Effect of Different Phosphates on Pyrolysis Temperature-Dependent Carbon Sequestration and Phosphorus Release Performance in Biochar
Abstract
1. Introduction
2. Results and Discussion
2.1. The Physical and Chemical Properties of Biochars
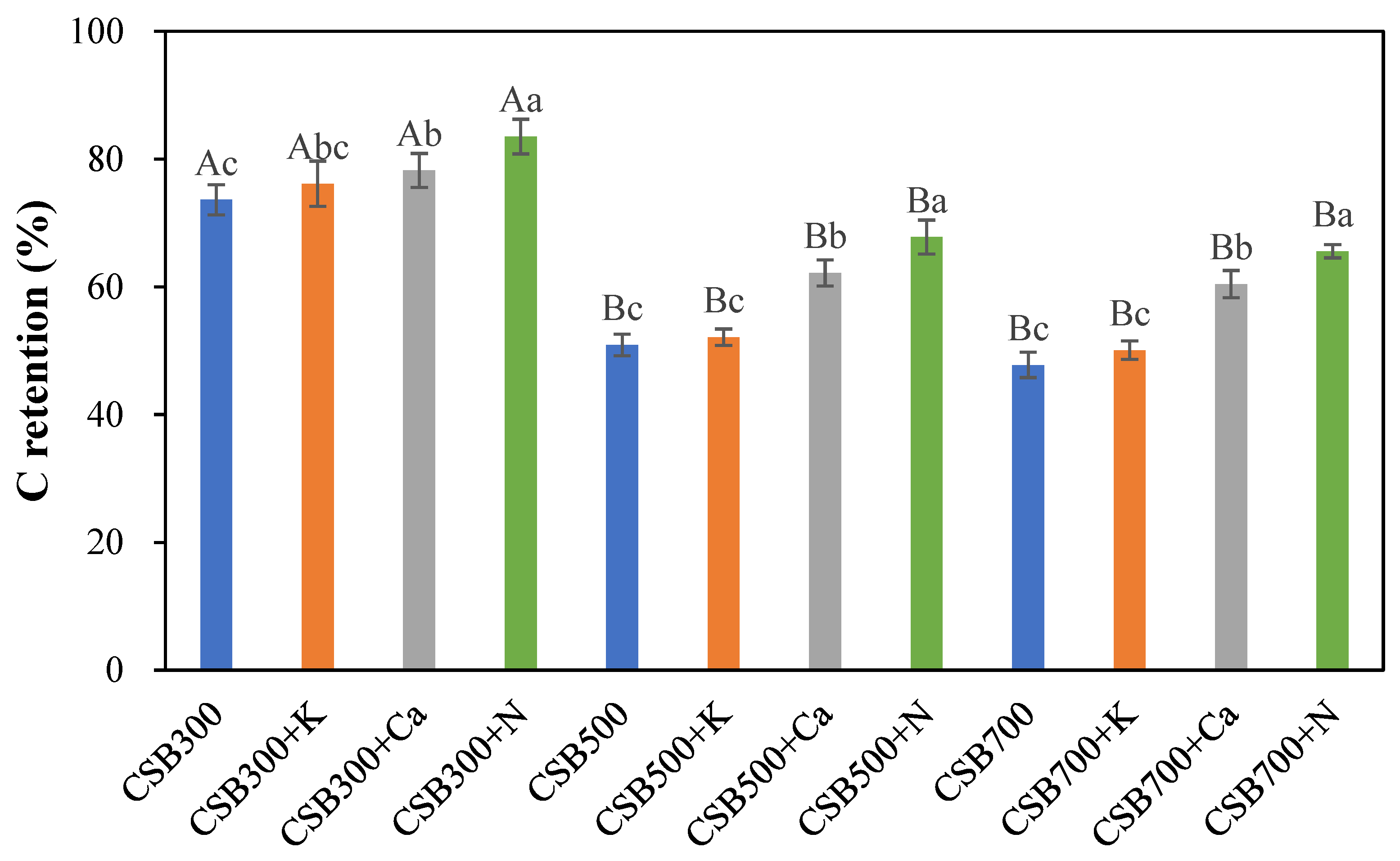
2.2. Carbon Retention of Biochar
2.2.1. Effect of P Addition and Pyrolysis Temperature on C Retention of Biochar
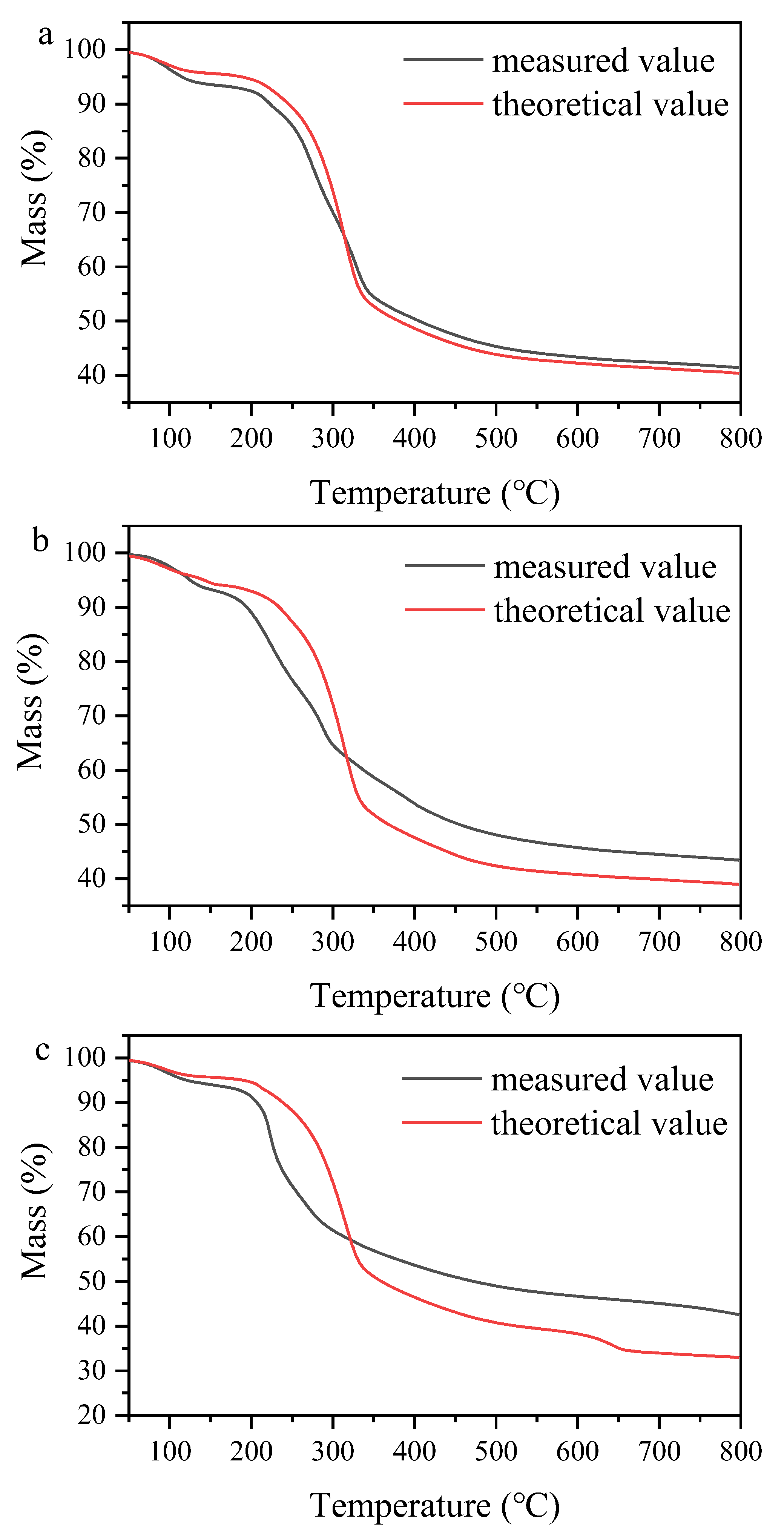
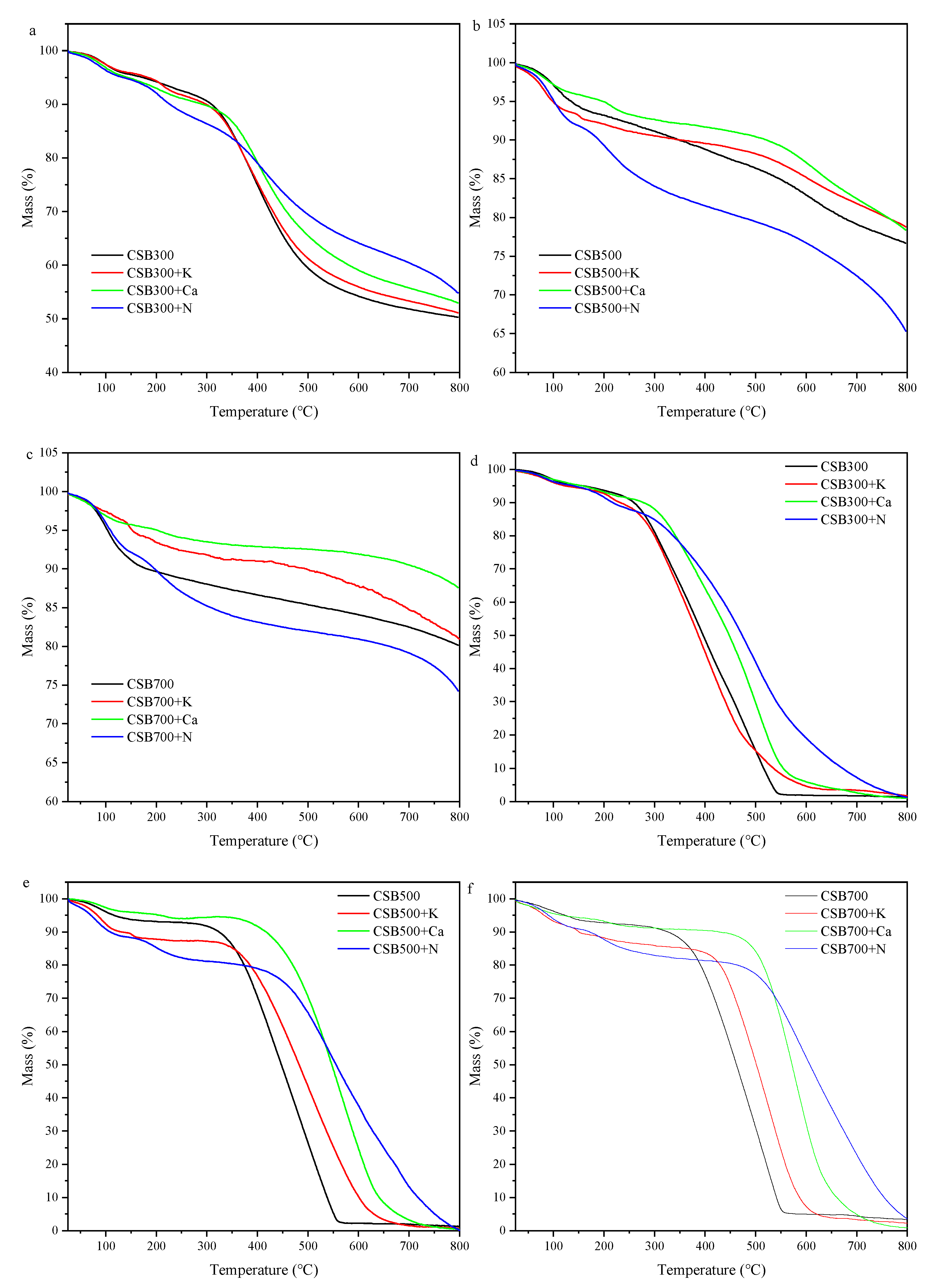
2.2.2. Thermogravimetric Analysis
2.2.3. SEM
2.2.4. XRD
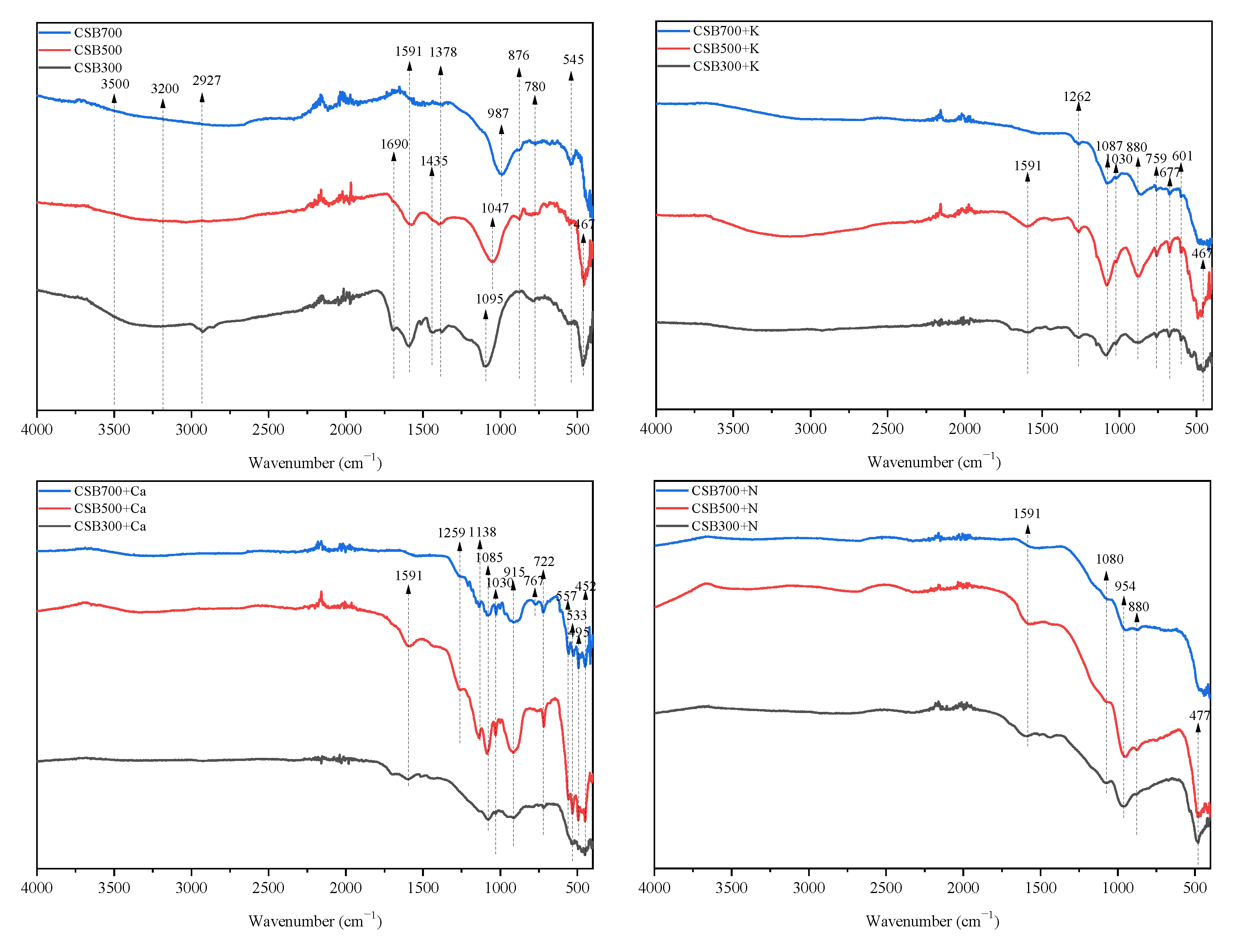
2.2.5. FTIR
2.2.6. XPS
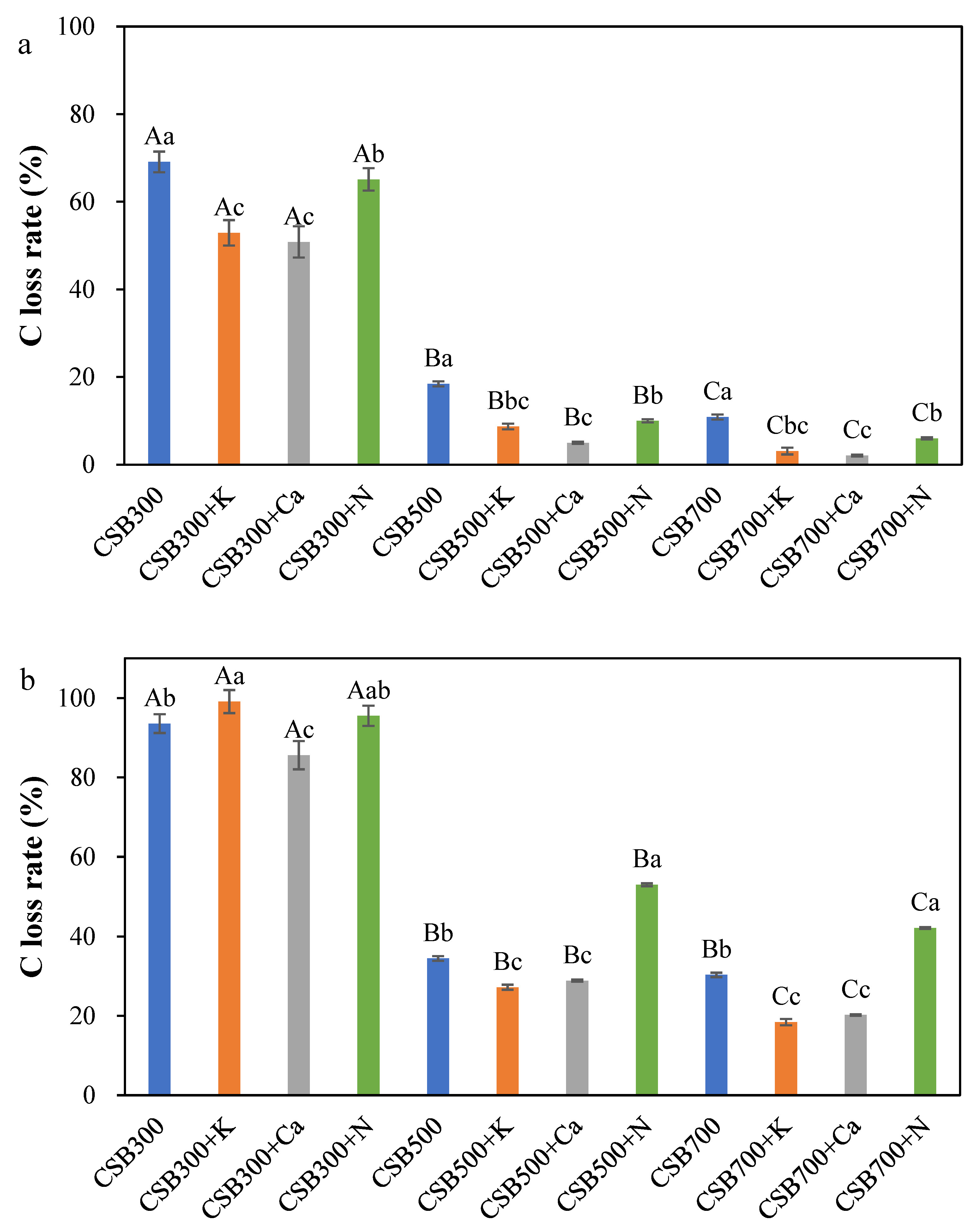
2.3. Chemical Stability of C in Biochar
2.4. Thermal and Oxidative Stability of Biochar
2.5. Phosphorus Release
3. Materials and Methods
3.1. Material
3.2. Biochar Production
3.3. Thermogravimetric Analysis (TGA)
3.4. Measurement of Biochars Properties
3.5. Measurement of C Stability in Biochar
3.6. Phosphorus Release from Biochar
3.7. Statistical Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Antar, M.; Lyu, D.; Nazari, M.; Shah, A.; Zhou, X.; Smith, D.L. Biomass for a sustainable bioeconomy: An overview of world biomass production and utilization. Renew. Sust. Energ. Rev. 2021, 139, 110691. [Google Scholar] [CrossRef]
- Fearnside, P.M. Global warming and tropical land-use change: Greenhouse gas emissions from biomass burning, decomposition and soils in forest conversion, shifting cultivation and secondary vegetation. Clim. Chang. 2000, 46, 115–158. [Google Scholar] [CrossRef]
- Leng, L.; Huang, H. An overview of the effect of pyrolysis process parameters on biochar stability. Bioresour. Technol. 2018, 270, 627–642. [Google Scholar] [CrossRef]
- Dai, Y.; Zheng, H.; Jiang, Z.; Xing, B. Combined effects of biochar properties and soil conditions on plant growth: A meta-analysis. Sci. Total Environ. 2020, 713, 136635. [Google Scholar] [CrossRef] [PubMed]
- Yuan, P.; Wang, J.; Pan, Y.; Shen, B.; Wu, C. Review of biochar for the management of contaminated soil: Preparation, application and prospect. Sci. Total Environ. 2019, 659, 473–490. [Google Scholar] [CrossRef]
- Yang, Y.; Sun, K.; Han, L.; Jin, J.; Sun, H.; Yang, Y.; Xing, B. Effect of minerals on the stability of biochar. Chemosphere 2018, 204, 310–317. [Google Scholar] [CrossRef]
- Zong, Y.; Xiao, Q.; Lu, S. Biochar derived from cadmium-contaminated rice straw at various pyrolysis temperatures: Cadmium immobilization mechanisms and environmental implication. Bioresour. Technol. 2021, 321, 124459. [Google Scholar] [CrossRef]
