N-, O- and S-Heterocycles Synthesis in Deep Eutectic Solvents
Abstract
1. Introduction
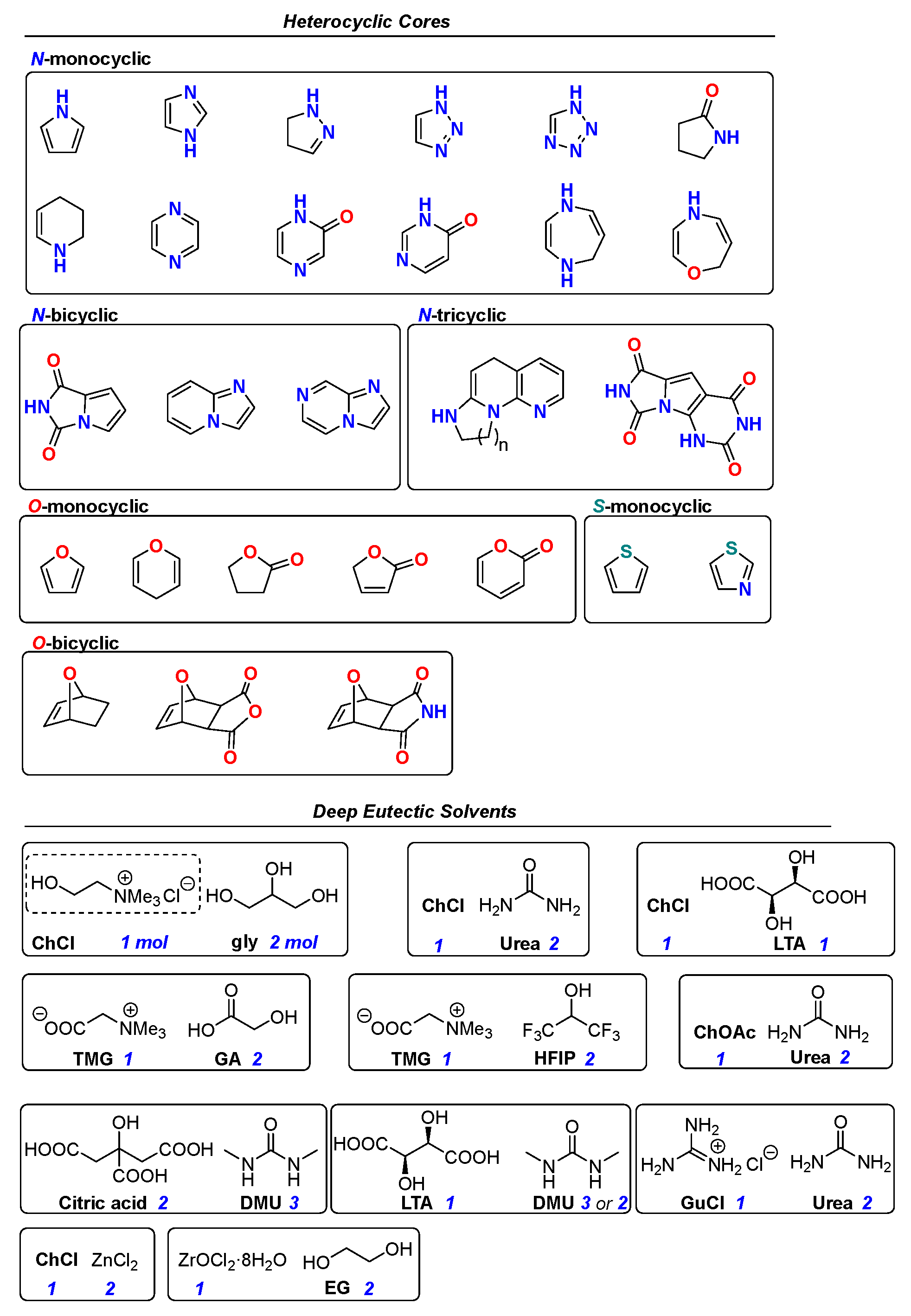
2. Preparation of N-Heterocycles in DES Mixtures
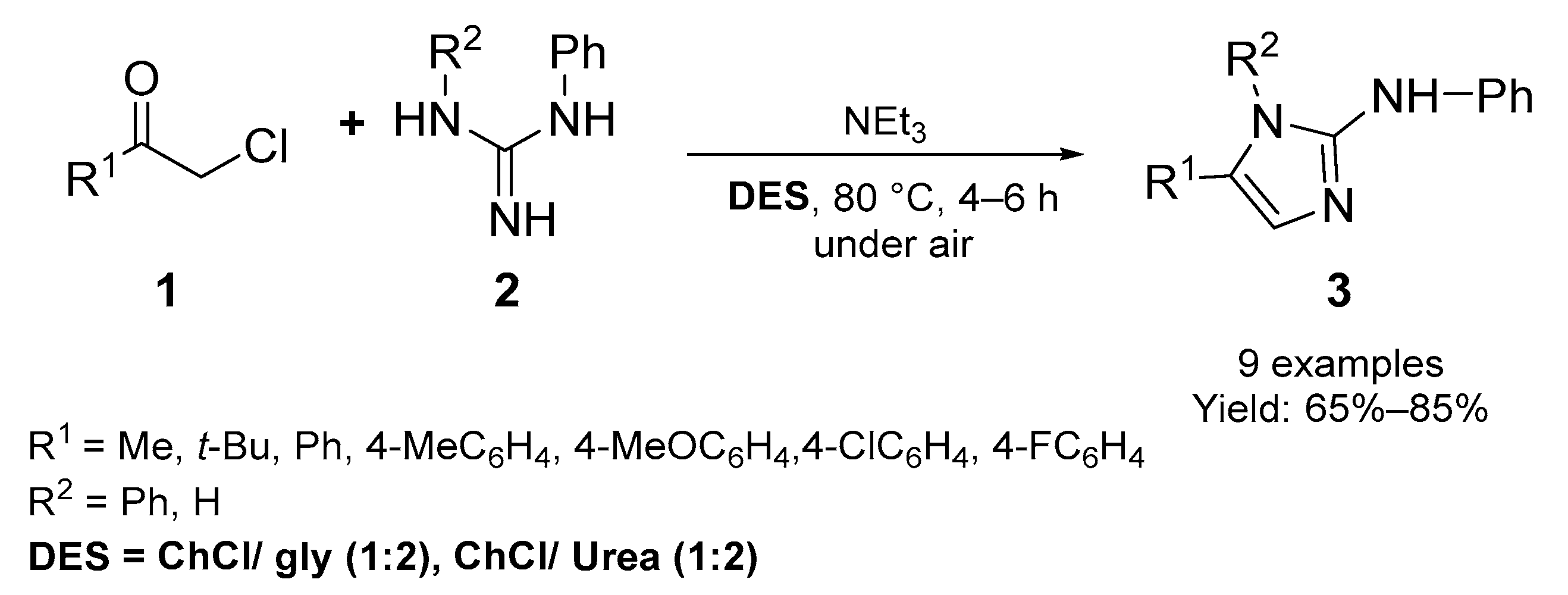
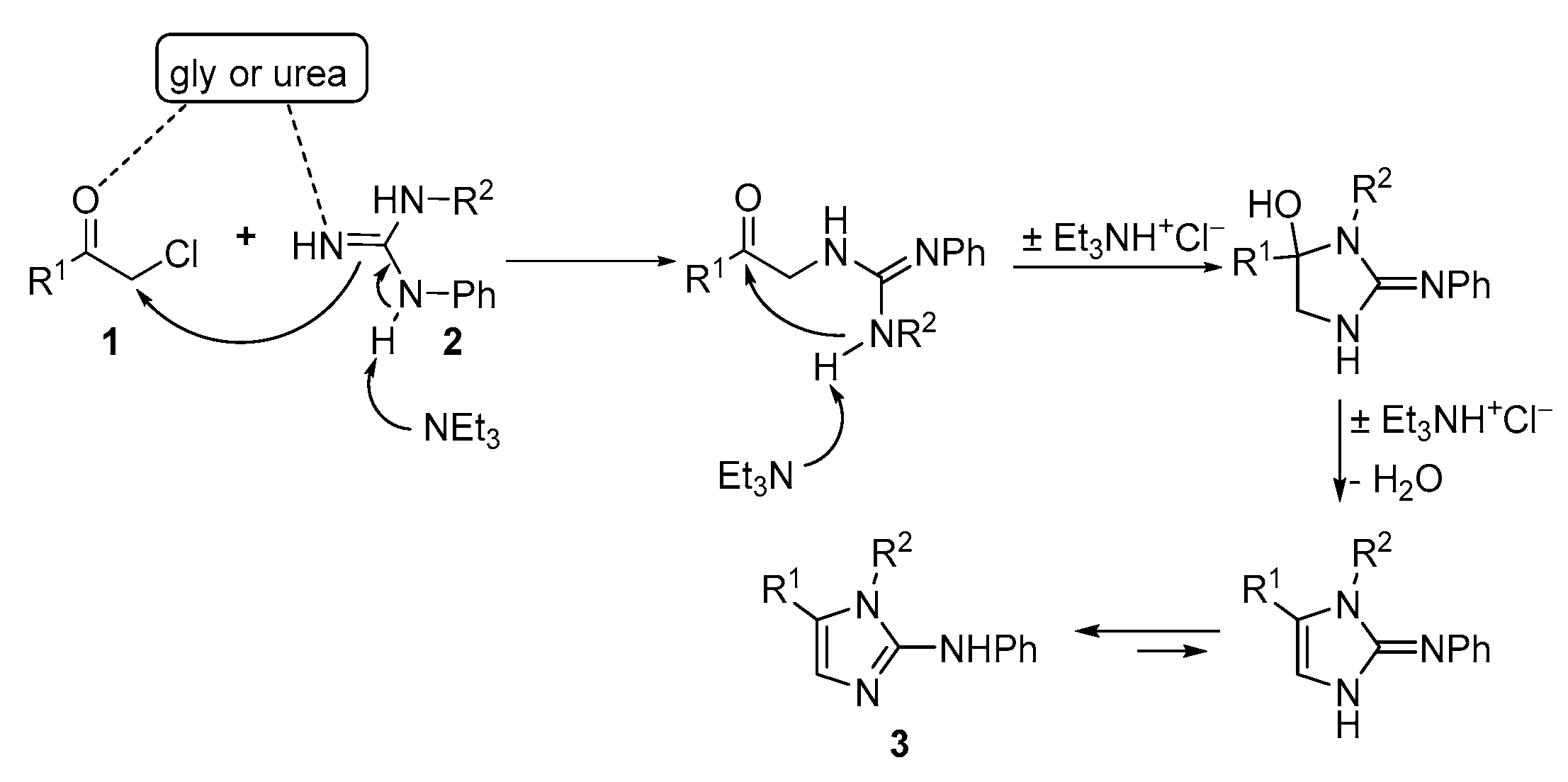
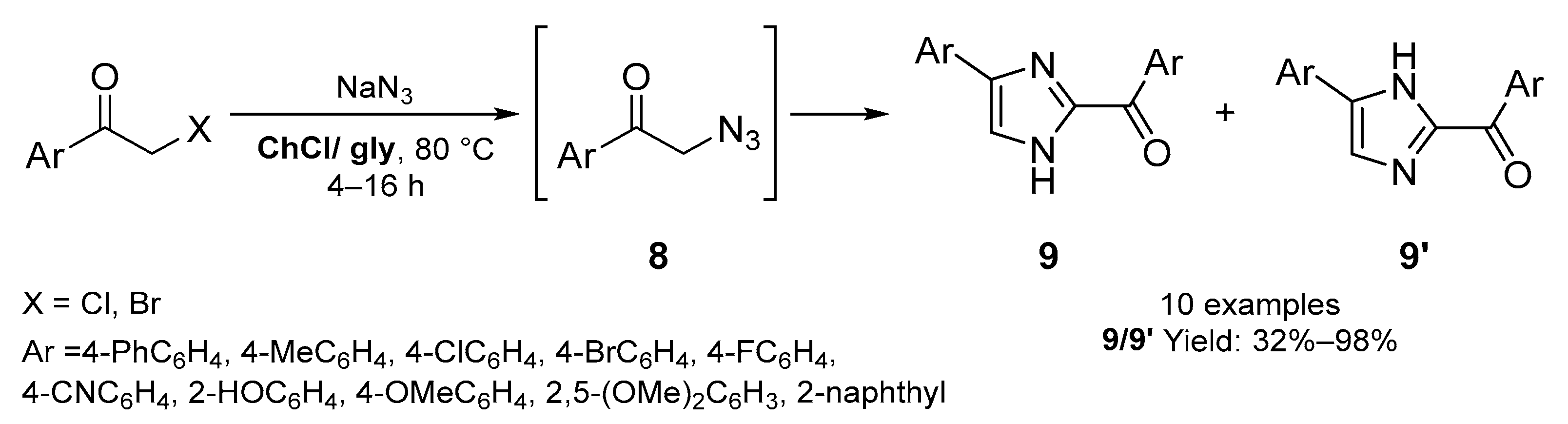
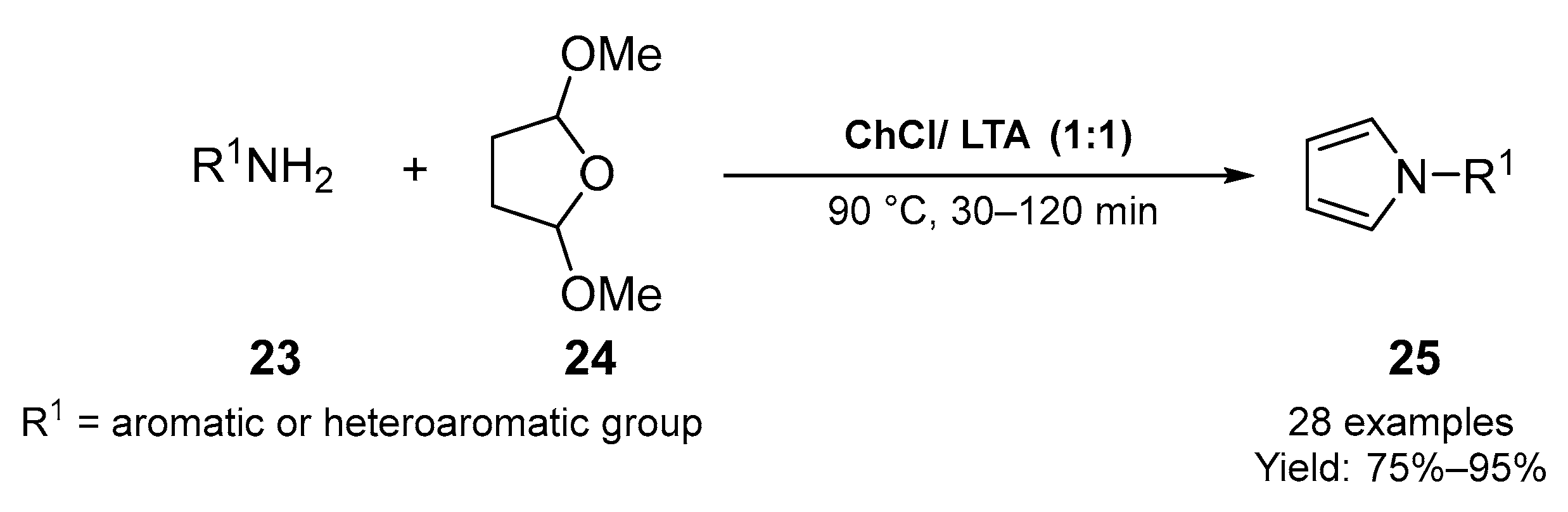
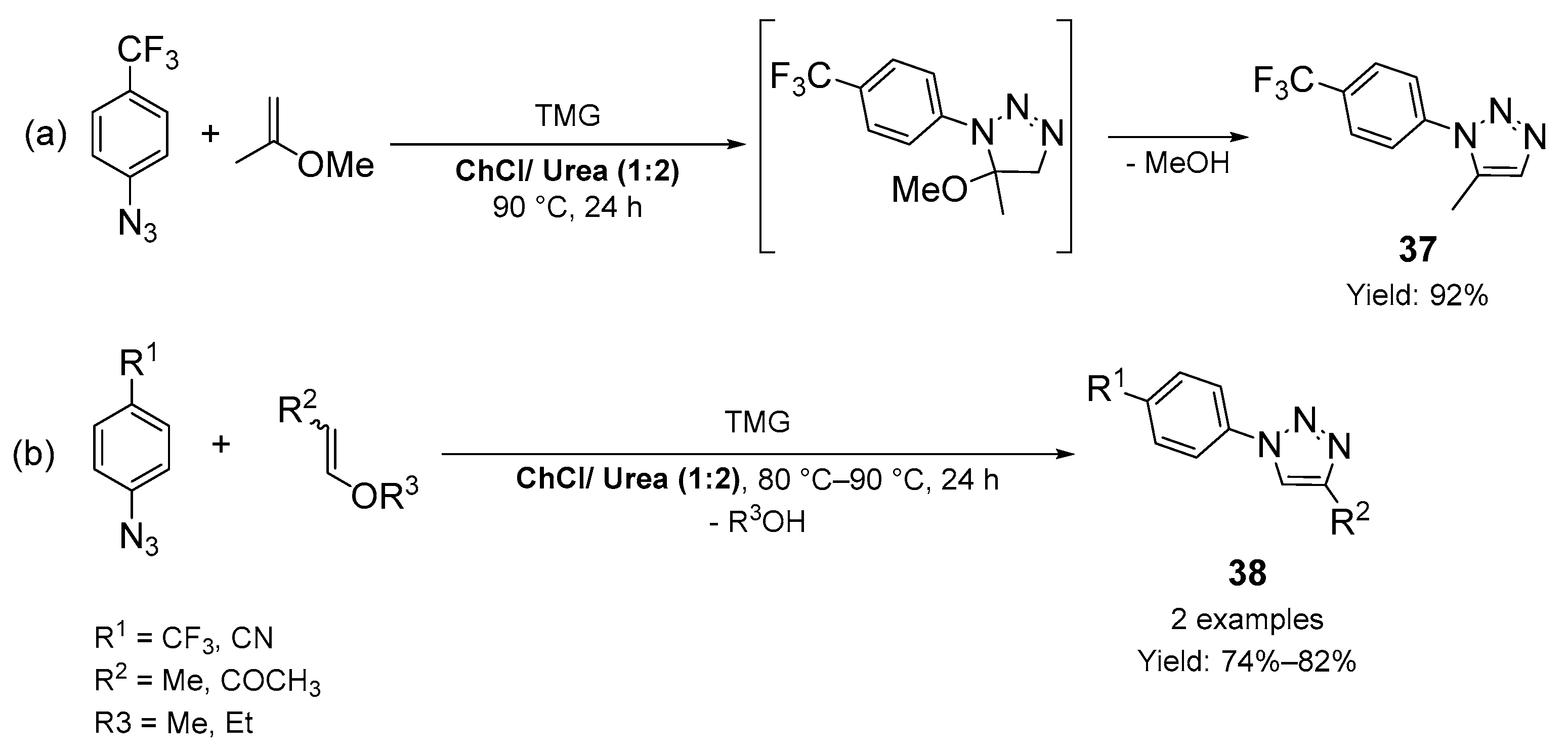
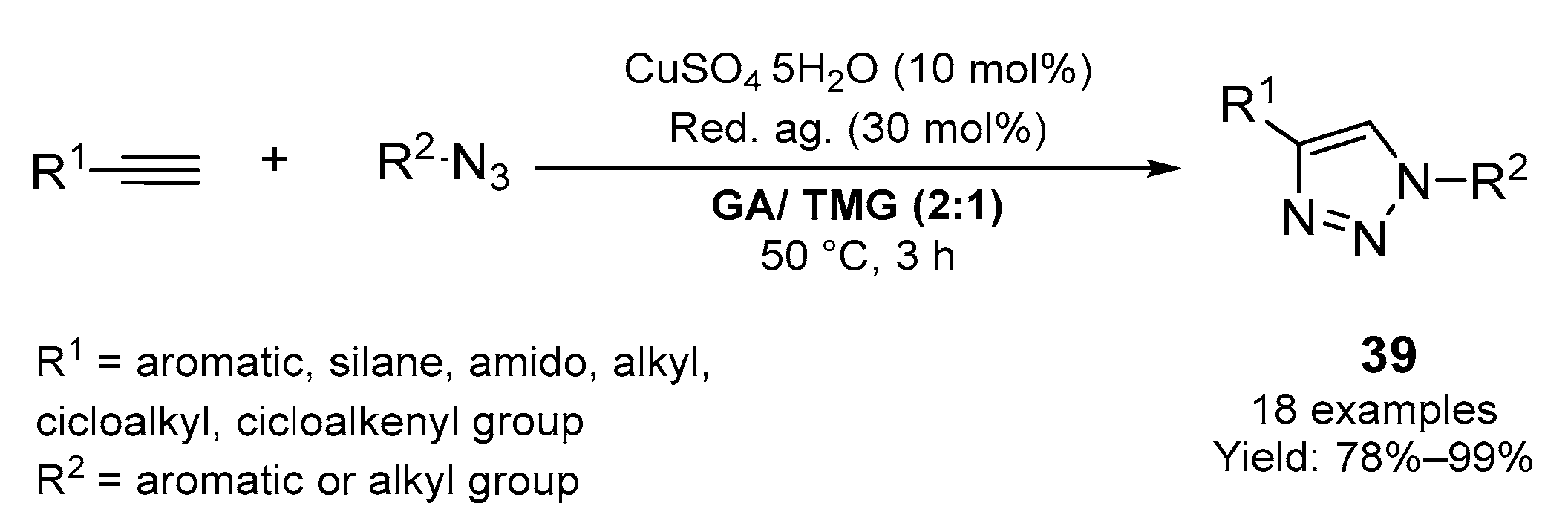
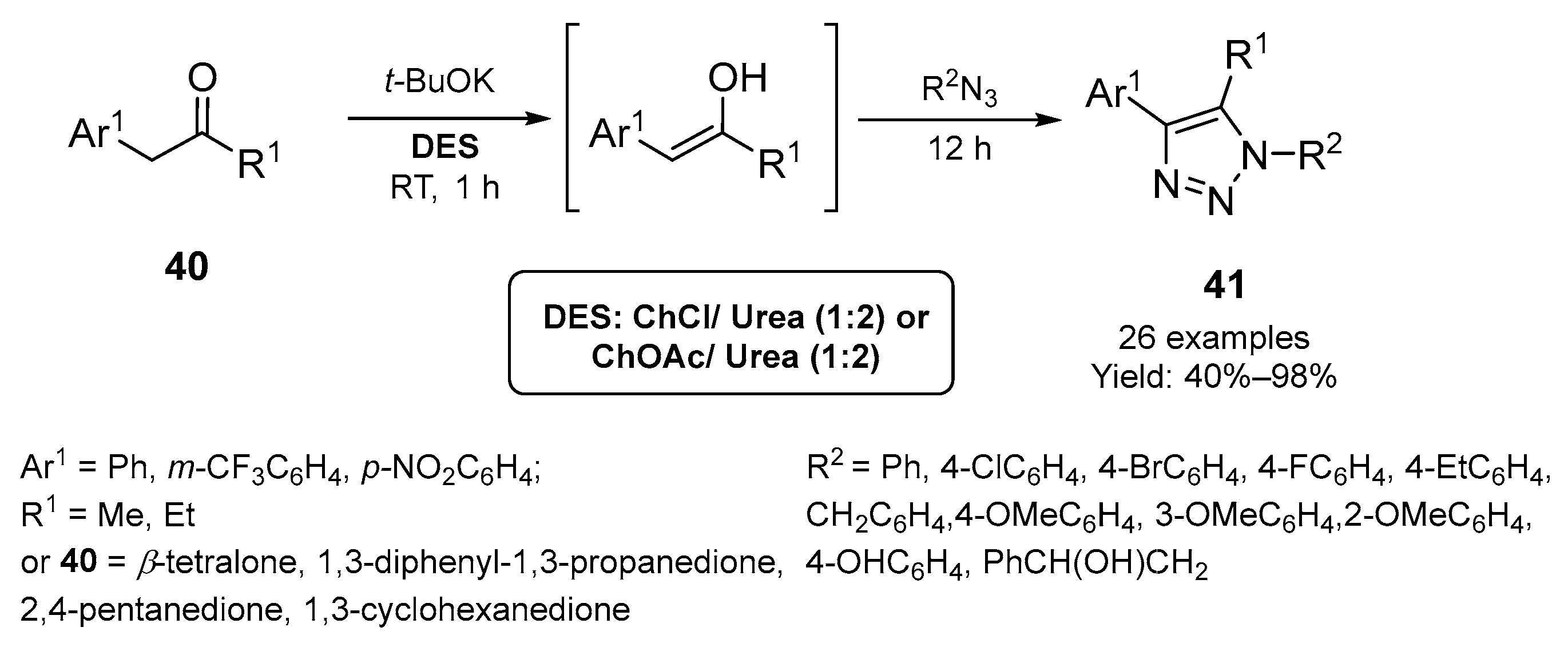
2.1. Synthesis of Five-Membered N-Heterocycles
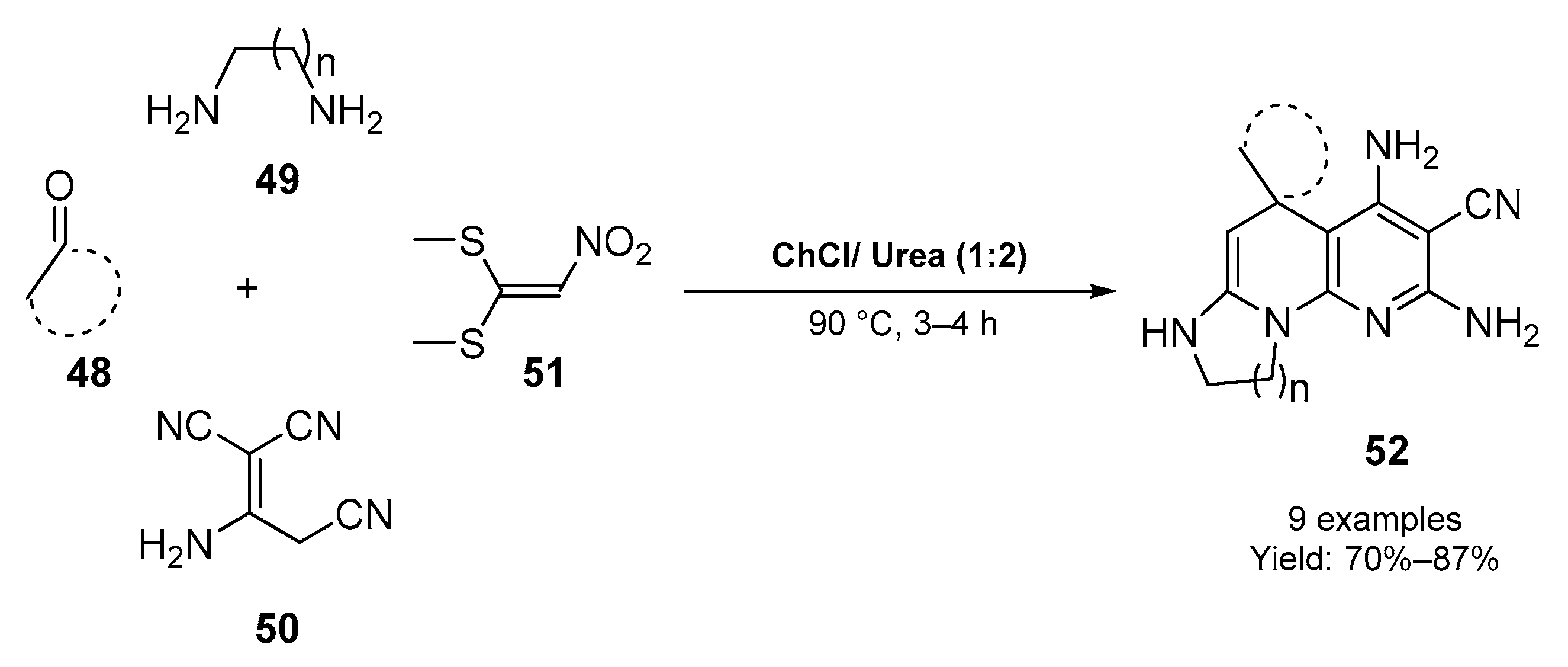
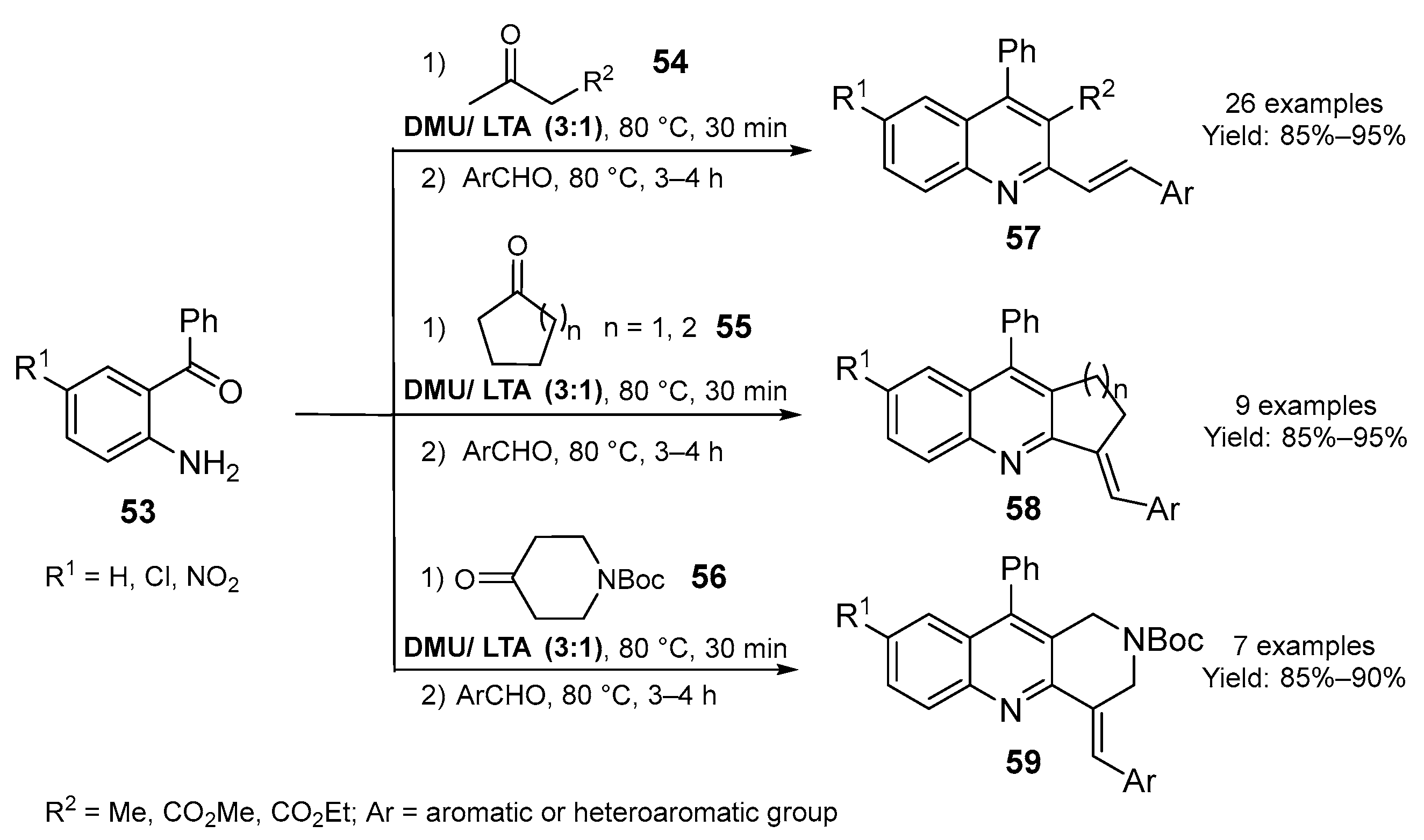
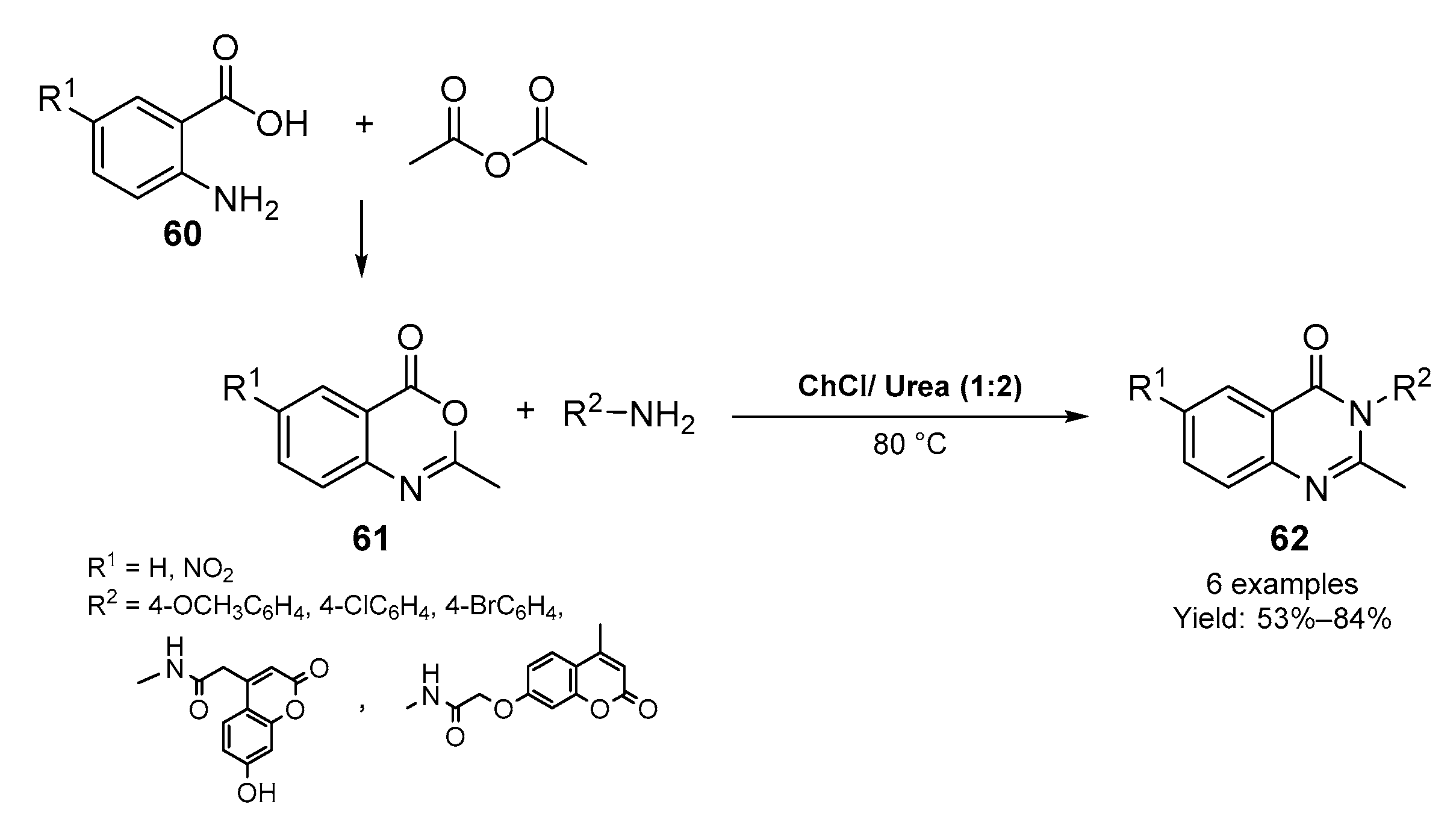
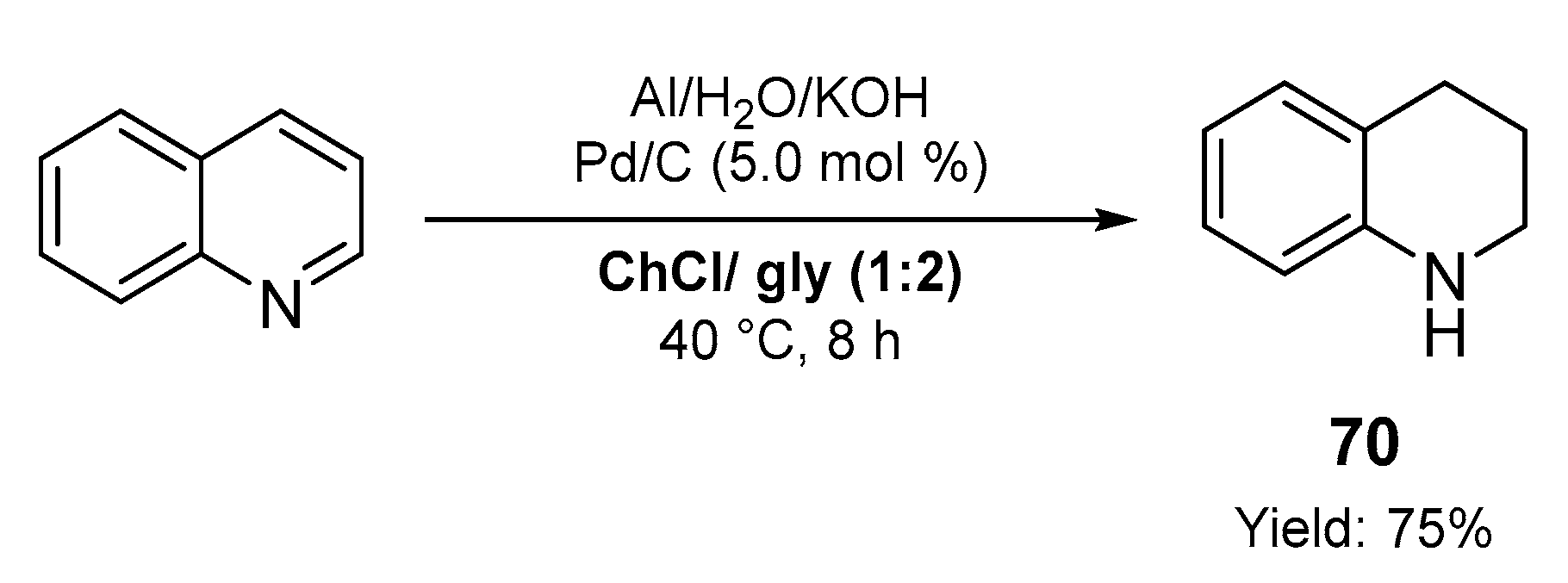
2.2. Synthesis of Six- and Seven- Membered N-Heterocycles
3. Preparation of O- and S-Heterocycles in DES Mixtures
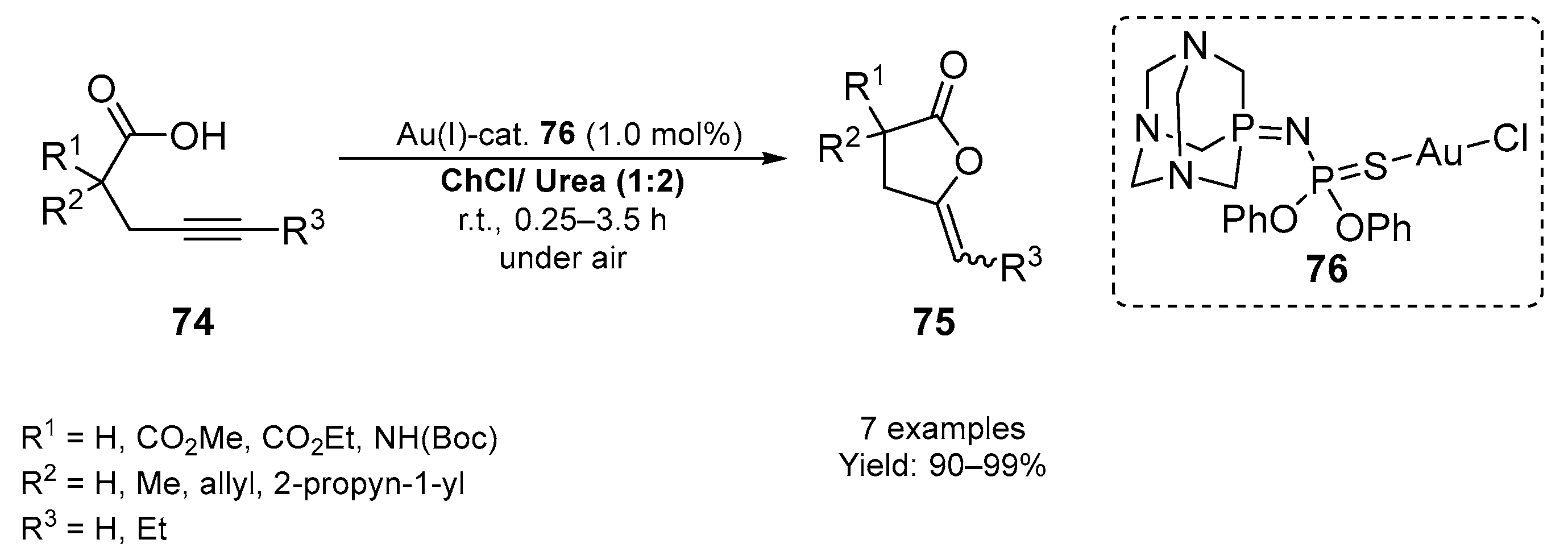
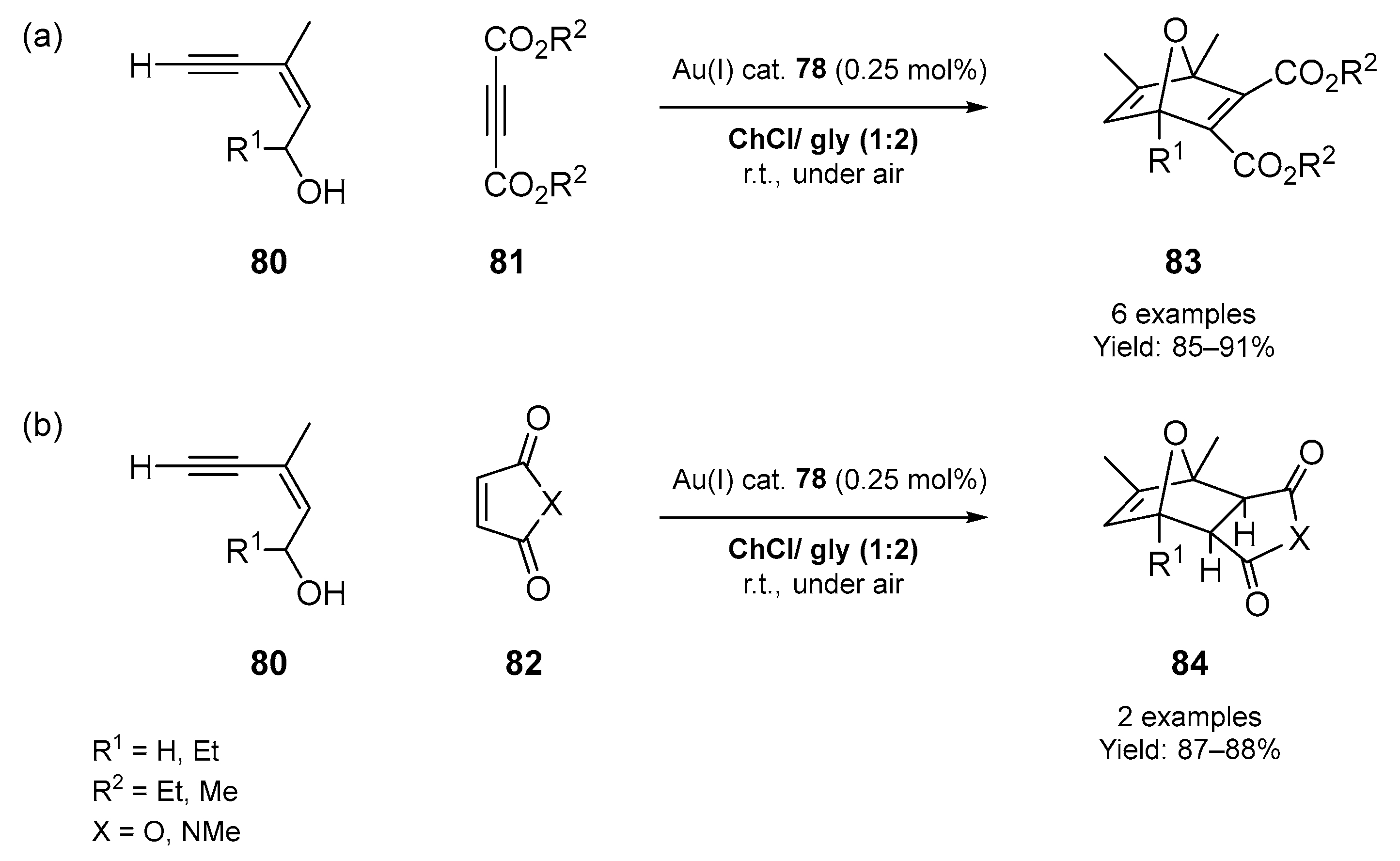
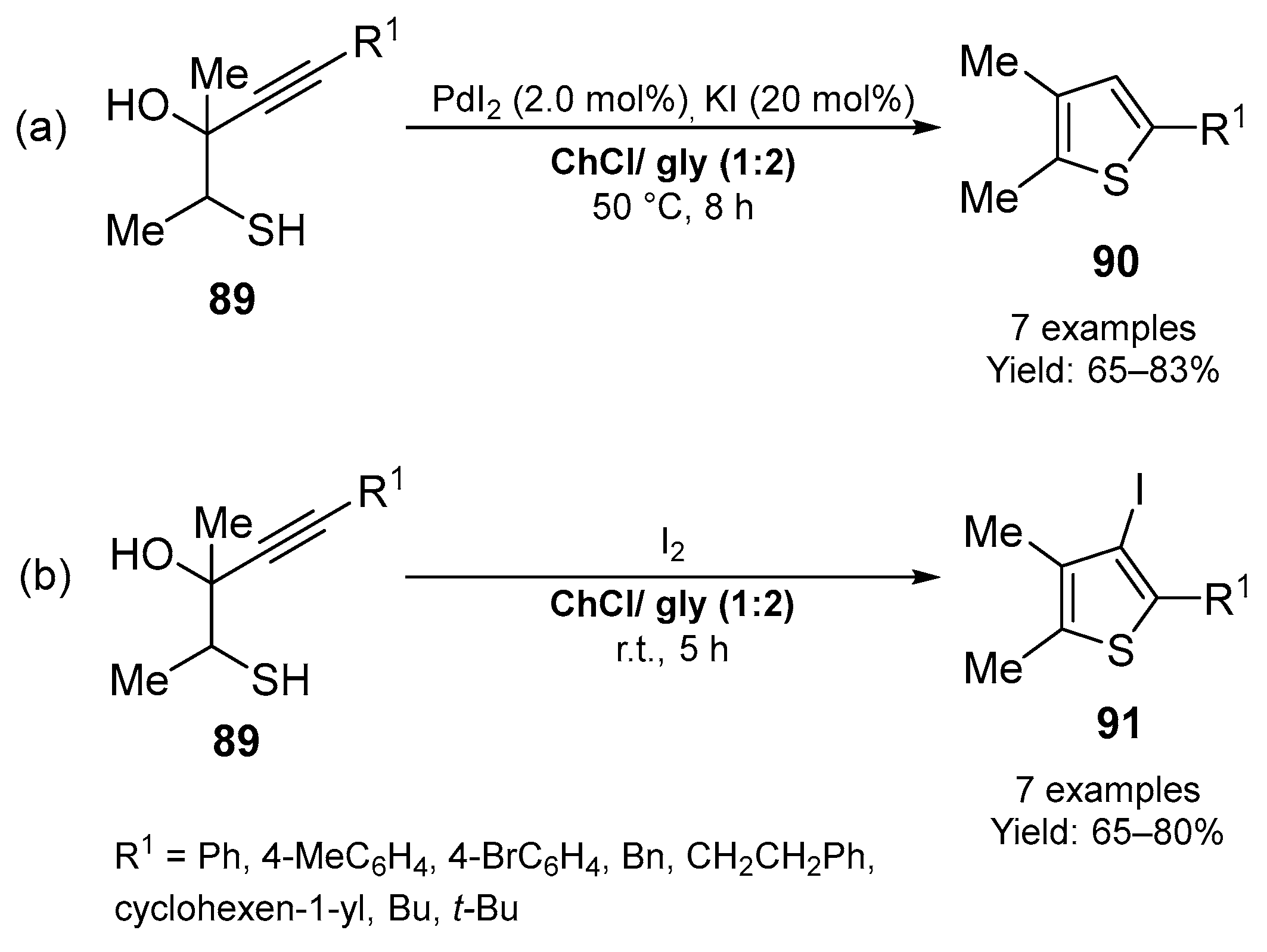
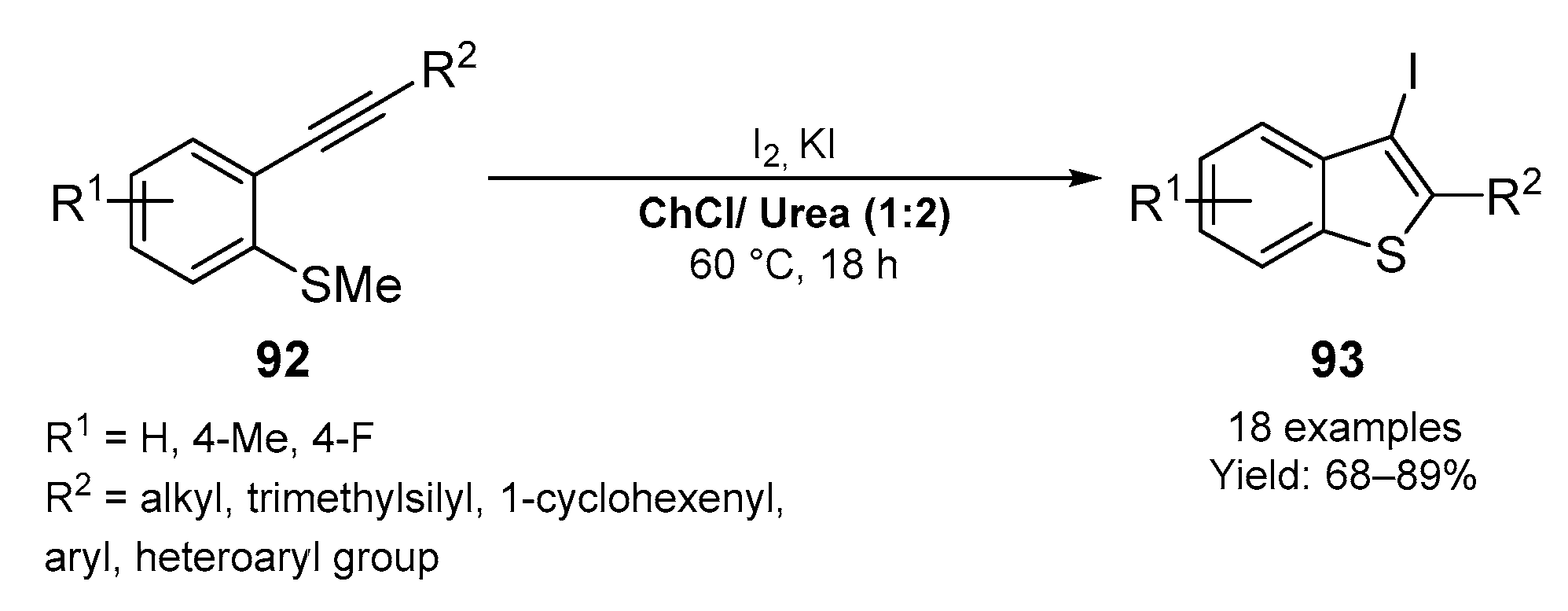
3.1. Synthesis of Five-Membered O- and S-Heterocycles
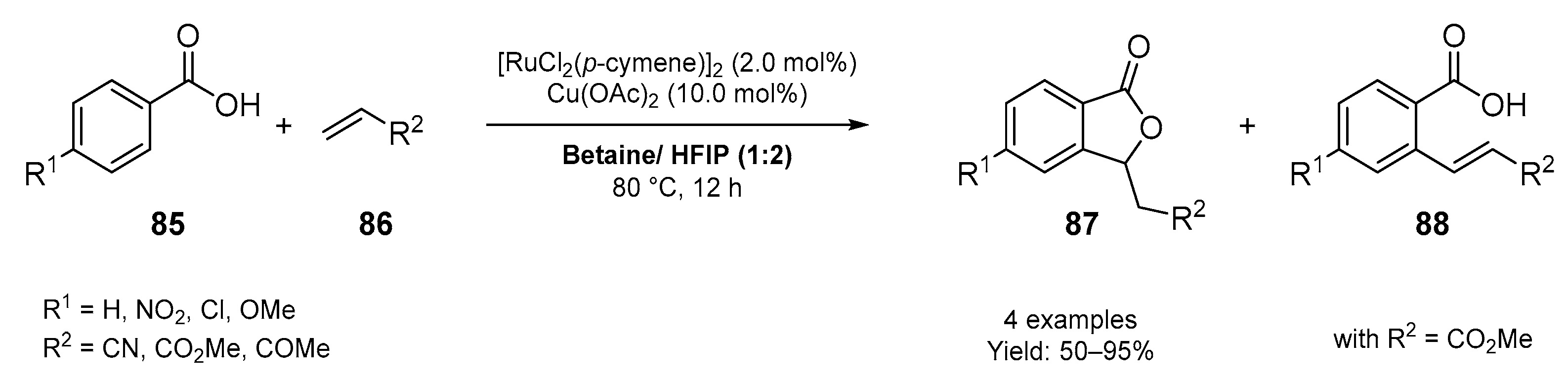
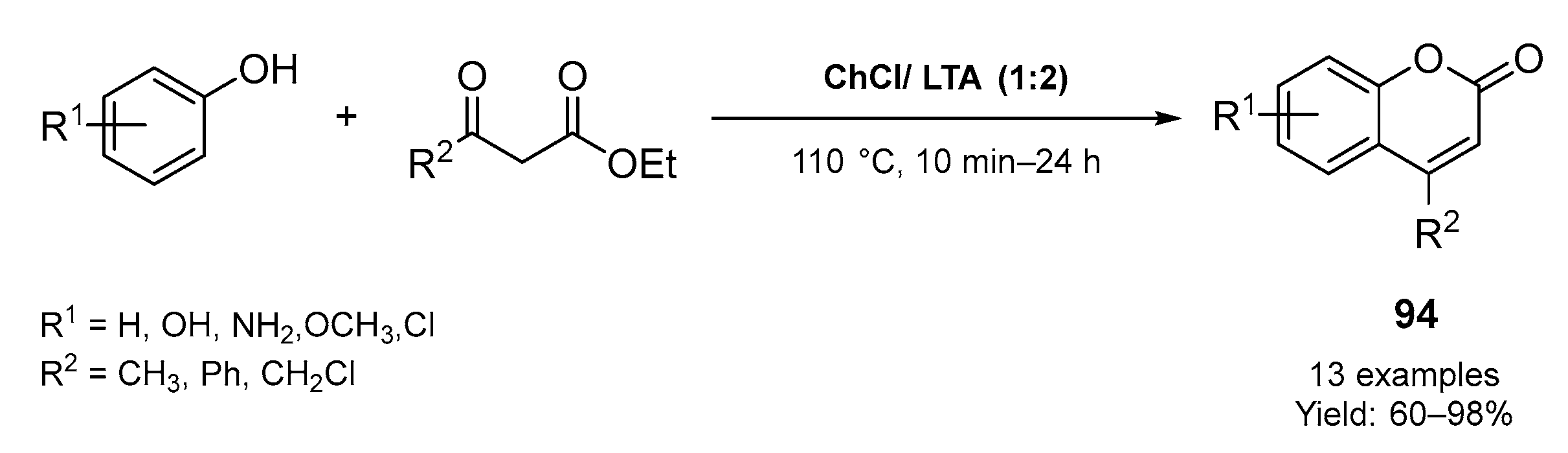
3.2. Synthesis of Six-Membered O-Heterocycles
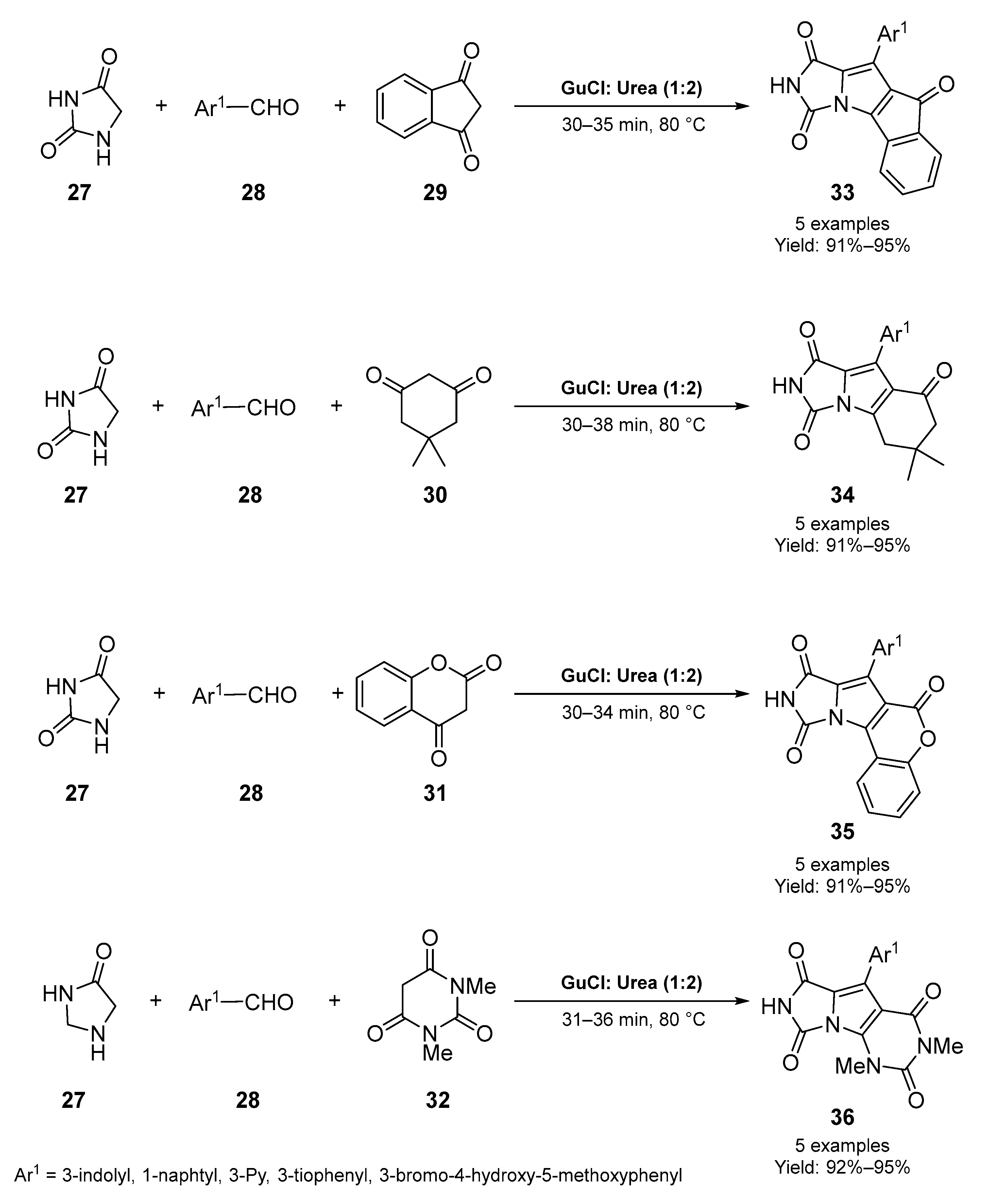
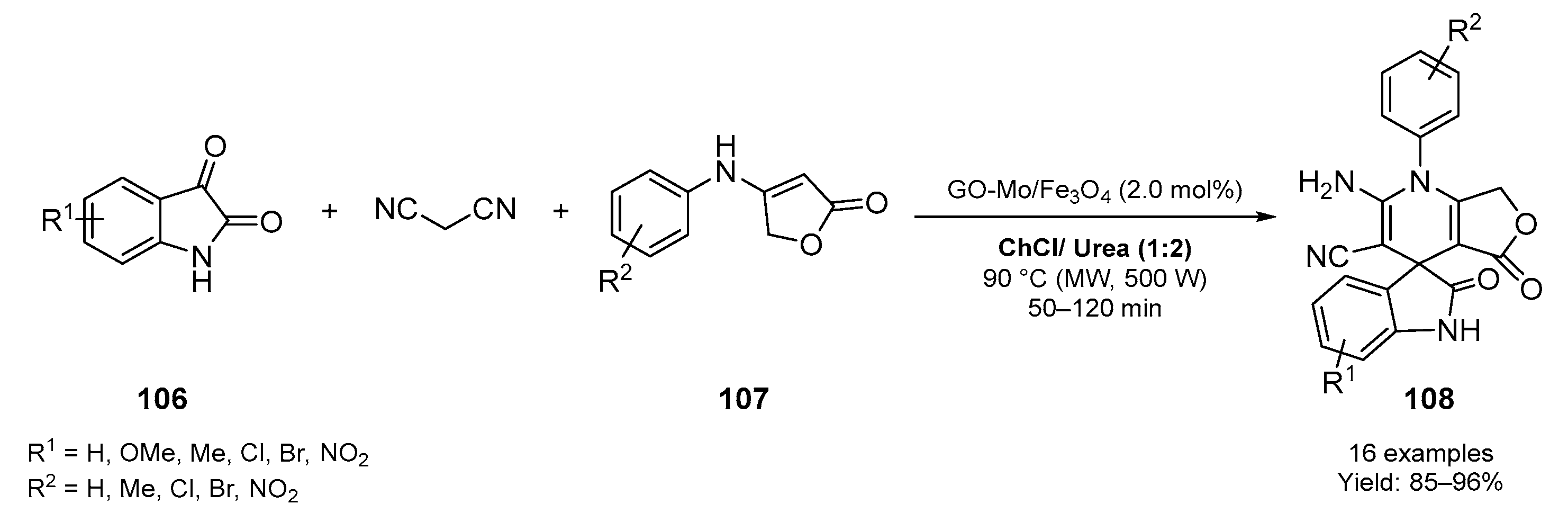
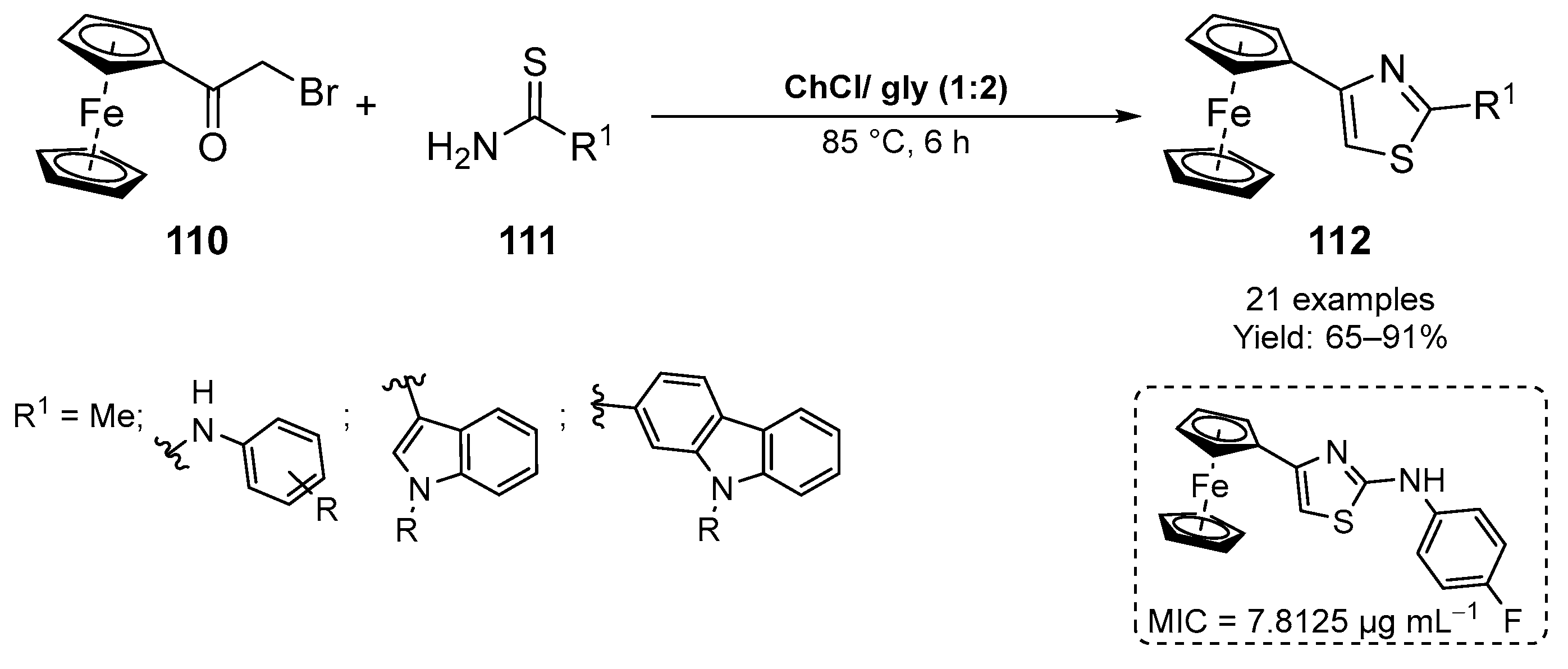
4. Miscellaneous
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Eicher, T.; Hauptmann, S.; Speicher, A. The Chemistry of Heterocycles: Structures, Reactions, Synthesis, and Applications; John Wiley & Sons: Hoboken, NJ, USA, 2003. [Google Scholar]
- Khandelwal, S.; Tailor, Y.K.; Kumar, M. Deep Eutectic Solvents (DESs) as Eco-Friendly and Sustainable Solvent/Catalyst Systems in Organic Transformations. J. Mol. Liq. 2016, 215, 345–386. [Google Scholar] [CrossRef]
- Smith, E.L.; Abbott, A.P.; Ryder, K.S. Deep Eutectic Solvents (DESs) and Their Applications. Chem. Rev. 2014, 114, 11060–11082. [Google Scholar] [CrossRef] [PubMed]
- Anastas, P.; Eghbali, N. Green Chemistry: Principles and Practice. Chem. Soc. Rev. 2009, 39, 301–312. [Google Scholar] [CrossRef] [PubMed]
- Abbott, A.P.; Capper, G.; Davies, D.L.; Rasheed, R.K.; Tambyrajah, V. Novel Solvent Properties of Choline Chloride/Urea Mixtures. Chem. Commun. 2003, 70–71. [Google Scholar] [CrossRef] [PubMed]
- Alonso, D.A.; Baeza, A.; Chinchilla, R.; Guillena, G.; Pastor, I.M.; Ramón, D.J. Deep Eutectic Solvents: The Organic Reaction Medium of the Century. Eur. J. Org. Chem. 2016, 2016, 612–632. [Google Scholar] [CrossRef]
- Abranches, D.O.; Coutinho, J.A.P. Type V Deep Eutectic Solvents: Design and Applications. Curr. Opin. Green Sustain. Chem. 2022, 35, 100612. [Google Scholar] [CrossRef]
- Ratnani, S.; Bargujar, S.; Khulbe, M.; Kathuria, A. Applications of Choline Chloride-Based Deep Eutectic Solvents as Sustainable Media and Catalyst in the Synthesis of Heterocyclic Scaffolds. Curr. Org. Chem. 2022, 26, 745–755. [Google Scholar]
- Siwach, A.; Verma, P.K. Synthesis and Therapeutic Potential of Imidazole Containing Compounds. BMC Chem. 2021, 15, 12. [Google Scholar] [CrossRef]
- Jung, F.; Olivier, A.; Boucherot, D.; Loftus, F. A New Approach to the Synthesis of Amino Imidazoles Application to the Cephalosporin Series. Tetrahedron Lett. 1989, 30, 2379–2382. [Google Scholar] [CrossRef]
