Theoretical Investigation of HER and OER Electrocatalysts Based on the 2D R-graphyne Completely Composed of Anti-Aromatic Carbon Rings
Abstract
1. Introduction
2. Results and Discussion
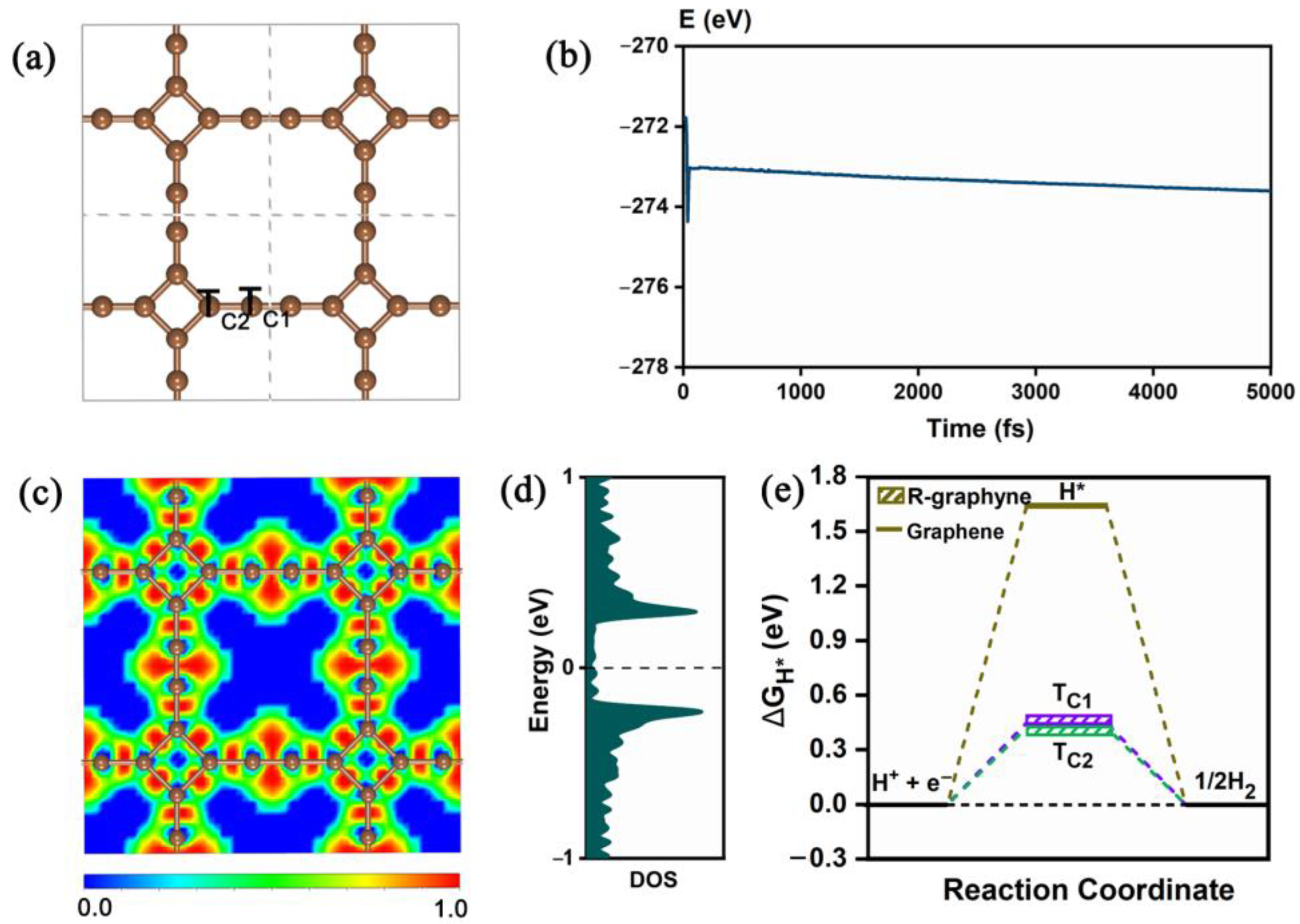
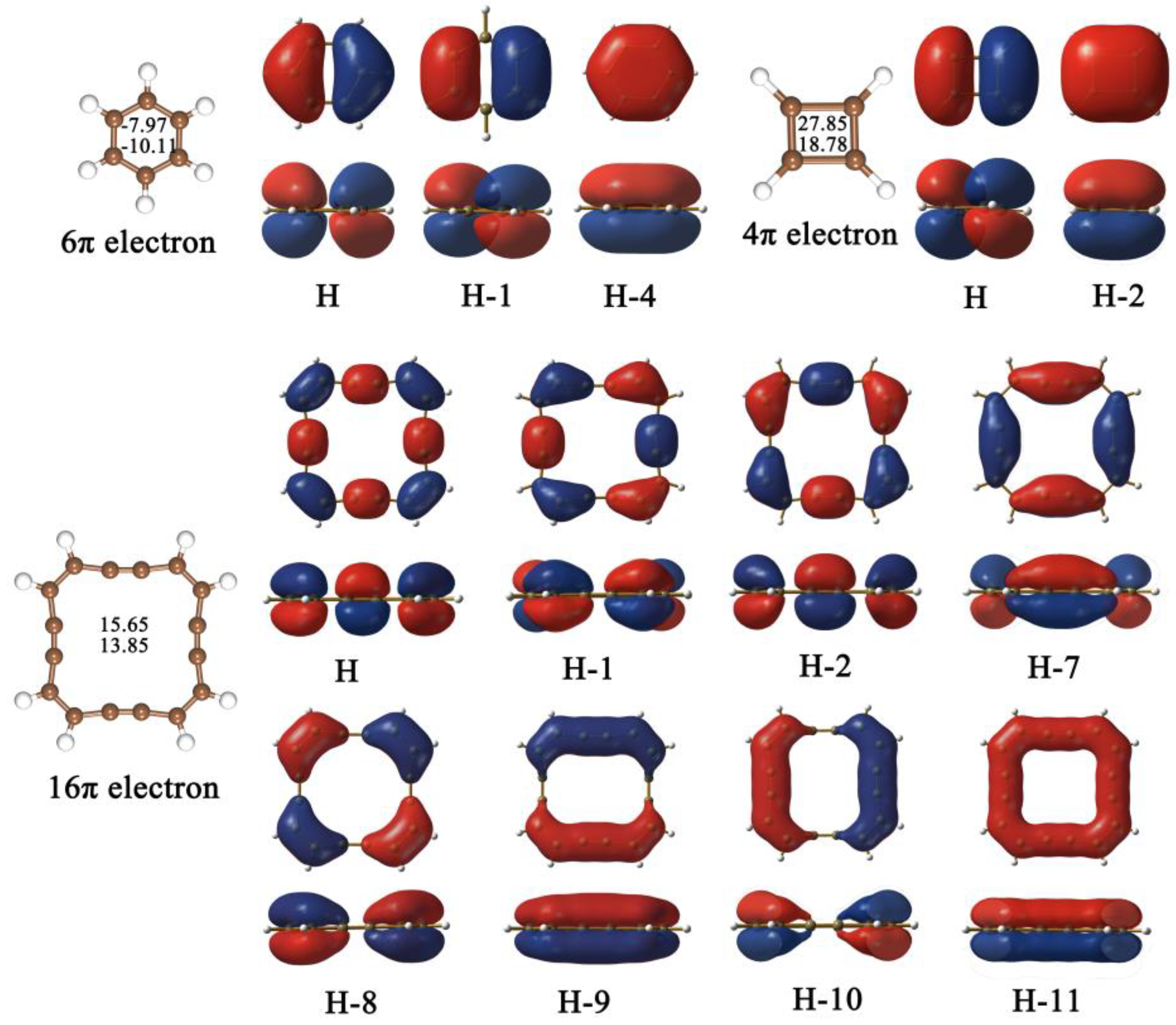
2.1. Structure, Stability, Electronic Property and HER Electrocatalytic Activity of Pristine R-graphyne
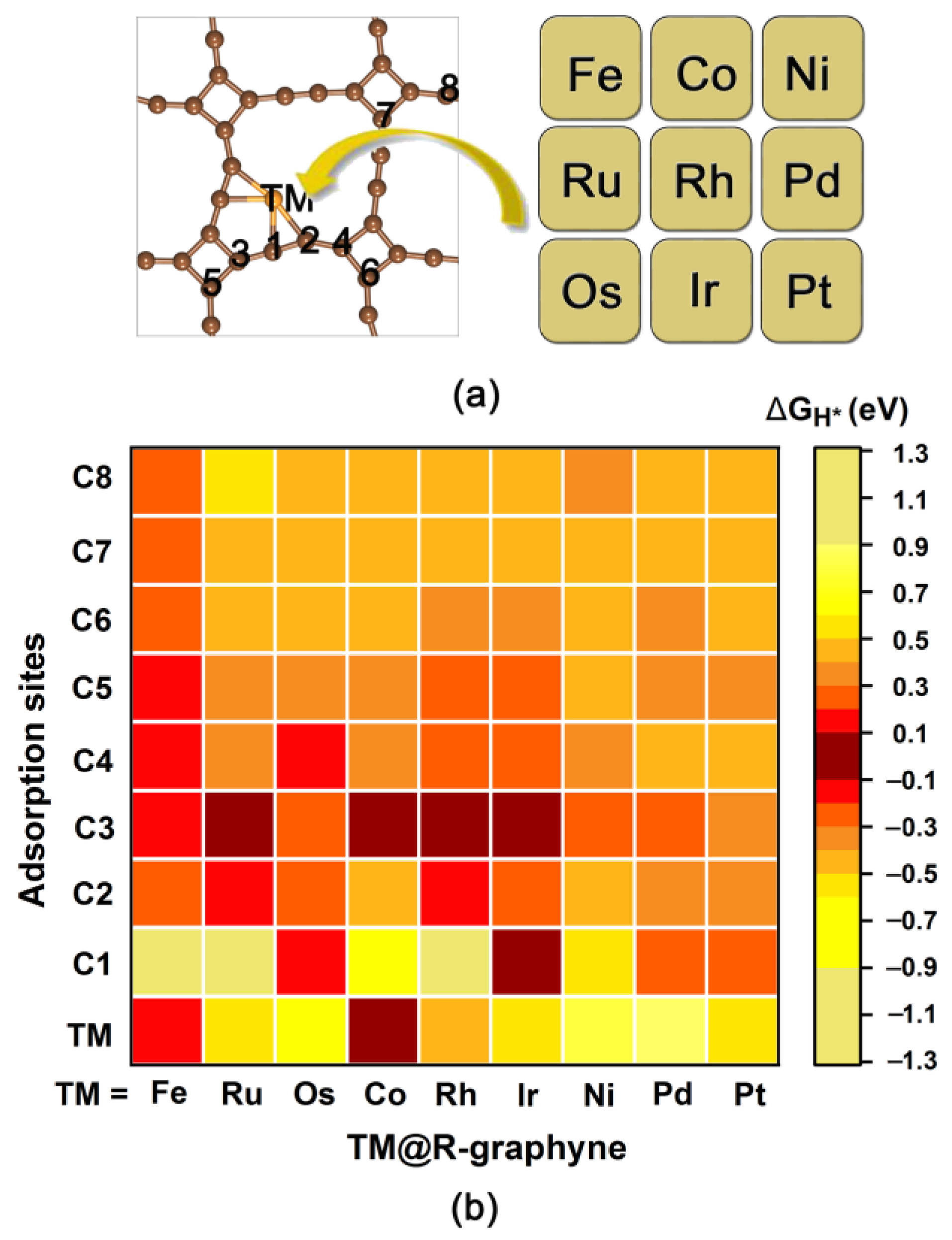
2.2. The HER Performance of TM@R-graphyne (TM = Fe, Ru, Os, Co, Rh, Ir, Ni, Pd and Pt)
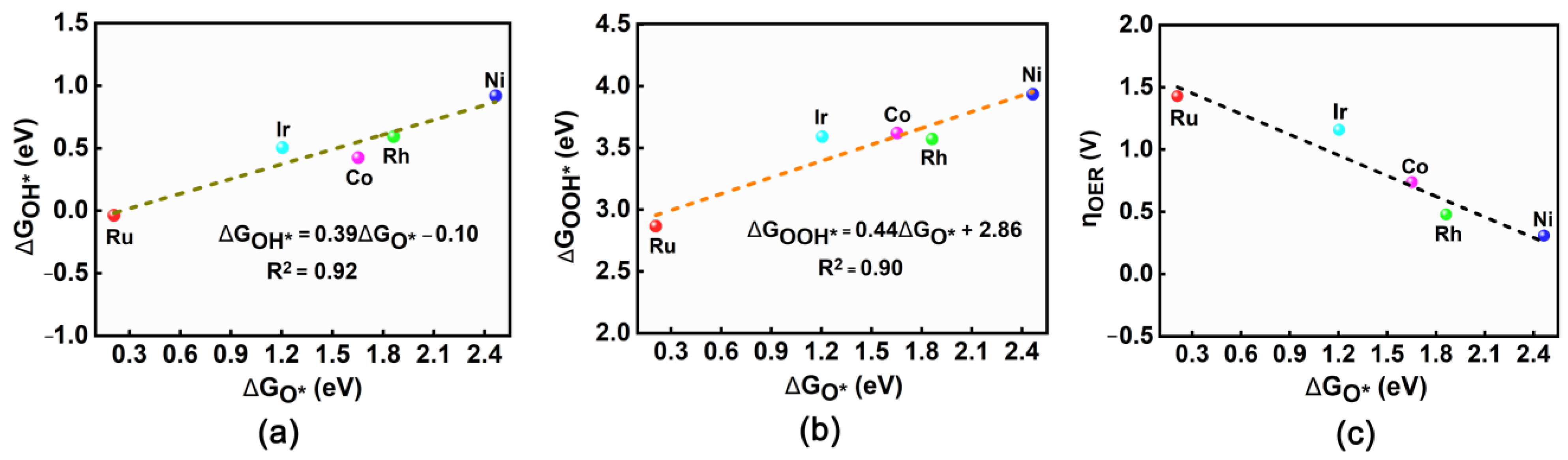
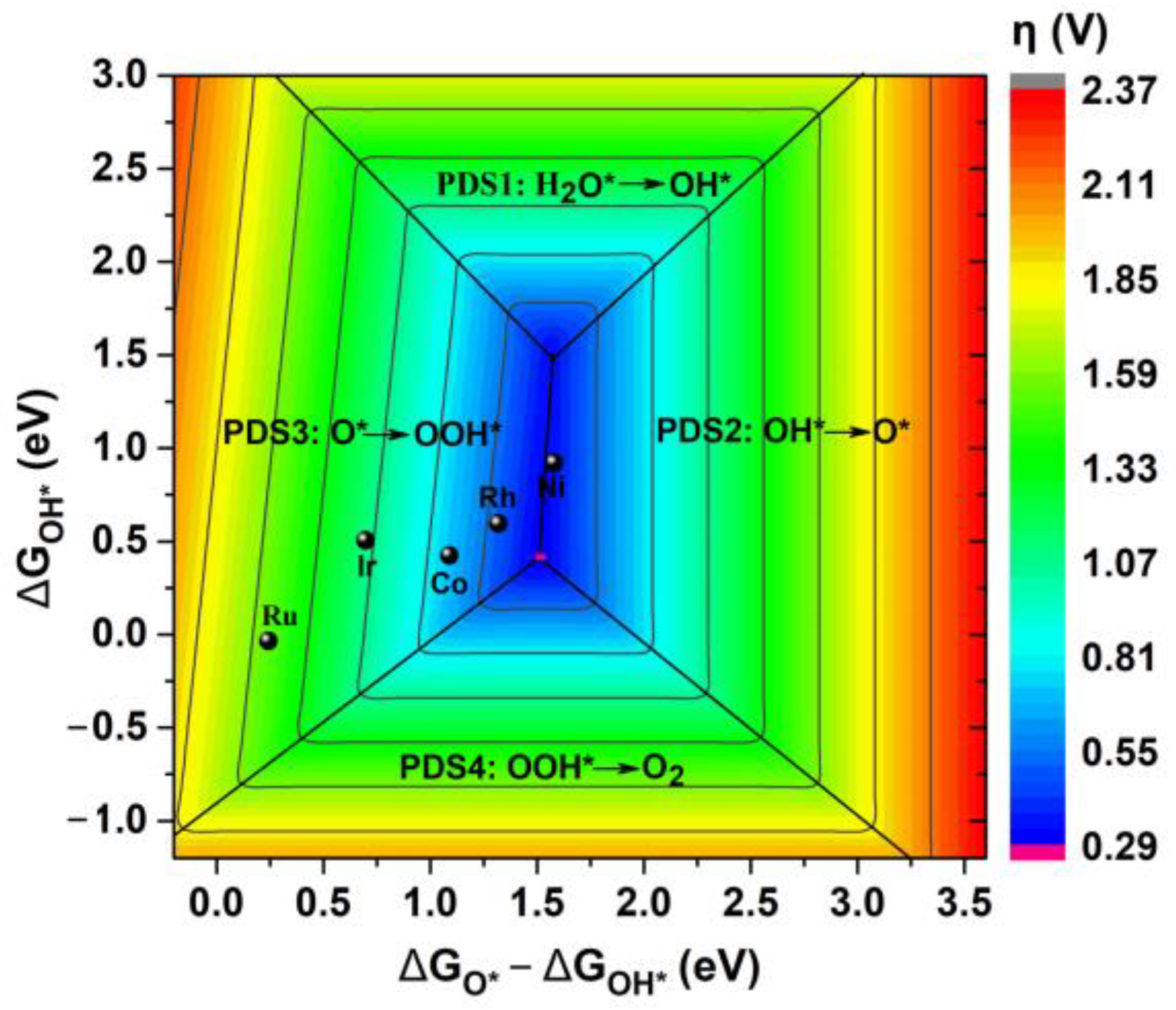
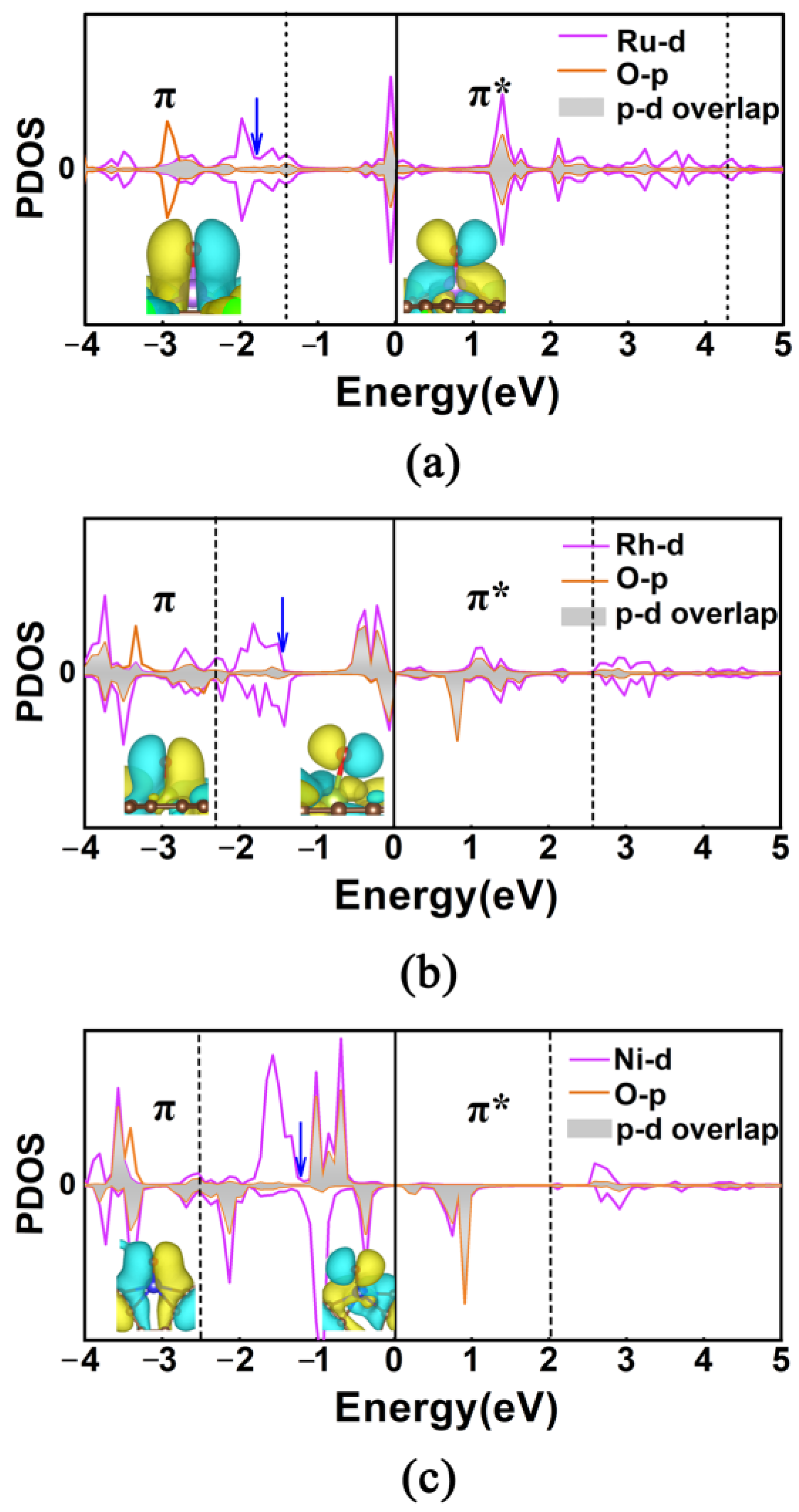
2.3. Evaluation of OER Catalytic Activity of TM@R-graphyne
3. Computational Methods
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Katsounaros, I.; Cherevko, S.; Zeradjanin, A.R.; Mayrhofer, K.J. Oxygen electrochemistry as a cornerstone for sustainable energy conversion. Angew. Chem. Int. Edit. 2014, 53, 102–121. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Zhao, Z.; Xia, Z.; Dai, L. A metal-free bifunctional electrocatalyst for oxygen reduction and oxygen evolution reactions. Nat. Nanotechnol. 2015, 10, 444–452. [Google Scholar] [CrossRef] [PubMed]
- Lewis, N.S. Research opportunities to advance solar energy utilization. Science 2016, 351, aad1920. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Yu, G.; Li, H.; Liu, J.; Huang, X.; Chen, W. Theoretical insights into the effective hydrogen evolution on Cu3P and its evident improvement by surface-doped Ni atoms. Phys. Chem. Chem. Phys. 2018, 20, 10407–10417. [Google Scholar] [CrossRef] [PubMed]
- Ma, Y.; Yu, G.; Wang, T.; Zhang, C.; Huang, X.; Chen, W. Highly efficient catalytic activity for the hydrogen evolution reaction on pristine and monovacancy defected WP systems: A first-principles investigation. Phys. Chem. Chem. Phys. 2018, 20, 13757–13764. [Google Scholar] [CrossRef] [PubMed]
- Qian, W.; Chen, Z.; Zhang, J.; Yin, L. Monolayer MoSi2N4−x as promising electrocatalyst for hydrogen evolution reaction: A DFT prediction. J. Mater. Sci. Technol. 2022, 99, 215–222. [Google Scholar] [CrossRef]