- Zhao, L.; Cao, X.; Zheng, W.; Kan, Y. Phosphorus-assisted biomass thermal conversion: Reducing carbon loss and improving biochar stability. PLoS ONE 2014, 9, 115373. [Google Scholar] [CrossRef]
- Nan, H.; Yin, J.; Yang, F.; Luo, Y.; Zhao, L.; Cao, X. Pyrolysis temperature-dependent carbon retention and stability of biochar with participation of calcium: Implications to carbon sequestration. Environ. Poll. 2021, 287, 117566. [Google Scholar] [CrossRef]
- Tumbure, A.; Bishop, P.; Bretherton, M.; Hedley, M. Co-pyrolysis of maize stover and igneous phosphate rock to produce potential biochar-based phosphate fertilizer with improved carbon retention and liming value. ACS Sustain. Chem. Eng. 2020, 8, 4178–4184. [Google Scholar] [CrossRef]
- Li, F.; Cao, X.; Zhao, L.; Wang, J.; Ding, Z. Effects of mineral additives on biochar formation: Carbon retention, stability, and properties. Environ. Sci. Technol. 2014, 48, 11211–11217. [Google Scholar] [CrossRef] [PubMed]
- Zhao, L.; Cao, X.; Zheng, W.; Scott, J.W.; Sharma, B.K.; Chen, X. Copyrolysis of biomass with phosphate fertilizers to improve biochar carbon retention, slow nutrient release, and stabilize heavy metals in soil. ACS Sustain. Chem. Eng. 2016, 4, 1630–1636. [Google Scholar] [CrossRef]
- Xu, Y.; Bai, T.; Li, Q.; Yang, H.; Yan, Y.; Sarkar, B.; Lam, S.S.; Bolan, N. Influence of pyrolysis temperature on the characteristics and lead (II) adsorption capacity of phosphorus-engineered poplar sawdust biochar. J. Anal. Appl. Pyrol. 2021, 154, 105010. [Google Scholar] [CrossRef]
- Xu, X.; Hu, X.; Ding, Z.; Chen, Y. Effects of copyrolysis of sludge with calcium carbonate and calcium hydrogen phosphate on chemical stability of carbon and release of toxic elements in the resultant biochars. Chemosphere 2017, 189, 76–85. [Google Scholar] [CrossRef]
- Carneiro, J.S.D.S.; Lustosa Filho, J.F.; Nardis, B.O.; Ribeiro-Soares, J.; Zinn, Y.L.; Melo, L.C.A. Carbon stability of engineered biochar-based phosphate fertilizers. ACS Sustain. Chem. Eng. 2018, 6, 14203–14212. [Google Scholar] [CrossRef]
- An, X.; Wu, Z.; Yu, J.; Cravotto, G.; Liu, X.; Li, Q.; Yu, B. Copyrolysis of biomass, bentonite, and nutrients as a new strategy for the synthesis of improved biochar-based slow-release fertilizers. ACS Sustain. Chem. Eng. 2020, 8, 3181–3190. [Google Scholar] [CrossRef]
- An, X.; Wu, Z.; Qin, H.; Liu, X.; He, Y.; Xu, X.; Li, T.; Yu, B. Integrated co-pyrolysis and coating for the synthesis of a new coated biochar-based fertilizer with enhanced slow-release performance. J. Clean. Prod. 2021, 283, 124642. [Google Scholar] [CrossRef]
