Glucosamine-Modified Reduction-Responsive Polymeric Micelles for Liver Cancer Therapy
Abstract
1. Introduction
2. Results and Discussion
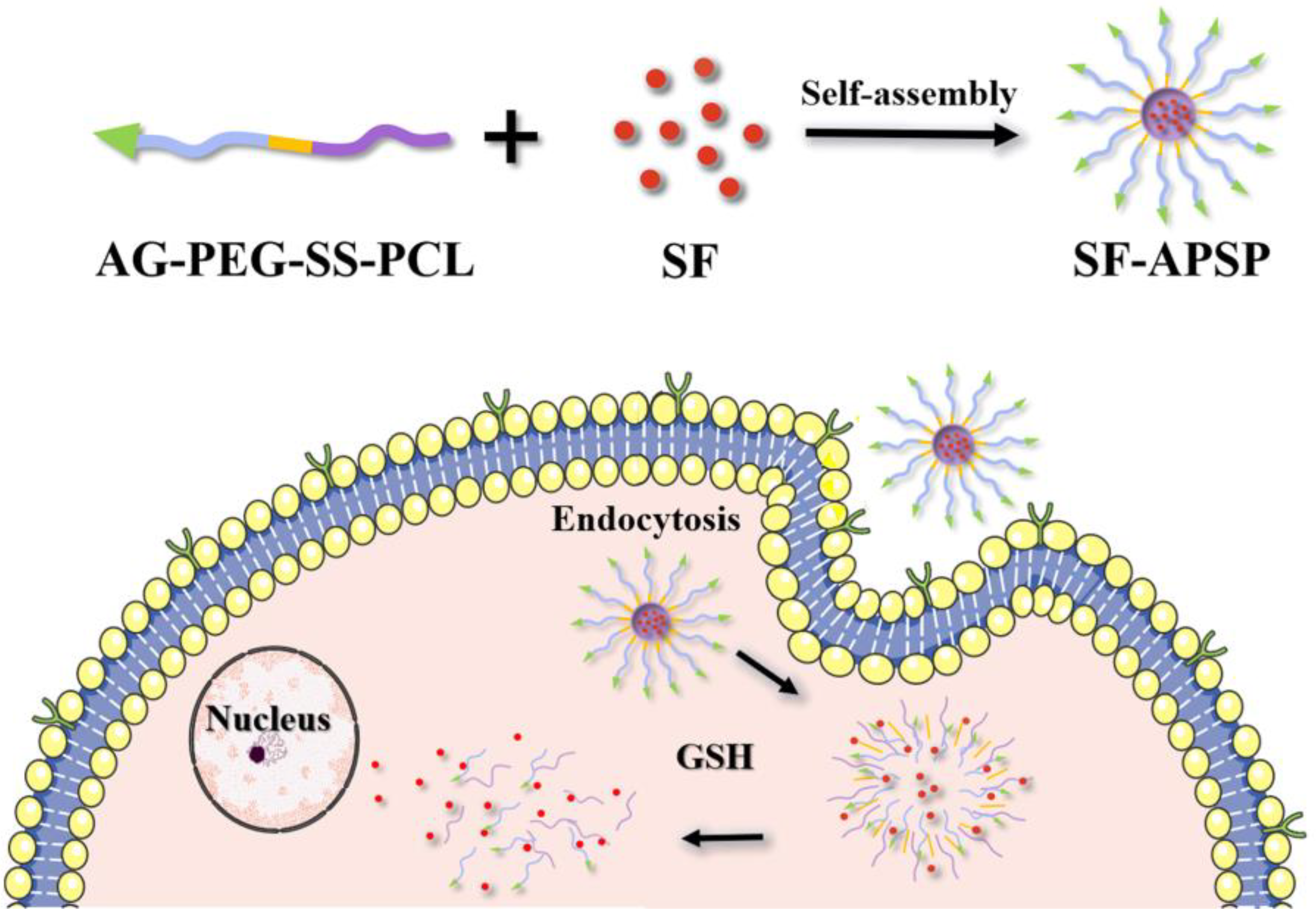
2.1. Synthesis and Characterization of APSP
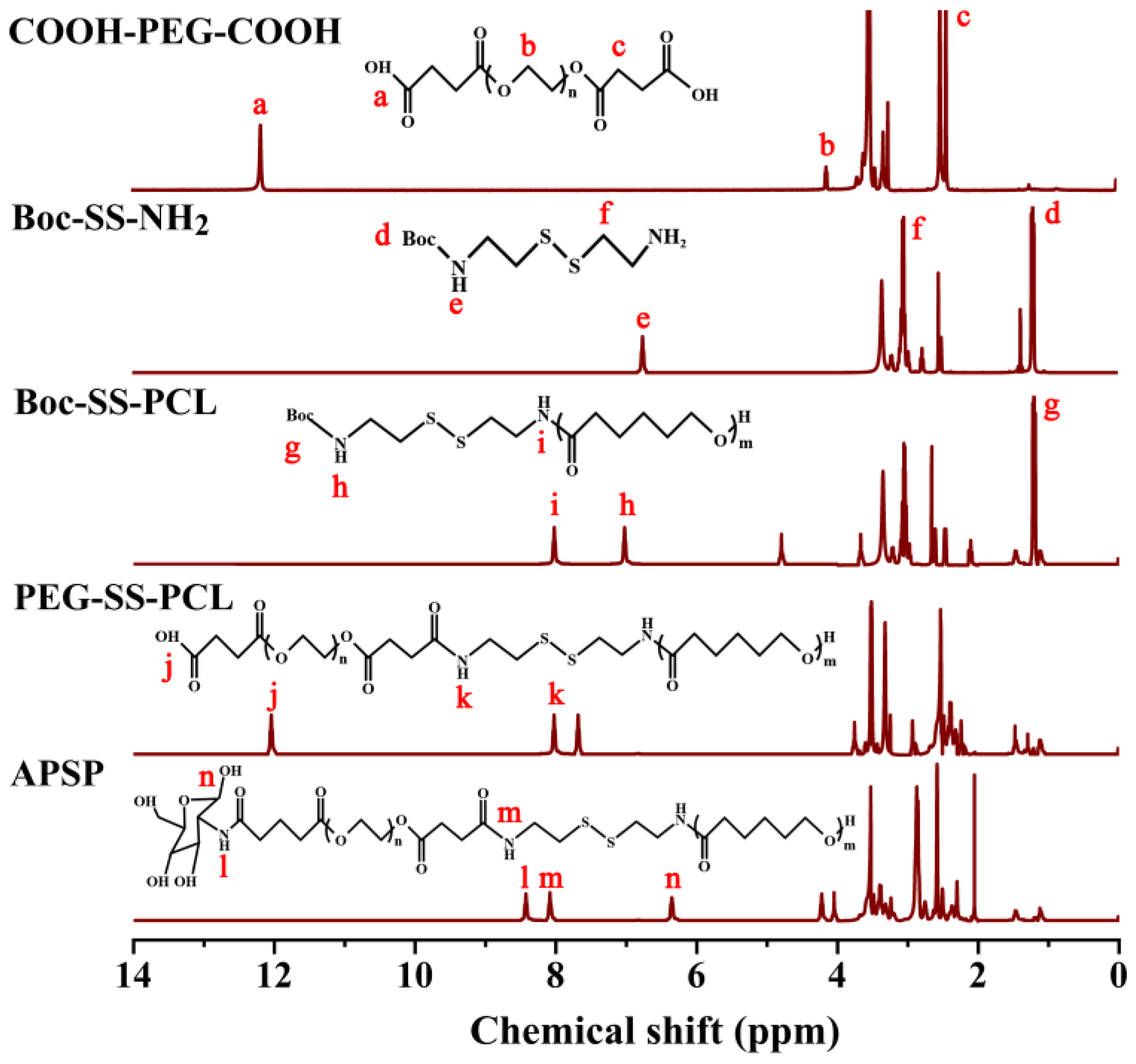
2.1.1. Proton Nuclear Magnetic Resonance (1H NMR) Analysis
2.1.2. Fourier-Transform Infrared (FT-IR) Spectroscopy Analysis
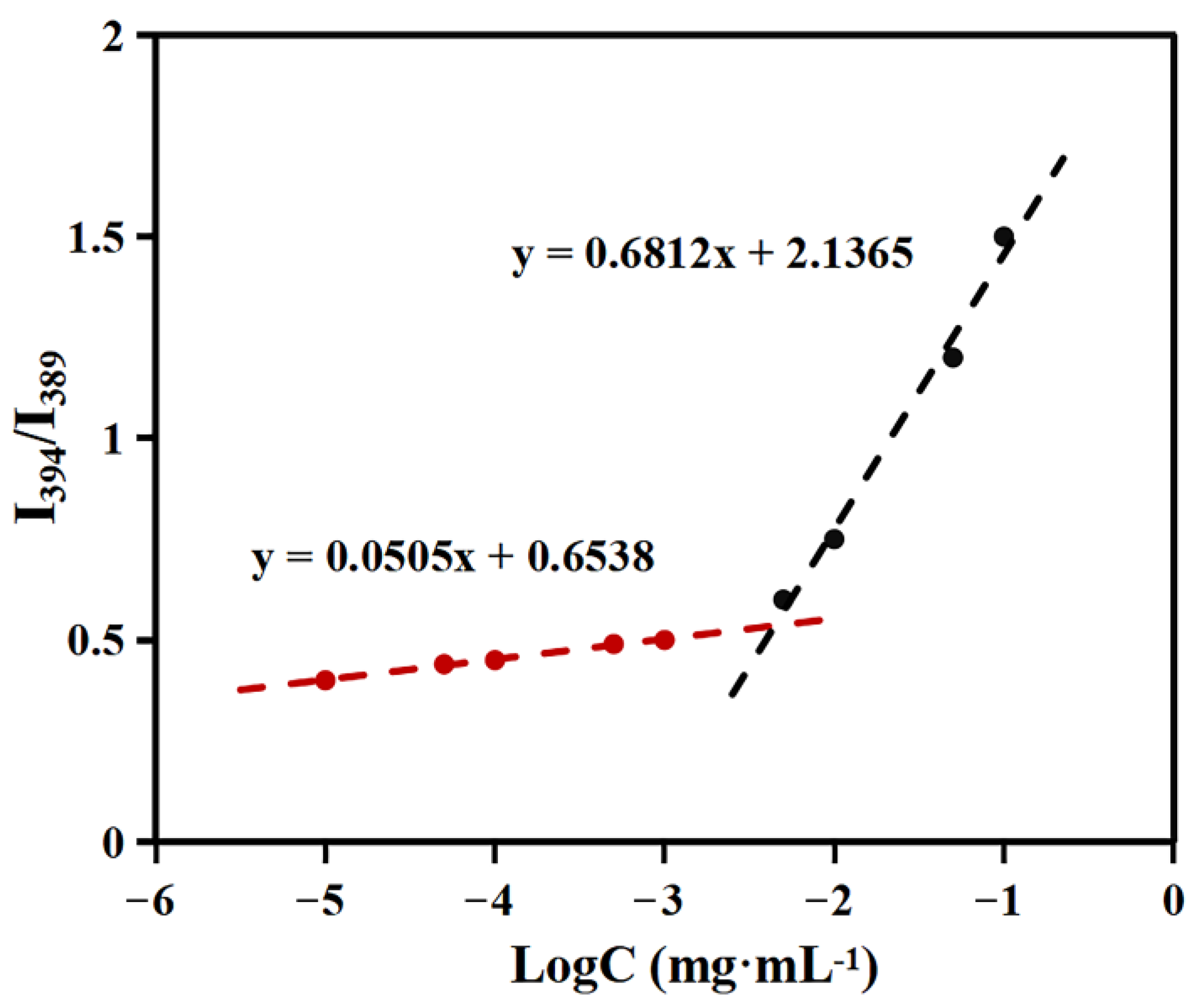
2.2. Critical Aggregation Concentration (CAC) Determination
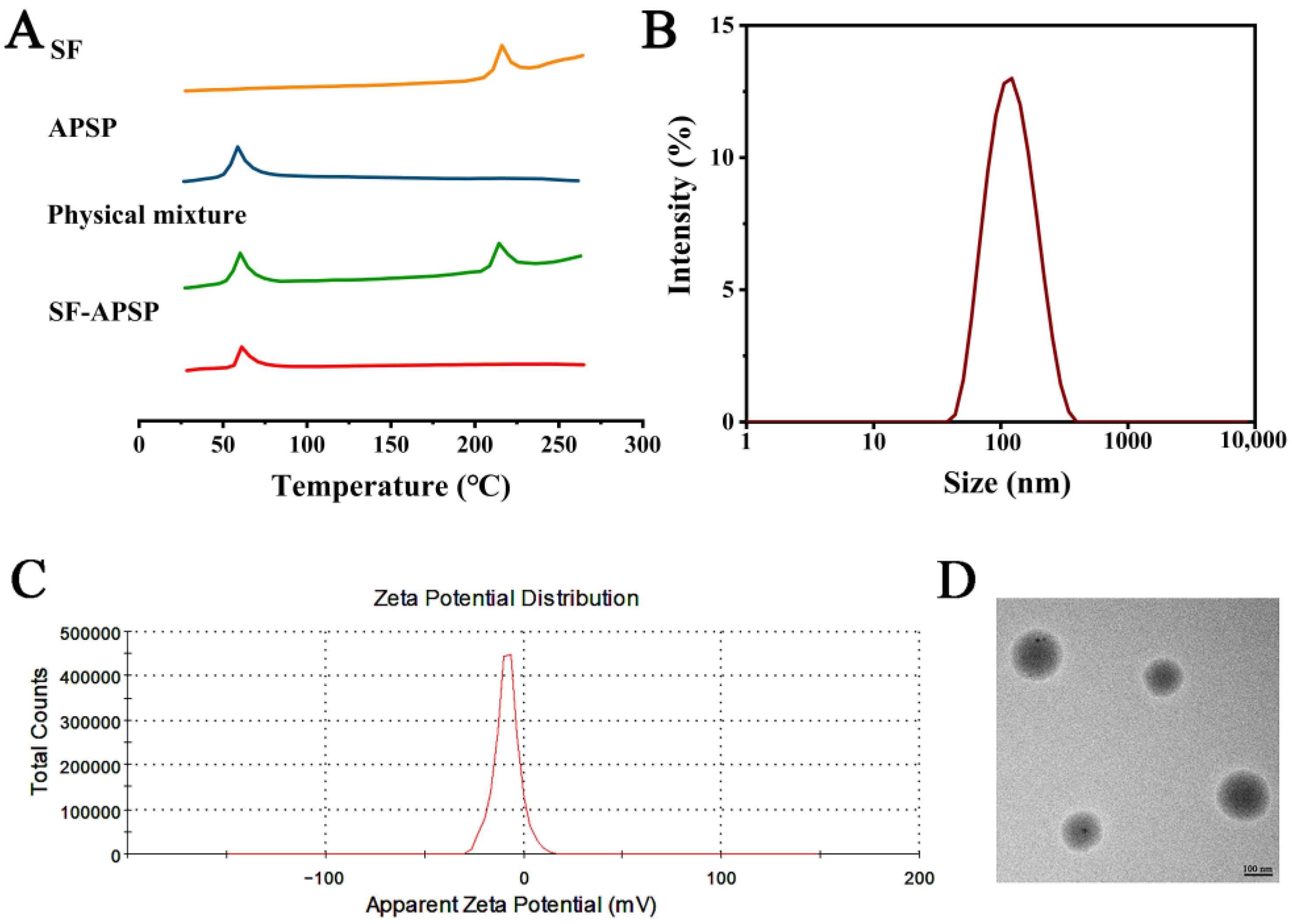
2.3. Preparation and Characterization of SF-APSP Micelles
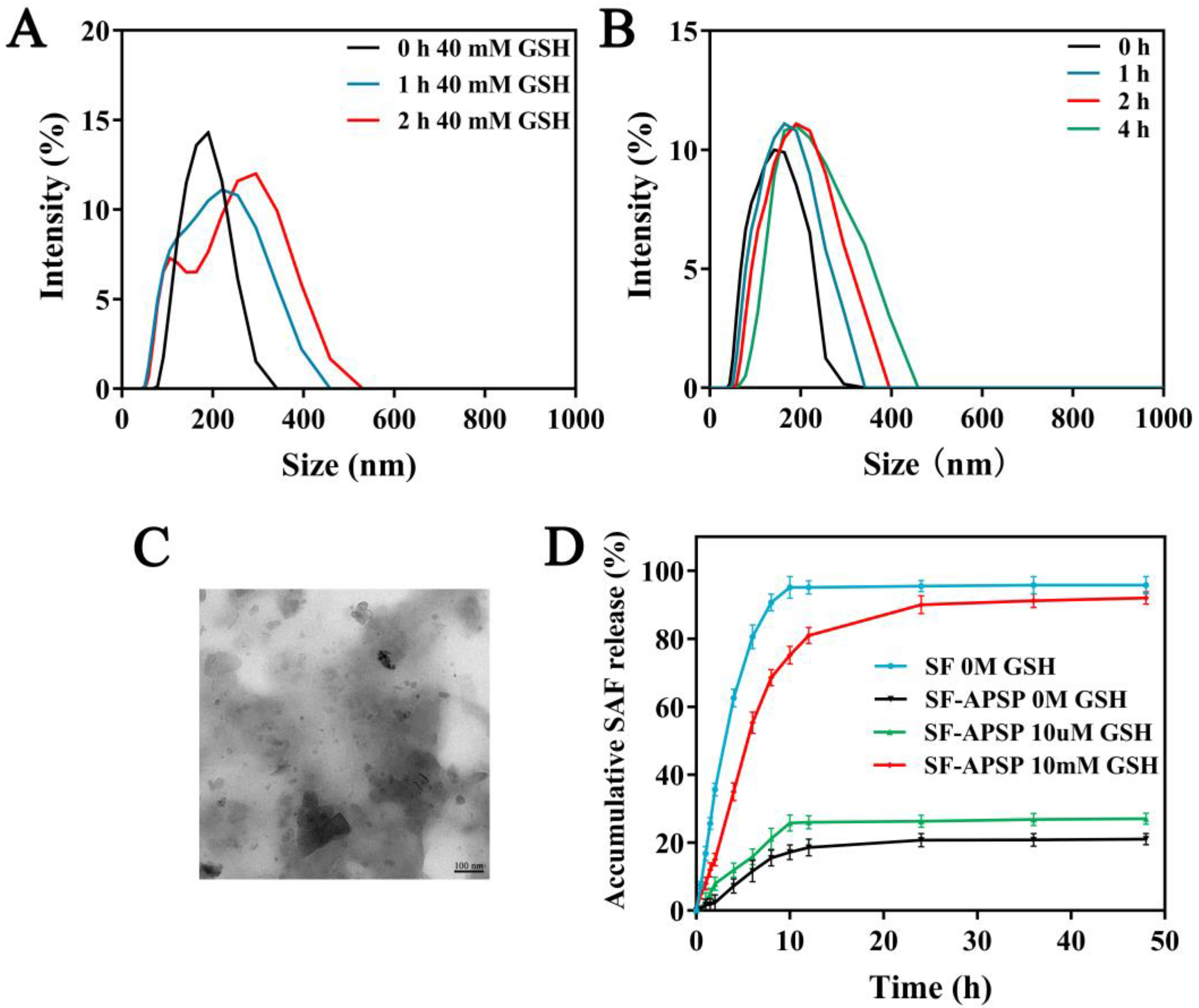
2.4. Stability of Micelles
2.5. GSH-Triggered Disassembly and Drug Release
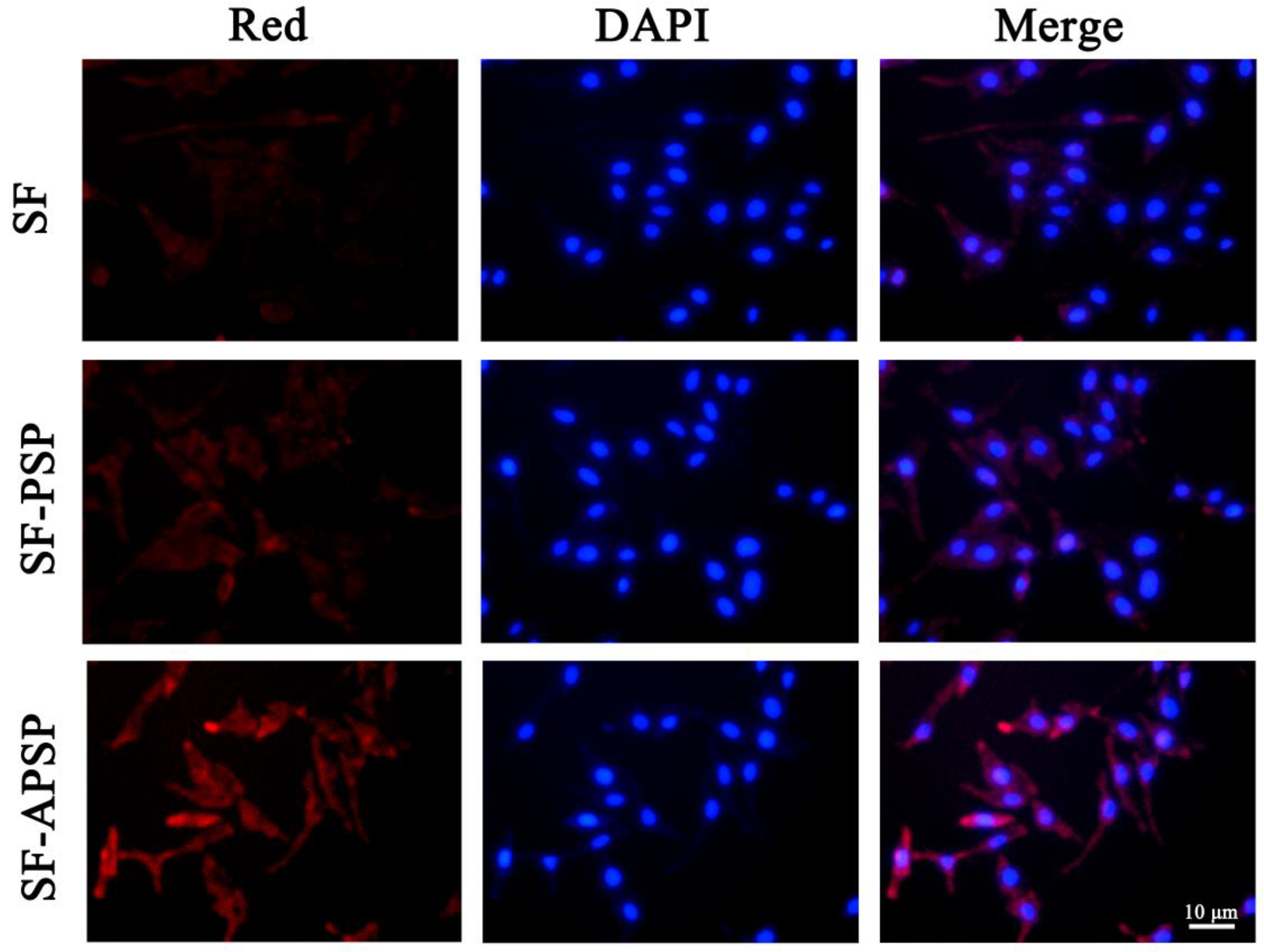
2.6. In Vitro Cellular Uptake
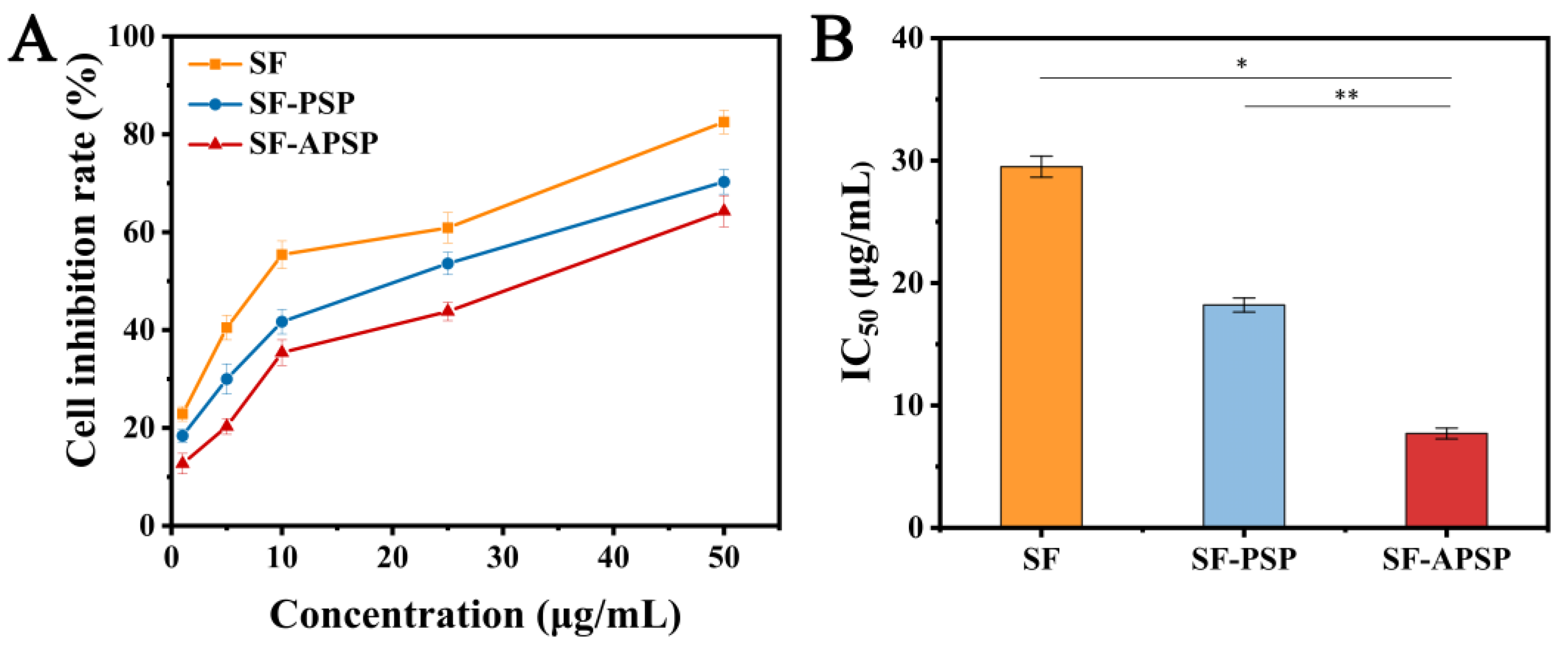
2.7. In Vitro Cytotoxicity Study
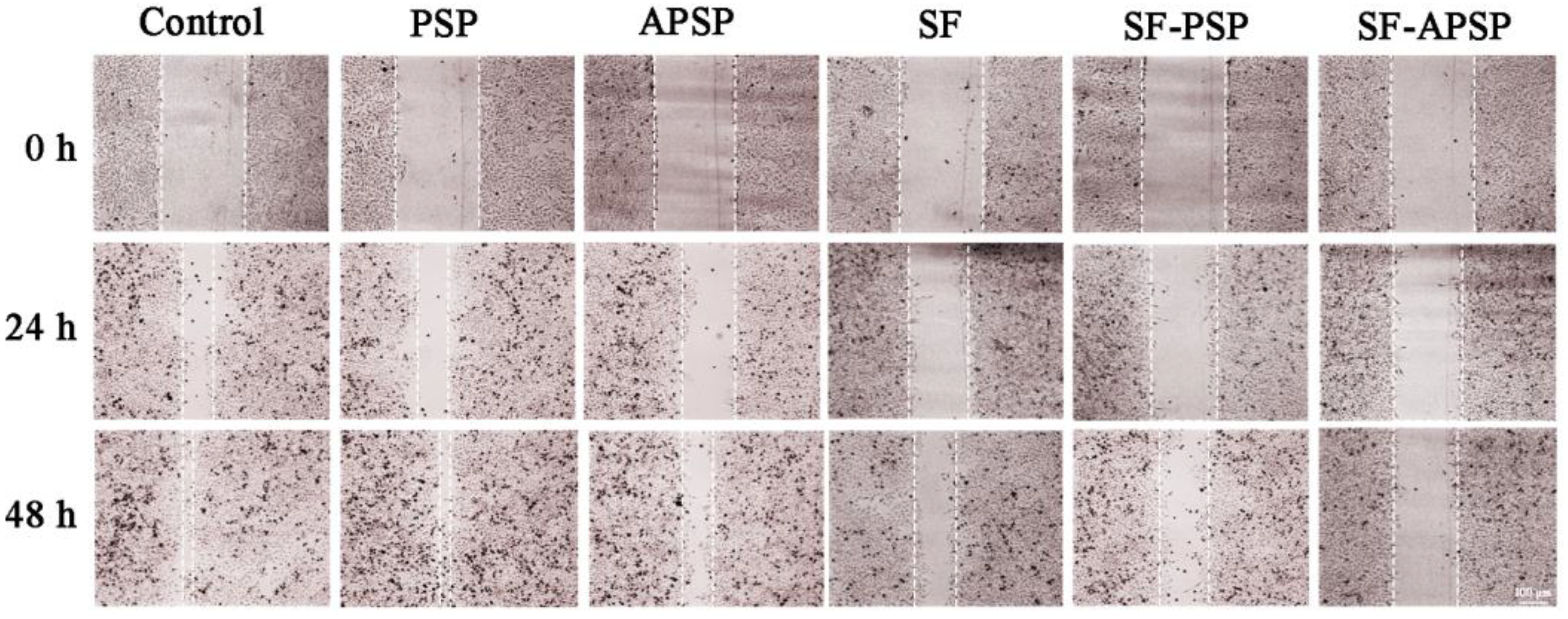
2.8. In Vitro Wound Healing Assay
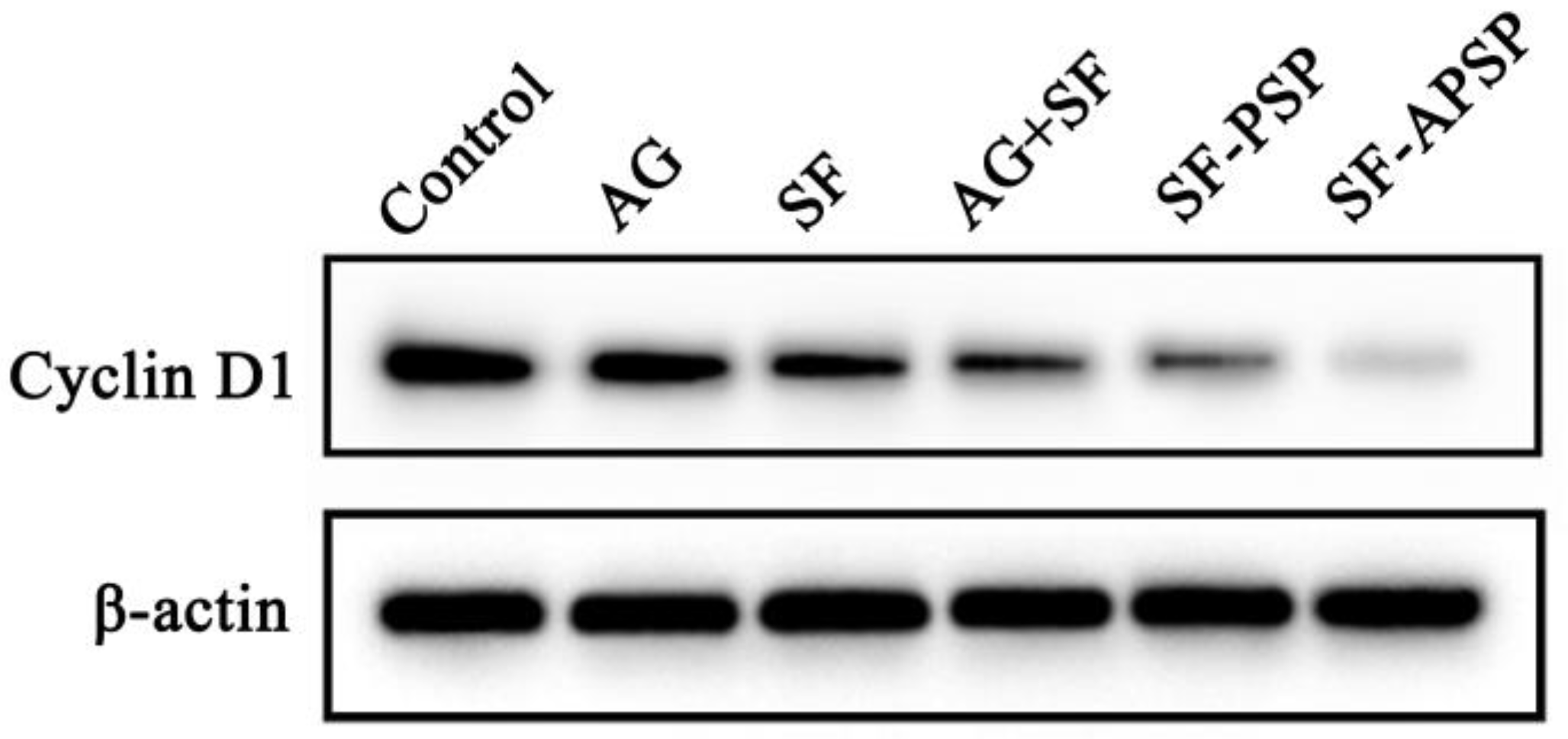
2.9. Western Blot Analysis
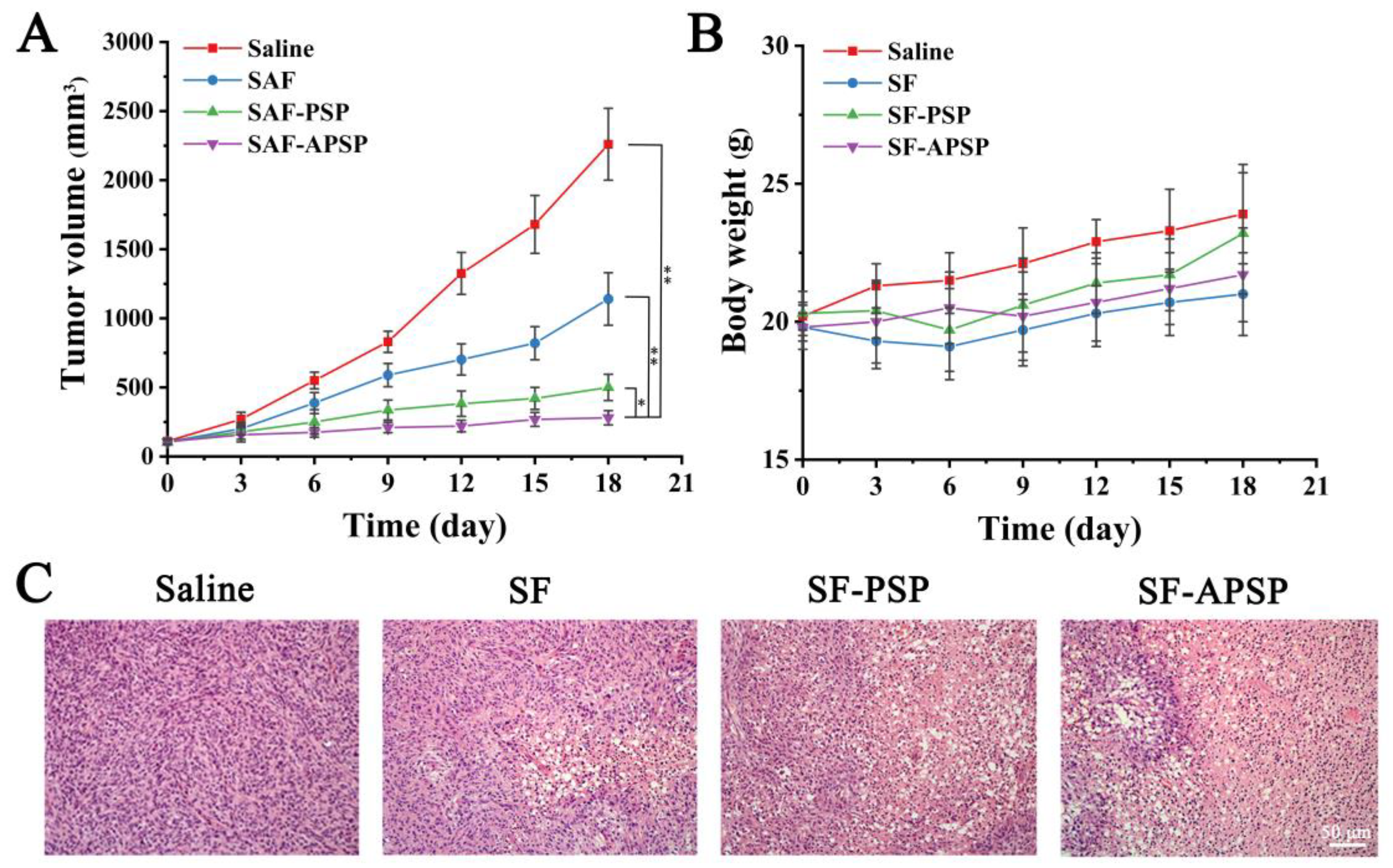
2.10. In Vivo Therapeutic Efficacy
2.11. Discussion
3. Materials and Methods
3.1. Materials
3.2. Cells and Animals
3.3. Synthesis of APSP
3.3.1. Synthesis of COOH-PEG-COOH
3.3.2. Synthesis of Boc-SS-NH2
3.3.3. Synthesis of Boc-SS-PCL
3.3.4. Synthesis of PEG-SS-PCL
3.3.5. Synthesis of APSP
3.4. Critical Aggregation Concentration (CAC) Determination
3.5. Preparation and Characterization of SF-APSP Micelles
