Comparison between Cashew-Based and Petrochemical Hydroxyoximes: Insights from Molecular Simulations
Abstract
1. Introduction
2. Results
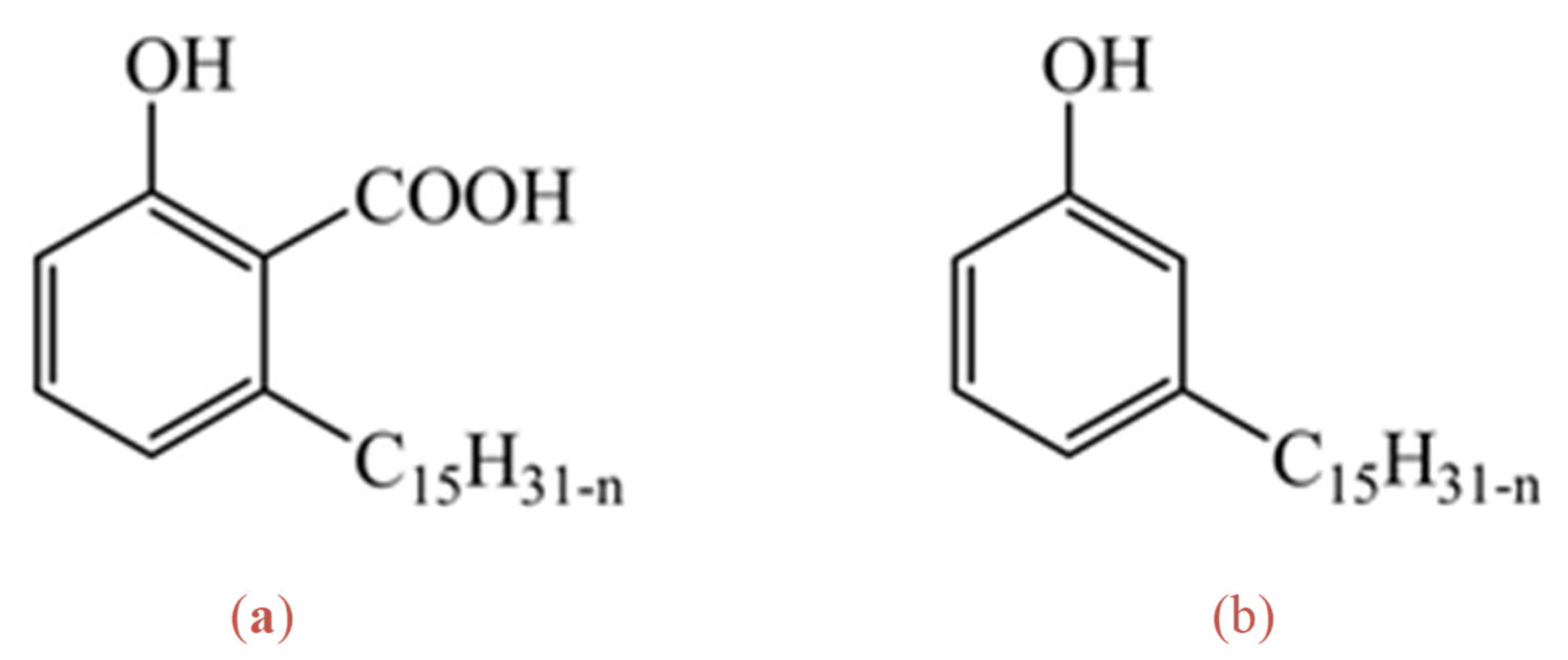
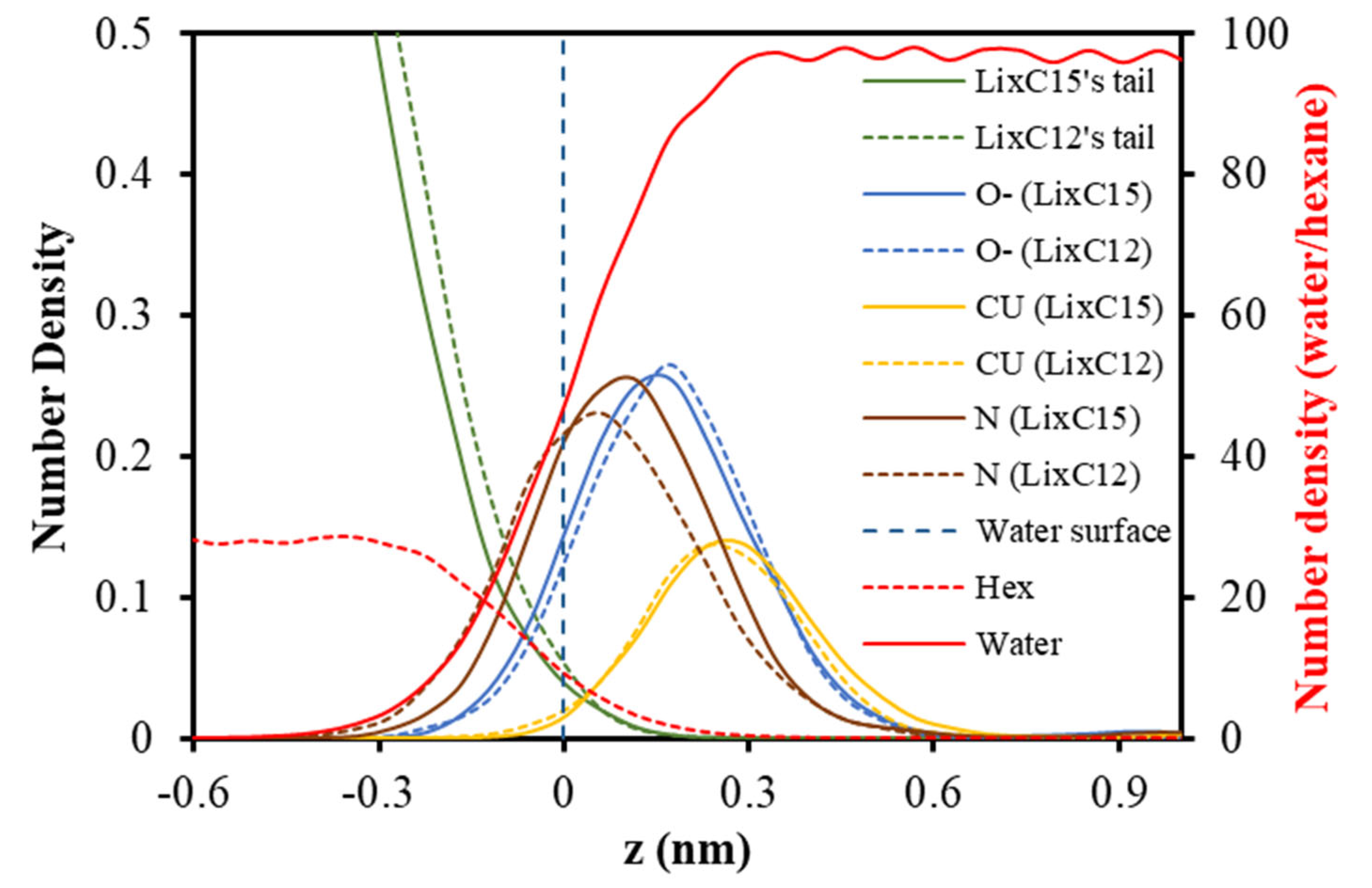
2.1. Interfacial Molecular Structure
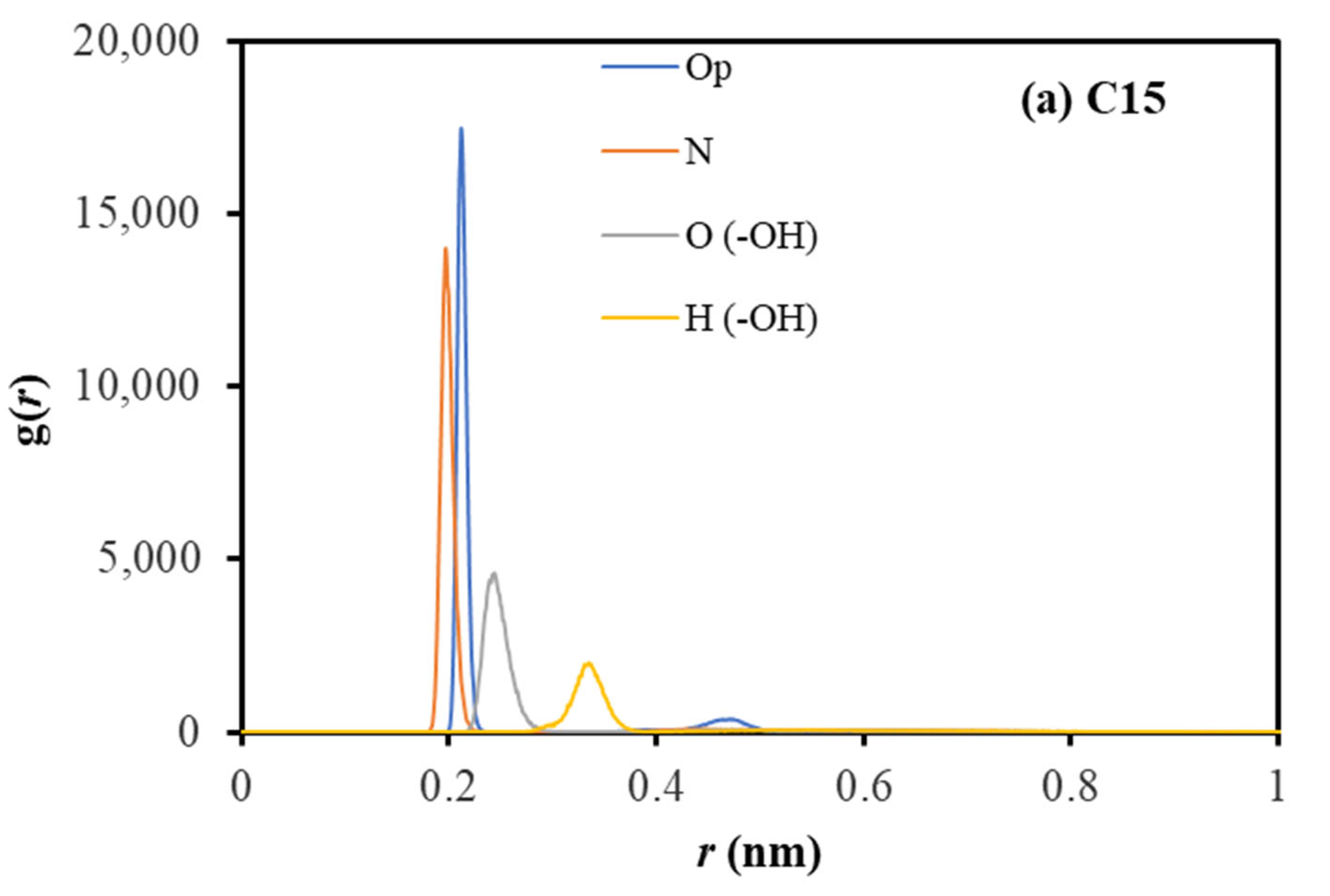
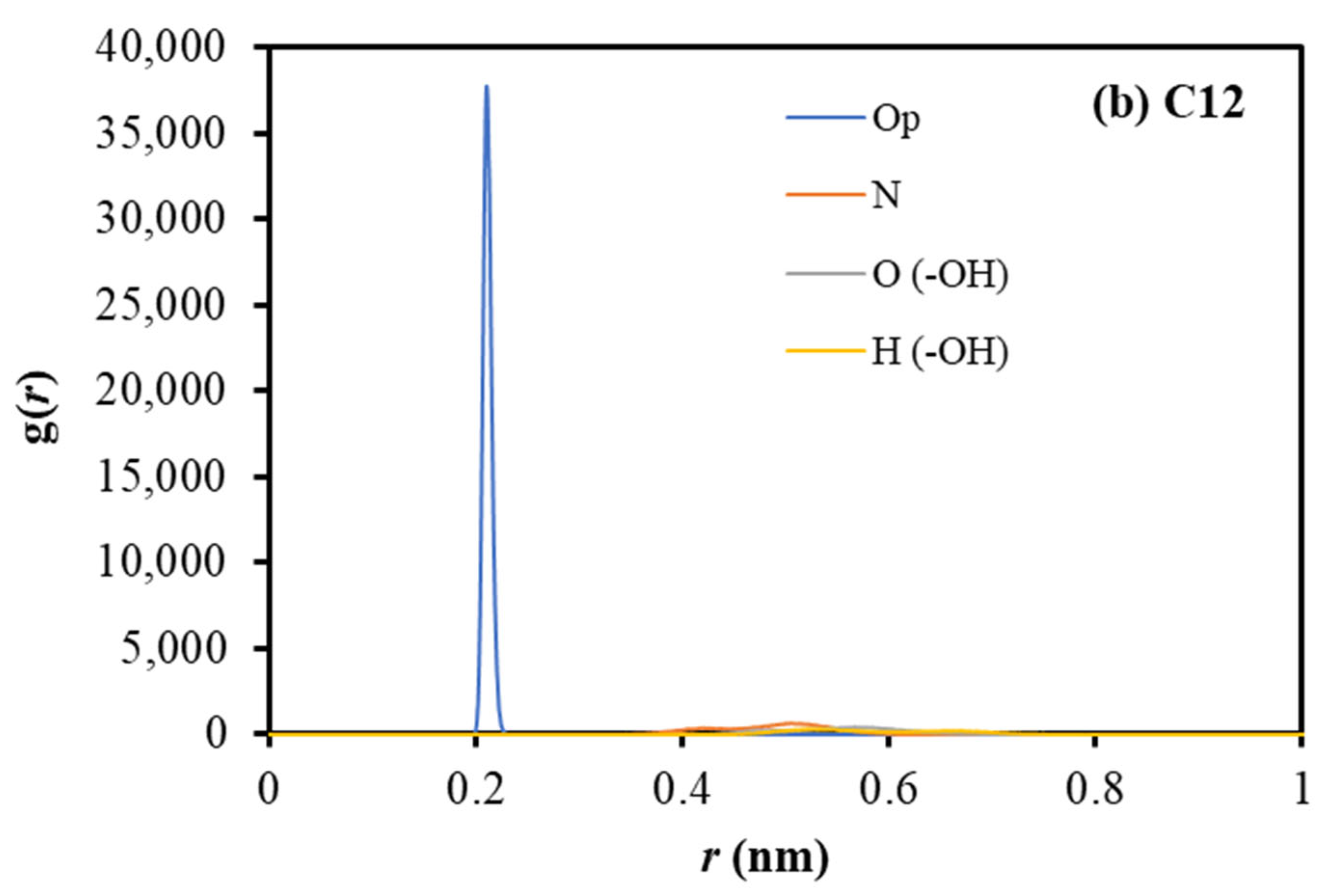
2.2. Coordination Structure of Extractant and Cu2+
3. Discussion
4. Materials and Methods
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Zeng, X.; Li, J.; Shen, B. Novel Approach to Recover Cobalt and Lithium from Spent Lithium-Ion Battery Using Oxalic Acid. J. Hazard. Mater. 2015, 295, 112–118. [Google Scholar] [CrossRef] [PubMed]
- Lèbre, É.; Stringer, M.; Svobodova, K.; Owen, J.R.; Kemp, D.; Côte, C.; Arratia-Solar, A.; Valenta, R.K. The social and environmental complexities of extracting energy transition metals. Nat. Commun. 2020, 11, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Seck, G.S.; Hache, E.; Bonnet, C.; Simoën, M.; Carcanague, S. Copper at the Crossroads: Assessment of the Interactions between Low-Carbon Energy Transition and Supply Limitations. Resour. Conserv. Recycl. 2020, 163, 105072. [Google Scholar] [CrossRef] [PubMed]
- Henckens, M.; Worrell, E. Reviewing the availability of copper and nickel for future generations. The balance between production growth, sustainability and recycling rates. J. Clean. Prod. 2020, 264, 121460. [Google Scholar] [CrossRef]