- Guan, D.; Shi, C.; Xu, H.; Gu, Y.; Zhong, J.; Sha, Y.; Hu, Z.; Ni, M.; Shao, Z. Simultaneously mastering operando strain and reconstruction effects via phase-segregation strategy for enhanced oxygen-evolving electrocatalysis. J. Energy Chem. 2023. [Google Scholar] [CrossRef]
- Huang, B.; Xu, H.; Jiang, N.; Wang, M.; Huang, J.; Guan, L. Tensile-Strained RuO2 Loaded on Antimony-Tin Oxide by Fast Quenching for Proton-Exchange Membrane Water Electrolyzer. Adv. Sci. 2022, 9, 2201654. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Gao, Y.; Xu, H.; Guan, D.; Hu, Z.; Jing, C.; Sha, Y.; Gu, Y.; Huang, Y.-C.; Chang, Y.-C.; et al. Combined Corner-Sharing and Edge-Sharing Networks in Hybrid Nanocomposite with Unusual Lattice-Oxygen Activation for Efficient Water Oxidation. Adv. Funct. Mater. 2022, 32, 2207618. [Google Scholar] [CrossRef]
- Baraiya, B.A.; Mankad, V.; Jha, P.K. Adsorption energetics of atoms and diatomic gases with electrocatalysis approach towards hydrogen and oxygen evolution reaction on Pt surfaces. ChemistrySelect 2018, 3, 10515–10525. [Google Scholar] [CrossRef]
- Antolini, E. Iridium as catalyst and cocatalyst for oxygen evolution/reduction in acidic polymer electrolyte membrane electrolyzers and fuel cells. ACS Catal. 2014, 4, 1426–1440. [Google Scholar] [CrossRef]
- Rossmeisl, J.; Qu, Z.-W.; Zhu, H.; Kroes, G.-J.; Nørskov, J.K. Electrolysis of water on oxide surfaces. J. Electroanal. Chem. 2007, 607, 83–89. [Google Scholar] [CrossRef]
- Yang, Y.; Liu, J.; Liu, F.; Wang, Z.; Wu, D. FeS2-anchored transition metal single atoms for highly efficient overall water splitting: A DFT computational screening study. J. Mater. Chem. A 2021, 9, 2438–2447. [Google Scholar] [CrossRef]
- Kumar, G.M.; Ilanchezhiyan, P.; Cho, H.D.; Lee, D.J.; Kim, D.Y.; Kang, T.W. Ultrathin VS2 nanodiscs for highly stable electro catalytic hydrogen evolution reaction. Int. J. Energy Res. 2020, 44, 811–820. [Google Scholar] [CrossRef]
- Yang, L.; Liu, P.; Li, J.; Xiang, B. Two-dimensional material molybdenum disulfides as electrocatalysts for hydrogen evolution. Catalysts 2017, 7, 285. [Google Scholar] [CrossRef]
