New System for Simultaneous Measurement of Oxygen Consumption and Changes in Wine Color
Abstract
:1. Introduction
2. Results
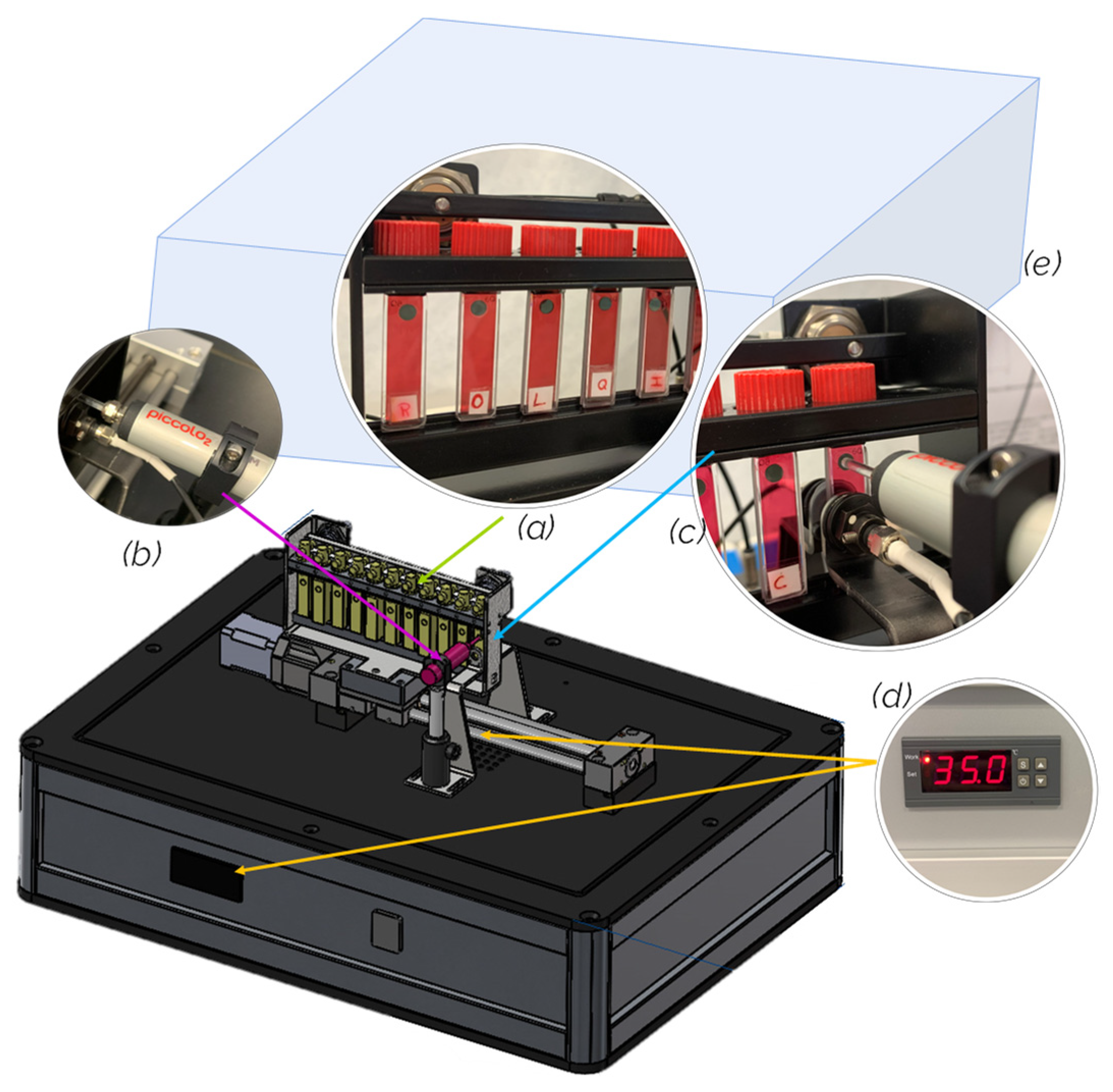
2.1. Characterization of the SpectrO2 unit
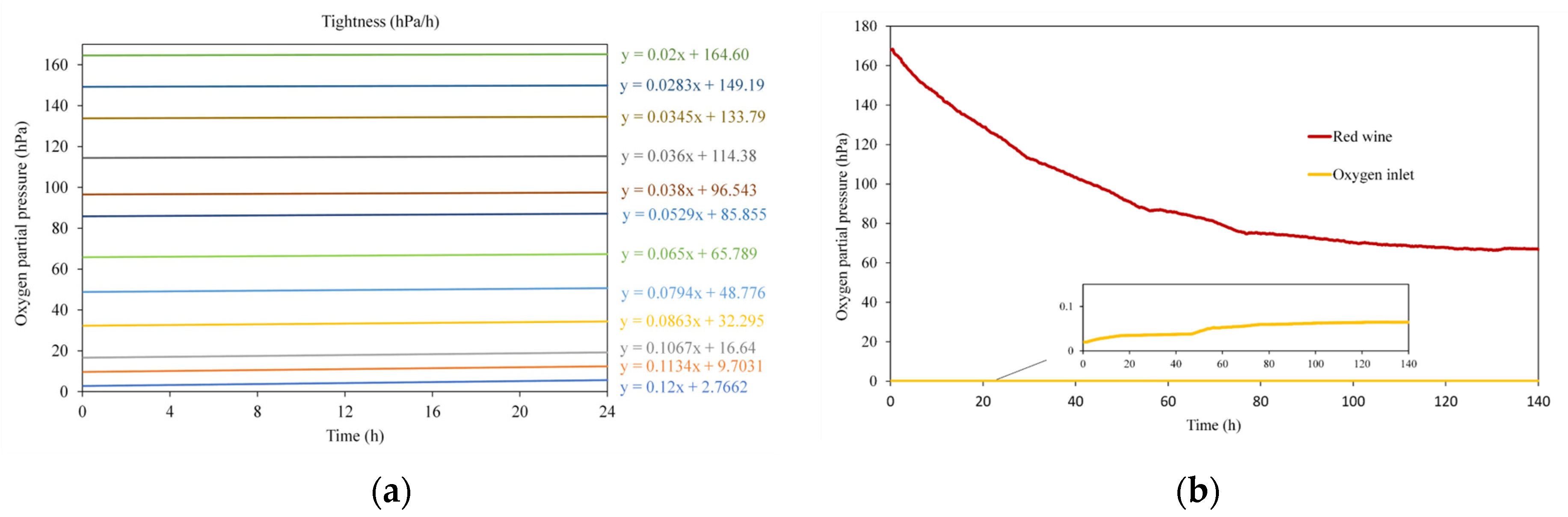
2.1.1. Tightness Tests
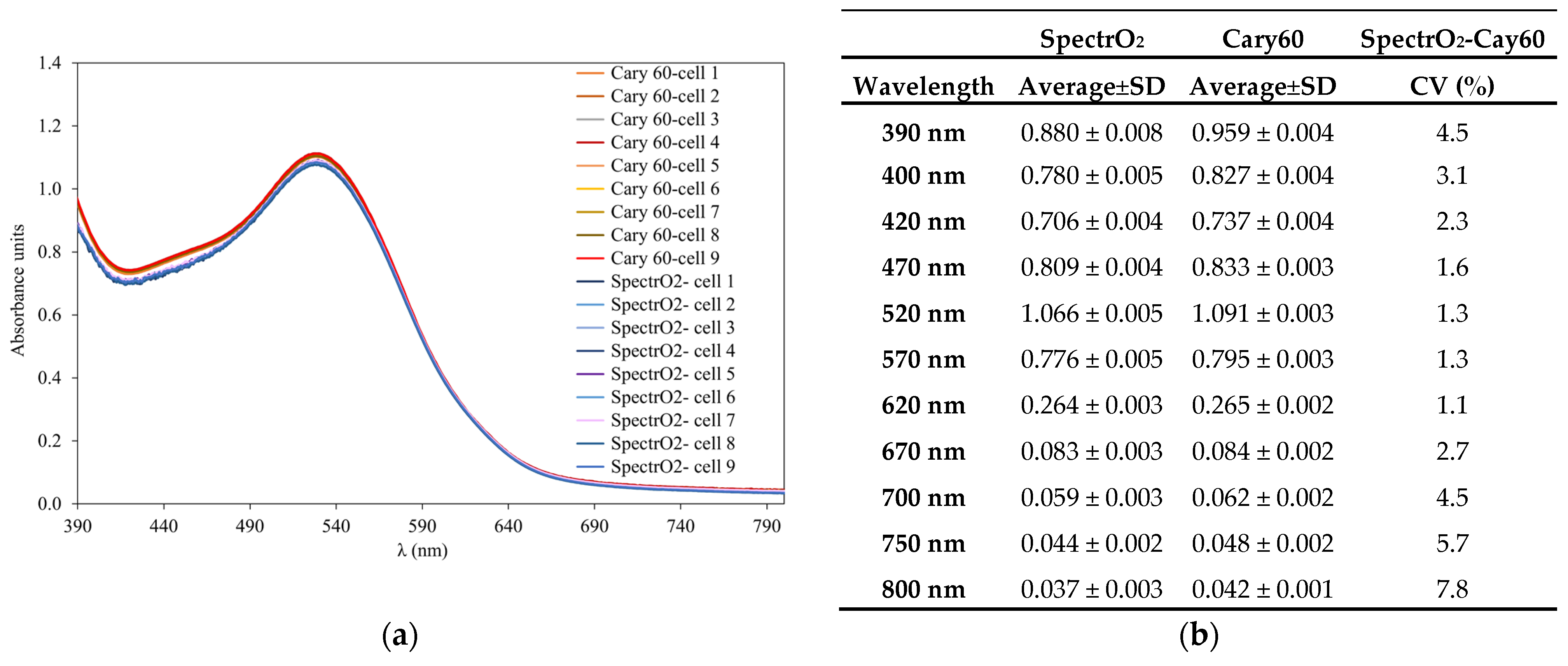
2.1.2. Measurement of UV-Vis Spectra
2.1.3. Measurement of Oxygen Kinetics
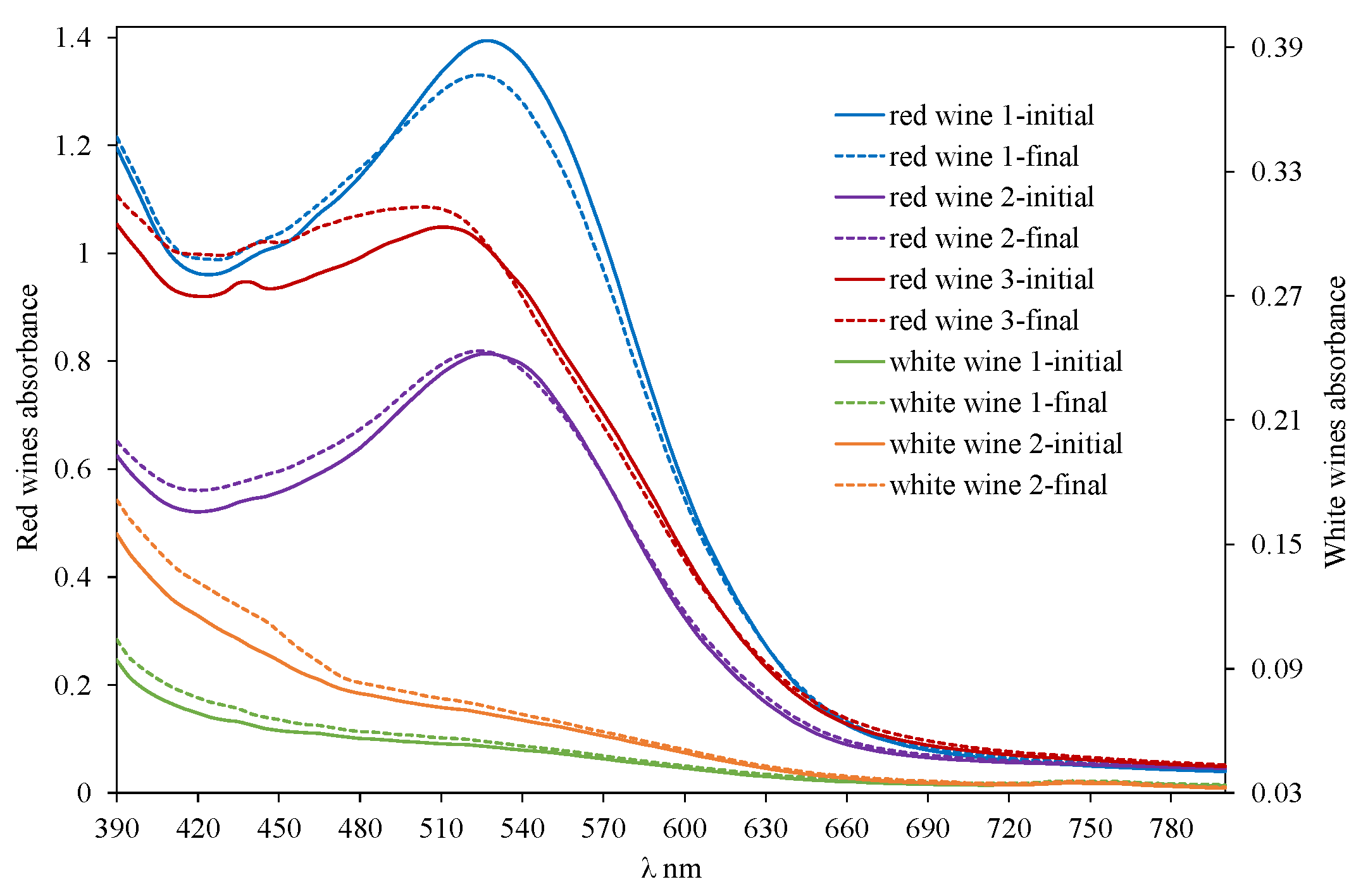
2.2. Measurements of Commercial Wines with SpectroO2
3. Materials and Methods
3.1. Wines Analyzed
3.2. SpectrO2 Device
3.3. Evaluation of Cell Quartz Tightness
3.4. Evaluation of Repeatability
3.4.1. Spectrum Repeatability
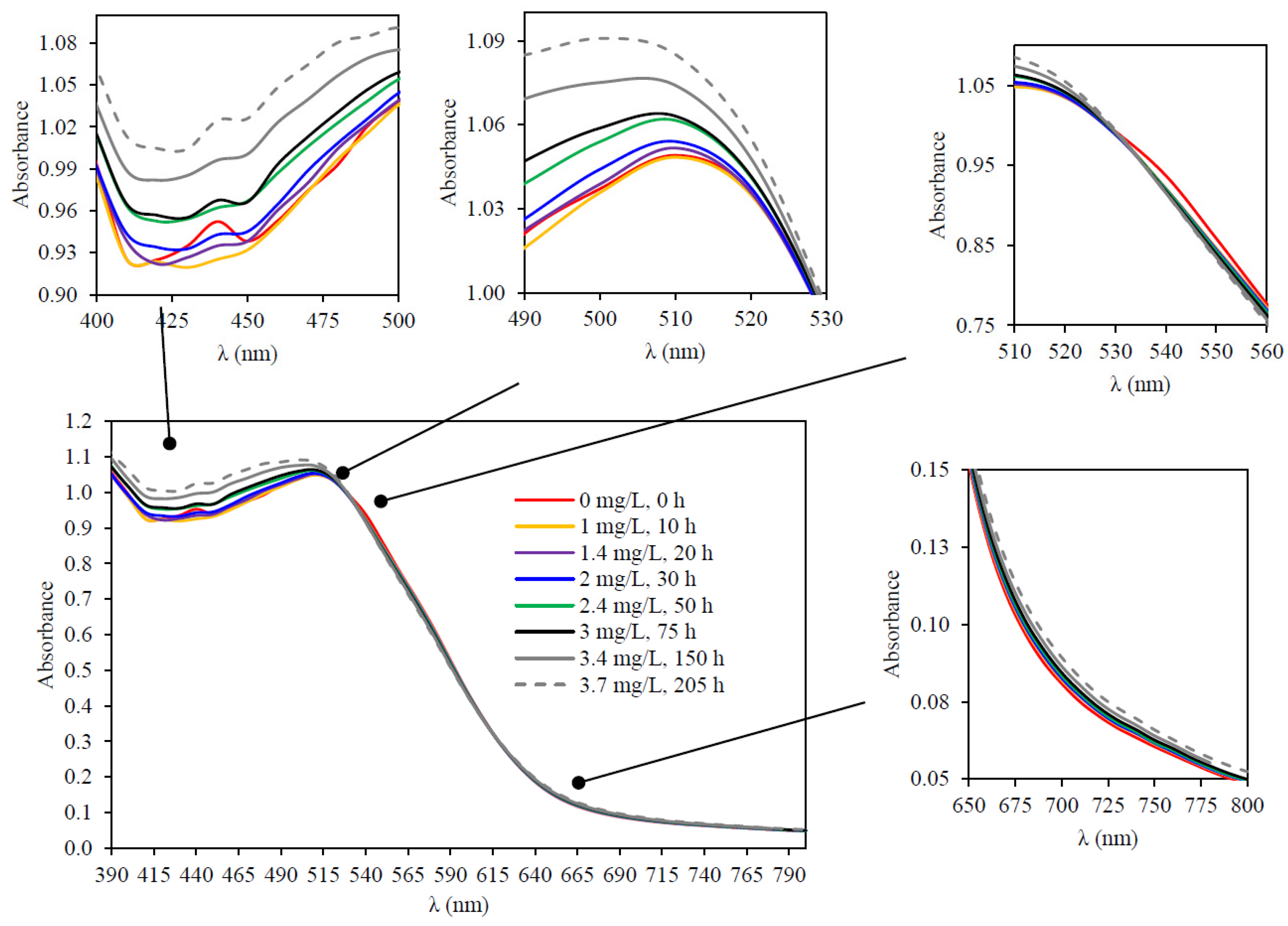
3.4.2. Repeatability of Consumption Kinetics
3.5. Simultaneous Measurement of Oxygen Consumption Kinetics and Spectra with SpectrO2 Equipment
3.6. Oxygen Consumption Kinetics Processing
3.7. Statistical Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sánchez-Gómez, R.; Nevares, I.; Martínez-Gil, A.; del Alamo-Sanza, M. Oxygen Consumption by Red Wines under Different Micro-Oxygenation Strategies and Q. Pyrenaica Chips. Effects on Color and Phenolic Characteristics. Beverages 2018, 4, 69. [Google Scholar] [CrossRef]