- Sastre, A.M.; Szymanowski, J. Discussion of the Physicochemical Effects of Modifiers on the Extraction Properties of Hydroxyoximes. A Review. Solvent Extr. Ion. Exch. 2004, 22, 737–759. [Google Scholar] [CrossRef]
- Li, L.; Zhai, L.; Zhang, X.; Lu, J.; Chen, R.; Wu, F.; Amine, K. Recovery of Valuable Metals from Spent Lithium-Ion Batteries by Ultrasonic-Assisted Leaching Process. J. Power Sources 2014, 262, 380–385. [Google Scholar] [CrossRef]
- Musariri, B.; Akdogan, G.; Dorfling, C.; Bradshaw, S. Evaluating organic acids as alternative leaching reagents for metal recovery from lithium ion batteries. Miner. Eng. 2019, 137, 108–117. [Google Scholar] [CrossRef]
- Szymanowski, J. Hydroxyoximes and Copper Hydrometallurgy; CRC Press: Boca Raton, FL, USA, 1993; ISBN 0849349400. [Google Scholar]
- Wilson, A.M.; Bailey, P.J.; Tasker, P.A.; Turkington, J.R.; Grant, R.A.; Love, J.B. Solvent Extraction: The Coordination Chemistry behind Extractive Metallurgy. Chem. Soc. Rev. 2014, 43, 123–134. [Google Scholar] [CrossRef]
- Flett, D.S. New Reagents or New Ways with Old Reagents. J. Chem. Technol. Biotechnol. 1999, 74, 99–105. [Google Scholar] [CrossRef]
- Lorenc, J.F.; Lambeth, G.; Scheffer, W. Alkylphenols. Kirk-Othmer Encyclopedia of Chemical Technology; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2000. [Google Scholar]
