Fe2O3 Embedded in N-Doped Porous Carbon Derived from Hemin Loaded on Active Carbon for Supercapacitors
Abstract
:1. Introduction
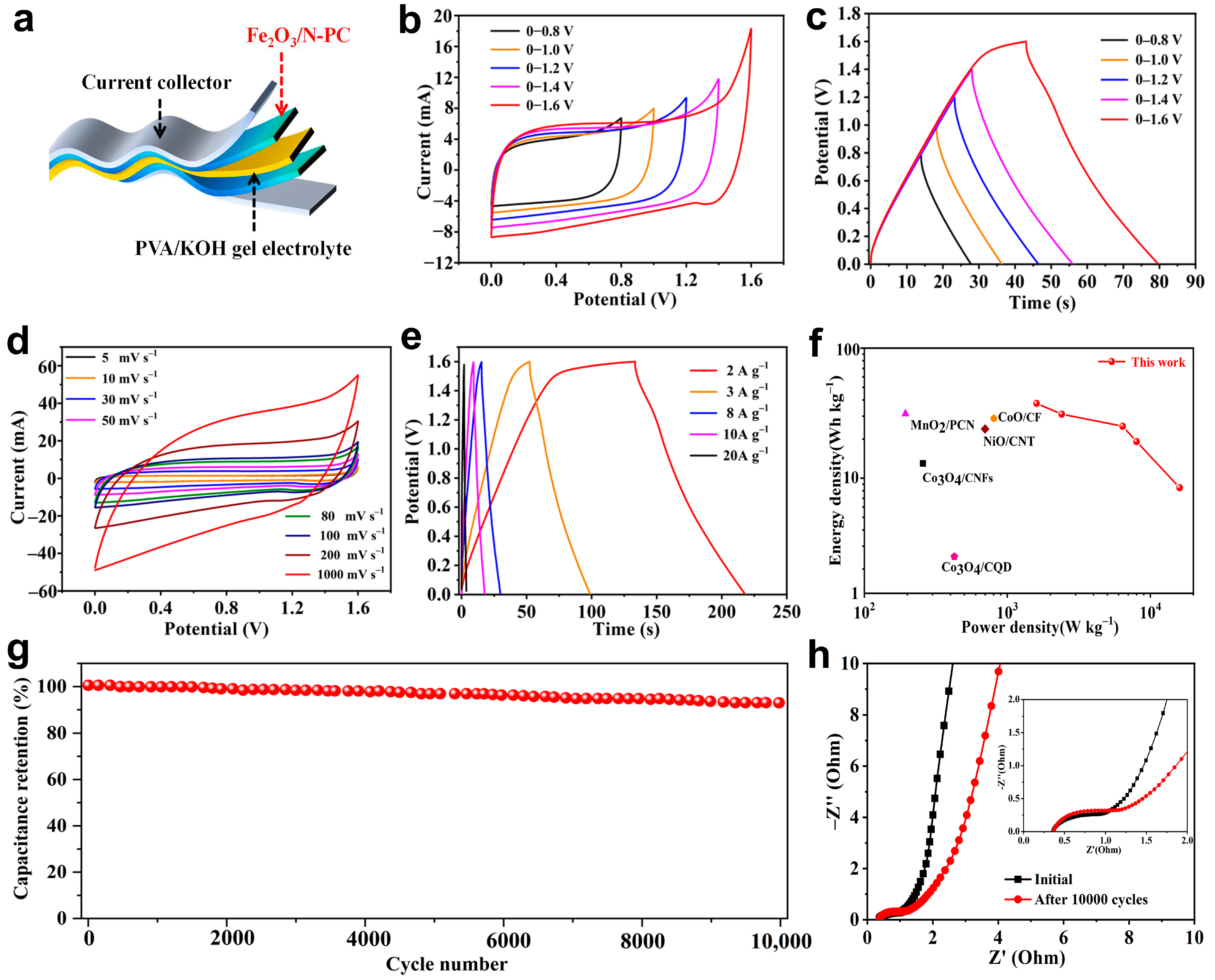
2. Results and Discussion
3. Experimental Section
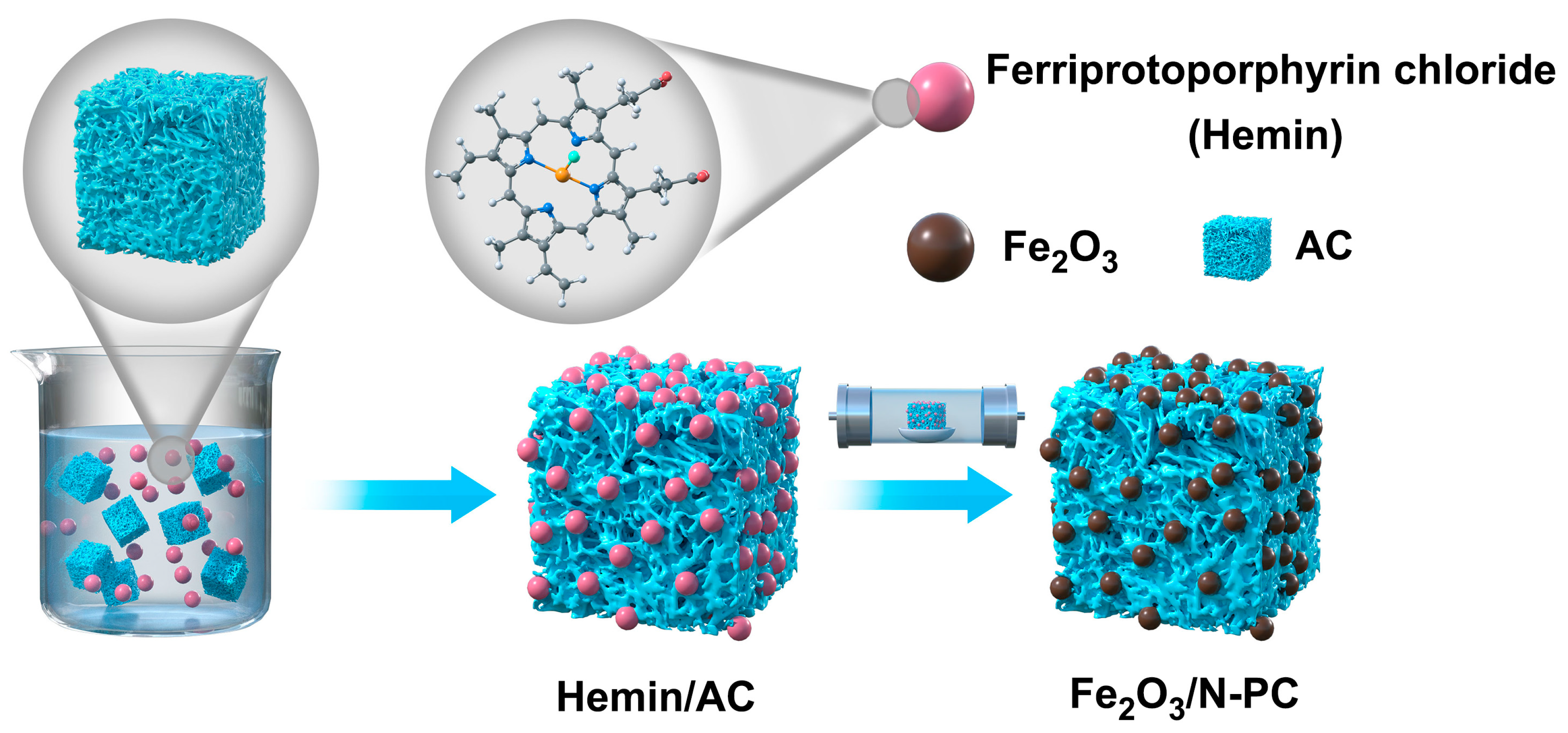
3.1. Preparation of Fe2O3 Embedded in N-Doped Porous Carbon (Fe2O3/N-PC)
3.2. Preparation of the Fe2O3/N-PC Electrode
3.3. Fabrication of Symmetric SCs
3.4. Characterization
3.5. Electrochemical Characterization
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Fu, M.; Chen, W.; Lei, Y.; Yu, H.; Lin, Y.X.; Terrones, M. Biomimetic Construction of Ferrite Quantum Dot/Graphene Heterostructure for Enhancing Ion/Charge Transfer in Supercapacitors. Adv. Mater. 2023, 35, 2300940. [Google Scholar] [CrossRef] [PubMed]
- Mansuer, M.; Miao, L.; Qin, Y.; Song, Z.Y.; Zhu, D.Z.; Duan, H.; Lv, Y.K.; Li, L.C.; Liu, M.X.; Gan, L.H. Trapping precursor-level functionalities in hierarchically porous carbons prepared by a pre-stabilization route for superior supercapacitors. Chin. Chem. Lett. 2023, 34, 107304. [Google Scholar] [CrossRef]
- Zhang, X.H.; Han, R.Y.; Liu, Y.Z.; Li, H.X.; Shi, W.J.; Yan, X.Y.; Zhao, X.X.; Li, Y.F.; Liu, B.S. Porous and graphitic structure optimization of biomass-based carbon materials from 0D to 3D for supercapacitors: A review. Chem. Eng. J. 2023, 460, 141607. [Google Scholar] [CrossRef]
- Huang, J.; Xie, Y.P.; You, Y.; Yuan, J.L.; Xu, Q.Q.; Xie, H.B.; Chen, Y.W. Rational Design of Electrode Materials for Advanced Supercapacitors: From Lab Research to Commercialization. Adv. Funct. Mater. 2023, 33, 2213095. [Google Scholar] [CrossRef]
- Deng, W.N.; Li, Y.H.; Xu, D.F.; Zhou, W.; Xiang, K.X.; Chen, H. Three-dimensional hierarchically porous nitrogen-doped carbon from water hyacinth as selenium host for high-performance lithium-selenium batteries. Rare Met. 2022, 41, 3432–3445. [Google Scholar] [CrossRef]
