Research Progress on Extraction, Isolation, Structural Analysis and Biological Activity of Polysaccharides from Panax Genus
Abstract
1. Introduction
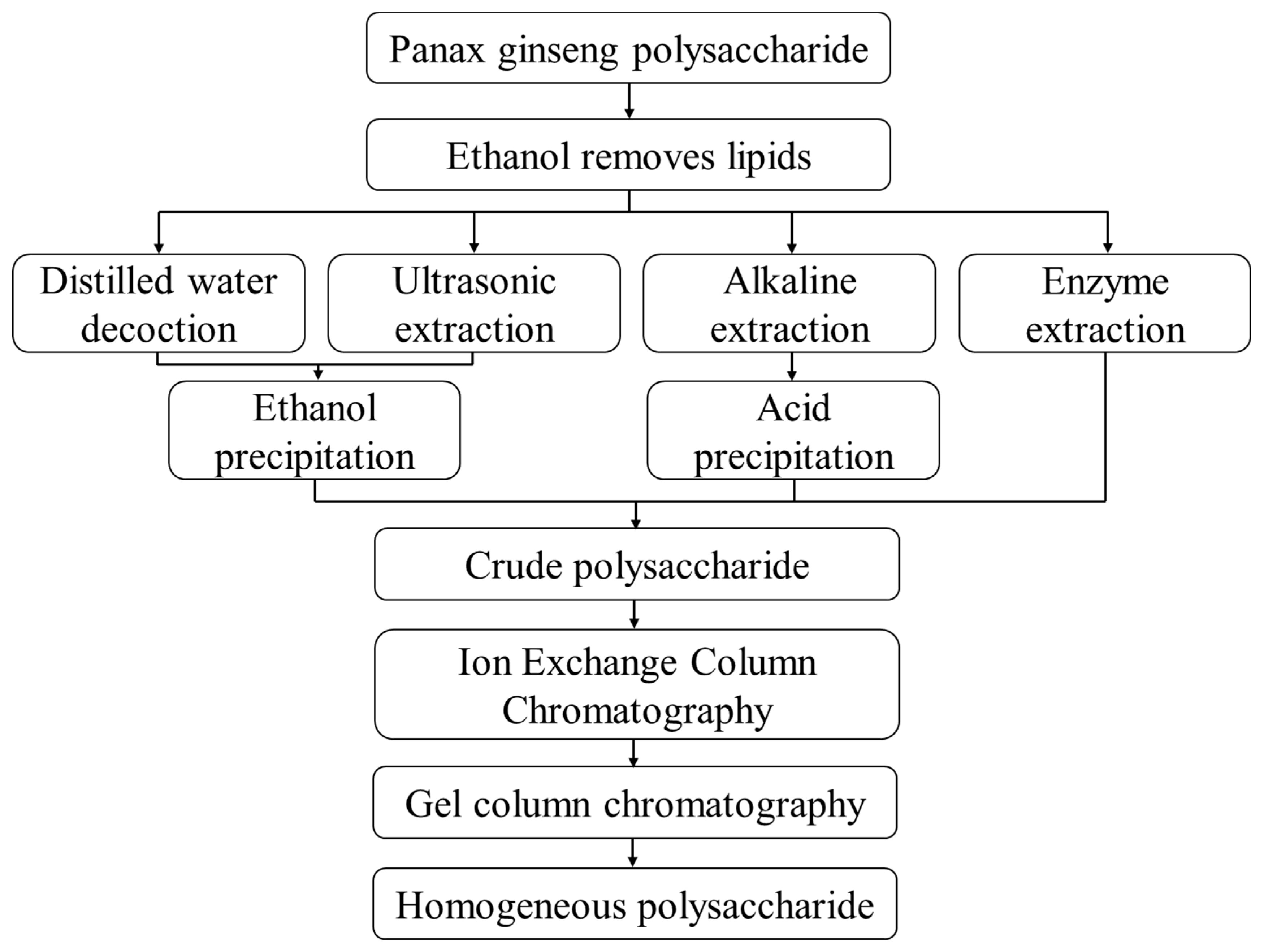
2. Separation and Extraction of Polysaccharides from Ginseng
3. Structural Analysis of Polysaccharides from the Panax Genus
3.1. Ginseng
3.2. American Ginseng
3.3. Panax notoginseng
4. Biological Activity of Ginseng Polysaccharides
4.1. Antioxidant Effect
4.2. Antitumor Effect
4.3. Immunomodulatory Effect
4.4. Antidiabetic Effect
4.5. Intestinal Protection
4.6. Skin Repair
4.7. Other Activities
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
References
- Li, X.; Liu, J.; Zuo, T.-T.; Hu, Y.; Li, Z.; Wang, H.-D.; Xu, X.-Y.; Yang, W.-Z.; Guo, D.-A. Advances and challenges in ginseng research from 2011 to 2020: The phytochemistry, quality control, metabolism, and biosynthesis. Nat. Prod. Rep. 2022, 39, 875–909. [Google Scholar] [CrossRef]
- Yang, Y.; Ju, Z.; Yang, Y.; Zhang, Y.; Yang, L.; Wang, Z. Phytochemical analysis of Panax species: A review. J. Ginseng Res. 2021, 45, 1–21. [Google Scholar] [CrossRef] [PubMed]
- Gurung, B.; Bhardwaj, P.K.; Rai, A.K.; Sahoo, D. Major ginsenoside contents in rhizomes of Panax sokpayensis and Panax bipinnatifidus. Nat. Prod. Res. 2018, 32, 234–238. [Google Scholar] [CrossRef] [PubMed]
- Hou, M.; Wang, R.; Zhao, S.; Wang, Z. Ginsenosides in Panax genus and their biosynthesis. Acta Pharm. Sin. B 2021, 11, 1813–1834. [Google Scholar] [CrossRef]
- Gao, X.-Y.; Liu, G.-C.; Zhang, J.-X.; Wang, L.-H.; Xu, C.; Yan, Z.-A.; Wang, A.; Su, Y.-F.; Lee, J.-J.; Piao, G.-C.; et al. Pharmacological Properties of Ginsenoside Re. Front. Pharmacol. 2022, 13, 754191. [Google Scholar] [CrossRef] [PubMed]
- Shi, Z.-Y.; Zeng, J.-Z.; Wong, A.S.T. Chemical Structures and Pharmacological Profiles of Ginseng Saponins. Molecules 2019, 24, 2443. [Google Scholar] [CrossRef]
- Chopra, P.; Chhillar, H.; Kim, Y.-J.; Jo, I.H.; Kim, S.T.; Gupta, R. Phytochemistry of ginsenosides: Recent advancements and emerging roles. Crit. Rev. Food Sci. Nutr. 2023, 63, 613–640. [Google Scholar] [CrossRef]
- Ahmadi, E.; Rezadoost, H.; Alilou, M.; Stuppner, H.; Farimani, M.M. Purification, structural characterization and antioxidant activity of a new arabinogalactan from Dorema ammoniacum gum. Int. J. Biol. Macromol. 2022, 194, 1019–1028. [Google Scholar] [CrossRef]
- Jiao, L.; Zhang, X.; Li, B.; Liu, Z.; Wang, M.; Liu, S. Anti-tumour and immunomodulatory activities of oligosaccharides isolated from Panax ginseng C.A. Meyer. Int. J. Biol. Macromol. 2014, 65, 229–233. [Google Scholar] [CrossRef]
- Hu, Y.; He, Y.; Niu, Z.; Shen, T.; Zhang, J.; Wang, X.; Hu, W.; Cho, J.Y. A review of the immunomodulatory activities of polysaccharides isolated from Panax species. J. Ginseng Res. 2021, 46, 23–32. [Google Scholar] [CrossRef]
- Chen, F.; Huang, G. Antioxidant activity of polysaccharides from different sources of ginseng. Int. J. Biol. Macromol. 2019, 125, 906–908. [Google Scholar] [CrossRef]
- Yu, X.-H.; Liu, Y.; Wu, X.-L.; Liu, L.-Z.; Fu, W.; Song, D.-D. Isolation, purification, characterization and immunostimulatory activity of polysaccharides derived from American ginseng. Carbohydr. Polym. 2017, 156, 9–18. [Google Scholar] [CrossRef]