- Yu, Y.; Zhou, J.; Sun, Z. Novel 2D Transition-Metal Carbides: Ultrahigh Performance Electrocatalysts for Overall Water Splitting and Oxygen Reduction. Adv. Funct. Mater. 2020, 30, 2000570. [Google Scholar] [CrossRef]
- Jia, J.; Wei, S.; Cai, Q.; Zhao, J. Two-dimensional IrN2 monolayer: An efficient bifunctional electrocatalyst for oxygen reduction and oxygen evolution reactions. J. Colloid Interface Sci. 2021, 600, 711–718. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Yu, G.; Ku, R.; Huang, X.; Chen, W. Theoretical investigation on the high HER catalytic activity of 2D layered GeP3 nanomaterials and its further enhancement by applying the surface strain or coupling with graphene. Appl. Surf. Sci. 2019, 481, 272–280. [Google Scholar] [CrossRef]
- Zhang, R.; Yu, G.; Gao, Y.; Huang, X.; Chen, W. Applying surface strain and coupling with pure or N/B-doped graphene to successfully achieve high HER catalytic activity in 2D layered SnP3-based nanomaterials: A first-principles investigation. Inorg. Chem. Front. 2020, 7, 647–658. [Google Scholar] [CrossRef]
- Zheng, S.; Yu, T.; Lin, J.; Lou, H.; Xu, H.; Yang, G. FeP3 monolayer as a high-efficiency catalyst for hydrogen evolution reaction. J. Mater. Chem. A 2019, 7, 25665–25671. [Google Scholar] [CrossRef]
- Qin, M.; Li, S.; Zhao, Y.; Lao, C.-Y.; Zhang, Z.; Liu, L.; Fang, F.; Wu, H.; Jia, B.; Liu, Z.; et al. Unprecedented Synthesis of Holey 2D Layered Double Hydroxide Nanomesh for Enhanced Oxygen Evolution. Adv. Energy Mater. 2019, 9, 1803060. [Google Scholar] [CrossRef]
- Chen, G.; Wang, T.; Zhang, J.; Liu, P.; Sun, H.; Zhuang, X.; Chen, M.; Feng, X. Accelerated hydrogen evolution kinetics on NiFe-layered double hydroxide electrocatalysts by tailoring water dissociation active sites. Adv. Mater. 2018, 30, 1706279. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Yu, G.; Li, Y.; Chen, W. Increasing Silicon Concentration and Doping Heteroatom to Successfully Realize High HER Catalytic Activity in 2D Metal-Free BSin (n = 1–4) Structures: A First-Principles Study. J. Electrochem. Soc. 2021, 168, 126527. [Google Scholar] [CrossRef]