- Gambuti, A.; Rinaldi, A.; Ugliano, M.; Moio, L. Evolution of Phenolic Compounds and Astringency during Aging of Red Wine: Effect of Oxygen Exposure before and after Bottling. J. Agric. Food Chem. 2013, 61, 1618–1627. [Google Scholar] [CrossRef] [PubMed]
- Ferreira, V.; Carrascon, V.; Bueno, M.; Ugliano, M.; Fernandez-Zurbano, P. Oxygen Consumption by Red Wines. Part I: Consumption Rates, Relationship with Chemical Composition, and Role of SO2. J. Agric. Food Chem. 2015, 63, 10928–10937. [Google Scholar] [CrossRef] [PubMed]
- Ranaweera, R.K.R.; Capone, D.L.; Bastian, S.E.P.; Cozzolino, D.; Jeffery, D.W. A Review of Wine Authentication Using Spectroscopic Approaches in Combination with Chemometrics. Molecules 2021, 26, 4334. [Google Scholar] [CrossRef] [PubMed]
- Cozzolino, D.; Cynkar, W.U.; Shah, N.; Smith, P.A. Can Spectroscopy Geographically Classify Sauvignon Blanc Wines from Australia and New Zealand? Food Chem. 2011, 126, 673–678. [Google Scholar] [CrossRef]
- Del Alamo Sanza, M.; Asensio Cuadrado, M.; Del Barrio Galán, R.; Bueno Herrera, M.; Cárcel Cárcel, L.M.; Carrasco Quiroz, M.; Mallen Pomes, J.; Martínez Gil, A.M.; Nevares Domínguez, I.; Pérez Magariño, S. Manual técnico. Buenas Prácticas para la Gestión del O2 en Bodega; Ediciones Universidad de Valladolid: Valladolid, Spain, 2023; ISBN 978-84-1320-264-8. Available online: https://uvadoc.uva.es/handle/10324/63572 (accessed on 2 December 2023).
