Fluorescent Dye-Doped Brightening Polymer-Stabilized Bistable Cholesteric Liquid Crystal Films
Abstract
1. Introduction
2. Results and Discussion
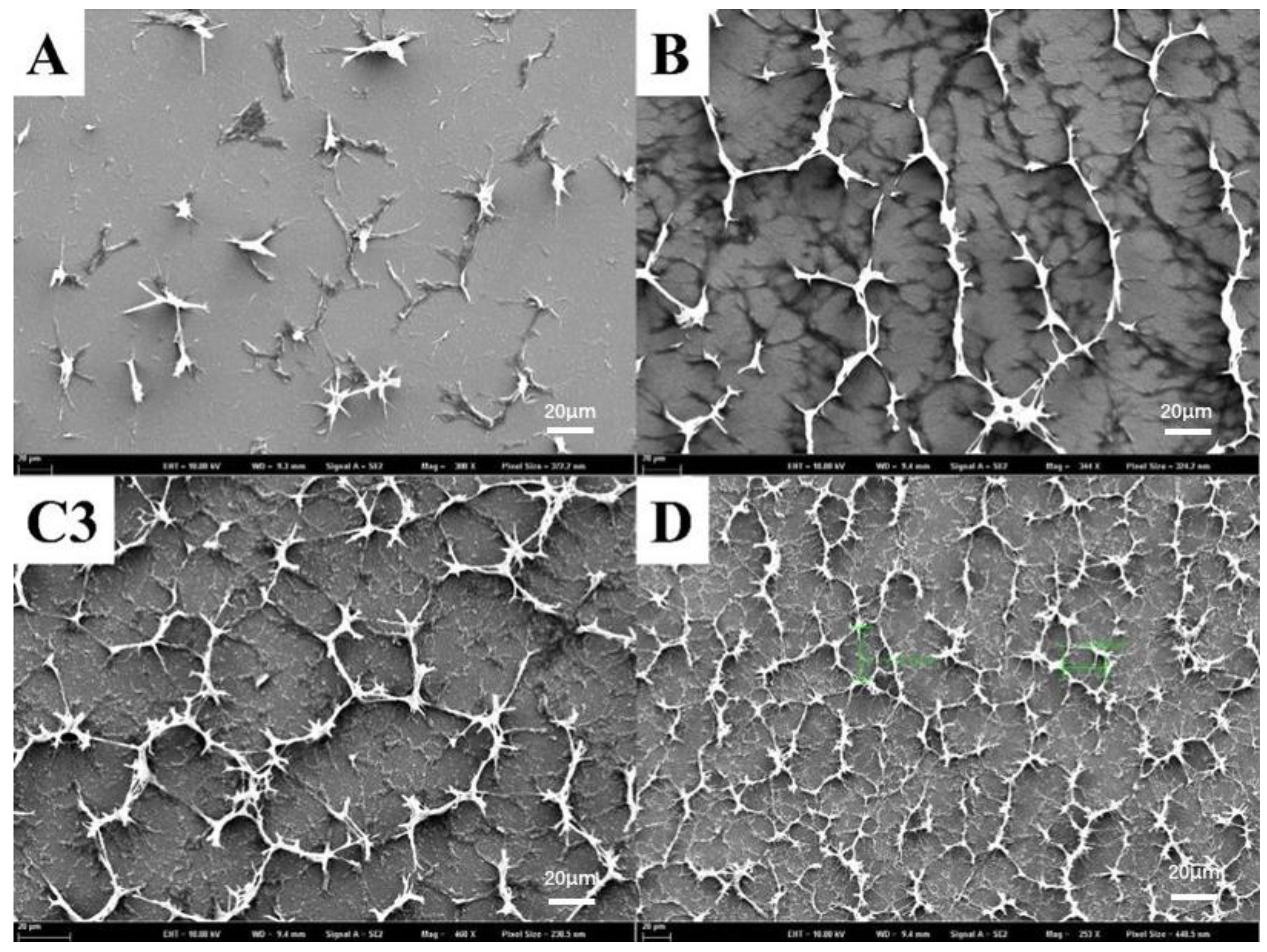
2.1. Building the Best PSBCLC Polymer Network
2.2. Study on Photoelectric Properties of Fluorescent Dye-Doped Liquid Crystal Composite System
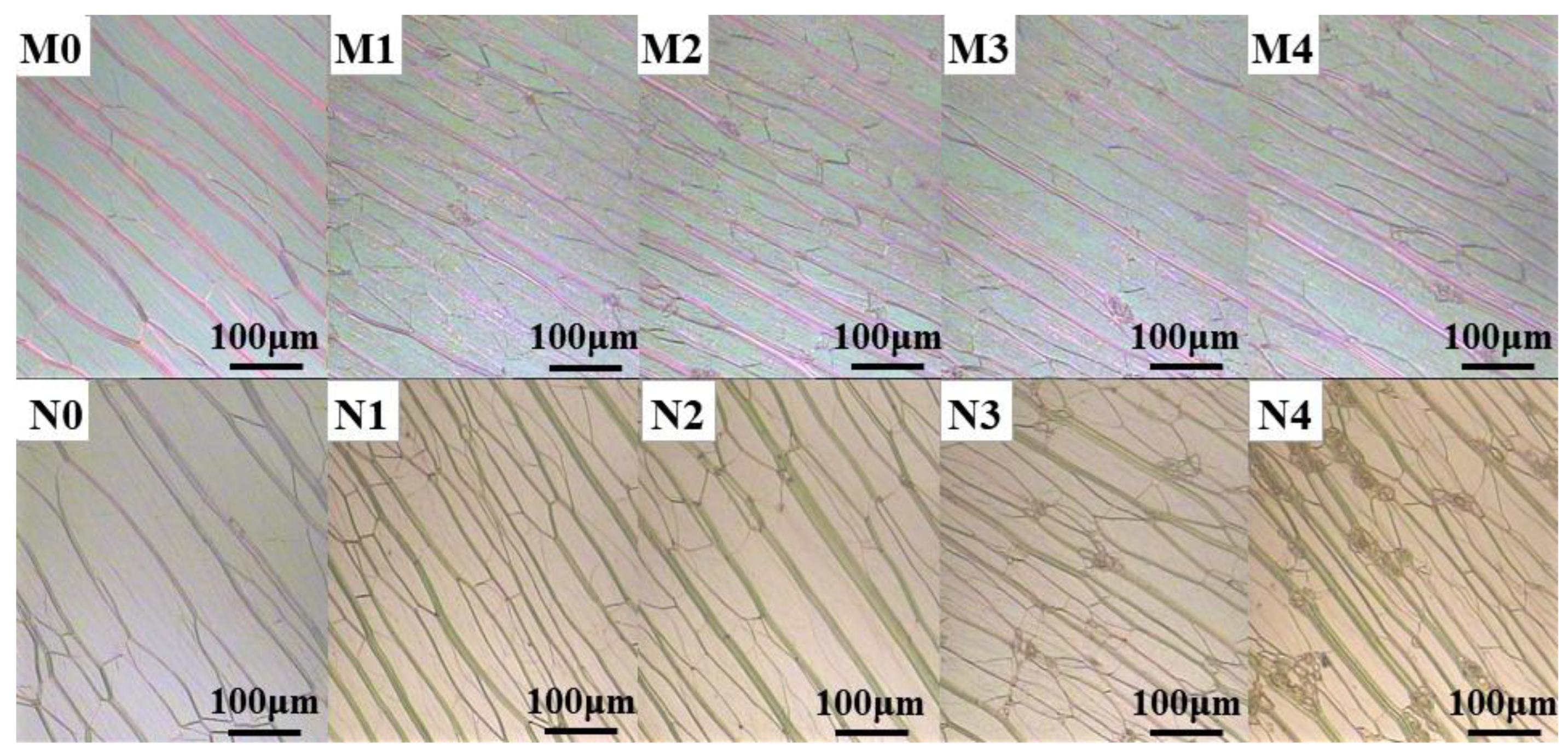
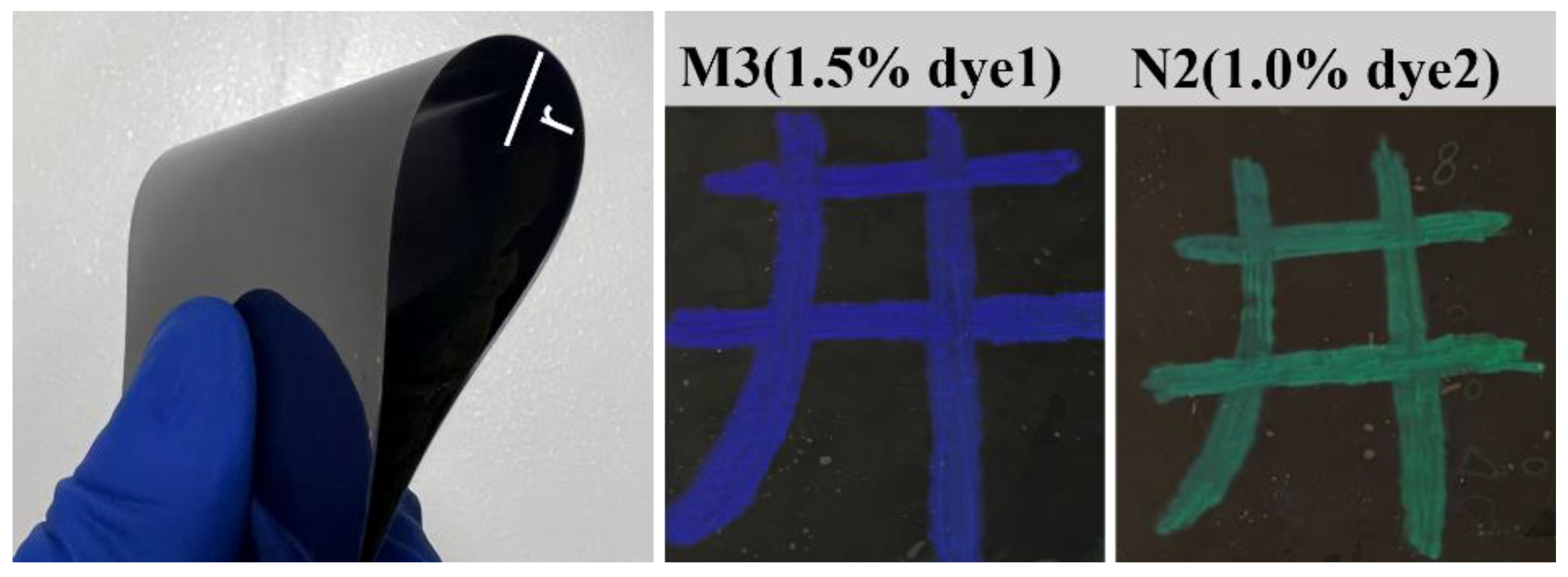
2.2.1. Dispersion of Fluorescent Dyes in Liquid Crystal Systems
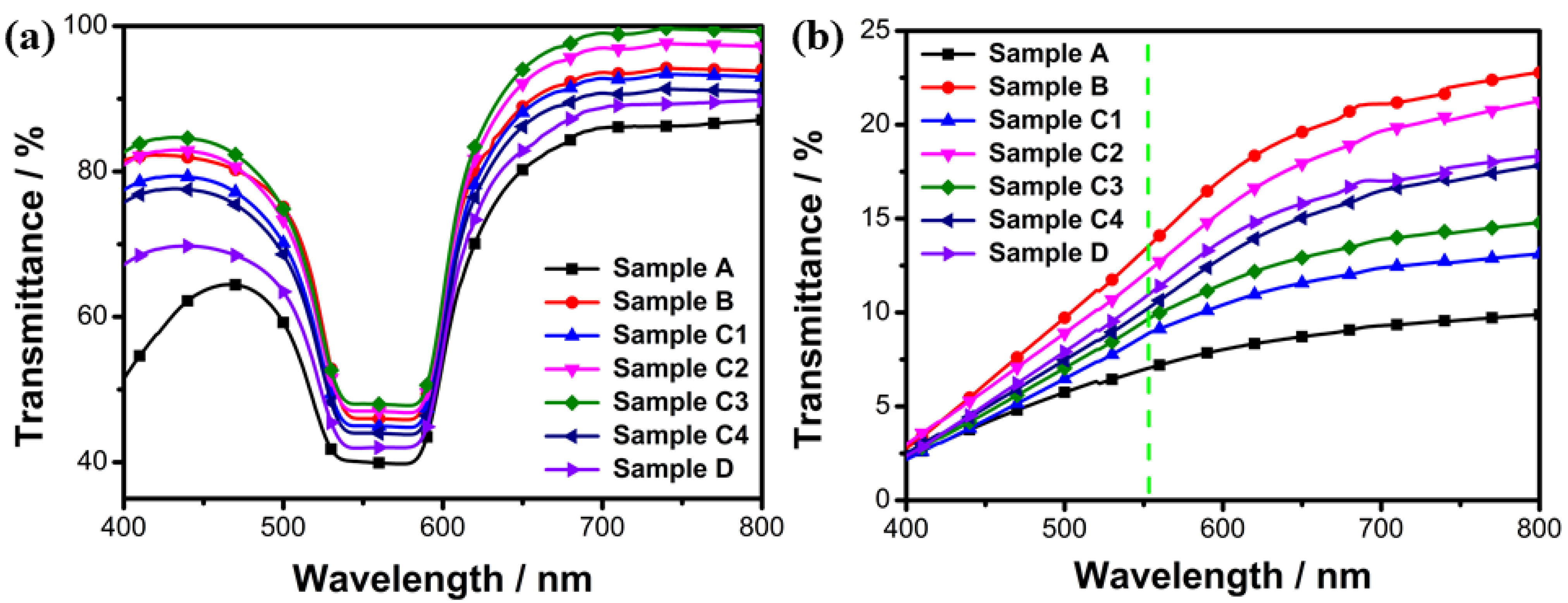
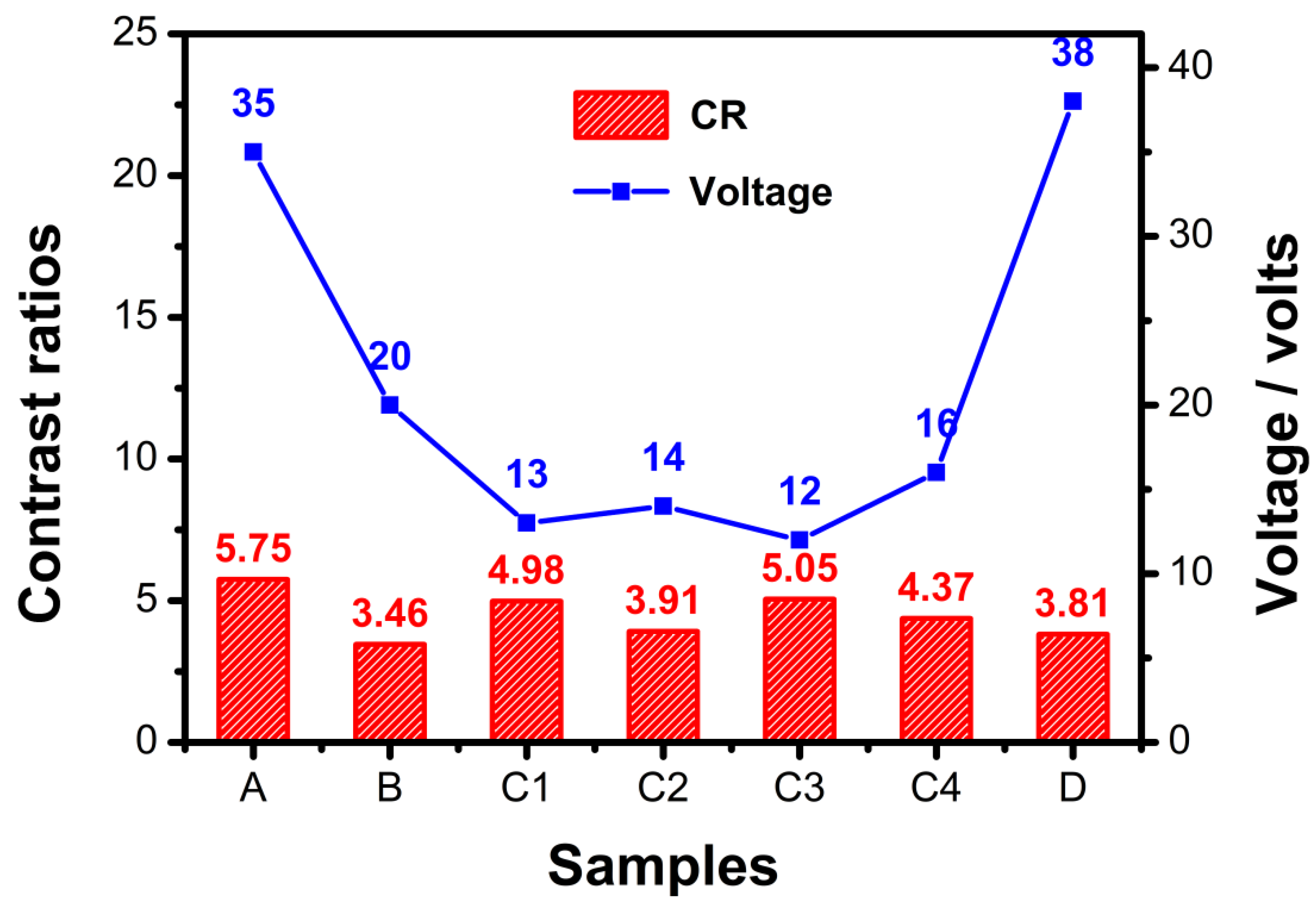
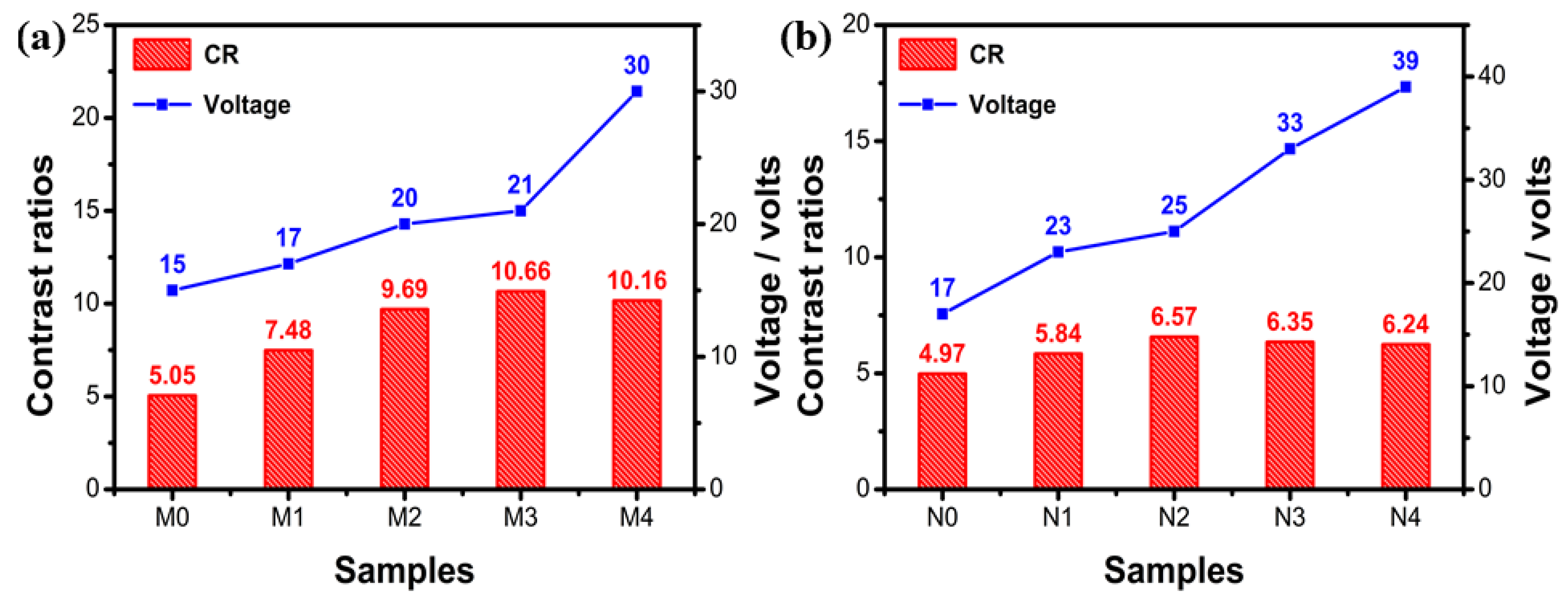
2.2.2. Effect of Fluorescent Dye Concentration on Transmission Spectra and Driving Voltage
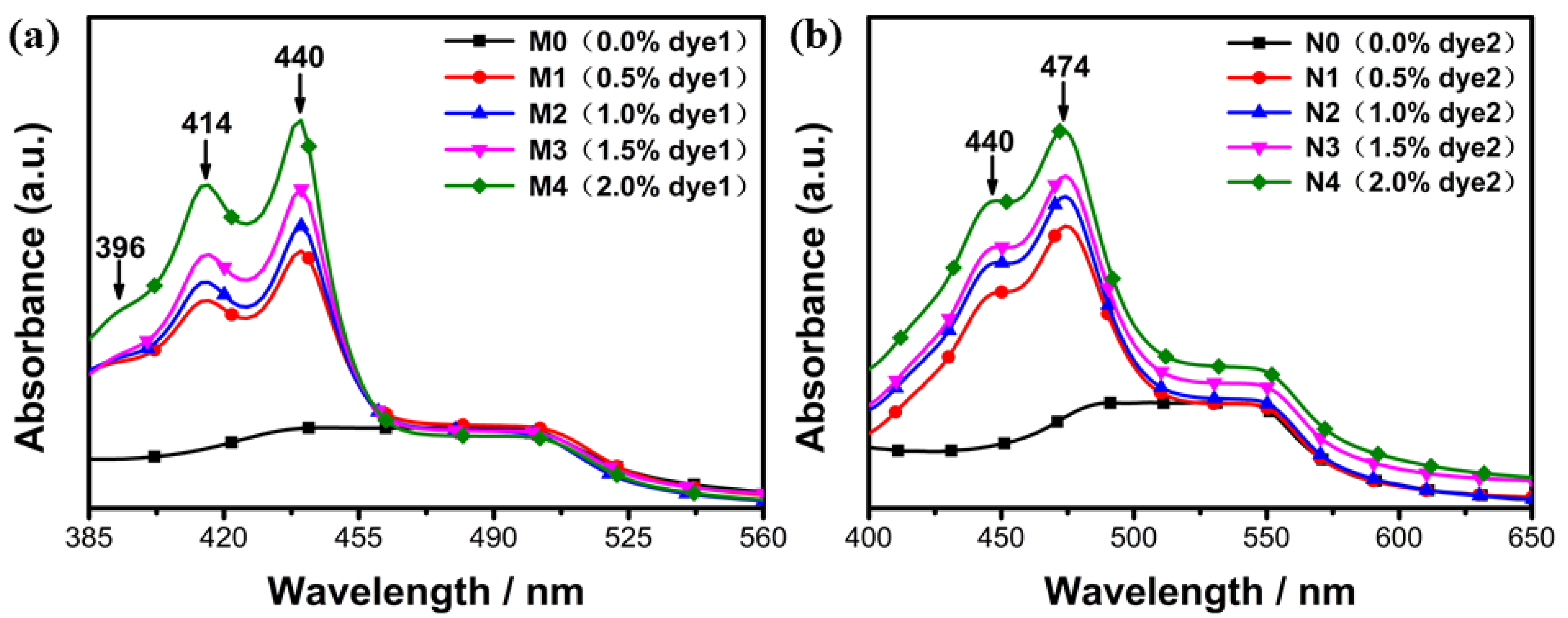
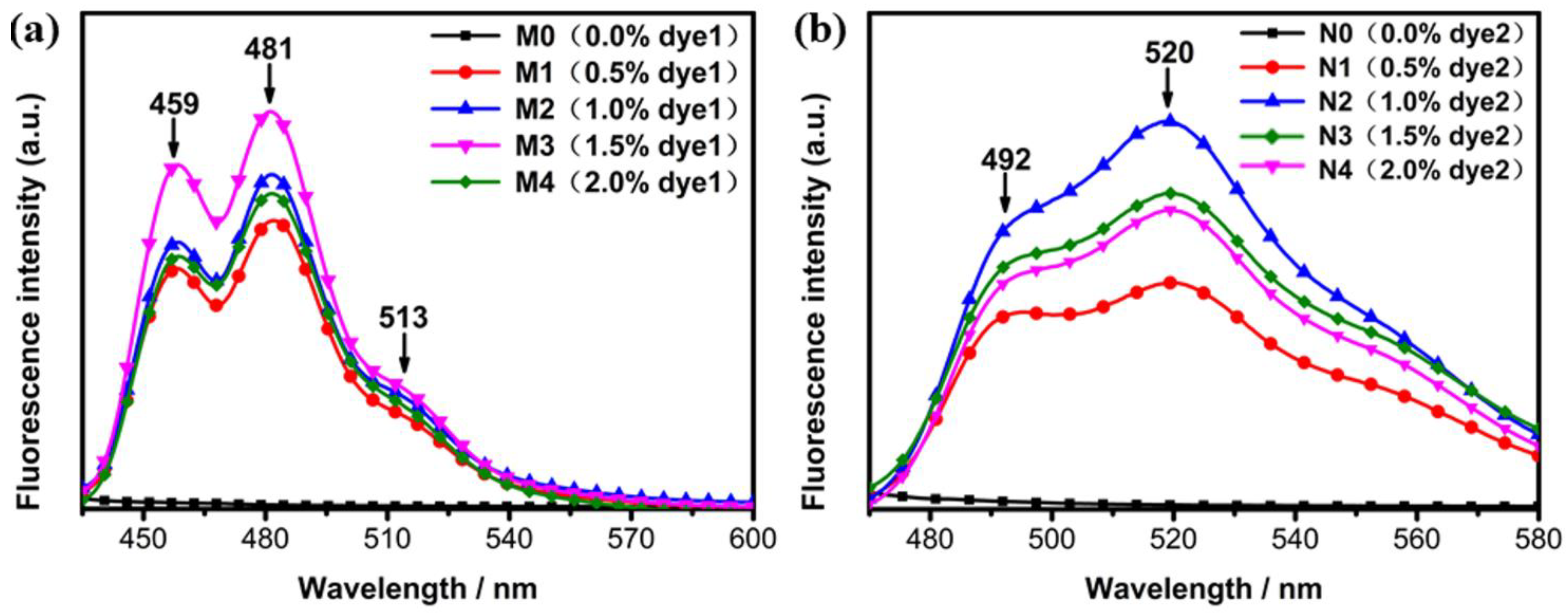
2.2.3. Effect of Fluorescent Dye Concentration on Absorption and Emission Spectra
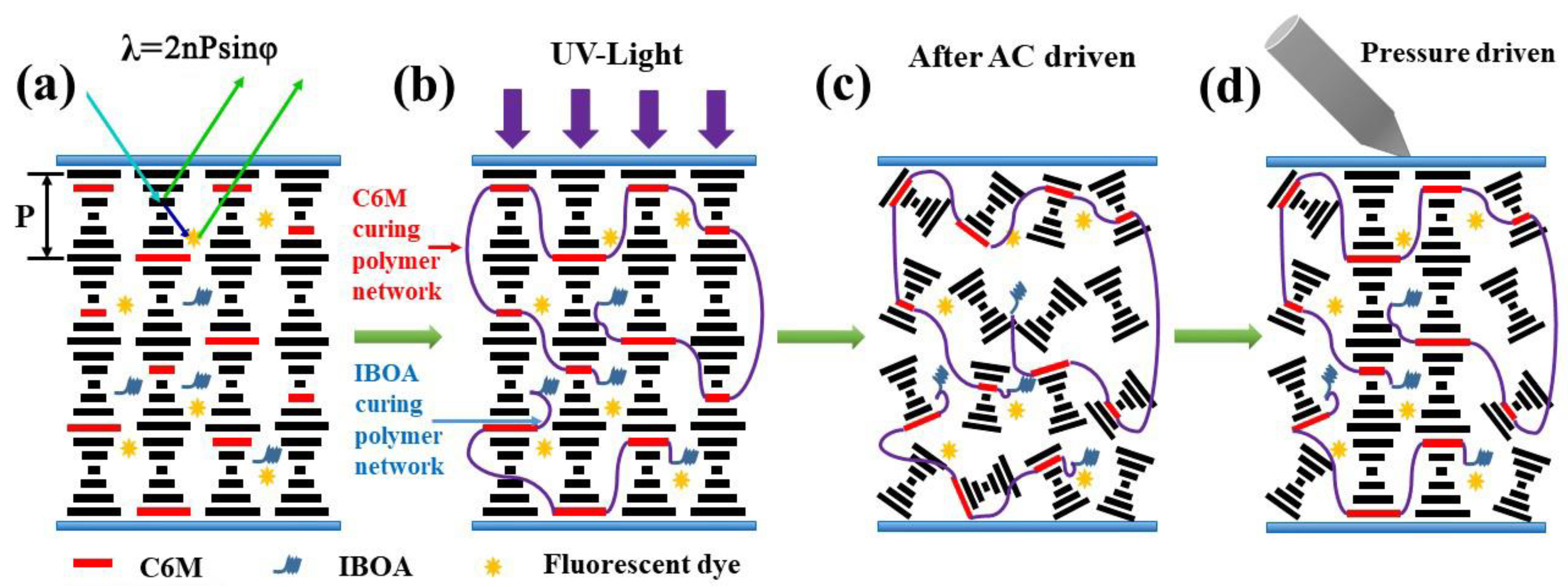
2.3. Mechanism
3. Experimental Section
3.1. Materials
3.2. Preparing the Samples
3.3. Measurements
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Shen, W.-B.; Cao, Y.-P.; Zhang, C.-H.; Yuan, X.-T.; Yang, Z.; Zhang, L.-Y. Network morphology and electro-optical characterisations of epoxy-based polymer stabilised liquid crystals. Liq. Cryst. 2020, 47, 481–488. [Google Scholar] [CrossRef]