- Li, C.; Yu, G.; Shen, X.; Li, Y.; Chen, W. Theoretical Study on the High HER/OER Electrocatalytic Activities of 2D GeSi, SnSi, and SnGe Monolayers and Further Improvement by Imposing Biaxial Strain or Doping Heteroatoms. Molecules 2022, 27, 5092. [Google Scholar] [CrossRef]
- Chen, X.; Zhang, H.; Zhang, Y. Transition metal doped graphene-like germanium carbide: Screening of high performance electrocatalysts for oxygen reduction, oxygen evolution, or hydrogen evolution. Colloid Surf. A 2021, 630, 127628. [Google Scholar] [CrossRef]
- Jing, T.; Liang, D.; Deng, M.; Cai, S.; Qi, X. Density Functional Theory Studies of Heteroatom-Doped Graphene-like GaN Monolayers as Electrocatalysts for Oxygen Evolution and Reduction. ACS Appl. Nano Mater. 2021, 4, 7125–7133. [Google Scholar] [CrossRef]
- Wang, E.; Zhang, B.; Zhou, J.; Sun, Z. High catalytic activity of MBenes-supported single atom catalysts for oxygen reduction and oxygen evolution reaction. Appl. Surf. Sci. 2022, 604, 154522. [Google Scholar] [CrossRef]
- Pang, S.-Y.; Wong, Y.-T.; Yuan, S.; Liu, Y.; Tsang, M.-K.; Yang, Z.; Huang, H.; Wong, W.-T.; Hao, J. Universal Strategy for HF-Free Facile and Rapid Synthesis of Two-dimensional MXenes as Multifunctional Energy Materials. J. Am. Chem. Soc. 2019, 141, 9610–9616. [Google Scholar] [CrossRef]
- Zhu, D.; Qiao, M.; Liu, J.; Tao, T.; Guo, C. Engineering pristine 2D metal–organic framework nanosheets for electrocatalysis. J. Mater. Chem. A 2020, 8, 8143–8170. [Google Scholar] [CrossRef]
- Ullah, F.; Ayub, K.; Mahmood, T. High performance SACs for HER process using late first-row transition metals anchored on graphyne support: A DFT insight. Int. J. Hydrogen Energy 2021, 46, 37814–37823. [Google Scholar] [CrossRef]
- Chen, Y.; Yue, Y.; Yang, C.; Zhang, X.; Qin, J.; Liu, R. Transition metal single-atom anchored g-CN monolayer for constructing high-activity multifunctional electrocatalyst. Appl. Surf. Sci. 2021, 565, 150547. [Google Scholar] [CrossRef]
