Organic-Cation Modulated Assembly Behaviors of a Ureidopyrimidone-Grafting Cluster
Abstract
1. Introduction
2. Results and Discussion
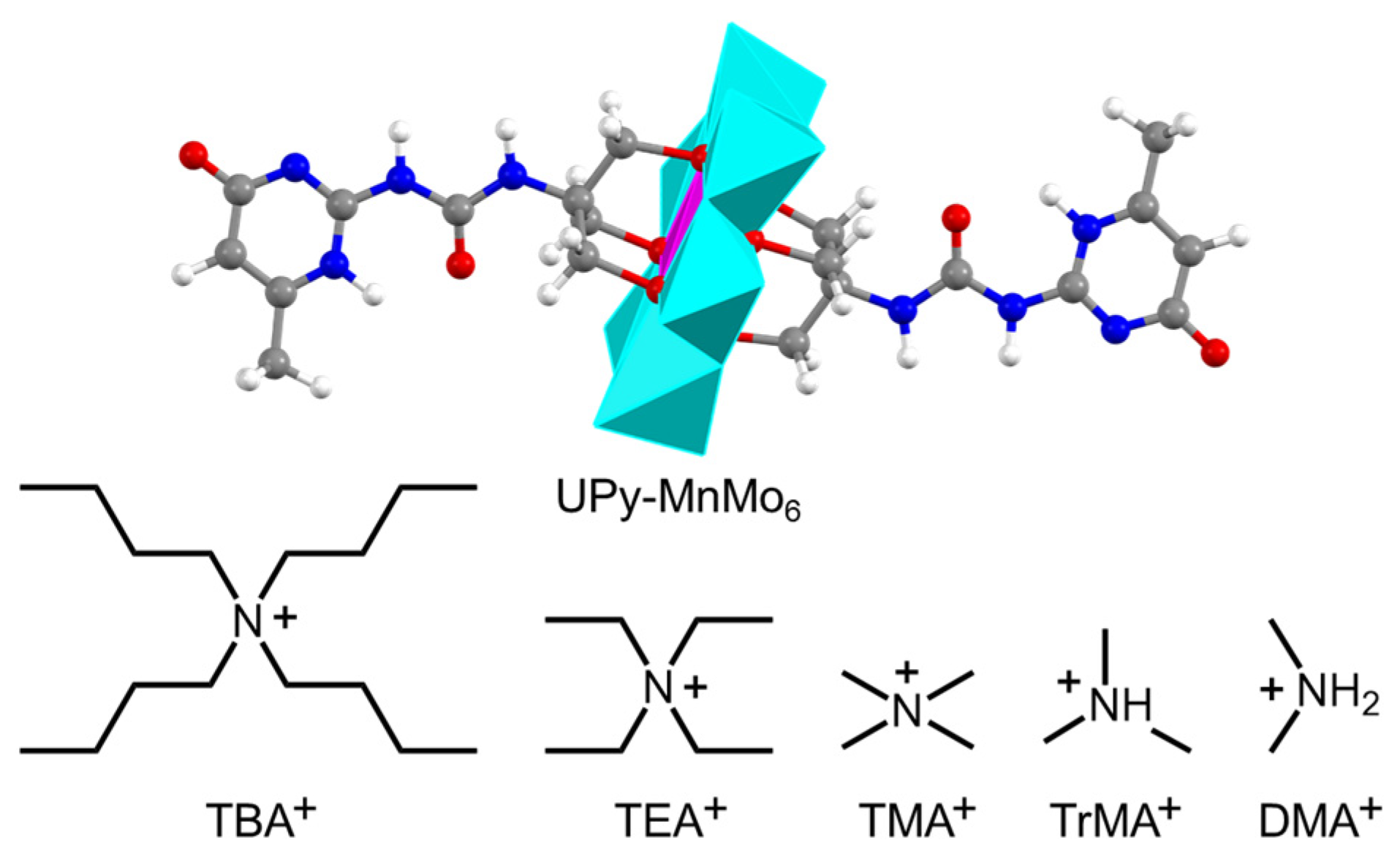
2.1. Synthesis of UPy-Containing Crystals
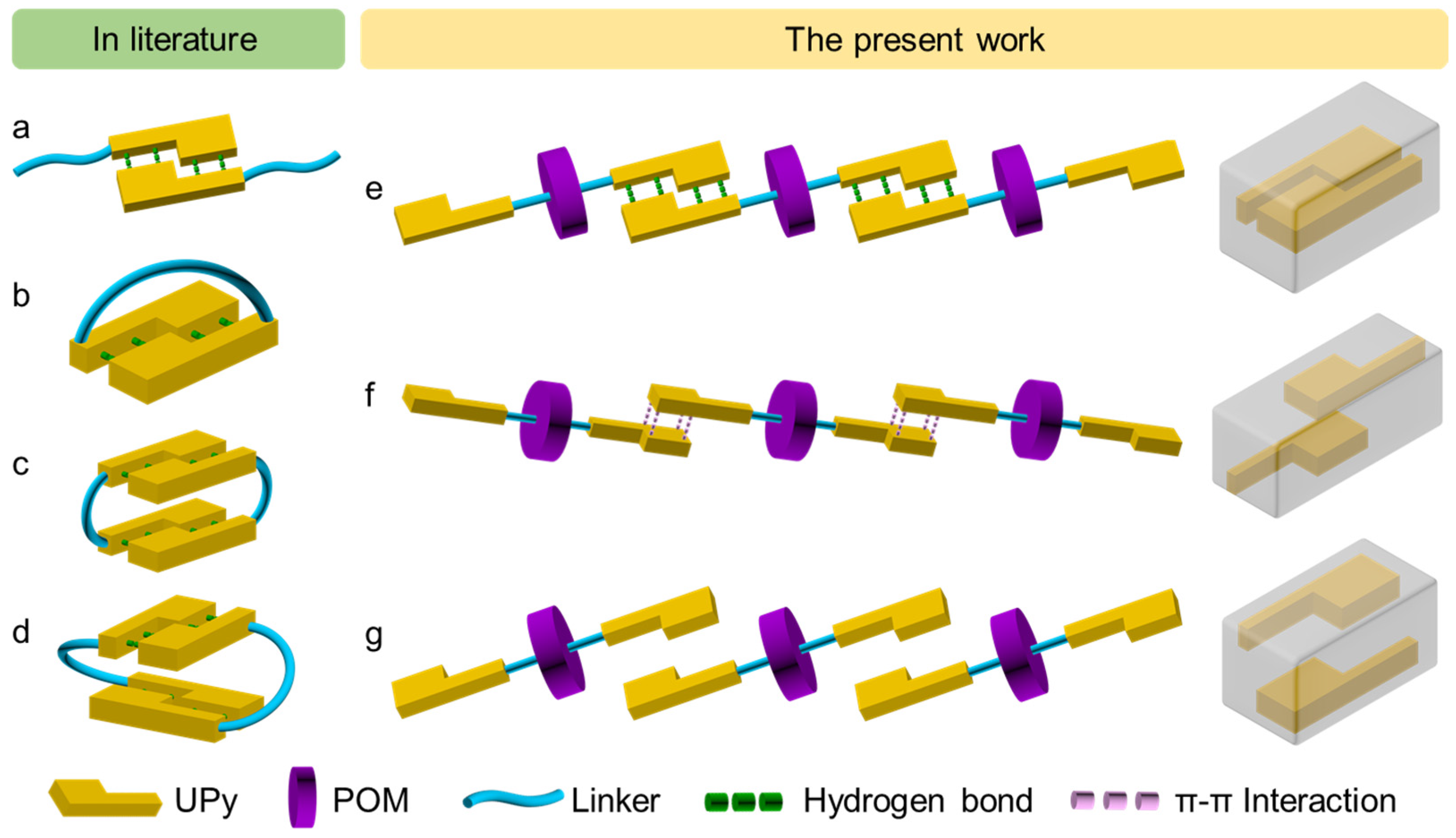
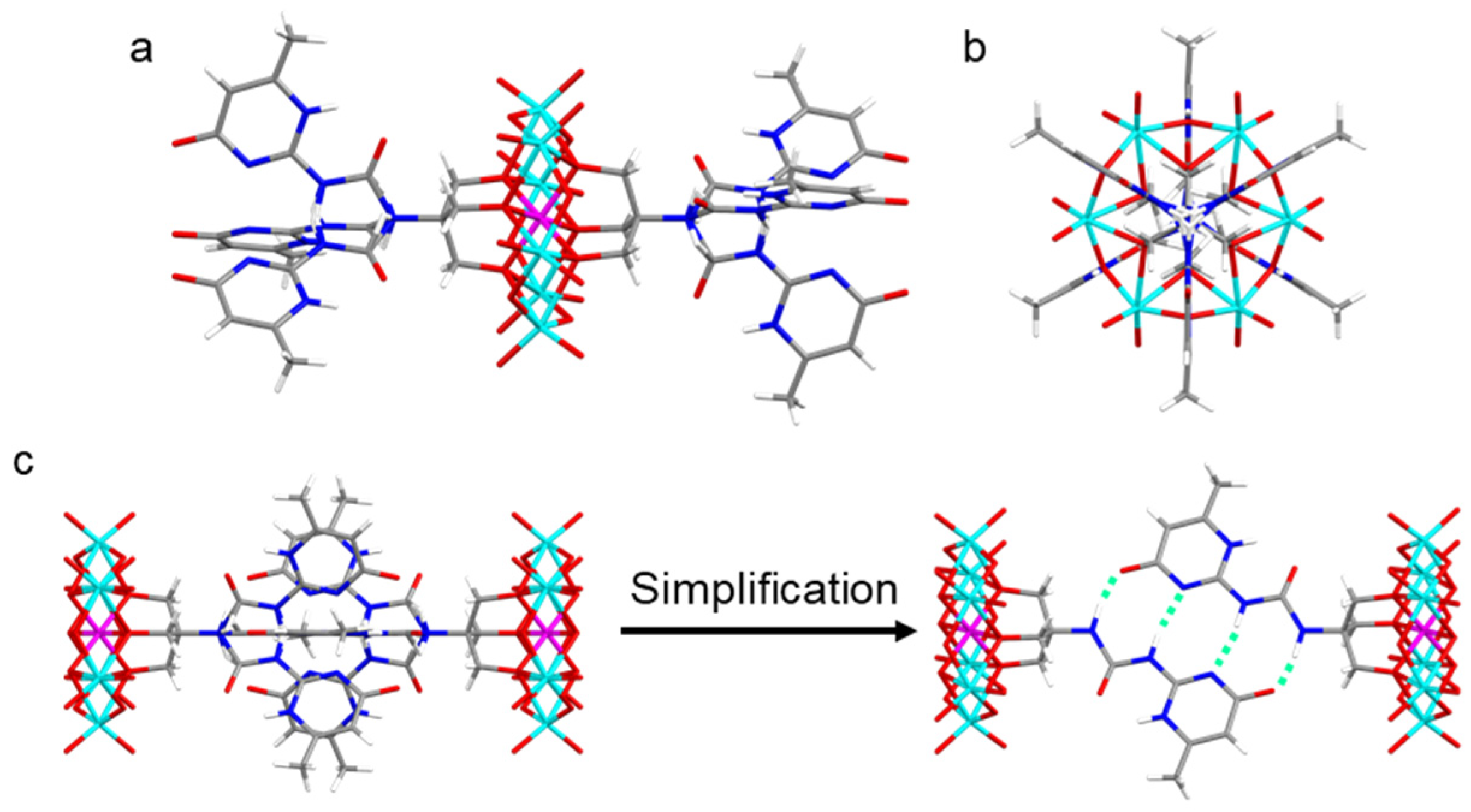
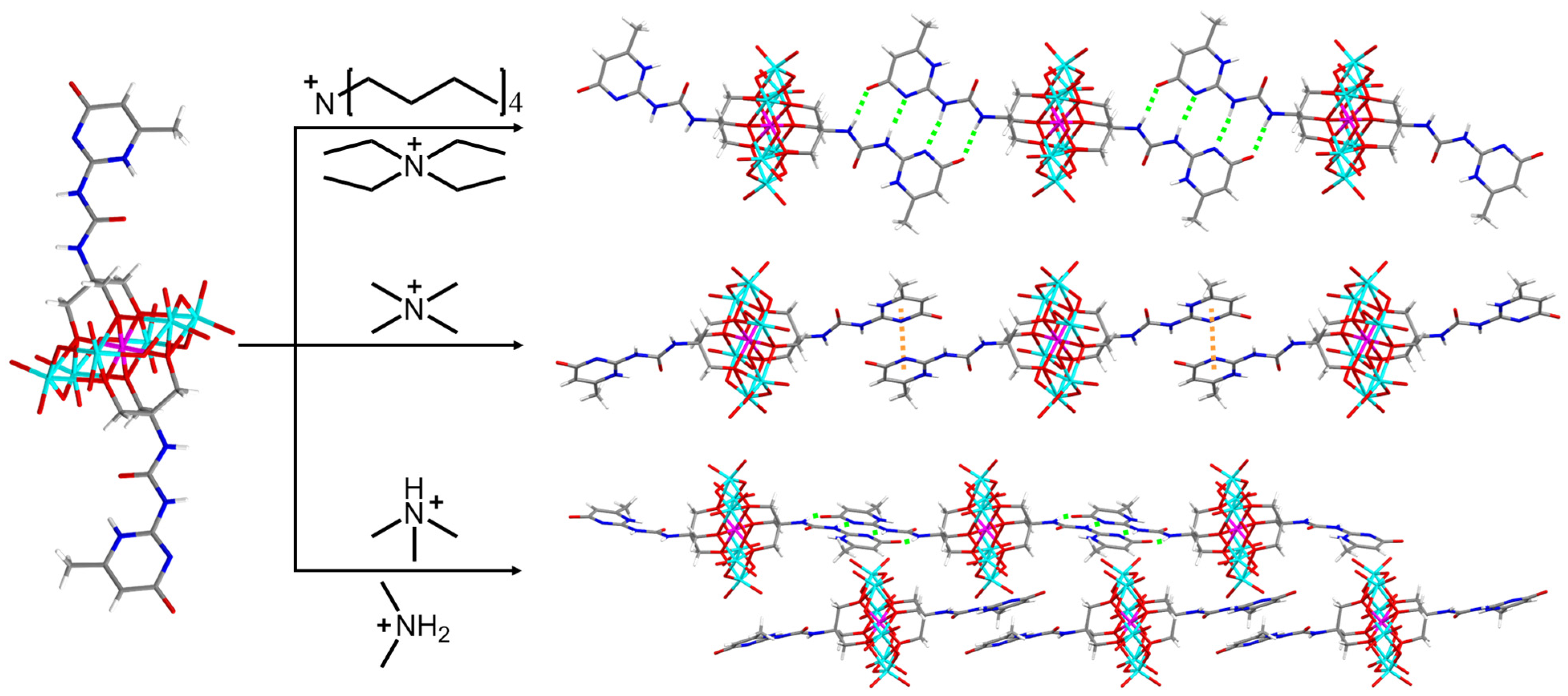
2.2. Combination Models of UPy in Crystals
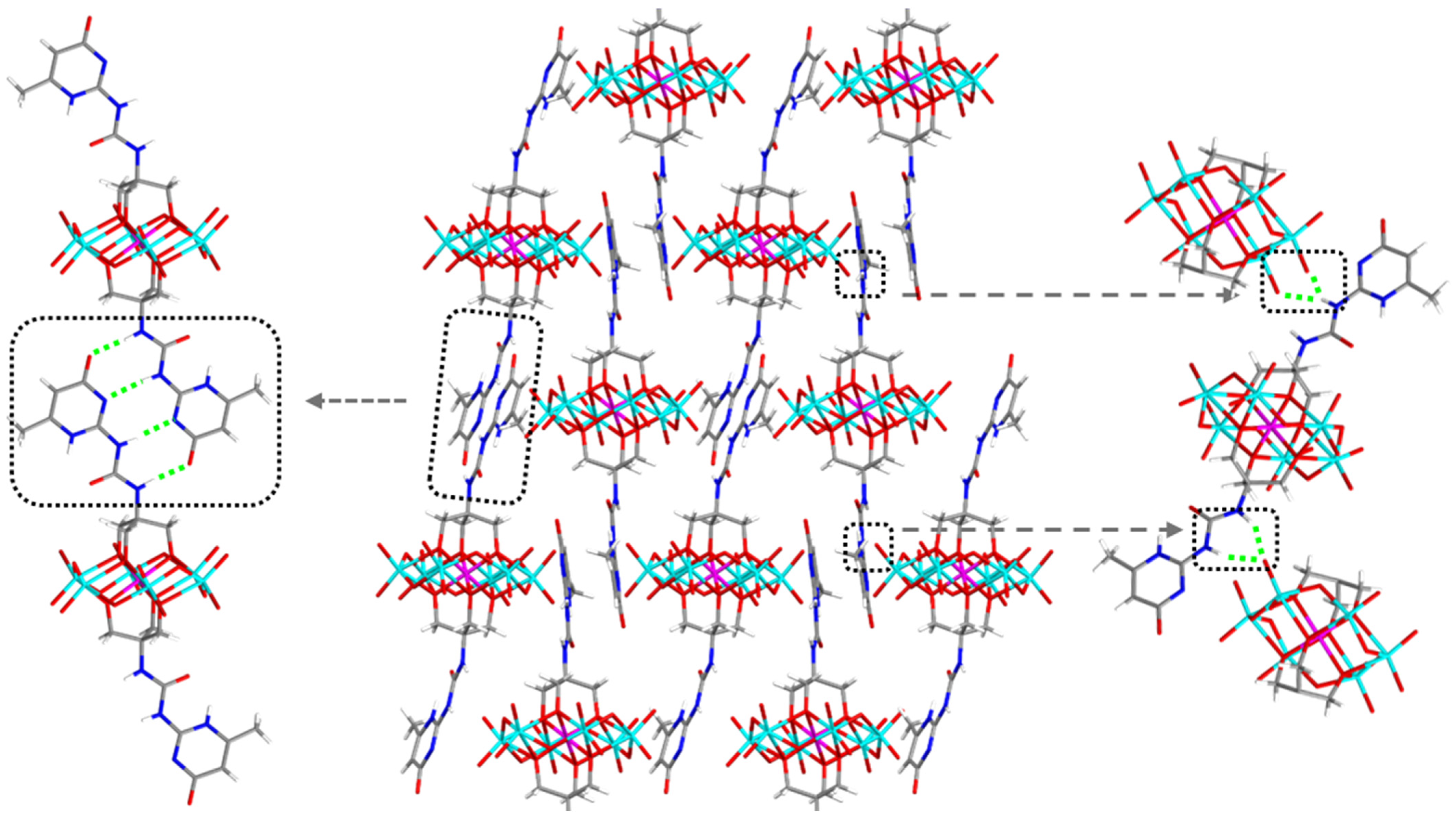
2.3. Organic-Cation Modulated Combination Model of UPy in Crystals
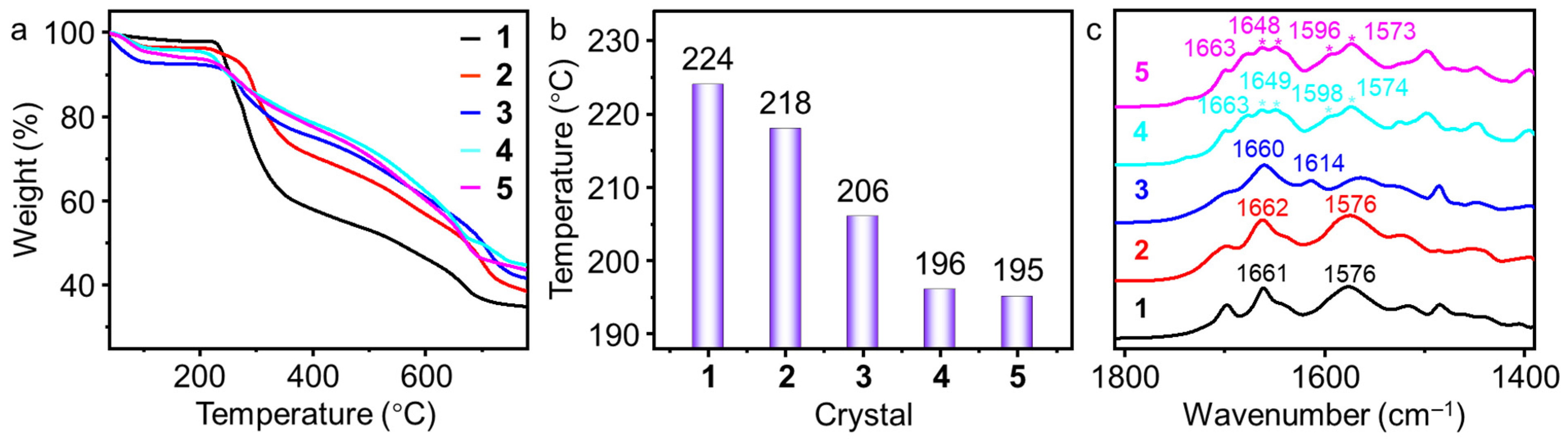
2.4. The Influence of Combination Models of UPy on the Thermal Stability and FT-IR Spectra
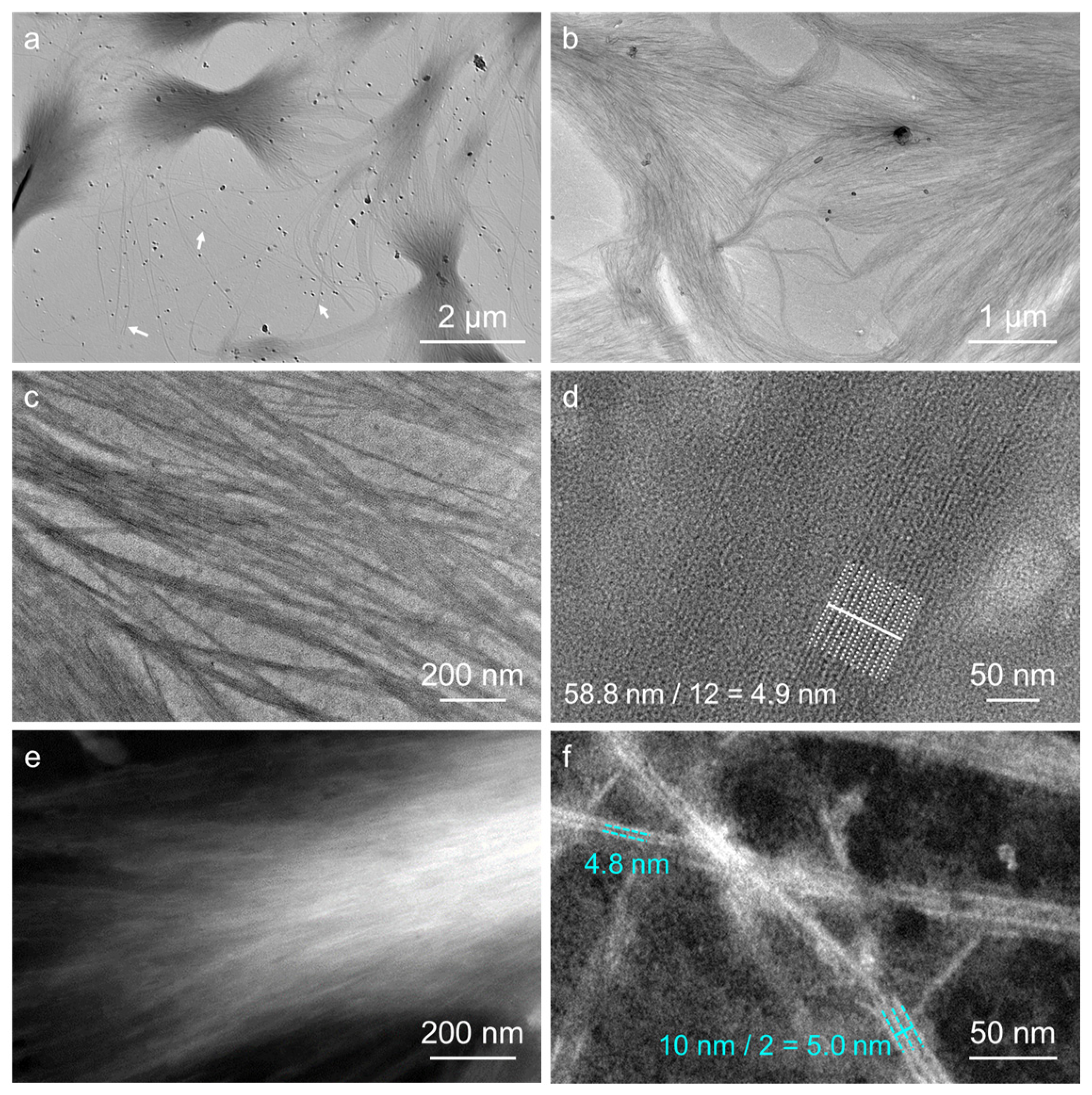
2.5. Assembled Behaviors in Aqueous Solution
3. Materials and Methods
3.1. General Methods and Materials
3.2. Synthesis of Five Crystals
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Aakeröy, C.B.; Seddon, K.S. The Hydrogen Bond and Crystal Engineering. Chem. Soc. Rev. 1993, 22, 397–407. [Google Scholar] [CrossRef]
- Sijbesma, R.P.; Beijer, F.H.; Brunsveld, L.; Folmer, B.J.B.; Hirschberg, J.H.K.K.; Lange, R.F.M.; Lowe, J.K.L.; Meijer, E.W. Reversible Polymers Formed from Self-Complementary Monomers Using Quadruple Hydrogen Bonding. Science 1997, 278, 1601–1604. [Google Scholar] [CrossRef] [PubMed]