- Agarwal, S.; Ferreira, A.E.; Santos, S.; Reis, T.; Ismael, R.; Correia, M.J.N.; Carvalho, J.M. Separation and recovery of copper from zinc leach liquor by solvent extraction using Acorga M5640. Int. J. Miner. Process. 2010, 97, 85–91. [Google Scholar] [CrossRef]
- Hoang, A.S.; Nguyen, H.N.; Bui, N.Q.; Vu, H.S.; Vo, T.P.; Nguyen, T.V.; Phan, C.M. Extraction of gallium from Bayer liquor using extractant produced from cashew nutshell liquid. Miner. Eng. 2015, 79, 88–93. [Google Scholar] [CrossRef]
- Phan, C.M.; Hoang, S.A.; Vu, S.H.; Nguyen, H.M.; Nguyen, C.V.; Hyde, A.E.; Yusa, S.-I. Application of a cashew-based oxime in extracting Ni, Mn and Co from aqueous solution. Chem. Biol. Technol. Agric. 2021, 8, 1–7. [Google Scholar] [CrossRef]
- Saygin, D.; Gielen, D. Zero-Emission Pathway for the Global Chemical and Petrochemical Sector. Energies 2021, 14, 3772. [Google Scholar] [CrossRef]
- Mgaya, J.; Shombe, G.B.; Masikane, S.C.; Mlowe, S.; Mubofu, E.B.; Revaprasadu, N. Cashew Nut Shell: A Potential Bio-Resource for the Production of Bio-Sourced Chemicals, Materials and Fuels. Green Chem. 2019, 21, 1186–1201. [Google Scholar] [CrossRef]
- Vasapollo, G.; Mele, G.; Del Sole, R. Cardanol-Based Materials as Natural Precursors for Olefin Metathesis. Molecules 2011, 16, 6871–6882. [Google Scholar] [CrossRef]
- Mota, J.P.F.; Ribeiro, V.G.P.; da Silva, F.L.F.; Junior, A.E.C.; Oliveira, D.R.; Kotzebue, L.R.V.; Mele, G.; Lomonaco, D.; Mazzetto, S.E. Developing Eco-Friendly Methods for Purification of Compounds Derived from Hydrogenated Cardanol. Sep. Sci. Technol. 2016, 51, 2473–2483. [Google Scholar] [CrossRef]
