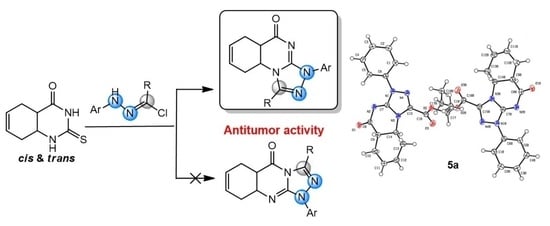
Angular Regioselective Synthesis of Varied Functionalized Hexahydro-1,2,4-triazolo[4,3-a]quinazolin-9-ones and Their Antiproliferative Action †
Abstract
1. Introduction
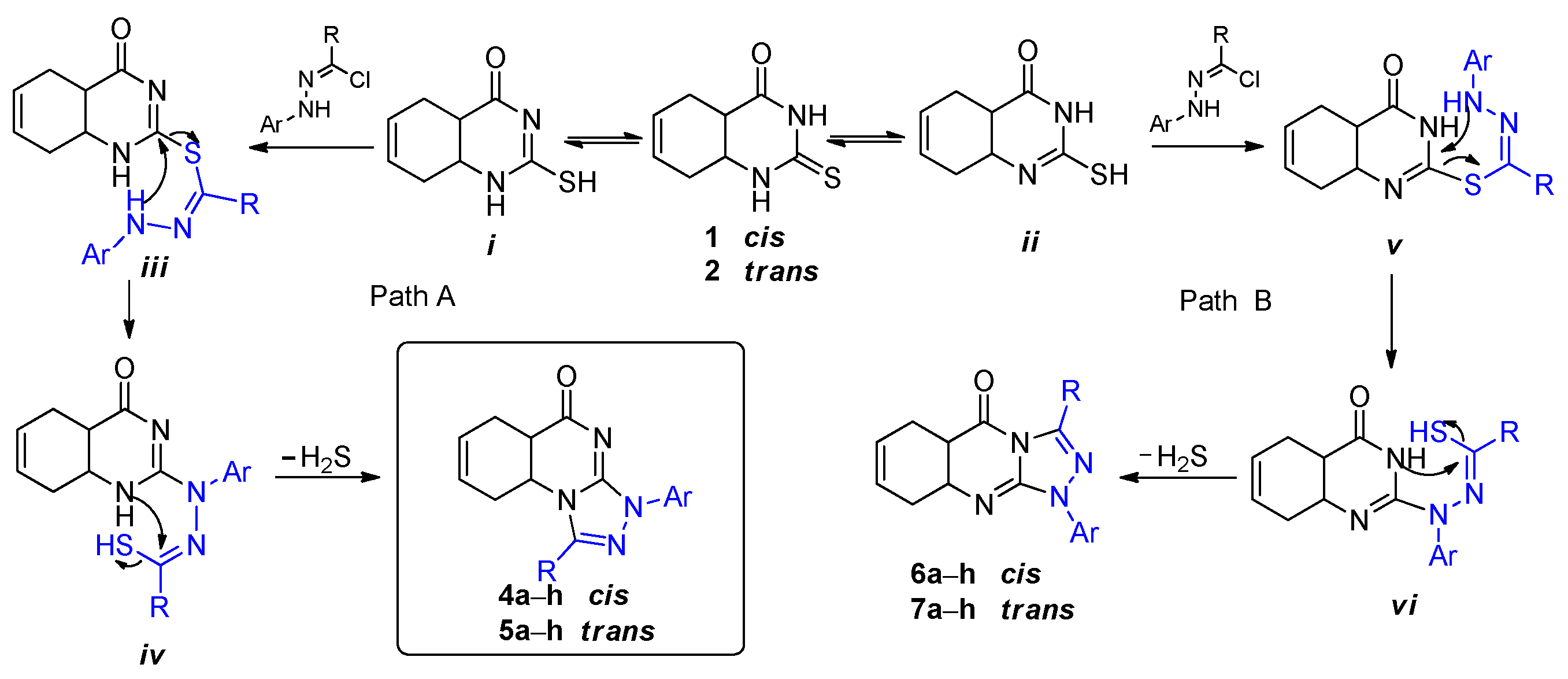
2. Results and Discussion
3. Materials and Methods
3.1. General Methods
3.2. Synthesis of Cis- and Trans-Hexahydro [1,2,4]triazolo[4,3-a]quinazolin-9(1H)-ones 4a–h and 5a–h
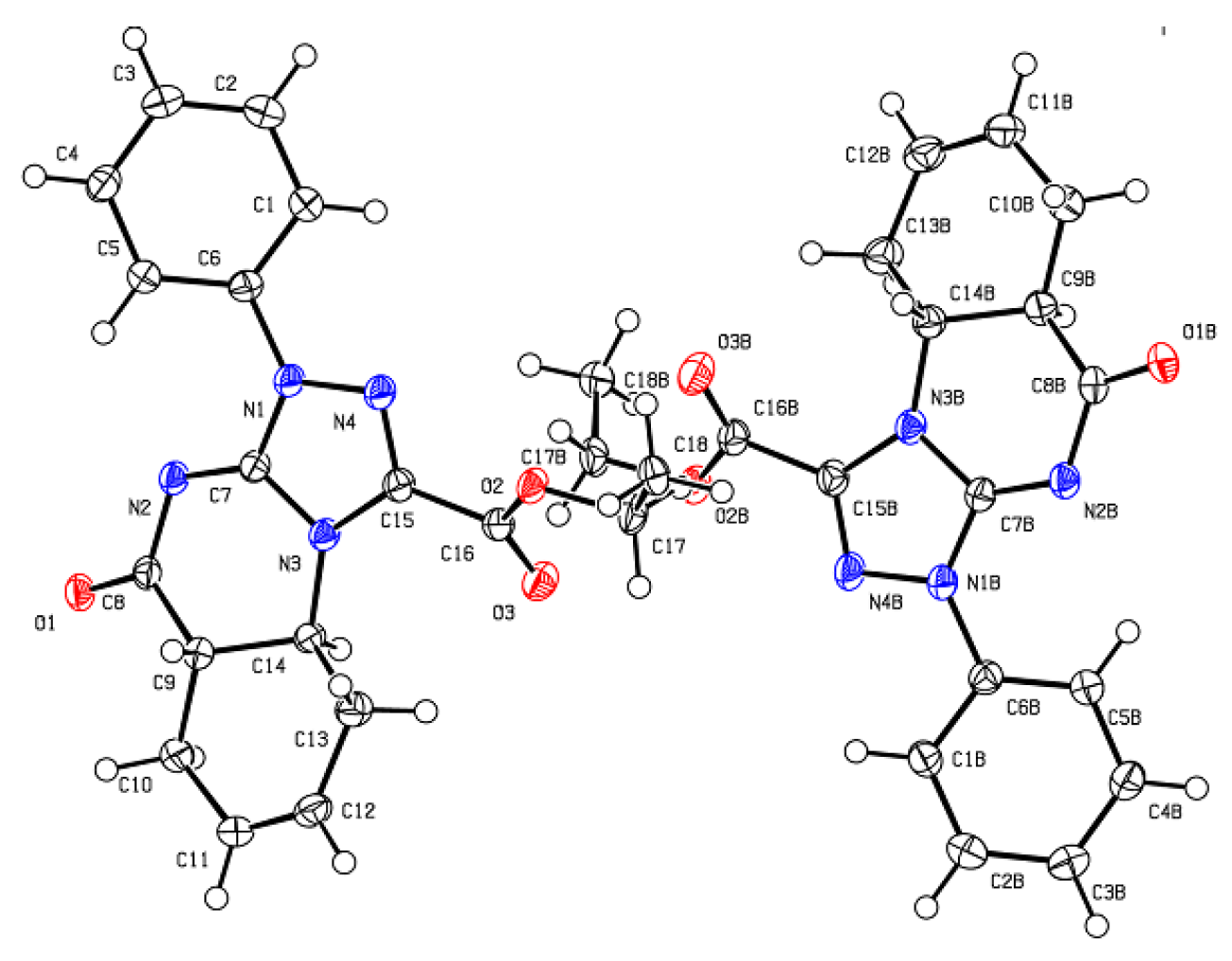
3.3. X-ray Structure Determinations
3.4. Determination of Antiproliferative Properties of the Prepared Compounds
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
Appendix A
References
- Al-Soliemy, M.A.; Sabour, R.; Thoraya, F.A. Pyrazoles and Fused Pyrimidines: Synthesis, Structure Elucidation, Antitubercular Activity and Molecular Docking Study. Med. Chem. 2022, 18, 181–198. [Google Scholar] [CrossRef]
- Sayed, A.R.M.; Ahmed, S.M.; Gomha, S.M. Efficient Methods for the Synthesis of Novel Arylazothiazoles Based on Acetylferrocene or Adamantane. Curr. Org. Synth. 2020, 17, 282–287. [Google Scholar] [CrossRef]
- Sayed, A.R.; Ali, S.H.; Al-Faiyz, Y.S. Recent progress of synthesis of new arylazoazoles based on bis(carbothioamides). Synth. Commun. 2019, 49, 3210–3217. [Google Scholar] [CrossRef]
- Shawali, A.S.; Mosselhi, M.A.; Farghaly, T.A. Synthesis and tautomeric structure of 2-arylazo-4H-imidazo [2,1-b][1,3,4]thiadiazines. J. Chem. Res. 2007, 8, 479–483. [Google Scholar] [CrossRef]
- Abdelriheem, N.A.; Mohamed, A.M.M.; Abdelhamid, A.O. Synthesis of Some New 1,3,4-Thiadiazole, Thiazole and Pyridine Derivatives Containing 1,2,3-Triazole Moiety. Molecules 2017, 22, 268. [Google Scholar] [CrossRef] [PubMed]
- Said, A.I.; Haukka, M.; Fülöp, F. Microwave-Assisted Regioselective Synthesis of Variously Functionalized [1,2,4]triazolo[3,4-b]quinazolin-5(1H)-ones. Curr. Org. Chem. 2020, 24, 1892–1896. [Google Scholar] [CrossRef]