- Chan, M.K.; Yu, Y.; Wulamu, S.; Wang, Y.; Wang, Q.; Zhou, Y.; Sun, L. Structural analysis of water-soluble polysaccharides isolated from Panax notoginseng. Int. J. Biol. Macromol. 2020, 155, 376–385. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Cai, J.; Geng, J.; Li, Y.; Wang, Z.; Li, R. Purification, characterization and anticancer activity of a polysaccharide from Panax ginseng. Int. J. Biol. Macromol. 2012, 51, 968–973. [Google Scholar] [CrossRef]
- Cui, L.; Wang, J.; Huang, R.; Tan, Y.; Zhang, F.; Zhou, Y.; Sun, L. Analysis of pectin from Panax ginseng flower buds and their binding activities to galectin-3. Int. J. Biol. Macromol. 2019, 128, 459–467. [Google Scholar] [CrossRef] [PubMed]
- Ji, L.; Jie, Z.; Ying, X.; Yue, Q.; Zhou, Y.; Sun, L. Structural characterization of alkali-soluble polysaccharides from Panax ginseng C. A. Meyer. R. Soc. Open Sci. 2018, 5, 171644. [Google Scholar] [CrossRef]
- Sun, L.; Wu, D.; Ning, X.; Yang, G.; Lin, Z.; Tian, M.; Zhou, Y. α-Amylase-assisted extraction of polysaccharides from Panax ginseng. Int. J. Biol. Macromol. 2015, 75, 152–157. [Google Scholar] [CrossRef] [PubMed]
- Zhao, J.-L.; Zhang, M.; Zhou, H.-L. Microwave-Assisted Extraction, Purification, Partial Characterization, and Bioactivity of Polysaccharides from Panax ginseng. Molecules 2019, 24, 1605. [Google Scholar] [CrossRef]
- Liu, Y.; Sui, D.; Fu, W.; Sun, L.; Li, Y.; Yu, P.; Yu, X.; Zhou, Y.; Xu, H. Protective effects of polysaccharides from Panax ginseng on acute gastric ulcers induced by ethanol in rats. Food Funct. 2021, 12, 2741–2749. [Google Scholar] [CrossRef]
- Bai, C.; Chen, R.; Tan, L.; Bai, H.; Tian, L.; Lu, J.; Gao, M.; Sun, H.; Chi, Y. Effects of multi-frequency ultrasonic on the physicochemical properties and bioactivities of polysaccharides from different parts of ginseng. Int. J. Biol. Macromol. 2022, 206, 896–910. [Google Scholar] [CrossRef]
- Byeon, S.E.; Lee, J.; Kim, J.H.; Yang, W.S.; Kwak, Y.-S.; Kim, S.Y.; Choung, E.S.; Rhee, M.H.; Cho, J.Y. Molecular mechanism of macrophage activation by red ginseng acidic polysaccharide from Korean red ginseng. Mediat. Inflamm. 2012, 2012, 732860. [Google Scholar] [CrossRef] [PubMed]
- Song, Y.-R.; Sung, S.-K.; Jang, M.; Lim, T.-G.; Cho, C.-W.; Han, C.-J.; Hong, H.-D. Enzyme-assisted extraction, chemical characteristics, and immunostimulatory activity of polysaccharides from Korean ginseng (Panax ginseng Meyer). Int. J. Biol. Macromol. 2018, 116, 1089–1097. [Google Scholar] [CrossRef] [PubMed]
- Yin, S.Y.; Kim, H.J.; Kim, H.J. A comparative study of the effects of whole red ginseng extract and polysaccharide and saponin fractions on influenza A (H1N1) virus infection. Biol. Pharm. Bull. 2013, 36, 1002–1007. [Google Scholar] [CrossRef]
- Ren, Y.; Bai, Y.; Zhang, Z.; Cai, W.; Del Rio Flores, A. The Preparation and Structure Analysis Methods of Natural Polysaccharides of Plants and Fungi: A Review of Recent Development. Molecules 2019, 24, 3122. [Google Scholar] [CrossRef] [PubMed]
- Zhang, T.; Liu, H.; Bai, X.; Liu, P.; Yang, Y.; Huang, J.; Zhou, L.; Min, X. Fractionation and antioxidant activities of the water-soluble polysaccharides from Lonicera japonica Thunb. Int. J. Biol. Macromol. 2020, 151, 1058–1066. [Google Scholar] [CrossRef]
- Qi, H.; Zhang, Z.; Liu, J.; Chen, Z.; Huang, Q.; Li, J.; Chen, J.; Wang, M.; Zhao, D.; Wang, Z.; et al. Comparisons of Isolation Methods, Structural Features, and Bioactivities of the Polysaccharides from Three Common Panax Species: A Review of Recent Progress. Molecules 2021, 26, 4997. [Google Scholar] [CrossRef]