- Liu, M.; Wang, Z.; Liu, P.; Wang, Z.; Yao, H.; Yao, X. Supramolecular Silicone Coating Capable of Strong Substrate Bonding, Readily Damage Healing, and Easy Oil Sliding. Sci. Adv. 2019, 5, eaaw5643. [Google Scholar] [CrossRef] [PubMed]
- Lin, R.-B.; He, Y.; Li, P.; Wang, H.; Zhou, W.; Chen, B. Multifunctional Porous Hydrogen-Bonded Organic Framework Materials. Chem. Soc. Rev. 2019, 48, 1362–1389. [Google Scholar] [CrossRef] [PubMed]
- Cao, J.; Lu, C.; Zhuang, J.; Liu, M.; Zhang, X.; Yu, Y.; Tao, Q. Multiple Hydrogen Bonding Enables the Self-Healing of Sensors for Human-Machine Interactions. Angew. Chem. Int. Ed. 2017, 56, 8795–8800. [Google Scholar] [CrossRef]
- Alkorta, I.; Elguero, J.; Frontera, A. Not Only Hydrogen Bonds: Other Noncovalent Interactions. Crystals 2020, 10, 180. [Google Scholar] [CrossRef]
- Desiraju, G.R. Reflections on the Hydrogen Bond in Crystal Engineering. Cryst. Growth Des. 2011, 11, 896–898. [Google Scholar] [CrossRef]
- Wang, X.; Li, Y.; Qian, Y.; Qi, H.; Li, J.; Sun, J. Mechanically Robust Atomic Oxygen-Resistant Coatings Capable of Autonomously Healing Damage in Low Earth Orbit Space Environment. Adv. Mater. 2018, 30, 1803854. [Google Scholar] [CrossRef]
