Scholar] [CrossRef]
- Kim, J.S.; Lee, Y.Y.; Kim, T.H. A review on alkaline pretreatment technology for bioconversion of lignocellulosic biomass. Bioresour. Technol. 2016, 199, 42–48. [Google Scholar] [CrossRef]
- Oriez, V.; Peydecastaing, J.; Pontalier, P.-Y. Lignocellulosic Biomass Mild Alkaline Fractionation and Resulting Extract Purification Processes: Conditions, Yields, and Purities. Clean Technol. 2020, 2, 91–115. [Google Scholar] [CrossRef]
- Fan, Y.; Li, L.; Yang, G.; Sun, Y.; He, L.; Wu, P.; Lam, C.H.; Song, B. Suppression Effect of Gamma-Valerolactone on the Mild Alkaline Pretreatment of Hybrid Pennisetum. ACS Sustain. Chem. Eng. 2021, 9, 14846–14856. [Google Scholar] [CrossRef]
- Solarte-Toro, J.C.; Romero-García, J.M.; Martínez-Patiño, J.C.; Ruiz-Ramos, E.; Castro-Galiano, E.; Cardona-Alzate, C.A. Acid pretreatment of lignocellulosic biomass for energy vectors production: A review focused on operational conditions and techno-economic assessment for bioethanol production. Renew. Sustain. Energy Rev. 2019, 107, 587–601. [Google Scholar] [CrossRef]
- Moe, S.T.; Janga, K.K.; Hertzberg, T.; Hägg, M.-B.; Øyaas, K.; Dyrset, N. Saccharification of Lignocellulosic Biomass for Biofuel and Biorefinery Applications—A Renaissance for the Concentrated Acid Hydrolysis? Energy Procedia 2012, 20, 50–58. [Google Scholar] [CrossRef]
- Saha, B.C.; Iten, L.B.; Cotta, M.A.; Wu, Y.V. Dilute acid pretreatment, enzymatic saccharification and fermentation of wheat straw to ethanol. Process Biochem. 2005, 40, 3693–3700. [Google Scholar] [CrossRef]
- Hrůzová, K.; Matsakas, L.; Rova, U.; Christakopoulos, P. Organosolv fractionation of spruce bark using ethanol–water mixtures: Towards a novel bio-refinery concept. Bioresour. Technol. 2021, 341, 125855. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Li, K.; Zhang, M. Lignin precipitation on the pulp fibers in the ethanol-based organosolv pulping. Colloids Surf. A Physicochem. Eng. Asp. 2007, 301, 255–263. [Google Scholar] [CrossRef]
- Smit, A.T.; Verges, M.; Schulze, P.; van Zomeren, A.; Lorenz, H. Laboratory-to Pilot-Scale Fractionation of Lignocellulosic Biomass Using an Acetone Organosolv Process. ACS Sustain. Chem. Eng. 2022, 10, 10503–10513. [Google Scholar] [CrossRef]
- Pascal, K.; Ren, H.; Sun, F.F.; Guo, S.; Hu, J.; He, J. Mild Acid-Catalyzed Atmospheric Glycerol Organosolv Pretreatment Effectively Improves Enzymatic Hydrolyzability of Lignocellulosic Biomass. ACS Omega 2019, 4, 20015–20023. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, M.A.; Lee, J.H.; Raja, A.A.; Choi, J.W. Effects of gamma-valerolactone assisted fractionation of ball-milled pine wood on lignin extraction and its characterization as well as its corresponding cellulose digestion. Appl. Sci. 2020, 10, 1599. [Google Scholar] [CrossRef]
- Mu, L.; Wu, J.; Matsakas, L.; Chen, M.; Vahidi, A.; Grahn, M.; Rova, U.; Christakopoulos, P.; Zhu, J.; Shi, Y. Lignin from Hardwood and Softwood Biomass as a Lubricating Additive to Ethylene Glycol. Molecules 2018, 23, 537. [Google Scholar] [CrossRef] [PubMed]
- El Hage, R.; Brosse, N.; Chrusciel, L.; Sanchez, C.; Sannigrahi, P.; Ragauskas, A. Characterization of milled wood lignin and ethanol organosolv lignin from miscanthus. Polym. Degrad. Stab. 2009, 94, 1632–1638. [Google Scholar] [CrossRef]
- Nitsos, C.; Stoklosa, R.; Karnaouri, A.; Vörös, D.; Lange, H.; Hodge, D.; Crestini, C.; Rova, U.; Christakopoulos, P. Isolation and Characterization of Organosolv and Alkaline Lignins from Hardwood and Softwood Biomass. ACS Sustain. Chem. Eng. 2016, 4, 5181–5193. [Google Scholar] [CrossRef]