- Li, S.; Huo, X.; Qi, Y.; Ren, D.; Li, Z.; Qu, D.; Sun, Y. The Protective Effects of Ginseng Polysaccharides and Their Effective Subfraction against Dextran Sodium Sulfate-Induced Colitis. Foods 2022, 11, 890. [Google Scholar] [CrossRef]
- Lü, Y.-L.; Liang, J.; Zhou, F.-Y.; Kuang, H.-X.; Xia, Y.-G. Discrimination and characterization of Panax polysaccharides by 2D COS-IR spectroscopy with chemometrics. Int. J. Biol. Macromol. 2021, 183, 193–202. [Google Scholar] [CrossRef]
- Zhao, B.; Wang, X.-Y.; Luo, W.; Lin, Y.; Lv, C.-N.; Lu, J.-C. Isolation and structural elucidation of a low-molecular-weight polysaccharide from the roots of Panax ginseng C. A. Meyer. Nat. Prod. Res. 2022, 36, 493–500. [Google Scholar] [CrossRef]
- Kim, H.M.; Song, Y.; Hyun, G.H.; Long, N.P.; Park, J.H.; Hsieh, Y.S.; Kwon, S.W. Characterization and Antioxidant Activity Determination of Neutral and Acidic Polysaccharides from Panax Ginseng C. A. Meyer. Molecules 2020, 25, 791. [Google Scholar] [CrossRef]
- Jia, H.; Zhao, B.; Zhang, F.; Santhanam, R.K.; Wang, X.; Lu, J. Extraction, Structural Characterization, and Anti-Hepatocellular Carcinoma Activity of Polysaccharides from Panax ginseng Meyer. Front. Oncol. 2021, 11, 785455. [Google Scholar] [CrossRef] [PubMed]
- Hwang, S.-H.; Shin, M.-S.; Yoon, T.J.; Shin, K.-S. Immunoadjuvant activity in mice of polysaccharides isolated from the leaves of Panax ginseng C.A. Meyer. Int. J. Biol. Macromol. 2018, 107, 2695–2700. [Google Scholar] [CrossRef] [PubMed]
- Wan, J.-Y.; Huang, W.-H.; Zheng, W.; Park, C.W.; Kim, S.H.; Seo, D.B.; Shin, K.-S.; Zeng, J.; Yao, H.; Sava-Segal, C.; et al. Multiple Effects of Ginseng Berry Polysaccharides: Plasma Cholesterol Level Reduction and Enteric Neoplasm Prevention. Am. J. Chin. Med. 2017, 45, 1293–1307. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Huang, M.; Sun, R.; Pan, L. Extraction, characterization of a Ginseng fruits polysaccharide and its immune modulating activities in rats with Lewis lung carcinoma. Carbohydr. Polym. 2015, 127, 215–221. [Google Scholar] [CrossRef]
- Kim, H.; Kim, H.-W.; Yu, K.-W.; Suh, H.-J. Polysaccharides fractionated from enzyme digests of Korean red ginseng water extracts enhance the immunostimulatory activity. Int. J. Biol. Macromol. 2018, 121, 913–920. [Google Scholar] [CrossRef]
- Jin, Y.R.; Oh, M.J.; Yuk, H.J.; An, H.J.; Kim, D.S. Novel analysis procedure for red ginseng polysaccharides by matrix-assisted laser desorption/ionization time-of-flight/time-of-flight mass spectrometry. J. Ginseng Res. 2021, 45, 539–545. [Google Scholar] [CrossRef]
- Wang, L.; Yu, X.; Yang, X.; Li, Y.; Yao, Y.; Lui, E.M.K.; Ren, G. Structural and anti-inflammatory characterization of a novel neutral polysaccharide from North American ginseng (Panax quinquefolius). Int. J. Biol. Macromol. 2015, 74, 12–17. [Google Scholar] [CrossRef]
- Yu, X.; Yang, X.; Cui, B.; Wang, L.; Ren, G. Antioxidant and immunoregulatory activity of alkali-extractable polysaccharides from North American ginseng. Int. J. Biol. Macromol. 2014, 65, 357–361. [Google Scholar] [CrossRef]
- Xia, Y.-G.; Li, X.; Yu, L.-S.; Liang, J.; Sun, H.-M.; Kuang, H.-X. Structural-fingerprinting of polysaccharides to discern Panax species by means of gas-liquid chromatography and mass spectrometry. Int. J. Biol. Macromol. 2020, 151, 932–943. [Google Scholar] [CrossRef]