- Lafitte, V.G.H.; Aliev, A.E.; Horton, P.N.; Hursthuse, M.B.; Bala, K.; Golding, P.; Hailes, H.C. Quadruply Hydrogen Bonded Cytosine Modules for Supramolecular Applications. J. Am. Chem. Soc. 2006, 128, 6544–6545. [Google Scholar] [CrossRef]
- Wang, L.; Gong, Z.-L.; Li, S.-Y.; Hong, W.; Zhong, Y.-W.; Wang, D.; Wan, L.-J. Molecular Conductance through a Quadruple-Hydrogen-Bond-Bridged Supramolecular Junction. Angew. Chem. Int. Ed. 2016, 55, 12393–12397. [Google Scholar] [CrossRef]
- Kheria, S.; Rayavarapu, S.; Kotmale, A.S.; Shinde, D.R.; Gonnade, R.G.; Sanjayan, G.J. Coumarin-Appended Stable Fluorescent Self-Complementary Quadruple-Hydrogen-Bonded Molecular Duplexes. J. Org. Chem. 2017, 82, 6403–6408. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Qi, S.; Zhang, C.; Fan, Z.; Ding, Q.; Mao, S.; Dong, Z. Controlling Keto-Enol Tautomerism of Ureidopyrimidinone to Generate a Single-Quadruple AADD-DDAA Dimeric Array. Org. Lett. 2020, 22, 7305–7309. [Google Scholar] [CrossRef]
- Xiao, T.; Feng, X.; Ye, S.; Guan, Y.; Li, S.-L.; Wang, Q.; Ji, Y.; Zhu, D.; Hu, X.; Lin, C.; et al. Highly Controllable Ring–Chain Equilibrium in Quadruply Hydrogen Bonded Supramolecular Polymers. Macromolecules 2012, 45, 9585–9594. [Google Scholar] [CrossRef]