- Zhang, X.; Chen, A.; Zhang, Z.; Jiao, M.; Zhou, Z. Transition metal anchored C2N monolayers as efficient bifunctional electrocatalysts for hydrogen and oxygen evolution reactions. J. Mater. Chem A 2018, 6, 11446–11452. [Google Scholar] [CrossRef]
- Zhou, Y.; Gao, G.; Kang, J.; Chu, W.; Wang, L.-W. Computational screening of transition-metal single atom doped C9N4 monolayers as efficient electrocatalysts for water splitting. Nanoscale 2019, 11, 18169–18175. [Google Scholar] [CrossRef]
- Zheng, Y.; Jiao, Y.; Zhu, Y.; Cai, Q.; Vasileff, A.; Li, L.H.; Han, Y.; Chen, Y.; Qiao, S.-Z. Molecule-level g-C3N4 coordinated transition metals as a new class of electrocatalysts for oxygen electrode reactions. J. Am. Chem. Soc. 2017, 139, 3336–3339. [Google Scholar] [CrossRef] [PubMed]
- Wang, T.; Yu, G.; Liu, J.; Huang, X.; Chen, W. Theoretical design of a series of 2D TM–C3N4 and TM–C3N4@graphene (TM = V, Nb and Ta) nanostructures with highly efficient catalytic activity for the hydrogen evolution reaction. Phys. Chem. Chem. Phys. 2019, 21, 1773–1783. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Yang, E.; Liu, R.; Lv, M.; Zhang, W.; Yu, G.; Chen, W. Embedding Group VIII Elements into a 2D Rigid pc-C3N2 Monolayer to Achieve Single-Atom Catalysts with Excellent OER Activity: A DFT Theoretical Study. Molecules 2023, 28, 254. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Y.; Jiao, Y.; Li, L.H.; Xing, T.; Chen, Y.; Jaroniec, M.; Qiao, S.Z. Toward Design of Synergistically Active Carbon-Based Catalysts for Electrocatalytic Hydrogen Evolution. ACS Nano 2014, 8, 5290–5296. [Google Scholar] [CrossRef]
- Fei, H.; Dong, J.; Arellano-Jiménez, M.J.; Ye, G.; Dong Kim, N.; Samuel, E.L.G.; Peng, Z.; Zhu, Z.; Qin, F.; Bao, J.; et al. Atomic cobalt on nitrogen-doped graphene for hydrogen generation. Nat. Commun. 2015, 6, 8668. [Google Scholar] [CrossRef]
- Zhao, X.; Liu, X.; Huang, B.; Wang, P.; Pei, Y. Hydroxyl group modification improves the electrocatalytic ORR and OER activity of graphene supported single and bi-metal atomic catalysts (Ni, Co, and Fe). J. Mater. Chem. A 2019, 7, 24583–24593. [Google Scholar] [CrossRef]
