Assessment of Cu(II) Removal from Aqueous Solutions by Modified Pomelo Peels: Experiments and Modelling
Abstract
1. Introduction
2. Results and Discussion
2.1. Effect of the Chemical Modification on Adsorption
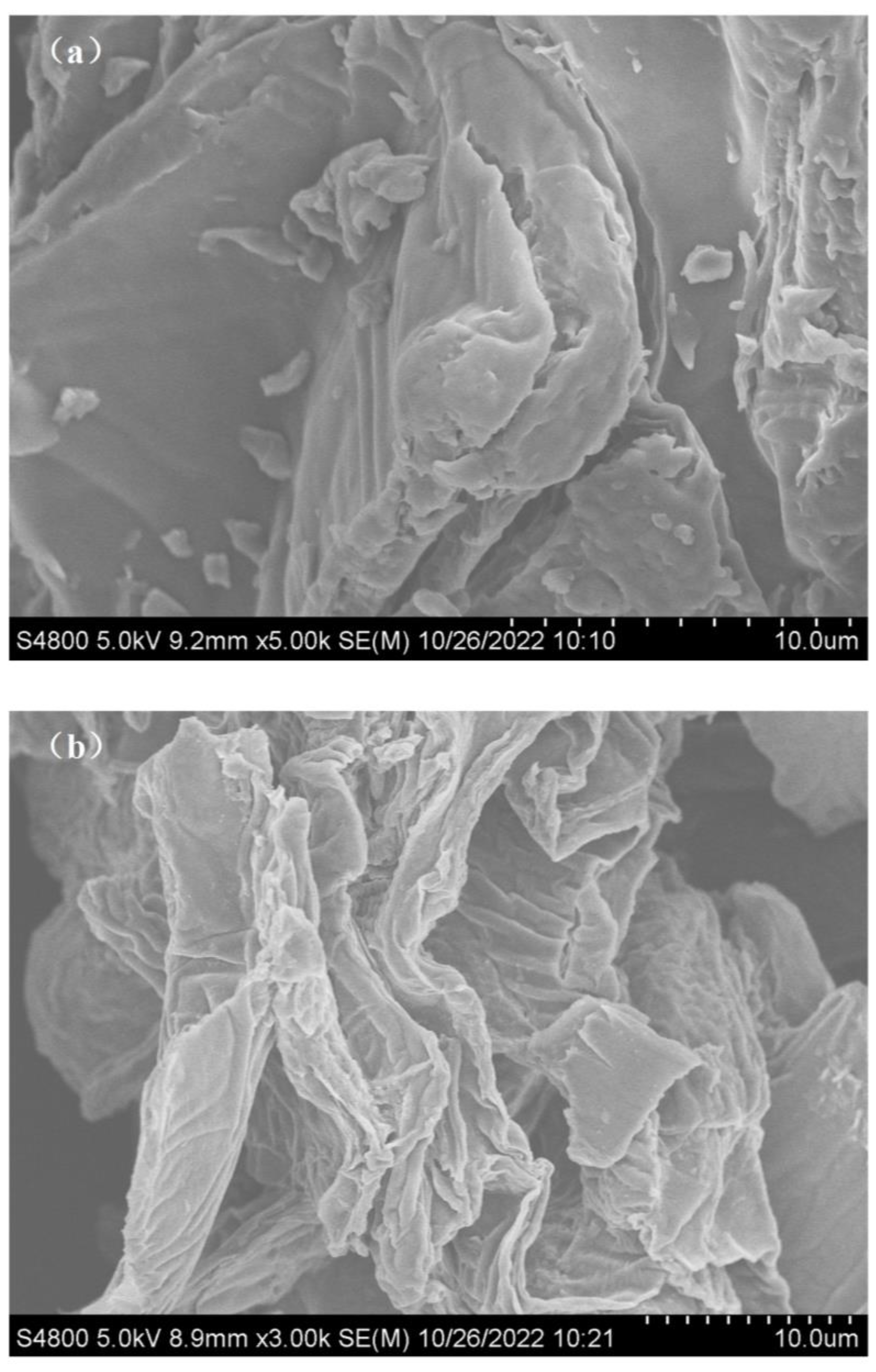
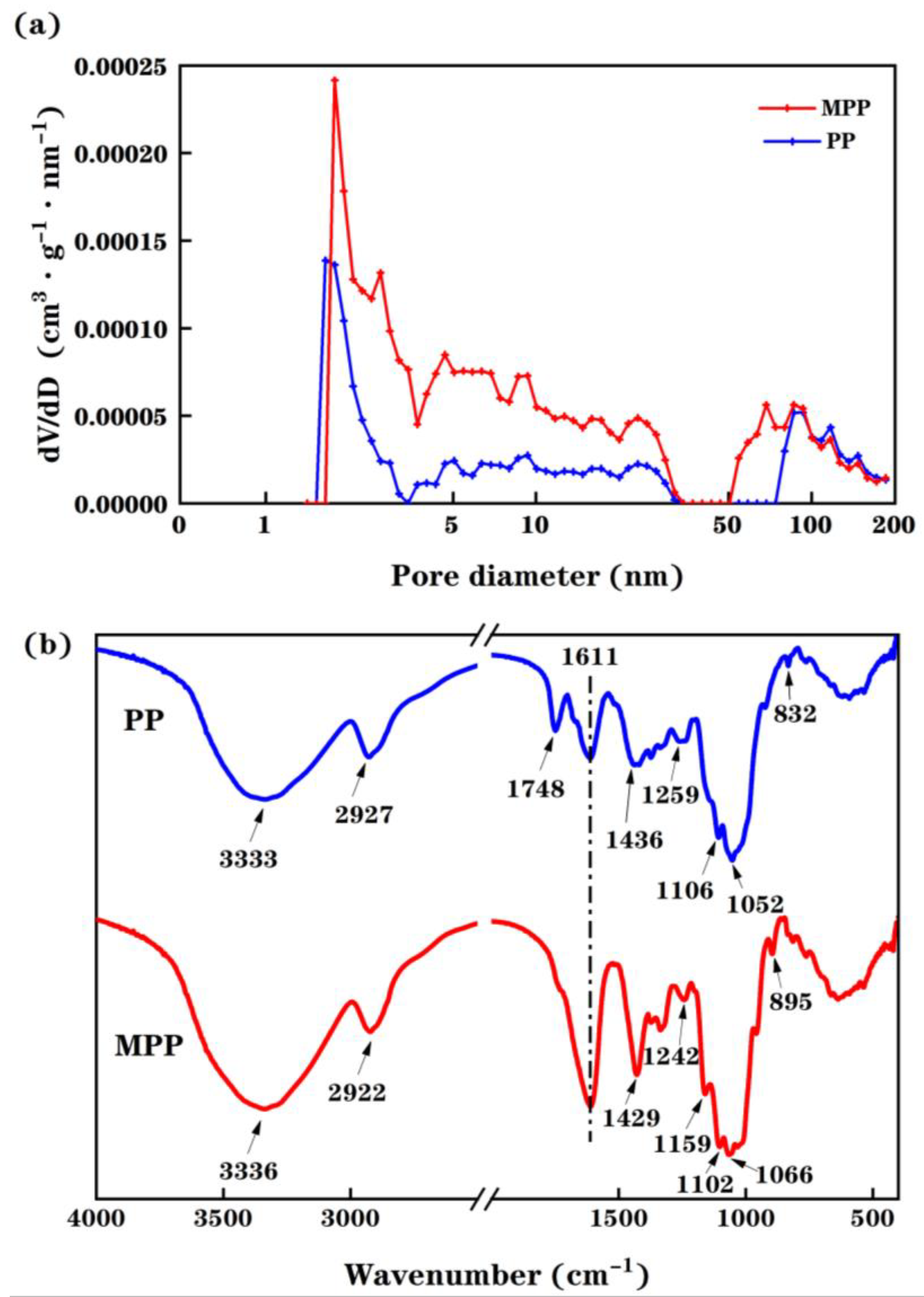
2.2. Adsorbent Characterization
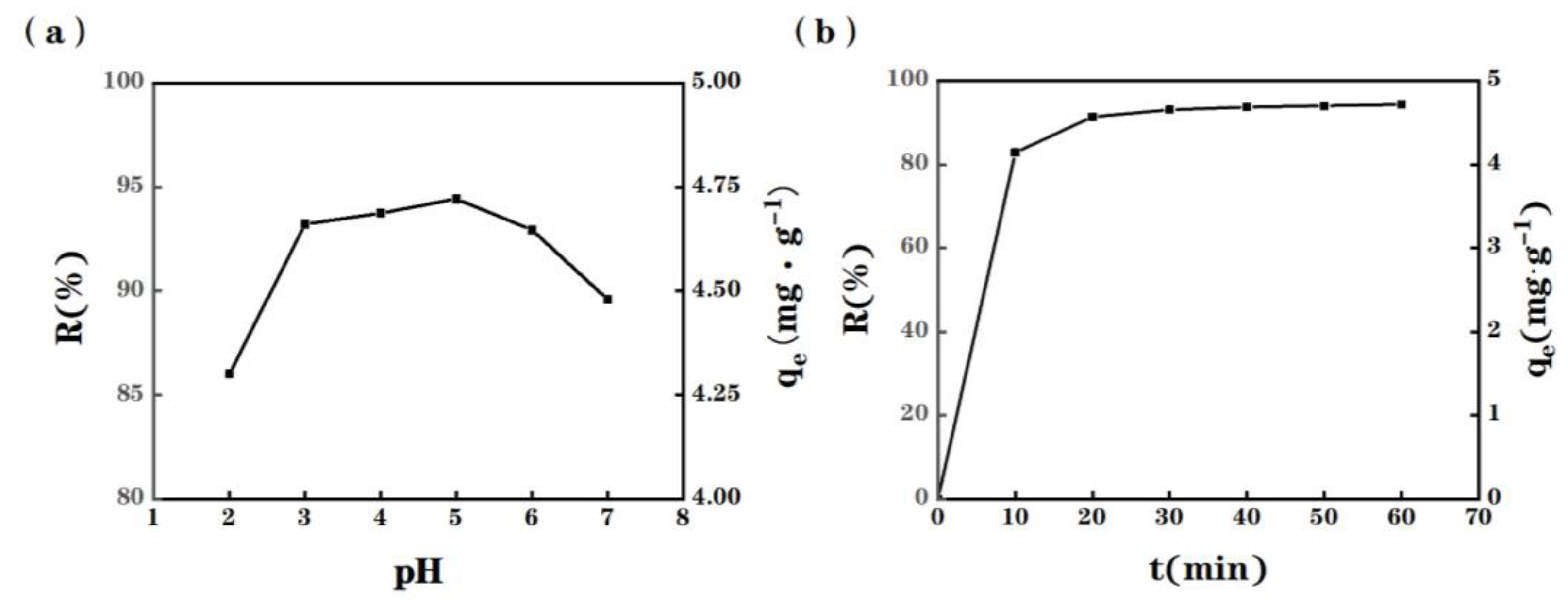
2.3. Effect of Initial pH
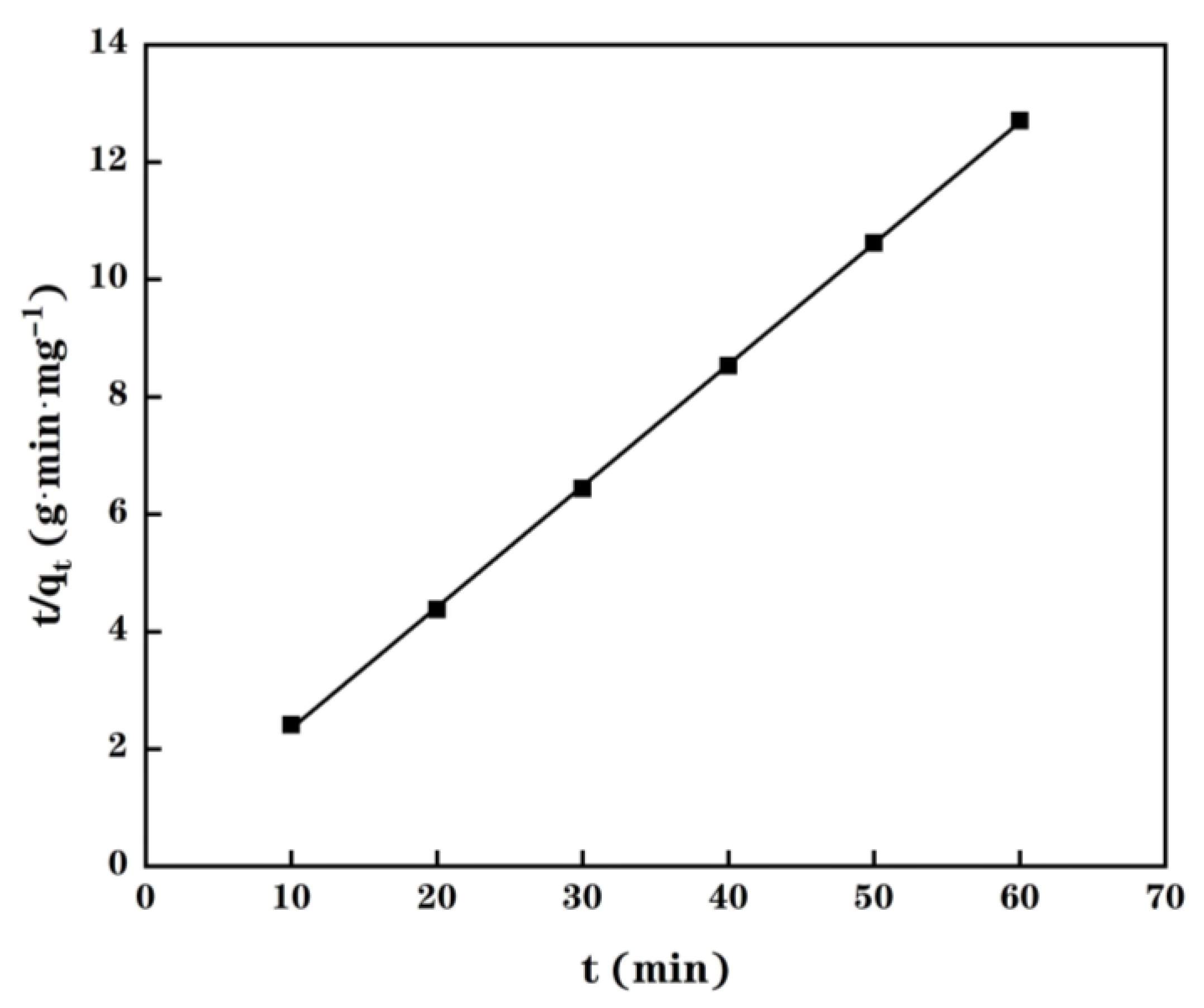
2.4. Adsorption Kinetics
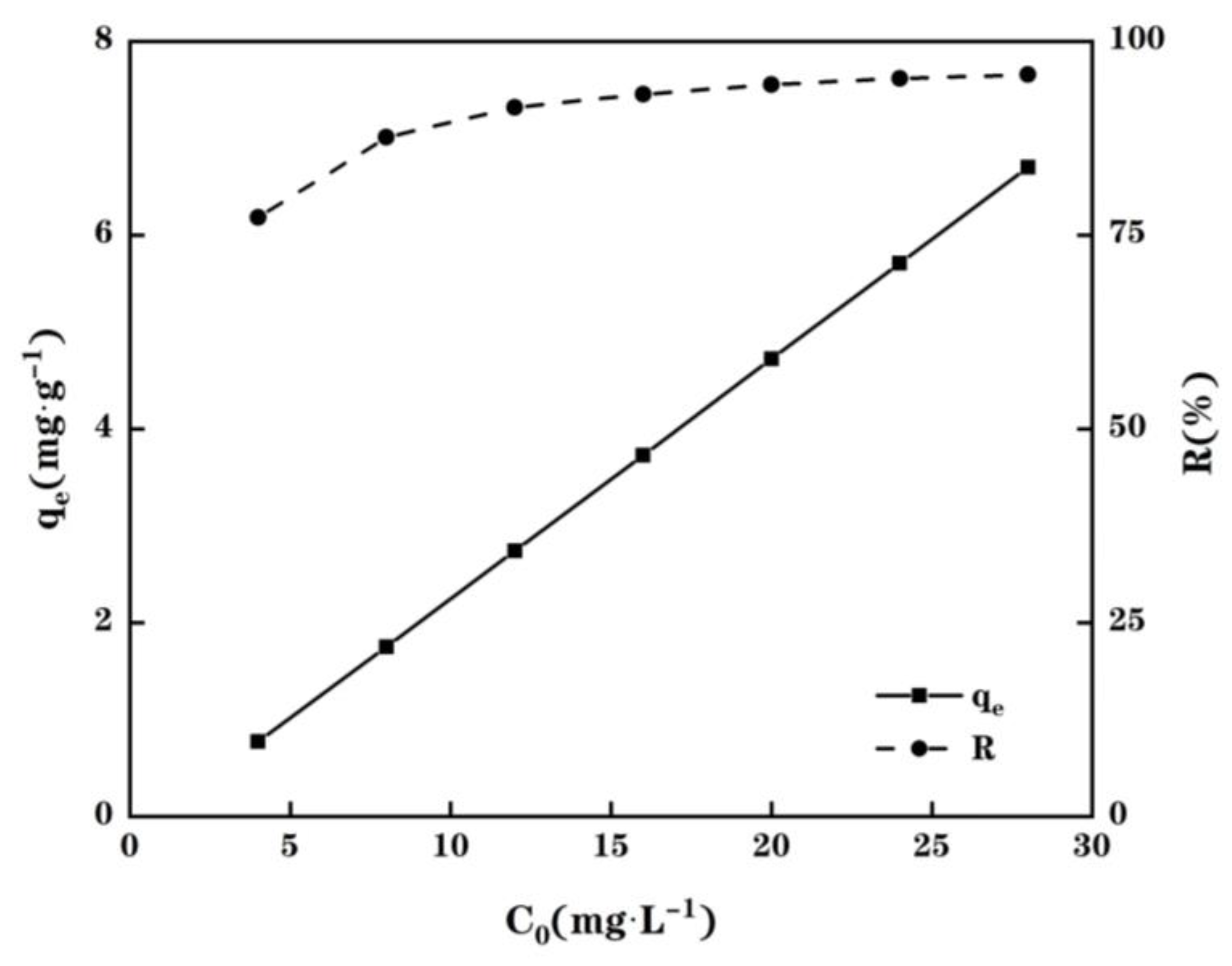
2.5. Adsorption Isotherms
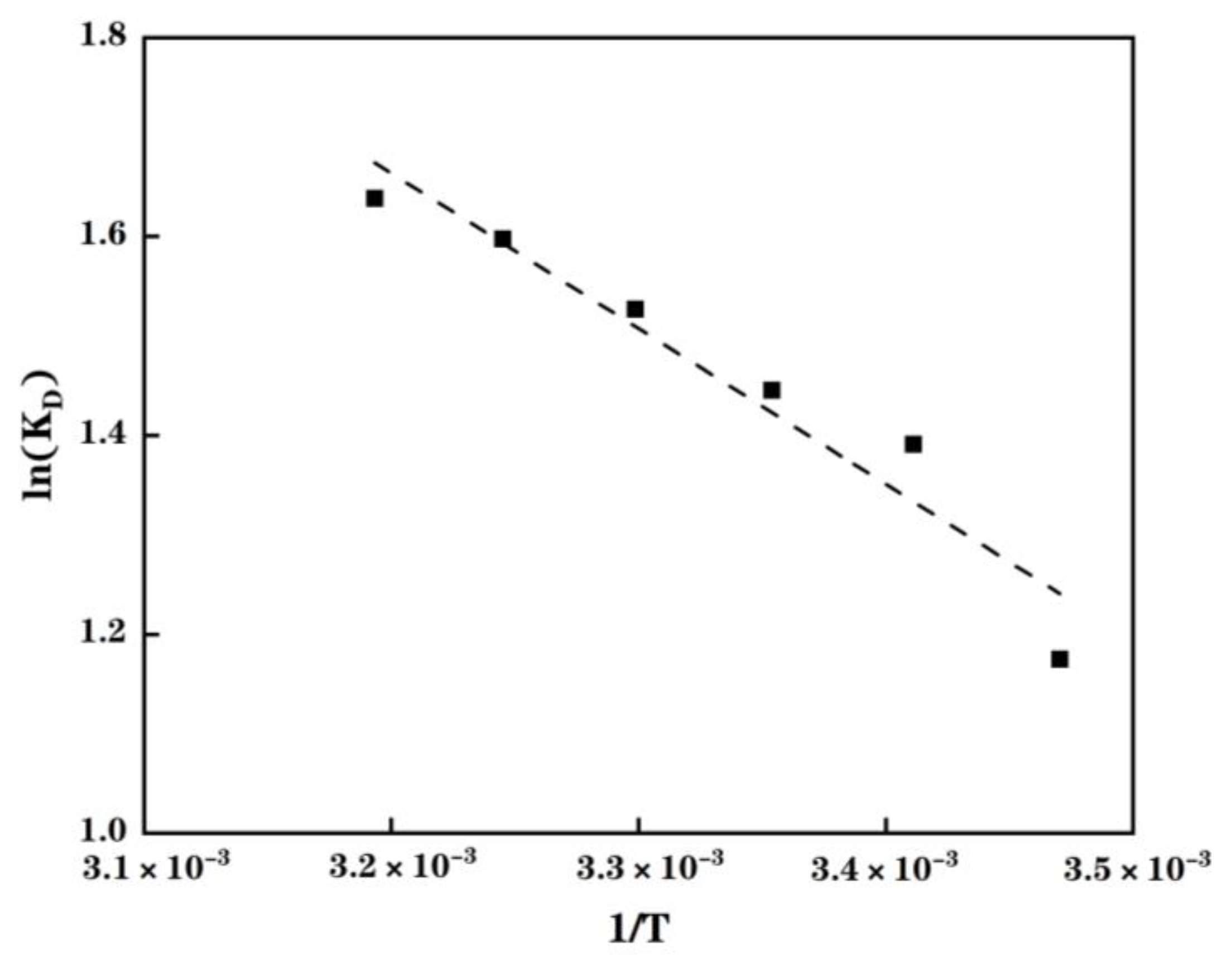
2.6. Effect of the Temperature
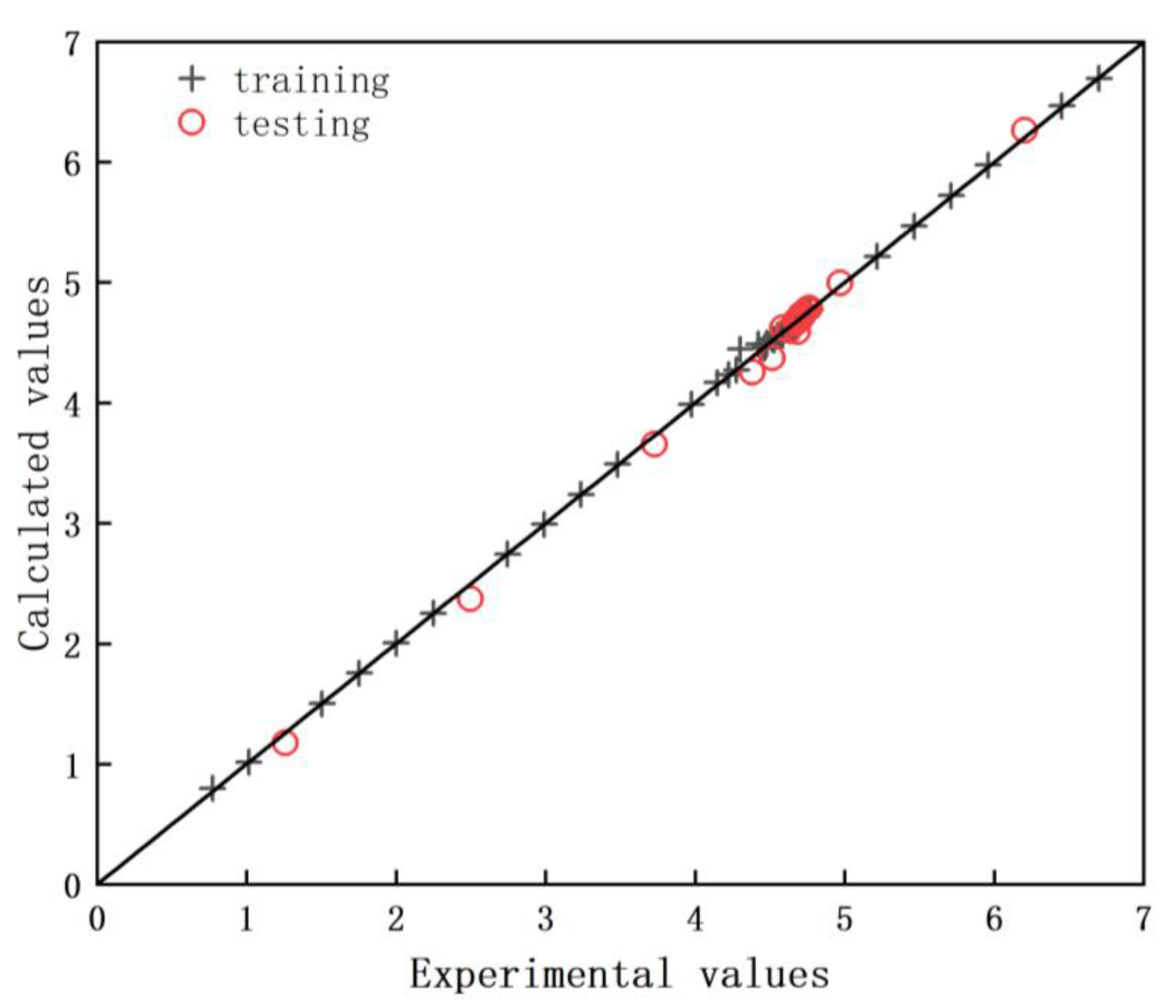
2.7. Performance of the ANN Model
3. Materials and Methods
3.1. Chemicals
3.2. Material Preparation
3.3. Characterization of the Tested Biosorbent
3.4. Cu2+ Adsorption Tests
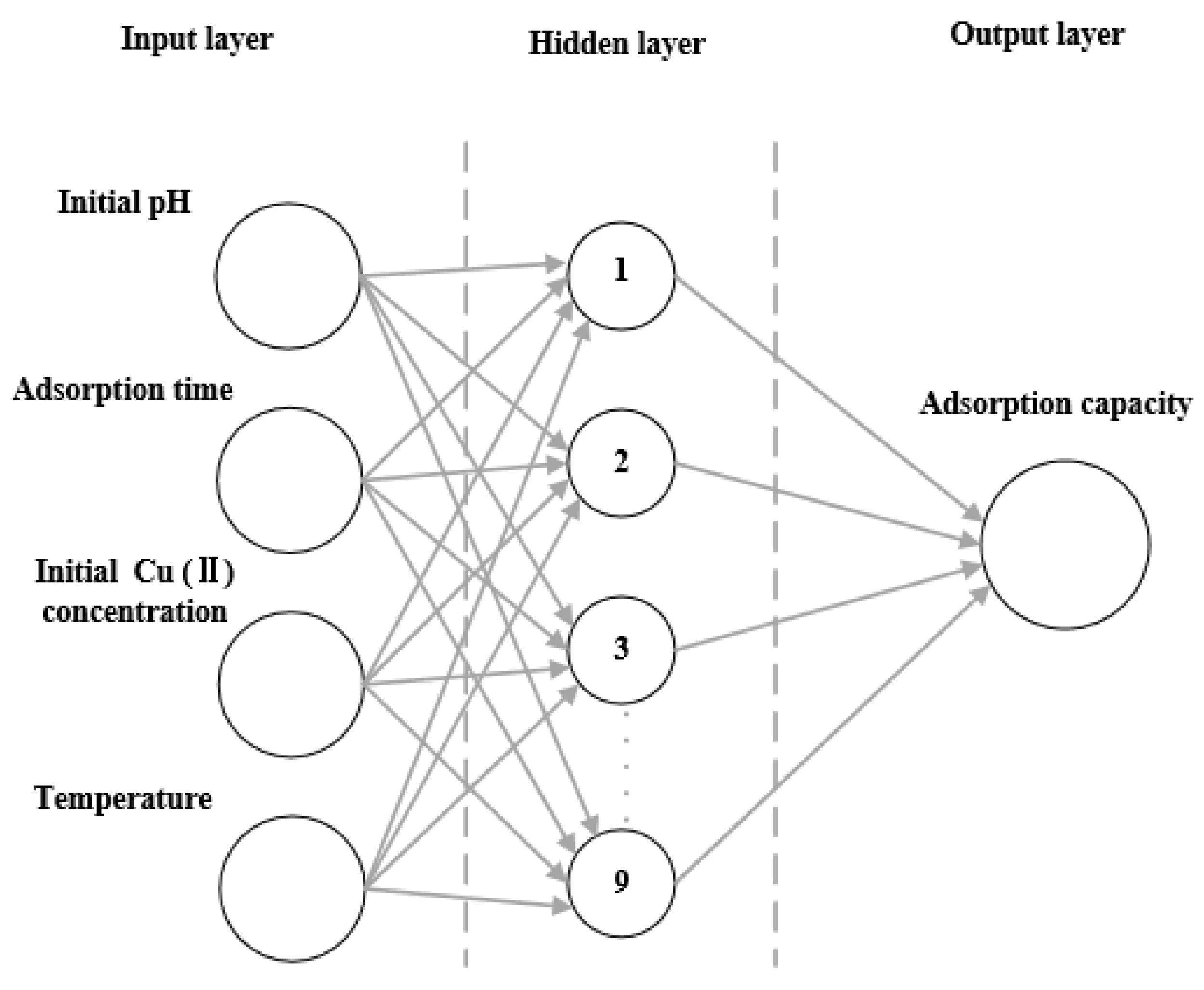
3.5. Artificial Neural Network (ANN) Model
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Kumar, P.S.; Gayathri, R.; Rathi, B.S. A review on adsorptive separation of toxic metals from aquatic system using biochar produced from agro-waste. Chemosphere 2021, 285, 131438. [Google Scholar] [CrossRef]
- Damodaran, D.; Shetty, K.V.; Mohan, B.R. Effect of chelaters on bioaccumulation of Cd(II), Cu(II), Cr(VI), Pb(II) and Zn(II) in Galerina vittiformis from soil. Int. Biodeterior. Biodegrad. 2013, 85, 182–188. [Google Scholar] [CrossRef]