- Da Silva Carneiro, J.S.; Ribeiro, I.C.A.; Nardis, B.O.; Barbosa, C.F.; Lustosa Filho, J.F.; Melo, L.C.A. Long-term effect of biochar-based fertilizers application in tropical soil: Agronomic efficiency and phosphorus availability. Sci. Total. Environ. 2021, 760, 143955. [Google Scholar] [CrossRef]
- Lustosa Filho, J.F.; Penido, E.S.; Castro, P.P.; Silva, C.A.; Melo, L.C. Co-pyrolysis of poultry litter and phosphate and magnesium generates alternative slow-release fertilizer suitable for tropical soils. ACS Sustain. Chem. Eng. 2017, 5, 9043–9052. [Google Scholar] [CrossRef]
- Ren, N.; Tang, Y.; Li, M. Mineral additive enhanced carbon retention and stabilization in sewage sludge-derived biochar. Process. Saf. Environ. 2018, 115, 70–78. [Google Scholar] [CrossRef]
- Wang, W.; Lemaire, R.; Bensakhria, A.; Luart, D. Review on the catalytic effects of alkali and alkaline earth metals (AAEMs) including sodium, potassium, calcium and magnesium on the pyrolysis of lignocellulosic biomass and on the co-pyrolysis of coal with biomass. J. Anal. Appl. Pyrol. 2022, 163, 105479. [Google Scholar] [CrossRef]
- Nan, H.; Zhao, L.; Yang, F.; Liu, Y.; Xiao, Z.; Cao, X.; Qiu, H. Different alkaline minerals interacted with biomass carbon during pyrolysis: Which one improved biochar carbon sequestration? J. Clean. Prod. 2020, 255, 120162. [Google Scholar] [CrossRef]
- Li, S.; Li, S.; Wang, C.; Zhu, X. Catalytic effects of ammonium dihydrogen phosphate on the pyrolysis of lignocellulosic biomass: Selective production of furfural and levoglucosenone. Fuel Process. Technol. 2020, 209, 106525. [Google Scholar] [CrossRef]
- Zhu, C.; Maduskar, S.; Paulsen, A.D.; Dauenhauer, P.J. Alkaline-Earth-Metal-Catalyzed Thin-Film Pyrolysis of Cellulose. ChemCatChem. 2016, 8, 818–829. [Google Scholar] [CrossRef]
- Safar, M.; Lin, B.J.; Chen, W.H.; Langauer, D.; Chang, J.S.; Raclavska, H.; Pétrissansd, A.; Rousset, P.; Pétrissans, M. Catalytic effects of potassium on biomass pyrolysis, combustion and torrefaction. Appl. Ener. 2019, 235, 346–355. [Google Scholar] [CrossRef]
- Vaimakis, T.C.; Sdoukos, A.T. The thermal dehydration of the Ca(H2PO4)2 2H2O SiO2 system. Part 1. Mechanism. Thermochim. Acta 1996, 277, 107–120. [Google Scholar] [CrossRef]
- Wang, Y.; Zuo, S.; Yang, J.; Yoon, S.H. Evolution of phosphorus-containing groups on activated carbons during heat treatment. Langmuir 2017, 33, 3112–3122. [Google Scholar] [CrossRef]