- Hou, G.; Song, Y.; Ma, X.; Chu, F.; Wu, M.; Wang, D.; Wu, J.; Qi, Y.; Wu, C.; Xiong, M. First principles study on electronic properties and oxygen evolution mechanism of 2D bimetallic N-doped graphene. J. Mol. Graph. Model. 2022, 111, 108101. [Google Scholar] [CrossRef]
- Zhang, D.; Mou, H.; Lu, F.; Song, C.; Wang, D. A novel strategy for 2D/2D NiS/graphene heterostructures as efficient bifunctional electrocatalysts for overall water splitting. Appl. Catal. B-Environ. 2019, 254, 471–478. [Google Scholar] [CrossRef]
- Li, T.; He, C.; Zhang, W. Rational design of porous carbon allotropes as anchoring materials for lithium sulfur batteries. J. Energy Chem. 2021, 52, 121–129. [Google Scholar] [CrossRef]
- Yin, W.-J.; Xie, Y.-E.; Liu, L.-M.; Wang, R.-Z.; Wei, X.-L.; Lau, L.; Zhong, J.-X.; Chen, Y.-P. R-graphyne: A new two-dimensional carbon allotrope with versatile Dirac-like point in nanoribbons. J. Mater. Chem. A 2013, 1, 5341–5346. [Google Scholar] [CrossRef]
- Tuckerman, M.; Berne, B.J.; Martyna, G.J. Reversible multiple time scale molecular dynamics. J. Chem. Phys. 1992, 97, 1990–2001. [Google Scholar] [CrossRef]
- Savin, A.; Nesper, R.; Wengert, S.; Fässler, T.F. ELF: The electron localization function. Angew. Chem. Int. Edit. 1997, 36, 1808–1832. [Google Scholar] [CrossRef]
- Nørskov, J.K.; Bligaard, T.; Logadottir, A.; Kitchin, J.; Chen, J.G.; Pandelov, S.; Stimming, U. Trends in the exchange current for hydrogen evolution. J. Electrochem. Soc. 2005, 152, 23–26. [Google Scholar] [CrossRef]
- Liao, W.; Yu, G.; Zhao, L.; Zhu, H.; Chen, W. Doping P atom with a lone pair: An effective strategy to realize high HER catalytic activity and avoid deactivation under wide H* coverage on 2D silicene and germanene by increasing the structural rigidity. Nanoscale 2022, 14, 10918–10928. [Google Scholar] [CrossRef]
- Corminboeuf, C.; Heine, T.; Weber, J. Evaluation of aromaticity: A new dissected NICS model based on canonical orbitals. Phys. Chem. Chem. Phys. 2003, 5, 246–251. [Google Scholar] [CrossRef]
- Man, I.C.; Su, H.Y.; Calle-Vallejo, F.; Hansen, H.A.; Martínez, J.I.; Inoglu, N.G.; Kitchin, J.; Jaramillo, T.F.; Nørskov, J.K.; Rossmeisl, J. Universality in oxygen evolution electrocatalysis on oxide surfaces. ChemCatChem 2011, 3, 1159–1165. [Google Scholar] [CrossRef]