- Munk, S.A.; Harcourt, D.A.; Arasasingham, P.N.; Burke, J.A.; Kharlamb, A.B.; Manlapaz, C.A.; Padillo, E.U.; Roberts, D.; Runde, E.; Williams, L.; et al. Synthesis and Evaluation of 2-(Arylamino)Imidazoles as A2-Adrenergic Agonists. J. Med. Chem. 1997, 40, 18–23. [Google Scholar] [CrossRef]
- Assmann, M.; Lichte, E.; Pawlik, J.R.; Köck, M. Chemical Defenses of the Caribbean Sponges Agelas Wiedenmayeri and Agelas Conifera. Mar. Ecol. Prog. Ser. 2000, 207, 255–262. [Google Scholar] [CrossRef]
- Yamada, A.; Kitamura, H.; Yamaguchi, K.; Fukuzawa, S.; Kamijima, C.; Yazawa, K.; Kuramoto, M.; Wang, G.Y.S.; Fujitani, Y.; Uemura, D. Development of Chemical Substances Regulating Biofilm Formation. BCSJ 1997, 70, 3061–3069. [Google Scholar] [CrossRef]
- Capua, M.; Perrone, S.; Perna, F.M.; Vitale, P.; Troisi, L.; Salomone, A.; Capriati, V. An Expeditious and Greener Synthesis of 2-Aminoimidazoles in Deep Eutectic Solvents. Molecules 2016, 21, 924. [Google Scholar] [CrossRef]
- Aziizi, N.; Manochehri, Z.; Nahayi, A.; Torkashvand, S. A Facile One-Pot Synthesis of Tetrasubstituted Imidazoles Catalyzed by Eutectic Mixture Stabilized Ferrofluid. J. Mol. Liq. 2014, 196, 153–158. [Google Scholar] [CrossRef]
- Vitale, P.; Cicco, L.; Cellamare, I.; Perna, F.M.; Salomone, A.; Capriati, V. Regiodivergent Synthesis of Functionalized Pyrimidines and Imidazoles through Phenacyl Azides in Deep Eutectic Solvents. Beilstein J. Org. Chem. 2020, 16, 1915–1923. [Google Scholar] [CrossRef] [PubMed]
- Shaabani, A.; Hooshmand, S.E. Choline Chloride/Urea as a Deep Eutectic Solvent/Organocatalyst Promoted Three-Component Synthesis of 3-Aminoimidazo-Fused Heterocycles via Groebke–Blackburn–Bienayme Process. Tetrahedron Lett. 2016, 57, 310–313. [Google Scholar] [CrossRef]
- Hamdouchi, C.; Ezquerra, J.; Vega, J.A.; Vaquero, J.J.; Alvarez-Builla, J.; Heinz, B.A. Short Synthesis and Anti-Rhinoviral Activity of Imidazo[1,2-a]Pyridines: The Effect of Acyl Groups at 3-Position. Bioorg. Med. Chem. Lett. 1999, 9, 1391–1394. [Google Scholar] [CrossRef]
- Gueiffier, A.; Mavel, S.; Lhassani, M.; Elhakmaoui, A.; Snoeck, R.; Andrei, G.; Chavignon, O.; Teulade, J.C.; Witvrouw, M.; Balzarini, J.; et al. Synthesis of Imidazo[1,2-a]Pyridines as Antiviral Agents. J. Med. Chem. 1998, 41, 5108–5112. [Google Scholar] [CrossRef]
- Rival, Y.; Grassy, G.; Taudou, A.; Ecalle, R. Antifungal Activity in Vitro of Some Imidazo[1,2-a]Pyrimidine Derivatives. Eur. J. Med. Chem. 1991, 26, 13–18. [Google Scholar] [CrossRef]
- Rodríguez-Álvarez, M.J.; García-Garrido, S.E.; Perrone, S.; García-Álvarez, J.; Capriati, V. Deep Eutectic Solvents and Heterogeneous Catalysis with Metallic Nanoparticles: A Powerful Partnership in Sustainable Synthesis. Curr. Opin. Green Sustain. Chem. 2023, 39, 100723. [Google Scholar] [CrossRef]
- Lu, J.; Li, X.-T.; Ma, E.-Q.; Mo, L.-P.; Zhang, Z.-H. Superparamagnetic CuFeO2 Nanoparticles in Deep Eutectic Solvent: An Efficient and Recyclable Catalytic System for the Synthesis of Imidazo[1,2-a]Pyridines. ChemCatChem 2014, 6, 2854–2859. [Google Scholar] [CrossRef]
- Varghese, B.; Al-Busafi, S.N.; Suliman, F.O.; Al-Kindy, S.M.Z. Unveiling a Versatile Heterocycle: Pyrazoline—A Review. RSC Adv. 2017, 7, 46999–47016. [Google Scholar] [CrossRef]
