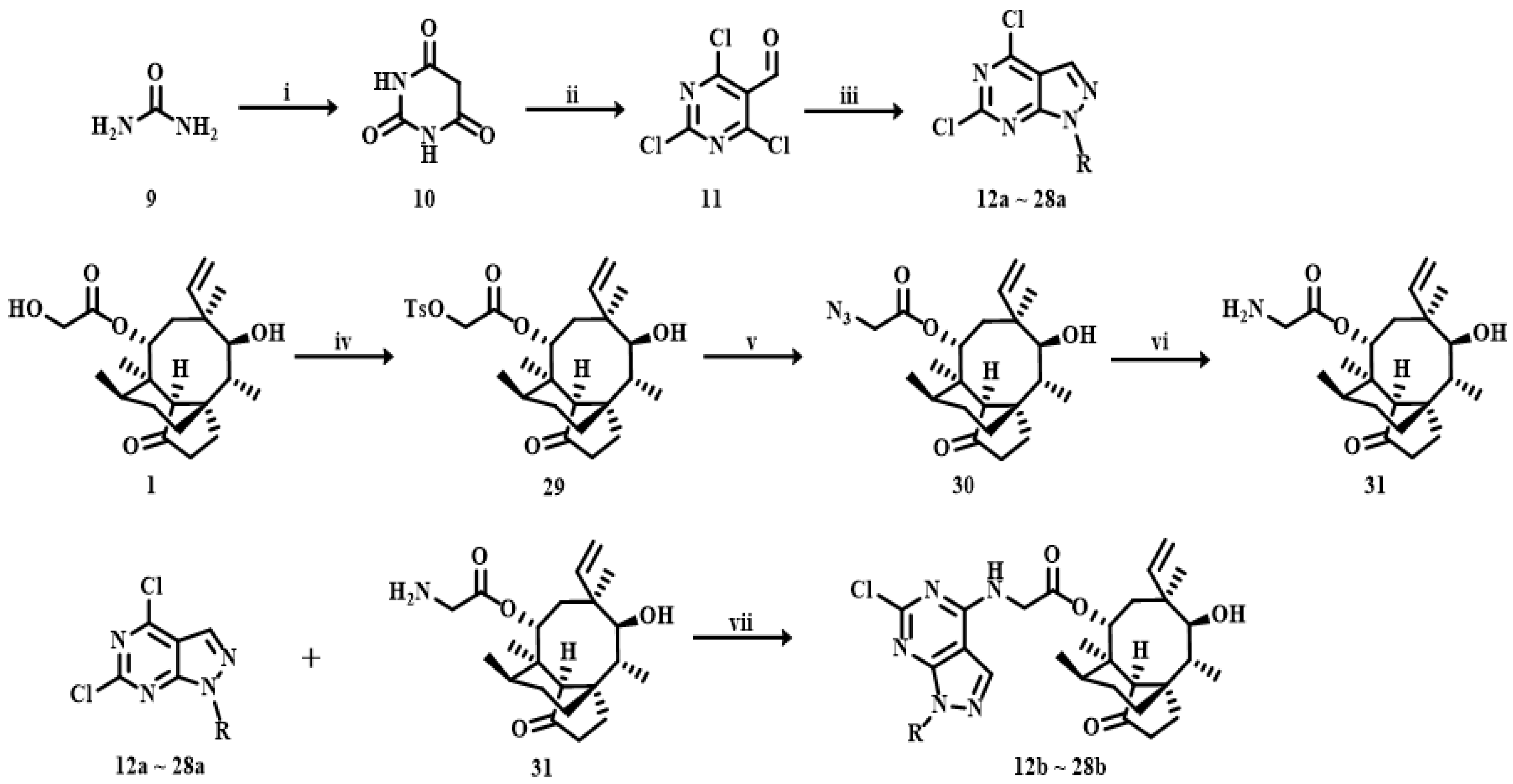
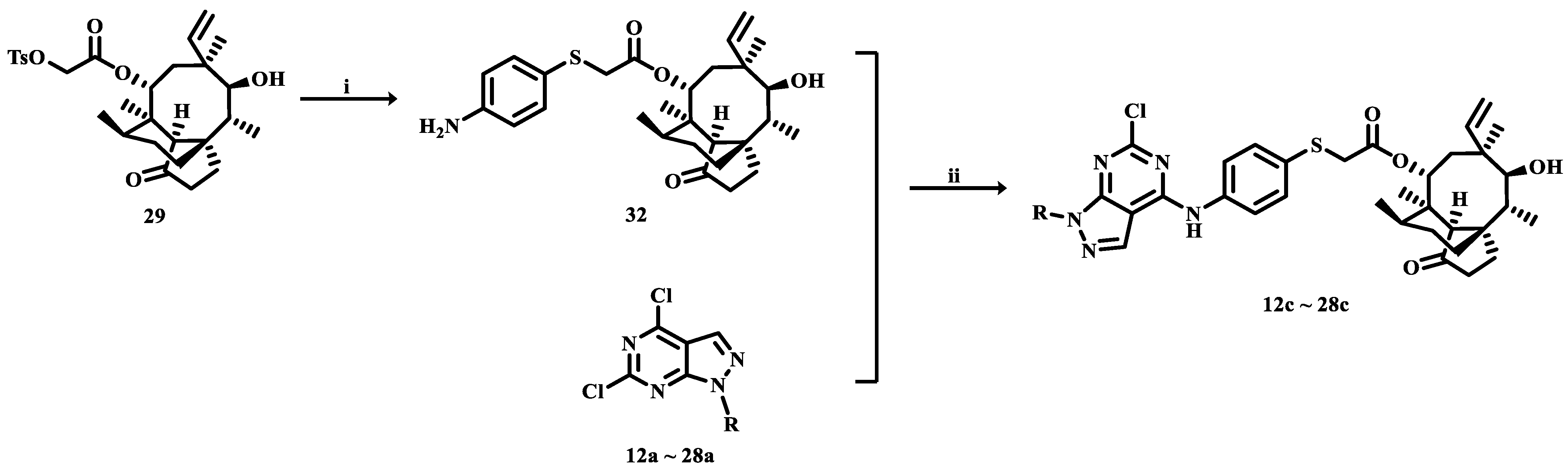
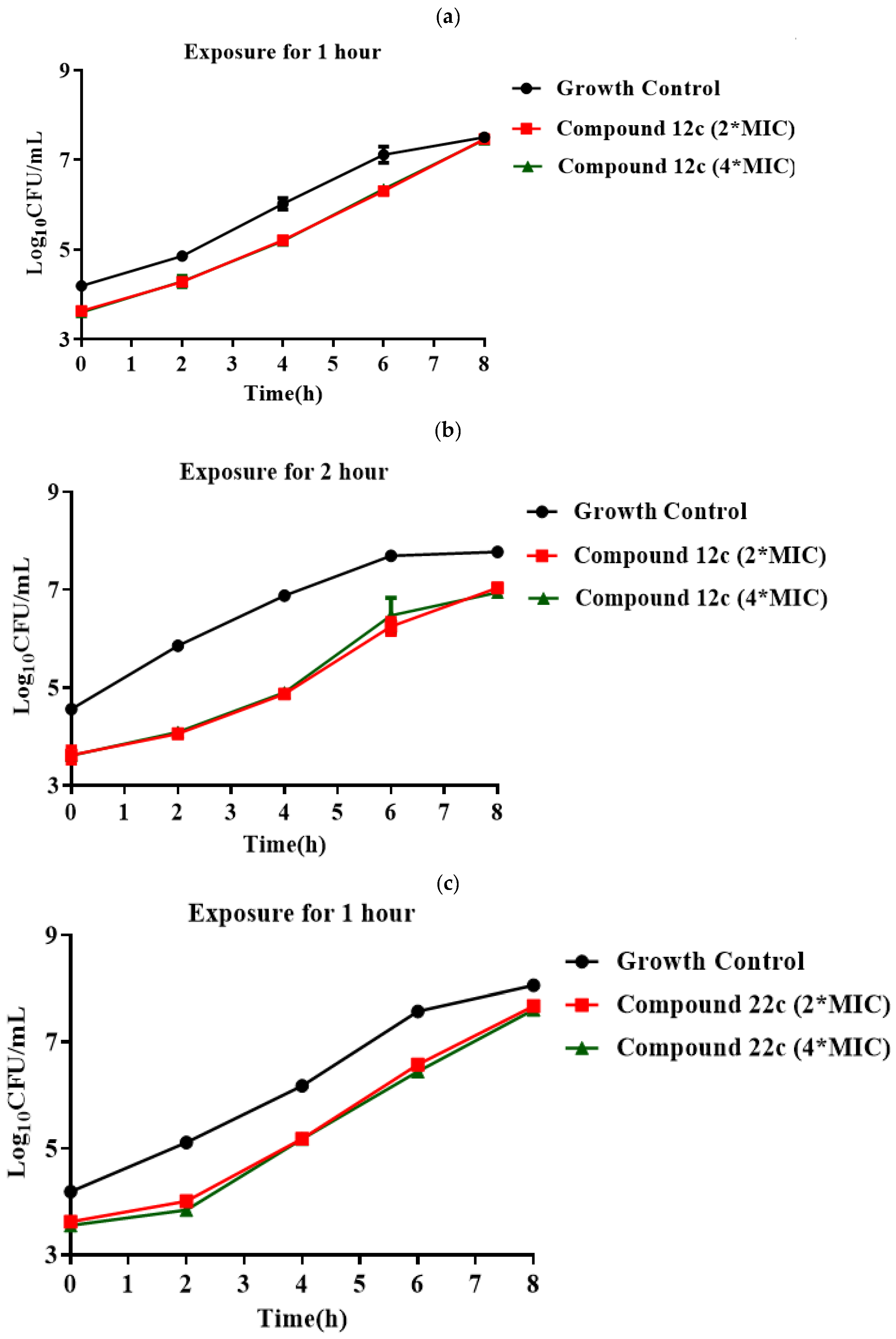
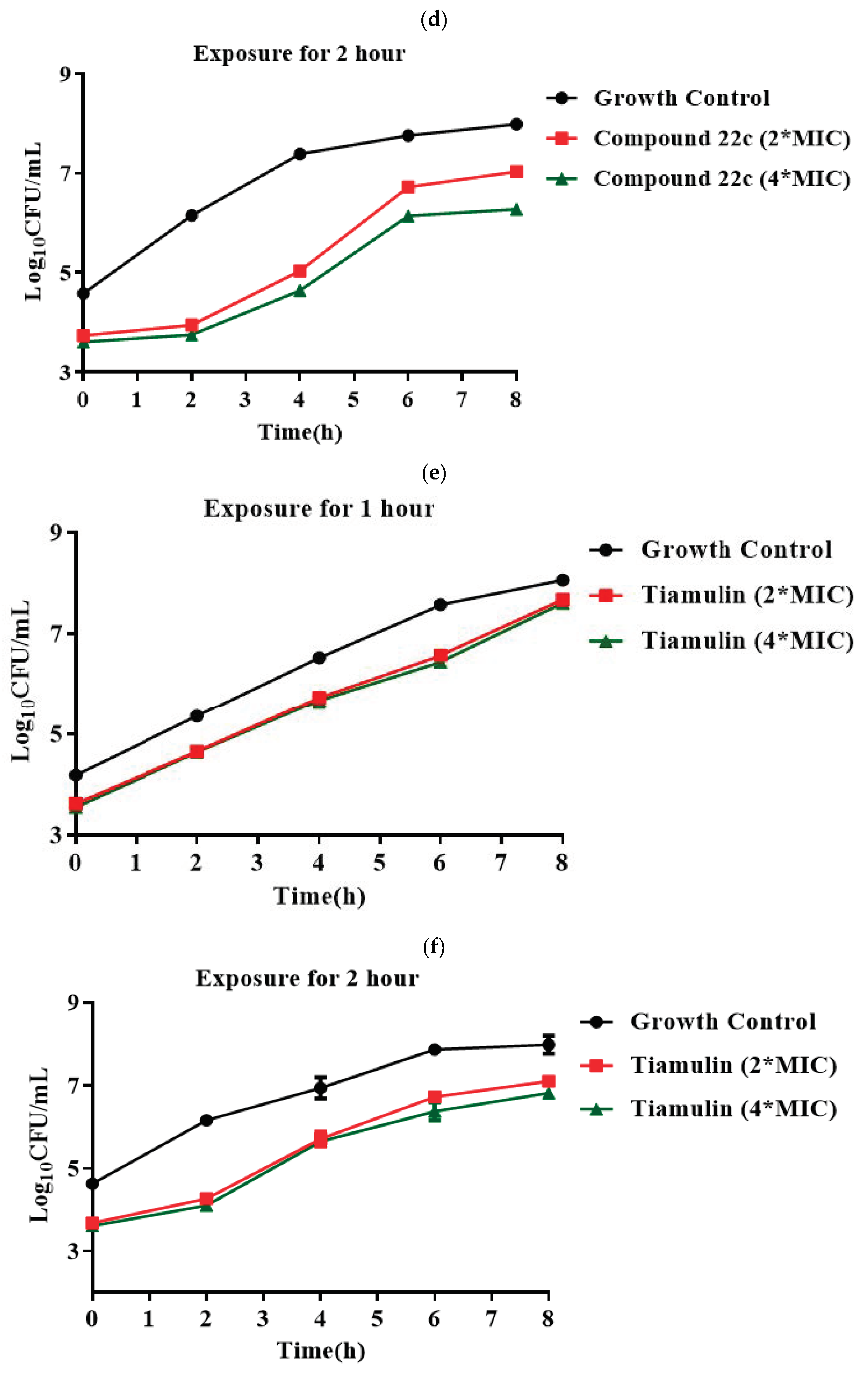
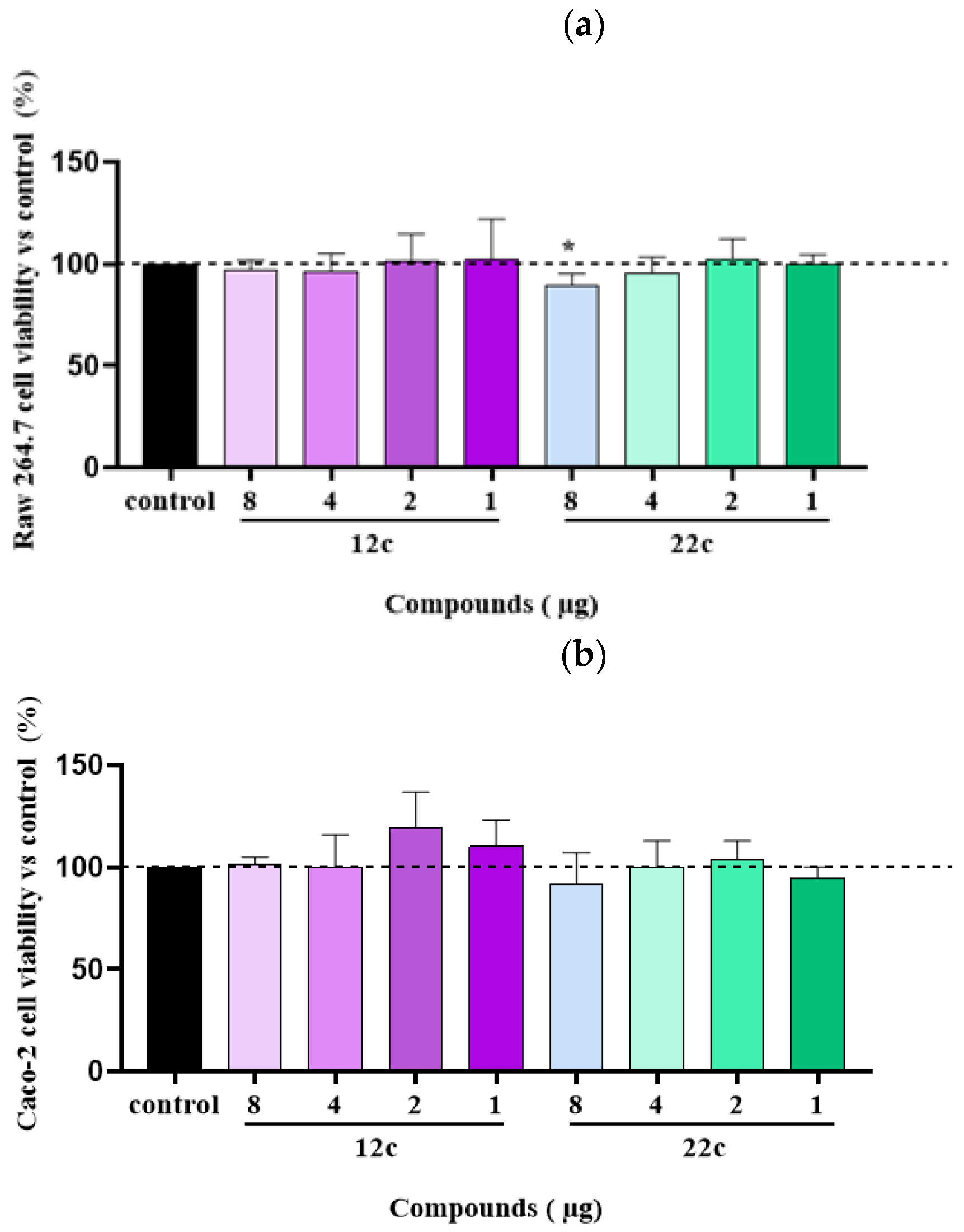
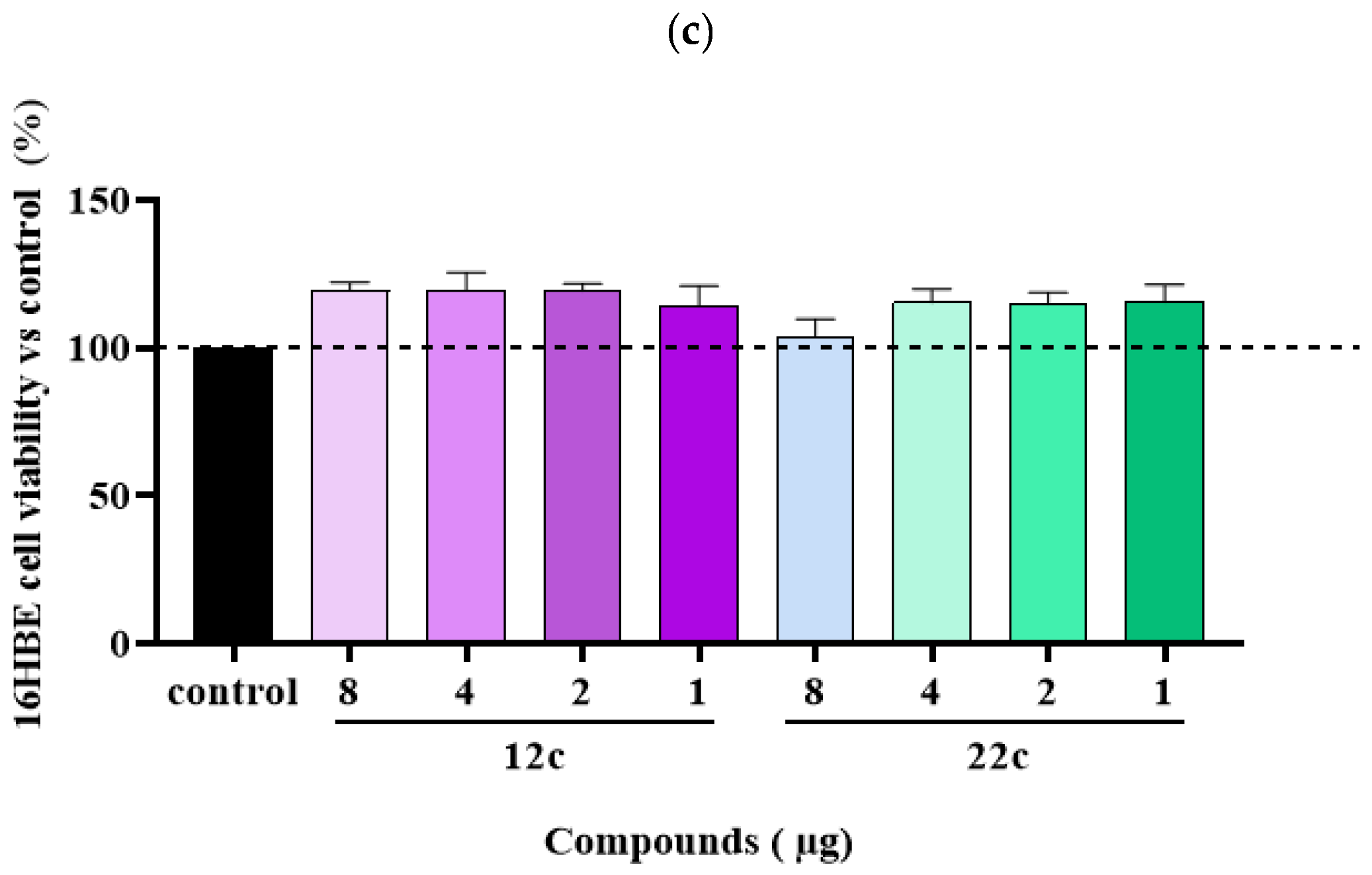
3.2. Synthesis
Two series of novel pleuromutilin derivatives containing 6-chloro-4-amino-1-R-1
H-pyrazolo[3,4-
d]pyrimidine were synthesized. The general synthetic routes are illustrated in
Scheme 1 and
Scheme 2.
3.2.1. 4,6-Dichloro-1-R-1H-pyrazolo[3,4-d]pyrimidine (12a~28a)
The raw material for synthesizing barbituric acid (compound 10) was urea (compound 9). Urea powder (6 g, 100 mmol) was dissolved in EtOH (50 mL), and then diethyl malonate (16 g, 120 mmol) was added to the solution. The reaction mixture was incubated with sodium methanolate (6.48 g, 120 mmol) for 48 h at 65 °C. The solution was acidified to pH 1~2, cooled down, crystallized and recrystallized to obtain pure barbituric acid. The Vilsmeier–Haack reaction of barbituric acid provided the product 2,4,6-trichloropyrimidine-5-carbaldehyde (compound 11). Phosphorus oxychloride (17.9 g, 117 mmol) was added into a 3-neck boiling flask at −10 °C, then N,N-dimethylformamide (15 mL) was dropped slowly into Phosphorus oxychloride, and then stirred for 1 h. Afterwards, Barbituric acid (5 g, 39 mmol) was added to the mixture and stirred for 48 h at 100 °C. After the reaction finished, the solution was slowly added into the ice water, a large number of solid precipitated out and then filtered, and the yield of compound 11 was 92%. The pyrazole ring was closed by treatment with hydrazine derivatives. Hydrazine derivatives (5.64 mmol) and compound 11 (1 g, 4.7 mmol) were dissolved in EtOH (10 mL) and then stirred for 2 h at −20 °C under alkaline conditions. After the reaction was completed, a large amount of solid was precipitated from the reaction solution. The crude products were purified using EtOH to obtain compounds 12a~28a. Yield: 83%~98%.