3.6. Stability Study
3.7. GSH-Triggered Drug Release and Micelle Disassembly
3.8. In Vitro Cellular Uptake
3.9. Cell Cytotoxicity Assays
3.10. Wound Healing Assay
3.11. Western Blot Analysis
3.12. In Vivo Therapeutic Efficacy
3.13. Statistical Analyses
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Manieri, E.; Herrera-Melle, L.; Mora, A.; Tomás-Loba, A.; Leiva-Vega, L.; Fernández, D.I.; Rodríguez, E.; Morán, L.; Hernández-Cosido, L.; Torres, J.L.; et al. Adiponectin accounts for gender differences in hepatocellular carcinoma incidence. J. Exp. Med. 2019, 216, 1108–1119. [Google Scholar] [CrossRef] [PubMed]
- Bray, F.; Ferlay, J.; Soerjomataram, I.; Siegel, R.L.; Torre, L.A.; Jemal, A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA A Cancer J. Clin. 2018, 68, 394–424. [Google Scholar] [CrossRef] [PubMed]
- Bruix, J.; Raoul, J.L.; Sherman, M.; Mazzaferro, V.; Bolondi, L.; Craxi, A.; Galle, P.R.; Santoro, A.; Beaugrand, M.; Sangiovanni, A.; et al. Efficacy and safety of sorafenib in patients with advanced hepatocellular carcinoma: Subanalyses of a phase III trial. J. Hepatol. 2012, 57, 821–829. [Google Scholar] [CrossRef]
- Gan, H.; Li, L.; Hu, X.; Cai, J.; Hu, X.; Zhang, H.; Zhao, N.; Xu, X.; Guo, H.; Pang, P. DDX24 regulates the chemosensitivity of hepatocellular carcinoma to sorafenib via mediating the expression of SNORA18. Cancer Biol. Ther. 2022, 23, 1–14. [Google Scholar] [CrossRef]