- Sebest, F.; Lachhani, K.; Pimpasri, C.; Casarrubios, L.; White, A.J.P.; Rzepa, H.S.; Díez-González, S. Cycloaddition Reactions of Azides and Electron-Deficient Alkenes in Deep Eutectic Solvents: Pyrazolines, Aziridines and Other Surprises. Adv. Synth. Catal. 2020, 362, 1877–1886. [Google Scholar] [CrossRef]
- Saavedra, B.; Pérez, J.M.; Rodríguez-Álvarez, M.J.; García-Álvarez, J.; Ramón, D.J. Impregnated Palladium on Magnetite as a Water Compatible Catalyst for the Cycloisomerization of Alkynoic Acid Derivatives. Green Chem. 2018, 20, 2151–2157. [Google Scholar] [CrossRef]
- Joshi, S.; More, U.; Kulkarni, V.; Aminabhavi, T. Pyrrole: Chemical Synthesis, Microwave Assisted Synthesis, Reactions and Applications: A Review. Curr. Org. Chem. 2013, 17, 2279–2304. [Google Scholar] [CrossRef]
- Wang, P.; Ma, F.P.; Zhang, Z.H. L-(+)-Tartaric Acid and Choline Chloride Based Deep Eutectic Solvent: An Efficient and Reusable Medium for Synthesis of N-Substituted Pyrroles via Clauson-Kaas Reaction. J. Mol. Liq. 2014, 198, 259–262. [Google Scholar] [CrossRef]
- Rushell, E.; Tailor, Y.K.; Khandewal, S.; Verma, K.; Agarwal, M.; Kumar, M. Deep Eutectic Solvent Promoted Synthesis of Structurally Diverse Hybrid Molecules with Privileged Heterocyclic Substructures. New J. Chem. 2019, 43, 12462–12467. [Google Scholar] [CrossRef]
- Dheer, D.; Singh, V.; Shankar, R. Medicinal Attributes of 1,2,3-Triazoles: Current Developments. Bioorg. Chem. 2017, 71, 30–54. [Google Scholar] [CrossRef]
- Bozorov, K.; Zhao, J.; Aisa, H.A. 1,2,3-Triazole-Containing Hybrids as Leads in Medicinal Chemistry: A Recent Overview. Bioorg. Med. Chem. 2019, 27, 3511–3531. [Google Scholar] [CrossRef]
- Sebest, F.; Haselgrove, S.; White, A.J.P.; Díez-González, S. Metal-Free 1,2,3-Triazole Synthesis in Deep Eutectic Solvents. Synlett 2020, 31, 605–609. [Google Scholar] [CrossRef]
- Giofrè, S.V.; Tiecco, M.; Ferlazzo, A.; Romeo, R.; Ciancaleoni, G.; Germani, R.; Iannazzo, D. Base-Free Copper-Catalyzed Azide-Alkyne Click Cycloadditions (CuAAc) in Natural Deep Eutectic Solvents as Green and Catalytic Reaction Media. Eur. J. Org. Chem. 2021, 2021, 4777–4789. [Google Scholar] [CrossRef]
- Cicco, L.; Perna, F.M.; Falcicchio, A.; Altomare, A.; Messa, F.; Salomone, A.; Capriati, V.; Vitale, P. 1,3-Dipolar Cycloaddition of Alkanone Enolates with Azides in Deep Eutectic Solvents for the Metal-Free Regioselective Synthesis of Densely Functionalized 1,2,3-Triazoles. Eur. J. Org. Chem. 2022, 2022, e202200843. [Google Scholar] [CrossRef]
- Popova, E.A.; Trifonov, R.E.; Ostrovskii, V.A. Tetrazoles for Biomedicine. Russ. Chem. Rev. 2019, 88, 644–676. [Google Scholar] [CrossRef]
- Xiong, X.; Yi, C.; Liao, X.; Lai, S. A Practical Multigram-Scale Method for the Green Synthesis of 5-Substituted-1H-Tetrazoles in Deep Eutectic Solvent. Tetrahedron Lett. 2019, 60, 402–406. [Google Scholar] [CrossRef]
- Padvi, S.A.; Dalal, D.S. Choline Chloride–ZnCl2: Recyclable and Efficient Deep Eutectic Solvent for the [2+3] Cycloaddition Reaction of Organic Nitriles with Sodium Azide. Synth. Commun. 2017, 47, 779–787. [Google Scholar] [CrossRef]
- Nantermet, P.G.; Barrow, J.C.; Newton, C.L.; Pellicore, J.M.; Young, M.B.; Lewis, S.D.; Lucas, B.J.; Krueger, J.A.; McMasters, D.R.; Yan, Y.; et al. Design and Synthesis of Potent and Selective Macrocyclic Thrombin Inhibitors. Bioorg. Med. Chem. Lett. 2003, 13, 2781–2784. [Google Scholar] [CrossRef]