3.2.2. 22-Amino-deoxypleuromutilin (31)
4-methylbenzene-1-sulfonyl chloride (5.6 g, 29.2 mmol) and pleuromutilin (10 g, 26.5 mmol) were dissolved in acetonitrile (50 mL), then sodium hydroxide granules (3 g, 52.84 mmol) were dissolved in water (15 mL) and dropped slowly into the above mixture solution and stirred in an ice bath for 3 h. The mixture was then vacuum-evaporated, extracted with 30 mL of dichloromethane and washed with water (30 mL). The organic layer was dried over anhydrous Na2SO4 and filtered. Afterwards, isopropanol (50 mL) was added, and the mixture was heated at 70 °C until the solid was completely dissolved. Stewing the solution at room temperature for 1–2 h, the white solid precipitated and the white solid was collected (compound 29), yield: 95%.
Compound 29 (1 g, 1.88 mmol) and sodium azide (0.37 g, 5.65 mmol) were added to 10 mL of acetone and 5 mL of water, respectively. The two solutions were mixed under continuous stirring and heated at 80 °C for 4 h. The mixture was then vacuum-evaporated, extracted with 30 mL of dichloromethane and washed with water (30 mL). The organic layer was dried over anhydrous Na2SO4, filtered and concentrated under reduced pressure to obtain the product compound 30, yield: 93%. Compound 30 (10 g, 25.67 mmol) and triphenylphosphine (7.14 g, 28.24 mmol) were dissolved in THF (80 mL) and H2O (20 mL) solution. The mixture was maintained in an ice bath for 2 h, then washed with dichloromethane (30 mL) and water (50 mL) 3 times. The organic phase was dried over anhydrous Na2SO4 and evaporated in vacuum. The crude product was purified by column chromatography (dichloromethane: methanol = 200:1) using silica gel to obtain compound 31.