- Sánchez-Gómez, R.; del Alamo-Sanza, M.; Martínez-Martínez, V.; Nevares, I. Study of the Role of Oxygen in the Evolution of Red Wine Colour under Different Ageing Conditions in Barrels and Bottles. Food Chem. 2020, 328, 127040. [Google Scholar] [CrossRef] [PubMed]
- Nevares, I.; Martínez-Martínez, V.; Martínez-Gil, A.; Martín, R.; Laurie, V.F.; del Álamo-Sanza, M. On-Line Monitoring of Oxygen as a Method to Qualify the Oxygen Consumption Rate of Wines. Food Chem. 2017, 229, 588–596. [Google Scholar] [CrossRef]
- Danilewicz, J.C.; Seccombe, J.T.; Whelan, J. Mechanism of Interaction of Polyphenols, Oxygen, and Sulfur Dioxide in Model Wine and Wine. Am. J. Enol. Vitic. 2008, 59, 128–136. [Google Scholar] [CrossRef]
- Laurie, V.F.; Waterhouse, A.L. Oxidation of Glycerol in the Presence of Hydrogen Peroxide and Iron in Model Solutions and Wine. Potential Effects on Wine Color. J. Agric. Food Chem. 2006, 54, 4668–4673. [Google Scholar] [CrossRef]
- Oliveira, C.M.; Ferreira, A.C.S.; De Freitas, V.; Silva, A.M.S. Oxidation Mechanisms Occurring in Wines. Food Res. Int. 2011, 44, 1115–1126. [Google Scholar] [CrossRef]
- du Toit, W.J.; Marais, J.; Pretorius, I.S.; du Toit, M. Oxygen in Must and Wine: A Review. S. Afr. J. Enol. Vitic. 2017, 27, 76–94. [Google Scholar] [CrossRef]
- Tarko, T.; Duda-Chodak, A.; Sroka, P.; Siuta, M. The Impact of Oxygen at Various Stages of Vinification on the Chemical Composition and the Antioxidant and Sensory Properties of White and Red Wines. Int. J. Food Sci. 2020, 27, 7902974. [Google Scholar] [CrossRef] [PubMed]
- Fernandez-Zurbano, P.; Ferreira, V.; Pena, C.; Escudero, A.; Serrano, F.; Cacho, J. Prediction of Oxidative Browning in White Wines as a Function of Their Chemical Composition. J. Agric. Food Chem. 1995, 43, 2813–2817. [Google Scholar] [CrossRef]
- He, J.; Oliveira, J.; Silva, A.M.S.; Mateus, N.; De Freitas, V. Oxovitisins: A New Class of Neutral Pyranone-Anthocyanin Derivatives in Red Wines. J. Agric. Food Chem. 2010, 58, 8814–8819. [Google Scholar] [CrossRef] [PubMed]
- Cheng, S.; Wu, T.; Gao, J.; Han, X.; Huang, W.; You, Y.; Zhan, J. Color Myth: Anthocyanins Reactions and Enological Approaches Achieving Their Stabilization in the Aging Process of Red Wine. Food Innov. Adv. 2023, 2, 255–271. [Google Scholar] [CrossRef]
- Es-Safi, N.-E.; Le Guernevé, C.; Fulcrand, H.; Cheynier, V.; Moutounet, M. Xanthylium Salts Formation Involved in Wine Colour Changes. Int. J. Food Sci. Technol. 2000, 35, 63–74. [Google Scholar] [CrossRef]
- Alcalde-Eon, C.; Escribano-Bailón, M.T.; Santos-Buelga, C.; Rivas-Gonzalo, J.C. Changes in the Detailed Pigment Composition of Red Wine during Maturity and Ageing. Anal. Chim. Acta 2006, 563, 238–254. [Google Scholar] [CrossRef]
- Benabdeljalil, C.; Cheynier, V.; Fulcrand, H.; Hafiki, A.; Mosaddak, M.; Moutounet, M. Mise En Évidence de Nouveaux Pigments Formés Par Réaction Des Anthocyanes Avec Des Métabolites de Levure. Sci. Aliment. 2000, 20, 203–220. [Google Scholar] [CrossRef]
- Sarni-manchado, P.; Fulcrand, H.; Souquet, J.-M.; Cheynier, V.; Moutounet, M. Stability and Color of Unreported Wine Anthocyanin-derived Pigments. J. Food Sci. 1996, 61, 938–941. [Google Scholar] [CrossRef]
- Francia-Aricha, E.M.; Guerra, M.T.; Rivas-Gonzalo, J.C.; Santos-Buelga, C. New Anthocyanin Pigments Formed after Condensation with Flavanols. J. Agric. Food Chem. 1997, 45, 2262–2266. [Google Scholar] [CrossRef]
- He, F.; Liang, N.-N.; Mu, L.; Pan, Q.-H.; Wang, J.; Reeves, M.J.; Duan, C.-Q. Anthocyanins and Their Variation in Red Wines II. Anthocyanin Derived Pigments and Their Color Evolution. Molecules 2012, 17, 1483–1519. [Google Scholar] [CrossRef] [PubMed]
- Monagas, M.; Núñez, V.; Bartolomé, B.; Gómez-Cordovés, C. Anthocyanin-Derived Pigments in Graciano, Tempranillo, and Cabernet Sauvignon Wines Produced in Spain. Am. J. Enol. Vitic. 2003, 54, 163–169. [Google Scholar] [CrossRef]
- de Freitas, V.; Mateus, N. Formation of Pyranoanthocyanins in Red Wines: A New and Diverse Class of Anthocyanin Derivatives. Anal. Bioanal. Chem. 2011, 401, 1463–1473. [Google Scholar] [CrossRef] [PubMed]
- Mateus, N.; de Pascual-Teresa, S.; Rivas-Gonzalo, J.C.; Santos-Buelga, C.; de Freitas, V. Structural Diversity of Anthocyanin-Derived Pigments in Port Wines. Food Chem. 2002, 76, 335–342. [Google Scholar] [CrossRef]
- De Villiers, A.; Vanhoenacker, G.; Majek, P.; Sandra, P. Determination of Anthocyanins in Wine by Direct Injection Liquid Chromatography–Diode Array Detection–Mass Spectrometry and Classification of Wines Using Discriminant Analysis. J. Chromatogr. A 2004, 1054, 195–204. [Google Scholar] [CrossRef] [PubMed]