- Wu, Y.; Wang, D. Structural characterization and DPPH radical scavenging activity of an arabinoglucogalactan from Panax notoginseng root. J. Nat. Prod. 2008, 71, 241–245. [Google Scholar] [CrossRef]
- Liu, Y.; Li, S.; Pu, M.; Qin, H.; Wang, H.; Zhao, Y.; Chen, T. Structural Characterization of Polysaccharides Isolated from Panax notoginseng Medicinal Residue and Its Protective Effect on Myelosuppression Induced by Cyclophosphamide. Chem. Biodivers. 2022, 19, e202100681. [Google Scholar] [CrossRef] [PubMed]
- Wang, P.; Zhang, L.; Yao, J.; Shi, Y.; Li, P.; Ding, K. An arabinogalactan from flowers of Panax notoginseng inhibits angiogenesis by BMP2/Smad/Id1 signaling. Carbohydr. Polym. 2015, 121, 328–335. [Google Scholar] [CrossRef] [PubMed]
- Zhao, B.; Wang, X.; Liu, H.; Lv, C.; Lu, J. Structural characterization and antioxidant activity of oligosaccharides from Panax ginseng C. A. Meyer. Int. J. Biol. Macromol. 2020, 150, 737–745. [Google Scholar] [CrossRef] [PubMed]
- Jiao, L.; Li, B.; Wang, M.; Liu, Z.; Zhang, X.; Liu, S. Antioxidant activities of the oligosaccharides from the roots, flowers and leaves of Panax ginseng C.A. Meyer. Carbohydr. Polym. 2014, 106, 293–298. [Google Scholar] [CrossRef] [PubMed]
- Lee, D.-Y.; Park, C.W.; Lee, S.J.; Park, H.-R.; Kim, S.H.; Son, S.-U.; Park, J.; Shin, K.-S. Anti-Cancer Effects of Panax ginseng Berry Polysaccharides via Activation of Immune-Related Cells. Front. Pharmacol. 2019, 10, 1411. [Google Scholar] [CrossRef]
- Shin, M.-S.; Hwang, S.-H.; Yoon, T.-J.; Kim, S.H.; Shin, K.-S. Polysaccharides from ginseng leaves inhibit tumor metastasis via macrophage and NK cell activation. Int. J. Biol. Macromol. 2017, 103, 1327–1333. [Google Scholar] [CrossRef]
- Llovet, J.M.; Di Bisceglie, A.M.; Bruix, J.; Kramer, B.S.; Lencioni, R.; Zhu, A.X.; Sherman, M.; Schwartz, M.; Lotze, M.; Talwalkar, J.; et al. Design and endpoints of clinical trials in hepatocellular carcinoma. Jnci-J. Natl. Cancer Inst. 2008, 100, 698–711. [Google Scholar] [CrossRef]
- Liu, Y.-H.; Qin, H.-Y.; Zhong, Y.-Y.; Li, S.; Wang, H.-J.; Wang, H.; Chen, L.-L.; Tang, X.; Li, Y.-L.; Qian, Z.-Y.; et al. Neutral polysaccharide from Panax notoginseng enhanced cyclophosphamide antitumor efficacy in hepatoma H22-bearing mice. BMC Cancer 2021, 21, 37. [Google Scholar] [CrossRef]
- Zhang, X.; Liu, Z.; Zhong, C.; Pu, Y.; Yang, Z.; Bao, Y. Structure characteristics and immunomodulatory activities of a polysaccharide RGRP-1b from radix ginseng Rubra. Int. J. Biol. Macromol. 2021, 189, 980–992. [Google Scholar] [CrossRef]
- Cui, L.; Chen, L.; Yang, G.; Li, Y.; Qiao, Z.; Liu, Y.; Meng, Y.; Zhou, Y.; Sun, L. Structural characterization and immunomodulatory activity of a heterogalactan from Panax ginseng flowers. Food Res. Int. 2021, 140, 109859. [Google Scholar] [CrossRef]
- Azike, C.G.; Charpentier, P.A.; Lui, E.M. Stimulation and suppression of innate immune function by American ginseng polysaccharides: Biological relevance and identification of bioactives. Pharm. Res. 2015, 32, 876–897. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.; Pettolino, F.; Mau, S.-L.; Shen, Y.-C.; Chen, C.-F.; Kuo, Y.-C.; Bacic, A. Immunoactive polysaccharide-rich fractions from Panax notoginseng. Planta Med. 2006, 72, 1193–1199. [Google Scholar] [CrossRef] [PubMed]