- Becker, J.W.; Rotonda, J.; Soisson, S.M.; Aspiotis, R.; Bayly, C.; Francoeur, S.; Gallant, M.; García-Calvo, M.; Giroux, A.; Grimm, E.; et al. Reducing the Peptidyl Features of Caspase-3 Inhibitors: A Structural Analysis. J. Med. Chem. 2004, 47, 2466–2474. [Google Scholar] [CrossRef]
- Hopkins, C.; Neuenschwander, K.; Scotese, A.; Jackson, S.; Nieduzak, T.; Pauls, H.; Liang, G.; Sides, K.; Cramer, D.; Cairns, J.; et al. Novel Pyrazinone Inhibitors of Mast Cell Tryptase: Synthesis and SAR Evaluation. Bioorg. Med. Chem. Lett. 2004, 14, 4819–4823. [Google Scholar] [CrossRef]
- Perrone, S.; Capua, M.; Messa, F.; Salomone, A.; Troisi, L. Green Synthesis of 2-Pyrazinones in Deep Eutectic Solvents: From α-Chloro Oximes to Peptidomimetic Scaffolds. Tetrahedron 2017, 73, 6193–6198. [Google Scholar] [CrossRef]
- Johns, B.A.; Weatherhead, J.G.; Allen, S.H.; Thompson, J.B.; Garvey, E.P.; Foster, S.A.; Jeffrey, J.L.; Miller, W.H. 1,3,4-Oxadiazole Substituted Naphthyridines as HIV-1 Integrase Inhibitors. Part 2: SAR of the C5 Position. Bioorg. Med. Chem. Lett. 2009, 19, 1807–1810. [Google Scholar] [CrossRef]
- Huang, S.; Qing, J.; Wang, S.; Wang, H.; Zhang, L.; Tang, Y. Design and Synthesis of Imidazo[1,2-α][1,8]Naphthyridine Derivatives as Anti-HCV Agents via Direct C-H Arylation. Org. Biomol. Chem. 2014, 12, 2344–2348. [Google Scholar] [CrossRef] [PubMed]
- Rudys, S.; Ríos-Luci, C.; Pérez-Roth, E.; Cikotiene, I.; Padrón, J.M. Antiproliferative Activity of Novel Benzo[b][1,6]Naphthyridines in Human Solid Tumor Cell Lines. Bioorg. Med. Chem. Lett. 2010, 20, 1504–1506. [Google Scholar] [CrossRef] [PubMed]
- Shaabani, A.; Hooshmand, S.E.; Tabatabaei, A.T. Synthesis of Fully Substituted Naphthyridines: A Novel Domino Four-Component Reaction in a Deep Eutectic Solvent System Based on Choline Chloride/Urea. Tetrahedron Lett. 2016, 57, 351–353. [Google Scholar] [CrossRef]
- Chung, P.Y.; Bian, Z.X.; Pun, H.Y.; Chan, D.; Chan, A.S.C.; Chui, C.H.; Tang, J.C.O.; Lam, K.H. Recent Advances in Research of Natural and Synthetic Bioactive Quinolines. Future Med. Chem. 2015, 7, 947–967. [Google Scholar] [CrossRef]
- Satyanarayana, N.; Sathish, K.; Nagaraju, S.; Pawar, R.; Faizan, M.; Arumugavel, M.; Shirisha, T.; Kashinath, D. Metal-Free, One-Pot Synthesis of 2-Styrylquinolines via Friedländer Annulation and Sp3 C–H Activation Using 1,3-Dimethylurea and L-Tartaric Acid (3:1) as a Deep Eutectic Solvent. New J. Chem. 2022, 46, 1637–1642. [Google Scholar] [CrossRef]
- Komar, M.; Molnar, M.; Jukić, M.; Glavaš-Obrovac, L.; Opačak-Bernardi, T. Green Chemistry Approach to the Synthesis of 3-Substituted-Quinazolin-4(3H)-Ones and 2-Methyl-3-Substituted-Quinazolin-4(3H)-Ones and Biological Evaluation. Green Chem. Lett. Rev. 2020, 13, 93–101. [Google Scholar] [CrossRef]
- Al-Suwaidan, I.A.; Abdel-Aziz, A.A.M.; Shawer, T.Z.; Ayyad, R.R.; Alanazi, A.M.; El-Morsy, A.M.; Mohamed, M.A.; Abdel-Aziz, N.I.; El-Sayed, M.A.A.; El-Azab, A.S. Synthesis, Antitumor Activity and Molecular Docking Study of Some Novel 3-Benzyl-4(3H)Quinazolinone Analogues. J. Enzyme Inhib. Med. Chem. 2016, 31, 78–89. [Google Scholar] [CrossRef]