- Boido, E.; Alcalde-Eon, C.; Carrau, F.; Dellacassa, E.; Rivas-Gonzalo, J.C. Aging Effect on the Pigment Composition and Color ofVitis ViniferaL. Cv. Tannat Wines. Contribution of the Main Pigment Families to Wine Color. J. Agric. Food Chem. 2006, 54, 6692–6704. [Google Scholar] [CrossRef] [PubMed]
- Schwarz, M.; Quast, P.; von Baer, D.; Winterhalter, P. Vitisin A Content in Chilean Wines from Vitis Vinifera CV. Cabernet Sauvignon and Contribution to the Color of Aged Red Wines. J. Agric. Food Chem. 2003, 51, 6261–6267. [Google Scholar] [CrossRef] [PubMed]
- Waterhouse, A.L.; Zhu, J. A Quarter Century of Wine Pigment Discovery. J. Sci. Food Agric. 2020, 100, 5093–5101. [Google Scholar] [CrossRef]
- Jackson, R.S. Wine Science: Principle and Applications; Elsevier-Academic Press: Oxford, UK, 2008. [Google Scholar]
- Ribéreau-Gayon, P.; Glories, Y.; Maujean, A.; Dubourdieu, D. Chemistry of Wine Stabilization and Treatments; John Wiley & Sons Ltd.: Chichester, UK, 2005; Volume 2. [Google Scholar]
- Berente, B.; De la Calle García, D.; Reichenbächer, M.; Danzer, K. Method Development for the Determination of Anthocyanins in Red Wines by High-Performance Liquid Chromatography and Classification of German Red Wines by Means of Multivariate Statistical Methods. J. Chromatogr. A 2000, 871, 95–103. [Google Scholar] [CrossRef]
- Lee, J.; Durst, R.W.; Wrolstad, R.E.; Eisele, T.; Giusti, M.M.; Hach, J.; Hofsommer, H.; Koswig, S.; Krueger, D.A.; Kupina, S.; et al. Determination of Total Monomeric Anthocyanin Pigment Content of Fruit Juices, Beverages, Natural Colorants, and Wines by the pH Differential Method: Collaborative Study. J. AOAC Int. 2005, 88, 1269–1278. [Google Scholar] [CrossRef]
- Es-Safı, N.-E.; Le Guernevé, C.; Fulcrand, H.; Cheynier, V.; Moutounet, M. New Polyphenolic Compounds with Xanthylium Skeletons Formed through Reaction between (+)-Catechin and Glyoxylic Acid. J. Agric. Food Chem. 1999, 47, 5211–5217. [Google Scholar] [CrossRef] [PubMed]
- Mateus, N.; Silva, A.M.S.; Rivas-Gonzalo, J.C.; Santos-Buelga, C.; de Freitas, V. A New Class of Blue Anthocyanin-Derived Pigments Isolated from Red Wines. J. Agric. Food Chem. 2003, 51, 1919–1923. [Google Scholar] [CrossRef] [PubMed]
- Oliveira, J.; Azevedo, J.; Silva, A.M.S.; Teixeira, N.; Cruz, L.; Mateus, N.; de Freitas, V. Pyranoanthocyanin Dimers: A New Family of Turquoise Blue Anthocyanin-Derived Pigments Found in Port Wine. J. Agric. Food Chem. 2010, 58, 5154–5159. [Google Scholar] [CrossRef] [PubMed]
- Burns, J.; Mullen, W.; Landrault, N.; Teissedre, P.-L.; Lean, M.E.J.; Crozier, A. Variations in the Profile and Content of Anthocyanins in Wines Made from Cabernet Sauvignon and Hybrid Grapes. J. Agric. Food Chem. 2002, 50, 4096–4102. [Google Scholar] [CrossRef]
- Mulinacci, N.; Santamaria, A.R.; Giaccherini, C.; Innocenti, M.; Valletta, A.; Ciolfi, G.; Pasqua, G. Anthocyanins and Flavan-3-Ols from Grapes and Wines of Vitis Vinifera Cv. Cesanese d’Affile. Nat. Prod. Res. 2008, 22, 1033–1039. [Google Scholar] [CrossRef]
- Cheynier, V.; Dueñas-Paton, M.; Salas, E.; Maury, C.; Souquet, J.-M.; Sarni-Manchado, P.; Fulcrand, H. Structure and Properties of Wine Pigments and Tannins. Am. J. Enol. Vitic. 2006, 57, 298–305. [Google Scholar] [CrossRef]
- Boulton, R. The Copigmentation of Anthocyanins and Its Role in the Color of Red Wine: A Critical Review. Am. J. Enol. Vitic. 2001, 52, 67–87. [Google Scholar] [CrossRef]
- Zhang, X.-K.; Jeffery, D.W.; Li, D.-M.; Lan, Y.-B.; Zhao, X.; Duan, C.-Q. Red Wine Coloration: A Review of Pigmented Molecules, Reactions, and Applications. Compr. Rev. Food Sci. Food Saf. 2022, 21, 3834–3866. [Google Scholar] [CrossRef]
- Oliveira, J.; Mateus, N.; de Freitas, V. Wine-Inspired Chemistry: Anthocyanin Transformations for a Portfolio of Natural Colors. Synlett 2017, 28, 898–906. [Google Scholar] [CrossRef]
- Del Alamo-Sanza, M.; Sánchez-Gómez, R.; Martínez-Martínez, V.; Martínez-Gil, A.; Nevares, I. Air Saturation Methodology Proposal for the Analysis of Wine Oxygen Consumption Kinetics. Food Res. Int. 2021, 147, 110535. [Google Scholar] [CrossRef]
- Pérez-Magariño, S.; Martínez-Gil, A.; Bueno-Herrera, M.; Nevares, I.; del Alamo-Sanza, M. Kinetics of Oxygen Consumption, a Key Factor in the Changes of Young Wines Composition. Lebenson. Wiss. Technol. 2023, 182, 114786. [Google Scholar] [CrossRef]
- Fulcrand, H.; dos Santos, P.-J.C.; Sarni-Manchado, P.; Cheynier, V.; Favre-Bonvin, J. Structure of New Anthocyanin-Derived Wine Pigments. J. Chem. Soc. Perkin Trans. 1 1996, 735–739. [Google Scholar] [CrossRef]
- OIV. Compendium of International Methods of Analysis of Wines and Musts. 2019. Available online: https://www.oiv.int (accessed on 12 November 2023).
- del Alamo Sanza, M.; Nevares Domínguez, I.; García Merino, S. Influence of Different Aging Systems and Oak Woods on Aged Wine Color and Anthocyanin Composition. Eur. Food Res. Technol. 2004, 219, 124–132. [Google Scholar] [CrossRef]