- Dierking, I. Polymer network–stabilized liquid crystals. Adv. Mater. 2000, 12, 167–181. [Google Scholar] [CrossRef]
- Miao, Z.-C.; Wang, D. An Electrically and thermally erasable liquid crystal film containing NIR absorbent carbon nanotube. Molecules 2022, 27, 562. [Google Scholar] [CrossRef]
- Saeed, M.-H.; Zhang, S.-F.; Cao, Y.-P.; Zhou, L.; Hu, J.-M.; Muhammad, I.; Xiao, J.-M.; Zhang, L.-Y.; Yang, H. Recent advances in the polymer dispersed liquid crystal composite and its applications. Molecules 2020, 25, 5510. [Google Scholar] [CrossRef]
- Deshmukh, R.-R.; Jain, A.-K. Effect of anti-parallel and twisted alignment techniques on various propertiesof polymer stabilised liquid crystal (PSLC) films. Liq. Cryst. 2016, 43, 436–447. [Google Scholar] [CrossRef]
- Parab, S.-S.; Malik, M.-K.; Bhatia, P.-G.; Deshmukha, R.-R. Investigation of liquid crystal dispersion and dielectric relaxation behavior in polymer dispersed liquid crystal composite films. J. Mol. Liq. 2014, 199, 287–293. [Google Scholar] [CrossRef]
- Lee, K.-M.; Bunning, T.-J.; White, T.-J.; McConney, M.-E.; Godman, N.-P. Effect of Ion Concentration on the Electro-Optic Response in Polymer-Stabilized Cholesteric Liquid Crystals. Crystals 2021, 11, 7. [Google Scholar] [CrossRef]