- Parot, M.; Rodrigue, D.; Stevanovic, T. High purity softwood lignin obtained by an eco-friendly organosolv process. Bioresour. Technol. Rep. 2021, 17, 100880. [Google Scholar] [CrossRef]
- Vaidya, A.A.; Murton, K.D.; Smith, D.A.; Dedual, G. A review on organosolv pretreatment of softwood with a focus on enzymatic hydrolysis of cellulose. Biomass-Convers. Biorefin. 2022, 12, 5427–5442. [Google Scholar] [CrossRef]
- Zhao, X.; Cheng, K.; Liu, D. Organosolv pretreatment of lignocellulosic biomass for enzymatic hydrolysis. Appl. Microbiol. Biotechnol. 2009, 82, 815–827. [Google Scholar] [CrossRef] [PubMed]
- Imman, S.; Arnthong, J.; Burapatana, V.; Champreda, V.; Laosiripojana, N. Fractionation of rice straw by a single-step solvothermal process: Effects of solvents, acid promoters, and microwave treatment. Renew. Energy 2015, 83, 663–673. [Google Scholar] [CrossRef]
- Hasanov, I.; Raud, M.; Kikas, T. The Role of Ionic Liquids in the Lignin Separation from Lignocellulosic Biomass. Energies 2020, 13, 4864. [Google Scholar] [CrossRef]
- Mora-Pale, M.; Meli, L.; Doherty, T.V.; Linhardt, R.J.; Dordick, J.S. Room temperature ionic liquids as emerging solvents for the pretreatment of lignocellulosic biomass. Biotechnol. Bioeng. 2011, 108, 1229–1245. [Google Scholar] [CrossRef] [PubMed]
- Glaser, S.J.; Al-Rudainy, B.; Hatti-Kaul, R.; Galbe, M. Wheat bran fractionation: Effect of steam explosion and hydrotropic extraction conditions on the recovery of sugars and lignin. Ind. Crop. Prod. 2023, 195. [Google Scholar] [CrossRef]
- Ghorbani, M.; Simone, M.I. Developing New Inexpensive Room-Temperature Ionic Liquids with High Thermal Stability and a Greener Synthetic Profile. ACS Omega 2020, 5, 12637–12648. [Google Scholar] [CrossRef] [PubMed]
- Brandt, A.; Gräsvik, J.; Hallett, J.P.; Welton, T. Green Chemistry CRITICAL REVIEW Deconstruction of lignocellulosic biomass with ionic liquids. Green Chem. 2012, 15, 550–583. [Google Scholar] [CrossRef]
- Usmani, Z.; Sharma, M.; Gupta, P.; Karpichev, Y.; Gathergood, N.; Bhat, R.; Gupta, V.K. Ionic liquid based pretreatment of lignocellulosic biomass for enhanced bioconversion. Bioresour. Technol. 2020, 304, 123003. [Google Scholar] [CrossRef]
- Xu, H.; Peng, J.; Kong, Y.; Liu, Y.; Su, Z.; Li, B.; Song, X.; Liu, S.; Tian, W. Key process parameters for deep eutectic solvents pretreatment of lignocellulosic biomass materials: A review. Bioresour. Technol. 2020, 310, 123416. [Google Scholar] [CrossRef]
- Sun, X.; Zhou, Z.; Tian, D.; Zhao, J.; Zhang, J.; Deng, P.; Zou, H.; Lu, C. Acidic deep eutectic solvent assisted mechanochemical delignification of lignocellulosic biomass at room temperature. Int. J. Biol. Macromol. 2023, 234, 123593. [Google Scholar] [CrossRef]
- Soares, B.; Lopes, A.M.d.C.; Silvestre, A.J.; Pinto, P.C.R.; Freire, C.S.; Coutinho, J.A. Wood delignification with aqueous solutions of deep eutectic solvents. Ind. Crop. Prod. 2020, 160, 113128. [Google Scholar] [CrossRef]
- Brodeur, G.; Yau, E.; Badal, K.; Collier, J.; Ramachandran, K.B.; Ramakrishnan, S. Chemical and Physicochemical Pretreatment of Lignocellulosic Biomass: A Review. Enzym. Res. 2011, 2011, 787532. [Google Scholar] [CrossRef] [PubMed]
- Montané, D.; Farriol, X.; Salvadó, J.; Jollez, P.; Chornet, E. Fractionation of wheat straw by steam-explosion pretreatment and alkali delignification. Cellulose pulp and byproducts from hemicellulose and lignin. J. Wood Chem. Technol. 1998, 18, 171–191. [Google Scholar] [CrossRef]