- Abdelhamid, A.O.; Shawali, A.S.; Gomha, S.M. Utility of N-aryl 2-aroylhydrazono-propanehydrazonoyl chlorides as precursors for synthesis of new functionalized 1,3,4-thiadiazoles with potential antimicrobial activity. J. Adv. Res. 2015, 6, 885–893. [Google Scholar] [CrossRef]
- Abdelhamid, A.O.; Gomha, S.M.; Abdelriheem, N.A. Utility of 2-(5-methyl-1-phenyl-1H-pyrazol-4-yl)-2-oxo-N′-phenylaceto-hydrazonoyl bromide as precursor for synthesis of new functionalized heterocycles. Synth. Commun. 2017, 47, 999–1005. [Google Scholar] [CrossRef]
- Farag, M.A.; Altalbawy, F.M.A.; Darwish, E.S.S. Synthesis and antimicrobial activity of 1,2,4-triazolo[4,3-b][1,2,4,5] tetrazines. Asian J. Chem. 2011, 23, 2951–2955. [Google Scholar]
- Su, N.N.; Li, Y.; Yu, S.J.; Zhang, X.; Liu, X.H.; Zhao, W.G. Microwave-assisted synthesis of some novel 1,2,3-triazoles by click chemistry, and their biological activity. Res. Chem. Intermed. 2013, 39, 759–766. [Google Scholar] [CrossRef]
- Su, N.N.; Xiong, L.X.; Yu, S.J.; Zhang, X.; Cui, C.; Li, Z.M.; Zhao, W.G. Larvicidal activity and click synthesis of 2-alkoxyl-2-(1,2,3-triazole-1-yl)acetamide library. Comb. Chem. High Throughput Screen. 2013, 16, 484–493. [Google Scholar] [CrossRef]
- Fan, W.-Q.; Katritzky, A.R. 1,2,3-Triazoles. In Comprehensive Heterocycle Chemistry II; Katritzky, A.R., Rees, C.W., Scriven, E.F.V., Eds.; Pergamon Press: New York, NY, USA, 1996; Volume 4, pp. 1–126. [Google Scholar]
- Katritzky, A.R.; Zhang, Y.; Singh, S.K. 1,2,3-Triazole formation under mild conditions via 1,3-dipolar cycloaddition of acetylenes with azides. Heterocycles 2003, 60, 1225–1239. [Google Scholar] [CrossRef]
- Christian, W.T.; Caspar, C.; Morten, M. Peptidotriazoles on solid phase: [1,2,3]-Triazoles by regiospecific copper(I)-catalyzed 1,3-dipolar cycloadditions of terminal alkynes to azides. J. Org. Chem. 2002, 67, 3057–3064. [Google Scholar] [CrossRef]