- Sadigh, M.-K.; Naziri, P.; Zakerhamidi, M.-S.; Ranjkesh, A.; Yoon, T.-H. Temperature dependent features of polymer stabilized cholesteric liquid crystals based on selected liquid crystal characteristics. Optik 2021, 230, 166354. [Google Scholar] [CrossRef]
- He, X.; Lu, H.; Wu, Z.; Song, Z.; Qiu, L.; Wang, X.; Zhang, J.; Hu, J.; Lv, G. Cell gap effects on domain size and electro-optical properties of normal-mode polymer-stabilised cholesteric texture. Liq. Cryst. 2015, 42, 255–260. [Google Scholar] [CrossRef]
- Yang, D.-K. Flexible bistable cholesteric reflective displays. J. Disp. Technol. 2006, 2, 32–37. [Google Scholar] [CrossRef]
- McConney, M.-E.; Tondiglia, V.-P.; Hurtubise, J.-M.; White, J.-M. Photoinduced hyper-reflective cholesteric liquid crystals enabled via surface initiated photopolymerization. Chem. Commun. 2011, 47, 505–507. [Google Scholar] [CrossRef]
- Hsiao, Y.-C.; Timofeev, I.-V.; Zyryanov, V.-Y.; Wei, L. Hybrid anchoring for a color-reflective dual-frequency cholesteric liquid crystal device switched by low voltages. Opt. Mater. Express 2015, 5, 2715–2720. [Google Scholar] [CrossRef]
- Oh, S.-W.; Baek, J.-M.; Heo, J.; Yoon, T.-H. Dye-doped cholesteric liquid crystal light shutter with a polymer-dispersed liquid crystal film. Dye. Pigment. 2016, 134, 36–40. [Google Scholar] [CrossRef]
- Bao, R.; Liu, C.; Yang, D.-K. Smart bistable polymer stabilized cholesteric texture light shutter. Appl. Phys. Express 2009, 2, 112401. [Google Scholar] [CrossRef]