- Komar, M.; Kraljević, T.G.; Jerković, I.; Molnar, M. Application of Deep Eutectic Solvents in the Synthesis of Substituted 2-Mercaptoquinazolin-4(3H)-Ones: A Comparison of Selected Green Chemistry Methods. Molecules 2022, 27, 558. [Google Scholar] [CrossRef]
- Shaabani, A.; Hooshmand, S.E.; Nazeri, M.T.; Afshari, R.; Ghasemi, S. Deep Eutectic Solvent as a Highly Efficient Reaction Media for the One-Pot Synthesis of Benzo-Fused Seven-Membered Heterocycles. Tetrahedron Lett. 2016, 57, 3727–3730. [Google Scholar] [CrossRef]
- Nardi, A.E.; Cosci, F.; Balon, R.; Weintraub, S.J.; Freire, R.C.; Krystal, J.H.; Roth, T.; Silberman, E.K.; Sonino, N.; Fava, G.A.; et al. The Prescription of Benzodiazepines for Panic Disorder: Time for an Evidence-Based Educational Approach. J. Clin. Psychopharmacol. 2018, 38, 283–285. [Google Scholar] [CrossRef]
- Strømme, M.F.; Mellesdal, L.S.; Bartz-Johannesen, C.A.; Kroken, R.A.; Krogenes, M.L.; Mehlum, L.; Johnsen, E. Use of Benzodiazepines and Antipsychotic Drugs Are Inversely Associated with Acute Readmission Risk in Schizophrenia. J. Clin. Psychopharmacol. 2022, 42, 37–42. [Google Scholar] [CrossRef] [PubMed]
- Blaser, H.U.; Malan, C.; Pugin, B.; Spindler, F.; Steiner, H.; Studer, M. Selective Hydrogenation for Fine Chemicals: Recent Trends and New Developments. Adv. Synth. Catal. 2003, 345, 103–151. [Google Scholar] [CrossRef]
- Karamé, I. (Ed.) Hydrogenation; BoD–Books on Demand: Norderstedt, Germany, 2012. [Google Scholar]
- Chen, B.; Dingerdissen, U.; Krauter, J.G.E.; Lansink Rotgerink, H.G.J.; Möbus, K.; Ostgard, D.J.; Panster, P.; Riermeier, T.H.; Seebald, S.; Tacke, T.; et al. New Developments in Hydrogenation Catalysis Particularly in Synthesis of Fine and Intermediate Chemicals. Appl. Catal. A Gen. 2005, 280, 17–46. [Google Scholar] [CrossRef]
- Perrone, S.; Messa, F.; Salomone, A. Towards Green Reductions in Bio-Derived Solvents. Eur. J. Org. Chem. 2023, 2023, e202201494. [Google Scholar] [CrossRef]
- Perrone, S.; Capua, M.; Cannazza, G.; Salomone, A.; Troisi, L. Synthesis of β-Enamino Acid and Heteroaryl Acetic Acid Derivatives by Pd-Catalyzed Carbonylation of α-Chloroimines and 2-Chloromethyl Aza-Heterocycles. Tetrahedron Lett. 2016, 57, 1421–1424. [Google Scholar] [CrossRef]
- Perrone, S.; Cannazza, G.; Caroli, A.; Salomone, A.; Troisi, L. Ring Opening of Heterocycles Containing a C–N Double Bond: A Simple Synthesis of Imides Promoted by Acyl Palladium Species. Tetrahedron 2014, 70, 6938–6943. [Google Scholar] [CrossRef]
- Perrone, S.; Capua, M.; Salomone, A.; Troisi, L. Multicomponent Synthesis of Uracil Analogues Promoted by Pd-Catalyzed Carbonylation of α-Chloroketones in the Presence of Isocyanates and Amines. J. Org. Chem. 2015, 80, 8189–8197. [Google Scholar] [CrossRef] [PubMed]
- Capua, M.; Perrone, S.; Bona, F.; Salomone, A.; Troisi, L. A Direct Synthesis of Isocytosine Analogues by Carbonylative Coupling of α-Chloro Ketones and Guanidines. Eur. J. Org. Chem. 2017, 2017, 1780–1787. [Google Scholar] [CrossRef]
- Capua, M.; Granito, C.; Perrone, S.; Salomone, A.; Troisi, L. Palladium-Catalyzed Carbonylative Coupling of α-Chloroketones with Hydrazines: A Simple Route to Pyrazolone Derivatives. Tetrahedron Lett. 2016, 57, 3363–3367. [Google Scholar] [CrossRef]