- Kumar, L.; Chandra, R.; Chung, P.A.; Saddler, J. Can the same steam pretreatment conditions be used for most softwoods to achieve good, enzymatic hydrolysis and sugar yields? Bioresour. Technol. 2010, 101, 7827–7833. [Google Scholar] [CrossRef] [PubMed]
- Hongzhang, C.; Liying, L. Unpolluted fractionation of wheat straw by steam explosion and ethanol extraction. Bioresour. Technol. 2007, 98, 666–676. [Google Scholar] [CrossRef] [PubMed]
- Ruiz, H.A.; Galbe, M.; Garrote, G.; Ramirez-Gutierrez, D.M.; Ximenes, E.; Sun, S.-N.; Lachos-Perez, D.; Rodríguez-Jasso, R.M.; Sun, R.-C.; Yang, B.; et al. Severity factor kinetic model as a strategic parameter of hydrothermal processing (steam explosion and liquid hot water) for biomass fractionation under biorefinery concept. Bioresour. Technol. 2021, 342, 125961. [Google Scholar] [CrossRef]
- Wojtasz-Mucha, J.; Hasani, M.; Theliander, H. Hydrothermal pretreatment of wood by mild steam explosion and hot water extraction. Bioresour. Technol. 2017, 241, 120–126. [Google Scholar] [CrossRef]
- Yan, L.; Ma, R.; Li, L.; Fu, J. Hot Water Pretreatment of Lignocellulosic Biomass: An Effective and Environmentally Friendly Approach to Enhance Biofuel Production. Chem. Eng. Technol. 2016, 39, 1759–1770. [Google Scholar] [CrossRef]
- Wojtasz-Mucha, J.; Hasani, M.; Theliander, H. Dissolution of wood components during hot water extraction of birch. Wood Sci. Technol. 2021, 55, 811–835. [Google Scholar] [CrossRef]
- Kim, D. Physico-Chemical Conversion of Lignocellulose: Inhibitor Effects and Detoxification Strategies: A Mini Review. Molecules 2018, 23, 309. [Google Scholar] [CrossRef]
- Taherzadeh, M.J.; Karimi, K. Pretreatment of lignocellulosic wastes to improve ethanol and biogas production: A review. Int. J. Mol. Sci. 2008, 9, 1621–1651. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Zhang, W.; Cai, Z.; Zhang, J.; Guan, D.; Ji, D.; Gao, W. Effect of ammonia fiber expansion combined with naoh pretreatment on the resource efficiency of herbaceous and woody lignocellulosic biomass. ACS Omega 2022, 7, 18761–18769. [Google Scholar] [CrossRef] [PubMed]
- Periyasamy, S.; Karthik, V.; Kumar, P.S.; Isabel, J.B.; Temesgen, T.; Hunegnaw, B.M.; Melese, B.B.; Mohamed, B.A.; Vo, D.-V.N. Chemical, physical and biological methods to convert lignocellulosic waste into value-added products. A review. Environ. Chem. Lett. 2022, 20, 1129–1152. [Google Scholar] [CrossRef]
- Zhou, M.; Tian, X. Development of different pretreatments and related technologies for efficient biomass conversion of lignocellulose. Int. J. Biol. Macromol. 2022, 202, 256–268. [Google Scholar] [CrossRef]
- Saritha, M.; Arora, A. Lata Biological Pretreatment of Lignocellulosic Substrates for Enhanced Delignification and Enzymatic Digestibility. Indian J. Microbiol. 2011, 52, 122–130. [Google Scholar] [CrossRef] [PubMed]
- Sharma, H.K.; Xu, C.; Qin, W. Biological Pretreatment of Lignocellulosic Biomass for Biofuels and Bioproducts: An Overview. Waste Biomass-Valorization 2017, 10, 235–251. [Google Scholar] [CrossRef]
- Lee, J.-W.; Gwak, K.-S.; Park, J.-Y.; Park, M.-J.; Choi, D.-H.; Kwon, M.; Choi, I.-G. Biological pretreatment of softwood Pinus densiflora by three white rot fungi. Artic. J. Microbiol. 2007, 45, 485–491. Available online: https://www.researchgate.net/publication/5672819 (accessed on 21 December 2023).