- Chen, Q.; Peng, Z.; Li, Y.; Liu, S.-X.; Zhou, P.-C.; Gu, J.-Y.; Lu, J.-G.; Yao, L.-S.; Wang, M.; Su, Y.-K. Multi-plane augmented reality display based on cholesteric liquid crystal reflective films. Opt. Express 2019, 27, 12039–12047. [Google Scholar] [CrossRef]
- Ma, J.; Shi, L.; Yang, D.-K. Bistable polymer stabilized cholesteric texture light shutter. Appl. Phys. Express 2010, 3, 021702. [Google Scholar] [CrossRef]
- Deshmukh, R.-R.; Jain, A.-K. The complete morphological, electro-optical and dielectric study of dichroic dye-doped polymer-dispersed liquid crystal. Liq. Cryst. 2014, 41, 960–975. [Google Scholar] [CrossRef]
- Huh, J.-W.; Ji, S.-M.; Heo, J.; Yu, B.-H.; Yoon, T.-H. Bistable light shutter using dye-doped cholesteric liquid crystals driven with crossed patterned electrodes. J. Disp. Technol. 2016, 12, 779–783. [Google Scholar] [CrossRef]
- Baliyan, V.-K.; Jeong, K.-U.; Kang, S.-W. Dichroic-dye-doped short pitch cholesteric liquid crystals for the application of electrically switchable smart windows. Dye. Pigment. 2019, 166, 403–409. [Google Scholar] [CrossRef]
- Ren, H.-W.; Xu, S.; Wu, S.-T. Gradient polymer network liquid crystal with a large refractive index change. Opt. Express 2012, 20, 26464–26472. [Google Scholar] [CrossRef]