- Biorn, A.C.; Cocklin, S.; Madani, N.; Si, Z.; Ivanovic, T.; Samanen, J.; Ryk, D.I.V.; Pantophlet, R.; Burton, D.R.; Freire, E.; et al. Mode of action for linear peptide inhibitors of HIV-1 gp120 interactions. Biochemistry 2004, 43, 1928–1938. [Google Scholar] [CrossRef]
- Whiting, M.; Muldoon, J.; Lin, Y.C.; Silverman, S.M.; Lindstrom, W.; Olson, A.J.; Kolb, H.C.; Finn, M.G.; Sharpless, B.K.; Elder, J.H.; et al. Inhibitors of HIV-1 protease by using in situ click chemistry. Angew. Chem. Int. Ed. 2006, 45, 1435–1439. [Google Scholar] [CrossRef]
- Wang, Z.J.; Gao, Y.; Hou, Y.L.; Zhang, C.; Yu, S.J.; Bian, Q.; Li, Z.M.; Zhao, W.G. Design, synthesis, and fungicidal evaluationof a series of novel 5-methyl-1H-1,2,3-trizole-4-carboxyl amide and ester analogues. Eur. J. Med. Chem. 2014, 86, 87–94. [Google Scholar] [CrossRef]
- Fares, M.; Abou-Seri, S.M.; Abdel-Aziz, H.A.; Abbas, S.E.S.; Youssef, M.M.; Eladwy, R.A. Synthesis and antitumor activity of pyrido [2,3-d]pyrimidine and pyrido [2,3-d][1,2,4]triazolo[4,3-a]pyrimidine derivatives that induce apoptosis through G(1) cell-cycle arrest. Eur. J. Med. Chem. 2014, 83, 155–166. [Google Scholar] [CrossRef] [PubMed]
- Pande, V.; Ramos, M.J. Structural basis for the GSK-3beta binding affinity and selectivity against CDK-2 of 1-(4-aminofurazan-3yl)-5-dialkylaminomethyl-1H-[1,2,3]triazole-4-carboxylic acid derivatives. Bioorg. Med. Chem. Lett. 2005, 15, 5129–5135. [Google Scholar] [CrossRef]
- Olesen, P.H.; Sørensen, A.R.; Ursö, B.; Kurtzhals, P.; Bowler, A.N.; Ehrbar, U.; Hansen, B.F. Synthesis and in vitro characterization of 1-(4-Aminofurazan-3-yl)-5-dialkylaminomethyl-1H-[1,2,3]triazole-4-carboxylic acid derivatives. A new class of selective GSK-3 inhibitors. J. Med. Chem. 2003, 46, 3333–3341. [Google Scholar] [CrossRef] [PubMed]