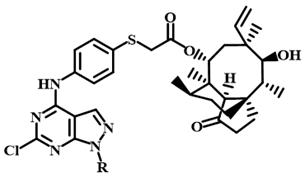
3.2.3. 22-(4-Amino-phenylsulfanyl)deoxypleuromutilin (32)
Compound 29 (10 g, 18.8 mmol) was dissolved in dichloromethane (100 mL), to which 4-aminothiophenol (2.5 g, 20 mmol) was added. Afterwards, 20% aqueous NaOH (10 mL) was added dropwise to the mixture and allowed to stir for 2 h at 70 °C. The crude product was purified by column chromatography (dichloromethane: methanol = 200:1) using silica gel to obtain compound 32. Yield: 79%.
3.2.4. General Procedure for the Synthesis of Compounds 12b~28b and 12c~28c
Compounds 12a~28a (0.25 mmol) and compound 31 (1.28 g, 2.65 mmol) were added to tetrahydrofuran (15 mL). Triethylamine (0.001 mmol) was added as a catalyst. The reaction proceeded for 2~12 h at room temperature. After completion of the reaction, the crude product was obtained by filtration. Compounds 12b~28b were purified by silica gel column chromatography (dichloromethane: methanol = 200:1). Compounds 12c~28c were obtained by subjecting compounds 12a~28a and compound 32 to the same procedures.
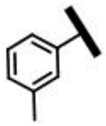
3.2.5. 22-[(6-Chloro-1-(3-methylphenyl)-1H-pyrazolo[3,4-d]pyrimidine-4-yl)amino]-22-deoxypleuromutilin (12b)
Yellow powder; yield: 79%; melting point: 97–99 °C; 1H NMR (600 MHz, Chloroform-d) δ 9.46 (1 H, s), 8.10 (1 H, s), 7.17 (1 H, t, J = 7.8 Hz), 7.01 (1 H, s), 6.79 (2 H, m), 6.52 (1 H, dd, J = 17.4, 11.0 Hz, H19), 5.95 (1 H, d, J = 8.5 Hz, H14), 5.29–5.18 (2 H, m, H20), 4.44–4.18 (2 H, m, H22), 3.39 (1 H, d, J = 6.5 Hz, H11), 2.38 (s, 3H), 2.36–2.06 (5 H, m, H2, H4, H10, 11-OH), 1.82–1.49 (6 H, m, H1, H6, H7, H8), 1.47 (3 H, s, H15), 1.43 (2 H, m, H13), 1.20 (3 H, s, H18), 1.15 (1 H, m, H8), 0.91 (3 H, d, J = 7.0 Hz, H17), 0.80 (3 H, d, J = 7.0 Hz, H16). 13C NMR (151 MHz, Chloroform-d) δ 216.98 (C3), 168.80 (C21), 159.47, 156.63, 156.31, 142.94, 139.50, 138.82 (C19), 132.32, 129.23, 122.07, 117.58 (C20), 113.27, 109.73, 107.12, 74.62 (C11), 70.06 (C14), 58.16 (C4), 45.49 (C9), 44.75 (C6), 44.37 (C13), 44.02 (C12), 41.9 (C5), 36.71 (C10), 36.20 (C2), 34.46 (C22), 30.44 (C8), 26.90 (C7), 26.45 (C18), 24.89 (C1), 21.57, 16.87 (C16), 14.84 (C15), 11.50 (C17). HR-MS (ESI): Calcd for C34H42ClN5O4 (M + Cl−): 654.2619; Found: 654.2618.
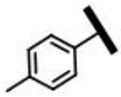
3.2.6. 22-[(6-Chloro-1-(4-methylphenyl)-1H-pyrazolo[3,4-d]pyrimidine-4-yl)amino]-22-deoxypleuromutilin (13b)
Yellow powder; yield: 62%; melting point: 93–95 °C; 1H NMR (600 MHz, Chloroform-d) δ 9.47 (1 H, s), 8.06 (1 H, s), 7.07 (2 H, d, J = 8.1 Hz), 6.97–6.85 (2 H, m), 6.54 (1 H, dd, J = 17.4, 11.0 Hz, H19), 5.91 (1 H, d, J = 8.5 Hz, H14), 5.37–5.17 (2 H, m, H20), 4.41–4.20 (2 H, m, H22), 3.40 (1 H, d, J = 6.5 Hz, H11), 2.38–2.12 (8 H, m, H2, H4, H10, 11-OH, H34), 1.83–1.48 (6 H, m, H1, H6, H7, H8), 1.47 (3 H, s, H15), 1.43 (2 H, m, H13), 1.21 (3 H, m, H18), 1.16 (1 H, m, H8), 0.92 (3 H, d, J = 7.0 Hz, H17), 0.81 (3 H, d, J = 7.1 Hz, H16). 13C NMR (151 MHz, Chloroform-d) δ 217.01 (C3), 168.63 (C21), 159.23, 157.89, 157.43, 146.47 138.76, 135.98, 132.58, 117.40 (C20), 115.42, 106.26, 74.61 (C11), 70.28 (C14), 58.16 (C4), 45.49 (C9), 44.78 (C13), 44.62 (C6), 43.96 (C12), 41.87 (C5), 36.72 (C10), 36.04 (C2), 34.46 (C22), 30.49 (C8), 26.71 (C7), 26.15 (C18), 24.87 (C1), 18.44, 16.82 (C16), 14.82 (C15), 11.46 (C17). HR-MS (ESI): Calcd for C34H42ClN5O4 (M + Cl−): 654.2619; Found: 654.2617.
3.2.7. 22-[(6-Chloro-1-(3,4-dimethylphenyl)-1H-pyrazolo[3,4-d]pyrimidine-4-yl)amino]-22-deoxypleuromutilin (14b)
Yellow powder; yield:72%; melting point: 99–103 °C; 1H NMR (600 MHz, Chloroform-d) δ 9.48 (1 H, s), 8.04 (1 H, s), 7.02 (1 H, d, J = 8.1 Hz), 6.96 (1 H, s), 6.75 (1 H, d, J = 9.9 Hz), 6.53 (1 H, dd, J = 17.4, 11.0 Hz, H19), 5.94 (1 H, d, J = 8.5 Hz, H14), 5.28–5.18 (2 H, m, H20), 4.42–4.24 (2 H, m, H22), 3.40 (1 H, d, J = 6.4 Hz, H11), 2.31–2.14 (11 H, m, H2, H4, H10, 11-OH, H34, H35), 1.75–1.55 (6 H, m, H1, H6, H7, H8), 1.47 (3 H, s, H15), 1.42 (2 H, m, H13), 1.20 (3 H, m, H18), 1.16 (1 H, m, H8), 0.92 (3 H, d, J = 7.0 Hz, H17), 0.80 (3 H, d, J = 7.0 Hz, H16). 13C NMR (151 MHz, DMSO-d6) δ 217.60 (C3), 168.41 (C21), 159.69, 155.53, 154.94, 142.09, 141.28, 137.63 (C19), 131.43, 130.59, 128.13, 115.81 (C20), 113.95, 110.47, 107.93, 73.07 (C11), 70.52 (C14), 57.64 (C4), 45.45 (C9), 44.57 (C6), 43.87 (C12), 41.98 (C5), 40.53 (C13), 37.00 (C10), 36.77 (C2), 34.46 (C22), 30.61 (C8), 29.02 (C7), 27.02 (C18), 24.95 (C1), 19.98, 19.12, 16.55 (C16), 14.80 (C15), 11.96 (C17). HR-MS (ESI): Calcd for C35H44ClN5O4 (M + Cl−): 668.2776; Found: 668.2778.
3.2.8. 22-[(6-Chloro-1-(3,5-dimethylphenyl)-1H-pyrazolo[3,4-d]pyrimidine-4-yl)amino]-22-deoxypleuromutilin (15b)
Yellow powder; yield: 66%; melting point: 95–100 °C; 1H NMR (600 MHz, DMSO-d6) δ 9.48 (1 H, s), 8.3 (1 H, s) 6.81 (2 H, s), 6.50 (1 H, s), 6.17 (1 H, dd, J = 17.7, 11.2 Hz, H19), 5.72 (1 H, d, J = 8.2 Hz, H14), 5.03 (2 H, m, H20), 4.36 (2 H, m, H22), 3.44 (1 H, m, H11), 2.43 (1 H, s, 11-OH), 2.26 (6 H, s), 2.20–2.01 (4 H, m, H2, H4, H10), 1.69–1.20 (11 H, m, H1, H6, H7, H8, H13, H15), 1.06 (4 H, m, H8, H18), 0.83 (3 H, d, J = 6.9 Hz, H17), 0.65 (3 H, d, J = 6.3 Hz, H16). 13C NMR (151 MHz, Chloroform-d) δ 216.84 (C3), 168.49 (C21), 159.37, 156.73, 156.41, 142.94, 139.28, 138.83 (C19), 132.25, 123.18, 117.55 (C20), 110.73, 107.03, 74.60 (C11), 69.82 (C14), 58.17 (C4), 45.47 (C9), 44.75 (C13), 44.54 (C6), 43.92 (C12), 41.88 (C5), 36.70 (C10), 36.28 (C2), 34.44 (C22), 30.45 (C8), 26.82 (C7), 26.37 (C18), 24.90 (C1), 21.40, 16.79 (C16), 14.82 (C15), 11.40 (C17). HR-MS (ESI): Calcd for C35H44ClN5O4 (M + Cl−): 668.2776; Found: 668.2773.
3.2.9. 22-[(6-Chloro-1-(4-ethylphenyl)-1H-pyrazolo[3,4-d]pyrimidine-4-yl)amino]-22-deoxypleuromutilin (16b)
Yellow powder; yield: 61%; melting point: 101–103 °C; 1H NMR (600 MHz, Chloroform-d) δ 9.49 (1 H, s), 8.07 (1 H, s), 7.11 (d, J = 8.4 Hz, 2H), 6.96 (d, J = 8.4 Hz, 2H), 6.55 (1 H, dd, J = 17.4, 11.0 Hz, H19), 5.92 (1 H, d, J = 8.5 Hz, H14), 5.31 (1 H, d, J = 11.0 Hz, H20), 5.24 (1 H, d, J = 18.7 Hz, H20), 4.43–4.22 (2 H, m, H22), 3.40 (1 H, d, J = 6.5 Hz, H11), 2.61 (2 H, q, J = 7.6 Hz), 2.39–2.12 (5 H, m, H2, H4, H10, 11-OH), 1.83–1.49 (6 H, m, H1, H6, H7, H8), 1.47 (3 H, s, H15), 1.44 (2 H, m, H13), 1.23 (t, J = 7.6 Hz, 3H), 1.21 (3 H, m, H18), 1.16 (1 H, m, H8), 0.91 (3 H, d, J = 7.0 Hz, H17), 0.81 (3 H, d, J = 7.0 Hz, H16). 13C NMR (151 MHz, Chloroform-d) δ 216.96 (C3), 168.71 (C21), 159.54, 156.51, 156.23, 140.92, 138.76 (C19), 137.09, 132.04, 128.71, 117.56 (C20), 112.75, 107.16, 74.61 (C11), 70.05 (C14), 58.13 (C4), 45.47 (C9), 44.68 (C13), 44.27 (C6), 44.04 (C12), 41.88 (C5), 36.70 (C10), 36.12 (C2), 34.45 (C22), 30.43 (C8), 28.09, 26.93 (C7), 26.40 (C18), 24.86 (C1), 16.87 (C16), 15.65, 14.82 (C15), 11.53 (C17). HR-MS (ESI): Calcd for C35H44ClN5O4 (M + Cl−): 668.2776; Found: 668.2773.