- Ahmad, F.; Jamil, M.; Lee, J.-W.; Kim, S.-R.; Jeon, Y.-J. The effect of UV intensities and curing time on polymer dispersed liquid crystal (PDLC) display: A detailed analysis study. Electron. Mater. Lett. 2016, 12, 685–692. [Google Scholar] [CrossRef]
- Wang, F.-F.; Li, K.-X.; Song, P.; Wu, X.-J.; Cao, H.; Yang, H. Photoinduced pitch gradients and the reflection behaviour of the broadband films: Influence of dye concentration, light intensity, temperature and monomer concentration. Liq. Cryst. 2012, 39, 707–714. [Google Scholar] [CrossRef]
- Yuan, W.-Z.; Lu, P.; Chen, S.-M.; Lam, J.-W.Y.; Wang, Z.-M.; Liu, Y.; Kwok, H.-S.; Ma, Y.-G.; Tang, B.-Z. Changing the behavior of chromophores from aggregation-caused quenching to aggregation-induced emission: Development of highly efficient light emitters in the solid state. Adv. Mater. 2010, 22, 2159–2163. [Google Scholar] [CrossRef]
- Brisk, J.-B. Photophysics of Aromatic Molecules; Wiley: London, UK, 1970. [Google Scholar]
- Xiao, J.-M.; Cao, H.; Zhao, D.-Y.; Yang, H. Effect of shearing stress on reflective properties of cholesteric liquid crystal films. J. Appl. Polym. Sci. 2010, 118, 1894–1897. [Google Scholar] [CrossRef]