- Grüter, R.; Trachsel, T.; Laube, P.; Jaisli, I. Expected Global Suitability of Coffee, Cashew and Avocado Due to Climate Change. PLoS ONE 2022, 17, e0261976. [Google Scholar] [CrossRef]
- Liang, Z.; Bu, W.; Schweighofer, K.J.; Walwark, D.J.; Harvey, J.S.; Hanlon, G.R.; Amoanu, D.; Erol, C.; Benjamin, I.; Schlossman, M.L. Nanoscale View of Assisted Ion Transport across the Liquid–Liquid Interface. Proc. Natl. Acad. Sci. USA 2019, 116, 18227–18232. [Google Scholar] [CrossRef]
- Danesi, P.R.; Chiarizia, R.; Coleman, C.F. The Kinetics of Metal Solvent Extraction. Crit. Rev. Anal. Chem. 1980, 10, 1–126. [Google Scholar] [CrossRef]
- Phan, C.M. Ionization of Surfactants at the Air-Water Interface; Elsevier Inc.: Amsterdam, The Netherlands, 2018; ISBN 9780128136423. [Google Scholar]
- Szymanowski, J.; Cox, M.; Hirons, C.G. The Determination of Hydrophilic Lipophilic Balance Values for Some Hydroxyoximes and Their Correlation with Rates of Copper Extraction. J. Chem. Technol. Biotechnol. Chem. Technol. 2007, 34, 218–226. [Google Scholar] [CrossRef]
- Shannon, R.D. Revised Effective Ionic Radii and Systematic Studies of Interatomic Distances in Halides and Chalcogenides. Acta Crystallogr. Sect. A 1976, 32, 751–767. [Google Scholar] [CrossRef]
- Smith, A.G.; Tasker, P.A.; White, D.J. The Structures of Phenolic Oximes and Their Complexes. Coord. Chem. Rev. 2003, 241, 61–85. [Google Scholar] [CrossRef]