- Sindhu, R.; Binod, P.; Pandey, A. Biological pretreatment of lignocellulosic biomass—An overview. Bioresour. Technol. 2016, 199, 76–82. [Google Scholar] [CrossRef]
- Nitsos, C.; Rova, U.; Christakopoulos, P. Organosolv fractionation of softwood biomass for biofuel and biorefinery applications. Energies 2017, 11, 50. [Google Scholar] [CrossRef]
- Inkrod, C.; Raita, M.; Champreda, V.; Laosiripojana, N. Characteristics of Lignin Extracted from Different Lignocellulosic Materials via Organosolv Fractionation. BioEnergy Res. 2018, 11, 277–290. [Google Scholar] [CrossRef]
- Lee, S.H.; Doherty, T.V.; Linhardt, R.J.; Dordick, J.S. Ionic liquid-mediated selective extraction of lignin from wood leading to enhanced enzymatic cellulose hydrolysis. Biotechnol. Bioeng. 2008, 102, 1368–1376. [Google Scholar] [CrossRef] [PubMed]
- Doherty, T.V.; Mora-Pale, M.; Foley, S.E.; Linhardt, R.J.; Dordick, J.S. Ionic liquid solvent properties as predictors of lignocellulose pretreatment efficacy. Green Chem. 2010, 12, 1967–1975. [Google Scholar] [CrossRef]
- Saha, K.; Dasgupta, J.; Chakraborty, S.; Antunes, F.A.F.; Sikder, J.; Curcio, S.; dos Santos, J.C.; Arafat, H.A.; da Silva, S.S. Optimization of lignin recovery from sugarcane bagasse using ionic liquid aided pretreatment. Cellulose 2017, 24, 3191–3207. [Google Scholar] [CrossRef]
- Jung, W.; Sharma-Shivappa, R.; Park, S.; Kolar, P. Effect of cellulolytic enzyme binding on lignin isolated from alkali and acid pretreated switchgrass on enzymatic hydrolysis. 3 Biotech 2019, 10, 1. [Google Scholar] [CrossRef] [PubMed]
- Peng, M.; Nakabayashi, M.; Kim, K.; Kamiya, K.; Qian, E.W. Lignin depolymerization with alkaline ionic liquids and ethylene glycol in a continuous flow reactor. Fuel 2023, 335, 126960. [Google Scholar] [CrossRef]
- Cequier, E.; Aguilera, J.; Balcells, M.; Canela-Garayoa, R. Extraction and characterization of lignin from olive pomace: A comparison study among ionic liquid, sulfuric acid, and alkaline treatments. Biomass-Convers. Biorefin. 2019, 9, 241–252. [Google Scholar] [CrossRef]
- Amidon, T.E.; Bujanovic, B.; Liu, S.; Howard, J.R. Commercializing biorefinery technology: A case for the multi-product pathway to a viable biorefinery. Forests 2011, 2, 929–947. [Google Scholar] [CrossRef]
- Mikulski, D.; Kłosowski, G. Hydrotropic pretreatment on distillery stillage for efficient cellulosic ethanol production. Bioresour. Technol. 2019, 300, 122661. [Google Scholar] [CrossRef]
- Wang, H.; Pu, Y.; Ragauskas, A.; Yang, B. From lignin to valuable products–strategies, challenges, and prospects. Bioresour. Technol. 2018, 271, 449–461. [Google Scholar] [CrossRef]
- Wang, Z.; Deuss, P.J. The isolation of lignin with native-like structure. Biotechnol. Adv. 2023, 68, 108230. [Google Scholar] [CrossRef]
- Mota, I.F.; Burgal, J.d.S.; Antunes, F.; Pintado, M.E.; Costa, P.S. High value-added lignin extracts from sugarcane by-products. Int. J. Biol. Macromol. 2023, 230, 123144. [Google Scholar] [CrossRef] [PubMed]