- Miao, Z.-C.; Chen, X.-L.; Zhang, Y.-T.; Wang, D.; Wang, L. Bistable Cholesteric Liquid Crystal Films with Excellent Electro-Optical Performance and Spacing Stability for Reflective Displays. ACS Appl. Polym. Mater. 2022, 5, 476–484. [Google Scholar] [CrossRef]
- Sheng, M.-F.; Li, J.-J.; Jiang, X.-J.; Wang, C.-C.; Li, J.-H.; Zhang, L.-P.; Fu, S.-H. Biomimetic solid-liquid transition structural dye-doped liquid crystal/phase-change-material microcapsules designed for wearable bistable electrochromic fabric. ACS Appl. Mater. Interfaces 2021, 13, 33282–33290. [Google Scholar] [CrossRef]
- Liu, C.-Y.; Yen, C.-F.; Hung, Y.-H.; Tu, C.-M.; Wu, G.-Y.; Chen, H.-Y. Polymer-stabilized bistable dual-frequency cholesteric liquid crystal devices assisted by a predesigned chiral dopant. J. Mater. Chem. C 2021, 9, 16672–16681. [Google Scholar] [CrossRef]
- Kocakülah, G.; Koysal, O.; Kahyaoglu, A. Electro-optical performance investigation of cholesteric liquid crystal containing azo dye: Light shutter device application. J. Electron. Mater. 2021, 50, 497–510. [Google Scholar] [CrossRef]
- Oh, S.-W.; Ji, S.-M.; Han, C.-H.; Yoon, T.-H. A cholesteric liquid crystal smart window with a low operating voltage. Dye. Pigment. 2022, 197, 109843. [Google Scholar] [CrossRef]
- He, Z.-M.; Zeng, J.-T.; Zhu, S.-T.; Zhang, D.-X.; Ma, C.; Zhang, C.-H.; Yu, P.; Miao, Z.-C. A bistable light shutter based on polymer stabilized cholesteric liquid crystals. Opt. Mater. 2023, 136, 113426. [Google Scholar] [CrossRef]