- Hassaneen, H.M.; Abdelhadi, H.A.; Abdallah, T.A. Novel synthesis of 1,2,4-triazolo[4,3-a]pyrimidin-5-one derivatives. Tetrahedron 2001, 57, 10133–10138. [Google Scholar] [CrossRef]
- Hassneen, H.M.; Abdallah, T.A. New Routes to Pyridino [2,3-d]pyrimidin-4-one and Pyridino [2,3-d]triazolino [4,5-a]pyrimidin-5-one Derivatives. Molecules 2003, 8, 333–341. [Google Scholar] [CrossRef]
- Abdel Hafez, N.A.; Farghaly, T.A.; Al-Omar, M.A.; Abdall, M.M. Synthesis of bioactive polyheterocyclic ring systems as 5α-reductase inhibitors. Eur. J. Med. Chem. 2010, 45, 4838–4844. [Google Scholar] [CrossRef] [PubMed]
- Riyadh, S.M. Enaminones as Building Blocks for the Synthesis of Substituted Pyrazoles with Antitumor and Antimicrobial Activities. Molecules 2011, 16, 1834–1853. [Google Scholar] [CrossRef] [PubMed]
- Abdallah, T.A.; Darwish, M.A.; Hassaneen, H.M. A Novel Synthesis of 1,2,4-Triazolopteridines. Molecules 2002, 7, 494–500. [Google Scholar] [CrossRef]
- Said, A.I.; Palkó, M.; Haukka, M.; Fülöp, F. Retro Diels Alder Protocol for Regioselective Synthesis of Novel [1,2,4]triazolo[4,3-a]pyrimidin-7(1H)-ones. RSC Adv. 2020, 10, 33937–33943. [Google Scholar] [CrossRef]
- Said, A.I.; Palkó, M.; Haukka, M.; Fülöp, F. Angular Regioselectivity in the Reactions of 2-Thioxopyrimidin-4-ones and Hydrazonoyl Chlorides: Synthesis of Novel Stereoisomeric Octahydro [1,2,4]triazolo[4,3-a]quinazolin-5-ones. Molecules 2020, 25, 5673. [Google Scholar] [CrossRef]
- Kamal, A.; Dastagiri, D.; Ramaiah, M.J.; Reddy, J.S.; Bharathi, E.V.; Reddy, M.K.; Sagar, M.V.; Reddy, T.L.; Pushpavalli, S.N.; Pal-Bhadra, M. Synthesis and apoptosis inducing ability of new anilino substituted pyrimidine sulfonamides as potential anticancer agents. Eur. J. Med. Chem. 2011, 46, 5817–5824. [Google Scholar] [CrossRef] [PubMed]
- Zheng, L.W.; Li, Y.; Ge, D.; Zhao, B.X.; Liu, Y.R.; Lv, H.S.; Ding, J.; Miao, J.Y. Synthesis of novel oxime-containing pyrazole derivatives and discovery of regulators for apoptosis and autophagy in A549 lung cancer cells. Bioorg. Med. Chem. Lett. 2010, 20, 4766–4770. [Google Scholar] [CrossRef] [PubMed]