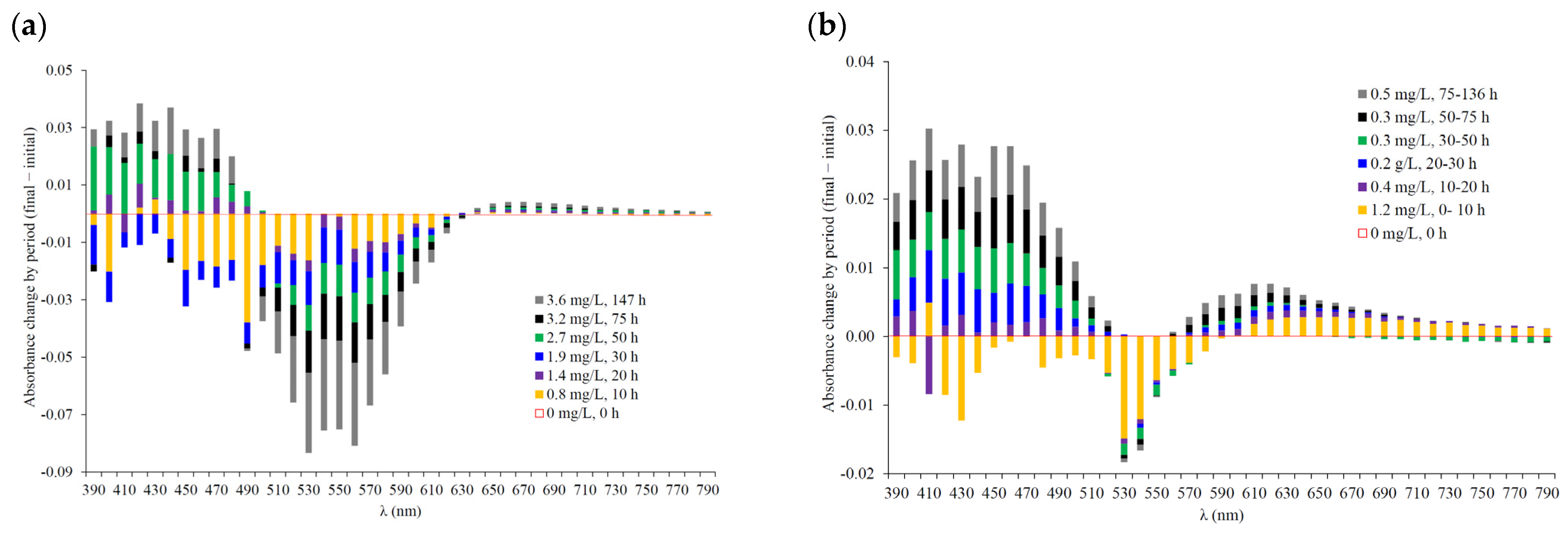
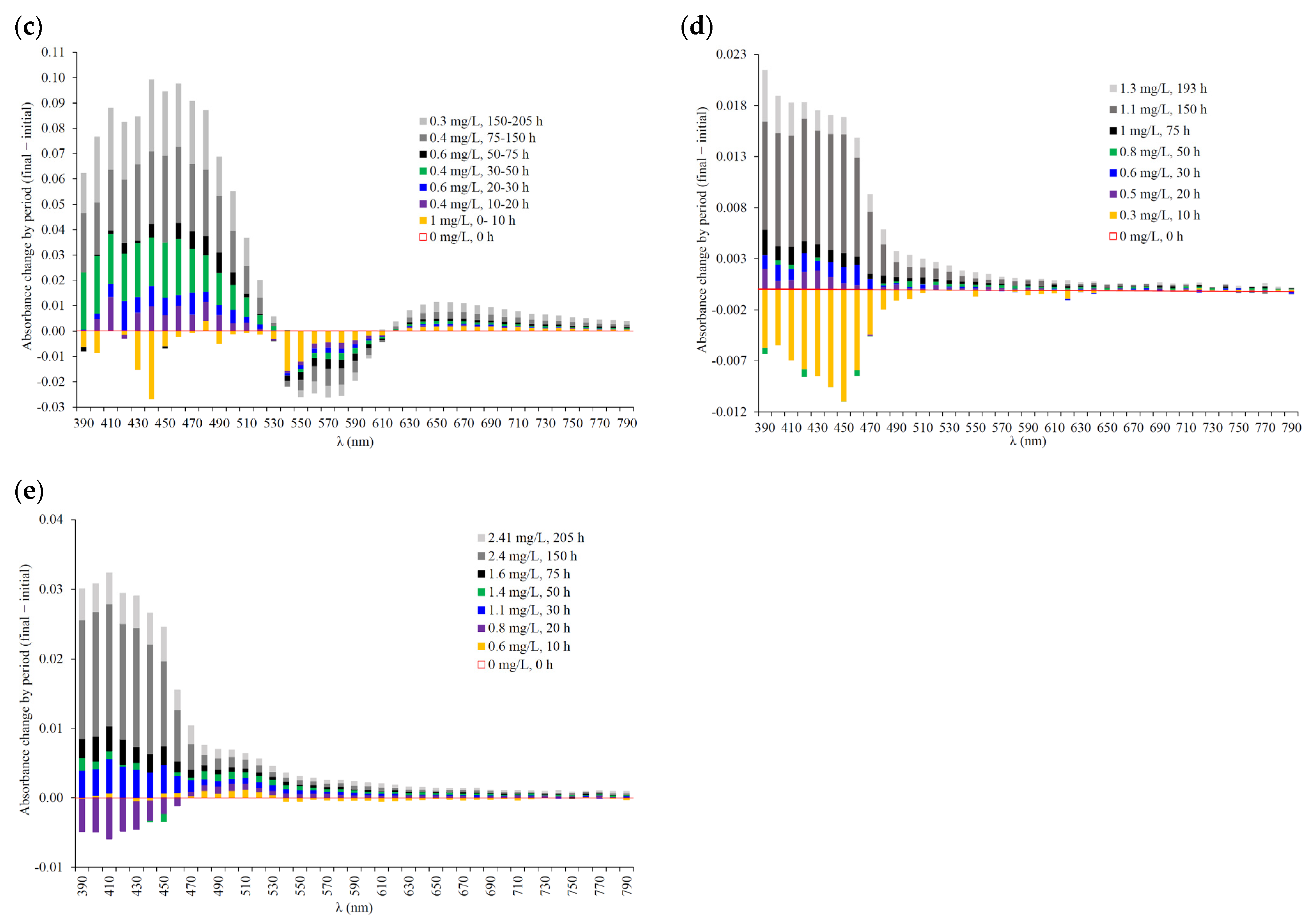
| Wine | Omax | Omin | ∆Omax_min | Oint | O90 | ∆O90_10 | O10 | tO_min | tO_90 | tO_10 | tO_int | AOmax_min | Amax_min |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Red wine 1 (n = 9) | 168.7 ± 1.9 b | 63.0 ± 1.8 a | 105.7 ± 2.2 d | 115.9 ± 1.5 a | 157.6 ± 1.9 d | 84.0 ± 1.9 d | 73.7 ± 1.7 a | 147.2 ± 3.1 a | 4.6 ± 0.7 c | 86.1 ± 6.5 a | 29.0 ± 1.4 ab | 4051 ± 143 bc | 13,468 ± 360 a |
| Red wine 2 (n = 3) | 167.2 ± 1.2 ab | 85.9 ± 8.8 b | 81.3 ± 7.7 c | 126.5 ± 5.0 b | 158.3 ± 6.5 c | 64.1 ± 6.5 c | 94.2 ± 8.1 b | 135.7 ± 11.8 a | 1.8 ± 0.4 ab | 104.3 ± 22.3 ab | 22.0 ± 11.3 a | 3215 ± 1416 b | 15,476 ± 286 b |
| Red wine 3 (n = 3) | 169.5 ± 4.7 b | 61.5 ± 5.8 a | 108.1 ± 4.7 d | 115.5 ± 4.7 a | 157.8 ± 3.8 d | 85.3 ± 3.8 d | 72.5 ± 5.7 a | 205.4 ± 4.5 b | 2.3 ± 0.9 ab | 132.5 ± 60.5 bc | 25.9 ± 3.4 ab | 5039 ± 1480 c | 17,841 ± 281 c |
| White wine 1 (n = 3) | 167.8 ± 0.8 ab | 129.9 ± 4.9 d | 37.9 ± 4.4 a | 148.8 ± 2.7 d | 163.9 ± 3.4 a | 30.2 ± 3.4 a | 133.7 ± 4.4 d | 193.2 ± 1.5 b | 3.8 ± 1.5 bc | 114.0 ± 4.1 abc | 33.4 ± 2.2 b | 1795 ± 296 a | 27,090 ± 656 d |
| White wine 2 (n = 3) | 165.0 ± 1.2 a | 102.8 ± 5.6 c | 62.3 ± 5.8 b | 133.9 ± 2.8 c | 158.6 ± 4.6 b | 49.3 ± 4.6 b | 109.4 ± 4.8 c | 205.3 ± 29.4 b | 2.6 ± 0.8 ab | 151.7 ± 13.8 c | 28.7 ± 4.7 b | 3270 ± 623 b | 27,884 ± 1001 d |
| Red Wine 1 | Red Wine 2 | Red Wine 3 | White Wine 1 | White Wine 2 | ||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| nm | 0–10 h | 0–20 h | 0–30 h | 0–50 h | 0–75 h | 0–147 h | 0–10 h | 0–20 h | 0–30 h | 0–50 h | 0–75 h | 0–136 h | 0–10 h | 0–20 h | 0–30 h | 0–50 h | 0–75 h | 0–150 h | 0–205 h | 0–10 h | 0–20 h | 0–30 h | 0–50 h | 0–75 h | 0–193 h | 0–10 h | 0–20 h | 0–30 h | 0–50 h | 0–75 h | 0–150 h | 0–205 h |
| 390 | −0.3% | −0.2% | −1.4% | 0.5% | 0.3% | 0.8% | −0.5% | −0.2% | 0.5% | 1.1% | 1.9% | 6.1% | −0.6% | −0.6% | −0.5% | 1.6% | 1.4% | 3.6% | 5.1% | −5.6% | −4.7% | −3.1% | −2.6% | −1.3% | 10.0% | −0.1% | −3.0% | −0.6% | 0.6% | 2.3% | 12.9% | 15.7% |
| 400 | −1.8% | −1.2% | −2.2% | −0.7% | −0.3% | 0.1% | −0.5% | 0.0% | 0.4% | 1.7% | 2.4% | 8.2% | −0.9% | −0.4% | −0.2% | 2.1% | 2.2% | 4.2% | 6.9% | −8.1% | −7.0% | −5.7% | −5.2% | −3.2% | 9.4% | 0.2% | −3.2% | −0.5% | 0.2% | 2.7% | 15.2% | 18.0% |
| 410 | 0.0% | −0.7% | −1.2% | 0.6% | 0.8% | 1.6% | −0.7% | 0.0% | 0.9% | 1.9% | 3.0% | 9.5% | 0.0% | 1.4% | 2.0% | 4.1% | 4.3% | 6.9% | 9.5% | −9.9% | −7.7% | −6.4% | −6.3% | −4.9% | 10.3% | 0.5% | −4.0% | −0.2% | 0.7% | 3.4% | 16.9% | 20.4% |
| 420 | 0.2% | 1.1% | −0.1% | 1.4% | 1.8% | 2.9% | 0.9% | −0.7% | 0.8% | 1.9% | 3.0% | 10.4% | −0.2% | −0.3% | 1.0% | 3.0% | 3.4% | 6.1% | 8.6% | −11.4% | −8.9% | −7.6% | −7.2% | −5.5% | 9.5% | 0.0% | −3.9% | −0.2% | 0.0% | 3.0% | 16.7% | 20.3% |
| 430 | 0.5% | 0.6% | −0.1% | 1.3% | 1.5% | 2.6% | −1.6% | −1.3% | 0.0% | 1.1% | 2.1% | 9.7% | −1.6% | −0.9% | −0.2% | 2.1% | 2.2% | 5.4% | 7.4% | −13.3% | −11.6% | −9.6% | −9.6% | −7.9% | 7.9% | −0.5% | −4.0% | −0.4% | 0.5% | 2.5% | 17.5% | 21.5% |
| 440 | −0.9% | −0.4% | −1.1% | 0.5% | 0.4% | 2.0% | −2.2% | −1.7% | −0.5% | 0.6% | 1.7% | 9.5% | −2.8% | −1.8% | −1.0% | 1.0% | 1.6% | 4.6% | 7.6% | −15.6% | −14.7% | −12.4% | −12.5% | −10.5% | 6.0% | −0.4% | −3.1% | 0.3% | 0.2% | 2.7% | 17.4% | 21.6% |
| 450 | −1.9% | −1.8% | −3.0% | −1.7% | −1.2% | −0.3% | −1.0% | −0.8% | 0.3% | 1.4% | 2.3% | 10.8% | −0.7% | 0.0% | 0.7% | 3.1% | 3.0% | 6.6% | 9.4% | −10.5% | −0.8% | 1.4% | 2.5% | 3.0% | 19.4% | 0.6% | −1.7% | 2.4% | 1.3% | 4.0% | 16.2% | 21.0% |
| 460 | −1.5% | −1.5% | −2.1% | −0.8% | −0.7% | 0.3% | −0.3% | 0.1% | 0.8% | 1.9% | 3.2% | 10.2% | −0.2% | 0.8% | 1.2% | 3.6% | 4.2% | 7.4% | 10.0% | −6.8% | −7.0% | −5.5% | −5.5% | −4.7% | 4.6% | 0.8% | −0.4% | 2.2% | 2.7% | 4.3% | 12.1% | 15.1% |
| 470 | −1.7% | −1.1% | −1.8% | −1.0% | −0.6% | 0.3% | −0.1% | 0.2% | 1.1% | 2.1% | 3.3% | 10.0% | −0.1% | 0.6% | 1.5% | 3.2% | 4.0% | 6.7% | 9.3% | −3.1% | −2.7% | −2.5% | −2.2% | −0.9% | 3.9% | 0.3% | 1.0% | 2.8% | 3.3% | 4.5% | 8.6% | 11.5% |
| 480 | −1.4% | −1.0% | −1.6% | −1.1% | −1.1% | −0.3% | 0.0% | 0.3% | 1.1% | 1.9% | 2.9% | 9.1% | 0.4% | 1.2% | 1.6% | 3.0% | 3.8% | 6.4% | 8.8% | −1.7% | −0.9% | −0.7% | −0.5% | 0.2% | 2.5% | 1.2% | 2.1% | 3.1% | 4.5% | 5.4% | 7.1% | 8.7% |
| 490 | −3.0% | −2.8% | −3.4% | −3.0% | −3.1% | −3.2% | −0.7% | −0.3% | 0.2% | 0.8% | 1.5% | 6.4% | −0.5% | 0.1% | 0.5% | 1.8% | 2.5% | 4.7% | 6.3% | −1.5% | −1.3% | −1.2% | −0.2% | 0.3% | 2.1% | 0.8% | 1.9% | 2.9% | 4.0% | 4.8% | 6.7% | 8.2% |