- Yang, Z.T.; Gao, H.Y.; Jia, Y.L.; Wu, A.; Zhao, C.Z.; Dong, Z.H.; Yu, J.G.; Zhao, Y.N.; Xie, H.J. Tetrakis(4-sulphophenyl) porphyrin cross-linked polypyrrole network with enhanced bulk conductivity and polysulfide regulation for improved Li-S battery performances. Chem. Eng. J. 2022, 447, 137428. [Google Scholar] [CrossRef]
- Deng, W.N.; Xu, Y.X.; Zhang, X.C.; Li, C.Y.; Liu, Y.X.; Xiang, K.X.; Chen, H. (NH4)2Co2V10O28·16H2O/(NH4)2V10O25·8H2O heterostructure as cathode for high-performance aqueous Zn-ion batteries. J. Alloys Compd. 2022, 903, 163824. [Google Scholar] [CrossRef]
- Wen, X.Y.; Luo, J.H.; Xiang, K.X.; Zhou, W.; Zhang, C.F.; Chen, H. High-performance monoclinic WO3 nanospheres with the novel NH4+diffusion behaviors for aqueous ammonium-ion batteries. Chem. Eng. J. 2023, 458, 141381. [Google Scholar] [CrossRef]
- Wan, L.; Chen, J.; Zhang, Y.; Du, C.; Xie, M.J.; Hu, S.X. High-mass-loading cobalt iron phosphide@nickel vanadium layered double hydroxide heterogeneous nanosheet arrays for hybrid supercapacitors. J. Colloid Interf. Sci. 2024, 654, 539–549. [Google Scholar] [CrossRef]
- Abdillah, O.B.; Bin Rus, Y.; Ulfa, M.; Dedi; Iskandar, F. Recent progress on reduced graphene oxide and polypyrrole composites for high performance supercapacitors: A review. J. Energy Storage 2023, 74, 109300. [Google Scholar] [CrossRef]
- Jia, X.T.; Du, C.K.; Qin, J.T.; Liu, C.S.; Su, Y.X.; Pang, Z.M.; Chen, W.Q.; Zhang, G.Y.; Li, G.P.; Cheng, C.W.; et al. Chemically bubbled 3D N-doped honeycomb-like carbon nanocage@carbon nanotube-based monolithic electrode for supercapacitor and lithium ion battery. Appl. Surf. Sci. 2023, 612, 155878. [Google Scholar] [CrossRef]
- Zhang, R.J.; Zhang, W.; Yang, Q.; Dong, J.D.; Ma, L.A.; Jiang, Z.X.; Huang, Y.D. 3D hierarchical oxygen-deficient AlCoNi-(oxy)hydroxides/N-doped carbon hybrids enable efficient battery-type asymmetric supercapacitor. J. Energy Chem. 2022, 72, 416–423. [Google Scholar] [CrossRef]
- Choi, J.; Lim, J.; Kim, D.; Park, S.; Yan, B.; Ko, D.; Cho, Y.; Lee, L.Y.S.; Piao, Y. CoFe Prussian blue analogues on 3D porous N-doped carbon nanosheets boost the intercalation kinetics for a high-performance quasi-solid-state hybrid capacitor. J. Mater. Chem. A 2022, 10, 14501–14512. [Google Scholar] [CrossRef]