- Randrup Hansen, C.; Grimm, D.; Bauer, J.; Wehland, M.; Magnusson, N.E. Effects and Side Effects of Using Sorafenib and Sunitinib in the Treatment of Metastatic Renal Cell Carcinoma. Int. J. Mol. Sci. 2017, 18, 461. [Google Scholar] [CrossRef] [PubMed]
- Abdel-Rahman, O.; Lamarca, A. Development of sorafenib-related side effects in patients diagnosed with advanced hepatocellular carcinoma treated with sorafenib: A systematic-review and meta-analysis of the impact on survival. Expert Rev. Gastroenterol. Hepatol. 2017, 11, 75–83. [Google Scholar] [CrossRef] [PubMed]
- Khalifa, A.M.; Elsheikh, M.A.; Khalifa, A.M.; Elnaggar, Y. Current strategies for different paclitaxel-loaded Nano-delivery Systems towards therapeutic applications for ovarian carcinoma: A review article. J. Control. Release Off. J. Control. Release Soc. 2019, 311–312, 125–137. [Google Scholar] [CrossRef]
- Deshmukh, A.S.; Chauhan, P.N.; Noolvi, M.N.; Chaturvedi, K.; Ganguly, K.; Shukla, S.S.; Nadagouda, M.N.; Aminabhavi, T.M. Polymeric micelles: Basic research to clinical practice. Int. J. Pharm. 2017, 532, 249–268. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.; Shi, Y.; Kim, J.Y.; Park, K.; Cheng, J.X. Overcoming the barriers in micellar drug delivery: Loading efficiency, in vivo stability, and micelle-cell interaction. Expert Opin. Drug Deliv. 2010, 7, 49–62. [Google Scholar] [CrossRef]
- Li, S.D.; Huang, L. Pharmacokinetics and biodistribution of nanoparticles. Mol. Pharm. 2008, 5, 496–504. [Google Scholar] [CrossRef]