- Sun, C.; Chen, Y.; Li, X.; Tai, G.; Fan, Y.; Zhou, Y. Anti-hyperglycemic and anti-oxidative activities of ginseng polysaccharides in STZ-induced diabetic mice. Food Funct. 2014, 5, 845–848. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Li, R.; Li, N.; Zheng, F.; Dai, Y.; Ge, Y.; Yue, H.; Yu, S. Mechanism of antidiabetic and synergistic effects of ginseng polysaccharide and ginsenoside Rb1 on diabetic rat model. J. Pharm. Biomed. Anal. 2018, 158, 451–460. [Google Scholar] [CrossRef] [PubMed]
- Xie, J.; Wu, J.; Mehendale, S.; Aung, H.; Yuan, C.-S. Anti-hyperglycemic effect of the polysaccharides fraction from American ginseng berry extract in ob/ob mice. Phytomedicine 2004, 11, 182–187. [Google Scholar] [CrossRef] [PubMed]
- Hikino, H.; Kobayashi, M.; Suzuki, Y.; Konno, C. Mechanisms of hypoglycemic activity of aconitan A, a glycan from Aconitum carmichaeli roots. J. Ethnopharmacol. 1989, 25, 295–304. [Google Scholar] [CrossRef]
- Rooks, M.G.; Garrett, W.S. Gut microbiota, metabolites and host immunity. Nat. Rev. Immunol. 2016, 16, 341–352. [Google Scholar] [CrossRef]
- Wang, D.; Shao, S.; Zhang, Y.; Zhao, D.; Wang, M. Insight into Polysaccharides from Panax ginseng C. A. Meyer in Improving Intestinal Inflammation: Modulating Intestinal Microbiota and Autophagy. Front. Immunol. 2021, 12, 683911. [Google Scholar] [CrossRef]
- Li, S.; Qi, Y.; Chen, L.; Qu, D.; Li, Z.; Gao, K.; Chen, J.; Sun, Y. Effects of Panax ginseng polysaccharides on the gut microbiota in mice with antibiotic-associated diarrhea. Int. J. Biol. Macromol. 2019, 124, 931–937. [Google Scholar] [CrossRef]
- Park, E.; Hwang, I.; Song, J.-Y.; Jee, Y. Acidic polysaccharide of Panax ginseng as a defense against small intestinal damage by whole-body gamma irradiation of mice. Acta Histochem. 2011, 113, 19–23. [Google Scholar] [CrossRef]
- Bing, S.J.; Kim, M.J.; Ahn, G.; Im, J.; Kim, D.S.; Ha, D.; Cho, J.; Kim, A.; Jee, Y. Acidic polysaccharide of Panax ginseng regulates the mitochondria/caspase-dependent apoptotic pathway in radiation-induced damage to the jejunum in mice. Acta Histochem. 2014, 116, 514–521. [Google Scholar] [CrossRef]
- Zhou, R.; He, D.; Xie, J.; Zhou, Q.; Zeng, H.; Li, H.; Huang, L. The Synergistic Effects of Polysaccharides and Ginsenosides from American Ginseng (Panax quinquefolius L.) Ameliorating Cyclophosphamide-Induced Intestinal Immune Disorders and Gut Barrier Dysfunctions Based on Microbiome-Metabolomics Analysis. Front. Immunol. 2021, 12, 665901. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Jiang, R.; Jing, C.; Liu, J.; Xu, X.; Sun, L.; Zhao, D. Protective effect of oligosaccharides isolated from Panax ginseng C. A. Meyer against UVB-induced skin barrier damage in BALB/c hairless mice and human keratinocytes. J. Ethnopharmacol. 2022, 283, 114677. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.; Jang, S.; Lee, C.H.; Lee, J.Y.; Park, H.; Lee, S.; Kim, S.H.; Park, E.; Lee, K.W.; Shin, H. Beneficial effects on skin health using polysaccharides from red ginseng by-product. J. Food Biochem. 2019, 43, e12961. [Google Scholar] [CrossRef] [PubMed]
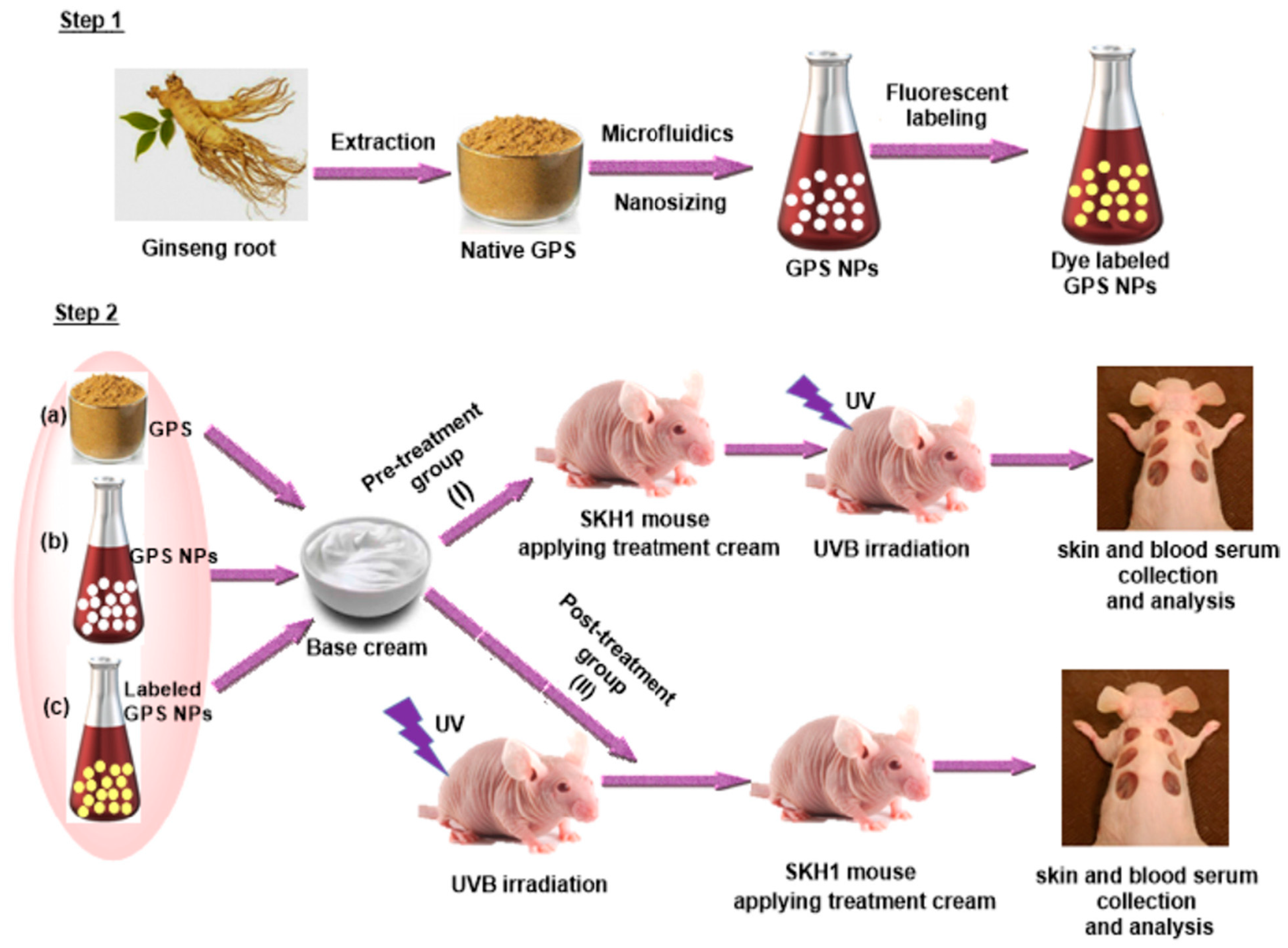
- Akhter, K.F.; Mumin, A.; Lui, E.M.; Charpentier, P.A. Transdermal nanotherapeutics: Panax quinquefolium polysaccharide nanoparticles attenuate UVB-induced skin cancer. Int. J. Biol. Macromol. 2021, 181, 221–231. [Google Scholar] [CrossRef] [PubMed]