- Tong, H.; Yue, S.H.; Jin, F.Q.; Lu, L.; Meng, Q.; Zhang, X.G. Honeycomb-like NiCo2O4@Ni(OH)2 supported on 3D N -doped graphene/carbon nanotubes sponge as an high performance electrode for Supercapacitor. Ceram. Int. 2018, 44, 3113–3121. [Google Scholar] [CrossRef]
- Liu, J.H.; He, X.; Cai, J.Y.; Zhou, J.; Liu, B.S.; Zhang, S.H.; Sun, Z.J.; Su, P.P.; Qu, D.Z.; Li, Y.D. 3D Porous VOx/N-Doped Carbon Nanosheet Hybrids Derived from Cross-Linked Dicyandiamide-Chitosan Hydrogels for Superior Supercapacitor Electrode Materials. Polymers 2023, 15, 3565. [Google Scholar] [CrossRef]
- Guan, C.; Zhao, W.; Hu, Y.T.; Lai, Z.C.; Li, X.; Sun, S.J.; Zhang, H.; Cheetham, A.K.; Wang, J. Cobalt oxide and N-doped carbon nanosheets derived from a single two-dimensional metal-organic framework precursor and their application in flexible asymmetric supercapacitors. Nanoscale Horiz. 2017, 2, 99–105. [Google Scholar] [CrossRef]
- Chen, Y.C.; Kang, C.X.; Ma, L.; Fu, L.K.; Li, G.H.; Hu, Q.; Liu, Q.M. MOF-derived Fe2O3 decorated with MnO2 nanosheet arrays as anode for high energy density hybrid supercapacitor. Chem. Eng. J. 2021, 417, 129243. [Google Scholar] [CrossRef]
- Wang, Y.; Du, Z.X.; Xiao, J.F.; Cen, W.L.; Yuan, S.J. Polypyrrole-encapsulated Fe2O3 nanotube arrays on a carbon cloth support: Achieving synergistic effect for enhanced supercapacitor performance. Electrochim. Acta 2021, 386, 138486. [Google Scholar] [CrossRef]
- Mummoorthi, G.; Arjunan, S.; Selvaraj, M.; Rokhum, S.L.; Mani, N.; Periyasamy, S.; Rajendran, R. High-performance solid-state asymmetric Supercapacitor based on a-Fe2O3/r-GO/GCN composite electrode material for energy storage application. Surf. Interfaces 2023, 41, 103166. [Google Scholar] [CrossRef]
- Qiao, Y.; Jiang, S.B.; Xia, R.; Xiao, Y.C.; Zhu, Z.X.; Liu, J.; Wang, A.C.; Gao, M.Z. High-Performance Lithium-Ion Capacitors Based on 3D Interconnected Nitrogen-Doped Ultrathin Carbon Frameworks with Encapsulated Fe2O3 Nanoparticles. Acs Appl. Energy Mater. 2022, 5, 4329–4339. [Google Scholar] [CrossRef]