- Razzak, S.A.; Faruque, M.O.; Alsheikh, Z.; Alsheikhmohamad, L.; Alkuroud, D.; Alfayz, A.; Hossain, S.M.Z.; Hossain, M.M. A comprehensive review on conventional and biological-driven heavy metals removal from industrial wastewater. Environ. Adv. 2022, 7, 100168. [Google Scholar] [CrossRef]
- Burakov, A.E.; Galunin, E.V.; Burakova, I.V.; Kucherova, A.E.; Agarwal, S.; Tkachev, A.G.; Gupta, V.K. Adsorption of heavy metals on conventional and nanostructured materials for wastewater treatment purposes: A review. Ecotoxicol. Environ. Saf. 2018, 148, 702–712. [Google Scholar] [CrossRef] [PubMed]
- Çoruh, S.; Geyikçi, F.; Kılıç, E.; Çoruh, U. The use of NARX neural network for modeling of adsorption of zinc ions using activated almond shell as a potential biosorbent. Bioresour. Technol. 2014, 151, 406–410. [Google Scholar] [CrossRef]
- Syeda, H.I.; Sultan, I.; Razavi, K.S.; Yap, P.S. Biosorption of heavy metals from aqueous solution by various chemically modified agricultural wastes: A review. J. Water Proc. Eng. 2022, 46, 102446. [Google Scholar] [CrossRef]
- Hokkanen, S.; Bhatnagar, A.; Sillanpää, M. A review on modification methods to cellulose-based adsorbents to improve adsorption capacity. Water Res. 2016, 91, 156–173. [Google Scholar] [CrossRef]