- Li, F.; Gui, X.; Ji, W.; Zhou, C. Effect of calcium dihydrogen phosphate addition on carbon retention and stability of biochars derived from cellulose, hemicellulose, and lignin. Chemosphere 2020, 251, 126335. [Google Scholar] [CrossRef]
- Jouini, A.; Férid, M.; Trabelsi-Ayadi, M. Equilibrium diagram of KPO3-Y(PO3)3 system, chemical preparation and characterization of KY(PO3)4. Thermochim. Acta. 2003, 400, 199–204. [Google Scholar] [CrossRef]
- Ning, C.Q.; Greish, Y.; El-Ghannam, A. Crystallization behavior of silica-calcium phosphate biocomposites: XRD and FTIR studies. J. Mater. Sci.-Mater. M 2004, 15, 1227–1235. [Google Scholar]
- Xiao, R.; Wang, J.J.; Gaston, L.A.; Zhou, B.; Park, J.H.; Li, R.; Dodla, S.K.; Zhang, Z. Biochar produced from mineral salt-impregnated chicken manure: Fertility properties and potential for carbon sequestration. Waste Manag. 2018, 78, 802–810. [Google Scholar] [CrossRef] [PubMed]
- Khabbouchi, M.; Hosni, K.; Zidi, R.; Srasra, E. Structural, conductive and dielectric properties of silicon phosphate SiP2O7 synthesis from activated clay. Appl. Clay. Sci. 2019, 178, 105139. [Google Scholar] [CrossRef]
- Huang, R.; Li, C.P.; Chen, D.; Zhao, G.; Cheng, W.; Zhang, Y.; Zhao, H. Preparation of phosphorylated starch by dry-heating in the presence of pyrophosphate and its calcium-phosphate solubilizing ability. J. Food. Sci. Technol. 2013, 50, 561–566. [Google Scholar] [CrossRef]
- Rol, F.; Sillard, C.; Bardet, M.; Yarava, J.R.; Emsley, L.; Gablin, C.; Léonarde, D.; Naceur Belgacem, N.; Bras, J. Cellulose phosphorylation comparison and analysis of phosphorate position on cellulose fibers. Carbohydr. Polym. 2020, 229, 115294. [Google Scholar] [CrossRef] [PubMed]
- Wu, X.; Radovic, L.R. Inhibition of catalytic oxidation of carbon/carbon composites by phosphorus. Carbon 2006, 44, 141–151. [Google Scholar] [CrossRef]
- Siow, K.S.; Britcher, L.; Kumar, S.; Griesser, H.J. XPS study of sulfur and phosphorus compounds with different oxidation states. Sains Malays. 2018, 47, 1913–1922. [Google Scholar]
- Somov, N.V.; Chausov, F.F.; Lomova, N.V.; Zakirova, R.M.; Petrov, V.G.; Zhirov, D.K.; Shumilova, M.A. Yttrium Coordination Compounds with Nitrilotris (Methylenephosphonic Acid). Russ. J. Coord. Chem. 2019, 45, 361–370. [Google Scholar] [CrossRef]
- Berenguer, R.; Ruiz-Rosas, R.; Gallardo, A.; Cazorla-Amorós, D.; Morallón, E.; Nishihara, H.; Kyotanid, J.T.; Rodríguez-Mirasol, J.; Cordero, T. Enhanced electro-oxidation resistance of carbon electrodes induced by phosphorus surface groups. Carbon 2015, 95, 681–689. [Google Scholar] [CrossRef]