- Hafez, H.N.; El-Gazzar, A.R. Synthesis and antitumor activity of substituted triazolo[4,3-a]pyrimidin-6-sulfonamide with an incorporated thiazolidinone moiety. Bioorg. Med. Chem. Lett. 2009, 19, 4143–4147. [Google Scholar] [CrossRef]
- Zhao, X.L.; Zhao, Y.F.; Guo, S.C.; Song, H.S.; Wang, D.; Gong, P. Synthesis and Anti-tumor Activities of Novel [1,2,4]triazolo[1,5-a]pyrimidines. Molecules 2007, 12, 1136–1146. [Google Scholar] [CrossRef]
- Shawali, A.S.; Sherif, S.M.; Darwish, M.A.; El-merzabani, M.M. Synthesis and antitumor screening of new 1,7-diphenyl-3-(1,3-disubstituted-1H-pyrazole-4-carbonyl)-[1,2,4]triazolo[4,3-a]pyrimidin-5(1H)-ones. Arch. Pharmacal Res. 2010, 33, 55–60. [Google Scholar] [CrossRef]
- Sohár, P.; Szöke-Molnár, Z.; Stájer, G.; Bernáth, G. Preparation and structure of cycloalkane-condensed [1,3]thiazino [3,2-a] pyrimidinones. Magn. Reson. Chem. 1989, 27, 959–963. [Google Scholar] [CrossRef]
- Elliott, A.J.; Callaghan, P.D.; Gibson, M.S.; Nemeth, S.T. The rearrangement of arylthiohydrazonates. Can. J. Chem. 1975, 53, 1484–1490. [Google Scholar] [CrossRef]
- Shawali, A.S.; Abbas, I.M.; Mahran, A.M. Facile Entries for Regioselective Synthesis of [1,2,4]Triazolo [4,3-a]pyrimidin-5(1H)-ones from 2-Thiouracil. J. Iran. Chem. Soc. 2004, 1, 33–39. [Google Scholar] [CrossRef]
- Stajer, G.; Szabo, A.E.; Sohar, P. Synthesis and structure of norbornane/ene-fused thiouracils and thiazino [3,2-a]pyrimidinones. Heterocycles 1999, 51, 1849–1854. [Google Scholar] [CrossRef]
- Stájer, G.; Szabó, A.E.; Pintye, J.; Bernáth, G.; Sohár, P. Stereochemical Studies. Part 86.Saturated Heterocycles. Part 81.I Preparation of New Thiouracils via Retrodiene Decomposition of Methylene-bridged QuinazoloneThiones. J. Chem. Soc. Perkin Trans. I 1985, 2483–2487. [Google Scholar] [CrossRef]