- Benis, K.Z.; Damuchali, A.M.; McPhedran, K.N.; Soltan, J. Treatment of aqueous arsenic—A review of biosorbent preparation methods. J. Environ. Manag. 2020, 273, 111126. [Google Scholar] [CrossRef]
- O’Connell, D.W.; Birkinshaw, C.; O’Dwyer, T.F. Heavy metal adsorbents prepared from the modification of cellulose: A review. Bioresour. Technol. 2008, 99, 6709–6724. [Google Scholar] [CrossRef]
- Chen, D.Z.; Xie, S.S.; Chen, C.Q.; Quan, H.Y.; Hua, L.; Luo, X.B.; Guo, L. Activated biochar derived from pomelo peel as a high-capacity sorbent for removal of carbamazepine from aqueous solution. RSC Adv. 2017, 7, 54969–54979. [Google Scholar] [CrossRef]
- Cheng, D.; Ngo, H.H.; Guo, W.S.; Chang, S.W.; Nguyen, D.D.; Zhang, X.B.; Varjani, S.; Liu, Y. Feasibility study on a new pomelo peel derived biochar for tetracycline antibiotics removal in swine wastewater. Sci. Total Environ. 2020, 720, 137662. [Google Scholar] [CrossRef]
- Dinh, V.P.; Huynh, T.D.T.; Le, H.M.; Nguyen, V.D.; Dao, V.A.; Hung, N.Q.; Tuyen, L.A.; Lee, S.; Yi, J.; Nguyen, T.D.; et al. Insight into the adsorption mechanisms of methylene blue and chromium(iii) from aqueous solution onto pomelo fruit peel. RSC Adv. 2019, 9, 25847–25860. [Google Scholar] [CrossRef]