- Xiao, T.; Xu, L.; Wang, J.; Li, Z.-Y.; Sun, X.-Q.; Wang, L. Biomimetic Folding of Small Organic Molecules Driven by Multiple Non-Covalent Interactions. Org. Chem. Front. 2019, 6, 936–941. [Google Scholar] [CrossRef]
- Lafitte, V.G.H.; Aliev, A.E.; Horton, P.N.; Hursthouse, M.B.; Hailes, H.C. Highly Stable Cyclic Dimers based on Non-Covalent Interactions. Chem. Commun. 2006, 20, 2173–2175. [Google Scholar] [CrossRef]
- Folmer, B.J.B.; Sijbesma, R.P.; Kooijman, H.; Spek, A.L.; Meijer, E.W. Cooperative Dynamics in Duplexes of Stacked Hydrogen-Bonded Moieties. J. Am. Chem. Soc. 1999, 121, 9001–9007. [Google Scholar] [CrossRef]
- Dolbecq, A.; Dumas, E.; Mayer, C.R.; Mialane, P. Hybrid Organic-Inorganic Polyoxometalate Compounds: From Structural Diversity to Applications. Chem. Rev. 2010, 110, 6009–6048. [Google Scholar] [CrossRef]
- Weinstock, I.A.; Schreiber, R.E.; Neumann, R. Dioxygen in Polyoxometalate Mediated Reactions. Chem. Rev. 2018, 118, 2680–2717. [Google Scholar] [CrossRef]
- Li, B.; Wu, L. Perspective of Polyoxometalate Complexes on Flexible Assembly and Integrated Potentials. Polyoxometalates 2023, 2, 9140016. [Google Scholar] [CrossRef]
- Harchani, A.; Kucerakova, M.; Dusek, M.; Haddad, A. Structures, Electronic Properties, Reactivity and Dynamic Studies of Three New Polyoxometalate Compounds. Dalton Trans. 2018, 47, 10965–10975. [Google Scholar] [CrossRef]