| 500 | −1.4% | −1.3% | −2.0% | −1.9% | −2.2% | −2.8% | −0.4% | −0.3% | 0.1% | 0.6% | 1.1% | 5.0% | −0.1% | 0.2% | 0.7% | 1.6% | 2.1% | 3.7% | 5.2% | −0.6% | −0.4% | 0.3% | 0.3% | 1.4% | 3.0% | 1.3% | 2.5% | 3.4% | 4.6% | 5.3% | 7.2% | 8.4% |
| 510 | −0.8% | −1.0% | −1.8% | −1.9% | −2.5% | −3.6% | −0.4% | −0.2% | 0.0% | 0.3% | 0.7% | 3.7% | −0.1% | 0.3% | 0.5% | 1.2% | 1.3% | 2.4% | 3.4% | −0.1% | 0.6% | 0.6% | 1.2% | 1.6% | 3.4% | 1.6% | 2.6% | 3.6% | 4.6% | 5.3% | 6.9% | 7.9% |
| 520 | −1.0% | −1.2% | −1.8% | −2.3% | −3.1% | −4.7% | −0.4% | −0.3% | −0.2% | −0.1% | 0.1% | 2.2% | −0.1% | −0.1% | 0.1% | 0.5% | 0.5% | 1.1% | 1.8% | −0.2% | 0.1% | 0.3% | 0.7% | 1.3% | 2.8% | 1.1% | 1.8% | 2.9% | 4.1% | 4.7% | 5.9% | 7.1% |
| 530 | −1.2% | −1.4% | −2.3% | −2.9% | −3.9% | −5.9% | −0.7% | −0.7% | −0.6% | −0.6% | −0.5% | 0.6% | −0.3% | −0.4% | −0.4% | −0.2% | −0.2% | −0.1% | 0.2% | −0.1% | 0.1% | 0.4% | 0.8% | 1.2% | 2.2% | 0.5% | 1.3% | 2.4% | 3.4% | 4.0% | 4.9% | 6.0% |
| 540 | 0.0% | −0.4% | −1.3% | −2.1% | −3.2% | −5.6% | −1.9% | −2.0% | −1.9% | −2.1% | −2.2% | −1.6% | −1.7% | −1.8% | −1.9% | −1.8% | −2.1% | −2.3% | −2.3% | −1.2% | −1.0% | −0.8% | −0.4% | 0.1% | 0.7% | −0.7% | 0.2% | 1.1% | 1.8% | 2.4% | 3.3% | 4.1% |
| 550 | −0.1% | −0.4% | −1.4% | −2.3% | −3.5% | −5.9% | −1.6% | −1.7% | −1.8% | −2.0% | −2.1% | −1.7% | −1.4% | −1.6% | −1.8% | −1.9% | −2.2% | −2.7% | −3.0% | −0.2% | 0.3% | 0.2% | 0.6% | 0.9% | 1.5% | −0.7% | 0.2% | 0.8% | 1.7% | 2.0% | 2.9% | 3.6% |
| 560 | −1.0% | −1.4% | −2.3% | −3.2% | −4.4% | −6.9% | −1.0% | −1.0% | −1.1% | −1.3% | −1.3% | −0.5% | −0.6% | −0.9% | −1.1% | −1.4% | −1.8% | −2.6% | −3.2% | −0.2% | 0.2% | 0.0% | 0.5% | 0.8% | 1.6% | −0.3% | 0.6% | 1.4% | 1.9% | 2.5% | 3.2% | 3.9% |
| 570 | −0.9% | −1.3% | −2.2% | −3.1% | −4.3% | −6.5% | −0.8% | −0.9% | −0.8% | −1.0% | −0.9% | 0.7% | −0.6% | −0.9% | −1.2% | −1.6% | −2.1% | −3.1% | −3.7% | −0.5% | −0.3% | −0.4% | 0.2% | 0.2% | 1.1% | −0.4% | 0.5% | 1.0% | 1.5% | 1.9% | 2.7% | 3.4% |
| 580 | −1.1% | −1.5% | −2.3% | −3.2% | −4.3% | −6.4% | −0.8% | −0.7% | −0.7% | −0.7% | −0.5% | 2.2% | −0.8% | −1.1% | −1.4% | −1.8% | −2.4% | −3.4% | −4.1% | −1.1% | −0.9% | −0.7% | −0.4% | −0.1% | 0.7% | −0.8% | 0.1% | 0.7% | 1.3% | 1.8% | 2.6% | 3.3% |
| 590 | −1.0% | −1.3% | −2.0% | −2.9% | −3.8% | −5.5% | −0.5% | −0.4% | −0.2% | −0.1% | 0.3% | 4.3% | −0.7% | −1.0% | −1.3% | −1.7% | −2.2% | −3.1% | −3.7% | −0.9% | −0.7% | −0.7% | −0.5% | −0.2% | 0.9% | −0.6% | 0.0% | 0.7% | 1.2% | 1.5% | 2.4% | 3.4% |
| 600 | −0.6% | −0.9% | −1.5% | −2.1% | −3.0% | −4.3% | −0.1% | 0.2% | 0.5% | 0.6% | 1.2% | 6.4% | −0.4% | −0.7% | −0.9% | −1.2% | −1.5% | −2.2% | −2.5% | −0.8% | −0.8% | −0.6% | −0.3% | 0.3% | 0.5% | −0.6% | 0.1% | 0.5% | 1.0% | 1.4% | 2.4% | 3.2% |
| 610 | −1.1% | −1.2% | −1.7% | −2.2% | −2.8% | −3.8% | 0.1% | 0.4% | 0.8% | 1.0% | 1.7% | 8.0% | −0.5% | −0.6% | −0.7% | −0.8% | −1.0% | −1.2% | −1.0% | −2.0% | −2.0% | −2.3% | −2.0% | −1.6% | −1.2% | −0.9% | −0.4% | 0.2% | 0.7% | 0.9% | 1.9% | 2.9% |
| 620 | −0.3% | −0.3% | −0.6% | −0.9% | −1.4% | −1.9% | 0.9% | 1.4% | 1.8% | 2.1% | 2.9% | 9.9% | 0.1% | 0.1% | 0.2% | 0.3% | 0.3% | 0.6% | 1.3% | −0.6% | −0.6% | −0.5% | −0.1% | 0.2% | 0.6% | −0.9% | −0.1% | 0.4% | 0.9% | 1.3% | 2.0% | 2.8% |
| 630 | 0.0% | 0.1% | −0.1% | −0.2% | −0.4% | −0.6% | 1.5% | 2.1% | 2.7% | 3.0% | 3.8% | 11.7% | 0.5% | 0.7% | 0.9% | 1.2% | 1.5% | 2.3% | 3.5% | −0.8% | −0.5% | −0.8% | −0.4% | −0.1% | 0.5% | −0.6% | 0.1% | 0.3% | 0.9% | 1.3% | 2.0% | 2.6% |
| 640 | 0.2% | 0.5% | 0.5% | 0.6% | 0.6% | 0.9% | 2.1% | 2.9% | 3.5% | 3.7% | 4.5% | 12.9% | 0.8% | 1.2% | 1.5% | 2.0% | 2.5% | 3.8% | 5.7% | −0.1% | −0.1% | −0.1% | 0.2% | 0.3% | 1.0% | −0.5% | 0.2% | 0.6% | 1.3% | 1.6% | 2.1% | 2.8% |
| 650 | 0.3% | 0.8% | 0.9% | 1.3% | 1.5% | 2.2% | 2.6% | 3.5% | 4.1% | 4.3% | 5.0% | 13.7% | 1.1% | 1.4% | 1.9% | 2.6% | 3.2% | 5.0% | 7.5% | −0.3% | −0.1% | 0.0% | 0.3% | 0.5% | 1.0% | −0.2% | 0.6% | 0.9% | 1.5% | 1.6% | 2.2% | 3.0% |
| 660 | 0.3% | 1.0% | 1.2% | 1.8% | 2.1% | 3.2% | 3.1% | 4.1% | 4.6% | 4.7% | 5.3% | 13.9% | 1.3% | 1.8% | 2.3% | 3.0% | 3.9% | 6.1% | 8.9% | −0.2% | −0.2% | 0.0% | 0.5% | 0.6% | 0.9% | −0.4% | 0.4% | 0.7% | 1.4% | 1.5% | 2.4% | 2.8% |
| 670 | 0.3% | 1.1% | 1.3% | 2.0% | 2.5% | 3.9% | 3.7% | 4.6% | 5.1% | 4.9% | 5.6% | 13.8% | 1.7% | 2.2% | 2.6% | 3.4% | 4.4% | 6.7% | 10.0% | −0.2% | −0.3% | 0.1% | 0.4% | 0.6% | 1.2% | −0.7% | 0.1% | 0.3% | 0.8% | 1.3% | 2.1% | 2.6% |
| 680 | 0.4% | 1.2% | 1.4% | 2.3% | 2.7% | 4.3% | 3.8% | 4.8% | 5.1% | 4.7% | 5.2% | 13.3% | 1.7% | 2.2% | 2.6% | 3.4% | 4.5% | 7.0% | 10.4% | 0.0% | 0.2% | −0.3% | 0.0% | 0.5% | 0.8% | −0.5% | 0.0% | 0.7% | 1.1% | 1.4% | 2.2% | 3.0% |
| 690 | 0.2% | 1.0% | 1.2% | 2.1% | 2.8% | 4.4% | 4.2% | 5.1% | 5.4% | 5.1% | 5.4% | 12.8% | 1.9% | 2.5% | 2.9% | 3.8% | 4.8% | 7.4% | 10.7% | −0.2% | −0.3% | −0.6% | −0.3% | 0.3% | 0.6% | −0.4% | 0.3% | 0.5% | 1.1% | 1.3% | 2.1% | 2.4% |