- Zhang, H.; Shao, J.; Zhang, S.; Zhang, X.; Chen, H. Effect of phosphorus-modified biochars on immobilization of Cu (II), Cd (II), and As(V) in paddy soil. J. Hazard. Mater. 2020, 390, 121349. [Google Scholar] [CrossRef]
- Rhim, Y.R.; Zhang, D.; Fairbrother, D.H.; Wepasnick, K.A.; Livi, K.J.; Bodnar, R.J.; Nagle, D.C. Changes in electrical and microstructural properties of microcrystalline cellulose as function of carbonization temperature. Carbon 2010, 48, 1012–1024. [Google Scholar] [CrossRef]
- Patwardhan, P.R.; Satrio, J.A.; Brown, R.C.; Shanks, B.H. Influence of inorganic salts on the primary pyrolysis products of cellulose. Bioresour. Technol. 2010, 101, 4646–4655. [Google Scholar] [CrossRef]
- Mašek, O.; Buss, W.; Brownsort, P.; Rovere, M.; Tagliaferro, A.; Zhao, L.; Cao, X.; Xu, G. Potassium doping increases biochar carbon sequestration potential by 45%, facilitating decoupling of carbon sequestration from soil improvement. Sci. Rep. 2019, 9, 5514. [Google Scholar] [CrossRef]
- Zhang, S.; Su, Y.; Ding, K.; Zhang, H. Impacts and release characteristics of K and Mg contained in rice husk during torrefaction process. Energy 2019, 186, 115888. [Google Scholar] [CrossRef]
- Liu, Y.; Gao, C.; Wang, Y.; He, L.; Lu, H.; Yang, S. Vermiculite modification increases carbon retention and stability of rice straw biochar at different carbonization temperatures. J. Clean. Prod. 2020, 254, 120111. [Google Scholar] [CrossRef]
- Chen, K.; Ma, D.; Yu, H.; Zhang, S.; Seyler, B.C.; Chai, Z.; Peng, S. Biosorption of V(V) onto Lantana camara biochar modified by H3PO4: Characteristics, mechanism, and regenerative capacity. Chemosphere 2022, 291, 132721. [Google Scholar] [CrossRef]
- Zolin, A.; Jensen, A.; Jensen, P.A.; Frandsen, F.; DamJohansen, K. The influence of inorganic materials on the thermal deactivation of fuel chars. Energy Fuel. 2001, 15, 1110−1122. [Google Scholar] [CrossRef]
- Al-Wabel, M.I.; Al-Omran, A.; El-Naggar, A.H.; Nadeem, M.; Usman, A.R. Pyrolysis temperature induced changes in characteristics and chemical composition of biochar produced from conocarpus wastes. Bioresour. Technol. 2013, 131, 374–379. [Google Scholar] [CrossRef]
- Chen, D.; Yu, X.; Song, C.; Pang, X.; Huang, J.; Li, Y. Effect of pyrolysis temperature on the chemical oxidation stability of bamboo biochar. Bioresour. Technol. 2016, 218, 1303–1306. [Google Scholar] [CrossRef]