- Dinh, V.P.; Xuan, D.T.; Hung, N.Q.; Luu, T.T.; Do, T.T.T.; Nguyen, T.D.; Nguyen, V.D.; Anh, T.T.K.; Tran, N.Q. Primary biosorption mechanism of lead(II) and cadmium(II) cations from aqueous solution by pomelo (Citrus maxima) fruit peels. Environ. Sci. Pollut. Res. 2021, 28, 63504–63515. [Google Scholar] [CrossRef] [PubMed]
- Podstawczyk, D.; Witek-Krowiak, A.; Dawiec, A.; Bhatnagar, A. Biosorption of copper(II) ions by flax meal: Empirical modeling and process optimization by response surface methodology (RSM) and artificial neural network (ANN) simulation. Ecol. Eng. 2015, 83, 364–379. [Google Scholar] [CrossRef]
- Ilavenil, K.K.; Pandian, P.; Kasthuri, A. Adsorption study of removal of lead ions using Prosopis juliflora and prediction by artificial neural network modeling. Mater. Today. Proc. 2023, 72, 2344–2350. [Google Scholar] [CrossRef]
- Raju, C.H.A.I.; Yugandhar, N.M.; Naidu, S.S.; Beena, C.H.; Bai, M.T. Removal of manganese from synthetic waste waters with Parthenium Hysteroporous bud and leaf powder and optimization using Artificial Neural Networks and Response Surface Methodology. Mater. Today. Proc. 2021, 42, 851–861. [Google Scholar] [CrossRef]
- Manunza, B.; Deiana, S.; Pintore, M.; Gessa, C. Interaction of Ca2+ and Na+ ions with polygalacturonate chains: A molecular dynamics study. Glycoconj. J. 1998, 15, 297–300. [Google Scholar] [CrossRef]