- Ni, Z.; Lv, H.; Yang, G. Recent Advances of Ti/Zr-Substituted Polyoxometalates: From Structural Diversity to Functional Applications. Molecules 2022, 27, 8799. [Google Scholar] [CrossRef] [PubMed]
- Cameron, J.M.; Guillemot, G.; Galambos, T.; Amin, S.S.; Hampson, E.; Mall Haidaraly, K.; Newton, G.N.; Izzet, G. Supramolecular Assemblies of Organo-functionalised Hybrid Polyoxometalates: From Functional Building Blocks to Hierarchical Nanomaterials. Chem. Soc. Rev. 2022, 51, 293–328. [Google Scholar] [CrossRef]
- Guan, W.; Wang, G.; Li, B.; Wu, L. Organic macrocycle-polyoxometalate hybrids. Coord. Chem. Rev. 2023, 481, 215039. [Google Scholar] [CrossRef]
- Blazevic, A.; Rompel, A. The Anderson–Evans Polyoxometalate: From Inorganic Building Blocks via Hybrid Organic–Inorganic Structures to Tomorrows “Bio-POM”. Coord. Chem. Rev. 2016, 307, 42–64. [Google Scholar]
- Wang, Y.; Duan, F.; Liu, X.; Li, B. Cations Modulated Assembly of Triol-Ligand Modified Cu-Centered Anderson-Evans Polyanions. Molecules 2022, 27, 2933. [Google Scholar] [CrossRef]
- Jiang, F.; Li, B.; Wu, L. Hydrogen-Bonded Framework of a Polyanionic Cluster and Its Growth from 2D to 3D for Dual-Selective Adsorption and pH-Controlled Oxidation. Inorg. Chem. 2022, 61, 20587–20595. [Google Scholar] [CrossRef]