| 700 | 0.3% | 1.3% | 1.4% | 2.3% | 2.9% | 4.5% | 3.5% | 4.5% | 4.8% | 4.2% | 4.6% | 11.8% | 1.5% | 2.1% | 2.5% | 3.4% | 4.4% | 6.9% | 10.5% | −0.3% | −0.1% | 0.1% | 0.6% | 0.6% | 0.9% | −0.2% | 0.4% | 0.6% | 1.1% | 1.3% | 1.9% | 2.5% |
| 710 | −0.4% | 0.6% | 0.6% | 1.5% | 2.2% | 3.7% | 4.2% | 4.7% | 4.9% | 4.2% | 4.4% | 11.2% | 2.1% | 2.4% | 2.7% | 3.6% | 4.5% | 7.1% | 10.4% | 0.3% | 0.3% | −0.5% | 0.0% | 0.4% | 0.5% | −0.8% | 0.1% | 0.3% | 0.6% | 0.8% | 1.3% | 1.9% |
| 720 | −0.5% | 0.2% | 0.4% | 1.3% | 1.7% | 3.2% | 3.7% | 4.4% | 4.3% | 3.4% | 3.6% | 9.8% | 1.7% | 2.0% | 2.3% | 3.0% | 4.0% | 6.4% | 9.8% | −0.3% | −0.4% | −0.5% | −0.2% | 0.0% | 0.0% | −0.4% | 0.3% | 0.6% | 1.3% | 1.4% | 1.7% | 2.1% |
| 730 | −0.7% | 0.0% | 0.1% | 1.2% | 1.6% | 2.9% | 3.4% | 4.0% | 4.1% | 3.1% | 3.2% | 9.5% | 1.5% | 1.8% | 2.1% | 2.9% | 3.9% | 6.3% | 9.8% | −0.4% | −0.5% | −0.3% | −0.1% | 0.2% | 0.7% | 0.2% | 0.9% | 0.9% | 1.4% | 1.5% | 1.9% | 2.4% |
| 740 | −0.8% | −0.2% | −0.3% | 0.6% | 0.9% | 2.2% | 3.7% | 4.2% | 4.2% | 3.1% | 3.1% | 9.0% | 1.9% | 2.2% | 2.4% | 3.1% | 4.1% | 6.4% | 9.7% | −0.7% | −0.9% | −0.6% | −0.5% | −0.4% | −0.4% | 0.1% | 0.7% | 0.5% | 1.1% | 1.3% | 1.7% | 2.5% |
| 750 | −1.1% | −0.3% | −0.4% | 0.8% | 1.1% | 1.6% | 3.2% | 3.8% | 3.6% | 2.2% | 2.3% | 8.0% | 1.5% | 1.9% | 2.1% | 2.4% | 3.5% | 5.8% | 9.0% | −0.3% | −0.7% | −0.7% | −0.3% | −0.1% | −0.2% | 0.2% | 0.6% | 0.7% | 1.2% | 1.1% | 1.8% | 2.3% |
| 760 | −1.1% | −0.6% | −0.9% | 0.1% | 0.3% | 1.3% | 3.1% | 3.6% | 3.4% | 2.2% | 2.3% | 7.2% | 1.5% | 1.8% | 2.0% | 2.8% | 3.7% | 5.8% | 8.7% | −0.3% | −0.3% | −0.6% | −0.3% | 0.2% | −0.3% | 0.3% | 0.6% | 0.9% | 1.3% | 1.6% | 2.4% | 2.5% |
| 770 | −0.9% | −0.3% | −0.6% | 0.1% | 0.5% | 1.0% | 2.7% | 3.1% | 2.9% | 1.8% | 1.6% | 6.5% | 1.3% | 1.4% | 1.7% | 2.4% | 3.2% | 5.3% | 8.2% | −0.4% | −0.4% | −0.5% | −0.4% | −0.5% | −0.4% | 0.4% | 1.0% | 0.9% | 1.4% | 1.6% | 2.1% | 2.5% |
| 780 | −0.9% | −0.3% | −0.3% | 0.1% | 0.4% | 0.9% | 2.7% | 3.3% | 2.9% | 1.5% | 1.5% | 6.2% | 1.3% | 1.7% | 1.6% | 2.3% | 3.3% | 5.2% | 8.2% | −0.6% | −0.6% | −1.2% | −1.1% | −0.8% | −0.7% | −0.1% | 0.3% | 0.3% | 0.8% | 1.1% | 1.7% | 2.4% |
| 790 | −1.5% | −1.1% | −1.0% | −0.4% | −0.3% | 0.1% | 2.8% | 3.1% | 3.0% | 1.4% | 1.2% | 6.2% | 1.4% | 1.6% | 1.6% | 2.1% | 3.0% | 4.9% | 8.0% | −0.4% | −0.3% | −0.6% | −0.4% | −0.1% | 0.1% | −0.7% | −0.5% | −0.1% | 0.4% | 0.5% | 1.1% | 1.8% |
| 800 | −1.6% | −0.9% | −1.2% | −0.6% | −0.6% | 0.0% | 2.5% | 2.6% | 2.4% | 0.9% | 0.7% | 5.8% | 0.9% | 1.0% | 1.1% | 1.6% | 2.3% | 4.2% | 7.4% | −0.3% | −0.6% | −1.4% | −1.3% | −0.9% | −0.1% | −0.5% | −0.1% | 0.3% | 0.4% | 0.7% | 0.7% | 1.2% |
| λ (nm) | Red Wine 1 | Red Wine 2 | Red Wine 3 | White Wine 1 | White Wine 2 | λ (nm) | Red Wine 1 | Red Wine 2 | Red Wine 3 | White Wine 1 | White Wine 2 |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 390 | 0.1629 | 0.5716 | 0.759 | 0.7064 | 0.8334 | 600 | −0.9561 | 0.5201 | −0.972 | 0.7022 | 0.9199 |
| 395 | 0.8428 | 0.5678 | 0.827 | 0.6826 | 0.8469 | 605 | −0.9505 | 0.5495 | −0.978 | 0.6883 | 0.9519 |
| 400 | 0.7816 | 0.6008 | 0.955 | 0.6431 | 0.8500 | 610 | −0.9853 | 0.5276 | −0.948 | −0.1849 | 0.8345 |
| 405 | 0.8625 | 0.4467 | 0.849 | 0.6030 | 0.8323 | 615 | −0.9736 | 0.5885 | 0.059 | 0.3676 | 0.8862 |
| 410 | 0.5082 | 0.6128 | 0.891 | 0.5993 | 0.8353 | 620 | −0.9375 | 0.6218 | 0.818 | 0.6377 | 0.9266 |
| 415 | 0.3637 | 0.5760 | 0.878 | 0.5902 | 0.8324 | 625 | −0.9247 | 0.6374 | 0.882 | 0.8301 | 0.8929 |
| 420 | 0.5120 | 0.5770 | 0.921 | 0.6042 | 0.8405 | 630 | −0.8603 | 0.6585 | 0.900 | 0.6131 | 0.9185 |
| 425 | 0.2329 | 0.5553 | 0.891 | 0.5951 | 0.8431 | 635 | 0.4612 | 0.6773 | 0.913 | 0.7496 | 0.9273 |
| 430 | 0.1923 | 0.5239 | 0.862 | 0.5430 | 0.8494 | 640 | 0.9497 | 0.6948 | 0.920 | 0.7543 | 0.9377 |
| 435 | 0.7628 | 0.3779 | 0.570 | 0.3465 | 0.7951 | 645 | 0.9760 | 0.6930 | 0.913 | 0.8647 | 0.9200 |
| 440 | 0.6096 | 0.5396 | 0.861 | 0.5110 | 0.8544 | 650 | 0.9587 | 0.7084 | 0.909 | 0.7246 | 0.9549 |
| 445 | 0.0135 | 0.5015 | 0.868 | 0.5216 | 0.8636 | 655 | 0.9677 | 0.7156 | 0.906 | 0.8001 | 0.9387 |
| 450 | −0.0714 | 0.5259 | 0.854 | 0.5364 | 0.8711 | 660 | 0.9642 | 0.7325 | 0.915 | 0.7444 | 0.9390 |
| 455 | 0.1190 | 0.5816 | 0.904 | 0.4380 | 0.8826 | 665 | 0.9592 | 0.7440 | 0.914 | 0.9012 | 0.9102 |
| 460 | −0.6625 | 0.5733 | 0.884 | 0.9105 | 0.9055 | 670 | 0.9555 | 0.7370 | 0.907 | 0.8210 | 0.9365 |
| 465 | 0.2303 | 0.6031 | 0.910 | 0.9236 | 0.9266 | 675 | 0.9467 | 0.7476 | 0.912 | 0.8981 | 0.9110 |
| 470 | 0.1104 | 0.6050 | 0.901 | 0.6431 | 0.9413 | 680 | 0.9555 | 0.7418 | 0.907 | 0.7330 | 0.9336 |
