Gadolinium Effects on Liposome Fluidity and Size Depend on the Headgroup and Side Chain Structure of Key Mammalian Brain Lipids
Abstract
1. Introduction
2. Results
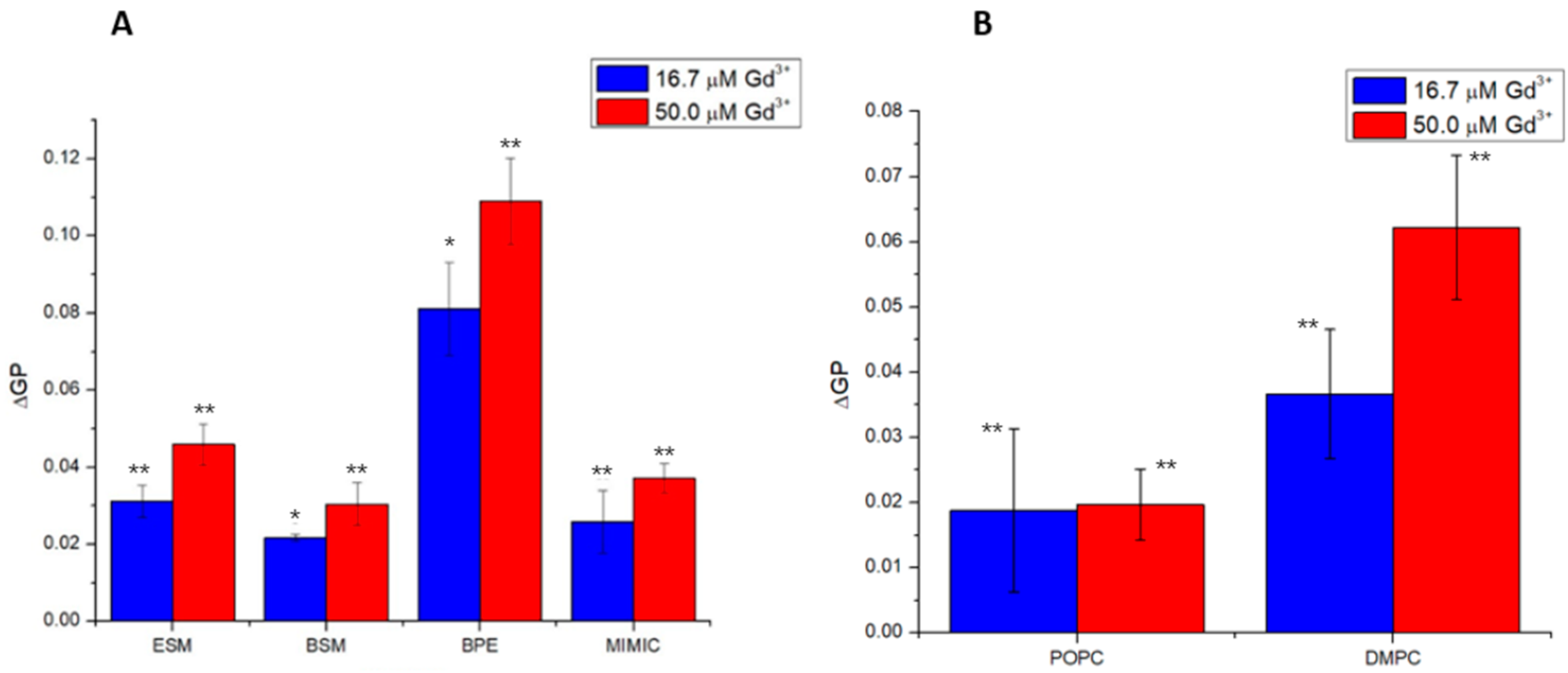
2.1. Impact of Gd3+ on the Fluidity of PC Membranes
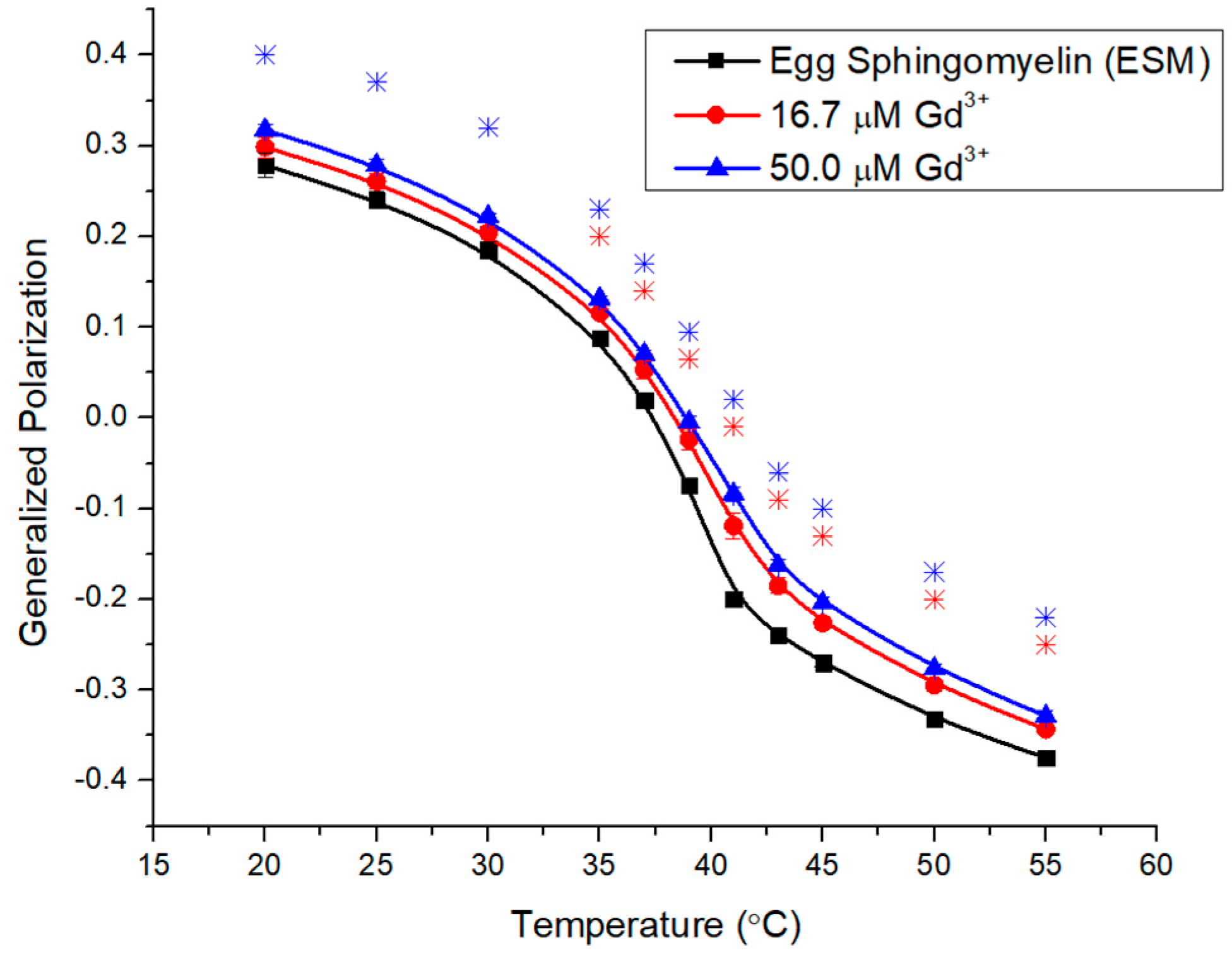
2.2. Impacts of Gd3+ on the Fluidity of SM Membranes
| System | Fatty Acid/Lipid Distribution | Percentage (%) |
|---|---|---|
| Brain sphingomyelin | Palmitic (16:0) | 2 |
| Stearic (18:0) | 50 | |
| Arachidic (20:0) | 5 | |
| Behenic (22:0) | 7 | |
| Lignoceric (24:0) | 5 | |
| Nervonic (24:1) | 21 | |
| Unknown | 10 | |
| Egg sphingomyelin | Palmitic (16:0) | 86 |
| Stearic (18:0) | 6 | |
| Behenic (22:0) | 3 | |
| Nervonic (24:1) | 3 | |
| Unknown | 2 | |
| Brain polar extract | PC | 12.6 |
| PE | 33.1 | |
| PI | 4.1 | |
| PS | 18.5 | |
| PA | 0.8 | |
| Unknown | 30.9 | |
| Myelin sheath mimic | Palmitoyl oleoyl phosphatidylcholine (POPC) | 10 |
| Ethanolamine plasmalogen | 12 | |
| Palmitoyl oleoyl phosphatidylserine (POPS) | 5 | |
| Glucosylceramide | 15 | |
| Cholesterol | 40 | |
| Brain SM | 18 |
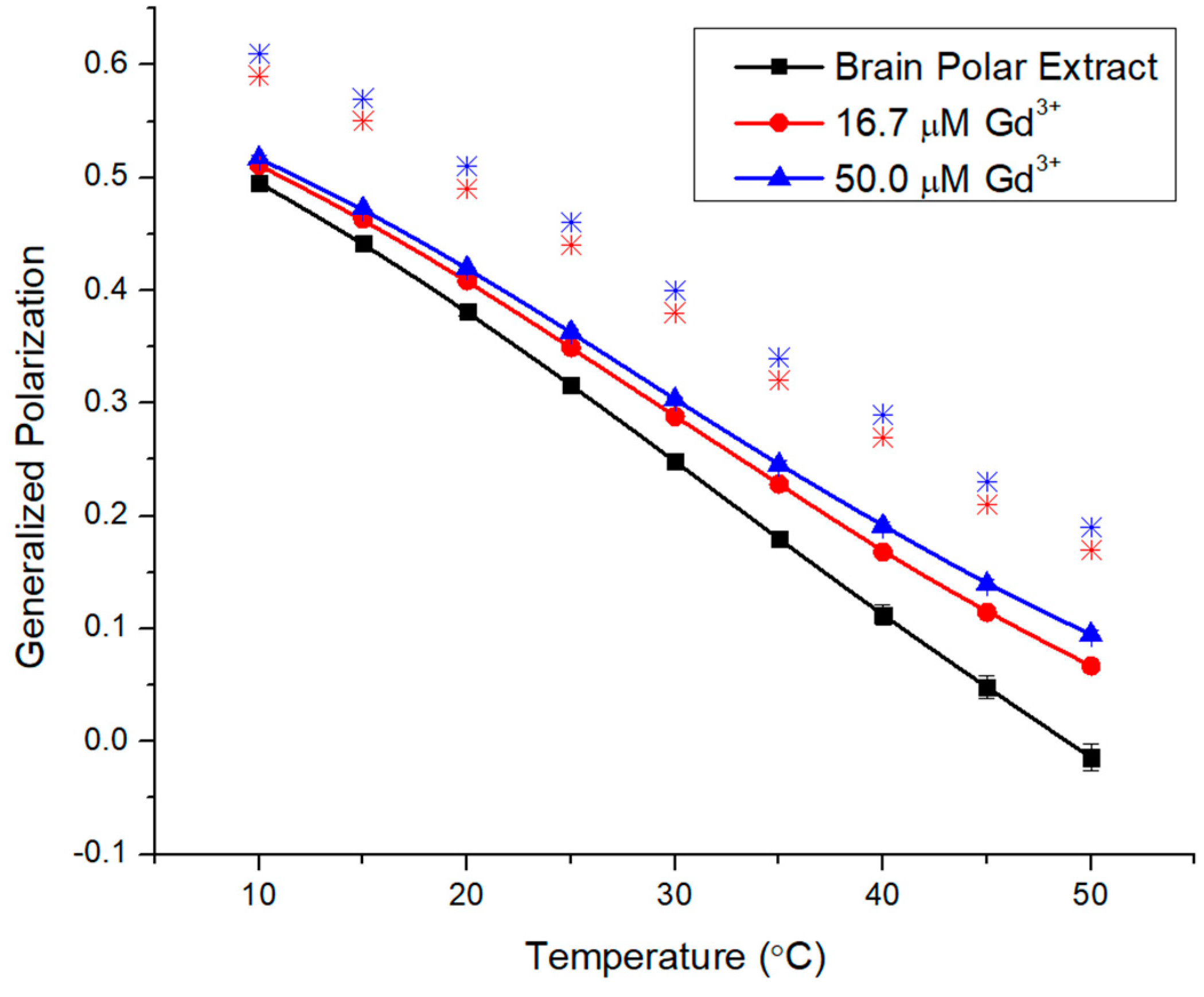
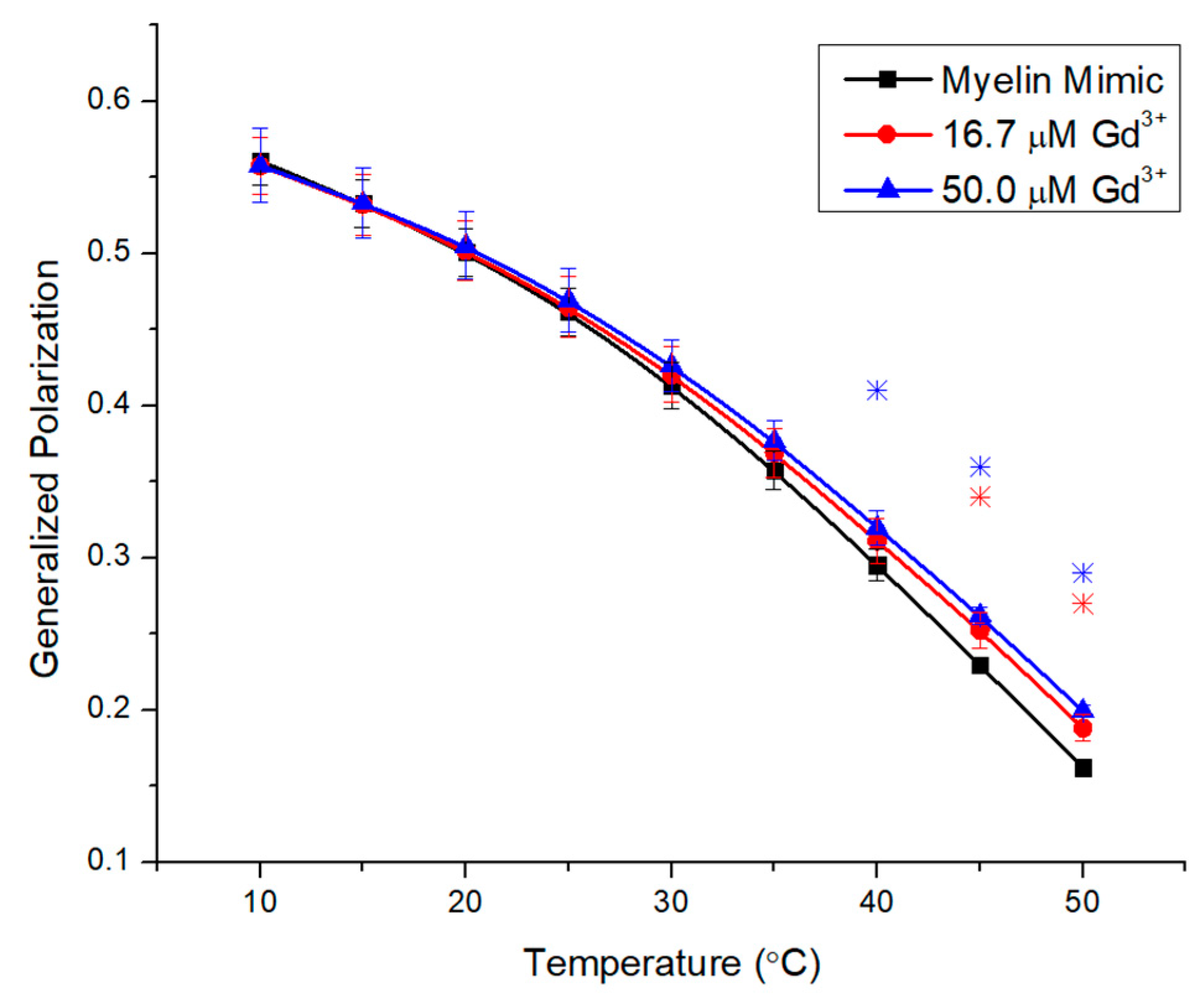
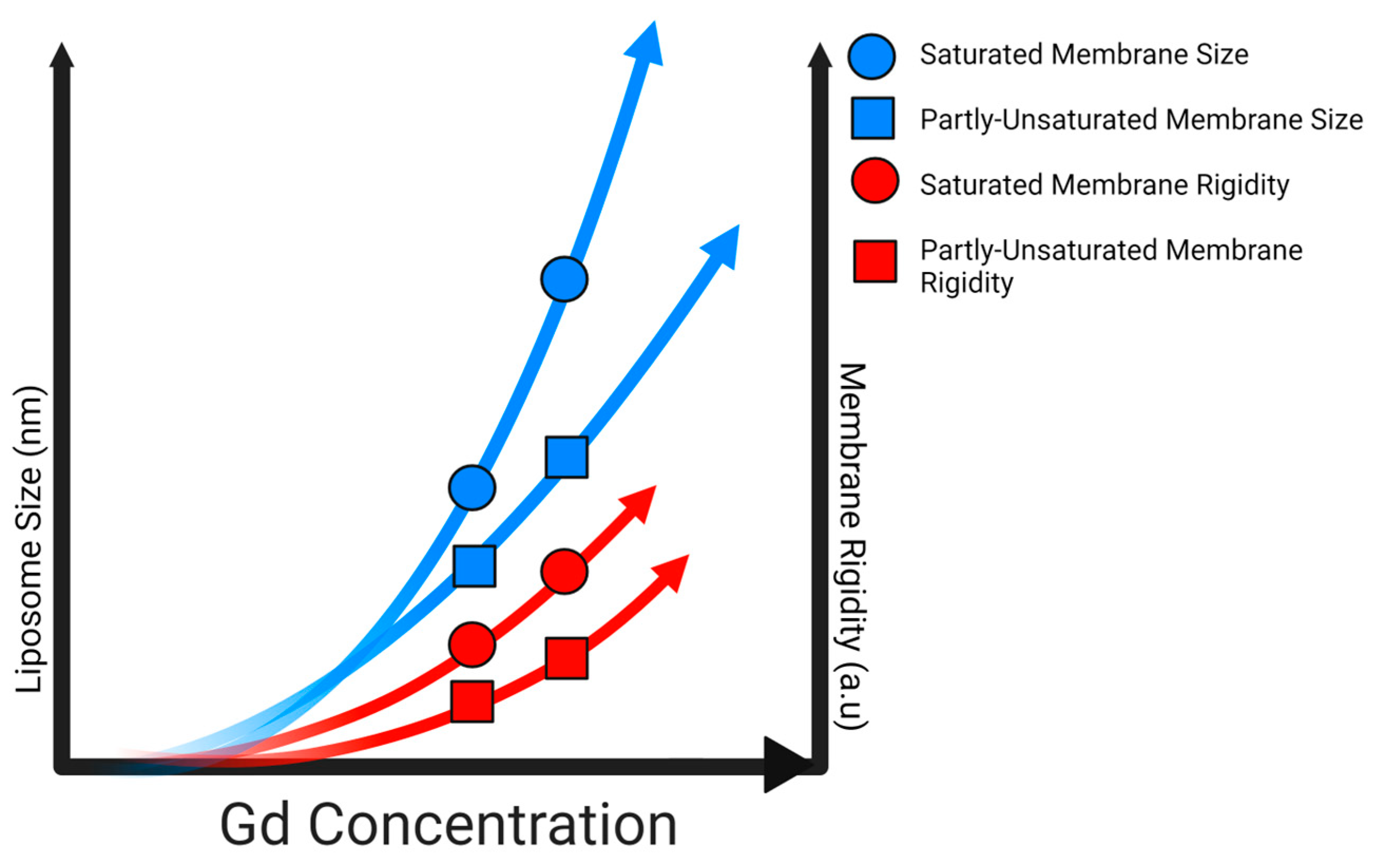
2.3. Impacts of Gd3+ on the Fluidity of Complex Membranes
2.4. Impacts of Gd3+ on Liposome Size
3. Discussion
4. Materials and Methods
4.1. Reagents
4.2. Liposome Preparation
4.3. Laurdan Generalized Polarization (Laurdan GP)
4.4. Dynamic Light Scattering
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
References
- Rogowska, J.; Olkowska, E.; Ratajczyk, W.; Wolska, L. Gadolinium as a New Emerging Contaminant of Aquatic Environments. Environ. Toxicol. Chem. 2018, 37, 1523–1534. [Google Scholar] [CrossRef] [PubMed]
- U.S. Geological Survey. Mineral Commodity Summaries 2019; U.S. Geological Survey: Reston, VA, USA, 2019. [CrossRef]
- Yang, M.; Liu, X.; Zhang, Z.; Song, Y.; Bai, L. Effect of Adding Rare Earth Elements Er and Gd on the Corrosion Residual Strength of Magnesium Alloy. Open Phys. 2019, 17, 373–380. [Google Scholar] [CrossRef]