- El-Hefny, N.E. Chemical Kinetics and Reaction Mechanisms in Solvent Extraction: New Trends and Applications. J. Phys. Sci. 2017, 28, 129–156. [Google Scholar] [CrossRef]
- Fatehi, M.; Mohebbi, A.; Moradi, A. Understanding the Structural, Dynamic and Thermodynamic Properties of 5-Nonylsalicylaldoxime: Molecular Dynamics and Experimental Studies. J. Mol. Liq. 2018, 271, 290–300. [Google Scholar] [CrossRef]
- Ciszewski, R.K.; Gordon, B.P.; Muller, B.N.; Richmond, G.L. Takes Two to Tango: Choreography of the Coadsorption of CTAB and Hexanol at the Oil-Water Interface. J. Phys. Chem. B. 2019, 123, 8519–8531. [Google Scholar] [CrossRef]
- Schabes, B.K.; Altman, R.M.; Richmond, G.L. Come Together: Molecular Details into the Synergistic Effects of Polymer-Surfactant Adsorption at the Oil/Water Interface. J. Phys. Chem. B. 2018, 122, 8582–8590. [Google Scholar] [CrossRef]
- Nguyen, C.V.; Nguyen, T.V.; Phan, C.M. Adsorption of alkyltrimethylammonium bromide surfactants at the air/water interface. Int. J. Heat Mass Transf. 2017, 106, 1035–1040. [Google Scholar] [CrossRef]
- Phan, C.M.; Le, T.N.; Nguyen, C.V.; Yusa, S. Modeling Adsorption of Cationic Surfactants at Air/Water Interface without Using the Gibbs Equation. Langmuir 2013, 29, 4743–4749. [Google Scholar] [CrossRef]
- Scoppola, E.; Micciulla, S.; Kuhrts, L.; Maestro, A.; Campbell, R.A.; Konovalov, O.V.; Fragneto, G.; Schneck, E. Reflectometry Reveals Accumulation of Surfactant Impurities at Bare Oil/Water Interfaces. Molecules 2019, 24, 4113. [Google Scholar] [CrossRef]