- Chang, T.; Qu, D.; Li, B.; Wu, L. Organic/Inorganic Species Synergistically Supported Unprecedented Vanadomolybdates. Molecules 2022, 27, 7447. [Google Scholar] [CrossRef]
- He, Z.; Li, B.; Ai, H.; Li, H.; Wu, L. A Processable Hybrid Supramolecular Polymer Formed by Base Pair Modified Polyoxometalate Clusters. Chem. Commun. 2013, 49, 8039–8041. [Google Scholar] [CrossRef] [PubMed]
- Marcoux, P.R.; Hasenknopf, B.; Vaissermann, J.; Gouzerh, P. Developing Remote Metal Binding Sites in Heteropolymolybdates. Eur. J. Inorg. Chem. 2003, 2003, 2406–2412. [Google Scholar]
- Teunissen, A.J.; Paffen, T.F.; Ercolani, G.; De Greef, T.F.; Meijer, E.W. Regulating Competing Supramolecular Interactions Using Ligand Concentration. J. Am. Chem. Soc. 2016, 138, 6852–6860. [Google Scholar] [CrossRef]
- Duan, F.; Liu, X.; Qu, D.; Li, B.; Wu, L. Polyoxometalate-Based Ionic Frameworks for Highly Selective CO2 Capture and Separation. CCS Chem. 2021, 3, 2676–2687. [Google Scholar] [CrossRef]
- Capici, C.; Cohen, Y.; D’Urso, A.; Gattuso, G.; Notti, A.; Pappalardo, A.; Pappalardo, S.; Parisi, M.F.; Purrello, R.; Slovak, S.; et al. Anion-Assisted Supramolecular Polymerization:From Achiral AB-Type Monomers to Chiral Assemblies. Angew. Chem. Int. Ed. 2011, 50, 11956–11961. [Google Scholar] [CrossRef] [PubMed]