- Feng, D.; Zhao, Y.; Zhang, Y.; Xu, H.; Zhang, L.; Sun, S. Catalytic mechanism of ion-exchanging alkali and alkaline earth metallic species on biochar reactivity during CO2/H2O gasification. Fuel 2018, 212, 523–532. [Google Scholar] [CrossRef]
- Rosas, J.M.; Ruiz-Rosas, R.; Rodríguez-Mirasol, J.; Cordero, T. Kinetic study of the oxidation resistance of phosphorus-containing activated carbons. Carbon 2012, 50, 1523–1537. [Google Scholar] [CrossRef]
- Valero-Romero, M.J.; García-Mateos, F.J.; Rodríguez-Mirasol, J.; Cordero, T. Role of surface phosphorus complexes on the oxidation of porous carbons. Fuel Process. Technol. 2017, 157, 116–126. [Google Scholar] [CrossRef]
- Islas-Espinoza, M.A.R.I.N.A.; Solís-Mejía, L.; Esteller, M.V. Phosphorus release kinetics in a soil amended with biosolids and vermicompost. Environ. Earth. Sci. 2014, 71, 1441–1451. [Google Scholar] [CrossRef]
- Liang, Y.; Cao, X.; Zhao, L.; Xu, X.; Harris, W. Phosphorus release from dairy manure, the manure-derived biochar, and their amended soil: Effects of phosphorus nature and soil property. J. Environ. Qual. 2014, 43, 1504–1509. [Google Scholar] [CrossRef] [PubMed]
- Gerente, C.; Lee, V.K.C.; Le Cloirec, P.; McKay, G. Application of chitosan for the removal of metals from wastewaters by adsorption-mechanisms and models. Rev. Crit. Rev. Environ. Sci. Technol. 2007, 37, 41–127. [Google Scholar] [CrossRef]


| pH | Yield Rate (%) | Ash Content (%) | FC (%) | VS (%) | C (%) | N (%) | O (%) | H (%) | P (%) | H/C | |
|---|---|---|---|---|---|---|---|---|---|---|---|
| CS | 6.95 ±0.06 | - | 11.35 ±0.20 | 6.96 ±0.06 | 81.69 ±0.79 | 41.45 ±0.26 | 1.25 ±0.03 | 15.26 ±0.09 | 5.67 ±0.06 | 0.24 ±0.00 | 1.64 ±0.02 |
| CSB300 | 7.51 ±0.08 Ca | 54.41 ±0.87 Ab | 16.98 ±0.11 Bd | 40.75 ±0.19 Ba | 42.27 ±0.28 Aa | 56.10 ±0.31 Ba | 1.82 ±0.02 Ab | 20.40 ±0.13 Ac | 4.35 ±0.05 Aa | 0.47 ±0.01 Bc | 0.93 ±0.01 Abc |
| CSB300+K | 6.64 ±0.05 Bb | 62.24 ±0.77 Aa | 39.20 ±0.12 Ba | 28.06 ±0.12 Bc | 32.74 ±0.36 Ab | 40.86 ±0.25 Ab | 1.51 ±0.03 Ac | 24.01 ±0.20 Ab | 3.21 ±0.07 Ab | 7.33 ±0.07 Bb | 0.95 ±0.02 Ab |
| CSB300+Ca | 3.55 ±0.07 Bc | 62.68 ±0.28 Aa | 35.88 ±0.25 Bb | 32.94 ±0.20 Bb | 31.18 ±0.40 Ab | 41.38 ±0.21 Ab | 1.44 ±0.02 Ac | 26.12 ±0.23 Aab | 3.05 ±0.02 Ab | 8.46 ±0.06 Ba | 0.88 ±0.01 Ac |
| CSB300+N | 2.38 ±0.02 Ad | 64.67 ±0.47 Aa | 27.87 ±0.14 Bc | 38.49 ±0.08 Ba | 33.64 ±0.21 Ab | 42.82 ±0.17 Ab | 4.4 ±0.01 Aa | 28.17 ±0.19 Aa | 4.11 ±0.03 Aa | 8.33 ±0.06 Ba | 1.15 ±0.03 Aa |