- Akutsu-Suyama, K.; Yamada, N.L.; Ueda, Y.; Motokawa, R.; Narita, H. New Design of a Sample Cell for Neutron Reflectometry in Liquid–Liquid Systems and Its Application for Studying Structures at Air–Liquid and Liquid–Liquid Interfaces. Appl. Sci. 2022, 12, 1215. [Google Scholar] [CrossRef]
- Jiang, F.; Pei, J.; Yin, S.; Zhang, L.; Peng, J.; Ju, S.; Miller, J.D.; Wang, X. Solvent extraction and stripping of copper in a Y-Y type microchannel reactor. Miner. Eng. 2018, 127, 296–304. [Google Scholar] [CrossRef]
- Lin, S.H.; Juang, R.S. Mass-Transfer in Hollow-Fiber Modules for Extraction and Back-Extraction of Copper(II) with LIX64N Carriers. J. Memb. Sci. 2001, 188, 251–262. [Google Scholar] [CrossRef]
- Bogacki, M.B.; Jakubiak, A.; Cote, G.; Szymanowski, J. Dialkyl Pyridinedicarboxylates’ Extraction Ability toward Copper(II) from Chloride Solutions and Its Modification with Alcohols. Ind. Eng. Chem. Res. 1997, 36, 838–845. [Google Scholar] [CrossRef]
- Barhoum, R.; Prochaska, K.; Szymanowski, J. Effect of alkyl chain length on 2-and 4-alkylphenol and 2-hydroxy-5-alkylbenzaldehyde oxime adsorption at the hydrocarbon/water interface. J. Colloid Interface Sci. 1998, 204, 394–397. [Google Scholar] [CrossRef]