- Jiao, L.; Li, J.; Liu, F.; Wang, J.; Jiang, P.; Li, B.; Li, H.; Chen, C.; Wu, W. Characterisation, Chain Conformation and Antifatigue Effect of Steamed Ginseng Polysaccharides with Different Molecular Weight. Front. Pharmacol. 2021, 12, 712836. [Google Scholar] [CrossRef]
- Wei, X.-M.; Jiang, S.; Li, S.-S.; Sun, Y.-S.; Wang, S.-H.; Liu, W.-C.; Wang, Z.; Wang, Y.-P.; Zhang, R.; Li, W. Endoplasmic Reticulum Stress-Activated PERK-eIF2α-ATF4 Signaling Pathway is Involved in the Ameliorative Effects of Ginseng Polysaccharides against Cisplatin-Induced Nephrotoxicity in Mice. ACS Omega 2021, 6, 8958–8966. [Google Scholar] [CrossRef]
- Kim, M.; Yi, Y.-S.; Kim, J.; Han, S.Y.; Kim, S.H.; Seo, D.B.; Cho, J.Y.; Shin, S.S. Effect of polysaccharides from a Korean ginseng berry on the immunosenescence of aged mice. J. Ginseng Res. 2018, 42, 447–454. [Google Scholar] [CrossRef]
- Wang, C.; Zheng, L.; Liu, S.; Guo, X.; Qu, Y.; Gao, M.; Cui, X.; Yang, Y. A novel acidic polysaccharide from the residue of Panax notoginseng and its hepatoprotective effect on alcoholic liver damage in mice. Int. J. Biol. Macromol. 2020, 149, 1084–1097. [Google Scholar] [CrossRef]
| Serial Number | Polysaccharide Name | Monosaccharide Composition and Ratio | Molecular Weight | Structure |
|---|---|---|---|---|
| 1 | WGPN | Glc:Gal:Ara =95.3:3.3:1.4 | ||
| 2 | WGPA | Glc:Gal:Ara:GalA:Rha = 13.6:18:15.4:44.2:3.8 | Contains RG-I and HG | |
| 3 | MCGP-3 | Glc:Gal:GlcA:GalA:Rha:Man:Ara = 33.17:22.88:0.687:15.67:6.005:0.631:20.96 | 1.572 × 105 | RG-I |
| 4 | MCGP-4 | Glc:Gal:GlcA:GalA:Rha:Man:Ara = 7.146:39.74:1.519:26.74:4.533:0.214:20.11 | 1.673 × 105 | RG-I |
| 5 | MCGPL | Glc:Gal:Man = 14.8:1:1.2 | 3 × 103 | The main chain is composed of (1→4)-α-D-Glcp |
| 6 | WGNP | Glc:Gal:Ara = 97.9:1.1:1 | 16.1–70.4 × 103 | |
| 7 | WGAP | Glc:Gal:GlcA:GalA:Ara = 24:24.4:32.2:1.3:18.1 | 50–80 × 103 | |
| 8 | GFP1 | Glc:Gal:Ara:Rha = 2:6.1:3.2:1.1 | 1.4 × 105 | The main chain is composed of (1→6)-Galp and (1→3,6)-. |
| 9 | RGCW-EZ-CP-4 | Gal:Ara:GalA = 29.9:19.8:38.6 | Contains RG-I and RG-II | |
| 10 | RG-CW-EZ-CP-8 | Gal:GalA:Ara = 10.3:12.3:64.3 | The main chain is arabinan or arabinogalactan. |
| Serial Number | Polysaccharide Name | Monosaccharide Composition and Ratio | Molecular Weight | Structure |
|---|---|---|---|---|
| 1 | WPS-1 | Ara:Rha:Man:Gal:Glc = 21.2:2.3:2.6:18.7:5.2 | 1.54 × 106 | Mainly composed of (1→6)-α-D-Glcp and (1→5)-α-L-Araf |
| WPS-2 | Ara:Rha:Man:Gal:Glc = 7.9:1.7:2.9:20.7:46.8 | 1.41 × 104 | ||
| 3 | SPS-1 | Ara:Xyl:Man:Gal:Glc:GalA:GlcA = 2.3:6.9:9.2:28.6:15.9:13.6:3.5 | 3.62 × 105 | Mainly composed of (1→6)-α-D-Glcp, (1→4)-α-D-Manp, (1→5)-α-L-Araf, β-D-Galp and β-D-xylose RG-I. |
| 4 | SPS-2 | Ara:Xyl:Man:Gal:Glc:GalA:GlcA = 14.2:5.3:7.9:22.5:25.3:16.9:7.9 | 9.7 × 105 | |
| 5 | SPS-3 | Ara:Rha:Xyl:Man:Gal:Glc:GalA:GlcA = 19.2:2.1:9.6:12.0:15.2:11.5:26.3:4.1 | 5.12 × 105 | |
| 6 | PPQN | Glc:Gal = 1:1.15 | 3.1 × 103 | |
| 7 | AEP-1 | Glc:Gal:GalA = 4.67:0.97:3.92 | ||
| 8 | AEP-2 | Ara:Man:Gal:Glc:GalA = 1.03:0.76:1.68:3.02:3.65 |
| Serial Number | Polysaccharide Name | Monosaccharide Composition and Ratio | Molecular Weight | Structure |
|---|---|---|---|---|