- Wu, C.X.; Zhang, Z.F.; Chen, Z.H.; Jiang, Z.M.; Li, H.Y.; Cao, H.J.; Liu, Y.S.; Zhu, Y.Y.; Fang, Z.B.; Yu, X.R. Rational design of novel ultra-small amorphous Fe2O3 nanodots/graphene heterostructures for all-solid-state asymmetric supercapacitors. Nano Res. 2021, 14, 953–960. [Google Scholar] [CrossRef]
- Zhang, Y.T.; Zhang, K.B.; Ren, S.Z.; Jia, K.L.; Dang, Y.Q.; Liu, G.Y.; Li, K.K.; Long, X.Y.; Qiu, J.S. 3D nanoflower-like composite anode of α-Fe2O3/coal-based graphene for lithium-ion batteries. J. Alloys Compd. 2019, 792, 828–834. [Google Scholar] [CrossRef]
- Ganguly, D.; Ramaprabhu, S. Facile synthesis and electrochemical properties of α-Fe2O3 nanoparticles/ etched carbon nanotube composites as anode for lithium-ion batteries. Mater. Chem. Phys. 2021, 267, 124664. [Google Scholar] [CrossRef]
- Liu, L.; Lang, J.W.; Zhang, P.; Hu, B.; Yan, X.B. Facile Synthesis of Fe2O3 Nano-Dots@Nitrogen-Doped Graphene for Supercapacitor Electrode with Ultralong Cycle Life in KOH Electrolyte. ACS Appl. Mater. Interfaces 2016, 8, 9335–9344. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.D.; Chu, L.; Zhang, C.H.; Ni, P.J.; Jiang, Y.Y.; Wang, B.; Lu, Y.Z.; Chen, C.X. Hemin-assisted synthesis of peroxidase-like Fe-N-C nanozymes for detection of ascorbic acid-generating bio-enzymes. Chem. Eng. J. 2021, 415, 128876. [Google Scholar] [CrossRef]
- Deng, Q.; Zhou, W.B.; Wang, H.R.; Fu, N.; Wu, X.W.; Wu, Y.P. Aspergillus Niger Derived Wrinkle-Like Carbon as Superior Electrode for Advanced Vanadium Redox Flow Batteries. Adv. Sci. 2023, 10, 2300640. [Google Scholar] [CrossRef] [PubMed]
- Sun, X.; Yan, X.; Su, H.J.; Sun, L.B.; Zhao, L.J.; Shi, J.J.; Wang, Z.F.; Niu, J.R.; Qian, H.L.; Duan, E.R. Non-Stacked γ-Fe2O3/C@TiO2 Double-Layer Hollow Nanoparticles for Enhanced Photocatalytic Applications under Visible Light. Nanomaterials 2022, 12, 201. [Google Scholar] [CrossRef]