- Hess, B.; Kutzner, C.; van der Spoel, D.; Lindahl, E.; van der Spoel, D.; Lindahl, E. GROMACS 4: Algorithms for Highly Efficient, Load-Balanced, and Scalable Molecular Simulation. J. Chem. Theory Comput. 2008, 4, 435–447. [Google Scholar] [CrossRef]
- Chen, F.; Smith, P.E. Simulated Surface Tensions of Common Water Models. J. Chem. Phys. 2007, 126, 221101. [Google Scholar] [CrossRef]
- Mark, P.; Nilsson, L. Structure and Dynamics of the TIP3P, SPC, and SPC/E Water Models at 298 K. J. Phys. Chem. A 2001, 105, 9954–9960. [Google Scholar] [CrossRef]
- Schüttelkopf, A.W.; van Aalten, D.M.F. PRODRG: A tool for high-throughput crystallography of protein–ligand complexes. Acta Crystallogr. Sect. D Biol. Crystallogr. 2004, 60, 1355–1363. [Google Scholar] [CrossRef]
- Evans, D.J.; Holian, B.L. The Nose–Hoover thermostat. J. Chem. Phys. 1985, 83, 4069–4074. [Google Scholar] [CrossRef]
- Hess, B.; Bekker, H.; Berendsen, H.J.C.; Fraaije, J.G.E.M. LINCS: A Linear Constraint Solver for Molecular Simulations. J. Comput. Chem. 1997, 18, 1463–1472. [Google Scholar] [CrossRef]
- Deserno, M.; Holm, C. How to mesh up Ewald sums. II. An accurate error estimate for the particle–particle–particle-mesh algorithm. J. Chem. Phys. 1998, 109, 7694–7701. [Google Scholar] [CrossRef]
- Lazarova, Z.; Lazarova, M. Hollow-Fiber Membrane Extraction of Copper from Aqueous Nitrate Media by LIX® Reagents: Comparison of Extraction Efficiency and Fractional Resistances. Solvent Extr. Ion. Exch. 2011, 29, 128–145. [Google Scholar] [CrossRef]