| Samples | IBOA | C6M | DYE10900 | IRG651 | UV Intensity (mW/cm2) |
|---|---|---|---|---|---|
| A | 7.50 | 0.50 | 91.68 | 0.32 | 3.00 |
| B | 4.50 | 1.50 | 93.76 | 0.24 | 3.00 |
| C1 | 4.00 | 1.00 | 94.80 | 0.20 | 1.00 |
| C2 | 4.00 | 1.00 | 94.80 | 0.20 | 2.00 333 |
| C3 | 4.00 | 1.00 | 94.80 | 0.20 | 3.00 |
| C4 | 4.00 | 1.00 | 94.80 | 0.20 | 4.00 |
| D | 5.00 | 3.00 | 91.68 | 0.32 | 3.00 |
| Reference | Materials | Dopants | Outcomes/Performance |
|---|---|---|---|
| M.-F. Sheng. (2021) [27] |
|
|
|
| C.-Y.Liu (2021) [28] |
|
|
|
| G. Kocakülah (2021) [29] |
|
|
|
| S.-W. Oh (2022) [30] |
|
|
|
| Z.-M. He (2023) [31] |
|
|
|
| Present (reported here) work |
|
|
|
| Sample | DYE10900 (wt%) | R5011 (wt%) | Bandwidth (nm) | Center Wavelength (nm) |
|---|---|---|---|---|
| LC1 | 99.6 | 0.4 | 440–510 | 475 |
| LC2 | 99.8 | 0.2 | 480–550 | 515 |
| Samples | IBOA | C6M | IRG651 | LC | Fluorescent Dyes | ||
|---|---|---|---|---|---|---|---|
| LC1 | LC2 | Dye1 | Dye2 | ||||
| M0 | 4.0 | 1.0 | 0.2 | 94.8 | 0.0 | 0.0 | 0.0 |
| M1 | 4.0 | 1.0 | 0.2 | 94.3 | 0.0 | 0.5 | 0.0 |
| M2 | 4.0 | 1.0 | 0.2 | 93.8 | 0.0 | 1.0 | 0.0 |
| M3 | 4.0 | 1.0 | 0.2 | 93.3 | 0.0 | 1.5 | 0.0 |
| M4 | 4.0 | 1.0 | 0.2 | 92.8 | 0.0 | 2.0 | 0.0 |
| N0 | 4.0 | 1.0 | 0.2 | 0.0 | 94.8 | 0.0 | 0.0 |