| 475 | 0.4174 | 0.6168 | 0.921 | 0.6897 | 0.9576 | 685 | 0.9357 | 0.7537 | 0.907 | 0.7201 | 0.9567 |
| 480 | −0.0579 | 0.6181 | 0.917 | 0.7997 | 0.9874 | 690 | 0.9420 | 0.7815 | 0.927 | 0.4937 | 0.9486 |
| 485 | −0.6642 | 0.5567 | 0.875 | 0.6929 | 0.9822 | 695 | 0.9355 | 0.7522 | 0.906 | 0.7669 | 0.9732 |
| 490 | −0.7202 | 0.5403 | 0.881 | 0.7814 | 0.9781 | 700 | 0.9316 | 0.7422 | 0.904 | 0.8902 | 0.9468 |
| 495 | −0.8686 | 0.5302 | 0.893 | 0.7388 | 0.9923 | 705 | 0.9284 | 0.7476 | 0.911 | 0.8001 | 0.8358 |
| 500 | −0.9729 | 0.5261 | 0.894 | 0.8390 | 0.9883 | 710 | 0.8846 | 0.7343 | 0.903 | 0.5769 | 0.8195 |
| 505 | −0.9808 | 0.5208 | 0.892 | 0.8940 | 0.9893 | 715 | 0.8923 | 0.7364 | 0.907 | 0.4309 | 0.9286 |
| 510 | −0.9770 | 0.5069 | 0.889 | 0.8986 | 0.9892 | 720 | 0.8473 | 0.7041 | 0.887 | 0.5509 | 0.9135 |
| 515 | −0.9595 | 0.4641 | 0.904 | 0.9184 | 0.9876 | 725 | 0.8779 | 0.7036 | 0.897 | −0.0467 | 0.9644 |
| 520 | −0.9536 | 0.3988 | 0.837 | 0.8758 | 0.9875 | 730 | 0.8405 | 0.6763 | 0.887 | 0.8210 | 0.9632 |
| 525 | −0.9541 | 0.2800 | 0.760 | 0.8837 | 0.9795 | 735 | 0.8232 | 0.6942 | 0.894 | 0.6751 | 0.8542 |
| 530 | −0.9573 | 0.0514 | 0.370 | 0.9119 | 0.9828 | 740 | 0.7450 | 0.6929 | 0.894 | 0.2369 | 0.9319 |
| 535 | −0.9725 | −0.5096 | −0.839 | 0.8518 | 0.9751 | 745 | 0.7563 | 0.6650 | 0.888 | −0.4675 | 0.9156 |
| 540 | −0.8912 | −0.8112 | −0.853 | 0.1640 | 0.9364 | 750 | 0.7684 | 0.6645 | 0.892 | 0.4518 | 0.9463 |
| 545 | 0.4292 | −0.8718 | −0.876 | −0.2214 | 0.8365 | 755 | 0.7100 | 0.5949 | 0.869 | 0.2467 | 0.9679 |
| 550 | −0.9791 | −0.8907 | −0.979 | 0.5491 | 0.9579 | 760 | 0.5872 | 0.6635 | 0.905 | 0.4134 | 0.9943 |
| 555 | −0.9588 | −0.8443 | −0.966 | 0.7447 | 0.9450 | 765 | 0.5850 | 0.6597 | 0.915 | −0.2791 | 0.9550 |
| 560 | −0.9466 | −0.6750 | −0.965 | 0.8990 | 0.9645 | 770 | 0.6833 | 0.5309 | 0.851 | −0.3695 | 0.9695 |
| 565 | −0.9471 | −0.4338 | −0.956 | 0.8314 | 0.9611 | 775 | 0.4832 | 0.6422 | 0.899 | 0.3983 | 0.9546 |
| 570 | −0.9494 | −0.0767 | −0.959 | 0.8084 | 0.9475 | 780 | 0.8649 | 0.5883 | 0.886 | −0.8028 | 0.8796 |
| 575 | −0.9551 | 0.0982 | −0.962 | 0.6888 | 0.9112 | 785 | 0.3836 | 0.6006 | 0.859 | 0.2559 | 0.9112 |
| 580 | −0.9600 | 0.2341 | −0.963 | 0.6291 | 0.9254 | 790 | 0.4205 | 0.5057 | 0.866 | 0.5779 | 0.8166 |
| 585 | −0.9664 | 0.3463 | −0.968 | 0.6232 | 0.9296 | 795 | 0.3400 | 0.4824 | 0.859 | −0.1861 | 0.8161 |
| 590 | −0.9623 | 0.4133 | −0.966 | 0.7037 | 0.9169 | 800 | 0.1590 | 0.5360 | 0.845 | 0.1647 | 0.8140 |
| 595 | −0.9590 | 0.4721 | −0.969 | 0.6613 | 0.9338 |
| Wine | Red Wine 1 | Red Wine 2 | Red Wine 3 | White Wine 1 | White Wine 2 |
|---|---|---|---|---|---|
| Variety | Tinta de Toro | Mandón | Cabernet Sauvignon | Sauvignon blanc | Verdejo |
| Vintage | 2021 | 2021 | 2016 | 2021 | 2019 |
| Type * | - | - | 6 | - | + |
| pH | 3.60 ± 0.0 | 3.30 ± 0.0 | 3.25 ± 0.0 | 3.10 ± 0.0 | 3.24 ± 0.0 |
| TA (g/L) | 5.4 ± 0.1 | 5.0 ± 0.0 | 4.8 ± 0.0 | 5.4 ± 0.0 | 5.3 ± 0.0 |
| CI | 13.62 ± 0.02 | 7.69 ± 0.01 | 11.25 ± 0.03 | 0.33 ± 0.01 | 0.63 ± 0.02 |
| %420 | 36 | 34 | 41 | 100 | 100 |
| %520 | 51 | 52 | 46 | - | - |
| %620 | 13 | 14 | 13 | - | - |
| AS (%vol) | 14.51 ± 0.01 | 10.51 ± 0.01 | 13.51 ± 0.01 | 13.01 ± 0.01 | 13.51 ± 0.01 |
| F–SO2 (mg/L) | 1 ± 2.1 | 1 ± 2.1 | 1 ± 2.1 | 17 ± 2.1 | 1.00 ± 2.1 |
| T–SO2 (mg/L) | 2 ± 3.5 | 25 ± 3.5 | 2 ± 3.5 | 152 ± 26.1 | 94.28 ± 3.5 |
| TPI | 60.5 ± 0.00 | 33.9 ± 0.28 | 65.53 ± 0.11 | 7.43 ± 0.67 | 7.43 ± 0.00 |
| ACY (mg/L) | 447 ± 11.9 | 197 ± 5.9 | 74 ± 6.9 | - | - |
| TP (mg/L) | 603 ± 4.2 | 468 ± 14.3 | 670 ± 101.5 | 88 ± 16.4 | 89 ± 2.1 |
| LPP (mg/L) | 397 ± 8.4 | 82 ± 3.4 | 373 ± 11.8 | 3 ± 0.5 | 6 ± 0.0 |
| HPP (mg/L) | 206 ± 4.2 | 387 ± 10.9 | 297 ± 89.7 | 85 ± 16.0 | 83 ± 2.1 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Carrasco-Quiroz, M.; Martínez-Gil, A.M.; Nevares, I.; del Alamo-Sanza, M. New System for Simultaneous Measurement of Oxygen Consumption and Changes in Wine Color. Molecules 2024, 29, 231. https://doi.org/10.3390/molecules29010231
Carrasco-Quiroz M, Martínez-Gil AM, Nevares I, del Alamo-Sanza M. New System for Simultaneous Measurement of Oxygen Consumption and Changes in Wine Color. Molecules. 2024; 29(1):231. https://doi.org/10.3390/molecules29010231
Chicago/Turabian StyleCarrasco-Quiroz, Marioli, Ana María Martínez-Gil, Ignacio Nevares, and Maria del Alamo-Sanza. 2024. "New System for Simultaneous Measurement of Oxygen Consumption and Changes in Wine Color" Molecules 29, no. 1: 231. https://doi.org/10.3390/molecules29010231
APA StyleCarrasco-Quiroz, M., Martínez-Gil, A. M., Nevares, I., & del Alamo-Sanza, M. (2024). New System for Simultaneous Measurement of Oxygen Consumption and Changes in Wine Color. Molecules, 29(1), 231. https://doi.org/10.3390/molecules29010231