- Chang, M.; Wu, M.; Li, H. Antitumor activities of novel glycyrrhetinic acid-modified curcumin-loaded cationic liposomes in vitro and in H22 tumor-bearing mice. Drug Deliv. 2018, 25, 1984–1995. [Google Scholar] [CrossRef] [PubMed]
- Plummer, R.; Wilson, R.H.; Calvert, H.; Boddy, A.V.; Griffin, M.; Sludden, J.; Tilby, M.J.; Eatock, M.; Pearson, D.G.; Ottley, C.J.; et al. A Phase I clinical study of cisplatin-incorporated polymeric micelles (NC-6004) in patients with solid tumours. Br. J. Cancer 2011, 104, 593–598. [Google Scholar] [CrossRef] [PubMed]
- Yang, T.; Lan, Y.; Cao, M.; Ma, X.; Cao, A.; Sun, Y.; Yang, J.; Li, L.; Liu, Y. Glycyrrhetinic acid-conjugated polymeric prodrug micelles co-delivered with doxorubicin as combination therapy treatment for liver cancer. Colloids Surf. B Biointerfaces 2019, 175, 106–115. [Google Scholar] [CrossRef] [PubMed]
- Brannon-Peppas, L.; Blanchette, J.O. Nanoparticle and targeted systems for cancer therapy. Adv. Drug Deliv. Rev. 2004, 56, 1649–1659. [Google Scholar] [CrossRef]
- Liu, S.; Huang, W.; Jin, M.J.; Fan, B.; Xia, G.M.; Gao, Z.G. Inhibition of murine breast cancer growth and metastasis by survivin-targeted siRNA using disulfide cross-linked linear PEI. Eur. J. Pharm. Sci. Off. J. Eur. Fed. Pharm. Sci. 2016, 82, 171–182. [Google Scholar] [CrossRef]