- Binnemans, K.; Jones, P.T.; Müller, T.; Yurramendi, L. Rare Earths and the Balance Problem: How to Deal with Changing Markets? J. Sustain. Metall. 2018, 4, 126–146. [Google Scholar] [CrossRef]
- Brünjes, R.; Hofmann, T. Anthropogenic Gadolinium in Freshwater and Drinking Water Systems. Water Res. 2020, 182, 115966. [Google Scholar] [CrossRef] [PubMed]
- Unruh, C.; Van Bavel, N.; Anikovskiy, M.; Prenner, E.J. Benefits and Detriments of Gadolinium from Medical Advances to Health and Ecological Risks. Molecules 2020, 25, 5762. [Google Scholar] [CrossRef] [PubMed]
- Laing, M. Gadolinium: Central Metal of the Lanthanoids. J. Chem. Educ. 2009, 86, 188–189. [Google Scholar] [CrossRef]
- Visual MINTEQ—Visual MINTEQ—A Free Equilibrium Speciation Model. Available online: https://vminteq.lwr.kth.se/ (accessed on 7 August 2023).
- Darnall, D.W.; Birnbaum, E.R. Lanthanide Ions Activate α-Amylase. Biochemistry 1973, 12, 3489–3491. [Google Scholar] [CrossRef]
- Sherry, A.D.; Caravan, P.; Lenkinski, R.E. Primer on Gadolinium Chemistry. J. Magn. Reson. Imaging 2009, 30, 1240–1248. [Google Scholar] [CrossRef]
- Ebrahimi, P.; Barbieri, M. Gadolinium as an Emerging Microcontaminant in Water Resources: Threats and Opportunities. Geosciences 2019, 9, 93. [Google Scholar] [CrossRef]
- Rogosnitzky, M.; Branch, S. Gadolinium-Based Contrast Agent Toxicity: A Review of Known and Proposed Mechanisms. BioMetals 2016, 29, 365–376. [Google Scholar] [CrossRef]
- Kanda, T.; Fukusato, T.; Matsuda, M.; Toyoda, K.; Oba, H.; Kotoku, J.; Haruyama, T.; Kitajima, K.; Furui, S. Gadolinium-Based Contrast Agent Accumulates in the Brain Even in Subjects without Severe Renal Dysfunction: Evaluation of Autopsy Brain Specimens with Inductively Coupled Plasma Mass Spectroscopy. Radiology 2015, 276, 228–232. [Google Scholar] [CrossRef] [PubMed]
- Idée, J.M.; Fretellier, N.; Robic, C.; Corot, C. The Role of Gadolinium Chelates in the Mechanism of Nephrogenic Systemic Fibrosis: A Critical Update. Crit. Rev. Toxicol. 2014, 44, 895–913. [Google Scholar] [CrossRef] [PubMed]