- Soliman, H.M.; Basuny, A.M.; Arafat, S.M. Utilization of Stearic acid Extracted from Olive Pomace for Production of Triazoles, Thiadiazoles and Thiadiazines Derivatives of Potential Biological Activities. J. Oleo Sci. 2015, 64, 1019–1032. [Google Scholar] [CrossRef]
- Matiychuk, V.S.; Potopnyk, M.A.; Luboradzki, R.; Obushak, M.D. New Method for the Synthesis of 1-Aryl-1,2,4-triazole Derivatives. Synthesis 2011, 43, 1799–1813. [Google Scholar] [CrossRef]
- Rikagu Oxford Diffraction. CrysAlisPro; Rikagu Oxford Diffraction Inc.: Oxfordshire, UK, 2020. [Google Scholar]
- Sheldrick, G.M. SHELXT—Integrated space-group and crystal-structure determination. Acta Cryst. 2015, A71, 3–8. [Google Scholar] [CrossRef] [PubMed]
- Hübschle, C.B.; Sheldrick, G.M.; Dittrich, B. ShelXle: A Qt graphical user interface for SHELXL. J. Appl. Cryst. 2011, 44, 1281–1284. [Google Scholar] [CrossRef] [PubMed]
- Mosmann, T. Rapid colorimetric assay for cellular growth and survival: Application to proliferation and cytotoxicity assays. J. Immunol. Methods 1983, 65, 55–63. [Google Scholar] [CrossRef] [PubMed]

| Comp. | Conc. | Inhibition of Cancer Growth (%) ± SEM | |||
|---|---|---|---|---|---|
| MDA-MB-231 | MCF-7 | SiHa | A2780 | ||
| 4c | 10 μM | 33.47 ± 0.90 | 41.80 ± 1.27 | 31.22 ± 0.57 | 24.37 ± 1.72 |
| 30 μM | 34.63 ± 2.55 | 46.85 ± 1.12 | 36.93 ± 0.48 | 30.55 ± 2.70 | |
| 4d | 10 μM | 14.65 ± 3.33 | – * | – | – |
| 30 μM | 14.75 ± 2.66 | – | – | – | |
| 4e | 10 μM | – | – | – | – |
| 30 μM | 12.02 ± 4.48 | – | – | – | |
| 4g | 10 μM | – | – | 17.29 ± 2.24 | – |
| 30 μM | – | – | 19.37 ± 1.29 | – | |
| 4h | 10 μM | – | – | – | – |
| 30 μM | – | 12.87 ± 1.16 | – | – | |
| 5b | 10 μM | – | – | 17.32 ± 1.90 | – |
| 30 μM | 13.52 ± 1.87 | 27.04 ± 2.39 | 27.34 ± 3.28 | 19.65 ± 2.09 | |
| 5c | 10 μM | – | 27.49 ± 2.70 | 16.58 ± 2.27 | 10.86 ± 2.32 |