- Amann, T.; Hellerbrand, C. GLUT1 as a therapeutic target in hepatocellular carcinoma. Expert Opin. Ther. Targets 2009, 13, 1411–1427. [Google Scholar] [CrossRef]
- Airley, R.; Evans, A.; Mobasheri, A.; Hewitt, S.M. Glucose transporter Glut-1 is detectable in peri-necrotic regions in many human tumor types but not normal tissues: Study using tissue microarrays. Ann. Anat. Anat. Anz. Off. Organ Anat. Ges. 2010, 192, 133–138. [Google Scholar] [CrossRef]
- Amann, T.; Kirovski, G.; Bosserhoff, A.K.; Hellerbrand, C. Analysis of a promoter polymorphism of the GLUT1 gene in patients with hepatocellular carcinoma. Mol. Membr. Biol. 2011, 28, 182–186. [Google Scholar] [CrossRef]
- Christgau, S.; Henrotin, Y.; Tankó, L.B.; Rovati, L.C.; Collette, J.; Bruyere, O.; Deroisy, R.; Reginster, J.Y. Osteoarthritic patients with high cartilage turnover show increased responsiveness to the cartilage protecting effects of glucosamine sulphate. Clin. Exp. Rheumatol. 2004, 22, 36–42. [Google Scholar]
- Dalirfardouei, R.; Karimi, G.; Jamialahmadi, K. Molecular mechanisms and biomedical applications of glucosamine as a potential multifunctional therapeutic agent. Life Sci. 2016, 152, 21–29. [Google Scholar] [CrossRef]
- Park, J.Y.; Park, J.W.; Suh, S.I.; Baek, W.K. D-glucosamine down-regulates HIF-1alpha through inhibition of protein translation in DU145 prostate cancer cells. Biochem. Biophys. Res. Commun. 2009, 382, 96–101. [Google Scholar] [CrossRef] [PubMed]