3.2.10. 22-[(6-Chloro-1-(3-methoxyphenyl)-1H-pyrazolo[3,4-d]pyrimidine-4-yl)amino]-22-deoxypleuromutilin (17b)
Yellow powder; yield: 72%; melting point: 99–104 °C; 1H NMR (600 MHz, Chloroform-d) δ 9.41 (1 H, t, J = 5.1 Hz), 8.27 (1 H, s), 8.09 (1 H, s), 7.18 (1 H, t, J = 8.1 Hz), 6.61 (1 H, dd, J = 8.0, 1.6 Hz), 6.57 (1 H, t, J = 2.1 Hz), 6.53 (1 H, dd, J = 17.4, 11.0 Hz), 6.49 (1 H, dd, J = 8.1, 2.1 Hz, H19), 5.89 (1 H, d, J = 8.5 Hz, H14), 5.32–5.15 (2 H, m, H20), 4.44–4.22 (2 H, m, H22), 3.81 (3 H, s), 3.39 (1 H, d, J = 6.5 Hz, H11), 2.38–2.09 (5 H, m, H1, H6, H7, 11-OH), 1.81–1.496 (6 H, m, H1, H7, H6, H8), 1.47 (3 H, s, H15), 1.43 (2 H, m, H13), 1.20 (3 H, s, H18), 1.16 (1 H, m, H8), 0.91 (3 H, d, J = 7.0 Hz, H17), 0.80 (3 H, d, J = 7.1 Hz, H16). 13C NMR (151 MHz, Chloroform-d) δ 216.84 (C3), 168.36 (C21), 159.46, 156.66, 156.39, 141.05, 138.80 (C19), 137.88, 132.04, 130.39, 129.52, 117.53 (C20), 114.42, 110.41, 107.07, 74.60 (C11), 69.85 (C14), 58.15 (C4), 45.48 (C9), 44.72 (C13), 44.48 (C6), 43.97 (C12), 41.88 (C5), 36.70 (C10), 36.21 (C2), 34.44 (C22), 30.46 (C8), 26.85 (C7), 26.36 (C18), 24.88 (C1), 19.88, 19.03, 16.82 (C16), 14.81 (C15), 11.47 (C17). HR-MS (ESI): Calcd for C34H42ClN5O5 (M + Cl−): 670.2568; Found: 670.2569.
3.2.11. 22-[(6-Chloro-1-(2-fluorophenyl)-1H-pyrazolo[3,4-d]pyrimidine-4-yl)amino]-22-deoxypleuromutilin (18b)
Yellow powder; yield: 52%; melting point: 107–110 °C; 1H NMR (600 MHz, Chloroform-d) δ 9.42 (1 H, t, J = 4.6 Hz), 8.28 (1 H, s), 7.68 (1 H, t, J = 8.8 Hz), 7.14 (1 H, t, J = 7.7 Hz), 7.07 (1 H, dd, J = 11.7, 8.2 Hz), 6.90 (1 H, q, J = 7.1, 6.6 Hz), 6.54 (1 H, dd, J = 17.4, 11.0 Hz, H19), 5.90 (1 H, d, J = 8.5 Hz, H14), 5.32–5.15 (2 H, m, H20), 4.44–4.23 (2 H, m, H22), 3.39 (1 H, d, J = 6.5 Hz, H11), 2.37–2.10 (5 H, m, H2, H4, H10, 11-OH), 1.80–1.54 (6 H, m, H1, H6, H7, H8), 1.47 (3 H, s, H15), 1.41 (2 H, m, H13), 1.20 (3 H, m, H18), 1.17 (1 H, m, H8), 0.92 (3 H, d, J = 7.0 Hz, H17), 0.78 (3 H, d, J = 7.1 Hz, H16). 13C NMR (151 MHz, Chloroform-d) δ 216.79 (C3), 168.36 (C21), 159.50, 157.58, 157.12, 138.69 (C19), 135.02, 131.60, 124.93, 120.87, 120.82, 117.59 (C20), 115.21, 114.39, 106.59, 74.60 (C11), 70.05 (C14), 58.09 (C4), 45.46 (C9), 44.70 (C13), 44.49 (C6), 44.04 (C12), 41.87 (C5), 36.66 (C10), 36.12 (C2), 34.43 (C22), 30.42 (C8), 26.90 (C7), 26.40 (C18), 24.86 (C1), 16.83 (C16), 14.80 (C15), 11.56 (C17). HR-MS (ESI): Calcd for C33H39ClFN5O4 (M + Cl−): 658.2369; Found:658.2367.
3.2.12. 22-[(6-Chloro-1-(4-fluorophenyl)-1H-pyrazolo[3,4-d]pyrimidine-4-yl)amino]-22-deoxypleuromutilin (19b)
Yellow powder; yield: 57%; melting point: 112–115 °C; 1H NMR (600 MHz, Chloroform-d) δ 9.40 (1 H, d, J = 4.5 Hz), 8.18 (1 H, s), 7.14 (2 H, dd, J = 8.9, 4.4 Hz), 7.03 (2 H, t, J = 8.6 Hz), 6.53 (1 H, dd, J = 17.4, 11.0 Hz, H19), 5.89 (1 H, d, J = 8.6 Hz, H14), 5.33–5.23 (2 H, m, H20), 4.40–4.23 (2 H, m, H22), 3.39 (1 H, dd, J = 6.5 Hz, H11), 2.41–2.10 (5 H, m, H2, H4, H10, 11-OH), 1.75–1.53 (6 H, m, H1, H6, H7, H8, H13), 1.46 (3 H, s, H15), 1.41 (2 H, m, H13), 1.20 (3 H, m, H18), 1.18 (1 H, m, H8), 0.93 (3 H, d, J = 7.0 Hz, H17), 0.77 (3 H, d, J = 7.1 Hz, H16). 13C NMR (151 MHz, Chloroform-d) δ 216.90 (C3), 168.44 (C21), 159.45, 157.09, 157.08, 139.53, 138.69 (C19), 133.06, 117.60 (C20), 116.01, 114.17, 106.85, 74.61 (C11), 70.11 (C14), 58.10 (C4), 45.48 (C9), 44.67 (C13), 44.47 (C6), 44.05 (C12), 41.89 (C5), 36.66 (C10), 36.14 (C2), 34.45 (C22), 30.41 (C8), 26.91 (C7), 26.42 (C18), 24.86 (C1), 16.85 (C16), 14.81 (C15), 11.55 (C17). HR-MS (ESI): Calcd for C33H39ClFN5O4 (M + Cl−): 658.2369; Found: 658.2369.
3.2.13. 22-[(6-Chloro-1-(2,4-difluorophenyl)-1H-pyrazolo[3,4-d]pyrimidine-4-yl)amino]-22-deoxypleuromutilin (20b)
Yellow powder; yield: 68%; melting point: 123–125 °C; 1H NMR (600 MHz, Chloroform-d) δ 9.38 (1 H, s), 8.33 (1 H, s), 7.70–7.53 (1 H, m), 6.89–6.71 (2 H, m), 6.52 (1 H, dd, J = 17.5, 11.0 Hz, H19), 5.90 (1 H, d, J = 8.6 Hz, H14), 5.28–5.20 (2 H, m, H20), 4.33 (2 H, ddd, J = 69.8, 18.9, 4.8 Hz, H22), 3.41 (1 H, dd, J = 10.2, 6.5 Hz, H11), 2.38–2.12 (5H, m, H2, H4, H10, 11-OH), 1.94–1.49 (6 H, m, H1, H6, H7, H8), 1.49 (3 H, s, H15), 1.43 (2 H, m, H13), 1.20 (3 H, m, H18), 1.16 (1 H, m, H8), 0.93 (3 H, d, J = 7.0 Hz, H17), 0.79 (3 H, d, J = 7.0 Hz, H16). 13C NMR (151 MHz, Chloroform-d) δ 216.81 (C3), 168.37 (C21), 159.41, 157.36, 156.99, 141.68, 138.65 (C19), 133.66, 129.43, 126.03, 117.64 (C20), 114.26, 106.67, 74.59 (C11), 70.17 (C14), 58.08 (C4), 45.47 (C9), 44.64 (C6), 44.53 (C13), 44.05 (C12), 41.89 (C5), 36.64 (C10), 36.14 (C2), 34.45 (C22), 30.40 (C8), 26.92 (C7), 26.39 (C18), 24.84 (C1), 16.87 (C16), 14.81 (C15), 11.54 (C17). HR-MS (ESI): Calcd for C33H38ClF2N5O4 (M + Cl−):676.2274; Found:676.2274.
3.2.14. 22-[(6-Chloro-1-(2,5-difluorophenyl)-1H-pyrazolo[3,4-d]pyrimidine-4-yl)amino]-22-deoxypleuromutilin (21b)
Yellow powder; yield: 62%; melting point: 121–125 °C; 1H NMR (600 MHz, Chloroform-d) δ 9.35 (1 H, s), 8.29 (1 H, s), 7.64–7.52 (1 H, m), 7.01–6.92 (1 H, m), 6.59 (1 H, dd, J = 17.3, 11.0 Hz), 6.55–6.47 (1 H, m, H18), 6.02 (1 H, d, J = 8.5 Hz, H14), 5.29–5.12 (2 H, m, H20), 4.33 (2 H, m, H22), 3.42–3.34 (1 H, m, H11), 2.46–2.11 (5 H, m, H2, H4, H10, 11- OH), 1.85–1.48 (6 H, m, H1, H6, H7, H8), 1.48 (3 H, s, H15), 1.40 (2 H, d, J = 16.1 Hz, H13), 1.19 (3 H, m, H18), 1.16 (1 H, m, H8), 0.92 (3 H, d, J = 7.0 Hz, H17), 0.78 (3 H, d, J = 7.1 Hz, H16). 13C NMR (151 MHz, Chloroform-d) δ 216.97 (C3), 168.77 (C21), 159.52, 156.45, 156.17, 140.72, 138.78 (C19), 131.98, 130.53, 129.89, 117.53 (C20), 112.63, 107.16, 74.61 (C11), 70.10 (C14), 58.11 (C4), 45.47 (C9), 44.67 (C6), 44.25 (C13), 44.06 (C12), 41.88 (C5), 36.69 (C10), 36.11 (C2), 34.46 (C22), 30.42 (C8), 26.93 (C7), 26.41 (C18), 24.85 (C1), 16.87 (C16), 14.82 (C15), 11.55 (C17). HR-MS (ESI): Calcd for C33H38ClF2N5O4 (M + Cl−):676.2274; Found:676.2274
3.2.15. 22-[(6-Chloro-1-(3,4-difluorophenyl)-1H-pyrazolo[3,4-d]pyrimidine-4-yl)amino]-22-deoxypleuromutilin (22b)
Yellow powder; yield: 61%; melting point: 130–132 °C; 1H NMR (600 MHz, Chloroform-d) δ 9.49 (1 H, t, J = 4.6 Hz), 8.22 (1 H, s), 7.28–7.21 (1 H, m), 7.06 (1 H, q, J = 8.9 Hz\), 6.73 (1 H, d, J = 8.9 Hz), 6.53 (1 H, dd, J = 17.4, 11.0 Hz, H19), 5.95 (1 H, d, J = 8.5 Hz, H14), 5.29–5.16 (2 H, m, H20), 4.32 (2 H, m, H22), 3.32 (1 H, m, H11), 2.42–2.08 (5 H, m, H2, H4, H10, 11-OH), 1.86–1.46 (6 H, m, H1, H6, H7, H8), 1.46 (3 H, s, H15), 1.41 (2 H, m, H13), 1.19 (3 H, s, H18), 1.16 (1 H, m, H8), 0.94 (3 H, d, J = 7.0 Hz, H17), 0.77 (3 H, d, J = 7.0 Hz, H16). 13C NMR (151 MHz, Chloroform-d) δ 216.74 (C3), 168.40 (C21), 159.40, 157.68, 157.25, 139.33, 138.67 (C19), 135.26, 132.09, 128.34, 117.58 (C20), 115.11, 111.57, 111.42, 106.50, 74.59 (C11), 70.17 (C14), 58.08 (C4), 45.46 (C9), 44.67 (C13), 44.58 (C6), 44.05 (C12), 41.88 (C5), 36.62 (C10), 36.16 (C2), 34.43 (C22), 30.40 (C8), 26.91 (C7), 26.43 (C18), 24.85 (C1), 16.84 (C16), 14.80 (C15), 11.54 (C17). HR-MS (ESI): Calcd for C33H38ClF2N5O4 (M + Cl−):676.2274; Found: 676.2274.