- Musa, A.; Mostafa, E.M.; Bukhari, S.N.A.; Alotaibi, N.H.; El-Ghorab, A.H.; Farouk, A.; Nayl, A.A.; Ghoneim, M.M.; Abdelgawad, M.A. EGFR and COX-2 Dual Inhibitor: The Design, Synthesis, and Biological Evaluation of Novel Chalcones. Molecules 2022, 27, 1158. [Google Scholar] [CrossRef]
- Yazdely, T.M.; Ghorbanloo, M.; Hosseini-Monfared, H. Polymeric ionic liquid material-anchored Mn-porphyrin anion: Heterogeneous catalyst for aerobic oxidation of olefins. Appl. Organomet. Chem. 2018, 32, e4388. [Google Scholar] [CrossRef]
- Wang, X.X.; Wang, B.; Zhong, J.; Zhao, F.P.; Han, N.; Huang, W.J.; Zeng, M.; Fan, J.; Li, Y.G. Iron polyphthalocyanine sheathed multiwalled carbon nanotubes: A high-performance electrocatalyst for oxygen reduction reaction. Nano Res. 2016, 9, 1497–1506. [Google Scholar] [CrossRef]
- Xiao, Z.B.; Li, L.Y.; Tang, Y.J.; Cheng, Z.B.; Pan, H.; Tian, D.X.; Wang, R.H. Covalent organic frameworks with lithiophilic and sulfiphilic dual linkages for cooperative affinity to polysulfides in lithium-sulfur batteries. Energy Storage Mater. 2018, 12, 252–259. [Google Scholar] [CrossRef]
- Shen, H.; Gele, A. Facile synthesis of N-doped lignin-based carbon nanofibers decorated with iron oxides for flexible supercapacitor electrodes. Inorg. Chem. Commun. 2021, 128, 108607. [Google Scholar] [CrossRef]
- Song, L.Q.; Shi, J.J.; Lu, J.; Lu, C. Structure observation of graphene quantum dots by single-layered formation in layered confinement space. Chem. Sci. 2015, 6, 4846–4850. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.Y.; Feng, X.G.; Wang, Y.J.; Shan, W.J.; Lou, Z.N.; Xiong, Y. In situ anchor of Na2Ti3O7 in nitrogen-rich carbon hollow red blood cell-like structure as a 0D-3D hierarchical electrode material for efficient electrochemical desalination. Chem. Sci. 2022, 13, 4545–4554. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Yang, Z.B.; Zhang, Z.H.; He, M. Corrosion-Engineered Morphology and Crystal Structure Regulation toward Fe-Based Efficient Oxygen Evolution Electrodes. Nanomaterials 2022, 12, 1975. [Google Scholar] [CrossRef] [PubMed]