- Pawar, S.; Vavia, P. Glucosamine anchored cancer targeted nano-vesicular drug delivery system of doxorubicin. J. Drug Target. 2016, 24, 68–79. [Google Scholar] [CrossRef] [PubMed]
- Oh, H.J.; Lee, J.S.; Song, D.K.; Shin, D.H.; Jang, B.C.; Suh, S.I.; Park, J.W.; Suh, M.H.; Baek, W.K. D-glucosamine inhibits proliferation of human cancer cells through inhibition of p70S6K. Biochem. Biophys. Res. Commun. 2007, 360, 840–845. [Google Scholar] [CrossRef] [PubMed]
- Meng, F.; Hennink, W.E.; Zhong, Z. Reduction-sensitive polymers and bioconjugates for biomedical applications. Biomaterials 2009, 30, 2180–2198. [Google Scholar] [CrossRef] [PubMed]
- Hong, W.; Chen, D.; Jia, L.; Gu, J.; Hu, H.; Zhao, X.; Qiao, M. Thermo- and pH-responsive copolymers based on PLGA-PEG-PLGA and poly(L-histidine): Synthesis and in vitro characterization of copolymer micelles. Acta Biomater. 2014, 10, 1259–1271. [Google Scholar] [CrossRef] [PubMed]
- Russo, A.; DeGraff, W.; Friedman, N.; Mitchell, J.B. Selective modulation of glutathione levels in human normal versus tumor cells and subsequent differential response to chemotherapy drugs. Cancer Res. 1986, 46, 2845–2848. [Google Scholar]
- Saito, G.; Swanson, J.A.; Lee, K.D. Drug delivery strategy utilizing conjugation via reversible disulfide linkages: Role and site of cellular reducing activities. Adv. Drug Deliv. Rev. 2003, 55, 199–215. [Google Scholar] [CrossRef]