- Newton, B.B.; Jimenez, S.A. Mechanism of NSF: New Evidence Challenging the Prevailing Theory. J. Magn. Reson. Imaging 2009, 30, 1277–1283. [Google Scholar] [CrossRef] [PubMed]
- Xia, Q.; Feng, X.; Huang, H.; Du, L.; Yang, X.; Wang, K. Gadolinium-Induced Oxidative Stress Triggers Endoplasmic Reticulum Stress in Rat Cortical Neurons. J. Neurochem. 2011, 117, 38–47. [Google Scholar] [CrossRef] [PubMed]
- Angeli, J.K.; Ramos, D.B.; Casali, E.A.; Souza, D.O.G.; Sarkis, J.J.F.; Stefanon, I.; Vassallo, D.V.; Fürstenau, C.R. Gadolinium Increases the Vascular Reactivity of Rat Aortic Rings. Braz. J. Med. Biol. Res. 2011, 44, 445–452. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Bau, M.; Dulski, P. Anthropogenic Origin of Positive Gadolinium Anomalies in River Waters. Earth Planet Sci. Lett. 1996, 143, 245–255. [Google Scholar] [CrossRef]
- Idée, J.M.; Port, M.; Raynal, I.; Schaefer, M.; Le Greneur, S.; Corot, C. Clinical and Biological Consequences of Transmetallation Induced by Contrast Agents for Magnetic Resonance Imaging: A Review. Fundam. Clin. Pharmacol. 2006, 20, 563–576. [Google Scholar] [CrossRef]
- Li, X.M.; Zhang, Y.F.; Ni, J.Z.; Chen, J.W.; Hwang, F. Effect of Lanthanide Ions on the Phase Behavior of Dipalmitoylphosphatidylcholine Multilamellar Liposomes. J. Inorg. Biochem. 1994, 53, 139–149. [Google Scholar] [CrossRef]
- Los, D.A.; Murata, N. Membrane Fluidity and Its Roles in the Perception of Environmental Signals. Biochim. Biophys. Acta (BBA)—Biomembr. 2004, 1666, 142–157. [Google Scholar] [CrossRef]
- Yu, W.; So, P.T.C.; French, T.; Gratton, E. Fluorescence Generalized Polarization of Cell Membranes: A Two-Photon Scanning Microscopy Approach. Biophys. J. 1996, 70, 626. [Google Scholar] [CrossRef]
- Sule, K.; Anikovskiy, M.; Prenner, E.J. Lipid Structure Determines the Differential Impact of Single Metal Additions and Binary Mixtures of Manganese, Calcium and Magnesium on Membrane Fluidity and Liposome Size. Int. J. Mol. Sci. 2023, 24, 1066. [Google Scholar] [CrossRef] [PubMed]