- Benaïssa, H.; Elouchdi, M.A. Removal of copper ions from aqueous solutions by dried sunflower leaves. Chem. Eng. Process. Process Intensif. 2007, 46, 614–622. [Google Scholar] [CrossRef]
- Elliot, H.A.; Huang, C.P. Adsorption characteristics of some Cu(II) complexes on aluminosilicates. Water Res. 1981, 15, 849–855. [Google Scholar] [CrossRef]
- Liang, S.; Guo, X.; Feng, N.; Tian, Q. Application of orange peel xanthate for the adsorption of Pb2+ from aqueous solutions. J. Hazard. Mater. 2009, 170, 425–429. [Google Scholar] [CrossRef]
- Shandi, S.G.; Ardejani, F.D.; Sharifi, F. Assessment of Cu(II) removal from an aqueous solution by raw Gundelia Tournefortii as a new low-cost biosorbent: Experiments and modelling. Chin. J. Chem. Eng. 2019, 27, 1945–1955. [Google Scholar] [CrossRef]
- Wu, J.; Dong, J.; Wang, J. Adsorptive removal of Cu(II) from aqueous solution by fermented sweet sorghum residues as a novel biosorbent. J. Mol. Liq. 2022, 367, 120362. [Google Scholar] [CrossRef]
- Agouborde, L.; Navia, R. Heavy metals retention capacity of a non-conventional sorbent developed from a mixture of industrial and agricultural wastes. J. Hazard. Mater. 2009, 167, 536–544. [Google Scholar] [CrossRef] [PubMed]