- Ma, Y.; Jin, F.; Hu, Y.H. Bifunctional electrocatalysts for oxygen reduction and oxygen evolution: A theoretical study on 2D metallic WO2-supported single atom (Fe, Co, or Ni) catalysts. Phys. Chem. Chem. Phys. 2021, 23, 13687–13695. [Google Scholar] [CrossRef]
- Ji, J.; Zhang, C.; Qin, S.; Jin, P. First-principles investigation of two-dimensional covalent–organic framework electrocatalysts for oxygen evolution/reduction and hydrogen evolution reactions. Sustain. Energ. Fuels 2021, 5, 5615–5626. [Google Scholar] [CrossRef]
- Koper, M.T. Thermodynamic theory of multi-electron transfer reactions: Implications for electrocatalysis. J. Electroanal. Chem. 2011, 660, 254–260. [Google Scholar] [CrossRef]
- Hu, M.; Li, S.; Zheng, S.; Liang, X.; Zheng, J.; Pan, F. Tuning single-atom catalysts of nitrogen-coordinated transition metals for optimizing oxygen evolution and reduction reactions. J. Phys. Chem. C 2020, 124, 13168–13176. [Google Scholar] [CrossRef]
- Li, X.; Liu, J.; Cai, Q.; Kan, Z.; Liu, S.; Zhao, J. Engineering d-band center of iron single atom site through boron incorporation to trigger the efficient bifunctional oxygen electrocatalysis. J. Colloid Interface Sci. 2022, 628, 331–342. [Google Scholar] [CrossRef] [PubMed]
- Wang, A.; Niu, H.; Wang, X.; Wan, X.; Xie, L.; Zhang, Z.; Wang, J.; Guo, Y. Two-dimensional metal–organic frameworks as efficient electrocatalysts for bifunctional oxygen evolution/reduction reactions. J. Mater. Chem. A 2022, 10, 13005–13012. [Google Scholar] [CrossRef]
- Perdew, J.P.; Burke, K.; Ernzerhof, M. Generalized Gradient Approximation Made Simple. Phys. Rev. Lett. 1996, 77, 3865–3868. [Google Scholar] [CrossRef]
- Kresse, G.; Hafner, J. Ab initio molecular dynamics for liquid metals. Phys. Rev. B 1993, 47, 558–561. [Google Scholar] [CrossRef]
- Kresse, G.; Hafner, J. Ab initio molecular-dynamics simulation of the liquid-metal–amorphous-semiconductor transition in germanium. Phys. Rev. B 1994, 49, 14251. [Google Scholar] [CrossRef]