- Messa, F.; Perrone, S.; Capua, M.; Tolomeo, F.; Troisi, L.; Capriati, V.; Salomone, A. Towards a Sustainable Synthesis of Amides: Chemoselective Palladium-Catalysed Aminocarbonylation of Aryl Iodides in Deep Eutectic Solvents. Chem. Commun. 2018, 54, 8100–8103. [Google Scholar] [CrossRef]
- Paparella, A.N.; Messa, F.; Dilauro, G.; Troisi, L.; Perrone, S.; Salomone, A. A Glycerol-Based Deep Eutectic Solvent as Natural Medium and Organic Reductant for Homocoupling of (Hetero)Aryl Chlorides: A Green Route to 2,2′-Bipyridine and Biaryl Scaffolds. ChemistrySelect 2022, 7, e202203438. [Google Scholar] [CrossRef]
- Messa, F.; Dilauro, G.; Paparella, A.N.; Silvestri, L.; Furlotti, G.; Iacoangeli, T.; Perrone, S.; Salomone, A. Deep Eutectic Solvents Meet Safe, Scalable and Sustainable Hydrogenations Enabled by Aluminum Powder and Pd/C. Green Chem. 2022, 24, 4388–4394. [Google Scholar] [CrossRef]
- Goli, N.; Mainkar, P.S.; Kotapalli, S.S.; Tejaswini, K.; Ummanni, R.; Chandrasekhar, S. Expanding the Tetrahydroquinoline Pharmacophore. Bioorg. Med. Chem. Lett. 2017, 27, 1714–1720. [Google Scholar] [CrossRef]
- Vitale, P.; Cicco, L.; Messa, F.; Perna, F.M.; Salomone, A.; Capriati, V. Streamlined Routes to Phenacyl Azides and 2,5-Diarylpyrazines Enabled by Deep Eutectic Solvents. Eur. J. Org. Chem. 2019, 2019, 5557–5562. [Google Scholar] [CrossRef]
- Dolezal, M.; Zitko, J. Pyrazine Derivatives: A Patent Review (June 2012–Present). Expert Opin. Ther. Patents 2015, 25, 33–47. [Google Scholar] [CrossRef] [PubMed]
- Schultheiss, N.; Powell, D.R.; Bosch, E. Silver(I) Coordination Chemistry of 2,6-Diarylpyrazines. π-Stacking, Anion Coordination, and Steric Control. Inorg. Chem. 2003, 42, 5304–5310. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez-álvarez, M.J.; Vidal, C.; Díez, J.; García-álvarez, J. Introducing Deep Eutectic Solvents as Biorenewable Media for Au(I)-Catalysed Cycloisomerisation of β-Alkynoic Acids: An Unprecedented Catalytic System. Chem. Commun. 2014, 50, 12927–12929. [Google Scholar] [CrossRef]
- Vidal, C.; Merz, L.; García-Álvarez, J. Deep Eutectic Solvents: Biorenewable Reaction Media for Au(i)-Catalysed Cycloisomerisations and One-Pot Tandem Cycloisomerisation/Diels-Alder Reactions. Green Chem. 2015, 17, 3870–3878. [Google Scholar] [CrossRef]
- Lipshutz, B.H. Five-Membered Heteroaromatic Rings as Intermediates in Organic Synthesis. Chem. Rev. 1986, 86, 795–819. [Google Scholar] [CrossRef]
- Ash, M.; Ash, I. Handbook of Flavors and Fragrances; Synapse Information Resources, Inc.: New York, NY, USA, 2006; ISBN 9781890595876. [Google Scholar]
- Curran, D.; Grimshaw, J.; Perera, S.D. Poly(Pyrrole) as a Support for Electrocatalytic Materials. Chem. Soc. Rev. 1991, 20, 391–404. [Google Scholar] [CrossRef]
- Spiegel, M.; Sroka, Z. Natural Dihydroisobenzofuran Derivatives as a Template for Promising Radical Scavengers: Theoretical Insights into Structure–Activity Relationships, Thermochemistry and Kinetics. Theor. Chem. Acc. 2022, 141, 61. [Google Scholar] [CrossRef]
- Harper, J.K.; Arif, A.M.; Ford, E.J.; Strobel, G.A.; Porco, J.A.; Tomer, D.P.; Oneill, K.L.; Heider, E.M.; Grant, D.M. Pestacin: A 1,3-Dihydro Isobenzofuran from Pestalotiopsis Microspora Possessing Antioxidant and Antimycotic Activities. Tetrahedron 2003, 59, 2471–2476. [Google Scholar] [CrossRef]