| 30 μM | – | 35.28 ± 2.09 | 21.43 ± 2.95 | 24.62 ± 1.73 | |
| 5d | 10 μM | – | – | – | – |
| 30 μM | 17.97 ± 2.44 | 25.51 ± 2.52 | 23.19 ± 2.39 | 17.34 ± 2.72 | |
| 5e | 10 μM | – | 21.01 ± 2.77 | 21.04 ± 2.90 | – |
| 30 μM | – | 23.38 ± 2.86 | 22.36 ± 2.58 | 14.53 ± 2.93 | |
| 5f | 10 μM | – | 12.75 ± 2.79 | – | – |
| 30 μM | – | 20.59 ± 2.79 | – | – | |
| 5g | 10 μM | – | 21.18 ± 2.55 | 10.08 ± 1.07 | – |
| 30 μM | – | 24.48 ± 2.82 | 10.02 ± 2.45 | 12.61 ± 2.53 | |
| 5h | 10 μM | – | – | – | – |
| 30 μM | 17.03 ± 2.36 | 39.01 ± 2.20 | 32.78 ± 2.89 | 31.90 ± 1.59 | |
| Cispl. | 10 μM | 42.72 ± 2.68 | 54.06 ± 1.17 | 88.64 ± 0.5 | 83.57 ± 1.21 |
| 30 μM | 88.43 ± 0.42 | 95.45 ± 0.28 | 90.18 ± 1.78 | 95.02 ± 0.28 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Said, A.I.; Gajdács, M.; Zupkó, I.; Haukka, M.; Palkó, M. Angular Regioselective Synthesis of Varied Functionalized Hexahydro-1,2,4-triazolo[4,3-a]quinazolin-9-ones and Their Antiproliferative Action. Molecules 2023, 28, 3718. https://doi.org/10.3390/molecules28093718
Said AI, Gajdács M, Zupkó I, Haukka M, Palkó M. Angular Regioselective Synthesis of Varied Functionalized Hexahydro-1,2,4-triazolo[4,3-a]quinazolin-9-ones and Their Antiproliferative Action. Molecules. 2023; 28(9):3718. https://doi.org/10.3390/molecules28093718
Chicago/Turabian StyleSaid, Awad I., Márió Gajdács, István Zupkó, Matti Haukka, and Márta Palkó. 2023. "Angular Regioselective Synthesis of Varied Functionalized Hexahydro-1,2,4-triazolo[4,3-a]quinazolin-9-ones and Their Antiproliferative Action" Molecules 28, no. 9: 3718. https://doi.org/10.3390/molecules28093718
APA StyleSaid, A. I., Gajdács, M., Zupkó, I., Haukka, M., & Palkó, M. (2023). Angular Regioselective Synthesis of Varied Functionalized Hexahydro-1,2,4-triazolo[4,3-a]quinazolin-9-ones and Their Antiproliferative Action. Molecules, 28(9), 3718. https://doi.org/10.3390/molecules28093718