| CSB500 | 9.23 ±0.05 Ba | 35.13 ±0.19 Bb | 26.13 ±0.13 Ad | 55.64 ±0.27 Aa | 18.23 ±0.19 Bb | 60.05 ±0.30 Aa | 1.54 ±0.03 Bb | 15.95 ±0.12 Bc | 2.72 ±0.04 Ba | 0.73 ±0.01 Ac | 0.54 ±0.00 Ba |
| CSB500+K | 7.25 ±0.06 ABb | 45.57 ±0.25 Ba | 51.09 ±0.29 Aa | 35.4 ±0.16 Ac | 13.51 ±0.11 Bc | 37.73 ±0.23 Ac | 1.02 ±0.02 Bc | 21.51 ±0.14 Bb | 1.50 ±0.01 Bc | 10.17 ±0.07 Ab | 0.47 ±0.01 Bb |
| CSB500+Ca | 3.90 ±0.03 Ac | 48.40 ±0.20 Ba | 46.49 ±0.24 Ab | 40.92 ±0.21 Ab | 12.59 ±0.09 Bc | 42.61 ±0.18 Aab | 1.46 ±0.03 Ab | 22.08 ±0.20 Bab | 1.32 ±0.01 Bd | 10.90 ±0.08 Aa | 0.37 ±0.01 Bc |
| CSB500+N | 2.42 ±0.04 Ad | 49.52 ±0.26 Ba | 35.62 ±0.18 Ac | 41.02 ±0.22 ABb | 23.36 ±0.22 Ba | 45.40 ±0.37 Ab | 3.57 ±0.06 Ba | 23.99 ±0.17 Ba | 1.81 ±0.02 Bb | 11.18 ±0.10 Aa | 0.48 ±0.01 Bb |
| CSB700 | 10.17 ±0.11 Aa | 32.18 ±0.31 Bb | 27.54 ±0.18 Ad | 57.06 ±0.16 Aa | 15.40 ±0.16 Bab | 61.54 ±0.45 Aa | 1.27 ±0.04 Cb | 10.98 ±0.08 Cc | 1.83 ±0.01 Ca | 0.79 ±0.03 Ac | 0.36 ±0.02 Ca |
| CSB700+K | 7.82 ±0.07 Ab | 42.74 ±0.33 Ba | 52.73 ±0.30 Aa | 35.01 ±0.13 Ac | 12.2 6±0.08 Bb | 38.85 ±0.37 Ad | 0.80 ±0.02 Cc | 19.85 ±0.11 Bb | 0.96 ±0.01 Cc | 10.67 ±0.09 Ab | 0.30 ±0.00 Cb |
| CSB700+Ca | 4.04 ±0.03 Ac | 44.63 ±0.25 Ba | 47.73 ±0.26 Ab | 44.92 ±0.14 Ab | 7.35 ±0.06 Cc | 44.89 ±0.30 Ac | 1.24 ±0.02 Bb | 21.15 ±0.13 Bab | 0.84 ±0.02 Cc | 11.88 ±0.11 Aa | 0.22 ±0.01 Cc |
| CSB700+N | 2.47 ±0.04 Ad | 45.30 ±0.12 Ba | 36.99 ±0.09 Ac | 45.33 ±0.20 Ab | 17.68 ±0.10 Ca | 48.00 ±0.24 Ab | 3.05 ±0.03 Ca | 23.92 ±0.22 Ba | 1.33 ±0.03 Cb | 11.93 ±0.12 Aa | 0.33 ±0.01 Cab |
| C–C/C=C (284.8 eV) | C–O (C-O-P) (285.9 eV) | C=O (286.8 eV) | O–C=O (288.9 eV) | |
|---|---|---|---|---|
| CSB300 | 76.92 | 13.85 | 6.15 | 3.08 |
| CSB300+K | 75.76 | 15.15 | 6.06 | 3.03 |
| CSB300+Ca | 71.94 | 18.71 | 6.47 | 2.88 |
| CSB300+N | 64.10 | 21.79 | 11.54 | 2.56 |
| CSB500 | 82.64 | 10.74 | 4.96 | 1.65 |
| CSB500+K | 81.97 | 11.48 | 4.10 | 2.46 |
| CSB500+Ca | 81.30 | 13.01 | 3.25 | 2.44 |
| CSB500+N | 77.52 | 15.50 | 5.43 | 1.55 |
| CSB700 | 86.21 | 7.76 | 4.31 | 1.72 |
| CSB700+K | 86.96 | 8.70 | 3.48 | 0.87 |
| CSB700+Ca | 84.03 | 10.92 | 3.36 | 1.68 |
| CSB700+N | 81.97 | 11.48 | 4.92 | 1.64 |
| Kinetics Models | |||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Pseudo-First | Pseudo-Second | Power Function | Elovich | Parabolic Diffusion | |||||||||||
| R2 | a | b | R2 | a | k | R2 | a | b | R2 | a | b | R2 | a | b | |
| CSB300 | 0.85 | 2.61 | 0.34 | 0.85 | 2.77 | 0.19 | 0.88 | 1.34 | 0.16 | 0.96 | 3.45 | 0.71 | 0.73 | 1.43 | 0.12 |
| CSB300+K | 0.47 | 59.18 | 0.27 | 0.70 | 63.14 | 0.01 | 1.00 | 27.78 | 0.18 | 0.97 | 3597.41 | 2.80 × 10−4 | 0.94 | 28.37 | 3.40 |