| 1 | PNPA-1A | GalA:Rha:Gal:Ara:Glc:Man = 5:0.8:63.2:27.7:2.4:0.9 | 8.8 × 104 | AG-Ⅱ Mainly HG, composed of different proportions of RG-I and RG-II. |
| 2 | PNPA-1B | GalA:Rha:Gal:Ara:GlcA:Man = 11.6:6:46:33.4:1:2 | 1.01 × 105 | |
| 3 | PNPA-2A | GalA:Rha:Gal:Ara:Glc:Man = 15.9:15.5:32.7:28.3:2.2:4 | 2.7 × 105 | AG-Ⅱ Mainly HG, composed of different proportions of RG-I and RG-II. RG-Ⅰ |
| PNPA-2B | GalA:Rha:Gal:Ara:Glc:GlcA:Man = 40.6:9.6:29.3:10.4:4.5:0.6:2.9 | 3 × 103 | ||
| 5 | PNPA-3A | GalA:Rha:Gal:Ara:GlcA:Man = 74.4:7.5:8.3:8.2:0.8:0.8 | 6 × 103 | |
| 6 | PNPA-3B | GalA:Rha:Gal:Ara:Glc:GlcA:Man = 75.8:5.2:8.8:5.1:1.6:0.9:1.4 | 1.8 × 104 | Mainly HG, composed of different proportions of RG-I and RG-II |
| 7 | Arabinogalactan | Ara:Glc:Gal = 1:1:8 | 6.7 × 104 | (1→3)-β-D-galactosyl residue is the backbone, α-L-Araf-(1→4)-β-D-Glcp-(1→is the branch. |
| 8 | NPPN | Ara:Gal:Glc:Man = 3.76:18.58:76.85:0.80 | 2.3 × 105 | |
| 9 | APPN-Ⅰ | Ara:Gal:Glc:Man:GalA:GlcA = 11.47:34.82:43.48:2.28:5.66:2.29 | 4.9 × 105 | The main chain is composed of α-1,4-Glcp glycosidic linkages. |
| 10 | APPNⅡ-A | Ara:Gal:Glc:GalA:GlcA = 11.04:39.59:39.80:7.03:2.54 | 4.5 × 105 | The main chain is composed of α-1,4-Glcp glycosidic linkages. |
| 11 | APPNⅡ-B | Ara:Gal:Glc:GalA = 1.49:1.64:2.50:94.36 | 2.8 × 104 | HG |
| 12 | APPNⅢ-A | Fuc:Ara:Gal:Glc:Xyl:Man:GalA:GlcA = 1.61:9.45:39.25:16.61:1.11:1.74:26.66:3.57 | 3.4 × 105 | Linked by β-pyranoside. |
| 13 | APPNⅢ-B | Ara:Gal:Glc:GalA = 1.22:1.52:2.90:94.36 | 5.6 × 104 | HG |
| 14 | RN1 | Gal:Ara = 43.7:56.3 | 2.1 × 104 | Consists of 1,6 linked Galp residues. |
| Serial Number | Source Plant | Biological Activity | Animal Model | Molecular Mechanism |
|---|---|---|---|---|
| 1 | ginseng | Anti-oxidation | In vitro | Determination of ABTS free radical scavenging rate, DPPH free radical scavenging rate, and ferric iron reducing ability |
| 2 | ginseng | Anti-oxidation | D-Gal-induced ICR mice | Increases the activity of SOD, CAT, GSH-Px, and T-AOC in mouse serum and liver, and reduces the level of MDA to play an antioxidant role |
| 3 | ginseng | Anti-oxidation | In vitro | Determination of DPPH free radical scavenging rate |
| 4 | American ginseng | Anti-oxidation | In vitro | Determination of ABTS free radical scavenging rate and oxygen free radical absorption capacity |
| 5 | Panax notoginseng | Anti-oxidation | In vitro | Determination of DPPH free radical scavenging rate |
| 6 | ginseng | Antitumor | B16-BL6 melanoma cells implanted in female BALB/c mice | Increased release of IL-6, IL-12, TNF-α, IFN-γ, and granzyme B from NK cells to inhibit tumor aggregation |
| 7 | ginseng | Antitumor | Colon 26-M3 cells and BALB/c mice | Promote the activation of macrophages and NK cells to play an antitumor role |
| 8 | American ginseng | Antitumor | HT29 cells | Inhibits cancer cell growth by causing decreased cell number, cell cycle arrest at G2/M, increased cell death, and increased expression of cleaved caspase-3 |
| 9 | Panax notoginseng | Antitumor | HT22 cells and tumor-bearing mice | Antitumor effect by enhancing host immune system and weak cytotoxicity against liver cancer cells |
| 10 | Panax notoginseng | Antitumor | HT22 cells and tumor-bearing mice | Inhibit the growth of H22 cells, combined with CTX to increase the tumor inhibition rate of tumor-bearing mice |
| 11 | ginseng | Immunomodulatory | RAW264.7 macrophages | Promote the phagocytosis of macrophages and the release of NO |