- Klemperer, W.G. Tetrabutylammonium isopolyoxometalates. Inorg. Syn. 1990, 27, 74–85. [Google Scholar]



| Item | 1 | 2 | 3 | 4 | 5 |
|---|---|---|---|---|---|
| Formula | [N(C4H9)4]3MnMo6O18[ C10H13N4O5]2·6(H2O) | Na[N(C2H5)4]2MnMo6O18[ C10H13N4O5]2·12H2O | H[N(CH3)4]2MnMo6O18[ C10H13N4O5]2·3H2O | H1.5[NH(CH3)3]1. 5MnMo6O18[C10H13N4O5]2· 4H2O | H1.5[NH2(CH3)2]1.5MnMo6O18[C10H13N4O5]2·8H2O |
| F.W. (g mol−1) | 2292.53 | 1956.74 | 1660.41 | 1620.82 | 1671.85 |
| Crystal system | Trigonal | Triclinic | Monoclinic | Triclinic | Triclinic |
| S.G. | R | P | P21/n | P | P |
| a (Å) | 22.1032 (5) | 12.3293 (9) | 14.112 (3) | 13.6400 (6) | 13.7866 (5) |
| b (Å) | 22.1032 (5) | 12.9719 (8) | 10.8510 (16) | 14.0770 (7) | 14.0074 (4) |
| c (Å) | 16.4882 (5) | 13.2711 (9) | 18.445 (3) | 16.4981 (8) | 16.4860 (6) |
| α (deg) | 90 | 111.614 (2) | 90 | 94.258 (2) | 80.2238 (13) |
| β (deg) | 90 | 114.857 (3) | 91.607 (8) | 95.937 (2) | 82.9721 (14) |
| γ (deg) | 120 | 99.082 (3) | 90 | 118.7227 (16) | 61.3974 (11) |
| V (Å3) | 6976.1 (4) | 1662.9 | 2823.4 (8) | 2735.1 (2) | 2751.46 (16) |
| Z | 3 | 1 | 2 | 2 | 2 |
| Dc (g cm−3) | 1.637 | 1.954 | 1.953 | 1.968 | 2.018 |
| F (000) | 3540 | 984 | 1640 | 1594 | 1650 |