- Milani, P.A.; Debs, K.B.; Carrilho, E.N.V.M. Agricultural solid waste for sorption of metal ions: Part I characterization and use of lettuce roots and sugarcane bagasse for Cu(II), Fe(II), Zn(II), and Mn(II) sorption from aqueous medium. Environ. Sci. Pollut. Res. 2018, 25, 35895–35905. [Google Scholar] [CrossRef] [PubMed]
- Hansen, H.K.; Arancibia, F.; Gutierrez, C. Adsorption of copper onto agriculture waste materials. J. Hazard. Mater. 2010, 180, 442–448. [Google Scholar] [CrossRef]
- Yadav, K.; Raphi, M.; Jagadevan, S. Adsorption of copper(II) on chemically modified biochar: A single-stage batch adsorber design and predictive modeling through artificial neural network. Biomass Conv. Bioref. 2021, 1–16. [Google Scholar] [CrossRef]
- Praksash, N.; Manikandan, S.A.; Govindarajan, L.; Vijayagopal, V. Prediction of biosorption efficiency for the removal of copper(II) using artificial neural networks. J. Hazard. Mater. 2008, 152, 1268–1275. [Google Scholar] [CrossRef]
- Asim, N.; Amin, M.H.; Samsudin, N.A.; Badiei, M.; Razali, H.; Akhtaruzzaman, M.; Amin, N.; Sopian, K. Development of effective and sustainable adsorbent biomaterial from an agricultural waste material: Cu(II) removal. Mater. Chem. Phys. 2020, 249, 123128. [Google Scholar] [CrossRef]