- Wang, F.F.; Zhang, N.; Zhao, X.D.; Wang, L.X.; Zhang, J.; Wang, T.S.; Liu, F.F.; Liu, Y.C.; Fan, L.Z. Realizing a High-Performance Na-Storage Cathode by Tailoring Ultrasmall Na2FePO4F Nanoparticles with Facilitated Reaction Kinetics. Adv. Sci. 2019, 6, 1900649. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.Y.; Pan, Z.H.; Li, Z.; Zhuang, H.H.; Zhao, L.; Li, Z.L.; Lei, Y.H.; Wang, G. CNT/copper hexacyanoferrate: A superior Faradic electrode for ammonium ion removal with stable performance and high capacity. Chem. Eng. J. 2023, 466, 143163. [Google Scholar] [CrossRef]
- Shen, S.; Zhai, Z.H.; Qin, J.Q.; Zhang, X.; Song, Y.J. Pyrolysis of self-assembled hemin on carbon for efficient oxygen reduction reaction. J. Porphyr. Phthalocyanines 2019, 23, 1013–1019. [Google Scholar] [CrossRef]
- Costa, A.A.; Ghesti, G.F.; de Macedo, J.L.; Braga, V.S.; Santos, M.M.; Dias, J.A.; Dias, S.C.L. Immobilization of Fe, Mn and Co tetraphenylporphyrin complexes in MCM-41 and their catalytic activity in cyclohexene oxidation reaction by hydrogen peroxide. J. Mol. Catal. A Chem. 2008, 282, 149–157. [Google Scholar] [CrossRef]
- Yuan, M.Y.; Que, H.A.; Yang, X.N.; Li, M. Nitrogen and oxygen co-doped glucose-based carbon materials with enhanced electrochemical performances as supercapacitors. Ionics 2019, 25, 4305–4314. [Google Scholar] [CrossRef]
- Zhou, M.; Yang, B.; Zhao, Y.P.; Jin, Z.H.; Li, K.; Tang, L.P.; Cai, Z.S. Facile preparation of N-doped carbon/FeOx-decorated carbon cloth for flexible symmetric solid-state supercapacitors. Cellulose 2020, 27, 1591–1601. [Google Scholar] [CrossRef]
- Xu, B.; Hou, S.S.; Cao, G.P.; Wu, F.; Yang, Y.S. Sustainable nitrogen-doped porous carbon with high surface areas prepared from gelatin for supercapacitors. J. Mater. Chem. 2012, 22, 19088–19093. [Google Scholar] [CrossRef]
- Cho, E.C.; Chang-Jian, C.W.; Lu, C.Z.; Huang, J.H.; Hsieh, T.H.; Wu, N.J.; Lee, K.C.; Hsu, S.C.; Weng, H.C. Bio-Phenolic Resin Derived Porous Carbon Materials for High-Performance Lithium-Ion Capacitor. Polymers 2022, 14, 575. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.S.; Hu, X.H.; Xiong, D.Q.; Li, B.L.; Wang, H.Q.; Li, Q.Y. Facile synthesis of bicontinuous microporous/mesoporous carbon foam with ultrahigh specific surface area for supercapacitor application. Electrochim. Acta 2016, 219, 339–349. [Google Scholar] [CrossRef]