| Reflections coll./unique | 36,466/3561 | 60,282/5850 | 119,356/6468 | 153,767/12,578 | 137,337/12,639 |
| Rint | 0.0643 | 0.1356 | 0.0501 | 0.0731 | 0.1376 |
| GOOF on F2 | 1.108 | 1.037 | 1.087 | 1.077 | 1.027 |
| a R1 [I > 2σ(I)] | 0.0745 | 0.0546 | 0.0302 | 0.0369 | 0.0504 |
| b wR2 b (all data) | 0.2736 | 0.1521 | 0.0994 | 0.1091 | 0.1312 |
| CCDC no. | 2,249,014 | 2,249,015 | 2,249,016 | 2,249,017 | 2,249,018 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jiang, F.; Wang, J.; Li, B.; Wu, L. Organic-Cation Modulated Assembly Behaviors of a Ureidopyrimidone-Grafting Cluster. Molecules 2023, 28, 3677. https://doi.org/10.3390/molecules28093677
Jiang F, Wang J, Li B, Wu L. Organic-Cation Modulated Assembly Behaviors of a Ureidopyrimidone-Grafting Cluster. Molecules. 2023; 28(9):3677. https://doi.org/10.3390/molecules28093677
Chicago/Turabian StyleJiang, Fengrui, Jiaxu Wang, Bao Li, and Lixin Wu. 2023. "Organic-Cation Modulated Assembly Behaviors of a Ureidopyrimidone-Grafting Cluster" Molecules 28, no. 9: 3677. https://doi.org/10.3390/molecules28093677
APA StyleJiang, F., Wang, J., Li, B., & Wu, L. (2023). Organic-Cation Modulated Assembly Behaviors of a Ureidopyrimidone-Grafting Cluster. Molecules, 28(9), 3677. https://doi.org/10.3390/molecules28093677