- Ma, X.M.; Guan, Y.L.; Mao, R.; Zheng, S.; Wei, Q. Modeling of lead removal by living Scenedesmus obliquus using backpropagation (BP) neural network algorithm. Environ. Technol. Innov. 2021, 22, 101410. [Google Scholar] [CrossRef]

| BET Surface Area (m2/g) | Average Pore Diameter (nm) | Pore Volume (10−3 cm3/g) | |
|---|---|---|---|
| PP | 0.4253 | 6.9269 | 0.737 |
| MPP | 0.9912 | 7.2912 | 1.807 |
| Bio-Sorbent | Adsorption Capacity (mg/g) | Reference |
|---|---|---|
| Pomelo peels | 3.38 | This work |
| Modified pomelo peels | 4.72 | This work |
| Sweet sorghum residues | 0.83 | [22] |
| Fermented sweet sorghum residues | 1.94 | [22] |
| Sawdust | 2.31 | [23] |
| Natura lettuce roots | 1.22 | [24] |
| Modified lettuce roots | 1.69 | [24] |
| Natura sugarcane bagasse | 0.58 | [24] |
| Modified sugarcane bagasse | 1.30 | [24] |
| Peach stones and pine sawdust | 10–15 | [25] |
| Raw coconut coir | 1.15 | [26] |
| CC NaOH 4.3M-RT-60 | 7.86 | [26] |
| T (K) | KD | ΔGΘ (KJ/mol) | ΔHΘ (KJ/mol) | ΔSΘ (J/(mol·K) |
|---|---|---|---|---|
| 288.15 | 3.2377 | −2.8146 | 13.01 | 55.46 |
| 293.15 | 4.0193 | −3.3905 | ||
| 298.15 | 4.2428 | −3.5825 | ||
| 303.15 | 4.6039 | −3.8484 | ||
| 308.15 | 4.9403 | −4.0925 | ||
| 313.15 | 5.1455 | −4.2649 |
| m | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 |