- Raikwar, D.; Majumdar, S.; Shee, D. Effects of solvents in the depolymerization of lignin into value-added products: A review. Biomass-Convers. Biorefin. 2021, 13, 11383–11416. [Google Scholar] [CrossRef]
- Banu, J.R.; Kavitha, S.; Kannah, R.Y.; Devi, T.P.; Gunasekaran, M.; Kim, S.-H.; Kumar, G. A review on biopolymer production via lignin valorization. Bioresour. Technol. 2019, 290, 121790. [Google Scholar] [CrossRef]
- Lu, X.; Gu, X. A review on lignin-based epoxy resins: Lignin effects on their synthesis and properties. Int. J. Biol. Macromol. 2023, 229, 778–790. [Google Scholar] [CrossRef] [PubMed]
- Miao, C.; Xinshu, Z.; Yang, L.; Li, H.; Lv, P.; Luo, W. Study on extraction of lignin and synthesis of lignin-based epoxy resins using ionic liquid. Biomass-Convers. Biorefin. 2021, 13, 1115–1126. [Google Scholar] [CrossRef]
- Zhang, Y.; Stepanova, S.; Van Aelst, K.; Sels, B.F. Consider lignin’s hydroxyl groups content and type, its molecular weight and content when converting it into epoxy resin. Curr. Opin. Green Sustain. Chem. 2023, 40, 100750. [Google Scholar] [CrossRef]
- Obasa, V.D.; Olanrewaju, O.A.; Gbenebor, O.P.; Ochulor, E.F.; Odili, C.C.; Abiodun, Y.O.; Adeosun, S.O. A Review on Lignin-Based Carbon Fibres for Carbon Footprint Reduction. Atmosphere 2022, 13, 1605. [Google Scholar] [CrossRef]
- Zhang, W.; Qiu, X.; Wang, C.; Zhong, L.; Fu, F.; Zhu, J.; Zhang, Z.; Qin, Y.; Yang, D.; Xu, C.C. Lignin derived carbon materials: Current status and future trends. Carbon Res. 2022, 1, 14. [Google Scholar] [CrossRef]
- Chatterjee, S.; Saito, T. Lignin-Derived Advanced Carbon Materials. ChemSusChem 2015, 8, 3941–3958. [Google Scholar] [CrossRef]
- Shu, R.; Li, R.; Lin, B.; Wang, C.; Cheng, Z.; Chen, Y. A review on the catalytic hydrodeoxygenation of lignin-derived phenolic compounds and the conversion of raw lignin to hydrocarbon liquid fuels. Biomass-Bioenergy 2019, 132, 105432. [Google Scholar] [CrossRef]
- Cao, Y.; Chen, S.S.; Zhang, S.; Ok, Y.S.; Matsagar, B.M.; Wu, K.C.-W.; Tsang, D.C. Advances in lignin valorization towards bio-based chemicals and fuels: Lignin biorefinery. Bioresour. Technol. 2019, 291, 121878. [Google Scholar] [CrossRef] [PubMed]
- Yunpu, W.; Leilei, D.; Liangliang, F.; Shaoqi, S.; Yuhuan, L.; Roger, R. Review of microwave-assisted lignin conversion for renewable fuels and chemicals. J. Anal. Appl. Pyrolysis 2016, 119, 104–113. [Google Scholar] [CrossRef]
- Sharma, V.; Tsai, M.-L.; Nargotra, P.; Chen, C.-W.; Sun, P.-P.; Singhania, R.R.; Patel, A.K.; Dong, C.-D. Journey of lignin from a roadblock to bridge for lignocellulose biorefineries: A comprehensive review. Sci. Total. Environ. 2023, 861, 160560. [Google Scholar] [CrossRef] [PubMed]