- O’Brien, J.S.; Sampson, E.L. Lipid Composition of the Normal Human Brain: Gray Matter, White Matter, and Myelin. J. Lipid Res. 1965, 6, 537–544. [Google Scholar] [CrossRef] [PubMed]
- Kerek, E.M.; Prenner, E.J. Inorganic Cadmium Affects the Fluidity and Size of Phospholipid Based Liposomes. Biochim. Biophys. Acta (BBA)—Biomembr. 2016, 1858, 3169–3181. [Google Scholar] [CrossRef] [PubMed]
- Leekumjorn, S.; Sum, A.K. Molecular Characterization of Gel and Liquid-Crystalline Structures of Fully Hydrated POPC and POPE Bilayers. J. Phys. Chem. B 2007, 111, 6026–6033. [Google Scholar] [CrossRef] [PubMed]
- Sule, K.; Prenner, E.J. Lipid Headgroup and Side Chain Architecture Determine Manganese-Induced Dose Dependent Membrane Rigidification and Liposome Size Increase. Eur. Biophys. J. 2022, 51, 205–223. [Google Scholar] [CrossRef] [PubMed]
- Umbsaar, J.; Kerek, E.; Prenner, E.J. Cobalt and Nickel Affect the Fluidity of Negatively-Charged Biomimetic Membranes. Chem. Phys. Lipids 2018, 210, 28–37. [Google Scholar] [CrossRef]
- Lewis, R.N.A.H.; Zhang, Y.P.; McElhaney, R.N. Calorimetric and Spectroscopic Studies of the Phase Behavior and Organization of Lipid Bilayer Model Membranes Composed of Binary Mixtures of Dimyristoylphosphatidylcholine and Dimyristoylphosphatidylglycerol. Biochim. Biophys. Acta (BBA)—Biomembr. 2005, 1668, 203–214. [Google Scholar] [CrossRef] [PubMed]
- Pokorny, A.; Yandek, L.E.; Elegbede, A.I.; Hinderliter, A.; Almeida, P.F.F. Temperature and Composition Dependence of the Interaction of Delta-Lysin with Ternary Mixtures of Sphingomyelin/Cholesterol/POPC. Biophys. J. 2006, 91, 2184–2197. [Google Scholar] [CrossRef]
- O’Brien, J.S. Stability of the myelin membrane. Science 1965, 147, 1099–1107. [Google Scholar] [CrossRef]
- Arsov, Z.; González-Ramírez, E.J.; Goñi, F.M.; Tristram-Nagle, S.; Nagle, J.F. Phase Behavior of Palmitoyl and Egg Sphingomyelin. Chem. Phys. Lipids 2018, 213, 102–110. [Google Scholar] [CrossRef]
- González-Ramírez, E.J.; Goñi, F.M.; Alonso, A. Mixing Brain Cerebrosides with Brain Ceramides, Cholesterol and Phospholipids. Sci. Rep. 2019, 9, 13326. [Google Scholar] [CrossRef] [PubMed]