- Gay, M.; Montaña, Á.M.; Batalla, C.; Mesas, J.M.; Alegre, M.T. Design, synthesis and SAR studies of novel 1,2-bis(aminomethyl)cyclohexane platinum (II) complexes with cytotoxic activity. Studies of interaction with DNA of iodinated seven-membered 1,4-diaminoplatinocycles. J. Inorg. Biochem. 2015, 142, 15–27. [Google Scholar] [CrossRef]
- Lee, S.H.; Hwang, H.S.; Yun, J.W. Antitumor activity of water extract of a mushroom, Inonotus obliquus, against HT-29 human colon cancer cells. Phytother. Res. PTR 2009, 23, 1784–1789. [Google Scholar] [CrossRef]
- Kolate, A.; Baradia, D.; Patil, S.; Vhora, I.; Kore, G.; Misra, A. PEG—A versatile conjugating ligand for drugs and drug delivery systems. J. Control. Release Off. J. Control. Release Soc. 2014, 192, 67–81. [Google Scholar] [CrossRef]
- Kheiri Manjili, H.; Ghasemi, P.; Malvandi, H.; Mousavi, M.S.; Attari, E.; Danafar, H. Pharmacokinetics and in vivo delivery of curcumin by copolymeric mPEG-PCL micelles. Eur. J. Pharm. Biopharm. 2017, 116, 17–30. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Liang, N.; Gong, X.; Kawashima, Y.; Cui, F.; Sun, S. Tumor-targeting micelles based on folic acid and α-tocopherol succinate conjugated hyaluronic acid for paclitaxel delivery. Colloids Surf. B Biointerfaces 2019, 177, 11–18. [Google Scholar] [CrossRef] [PubMed]