| 12 | ginseng | Immunomodulatory | RAW264.7 macrophages | Increased TNF-α, IL-6, IFN-γ, and IL-1β levels and release of NO |
| 13 | ginseng | Immunomodulatory | CTX-induced BALB/c mice | Enhance immunity by activating macrophages |
| 14 | American ginseng | Immunomodulatory | LPS-induced rats | Increased TNF-α level and NO release from isolated alveolar macrophages |
| 15 | Panax notoginseng | Immunomodulatory | Human polymorphonuclear neutrophils | Enhancing complement fixation activity and promoting mitosis by regulating ROS and IFN-γ |
| 16 | Panax notoginseng | Immunomodulatory | Mouse spleen lymphocytes and peritoneal macrophages | Induces production of interferon-γ and TNF-α |
| 17 | ginseng | Antidiabetic | STZ-induced ICR mice | Reduce serum MDA level, increase serum insulin, SOD activity, and liver glycogen level |
| 18 | ginseng | Antidiabetic | STZ-induced rats | Upregulates the relative abundance of Bacteroides and increases fecal β-D-glucosidase activity |
| 19 | American ginseng | Antidiabetic | ob/ob mice | Reduce fasting blood glucose in mice |
| 20 | ginseng | Gut protection | SD rats induced by DSS | Regulation of intestinal flora structure and blocking of TLR4-MyD88 pathway to inhibit NF-κB, oxidative stress, and cytokine release inhibit inflammation |
| 21 | ginseng | Gut protection | Balb/c mice induced by lincomycin hydrochloride | Regulate the number of intestinal flora, balance the metabolic process |
| 22 | ginseng | Gut protection | Irradiated C57BL/6 mice | Inhibition of p53-dependent and mitochondrial/caspase pathways reduces apoptosis. |
| 23 | American ginseng | Gut protection | CTX-induced C57BL/6 mice | Regulating gut microbiota and metabolites |
| 24 | ginseng | Skin repair | NC/Nga mice | Suppression of solar ultraviolet-induced matrix MMP-1 protein expression by stimulating AP-1 |
| 25 | American ginseng | Skin repair | SKH1 hairless mice. | Reduces the level of pro-inflammatory cytokines and inhibits the initiation of pro-inflammatory cascades |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, S.; Ding, C.; Liu, X.; Zhao, Y.; Ding, Q.; Sun, S.; Zhang, J.; Yang, J.; Liu, W.; Li, W. Research Progress on Extraction, Isolation, Structural Analysis and Biological Activity of Polysaccharides from Panax Genus. Molecules 2023, 28, 3733. https://doi.org/10.3390/molecules28093733
Zhang S, Ding C, Liu X, Zhao Y, Ding Q, Sun S, Zhang J, Yang J, Liu W, Li W. Research Progress on Extraction, Isolation, Structural Analysis and Biological Activity of Polysaccharides from Panax Genus. Molecules. 2023; 28(9):3733. https://doi.org/10.3390/molecules28093733
Chicago/Turabian StyleZhang, Shuai, Chuanbo Ding, Xinglong Liu, Yingchun Zhao, Qiteng Ding, Shuwen Sun, Jinping Zhang, Jiali Yang, Wencong Liu, and Wei Li. 2023. "Research Progress on Extraction, Isolation, Structural Analysis and Biological Activity of Polysaccharides from Panax Genus" Molecules 28, no. 9: 3733. https://doi.org/10.3390/molecules28093733
APA StyleZhang, S., Ding, C., Liu, X., Zhao, Y., Ding, Q., Sun, S., Zhang, J., Yang, J., Liu, W., & Li, W. (2023). Research Progress on Extraction, Isolation, Structural Analysis and Biological Activity of Polysaccharides from Panax Genus. Molecules, 28(9), 3733. https://doi.org/10.3390/molecules28093733