- Al Osman, M.; Yang, F.; Massey, I.Y. Exposure Routes and Health Effects of Heavy Metals on Children. BioMetals 2019, 32, 563–573. [Google Scholar] [CrossRef] [PubMed]
- Yoon, J.H.; Seo, Y.; Jo, Y.S.; Lee, S.; Cho, E.; Cazenave-Gassiot, A.; Shin, Y.S.; Moon, M.H.; An, H.J.; Wenk, M.R.; et al. Brain Lipidomics: From Functional Landscape to Clinical Significance. Sci. Adv. 2022, 8, eadc9317. [Google Scholar] [CrossRef] [PubMed]
- Naudí, A.; Cabré, R.; Jové, M.; Ayala, V.; Gonzalo, H.; Portero-Otín, M.; Ferrer, I.; Pamplona, R. Lipidomics of Human Brain Aging and Alzheimer’s Disease Pathology. Int. Rev. Neurobiol. 2015, 122, 133–189. [Google Scholar] [CrossRef]
- Foglia, F.; Lawrence, M.J.; Lorenz, C.D.; McLain, S.E. On the Hydration of the Phosphocholine Headgroup in Aqueous Solution. J. Chem. Phys. 2010, 133, 145103. [Google Scholar] [CrossRef]
- Guo, F.; Friedman, J.M. Charge Density-Dependent Modifications of Hydration Shell Waters by Hofmeister Ions. J. Am. Chem. Soc. 2009, 131, 11010–11018. [Google Scholar] [CrossRef]
- Van Meer, G.; Voelker, D.R.; Feigenson, G.W. Membrane Lipids: Where They Are and How They Behave. Nat. Rev. Mol. Cell Biol. 2008, 9, 112–124. [Google Scholar] [CrossRef]
- Lange, Y.; Swaisgood, M.H.; Ramos, B.V.; Steck, T.L. Plasma Membranes Contain Half the Phospholipid and 90% of the Cholesterol and Sphingomyelin in Cultured Human Fibroblasts. J. Biol. Chem. 1989, 264, 3786–3793. [Google Scholar] [CrossRef]
- Slotte, J.P.; Ramstedt, B. The Functional Role of Sphingomyelin in Cell Membranes. Eur. J. Lipid Sci. Technol. 2007, 109, 977–981. [Google Scholar] [CrossRef]
- Sezgin, E.; Levental, I.; Mayor, S.; Eggeling, C. The Mystery of Membrane Organization: Composition, Regulation and Roles of Lipid Rafts. Nat. Rev. Mol. Cell Biol. 2017, 18, 361–374. [Google Scholar] [CrossRef]
- Slotte, J.P.; Bierman, E.L. Depletion of Plasma-Membrane Sphingomyelin Rapidly Alters the Distribution of Cholesterol between Plasma Membranes and Intracellular Cholesterol Pools in Cultured Fibroblasts. Biochem. J. 1988, 250, 653–658. [Google Scholar] [CrossRef] [PubMed]