3.2.16. 22-[(6-Chloro-1-(4-(trifluoromethoxy)Phenyl)-1H-pyrazolo[3,4-d]pyrimidine-4-yl)amino]-22-deoxypleuromutilin (23b)
Yellow powder; yield: 62%; melting point: 132–125 °C; 1H NMR (600 MHz, Chloroform-d) δ 9.39 (1 H, s), 8.19 (1 H, s), 7.20-7.11 (4 H, m), 6.53 (1 H, dd, J = 17.5, 11.0 Hz, H19), 5.92 (1 H, d, J = 8.5 Hz, H14), 5.29-5.20 (2 H, m, H20), 4.43–4.24 (2 H, m, H22), 3.40 (1 H, d, J = 6.5 Hz, H11), 2.37–2.11 (5 H, m, H2, H4, H10, 11-OH), 1.84–1.51 (7 H, m, H1, H6, H7, H8), 1.48 (3 H, s, H15), 1.43 (2 H, m, H13), 1.21 (3 H, s, H18), 1.16 (1 H, m, H8), 0.93 (3 H, d, J = 7.2 Hz, H17), 0.78 (3 H, d, J = 7.0 Hz, H16). 13C NMR (151 MHz, Chloroform-d) δ 216.97 (C3), 168.62 (C21), 159.41, 157.29, 156.93, 143.14, 141.82, 138.64 (C19), 133.75, 122.42, 117.55 (C20), 113.61, 106.74, 74.60 (C11), 70.23 (C14), 58.13 (C4), 45.49 (C13), 44.66 (C6), 44.50 (C12), 44.04, 41.89 (C5), 36.67 (C10), 36.17 (C2), 34.46 (C22), 30.40 (C8), 26.90 (C7), 26.41 (C18), 24.86 (C1), 16.84 (C16), 14.83 (C15), 11.44 (C17). HR-MS (ESI): Calcd for C34H39ClF3N5O5 (M + Cl−): 724.2286; Found: 724.2290.
3.2.17. 22-[(6-Chloro-1-(3-chlorophenyl)-1H-pyrazolo[3,4-d]pyrimidine-4-yl)amino]-22-deoxypleuromutilin (24b)
Yellow powder; yield: 69%; melting point: 117–119 °C; 1H NMR (600 MHz, Chloroform-d) δ 9.37 (1 H, s), 8.24 (1 H, s), 7.39 (1 H, s), 7.19 (1 H, t, J = 8.0 Hz), 6.91 (1 H, d, J = 8.9 Hz), 6.84 (1 H, d, J = 9.5 Hz\), 6.61 (1 H, dd, J = 17.4, 11.0 Hz, H19), 6.00 (1 H, d, J = 8.5 Hz, H14), 5.27–5.14 (2 H, m, H20), 4.32 (2 H, m, H22), 3.38 (1 H, d, J = 6.0 Hz, H11), 2.37–2.12 (5 H, m, H2, H4, H10, 11-OH), 1.67 (6 H, m, H1, H6, H7, H8), 1.48 (3 H, s, H15), 1.41 (2 H, m, H13), 1.19 (3 H, s, H18), 1.16 (1 H, m, H8), 0.91 (3 H, d, J = 7.0 Hz, H17), 0.80 (3 H, d, J = 7.0 Hz, H16). 13C NMR (151 MHz, Chloroform-d) δ 217.01 (C3), 168.50 (C21), 159.31, 157.39, 157.04, 144.17, 138.98 (C19), 135.66, 133.98, 130.28, 121.14, 117.45 (C20), 112.95, 111.10, 106.58, 74.60 (C11), 70.15 (C14), 58.16 (C4), 45.49 (C9), 44.76 (C13), 44.55 (C6), 43.98 (C12), 41.87 (C5), 36.74 (C10), 36.15 (C2), 34.47 (C22), 30.47 (C8), 26.82 (C7), 26.19 (C18), 24.87 (C1), 16.87 (C16), 14.82 (C15), 11.48 (C17). HR-MS (ESI): Calcd for C33H39Cl2N5O4 (M + Cl−): 676.2042; Found: 676.2042.
3.2.18. 22-[(6-Chloro-1-(4-chlorophenyl)-1H-pyrazolo[3,4-d]pyrimidine-4-yl)amino]-22-deoxypleuromutilin (25b)
Yellow powder; yield: 66%; melting point: 107–111 °C, 1H NMR (600 MHz, Chloroform-d) δ 9.37 (1 H, s), 8.18 (1 H, s), 7.28 (2 H, d, J = 8.5Hz), 7.13 (2 H, d, J = 8.8 Hz), 6.54 (1 H, dd, J = 17.4, 11.0 Hz, H19), 5.90 (2 H, d, J = 8.6 Hz, H14), 5.32 (1 H, d, J = 11.3 Hz, H20), 5.24 (1 H, d, J = 17.4 Hz, H20), 4.47–4.18 (2 H, m, H22), 3.44–3.34 (1 H, m, H11), 2.40–2.10 (5 H, m, H2, H4, H10, 11-OH), 1.75–1.56 (7 H, m, H1, H6, H7, H8), 1.47 (3 H, s, H15), 1.42 (2 H, m, H13), 1.20 (3 H, d, J = 7.0 Hz, H18), 1.16 (1 H, m, H8), 0.93 (3 H, d, J = 7.0 Hz, H17), 0.77 (3 H, d, J = 7.1 Hz, H16). 13C NMR (151 MHz, Chloroform-d) δ 216.92 (C3), 168.45 (C21), 159.24, 157.53, 157.19, 144.44, 138.71 (C19), 134.07, 117.46 (C20), 108.20, 106.47, 102.79, 102.64, 74.62 (C11), 70.20 (C14), 58.14 (C4), 45.49 (C9), 44.63 (C13), 43.98 (C12), 41.88 (C5), 36.69 (C10), 36.10 (C2), 34.46 (C22), 30.46 (C8), 29.71 (C6), 26.77 (C7), 26.24 (C18), 24.87 (C1), 16.82 (C16), 14.81 (C15), 11.48 (C17). HR-MS (ESI): Calcd for C33H39Cl2N5O4 (M + Cl−): 676.2042; Found: 676.2042.
3.2.19. 22-[(6-Chloro-1-(3,5-dichlorophenyl)-1H-pyrazolo[3,4-d]pyrimidine-4-yl)amino]-22-deoxypleuromutilin (26b)
Yellow powder; yield: 56%; melting point: 151–156 °C; 1H NMR (600 MHz, Chloroform-d) δ 9.33 (1 H, s), 8.21 (1 H, s), 7.22 (1 H, s), 6.94 (1 H, s), 6.64 (1 H, dd, J = 17.3, 11.0 Hz, H19), 6.06 (1 H, d, J = 8.4 Hz, H14), 5.28–5.10 (2 H, m, H20), 4.30 (2 H, m, H22), 3.41–3.33 (1 H, m, H11), 2.40–2.08 (5 H, m, H2, H4, H10, 11-OH), 1.89–1.58 (6 H, m, H1, H6, H7, H8), 1.47 (3 H, s, H15), 1.36 (2 H, m, H13), 1.18 (3 H, s, H18), 1.16 (1 H, m, H8), 0.90 (3 H, d, J = 7.0 Hz, H17), 0.77 (3 H, d, J = 7.0 Hz, H16). 13C NMR (151 MHz, Chloroform-d) δ 216.92 (C3), 168.40 (C21), 159.13, 157.56, 144.74, 139.11 (C19), 135.93, 135.58, 135.24, 121.03, 117.39 (C20), 111.50, 106.16, 74.58 (C11), 70.16 (C14), 58.18 (C4), 45.50 (C9), 44.80 (C13), 44.72 (C6), 43.91 (C12), 41.85 (C10), 36.74 (C10), 36.23 (C2), 34.46 (C22), 30.50 (C8), 26.75 (C7), 26.07 (C18), 24.89 (C1), 16.85 (C16), 14.82 (C15), 11.41 (C17). HR-MS (ESI): Calcd for C33H38Cl3N5O4 (M + Cl−): 710.1654; Found: 710.1656.
3.2.20. 22-[(6-Chloro-1-(3-nitrophenyl)-1H-pyrazolo[3,4-d]pyrimidine-4-yl)amino]-22-deoxypleuromutilin (27b)
Yellow powder; yield: 69%; melting point: 131–135 °C; 1H NMR (600 MHz, Chloroform-d) δ 9.39 (1 H, s), 8.35 (1 H, s), 8.01 (1 H, s), 7.78 (2 H, d, J = 7.8 Hz), 7.44 (2 H, dt, J = 16.1, 8.3 Hz), 6.50 (1 H, dd, J = 17.4, 11.0 Hz, H19), 5.91 (1 H, d, J = 8.6 Hz, H14), 5.19–5.08 (2 H, m, H20), 4.41–4.26 (2 H, m, H22), 3.44–3.34 (1 H, m, H11), 2.41–2.09 (5 H, m, H2, H4, H10, 11-OH), 1.88–1.60 (6 H, m, H1, H6, H7, H8), 1.48 (3 H, s, H15), 1.40 (2 H, m, H13), 1.18 (3 H, m, H18), 1.14 (1 H, m, H8), 0.94 (3 H, d, J = 7.0 Hz, H17), 0.80 (3 H, d, J = 7.0 Hz, H16). 13C NMR (151 MHz, Chloroform-d) δ 216.91 (C3), 168.43 (C21), 159.42, 157.60, 149.65, 144.07, 138.89 (C19), 135.47, 130.15, 118.55, 117.13 (C20), 115.71, 107.61, 106.23, 98.57, 74.61 (C11), 70.55 (C14), 58.08 (C4), 45.50 (C9), 44.66 (C13), 44.51 (C6), 44.11 (C12), 41.90 (C5), 36.69 (C10), 35.98 (C2), 34.48 (C22), 30.45 (C8), 26.78 (C7), 26.24 (C18), 24.84 (C1), 16.95 (C16), 14.81 (C15), 11.48 (C17). HR-MS (ESI): Calcd for C33H39ClN6O6 (M + Cl−): 685.2314; Found: 685.2310.
3.2.21. 22-[(6-Chloro-1-(naphthalen-2-yl)-1H-pyrazolo[3,4-d]pyrimidine-4-yl)amino]-22-deoxypleuromutilin (28b)
Yellow powder; yield:63%; melting point: 123–125 °C; 1H NMR (600 MHz, Chloroform-d) δ 9.55 (1 H, s), 8.21 (1 H, s), 7.92 (1 H, d, J = 8.2 Hz), 7.84 (1 H, s), 7.77 (2 H, t, J = 8.7 Hz), 7.36 (2 H, m, 7.3 Hz), 7.20 (1 H, dd, J = 8.8, 2.1 Hz), 6.54 (1 H, dd, J = 17.4, 11.0 Hz, H19), 6.01 (1 H, d, J = 8.5 Hz, H14), 5.18-4.98 (2 H, m, H20), 4.48–4.27 (2 H, m, H22), 3.40 (1 H, d, J = 6.4 Hz, H11), 2.48-2.14 (5 H, m, H2, H4, H10, 11-OH), 1.68–1.49 (6 H, m, H1, H6, H7, H8), 1.49 (3 H, s, H15), 1.42 (2 H, m, H13), 1.19 (1 H, m, H8), 1.17 (3 H, s, H16), 0.98 (3 H, d, J = 6.7 Hz, H17), 0.81 (3 H, d, J =6.8 Hz, H16). 13C NMR (151 MHz, Chloroform-d) δ 216.83 (C3), 168.43 (C21), 159.34, 157.13, 156.76, 140.42, 138.60 (C19), 134.81, 133.33, 129.38, 129.29, 127.77, 126.93, 126.54, 123.64, 117.63 (C20), 115.15, 107.83, 106.88, 74.61 (C11), 70.03 (C14), 58.14 (C4), 45.48(C9), 44.82 (C6), 44.74 (C13), 43.96 (C12), 41.91 (C5), 36.70 (C10), 36.26 (C2), 34.44 (C22), 30.51 (C8), 26.92 (C7), 26.38 (C18), 24.91 (C1), 16.84 (C16), 14.83 (C15), 11.75 (C17). HR-MS (ESI): Calcd for C37H42ClN5O4 (M + Cl−): 690.2619; Found: 690.2616.