- Patel, R.; Dhar, P.; Babaei-Ghazvini, A.; Dafchahi, M.N.; Acharya, B. Transforming lignin into renewable fuels, chemicals, and materials: A review. Bioresour. Technol. Rep. 2023, 22, 101463. [Google Scholar] [CrossRef]
- Suresh, S.; Viswanathan, V.; Angamuthu, M.; Dhakshinamoorthy, G.P.; Gopinath, K.P.; Bhatnagar, A. Lignin waste processing into solid, liquid, and gaseous fuels: A comprehensive review. Biomass-Convers. Biorefin. 2021, 13, 4515–4553. [Google Scholar] [CrossRef]
- Luo, Z.; Qian, Q.; Sun, H.; Wei, Q.; Zhou, J.; Wang, K. Lignin-First Biorefinery for Converting Lignocellulosic Biomass into Fuels and Chemicals. Energies 2022, 16, 125. [Google Scholar] [CrossRef]
- Das, P.; Stoffel, R.B.; Area, M.C.; Ragauskas, A.J. Effects of one-step alkaline and two-step alkaline/dilute acid and alkaline/steam explosion pretreatments on the structure of isolated pine lignin. Biomass-Bioenergy 2019, 120, 350–358. [Google Scholar] [CrossRef]
- Watkins, D.; Nuruddin, M.; Hosur, M.; Tcherbi-Narteh, A.; Jeelani, S. Extraction and characterization of lignin from different biomass resources. J. Mater. Res. Technol. 2015, 4, 26–32. [Google Scholar] [CrossRef]
- Zhang, X.; Zhu, J.; Sun, L.; Yuan, Q.; Cheng, G.; Argyropoulos, D.S. Extraction and characterization of lignin from corncob residue after acid-catalyzed steam explosion pretreatment. Ind. Crop. Prod. 2019, 133, 241–249. [Google Scholar] [CrossRef]
- Lê, H.Q.; Ma, Y.; Borrega, M.; Sixta, H. Wood biorefinery based on γ-valerolactone/water fractionation. Green Chem. 2016, 18, 5466–5476. [Google Scholar] [CrossRef]
- Wang, G.; Liu, X.; Yang, B.; Si, C.; Parvez, A.M.; Jang, J.; Ni, Y. Using Green γ-Valerolactone/Water Solvent to Decrease Lignin Heterogeneity by Gradient Precipitation. ACS Sustain. Chem. Eng. 2019, 7, 10112–10120. [Google Scholar] [CrossRef]
- Zeng, S.; Ma, Q.; Zhang, S.; Shen, C.; Li, J.; Zhao, H.; Guo, D.; Zhang, Y.; Yang, H. Evaluation of oxy-organosolv pretreatment on lignin extraction from wheat straw. Int. J. Biol. Macromol. 2023, 229, 861–872. [Google Scholar] [CrossRef] [PubMed]
- Jiang, X.; Wan, S.; Kollman, M.; Jiang, H.; Wu, S.; Jameel, H.; Chang, H.-M. Role of extractable lignin in enzymatic hydrolysis of hydrothermally pretreated hardwood. Ind. Crop. Prod. 2023, 193, 116150. [Google Scholar] [CrossRef]
- Chen, Y.; Peng, N.; Gao, Y.; Li, Q.; Wang, Z.; Yao, B.; Li, Y. Two-Stage Pretreatment of Jerusalem Artichoke Stalks with Wastewater Recycling and Lignin Recovery for the Biorefinery of Lignocellulosic Biomass. Processes 2023, 11, 127. [Google Scholar] [CrossRef]
- Madadi, M.; Elsayed, M.; Sun, F.; Wang, J.; Karimi, K.; Song, G.; Tabatabaei, M.; Aghbashlo, M. Sustainable lignocellulose fractionation by integrating p-toluenesulfonic acid/pentanol pretreatment with mannitol for efficient production of glucose, native-like lignin, and furfural. Bioresour. Technol. 2023, 371, 128591. [Google Scholar] [CrossRef] [PubMed]