- Ramstedt, B.; Slotte, J.P. Interaction of Cholesterol with Sphingomyelins and Acyl-Chain-Matched Phosphatidylcholines: A Comparative Study of the Effect of the Chain Length. Biophys. J. 1999, 76, 908. [Google Scholar] [CrossRef] [PubMed]
- Vázquez, R.F.; Daza Millone, M.A.; Pavinatto, F.J.; Fanani, M.L.; Oliveira, O.N.; Vela, M.E.; Maté, S.M. Impact of Sphingomyelin Acyl Chain (16:0 vs 24:1) on the Interfacial Properties of Langmuir Monolayers: A PM-IRRAS Study. Colloids Surf. B Biointerfaces 2019, 173, 549–556. [Google Scholar] [CrossRef] [PubMed]
- Prenner, E.; Honsek, G.; Hönig, D.; Möbius, D.; Lohner, K. Imaging of the Domain Organization in Sphingomyelin and Phosphatidylcholine Monolayers. Chem. Phys. Lipids 2007, 145, 106–118. [Google Scholar] [CrossRef] [PubMed]
- Petruzielo, R.S.; Heberle, F.A.; Drazba, P.; Katsaras, J.; Feigenson, G.W. Phase Behavior and Domain Size in Sphingomyelin-Containing Lipid Bilayers. Biochim. Biophys. Acta (BBA)—Biomembr. 2013, 1828, 1302–1313. [Google Scholar] [CrossRef] [PubMed]
- Mulukutla, S.; Shipley, G.G. Structure and thermotropic properties of phosphatidylethanolamine and its N-methyl derivatives. Biochemistry 1984, 23, 2514–2519. [Google Scholar] [CrossRef] [PubMed]
- Sule, K.; Umbsaar, J.; Prenner, E.J. Mechanisms of Co, Ni, and Mn Toxicity: From Exposure and Homeostasis to Their Interactions with and Impact on Lipids and Biomembranes. Biochim. Biophys. Acta Biomembr. 2020, 1862, 183250. [Google Scholar] [CrossRef] [PubMed]
- Ermakov, Y.A.; Averbakh, A.Z.; Yusipovich, A.I.; Sukharev, S. Dipole Potentials Indicate Restructuring of the Membrane Interface Induced by Gadolinium and Beryllium Ions. Biophys. J. 2001, 80, 1851. [Google Scholar] [CrossRef]
- Le, C.T.M.; Houri, A.; Balage, N.; Smith, B.J.; Mechler, A. Interaction of Small Ionic Species with Phospholipid Membranes: The Role of Metal Coordination. Front. Mater. 2019, 5, 401354. [Google Scholar] [CrossRef]
- Daear, W.; Mundle, R.; Sule, K.; Prenner, E.J. The Degree and Position of Phosphorylation Determine the Impact of Toxic and Trace Metals on Phosphoinositide Containing Model Membranes. BBA Adv. 2021, 1, 100021. [Google Scholar] [CrossRef]