|---|---|---|---|---|---|---|---|---|---|
| RAAD (%) | 0.255 | 0.644 | 0.621 | 1.183 | 0.713 | 0.038 | 0.324 | 0.533 | 0.417 |
| R2 | 0.9998 | 0.9997 | 0.9997 | 0.9991 | 0.9978 | 0.9999 | 0.9999 | 0.9999 | 0.9989 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, R.; Jiao, M.; Zhao, N.; Jacquemin, J.; Zhang, Y.; Liu, H. Assessment of Cu(II) Removal from Aqueous Solutions by Modified Pomelo Peels: Experiments and Modelling. Molecules 2023, 28, 3438. https://doi.org/10.3390/molecules28083438
Zhang R, Jiao M, Zhao N, Jacquemin J, Zhang Y, Liu H. Assessment of Cu(II) Removal from Aqueous Solutions by Modified Pomelo Peels: Experiments and Modelling. Molecules. 2023; 28(8):3438. https://doi.org/10.3390/molecules28083438
Chicago/Turabian StyleZhang, Ruixue, Mengqing Jiao, Nan Zhao, Johan Jacquemin, Yinqin Zhang, and Honglai Liu. 2023. "Assessment of Cu(II) Removal from Aqueous Solutions by Modified Pomelo Peels: Experiments and Modelling" Molecules 28, no. 8: 3438. https://doi.org/10.3390/molecules28083438
APA StyleZhang, R., Jiao, M., Zhao, N., Jacquemin, J., Zhang, Y., & Liu, H. (2023). Assessment of Cu(II) Removal from Aqueous Solutions by Modified Pomelo Peels: Experiments and Modelling. Molecules, 28(8), 3438. https://doi.org/10.3390/molecules28083438