- Yue, X.; Suopajärvi, T.; Sun, S.; Mankinen, O.; Mikkelson, A.; Huttunen, H.; Komulainen, S.; Romakkaniemi, I.; Ahola, J.; Telkki, V.-V.; et al. High-purity lignin fractions and nanospheres rich in phenolic hydroxyl and carboxyl groups isolated with alkaline deep eutectic solvent from wheat straw. Bioresour. Technol. 2022, 360, 127570. [Google Scholar] [CrossRef] [PubMed]
- Wufuer, A.; Wang, Y.; Dai, L. Enhanced lignin degradation by a two-step acidic protic bio-based ionic liquid pretreatment method. Biomass-Convers. Biorefin. 2021, 1–10. [Google Scholar] [CrossRef]
- Yang, X.; Song, Y.; Ma, S.; Zhang, X.; Tan, T. Using γ-valerolactone and toluenesulfonic acid to extract lignin efficiently with a combined hydrolysis factor and structure characteristics analysis of lignin. Cellulose 2020, 27, 3581–3590. [Google Scholar] [CrossRef]
- Wu, Y.; Ji, H.; Ji, X.; Tian, Z.; Chen, J. Fibrillating wood chips to facilitate high-valued lignin extraction and high titer ethanol production. Ind. Crop. Prod. 2020, 146, 112153. [Google Scholar] [CrossRef]
- Charisteidis, I.; Lazaridis, P.; Fotopoulos, A.; Pachatouridou, E.; Matsakas, L.; Rova, U.; Christakopoulos, P.; Triantafyllidis, K. Catalytic fast pyrolysis of lignin isolated by hybrid organosolv—Steam explosion pretreatment of hardwood and softwood biomass for the production of phenolics and aromatics. Catalysts 2019, 9, 935. [Google Scholar] [CrossRef]
- Liu, Z.-H.; Xie, S.; Lin, F.; Jin, M.; Yuan, J.S. Combinatorial pretreatment and fermentation optimization enabled a record yield on lignin bioconversion. Biotechnol. Biofuels 2018, 11, 21. [Google Scholar] [CrossRef] [PubMed]
- Kumar, N.; Vijayshankar, S.; Pasupathi, P.; Kumar, S.N.; Elangovan, P.; Rajesh, M.; Tamilarasan, K. Optimal extraction, sequential fractionation and structural characterization of soda lignin. Res. Chem. Intermed. 2018, 44, 5403–5417. [Google Scholar] [CrossRef]
- Kim, J.-W.; Kim, K.S.; Lee, J.-S.; Park, S.M.; Cho, H.-Y.; Park, J.C.; Kim, J.S. Two-stage pretreatment of rice straw using aqueous ammonia and dilute acid. Bioresour. Technol. 2011, 102, 8992–8999. [Google Scholar] [CrossRef] [PubMed]
- Toscan, A.; Fontana, R.C.; Andreaus, J.; Camassola, M.; Lukasik, R.M.; Dillon, A.J.P. New two-stage pretreatment for the fractionation of lignocellulosic components using hydrothermal pretreatment followed by imidazole delignification: Focus on the polysaccharide valorization. Bioresour. Technol. 2019, 285, 121346. [Google Scholar] [CrossRef] [PubMed]
- Wang, K.; Jiang, J.-X.; Xu, F.; Sun, R.-C. Effects of Incubation Time on the Fractionation and Characterization of Lignin During Steam Explosion Pretreatment. Ind. Eng. Chem. Res. 2012, 51, 2704–2713. [Google Scholar] [CrossRef]
- Panagiotopoulos, I.; Chandra, R.; Saddler, J. A two-stage pretreatment approach to maximise sugar yield and enhance reactive lignin recovery from poplar wood chips. Bioresour. Technol. 2013, 130, 570–577. [Google Scholar] [CrossRef] [PubMed]