- Kuipa, P.K.; Hughes, M.A. Diluent Effect on the Solvent Extraction Rate of Copper. Sep. Sci. Technol. 2002, 37, 1135–1152. [Google Scholar] [CrossRef]

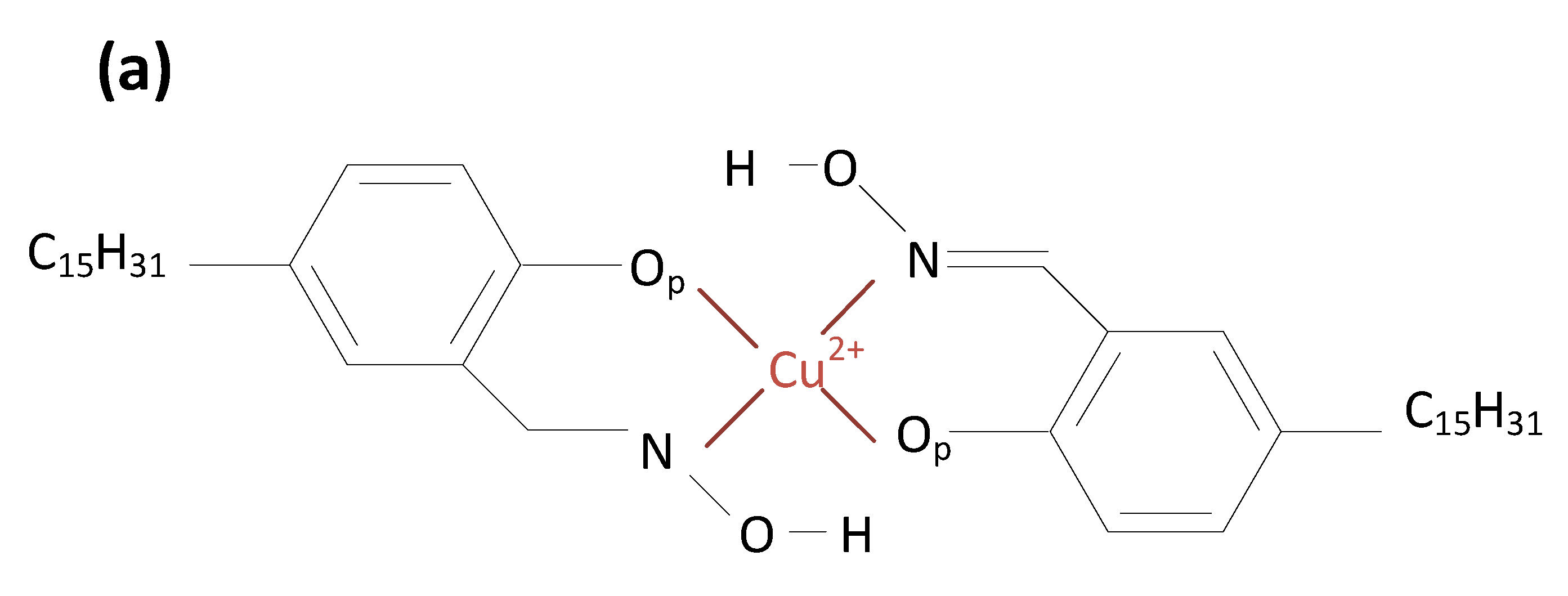
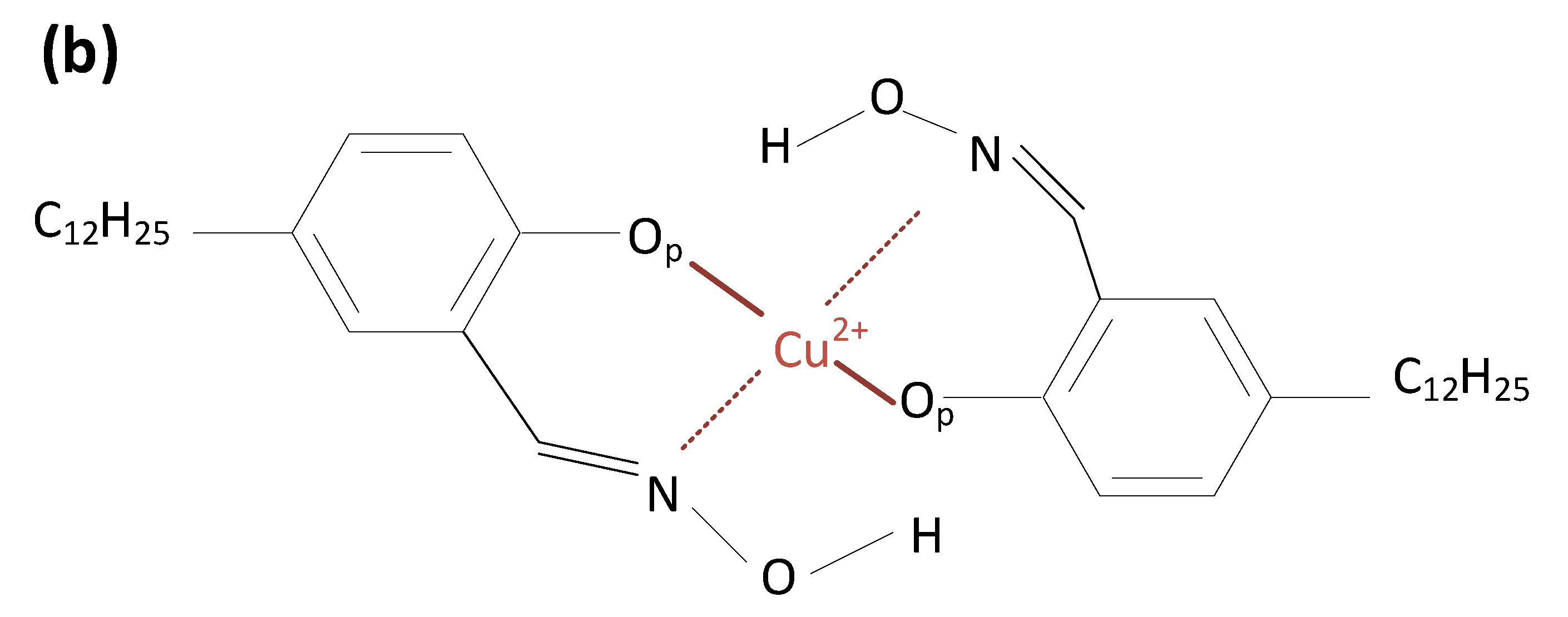

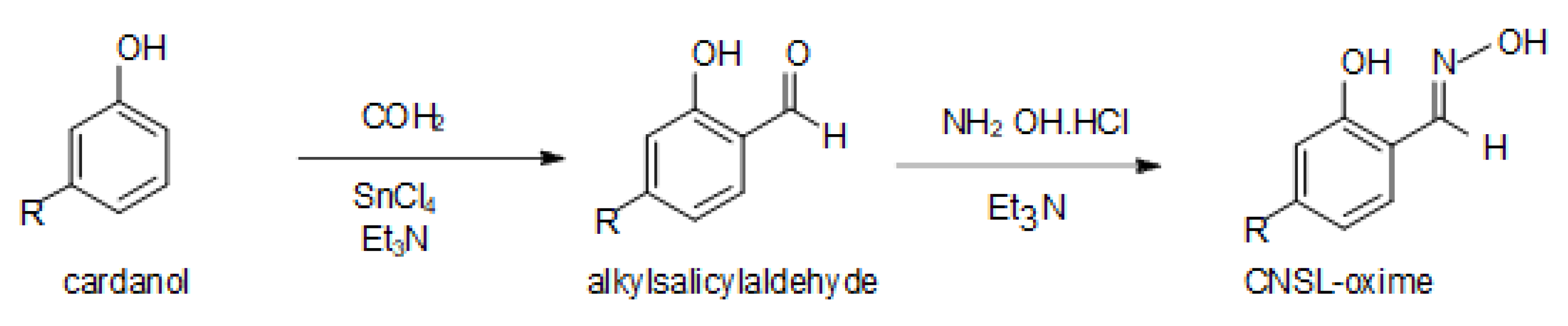
| United Atoms/Ions | Charge |
|---|---|
| CHx (Hexane) | 0.0000 |
| CHx (CnOX) | 0.0000 |
| CH (=N) | 0.3381 |
| N | −0.2845 |
| O (-OH) | −0.5159 |
| H (-OH) | 0.4623 |
| Op | −1.0000 |
| Cu2+ | 2.0000 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nguyen, C.V.; Phan, C.M.; Hoang, S.A.; Yusa, S.-i. Comparison between Cashew-Based and Petrochemical Hydroxyoximes: Insights from Molecular Simulations. Molecules 2023, 28, 3971. https://doi.org/10.3390/molecules28093971
Nguyen CV, Phan CM, Hoang SA, Yusa S-i. Comparison between Cashew-Based and Petrochemical Hydroxyoximes: Insights from Molecular Simulations. Molecules. 2023; 28(9):3971. https://doi.org/10.3390/molecules28093971
Chicago/Turabian StyleNguyen, Cuong V., Chi M. Phan, Son A. Hoang, and Shin-ichi Yusa. 2023. "Comparison between Cashew-Based and Petrochemical Hydroxyoximes: Insights from Molecular Simulations" Molecules 28, no. 9: 3971. https://doi.org/10.3390/molecules28093971
APA StyleNguyen, C. V., Phan, C. M., Hoang, S. A., & Yusa, S.-i. (2023). Comparison between Cashew-Based and Petrochemical Hydroxyoximes: Insights from Molecular Simulations. Molecules, 28(9), 3971. https://doi.org/10.3390/molecules28093971