| CSB300+Ca | 0.43 | 42.66 | 2.27 | - | - | - | 0.96 | 35.22 | 0.06 | 0.98 | 181.83 | 0.07 | 0.75 | 36.19 | 0.95 |
| CSB300+N | 0.44 | 64.98 | 3.13 | - | - | - | 0.94 | 57.51 | −2.57 | 0.95 | 1082.39 | 0.07 | 0.70 | 58.84 | 0.92 |
| CSB500 | 0.58 | 2.75 | 0.78 | 0.76 | 2.97 | 0.34 | 0.98 | 1.70 | 0.14 | 0.95 | 4.82 | 0.78 | 0.74 | 1.82 | 0.09 |
| CSB500+K | 0.39 | 54.99 | 0.91 | 0.69 | 59.74 | 0.02 | 0.99 | 35.16 | 0.13 | 0.99 | 696.78 | 15.1 × 10−4 | 0.89 | 36.32 | 2.56 |
| CSB500+Ca | 0.57 | 40.40 | 1.58 | - | - | - | 0.93 | 31.07 | 0.08 | 0.96 | 87.46 | 0.04 | 0.73 | 32.12 | 1.13 |
| CSB500+N | 0.36 | 74.42 | 2.55 | - | - | - | 0.99 | 62.03 | −4.28 | 0.99 | 170.38 | 0.01 | 0.82 | 63.86 | 1.62 |
| CSB700 | 0.63 | 2.50 | 1.41 | 0.86 | 2.63 | 0.80 | 0.92 | 1.88 | 0.09 | 0.96 | 5.28 | 0.79 | 0.81 | 1.90 | 0.09 |
| CSB700+K | 0.34 | 77.37 | 1.46 | - | - | - | 0.99 | 56.11 | 0.10 | 0.99 | 1415.30 | 7.36 × 10−4 | 0.85 | 57.96 | 2.82 |
| CSB700+Ca | 0.62 | 42.59 | 1.62 | - | - | - | 0.90 | 32.24 | 0.07 | 0.94 | 96.38 | 0.04 | 0.67 | 34.46 | 1.09 |
| CSB700+N | 0.22 | 56.28 | 1.73 | - | - | - | 0.99 | 48.17 | −2.86 | 0.99 | 273.24 | 0.06 | 0.84 | 49.30 | 1.10 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bai, T.; Ma, W.; Li, W.; Jiang, J.; Chen, J.; Cao, R.; Yang, W.; Dong, D.; Liu, T.; Xu, Y. Effect of Different Phosphates on Pyrolysis Temperature-Dependent Carbon Sequestration and Phosphorus Release Performance in Biochar. Molecules 2023, 28, 3950. https://doi.org/10.3390/molecules28093950
Bai T, Ma W, Li W, Jiang J, Chen J, Cao R, Yang W, Dong D, Liu T, Xu Y. Effect of Different Phosphates on Pyrolysis Temperature-Dependent Carbon Sequestration and Phosphorus Release Performance in Biochar. Molecules. 2023; 28(9):3950. https://doi.org/10.3390/molecules28093950
Chicago/Turabian StyleBai, Tianxia, Wenge Ma, Wenhui Li, Jinling Jiang, Jiamin Chen, Rui Cao, Wenjie Yang, Dan Dong, Tingwu Liu, and Yonggang Xu. 2023. "Effect of Different Phosphates on Pyrolysis Temperature-Dependent Carbon Sequestration and Phosphorus Release Performance in Biochar" Molecules 28, no. 9: 3950. https://doi.org/10.3390/molecules28093950
APA StyleBai, T., Ma, W., Li, W., Jiang, J., Chen, J., Cao, R., Yang, W., Dong, D., Liu, T., & Xu, Y. (2023). Effect of Different Phosphates on Pyrolysis Temperature-Dependent Carbon Sequestration and Phosphorus Release Performance in Biochar. Molecules, 28(9), 3950. https://doi.org/10.3390/molecules28093950