- Luo, W.L.; Liu, Q.W.; Zhang, B.Z.; Li, J.; Li, R.D.; Li, T.X.; Sun, Z.Q.; Ma, Y. Binder-free flexible Ti3C2Tx MXene/reduced graphene oxide/carbon nanotubes film as electrode for asymmetric supercapacitor. Chem. Eng. J. 2023, 474, 145553. [Google Scholar] [CrossRef]
- Feng, M.Y.; Zhang, Y.; Zhu, X.L.; Chen, W.X.; Lu, W.Y.; Wu, G. Interface-Anchored Covalent Organic Frameworks@Amino-Modified Ti3C2Tx MXene on Nylon 6 Film for High-Performance Deformable Supercapacitors. Angew. Chem.-Int. Edit. 2023, 62, e202307195. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.K.; Niu, H.; Qin, F.F.; Guo, Z.Y.; Wang, J.S.; Ni, G.S.; Zuo, P.P.; Qu, S.J.; Shen, W.Z. MnO2 doped carbon nanosheets prepared from coal tar pitch for advanced asymmetric supercapacitor. Electrochim. Acta 2020, 354, 136667. [Google Scholar] [CrossRef]
- Zhao, Y.R.; Liu, Y.; Du, J.W.; Zhang, X.D.; Zhou, J.Y.; Li, X.P.; Wu, C.X.; Zhu, Z.R.; Xie, E.Q.; Pan, X.J. Facile synthesis of interconnected carbon network decorated with Co3O4 nanoparticles for potential supercapacitor applications. Appl. Surf. Sci. 2019, 487, 442–451. [Google Scholar] [CrossRef]
- Lai, Y.H.; Gupta, S.; Hsiao, C.H.; Lee, C.Y.; Tai, N.H. Multilayered nickel oxide/carbon nanotube composite paper electrodes for asymmetric supercapacitors. Electrochim. Acta 2020, 354, 136744. [Google Scholar] [CrossRef]
- Chen, H.; Zhu, Y.H.; Yan, X.H.; Zhang, W.J.; Zhang, M.Y.; Huang, X.P.; Pan, J.M.; Shahnavaz, Z.; Moradian, J.M. Porous CoO/carbon foam composites synthesized by solvothermal method for supercapacitor and enhanced microwave absorption applications. Diam. Relat. Mater. 2023, 138, 110270. [Google Scholar] [CrossRef]
- Prabakaran, P.; Arumugam, G.; Ramu, P.; Selvaraj, M.; Assiri, M.A.; Rokhum, S.L.; Periyasamy, S.; Arjunan, S.; Rajendran, R. Preparation and characterization of carbon quantum dots grafted Co3O4 nanocomposite for supercapacitors application. Surf. Interfaces 2023, 40, 103153. [Google Scholar] [CrossRef]