- Jiménez-Rojo, N.; Riezman, H. On the Road to Unraveling the Molecular Functions of Ether Lipids. FEBS Lett. 2019, 593, 2378–2389. [Google Scholar] [CrossRef] [PubMed]
- Lohner, K.; Hermetter, A.; Paltauf, F. Phase Behavior of Ethanolamine Plasmalogen. Chem. Phys. Lipids 1984, 34, 163–170. [Google Scholar] [CrossRef]
- Waheed, Q.; Tjörnhammar, R.; Edholm, O. Phase Transitions in Coarse-Grained Lipid Bilayers Containing Cholesterol by Molecular Dynamics Simulations. Biophys. J. 2012, 103, 2125. [Google Scholar] [CrossRef] [PubMed]
- Almeida, P.F. A Simple Thermodynamic Model of the Liquid-Ordered State and the Interactions between Phospholipids and Cholesterol. Biophys. J. 2011, 100, 420. [Google Scholar] [CrossRef]
- Dietschy, J.M.; Turley, S.D. Cholesterol Metabolism in the Brain. Curr. Opin. Lipidol. 2001, 12, 105–112. [Google Scholar] [CrossRef]
- Marquardt, D.; Geier, B.; Pabst, G. Asymmetric Lipid Membranes: Towards More Realistic Model Systems. Membranes 2015, 5, 180–196. [Google Scholar] [CrossRef]
- Chanaday, N.L.; Kavalali, E.T. Time Course and Temperature Dependence of Synaptic Vesicle Endocytosis. FEBS Lett. 2018, 592, 3606–3614. [Google Scholar] [CrossRef]
- Mahadeo, M.; Furber, K.L.; Lam, S.; Coorssen, J.R.; Prenner, E.J. Secretory Vesicle Cholesterol: Correlating Lipid Domain Organization and Ca2+ Triggered Fusion. Biochim. Biophys. Acta (BBA)—Biomembr. 2015, 1848, 1165–1174. [Google Scholar] [CrossRef]
- Parasassi, T.; Krasnowska, E.K.; Bagatolli, L.; Gratton, E. Laurdan and Prodan as Polarity-Sensitive Fluorescent Membrane Probes. J. Fluoresc. 1998, 8, 365–373. [Google Scholar] [CrossRef]
- Ames, B.N. [10] Assay of Inorganic Phosphate, Total Phosphate and Phosphatases. Methods Enzym. 1966, 8, 115–118. [Google Scholar] [CrossRef]
- Sanchez, S.A.; Tricerri, M.A.; Gunther, G.; Gratton, E. Laurdan Generalized Polarization: From Cuvette to Microscope. Mod. Res. Educ. Top. Microsc. 2007, 2, 1007–1014. [Google Scholar]