- Tanizawa, Y.; Kaku, K.; Araki, E.; Tobe, K.; Terauchi, Y.; Utsunomiya, K.; Iwamoto, Y.; Watada, H.; Ohtsuka, W.; Watanabe, D.; et al. Long-Term Safety and Efficacy of Tofogliflozin, a Selective Inhibitor of Sodium-Glucose Cotransporter 2, as Monotherapy or in Combination with Other Oral Antidiabetic Agents in Japanese Patients with Type 2 Diabetes Mellitus: Multicenter, Open-Label, Randomized Controlled Trials. Expert Opin. Pharmacother. 2014, 15, 749–766. [Google Scholar] [PubMed]
- Navarro, S.D.; Pessatto, L.R.; Meza, A.; de Oliveira, E.J.T.; Auharek, S.A.; Vilela, L.C.; de Lima, D.P.; de Azevedo, R.B.; Kassuya, C.A.L.; Cáceres, O.I.A.; et al. Resorcinolic Lipid 3-Heptyl-3,4,6-Trimethoxy-3H-Isobenzofuran-1-One Is a Strategy for Melanoma Treatment. Life Sci. 2018, 209, 300–312. [Google Scholar] [CrossRef]
- González-Gallardo, N.; Saavedra, B.; Guillena, G.; Ramón, D.J. A Jackpot C-H Activation Protocol Using Simple Ruthenium Catalyst in Deep Eutectic Solvents. Green Chem. 2022, 24, 4941–4951. [Google Scholar] [CrossRef]
- Mancuso, R.; Maner, A.; Cicco, L.; Perna, F.M.; Capriati, V.; Gabriele, B. Synthesis of Thiophenes in a Deep Eutectic Solvent: Heterocyclodehydration and Iodocyclization of 1-Mercapto-3-Yn-2-Ols in a Choline Chloride/Glycerol Medium. Tetrahedron 2016, 72, 4239–4244. [Google Scholar] [CrossRef]
- Mancuso, R.; Lettieri, M.; Strangis, R.; Russo, P.; Piccionello, A.P.; De Angelis, S.; Gabriele, B. Iodocyclization of 2-Methylthiophenylacetylenes to 3-Iodobenzothiophenes and Their Coupling Reactions under More Sustainable Conditions. Asian J. Org. Chem. 2022, 11, e202200353. [Google Scholar] [CrossRef]
- Yang, C.; Pohl, R.; Tichý, M.; Gurská, S.; Pavliš, P.; Džubák, P.; Hajdúch, M.; Hocek, M. Synthesis, Photophysical Properties, and Biological Profiling of Benzothieno-Fused 7-Deazapurine Ribonucleosides. J. Org. Chem. 2020, 85, 8085–8101. [Google Scholar] [CrossRef]
- Christie, R.M.; Lui, C.H. Studies of Fluorescent Dyes: Part 2. An Investigation of the Synthesis and Electronic Spectral Properties of Substituted 3-(2′-Benzimidazolyl)Coumarins. Dye Pigment. 2000, 47, 79–89. [Google Scholar] [CrossRef]
- Kostova, I. Coumarins as Inhibitors of HIV Reverse Transcriptase. Curr. HIV Res. 2006, 4, 347–363. [Google Scholar] [CrossRef]
- Wang, C.J.; Hsieh, Y.J.; Chu, C.Y.; Lin, Y.L.; Tseng, T.H. Inhibition of Cell Cycle Progression in Human Leukemia HL-60 Cells by Esculetin. Cancer Lett. 2002, 183, 163–168. [Google Scholar] [CrossRef] [PubMed]
- Rather, I.A.; Ali, R. An Efficient and Versatile Deep Eutectic Solvent-Mediated Green Method for the Synthesis of Functionalized Coumarins. ACS Omega 2022, 7, 10649–10659. [Google Scholar] [CrossRef]
- Pal, S.; Pal, M. Isocoumarin, Thiaisocoumarin and Phosphaisocoumarin: Natural Occurrences, Synthetic Approaches and Pharmaceutical Applications; Elsevier: Amsterdam, The Netherlands, 2018; ISBN 9780128154113. [Google Scholar]
- Wright, P.; Staff, U. Xanthene Dyes. In Kirk-Othmer Encyclopedia of Chemical Technology; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2014; pp. 1–19. [Google Scholar]
- Ghahsare, A.G.; Nazifi, Z.S.; Nazifi, S.M.R. Structure-Bioactivity Relationship Study of Xanthene Derivatives: A Brief Review. Curr. Org. Synth. 2019, 16, 1071–1077. [Google Scholar] [CrossRef] [PubMed]