| N1 | 4.0 | 1.0 | 0.2 | 0.0 | 94.3 | 0.0 | 0.5 |
| N2 | 4.0 | 1.0 | 0.2 | 0.0 | 93.8 | 0.0 | 1.0 |
| N3 | 4.0 | 1.0 | 0.2 | 0.0 | 93.3 | 0.0 | 1.5 |
| N4 | 4.0 | 1.0 | 0.2 | 0.0 | 92.8 | 0.0 | 2.0 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhao, Y.; Li, C.; Lang, T.; Sun, Y.; Li, Q.; Shi, X.; Yao, R.; Zhang, H.; Zhao, Y. Fluorescent Dye-Doped Brightening Polymer-Stabilized Bistable Cholesteric Liquid Crystal Films. Molecules 2023, 28, 3509. https://doi.org/10.3390/molecules28083509
Zhao Y, Li C, Lang T, Sun Y, Li Q, Shi X, Yao R, Zhang H, Zhao Y. Fluorescent Dye-Doped Brightening Polymer-Stabilized Bistable Cholesteric Liquid Crystal Films. Molecules. 2023; 28(8):3509. https://doi.org/10.3390/molecules28083509
Chicago/Turabian StyleZhao, Yuzhen, Chaonian Li, Tingting Lang, Yitian Sun, Qingbo Li, Xinli Shi, Ruijuan Yao, Huimin Zhang, and Yang Zhao. 2023. "Fluorescent Dye-Doped Brightening Polymer-Stabilized Bistable Cholesteric Liquid Crystal Films" Molecules 28, no. 8: 3509. https://doi.org/10.3390/molecules28083509
APA StyleZhao, Y., Li, C., Lang, T., Sun, Y., Li, Q., Shi, X., Yao, R., Zhang, H., & Zhao, Y. (2023). Fluorescent Dye-Doped Brightening Polymer-Stabilized Bistable Cholesteric Liquid Crystal Films. Molecules, 28(8), 3509. https://doi.org/10.3390/molecules28083509