- Gelosia, M.; Ingles, D.; Pompili, E.; D’antonio, S.; Cavalaglio, G.; Petrozzi, A.; Coccia, V. Fractionation of lignocellulosic residues coupling steam explosion and organosolv treatments using green solvent γ-valerolactone. Energies 2017, 10, 1264. [Google Scholar] [CrossRef]
- Sathitsuksanoh, N.; Holtman, K.M.; Yelle, D.J.; Morgan, T.; Stavila, V.; Pelton, J.; Blanch, H.; Simmons, B.A.; George, A. Lignin fate and characterization during ionic liquid biomass pretreatment for renewable chemicals and fuels production. Green Chem. 2013, 16, 1236–1247. [Google Scholar] [CrossRef]
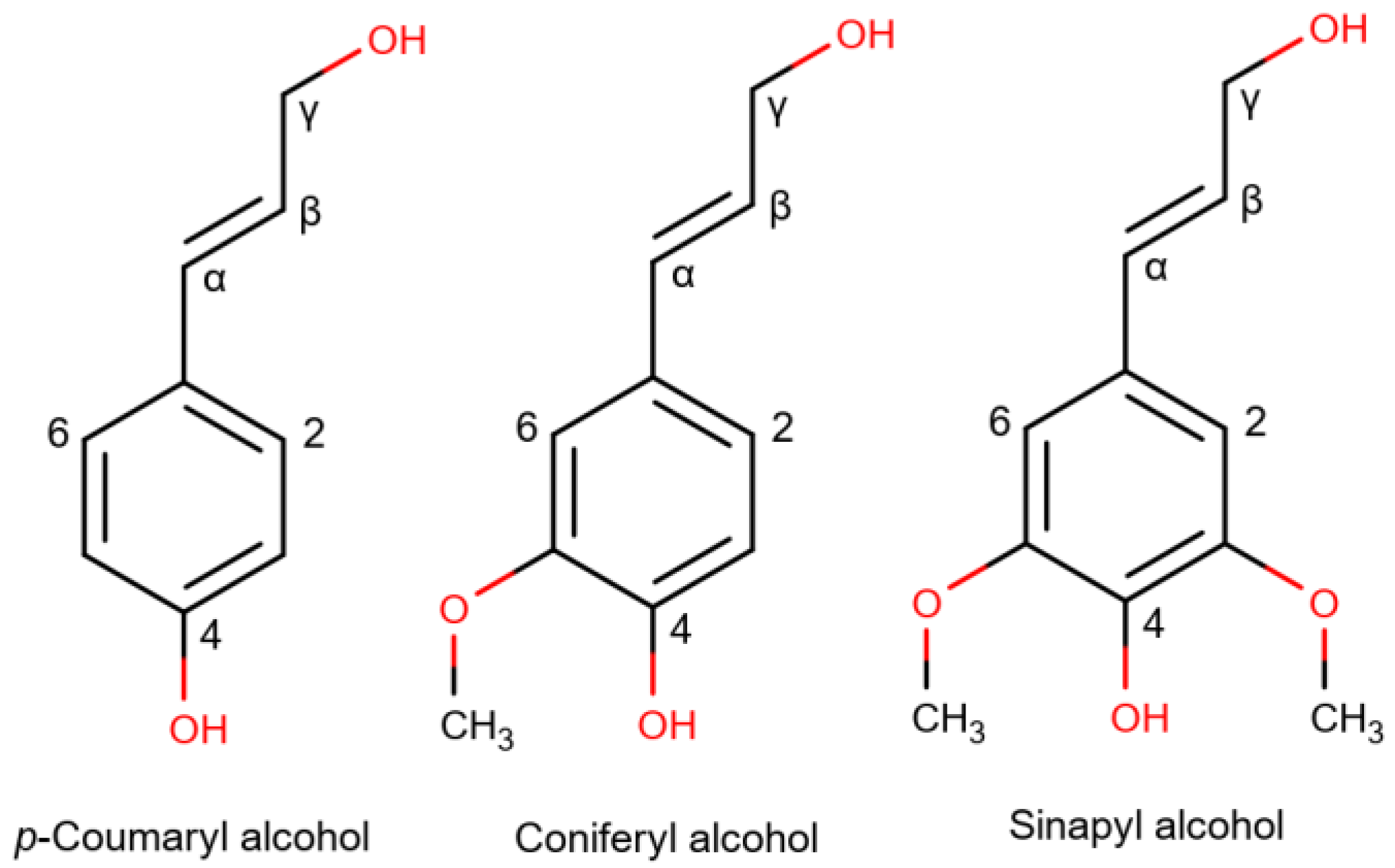

| Biomass | Type | Cellulose | Hemicellulose | Lignin |
|---|---|---|---|---|
| Spruce | Softwood | 44 | 29 | 27 |
| Birch * | Hardwood | 42 | 38 | 19 |
| Wheat straw * | Herbaceous | 40 | 21 | 20 |
| Aspen * | Hardwood | 53 | 22 | 20 |
| Oak * | Hardwood | 38 | 29 | 25 |
| Pine * | Softwood | 41 | 26 | 27 |
| Hemlock | Softwood | 42 | 32 | 26 |
| Bagasse * | Herbaceous | 39 | 29 | 19 |
| Content (%, w/w) | |||
|---|---|---|---|
| Type of Lignin | H-units | G-units | S-units |
| Herbaceous | 5–30 | 35–80 | 20–55 |
| Softwood | - | 90–95 | 5–10 |
| Hardwood | 0–8 | 25–50 | 50–75 |
| Pretreatment Method | Advantages | Drawbacks |
|---|---|---|
| Physical | Minimize the structural recalcitrance | Lack of ability to remove lignin |
| Reduce particle size and moisture content | Higher energy-demand | |
| Increase the accessibility and storage availability | Insufficient separation of components | |
| Chemical | Room temperature | Higher cost |
| Higher delignification rates | Certain effects on the environment and fermentation | |
| Maximize conversion of polysaccharides into sugars | ||
| Fast | ||
| Physicochemical | Improve the accessibility of the lignocellulosic matrix | High demand for energy |
| Lack of formation of inhibitors | High cost | |
| Lignin removal efficiency | Higher temperature and pressure | |
| Biological | Lower energy consumption | Low efficiency |
| Lignin and hemicellulose degradation | Low rate of hydrolysis |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tanis, M.H.; Wallberg, O.; Galbe, M.; Al-Rudainy, B. Lignin Extraction by Using Two-Step Fractionation: A Review. Molecules 2024, 29, 98. https://doi.org/10.3390/molecules29010098
Tanis MH, Wallberg O, Galbe M, Al-Rudainy B. Lignin Extraction by Using Two-Step Fractionation: A Review. Molecules. 2024; 29(1):98. https://doi.org/10.3390/molecules29010098
Chicago/Turabian StyleTanis, Medya Hatun, Ola Wallberg, Mats Galbe, and Basel Al-Rudainy. 2024. "Lignin Extraction by Using Two-Step Fractionation: A Review" Molecules 29, no. 1: 98. https://doi.org/10.3390/molecules29010098
APA StyleTanis, M. H., Wallberg, O., Galbe, M., & Al-Rudainy, B. (2024). Lignin Extraction by Using Two-Step Fractionation: A Review. Molecules, 29(1), 98. https://doi.org/10.3390/molecules29010098