- Shchekotikhin, Y.M.; Nikolaeva, T.G. Transformations of Sym-Octahydroxanthene-1,8-Diones and 1,8-Dioxo-Sym-Octahydroxanthylium Salts in Recyclization under the Influence of Amines. Chem. Heterocycl. Compd. 2006, 42, 28–33. [Google Scholar] [CrossRef]
- Shaibuna, M.; Abbas, A.; Kariyottu Kuniyil, M.J.; Sreekumar, K. Sustainable Synthesis of 1,8-Dioxooctahydroxanthenes in Deep Eutectic Solvents (DESs). New J. Chem. 2021, 45, 8335–8344. [Google Scholar] [CrossRef]
- Katiyar, M.K.; Dhakad, G.K.; Shivani; Arora, S.; Bhagat, S.; Arora, T.; Kumar, R. Synthetic Strategies and Pharmacological Activities of Chromene and Its Derivatives: An Overview. J. Mol. Struct. 2022, 1263, 133012. [Google Scholar] [CrossRef]
- Sathish, K.; Nagaraju, S.; Kashinath, D. Dimethylurea/L-Tartaric Acid as Deep Eutectic Solvent for One-Pot Synthesis of 2-(Methylamino)-3-Nitrospiro-[Chromene] and N-Methyl-3-Nitro-4H Chromen-2-Amines. Synth. Commun. 2021, 51, 1242–1251. [Google Scholar] [CrossRef]
- Maiti, B.; Chanda, K. Diversity Oriented Synthesis of Benzimidazole-Based Biheterocyclic Molecules by Combinatorial Approach: A Critical Review. RSC Adv. 2016, 6, 50384–50413. [Google Scholar] [CrossRef]
- Zhang, M.; Liu, Y.H.; Shang, Z.R.; Hu, H.C.; Zhang, Z.H. Supported Molybdenum on Graphene Oxide/Fe3O4: An Efficient, Magnetically Separable Catalyst for One-Pot Construction of Spiro-Oxindole Dihydropyridines in Deep Eutectic Solvent under Microwave Irradiation. Catal. Commun. 2017, 88, 39–44. [Google Scholar] [CrossRef]
- Nguyen, H.T.; Van Le, T.; Tran, P.H. AC-SO3H/[CholineCl][Urea]2 as a Green Catalytic System for the Synthesis of Pyrano[2,3-c]Pyrazole Scaffolds. J. Environ. Chem. Eng. 2021, 9, 105228. [Google Scholar] [CrossRef]
- Kuo, S.C.; Huang, L.J.; Nakamura, H. Studies on Heterocyclic Compounds. 6.112Synthesis and Analgesic and Antiinflammatory Activities of 3,4-Dimethylpyrano[2,3-c]Pyrazol-6-One Derivatives. J. Med. Chem. 1984, 27, 539–544. [Google Scholar] [CrossRef] [PubMed]
- Zhao, D.; Liu, Y.; Li, Y.; Chen, Y. A Green Synthesis and Antibacterial Activity of Ferrocene-Based Thiazole Derivatives in Choline Chloride/Glycerol Eutectic Solvent. RSC Adv. 2022, 12, 22054–22059. [Google Scholar] [CrossRef] [PubMed]
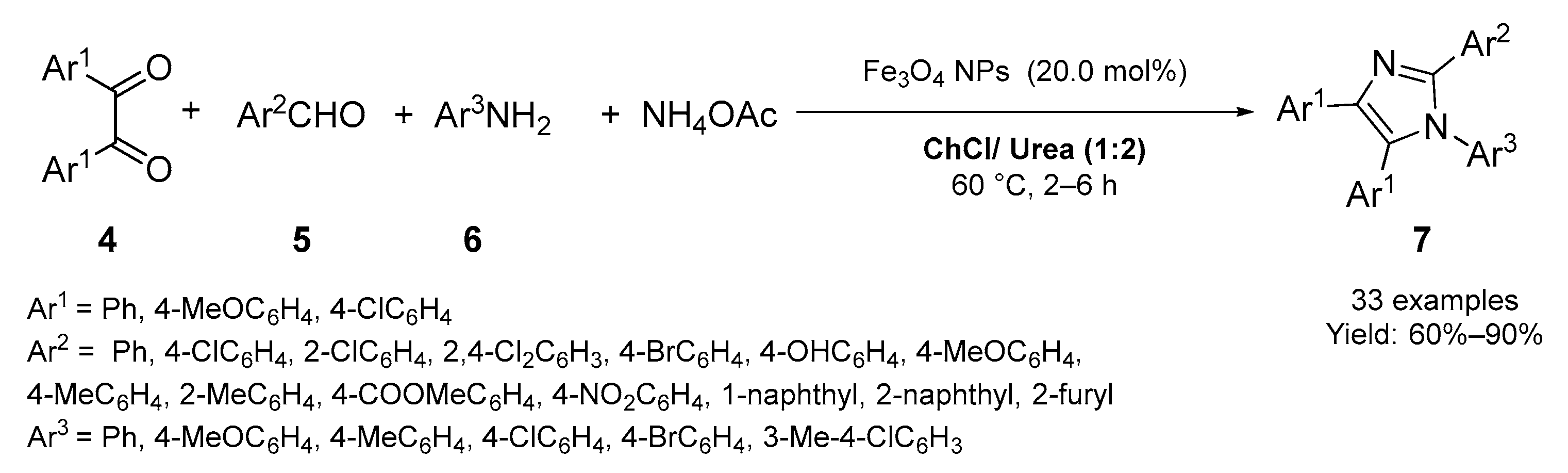



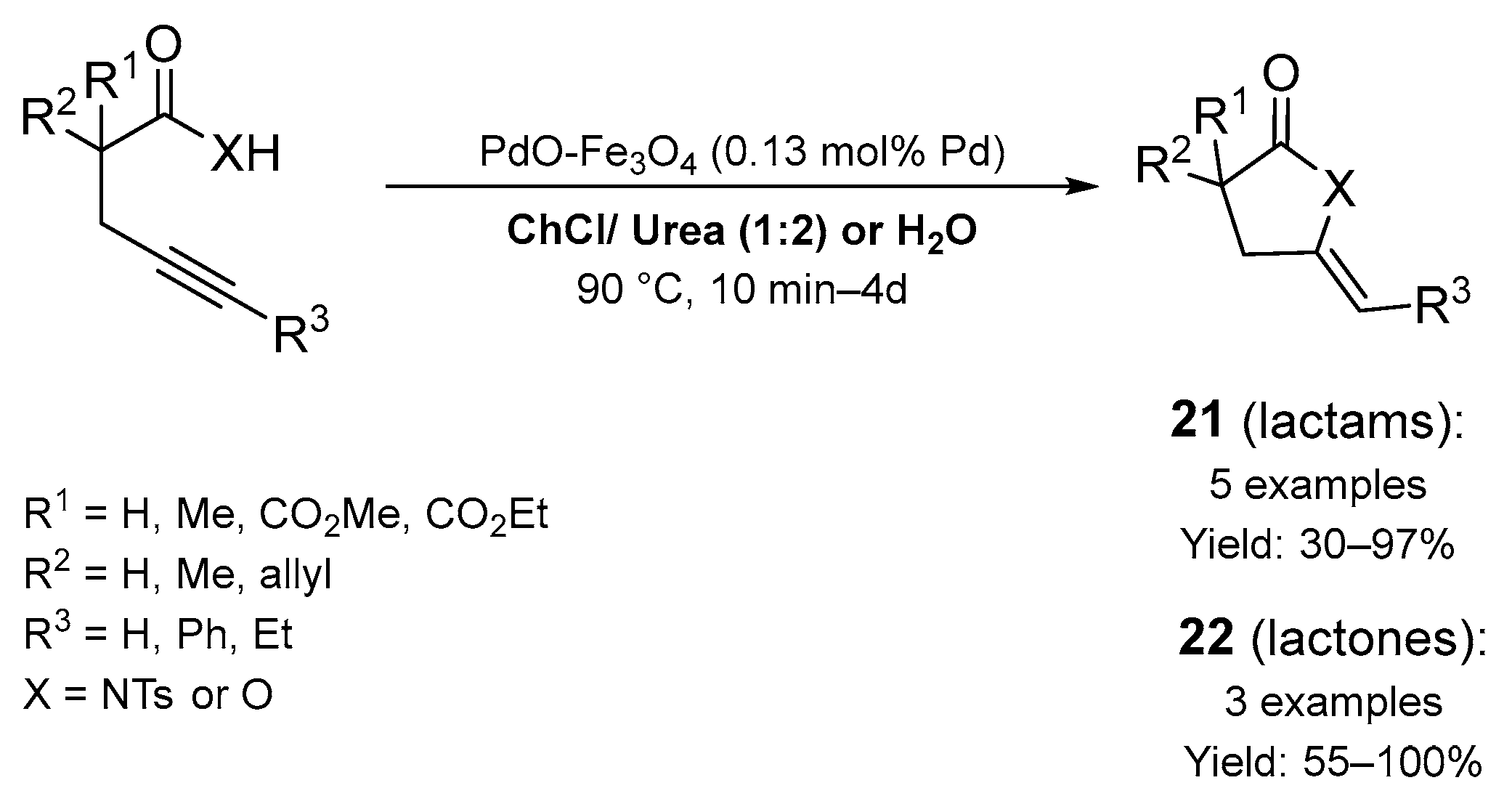



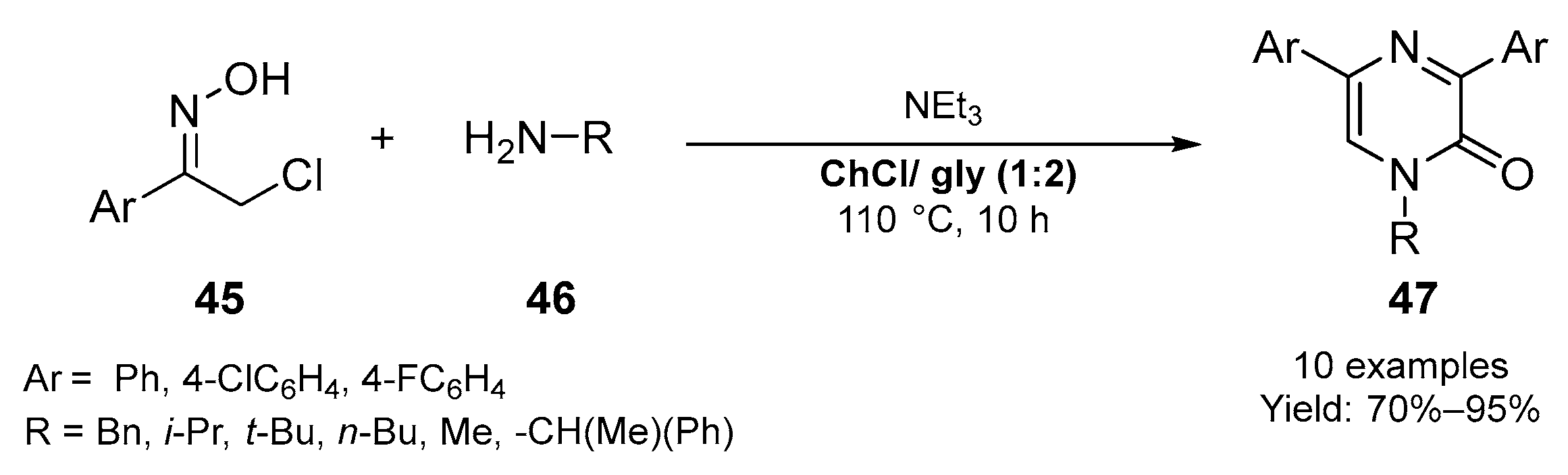









Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Perrone, S.; Messa, F.; Troisi, L.; Salomone, A. N-, O- and S-Heterocycles Synthesis in Deep Eutectic Solvents. Molecules 2023, 28, 3459. https://doi.org/10.3390/molecules28083459
Perrone S, Messa F, Troisi L, Salomone A. N-, O- and S-Heterocycles Synthesis in Deep Eutectic Solvents. Molecules. 2023; 28(8):3459. https://doi.org/10.3390/molecules28083459
Chicago/Turabian StylePerrone, Serena, Francesco Messa, Luigino Troisi, and Antonio Salomone. 2023. "N-, O- and S-Heterocycles Synthesis in Deep Eutectic Solvents" Molecules 28, no. 8: 3459. https://doi.org/10.3390/molecules28083459
APA StylePerrone, S., Messa, F., Troisi, L., & Salomone, A. (2023). N-, O- and S-Heterocycles Synthesis in Deep Eutectic Solvents. Molecules, 28(8), 3459. https://doi.org/10.3390/molecules28083459