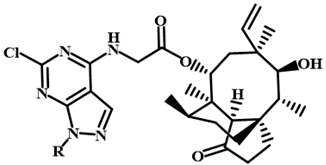
3.2.22. 22-[4-(6-Chloro-1-(3-methylphenyl)-1H-pyrazolo[3,4-d]pyrimidine-4-yl)amino-Phenylsulfanyl]-22-deoxypleuromutilin (12c)
Yellow powder; yield: 52%; melting point: 95–97 °C; 1H NMR (600 MHz, DMSO-d6) δ 11.42 (1 H, s), 8.35 (1 H, s), 7.66–7.60 (2 H, m), 7.47 (1 H, d, J = 8.2 Hz), 7.19 (1 H, t, J = 7.7 Hz), 6.86 (1 H, s), 6.75 (1 H, dd, J = 34.4, 7.4 Hz), 6.08–5.99 (1 H, m, H19), 5.51 (1 H, d, J = 8.3 Hz, H14), 4.96 (2 H, m, H20), 3.90–3.75 (2 H, m, H22), 3.37 (1 H, t, J = 5.7 Hz, H11), 2.29 (3 H, s), 2.22-1.99 (5 H, m, H2, H4, H10, 11-OH), 1.70–1.34 (5 H, m, H1, H6, H7), 1.33 (3 H, s, H15), 1.25 (3 H, m, H8, H13), 0.98 (4 H, m, H8, H18), 0.79 (3 H, d, J = 7.0 Hz, H17), 0.58 (3 H, d, J = 7.1 Hz, H16). 13C NMR (151 MHz, DMSO-d6) δ 217.59 (C3), 168.11 (C21), 157.33, 156.67, 154.91, 144.06, 141.17 (C19), 139.37, 136.40, 132.14, 131.26, 130.24, 129.87, 122.45, 121.73, 115.63 (C20), 113.12, 110.26, 108.50, 73.04 (C11), 70.23 (C14), 57.70 (C4), 45.40 (C9), 44.42 (C13), 44.14 (C12), 41.92 (C5), 36.83 (C6), 36.81 (C10), 36.20 (C2), 34.44 (C22), 30.55 (C8), 28.96 (C7), 27.04 (C18), 24.91 (C1), 21.79, 16.55 (C16), 14.98 (C15), 11.97 (C17). HR-MS (ESI): Calcd for C40H46ClN5O4S (M + Cl−): 762.2653; Found: 762.2654.
3.2.23. 22-[4-(6-Chloro-1-(4-methylphenyl)-1H-pyrazolo[3,4-d]pyrimidine-4-yl)amino-phenylsulfanyl]-22-deoxypleuromutilin (13c)
Yellow powder; yield: 61%; melting point: 101–105 °C; 1H NMR (600 MHz, Chloroform-d) δ 11.26 (1 H, s), 8.29 (1 H, s), 7.64 (2 H, d, J = 8.6 Hz), 7.41 (2 H, d, J = 8.6 Hz), 7.12 (2 H, d, J = 8.1 Hz,), 6.90 (2 H, d, J = 8.3 Hz), 6.45–6.36 (1 H, m, H19), 5.74 (1 H, d, J = 8.5 Hz, H14), 5.31 (1 H, m, H20), 5.16 (1 H, d, J = 18.5 Hz, 1H20), 3.62-3.47 (2 H, m, H22), 3.32 (1 H, m, H11), 2.32 (s, 3H), 2.30–1.98 (5 H, m, H2, H4, H10, 11-OH), 1.78–1.43 (6 H, m, H1, H6, H7, H8, H13), 1.43 (3 H, s, H15), 1.35 (2 H, m, H13), 1.12 (4 H, m, H8, H18), 0.86 (3 H, d, J = 7.0 Hz, H17), 0.70 (3 H, d, J = 7.1 Hz, H16). 13C NMR (151 MHz, Chloroform-d) δ 217.16 (C3), 168.36 (C21), 157.10, 156.29, 140.71, 138.87 (C19), 137.13, 132.32, 131.63, 131.27, 130.29, 130.17, 121.99, 117.33 (C20), 113.08, 113.06, 107.41, 74.60 (C11), 69.64 (C14), 58.18 (C4), 45.43 (C9), 44.76 (C13), 43.87 (C12), 41.77 (C5), 37.77 (C6), 36.77 (C10), 35.97 (C2), 34.48 (C22), 30.41 (C8), 26.84 (C7), 26.37 (C18), 24.83 (C1), 20.66, 16.79 (C16), 14.89 (C15), 11.51 (C17). HR-MS (ESI): Calcd for C40H46ClN5O4S (M + Cl−): 762.2653; Found: 762.2654.
3.2.24. 22-[4-(6-Chloro-1-(3,4-dimethylphenyl)-1H-pyrazolo[3,4-d]pyrimidine-4-yl)amino-Phenylsulfanyl]-22-deoxypleuromutilin (14c)
Yellow powder; yield: 58.58%; melting point: 103–105 °C; 1H NMR (600 MHz, Chloroform-d) δ 11.09 (1 H, s), 8.20 (1 H, s), 7.88 (1 H, d, J = 8.8 Hz), 7.54 (1 H, d, J = 9.1 Hz), 7.37 (1 H, t, J = 8.4 Hz), 7.16 (1 H, t, J = 8.2 Hz), 6.77 (2 H, s), 6.59 (1 H, s), 6.36 (1 H, dd, J = 17.4, 11.0 Hz, H19), 5.62 (1 H, d, J = 8.5 Hz, H14), 5.27 (1 H, d, J = 11.0 Hz, H20), 5.13 (1 H, d, J = 17.4 Hz, H20), 3.40–3.32 (2 H, m, H22), 3.29 (1 H, d, J = 6.5 Hz, H11), 2.25 (6 H, s), 2.18–1.89 (5 H, m, H2, H4, H10, 11-OH), 1.70–1.43 (6 H, m, H1, H6, H7, H8), 1.28 (3 H, s, H15), 1.26 (2 H, m, H13), 1.09 (4 H, m, H8, H18), 0.85 (3 H, d, J = 7.0 Hz, H17), 0.53 (3 H, d, J = 7.0 Hz, H16). 13C NMR (151 MHz, Chloroform-d) δ 217.05 (C3), 168.27 (C21), 157.51, 157.26, 140.96, 138.88, 138.12 (C19), 137.21, 132.25, 131.69, 130.72, 130.23, 122.05, 117.35 (C20), 114.57, 110.59, 107.37, 74.62 (C11), 69.60 (C14), 58.17 (C4), 45.44 (C9), 44.78 (C13), 43.881 (C12), 41.78 (C5), 37.86 (C6), 36.77 (C10), 35.99 (C2), 34.46 (C22), 30.43 (C8), 26.84 (C7), 26.31 (C18), 24.84 (C1), 20.14, 19.02, 16.78 (C16), 14.88 (C15), 11.50 (C17). HR-MS (ESI): Calcd for C41H48ClN5O4S (M + Cl−): 776.2810; Found: 776.2810.
3.2.25. 22-[4-(6-Chloro-1-(3,5-dimethylphenyl)-1H-pyrazolo[3,4-d]pyrimidine-4-yl)amino-Phenylsulfanyl]-22-deoxypleuromutilin (15c)
Yellow powder; yield: 59%; melting point: 92–95 °C; 1H NMR (600 MHz, Chloroform-d) δ 11.36 (1 H, s), 8.22 (1 H, s), 7.73 (2 H, d, J = 8.6 Hz), 7.44 (2 H, d, J = 8.6 Hz), 6.68 (3 H, s), 6.50–6.37 (1 H, m, H19), 5.74 (1 H, d, J = 8.1 Hz, H14), 5.35–5.11 (2 H, m, H20), 3.62–3.51 (2 H, s, H22), 3.32 (1 H, m, H11), 2.33 (6 H, s), 2.21–2.02 (5 H, m, H2, H4, H10, 11-OH), 1.69–1.44 (6 H, m, H1, H6, H7, H8), 1.42 (3 H, s, H15), 1.35 (2 H, m, H13), 1.13 (1 H, m, H8), 1.11 (3 H, m, H18), 0.86 (3 H, d, J = 6.9 Hz, H17), 0.69 (3 H, d, J = 6.8 Hz, H16). 13C NMR (151 MHz, Chloroform-d) δ 217.04 (C3), 168.24 (C21), 157.65, 157.17, 156.57, 142.93, 139.63, 138.86 (C19), 137.23, 132.51, 131.68, 130.41, 123.76, 121.96, 117.36 (C20), 110.99, 107.32, 74.61 (C11), 69.60 (C14), 58.17 (C4), 45.44 (C9), 44.79 (C13), 43.88 (C12), 41.77 (C5), 37.89 (C6), 36.77 (C10), 35.99 (C2), 34.46 (C22), 30.43 (C8), 26.84 (C7), 26.30 (C18), 24.84 (C1), 21.53, 16.77 (C16), 14.88 (C15), 11.50 (C17). HR-MS (ESI): Calcd for C41H48ClN5O4S (M + Cl−): 776.2810; Found: 776.2812.
3.2.26. 22-[4-(6-Chloro-1-(4-ethylphenyl)-1H-pyrazolo[3,4-d]pyrimidine-4-yl)amino-Phenylsulfanyl]-22-deoxypleuromutilin (16c)
Yellow powder; yield: 74%; melting point: 97–99 °C; 1H NMR (600 MHz, Chloroform-d) δ 11.26 (1 H, s), 8.23 (1 H, s), 7.67 (2 H, d, J = 8.6 Hz), 7.45 (2 H, d, J = 10.9 Hz), 7.19 (2 H, d, J = 7.7 Hz), 6.97 (2 H, d, J = 7.5 Hz), 6.48–6.37 (1 H, m, H19), 5.74 (1 H, d, J = 8.1 Hz, H14), 5.33 (1 H, d, J = 11.0 Hz, H20), 5.17 (1 H, d, J = 17.4 Hz, H20), 3.58 (2 H, d, J = 15.7 Hz, H22), 3.32 (1 H, m, H11), 2.65 (2 H, d, J = 7.4 Hz), 2.33–2.03 (5 H, m, H2, H4, H10, 11-OH), 1.78–1.43 (6 H, m, H1, H6, H7, H8), 1.43 (3 H, s, H15), 1.34 (2 H, m, H13), 1.24 (3 H, m), 1.12 (3 H, m, H18), 1.06 (1 H, m, H8), 0.86 (3 H, d, J = 7.0 Hz, H17), 0.70 (3 H, d, J = 6.9 Hz, H16). 13C NMR (151 MHz, Chloroform-d) δ 217.05 (C3), 168.31 (C21), 157.61, 157.26, 156.53, 140.85, 138.90, 138.01 (C19), 137.10, 132.55, 131.67, 130.48, 129.09, 122.19, 117.35 (C20), 113.16, 107.31, 74.62 (C11), 69.61 (C14), 58.18 (C4), 45.45 (C9), 44.79 (C13), 43.89 (C12), 41.79 (C5), 37.81 (C6), 36.78 (C10), 36.00 (C2), 34.46 (C22), 30.43 (C8), 28.14, 26.85 (C7), 26.33 (C18), 24.84 (C1), 16.78 (C16), 15.78, 14.89 (C15), 11.51 (C17). HR-MS (ESI): Calcd for C41H48ClN5O4S (M + Cl−): 776.2810; Found: 776.2814.
3.2.27. 22-[4-(6-Chloro-1-(3-methoxyphenyl)-1H-pyrazolo[3,4-d]pyrimidine-4-yl)amino-Phenylsulfanyl]-22-deoxypleuromutilin (17c)
Yellow powder; yield: 73%; melting point: 97–102 °C; 1H NMR (600 MHz, Chloroform-d) δ 11.20 (1 H, s), 8.24 (1 H, s), 7.68 (2 H, d, J = 8.7 Hz), 7.45 (2 H, d, J = 8.7 Hz), 7.25 (1 H, t), 6.64–6.53 (3 H, m), 6.42 (1 H, dd, J = 17.4, 11.0 Hz, H19), 5.74 (1 H, d, J = 8.5 Hz, H14), 5.33 (1 H, dd, J = 11.0, 1.5 Hz, H20), 5.17 (1 H, dd, J = 17.4, 1H), 3.59–3.51 (2 H, m, H22), 3.35–3.29 (1 H, m, H11), 2.32–1.99 (5 H, m, H1, H6, H7, 11-OH), 1.77–1.46 (9 H, m, H1, H7, H6, H8, H40), 1.42 (3 H, s, H15), 1.39–1.27 (2H, m, H13), 1.11 (4 H, s, H8, H18), 0.86 (3 H, d, J = 7.0 Hz, H17), 0.70 (3 H, d, J = 7.1 Hz, H16). 13C NMR (151 MHz, Chloroform-d) δ 217.06 (C3), 168.26 (C21), 161.09, 157.93, 157.28, 156.79, 144.21, 138.87 (C19), 137.00, 133.24, 131.65, 130.66, 130.59, 122.23, 117.36 (C20), 107.13, 106.89, 105.67, 99.40, 74.62 (C11), 69.60 (C14), 58.17 (C4), 55.38, 45.44 (C9), 44.78 (C13), 43.88 (C12), 41.78 (C5), 37.81 (C6), 36.77 (C10), 35.99 (C2), 34.46 (C22), 30.43 (C8), 26.84 (C7), 26.31 (C18), 24.84 (C1), 16.77 (C16), 14.88 (C15), 11.50 (C17). HR-MS (ESI): Calcd for C40H46ClN5O5S (M + Cl−): 778.2602; Found: 778.2604.