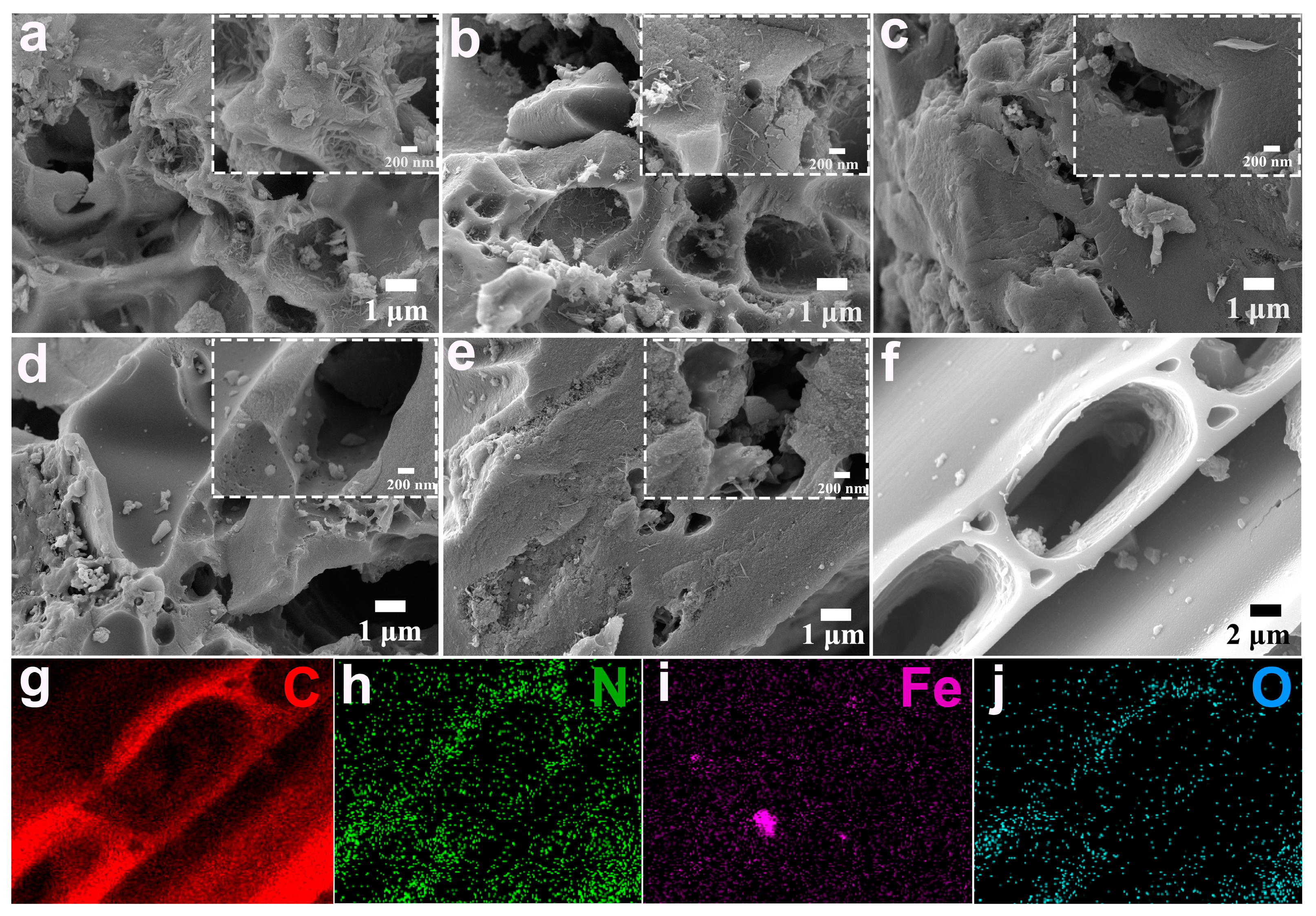


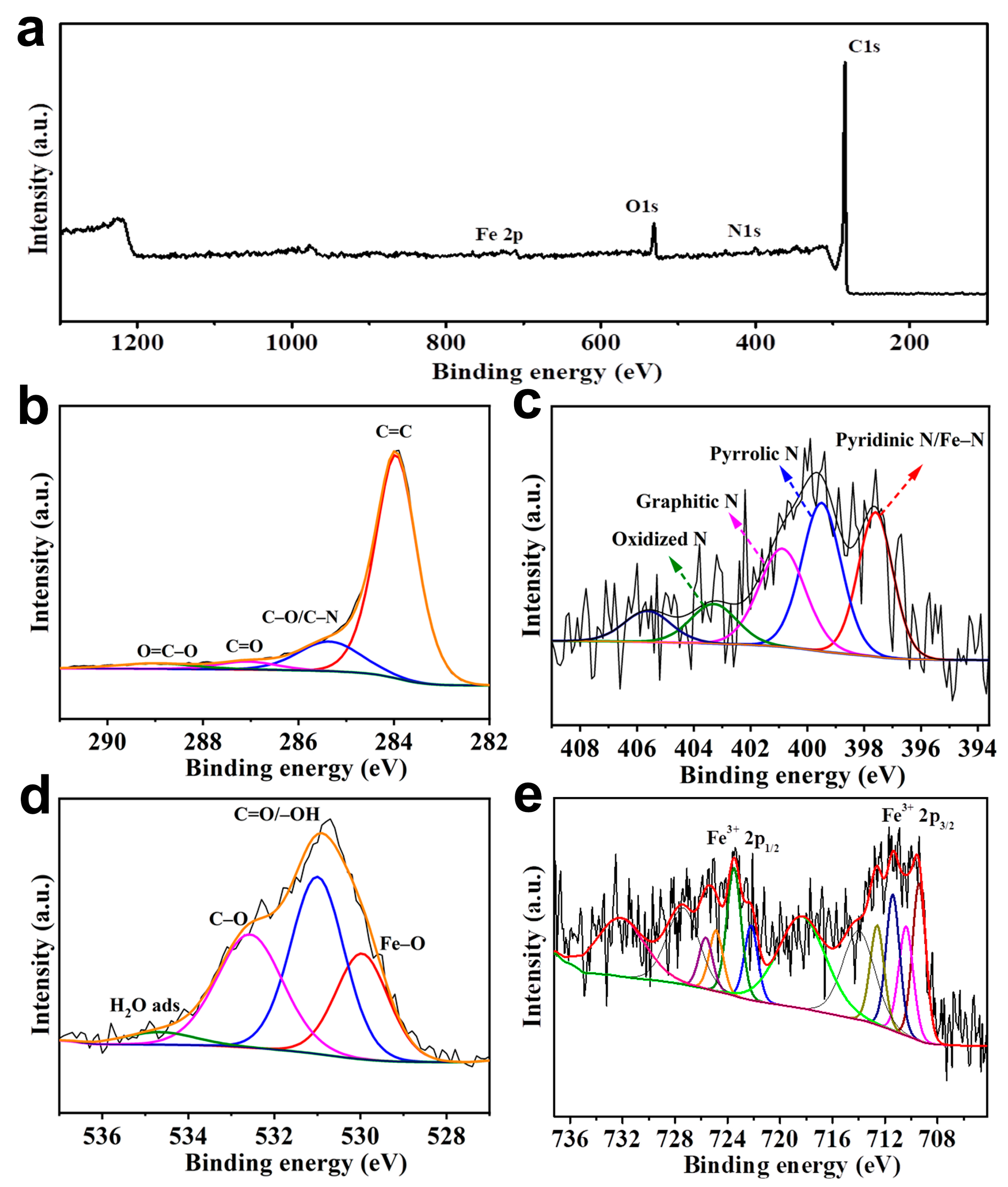

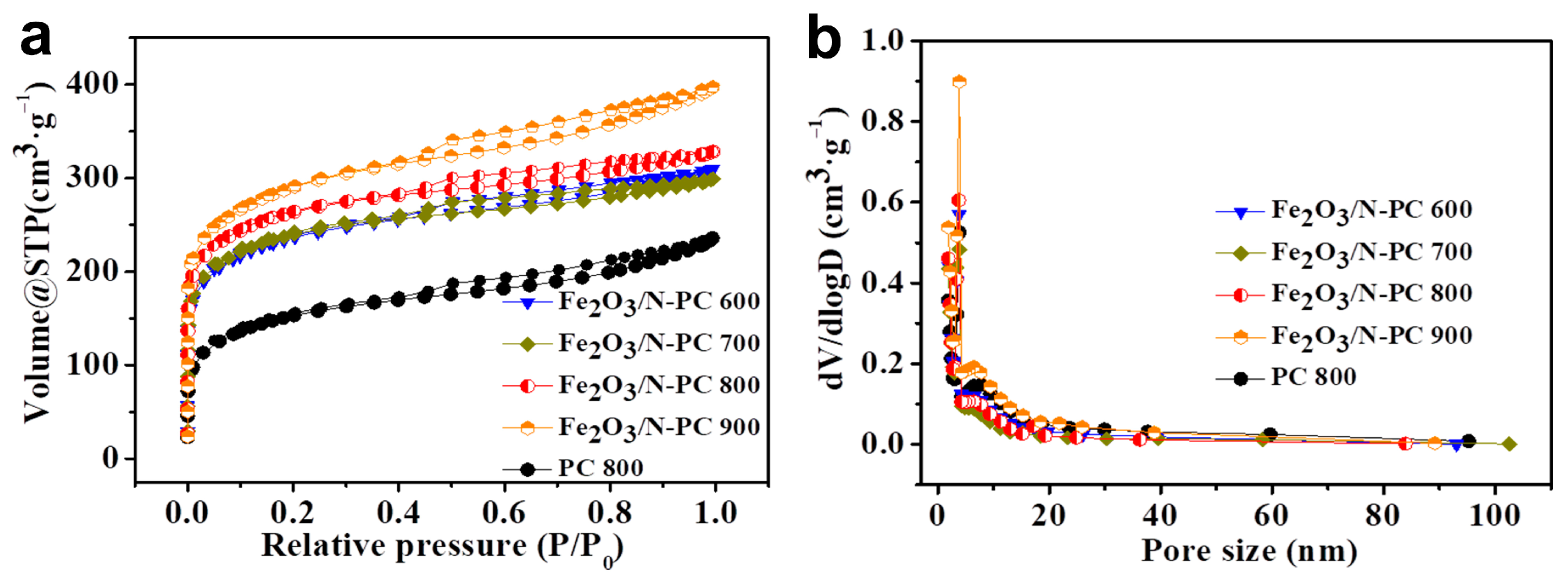
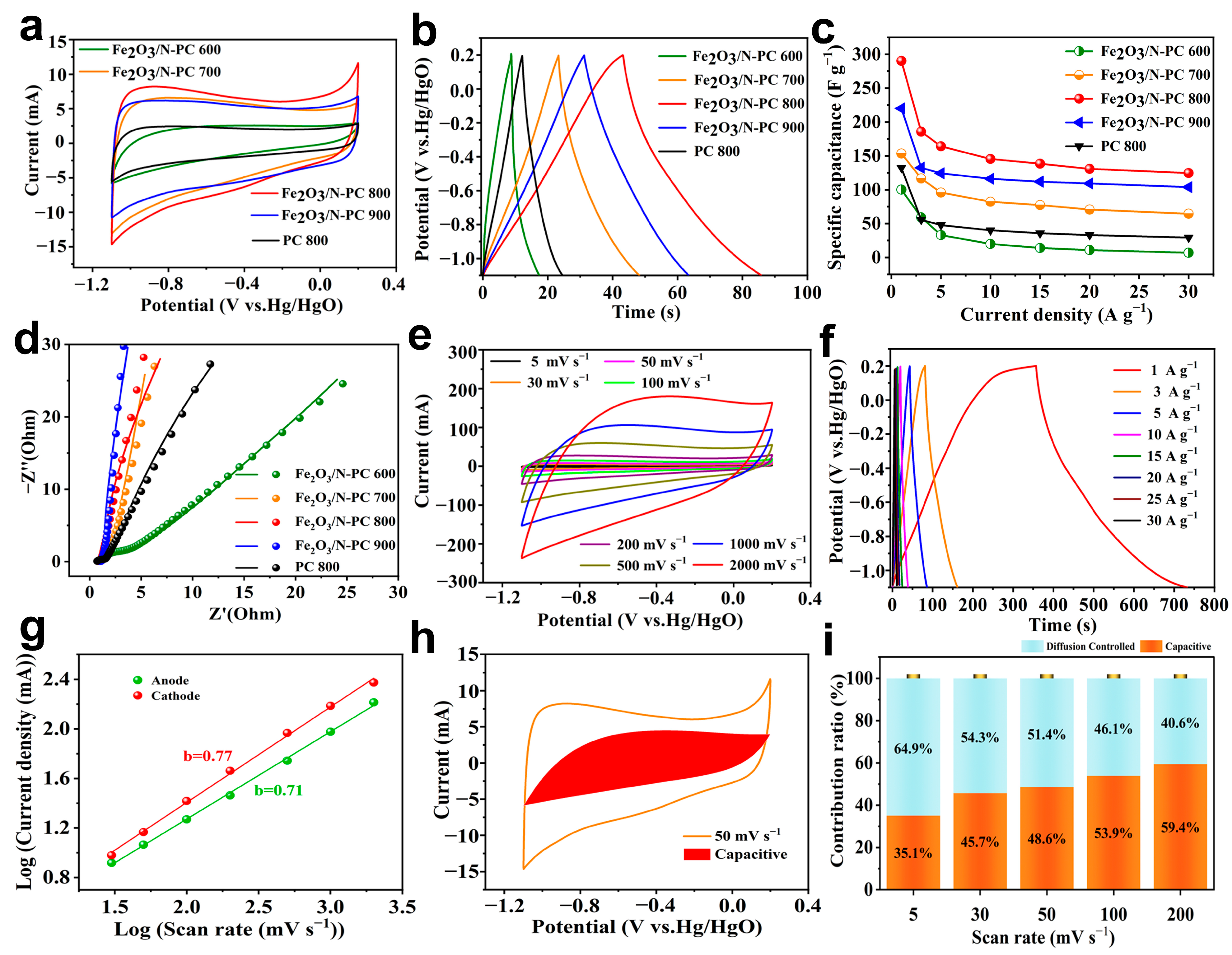
| Sample | C (at%) | N (at%) | O (at%) | Fe (at%) |
|---|---|---|---|---|
| PC 800 | 91.44 | / | 8.56 | / |
| Fe2O3/N-PC 600 | 83.33 | 2.95 | 12.85 | 0.87 |
| Fe2O3/N-PC 700 | 85.09 | 3.12 | 10.88 | 0.91 |
| Fe2O3/N-PC 800 | 87.56 | 3.56 | 7.84 | 1.04 |
| Fe2O3/N-PC 900 | 90.46 | 1.92 | 7.23 | 0.39 |
| Sample | SBET (m2 g−1) | VBET (cm3 g−1) | SBET-micro (m2 g−1) | VBET-micro (cm3 g−1) | Dave (nm) |
|---|---|---|---|---|---|
| PC 800 | 539.9 | 0.36 | 274.0 | 0.12 | 2.7 |
| Fe2O3/N-PC 600 | 852.7 | 0.48 | 542.8 | 0.23 | 2.3 |
| Fe2O3/N-PC 700 | 866.2 | 0.46 | 571.4 | 0.24 | 2.1 |
| Fe2O3/N-PC 800 | 953.3 | 0.51 | 614.5 | 0.26 | 2.1 |
| Fe2O3/N-PC 900 | 1044.5 | 0.61 | 673.5 | 0.28 | 2.4 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, Z.; Luo, C.; Wang, N.; Liu, J.; Zhang, M.; Xu, J.; Zhao, Y. Fe2O3 Embedded in N-Doped Porous Carbon Derived from Hemin Loaded on Active Carbon for Supercapacitors. Molecules 2024, 29, 146. https://doi.org/10.3390/molecules29010146
Yang Z, Luo C, Wang N, Liu J, Zhang M, Xu J, Zhao Y. Fe2O3 Embedded in N-Doped Porous Carbon Derived from Hemin Loaded on Active Carbon for Supercapacitors. Molecules. 2024; 29(1):146. https://doi.org/10.3390/molecules29010146
Chicago/Turabian StyleYang, Zitao, Cunhao Luo, Ning Wang, Junshao Liu, Menglong Zhang, Jing Xu, and Yongnan Zhao. 2024. "Fe2O3 Embedded in N-Doped Porous Carbon Derived from Hemin Loaded on Active Carbon for Supercapacitors" Molecules 29, no. 1: 146. https://doi.org/10.3390/molecules29010146
APA StyleYang, Z., Luo, C., Wang, N., Liu, J., Zhang, M., Xu, J., & Zhao, Y. (2024). Fe2O3 Embedded in N-Doped Porous Carbon Derived from Hemin Loaded on Active Carbon for Supercapacitors. Molecules, 29(1), 146. https://doi.org/10.3390/molecules29010146