- Hassanin, M.; Kerek, E.; Chiu, M.; Anikovskiy, M.; Prenner, E.J. Binding Affinity of Inorganic Mercury and Cadmium to Biomimetic Erythrocyte Membranes. J. Phys. Chem. B 2016, 120, 12872–12882. [Google Scholar] [CrossRef]
- Kanda, T.; Nakai, Y.; Oba, H.; Toyoda, K.; Kitajima, K.; Furui, S. Gadolinium Deposition in the Brain. Magn. Reson. Imaging 2016, 34, 1346–1350. [Google Scholar] [CrossRef]
- Puchkov, D.; Haucke, V. Greasing the Synaptic Vesicle Cycle by Membrane Lipids. Trends Cell Biol. 2013, 23, 493–503. [Google Scholar] [CrossRef]
- Postila, P.A.; Róg, T. A Perspective: Active Role of Lipids in Neurotransmitter Dynamics. Mol. Neurobiol. 2019, 57, 910–925. [Google Scholar] [CrossRef]




| System | Δ Size (nm) (16.7 µM Gd) | Δ Size (nm) (50.0 µM Gd) |
|---|---|---|
| POPC | 14.2 ± 1.3 | 17.5 ± 2.8 |
| DMPC | 17.3 ± 2.1 | 56.3 ± 12.4 |
| BPE | 39.6 ± 15.9 | 119.7 ± 18.1 |
| ESM | −2.9 ± 3.7 | 1113.1 ± 108.4 |
| BSM | 87.9 ± 11.4 | 80.5 ± 7.6 |
| MM | 25.9 ± 14.4 | ± 18.8 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Farzi, K.; Issler, T.; Unruh, C.; Prenner, E.J. Gadolinium Effects on Liposome Fluidity and Size Depend on the Headgroup and Side Chain Structure of Key Mammalian Brain Lipids. Molecules 2024, 29, 135. https://doi.org/10.3390/molecules29010135
Farzi K, Issler T, Unruh C, Prenner EJ. Gadolinium Effects on Liposome Fluidity and Size Depend on the Headgroup and Side Chain Structure of Key Mammalian Brain Lipids. Molecules. 2024; 29(1):135. https://doi.org/10.3390/molecules29010135
Chicago/Turabian StyleFarzi, Kianmehr, Travis Issler, Colin Unruh, and Elmar J. Prenner. 2024. "Gadolinium Effects on Liposome Fluidity and Size Depend on the Headgroup and Side Chain Structure of Key Mammalian Brain Lipids" Molecules 29, no. 1: 135. https://doi.org/10.3390/molecules29010135
APA StyleFarzi, K., Issler, T., Unruh, C., & Prenner, E. J. (2024). Gadolinium Effects on Liposome Fluidity and Size Depend on the Headgroup and Side Chain Structure of Key Mammalian Brain Lipids. Molecules, 29(1), 135. https://doi.org/10.3390/molecules29010135