3.2.28. 22-[4-(6-Chloro-1-(2-fluorophenyl)-1H-pyrazolo[3,4-d]pyrimidine-4-yl)amino-Phenylsulfanyl]-22-deoxypleuromutilin (18c)
Yellow powder; yield: 68%; melting point: 123–127 °C; 1H NMR (600 MHz, Chloroform-d) δ 11.12 (1 H, s), 8.32 (1 H, s), 7.63 (2 H, d, J = 8.6 Hz), 7.42 (2 H, d, J = 8.6 Hz), 7.26 (1 H, d, J = 12.9 Hz), 7.21–7.06 (2 H, m), 6.93 (1 H, t, J = 10.9 Hz), 6.42 (1 H, dd, J = 17.4, 11.0 Hz, H19), 5.74 (1 H, d, J = 8.5 Hz, H14), 5.31 (1 H, m, H20), 5.16 (1 H, d, J = 18.6 Hz, H20), 3.62–3.45 (2 H, m, H22), 3.33 (1 H, t, J = 7.6 Hz, H11), 2.32–1.99 (5 H, m, H2, H4, H10, 11-OH), 1.83–1.44 (6 H, m, H1, H6, H7, H8), 1.43 (3 H, s, H15), 1.39–1.18 (2 H, m, H13), 1.12 (3 H, m, H18), 1.08 (1 H, m, H8), 0.86 (3 H, d, J = 7.0 Hz, H17), 0.70 (3 H, d, J = 7.1 Hz, H16). 13C NMR (151 MHz, Chloroform-d) δ 217.15 (C3), 168.30 (C21), 158.28, 157.28, 157.03, 150.84, 149.24, 138.90 (C19), 136.88, 135.25, 131.58, 130.68, 125.06, 122.14, 121.44, 117.27 (C20), 115.76, 115.64, 113.76, 106.92, 74.59 (C11), 69.65 (C14), 58.16 (C4), 45.43 (C9), 44.78 (C13), 43.88 (C12), 41.77 (C5), 37.68 (C6), 36.76 (C10), 35.97 (C2), 34.45 (C22), 30.40 (C8), 26.83 (C7), 26.35 (C18), 24.82 (C1), 16.78 (C16), 14.88 (C15), 11.49 (C17). HR-MS (ESI): Calcd for C39H43ClFN5O4S (M + Cl−): 766.2402; Found: 766.2405.
3.2.29. 22-[4-(6-Chloro-1-(4-fluorophenyl)-1H-pyrazolo[3,4-d]pyrimidine-4-yl)amino-Phenylsulfanyl]-22-deoxypleuromutilin (19c)
Yellow powder; yield: 74%; melting point: 141–144 °C; 1H NMR (600 MHz, Chloroform-d) δ 11.19 (1 H, s), 8.64 (1 H, s), 7.60 (2 H, d, J = 8.7 Hz), 7.41 (d, J = 8.6 Hz, 2H), 7.04 (2 H, t, J = 8.5 Hz), 6.99-6.92 (2 H, m), 6.41 (1 H, dd, J = 17.4, 11.2 Hz, H19), 5.74 (1 H, d, J = 8.5 Hz, H14), 5.29 (1 H, m, H20), 5.16 (1 H, d, J = 18.6 Hz, H20), 3.57 (2 H, m, H22), 3.34 (1 H, t, J = 5.9 Hz, H11), 2.33–2.08 (5 H, m, H2, H4, H10, 11-OH), 1.77–1.44 (6 H, m, H1, H6, H7, H8), 1.43 (3 H, s, H15), 1.36 (2 H, m, H13), 1.12 (4 H, m, H8, H18), 0.86 (3 H, d, J = 7.0 Hz, H17), 0.70 (3 H, d, J = 7.1 Hz, H16). 13C NMR (151 MHz, Chloroform-d) δ 217.45 (C3), 168.39 (C21), 157.20, 156.97, 156.03, 139.72, 138.98 (C19), 136.94, 132.63, 131.38, 130.33, 121.80, 117.12, 116.27 (C20), 116.12, 114.03, 107.47, 74.56 (C11), 69.75 (C14), 58.18 (C4), 45.43 (C9), 44.77 (C13), 43.87 (C12), 41.78 (C5), 37.59 (C6), 36.77 (C10), 35.99 (C2), 34.49 (C22), 30.37 (C8), 26.82 (C7), 26.56 (C18), 24.81 (C1), 16.74 (C16), 14.88 (C15), 11.50 (C17). HR-MS (ESI): Calcd for C39H43ClFN5O4S (M + Cl−): 766.2402; Found: 766.2405.
3.2.30. 22-[4-(6-Chloro-1-(2,4-difluorophenyl)-1H-pyrazolo[3,4-d]pyrimidine-4-yl)amino-Phenylsulfanyl]-22-deoxypleuromutilin (20c)
Yellow powder; yield: 51%; melting point: 132–134 °C; 1H NMR (600 MHz, Chloroform-d) δ 11.04 (1 H, s), 8.37 (1 H, s), 7.61 (2 H, d, J = 8.7 Hz), 7.45 (2 H, d, J = 8.6 Hz), 7.25–7.17 (1 H, m, 1H), 6.99–6.87 (2 H, m), 6.43 (1 H, dd, J = 17.4, 11.0 Hz, H19), 5.74 (1 H, d, J = 8.5 Hz, H14), 5.33 (1 H, d, J = 11.0 Hz, H20), 5.17 (1 H, d, J = 18.8 Hz, H20), 3.56 (2 H, d, J = 3.7 Hz, H22), 3.36–3.29 (1 H, m, H11), 2.29-1.97 (5H, m, H2, H4, H10, 11-OH), 1.79–1.58 (6 H, m, H1, H6, H7, H8), 1.42 (3 H, s, H15), 1.35–1.25 (2 H, m, H13), 1.12 (4 H, m, H8, H18), 0.86 (3 H, d, J = 7.0 Hz, H17), 0.70 (3 H, d, J = 7.0 Hz, H16). 13C NMR (151 MHz, DMSO-d6) δ 217.59 (C3), 168.32 (C21), 160.01, 156.79, 155.80, 155.77, 148.48, 148.40, 141.29 (C19), 135.63, 129.45, 129.39, 115.79 (C20), 115.03, 115.00, 111.84, 111.67, 107.51, 104.62, 73.05 (C11), 70.51 (C14), 57.59 (C4), 45.43 (C9), 44.59 (C13), 44.44 (C12), 43.86 (C5), 41.94 (C6), 36.93 (C10), 36.74 (C2), 34.45 (C22), 30.57 (C8), 29.00 (C7), 27.07 (C18), 24.93 (C1), 16.61 (C16), 14.76 (C15), 12.00 (C17). HR-MS (ESI): Calcd for C39H42ClF2N5O4S (M + Cl−): 784.2308; Found: 784.2314
3.2.31. 22-[4-(6-Chloro-1-(2,5-difluorophenyl)-1H-pyrazolo[3,4-d]pyrimidine-4-yl)amino-Phenylsulfanyl]-22-deoxypleuromutilin (21c)
Yellow powder; yield: 77%; melting point: 115–118 °C; 1H NMR (600 MHz, Chloroform-d) δ 11.14 (1 H, s), 8.75 (1 H, s), 7.68 (2 H, d, J = 8.7 Hz), 7.51 (2 H, d, J = 8.4 Hz), 7.37–7.28 (1 H, m), 7.00 (1 H, t, J = 8.2 Hz), 6.75 (1 H, t, J = 8.4 Hz), 6.17 (1 H, dd, J = 17.2, 11.7 Hz, H19), 5.63 (1 H, d, J = 8.3 Hz, H14), 5.11–5.05 (2 H, m, H20), 3.98–3.82 (2 H, s, H22), 3.58–3.48 (1 H, m, H11), 2.34–2.10 (5 H, m, H2, H4, H10, 11- OH), 1.73–1.26 (11 H, m, H1, H6, H7, H8, H13, H15), 1.09 (4 H, m, H8, H18), 0.90 (3 H, d, J = 7.0 Hz, H17), 0.70 (3 H, d, J = 7.1 Hz, H16). 13C NMR (151 MHz, DMSO-d6) δ 217.54 (C3), 168.09 (C21), 160.27, 158.68, 157.96, 157.51, 155.83, 146.91, 145.34, 141.12 (C19), 136.58, 136.16, 133.53, 131.51, 130.02, 122.66, 117.11, 115.61 (C20), 107.89, 73.08 (C11), 70.22 (C14), 57.71 (C4), 45.40 (C9), 44.39 (C13), 44.17 (C12), 41.91 (C5), 36.81 (C6, C10), 36.23 (C2), 34.43 (C22), 30.55 (C8), 28.89 (C7), 27.02 (C18), 24.91 (C1), 16.52 (C16), 14.54 (C15), 11.94 (C17). HR-MS (ESI): Calcd for C39H42ClF2N5O4S (M + Cl−): 784.2308; Found: 784.2311.
3.2.32. 22-[4-(6-Chloro-1-(3,4-difluorophenyl)-1H-pyrazolo[3,4-d]pyrimidine-4-yl)amino-Phenylsulfanyl]-22-deoxypleuromutilin (22c)
Yellow powder; yield: 62%; melting point: 109–113 °C; 1H NMR (600 MHz, Chloroform-d) δ 11.05 (1 H, s), 8.58 (1 H, s), 7.62 (2 H, d, J = 8.6 Hz), 7.43 (2 H, d, J = 8.6 Hz), 7.12 (1 H, d, J = 9.2 Hz), 6.89–6.82 (1 H, m), 6.68 (1 H, d, J = 8.5 Hz), 6.42 (1 H, dd, J = 17.4, 11.0 Hz, H19), 5.74 (1 H, d, J = 8.6 Hz, H14), 5.31 (1 H, d, J = 10.8 Hz, H20), 5.17 (1 H, d, J = 17.4 Hz, H20), 3.62–3.51 (2 H, m, H22), 3.34 (1 H, d, J = 6.3 Hz, H11), 2.35–2.11 (5 H, m, H2, H4, H10, 11-OH), 1.81–1.50 (6 H, m, H1, H6, H7, H8), 1.42 (3 H, s, H15), 1.35 (2 H, m, H13), 1.13 (3 H, s, H18), 1.07 (1 H, m, H8), 0.86 (3 H, d, J = 7.0 Hz, H17), 0.70 (3 H, d, J = 7.0 Hz, H16). 13C NMR (151 MHz, Chloroform-d) δ 217.29 (C3), 168.39 (C21), 158.10, 157.19, 156.90, 139.07, 138.90 (C19), 136.79, 134.39, 134.20, 131.58 130.79, 122.01, 118.26, 118.14, 117.30 (C20), 117.15, 106.98, 102.19, 74.62 (C11), 69.73 (C14), 58.18 (C4), 45.46 (C9), 44.79 (C13), 43.89 (C12), 41.75 (C5), 37.66 (C6), 36.79 (C10), 35.99 (C2), 34.49 (C22), 30.42 (C8), 26.84 (C7), 26.37 (C18), 24.83 (C1), 16.79 (C16), 14.88 (C15), 11.50 (C17). HR-MS (ESI): Calcd for C39H42ClF2N5O4S (M + Cl−): 784.2308; Found: 784.2313.
3.2.33. 22-[4-(6-Chloro-1-(4-(yrifluoromethoxy)Phenyl)-1H-pyrazolo[3,4-d]pyrimidine-4-yl)amino-Phenylsulfanyl]-22-deoxypleuromutilin (23c)
Yellow powder; yield: 69%; melting point: 144–147 °C; 1H NMR (600 MHz, DMSO-d6) δ 11.17 (1 H, s), 8.40 (1 H, s), 7.63 (2 H, d, J = 8.8 Hz), 7.47 (2 H, d, J = 8.8 Hz), 7.32 (2 H, d, J = 8.5 Hz), 7.11–7.06 (2 H, d, J = 8.5 Hz), 6.10–5.99 (1 H, m, H19), 5.52 (1 H, d, J = 8.3 Hz, H14), 4.99–4.95 (2 H, m, H20), 3.91–3.76 (2 H, m, H22),3.34 (1 H, dd, H11), 2.19–1.99 (5 H, m, H2, H4, H10, 11-OH), 1.68-1.36 (5 H, m, H1, H6, H7), 1.34 (3 H, s, H15), 1.31–1.19 (3 H, m, H8, H13), 0.98 (4 H, s, H8, H18), 0.80 (3 H, d, J = 7.2 Hz, H17), 0.59 (3 H, d, J = 7.0 Hz, H16). 13C NMR (151 MHz, DMSO-d6) δ 217.60 (C3), 168.12 (C21), 157.54, 157.27, 155.38, 143.34, 142.02, 141.17 (C19), 136.16, 133.70, 131.43, 130.20, 123.19, 115.61 (C20), 113.57, 108.19, 73.05 (C11), 70.24 (C14), 57.71 (C4), 45.40 (C13), 44.42 (C12), 44.13, 41.99 (C5), 36.84 (C6), 36.81 (C10), 36.12 (C2), 34.43 (C22), 30.53 (C8), 28.95 (C7), 27.03 (C18), 24.91 (C1), 16.54 (C16), 14.97 (C15), 11.94 (C17). HR-MS (ESI): Calcd for C40H43ClF3N5O5S (M + Cl−): 832.2320; Found: 832.2327.