- Zahedipour, F.; Dalirfardouei, R.; Karimi, G.; Jamialahmadi, K. Molecular mechanisms of anticancer effects of Glucosamine. Biomed. Pharmacother. Biomed. Pharmacother. 2017, 95, 1051–1058. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.K.; Diehl, J.A. Nuclear cyclin D1: An oncogenic driver in human cancer. J. Cell. Physiol. 2009, 220, 292–296. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.; Sun, W.; Wang, X.; Wang, Y.; Wang, P. pH-responsive nanoreservoirs based on hyaluronic acid end-capped mesoporous silica nanoparticles for targeted drug delivery. Int. J. Biol. Macromol. 2018, 111, 1106–1115. [Google Scholar] [CrossRef]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Meng, L.; Liu, F.; Du, C.; Zhu, J.; Xiong, Q.; Li, J.; Sun, W. Glucosamine-Modified Reduction-Responsive Polymeric Micelles for Liver Cancer Therapy. Molecules 2023, 28, 3824. https://doi.org/10.3390/molecules28093824
Meng L, Liu F, Du C, Zhu J, Xiong Q, Li J, Sun W. Glucosamine-Modified Reduction-Responsive Polymeric Micelles for Liver Cancer Therapy. Molecules. 2023; 28(9):3824. https://doi.org/10.3390/molecules28093824
Chicago/Turabian StyleMeng, Lei, Fangshu Liu, Chenchen Du, Jiaying Zhu, Qian Xiong, Jing Li, and Weitong Sun. 2023. "Glucosamine-Modified Reduction-Responsive Polymeric Micelles for Liver Cancer Therapy" Molecules 28, no. 9: 3824. https://doi.org/10.3390/molecules28093824
APA StyleMeng, L., Liu, F., Du, C., Zhu, J., Xiong, Q., Li, J., & Sun, W. (2023). Glucosamine-Modified Reduction-Responsive Polymeric Micelles for Liver Cancer Therapy. Molecules, 28(9), 3824. https://doi.org/10.3390/molecules28093824