- Grimme, S. Semiempirical GGA-type density functional constructed with a long-range dispersion correction. J. Comput. Chem. 2006, 27, 1787–1799. [Google Scholar] [CrossRef]
- Blöchl, P.; Jepsen, O.; Andersen, O. Projector augmented-wave method. Phys. Rev. B 1994, 50, 17953–17979. [Google Scholar] [CrossRef]
- Chen, Z.; Wannere, C.S.; Corminboeuf, C.; Puchta, R.; Schleyer, P.v.R. Nucleus-independent chemical shifts (NICS) as an aromaticity criterion. Chem. Rev. 2005, 105, 3842–3888. [Google Scholar] [CrossRef] [PubMed]
- Schleyer, P.V.R.; Manoharan, M.; Wang, Z.-X.; Kiran, B.; Jiao, H.; Puchta, R.; van Eikema Hommes, N.J. Dissected nucleus-independent chemical shift analysis of π-aromaticity and antiaromaticity. Org. Lett. 2001, 3, 2465–2468. [Google Scholar] [CrossRef] [PubMed]
- Frisch, M.J.; Trucks, G.W.; Schlegel, H.B.; Scuseria, G.E.; Robb, M.A.; Cheeseman, J.R.; Scalmani, G.; Barone, V.; Mennucci, B.; Petersson, G.A.; et al. Gaussian 09 (Revision D.01); Gaussian, Inc.: Wallingford, CT, USA, 2013. [Google Scholar]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, C.; Li, T.; Yu, G.; Chen, W. Theoretical Investigation of HER and OER Electrocatalysts Based on the 2D R-graphyne Completely Composed of Anti-Aromatic Carbon Rings. Molecules 2023, 28, 3888. https://doi.org/10.3390/molecules28093888
Li C, Li T, Yu G, Chen W. Theoretical Investigation of HER and OER Electrocatalysts Based on the 2D R-graphyne Completely Composed of Anti-Aromatic Carbon Rings. Molecules. 2023; 28(9):3888. https://doi.org/10.3390/molecules28093888
Chicago/Turabian StyleLi, Cuimei, Tianya Li, Guangtao Yu, and Wei Chen. 2023. "Theoretical Investigation of HER and OER Electrocatalysts Based on the 2D R-graphyne Completely Composed of Anti-Aromatic Carbon Rings" Molecules 28, no. 9: 3888. https://doi.org/10.3390/molecules28093888
APA StyleLi, C., Li, T., Yu, G., & Chen, W. (2023). Theoretical Investigation of HER and OER Electrocatalysts Based on the 2D R-graphyne Completely Composed of Anti-Aromatic Carbon Rings. Molecules, 28(9), 3888. https://doi.org/10.3390/molecules28093888