3.2.34. 22-[4-(6-Chloro-1-(3-chlorophenyl)-1H-pyrazolo[3,4-d]pyrimidine-4-yl)amino-Phenylsulfanyl]-22-deoxypleuromutilin (24c)
Yellow powder; yield: 73%; melting point: 121–124 °C; 1H NMR (600 MHz, Chloroform-d) δ 11.09 (1 H, s), 8.60 (1 H, s), 7.66 (2 H, d, J = 8.6 Hz), 7.40 (2 H, d, J = 8.6 Hz), 7.20 (1 H, t, J = 8.0 Hz), 7.03 (1 H, s), 6.91 (1 H, d, J = 7.9 Hz, 1H), 6.79 (1 H, d, J = 9.5 Hz), 6.41 (1 H, dd, J = 17.4, 11.0 Hz, H19), 5.73 (1 H, d, J = 8.5 Hz, H14), 5.29 (1 H, d, J = 9.5 Hz, H20), 5.16 (1 H, d, J = 17.5 Hz, H20), 3.63–3.52 (2 H, m, H22), 3.33 (1 H, t, J = 7.3 Hz, H11), 2.31–2.00 (5 H, m, H2, H4, H10, 11-OH), 1.79–1.43 (6 H, m, H1, H6, H7, H8), 1.42 (3 H, s, H15), 1.34 (2 H, m, H13), 1.13 (3 H, s, H18), 1.07 (1 H, m, H8), 0.86 (3 H, d, J = 7.0 Hz, H17), 0.71 (3 H, d, J = 7.0 Hz, H16). 13C NMR (151 MHz, Chloroform-d) δ 217.17 (C3), 168.35 (C21), 158.27, 157.18, 157.05, 144.12, 138.86 (C19), 136.92, 135.67, 134.29, 131.70, 130.77, 130.65, 121.98, 121.56, 117.38 (C20), 112.99, 111.19, 106.93, 74.63 (C11), 69.67 (C14), 58.19 (C4), 45.45 (C9), 44.79 (C13), 43.89 (C12), 41.79 (C5), 37.78 (C6), 36.79 (C10), 35.99 (C2), 34.49 (C22), 30.43 (C8), 26.85 (C7), 26.34 (C18), 24.84 (C1), 16.81 (C16), 14.90 (C15), 11.51 (C17). HR-MS (ESI): Calcd for C39H43Cl2N5O4S (M + Cl−): 784.2077; Found: 784.2079.
3.2.35. 22-[4-(6-Chloro-1-(4-chlorophenyl)-1H-pyrazolo[3,4-d]pyrimidine-4-yl)amino-Phenylsulfanyl]-22-deoxypleuromutilin (25c)
Yellow powder; yield: 65%; melting point: 124–129 °C; 1H NMR (600 MHz, Chloroform-d) δ 11.11 (1 H, s), 8.33 (1 H, s), 7.61 (2 H, d, J = 8.6 Hz), 7.42 (2 H, d, J = 8.6 Hz), 7.29 (2 H, d, J = 8.7 Hz), 6.95 (2 H, d, J = 8.8 Hz), 6.42 (1 H, dd, J = 17.4, 11.0 Hz, H19), 5.74 (1 H, d, J = 8.5 Hz, H14), 5.31 (1 H, d, J = 12.3 Hz, H20), 5.17 (1 H, d, J = 17.4 Hz, H20), 3.62–3.54 (2 H, m, H22), 3.33 (1 H, d, J = 6.3 Hz, H11), 2.34–2.08 (5 H, m, H2, H4, H10, 11-OH), 1.81–1.51 (6 H, m, H1, H6, H7, H8), 1.42 (3 H, s, H15), 1.39–1.30 (2 H, m, H13), 1.12 (3 H, d, J = 7.0 Hz, H18), 1.08 (1 H, m, H8), 0.86 (3 H, d, J = 7.0 Hz, H17), 0.70 (3 H, d, J = 7.1 Hz, H16). 13C NMR (151 MHz, Chloroform-d) δ 217.16 (C3), 168.35 (C21), 158.03, 157.21, 156.87, 141.66 (C19), 138.91, 136.87, 133.85, 131.60, 130.73, 129.70, 126.52, 122.09, 117.33 (C20), 114.13, 107.02, 74.62 (C11), 69.69 (C14), 58.18 (C4), 45.45 (C9), 44.79 (C13), 43.90 (C12), 41.78 (C5), 37.71 (C6), 36.77 (C10), 35.99 (C2), 34.48 (C22), 30.41 (C8), 26.85 (C7), 26.37 (C18), 24.83 (C1), 16.80 (C16), 14.89 (C15), 11.50 (C17). HR-MS (ESI): Calcd for C39H43Cl2N5O4S (M + Cl−): 784.2077; Found: 784.2076.
3.2.36. 22-[4-(6-Chloro-1-(3,5-dichlorophenyl)-1H-pyrazolo[3,4-d]pyrimidine-4-yl)amino-Phenylsulfanyl]-22-deoxypleuromutilin (26c)
Yellow powder; yield: 73%; melting point: 137–139 °C; 1H NMR (600 MHz, Chloroform-d) δ 11.01 (1 H, s), 8.29 (1 H, s), 7.71 (2 H, d, J = 8.7 Hz), 7.46 (2 H, d, J = 8.7 Hz), 6.97 (1 H, t, J = 1.7 Hz), 6.93 (2 H, d, J = 1.7 Hz), 6.42 (1 H, dd, J = 17.4, 11.0 Hz, H19), 5.74 (1 H d, J = 8.5 Hz, H14), 5.32 (1 H, d, J = 11.0 Hz, H20), 5.17 (1 H, d, J = 16.1 Hz, H20), 3.61–3.53 (2 H, m, H22), 3.38–3.28 (1 H, m, H11), 2.31–1.99 (5 H, m, H2, H4, H10, 11-OH), 1.73–1.42 (6 H, m, H1, H6, H7, H8), 1.41 (3 H, s, H15), 1.36 (2 H, m, H13), 1.12 (4 H, s, H8, H18), 0.86 (3 H, d, J = 7.0 Hz, H17), 0.70 (3 H, d, J = 7.0 Hz, H16). 13C NMR (151 MHz, Chloroform-d) δ 217.10 (C3), 168.28 (C21), 158.82, 157.54, 157.22, 144.66, 138.86 (C19), 136.75, 136.23, 135.55, 131.72, 130.90, 121.93, 121.38, 117.37 (C20), 111.40, 106.58, 74.63 (C11), 69.65 (C14), 58.18 (C4), 45.45 (C9), 44.80 (C13), 43.89 (C12), 41.78 (C5), 37.77 (C6), 36.78 (C10), 35.99 (C2), 34.47 (C22), 30.43 (C8), 26.85 (C7), 26.32 (C18), 24.84 (C1), 16.80 (C16), 14.88 (C15), 11.50 (C17). HR-MS (ESI): Calcd for C39H42Cl3N5O4S (M + Cl−): 818.1688; Found: 818.1698.
3.2.37. 22-[4-(6-Chloro-1-(3-nitrophenyl)-1H-pyrazolo[3,4-d]pyrimidine-4-yl)amino-Phenylsulfanyl]-22-deoxypleuromutilin (27c)
Yellow powder; yield: 74%; melting point: 137–139 °C; 1H NMR (600 MHz, Chloroform-d) δ 10.90 (1 H, s), 8.79 (1 H, s), 7.87 (1 H, s), 7.75 (3 H, d, J = 8.5 Hz), 7.43 (3 H, d, J = 8.5 Hz), 7.16 (1 H, d, J = 9.3 Hz), 6.41 (1 H, dd, J = 17.4, 11.0 Hz, H19), 5.73 (1 H, d, J = 8.5 Hz, H14), 5.27 (1 H, d, J = 11.4 Hz, H20), 5.17 (1 H, d, J = 17.4 Hz, H20), 3.62-3.52 (2 H, m, H22), 3.35 (1 H, s, H11), 2.31–2.03 (5 H, m, H2, H4, H10, 11-OH), 1.80–1.46 (6 H, m, H1, H6, H7, H8), 1.43 (3 H, s, H15), 1.40–1.26 (2H, m, H13), 1.13 (4 H, m, H8, H18), 0.86 (3 H, d, J = 7.0 Hz, H17), 0.73 (3 H, d, J = 7.0 Hz, H16). 13C NMR (151 MHz, DMSO-d6) δ 216.83 (C3), 168.12 (C21), 158.32, 156.96, 156.64, 148.96, 143.60, 138.43 (C19), 136.25, 135.29, 131.21, 130.40, 130.02, 121.52, 118.17, 116.92 (C20), 115.42, 106.19, 74.19 (C11), 69.42 (C11), 57.73 (C4), 45.03 (C9), 44.32 (C13), 43.51 (C12), 41.38 (C5), 37.21 (C6), 36.37 (C10), 35.55 (C2), 34.08 (C22), 30.00 (C8), 26.44 (C7), 25.90 (C18), 24.40 (C1), 16.45 (C16), 14.46 (C15), 11.06 (C17). HR-MS (ESI): Calcd for C39H43ClN6O6S (M + Cl−): 793.2347; Found: 793.2353.
3.2.38. 22-[4-(6-Chloro-1-(naphthalen-2-yl)-1H-pyrazolo[3,4-d]pyrimidine-4-yl-yl)amino-Phenylsulfanyl]-22-deoxypleuromutilin (28c)
Yellow powder; yield: 63%; melting point: 115–118 °C; 1H NMR (600 MHz, Chloroform-d) δ 11.32 (1 H, s), 8.33 (1 H, s), 7.79–7.70 (4 H, m, 4H), 7.64 (1 H, d, J = 8.2 Hz), 7.45 (3 H, d, J = 8.0 Hz), 7.34 (2 H, s), 7.15 (1 H, d, J = 8.6 Hz), 6.42 (1 H, dd, J = 17.4, 11.0 Hz, H19), 5.74 (1 H, d, J = 8.4 Hz, H14), 5.28(1 H, m, H20), 5.14 (1 H, d, J = 17.4 Hz, H20), 3.63–3.50 (2 H, m, H22), 3.31 (1 H, d, J = 5.3 Hz, H11), 2.31–2.02 (5 H, m, H2, H4, H10, 11-OH), 1.77–1.48 (6 H, m, H1, H6, H7, H8), 1.43 (3 H, s, H15), 1.30 (2 H, m, H13), 1.14 (1 H, m, H8), 1.10 (3 H, s, H18), 0.84 (3 H, d, J = 6.9 Hz, H17), 0.71 (3 H, d, J = 7.1 Hz, H16). 13C NMR (151 MHz, Chloroform-d) δ 217.18 (C3), 168.33 (C21), 157.74, 157.10, 156.58, 140.52, 138.84 (C19), 137.14, 134.38, 134.02, 133.33, 131.72, 130.43, 129.79, 129.45, 127.92, 127.21, 126.39, 124.03, 121.98, 117.36 (C20), 114.96, 107.51, 74.59 (C11), 69.65 (C14), 58.17 (C4), 45.43 (C9), 44.77 (C13), 43.87 (C12), 41.77 (C5), 37.80 (C6), 36.77 (C10), 35.97 (C2), 34.46 (C22), 30.40 (C8), 26.84 (C7), 26.34 (C18), 24.81 (C1), 16.80 (C16), 14.90 (C15), 11.49 (C17). HR-MS (ESI): Calcd for C43H46ClN5O4S (M + Cl−): 798.2653; Found: 798.2651.