Activated Carbon with Ultrahigh Specific Surface Derived from Bamboo Shoot Shell through K2FeO4 Oxidative Pyrolysis for Adsorption of Methylene Blue
Abstract
1. Introduction
2. Results and Discussion
2.1. Effect of Activation Parameters on Preparation of BACs
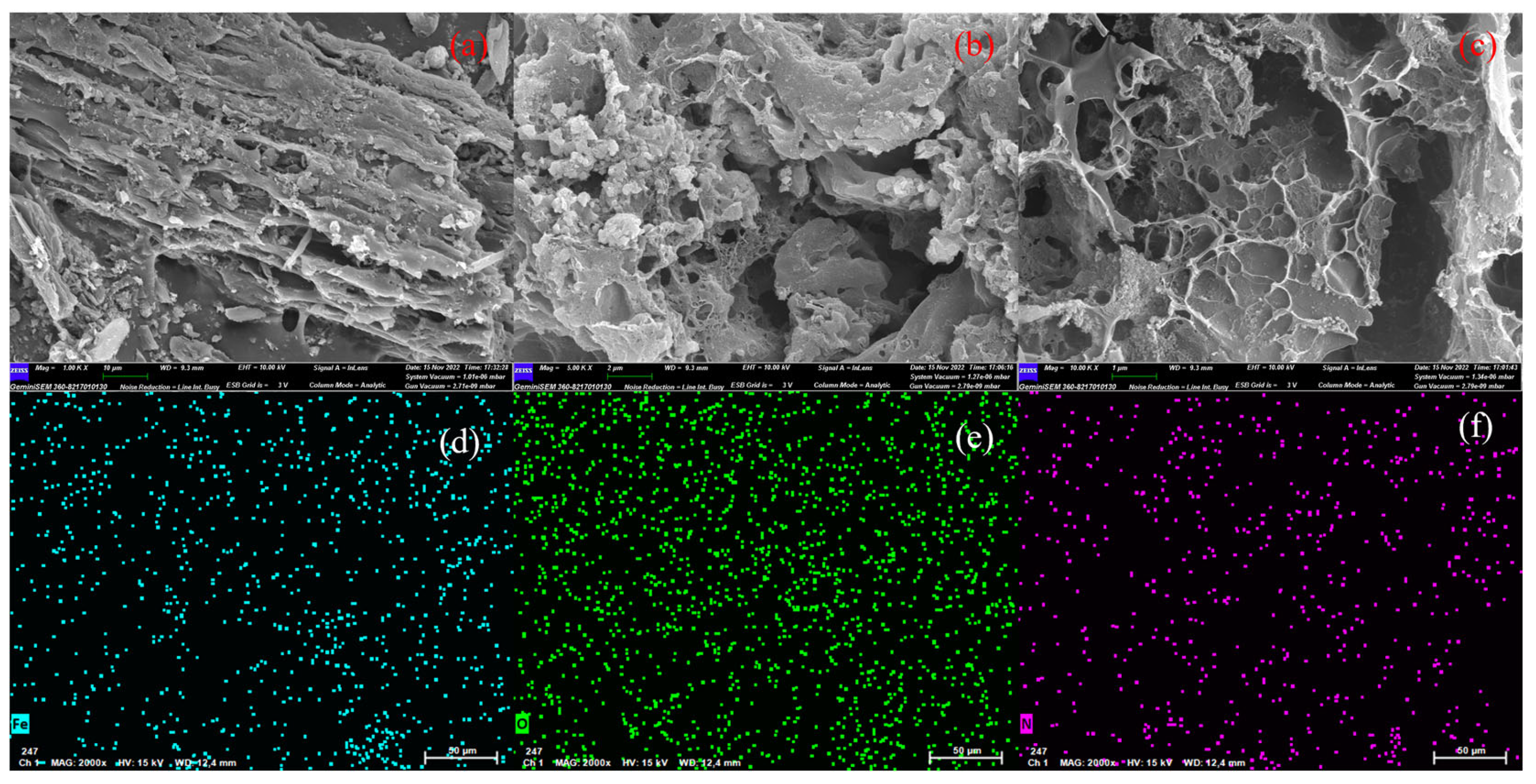
2.2. Characterizations of BACs
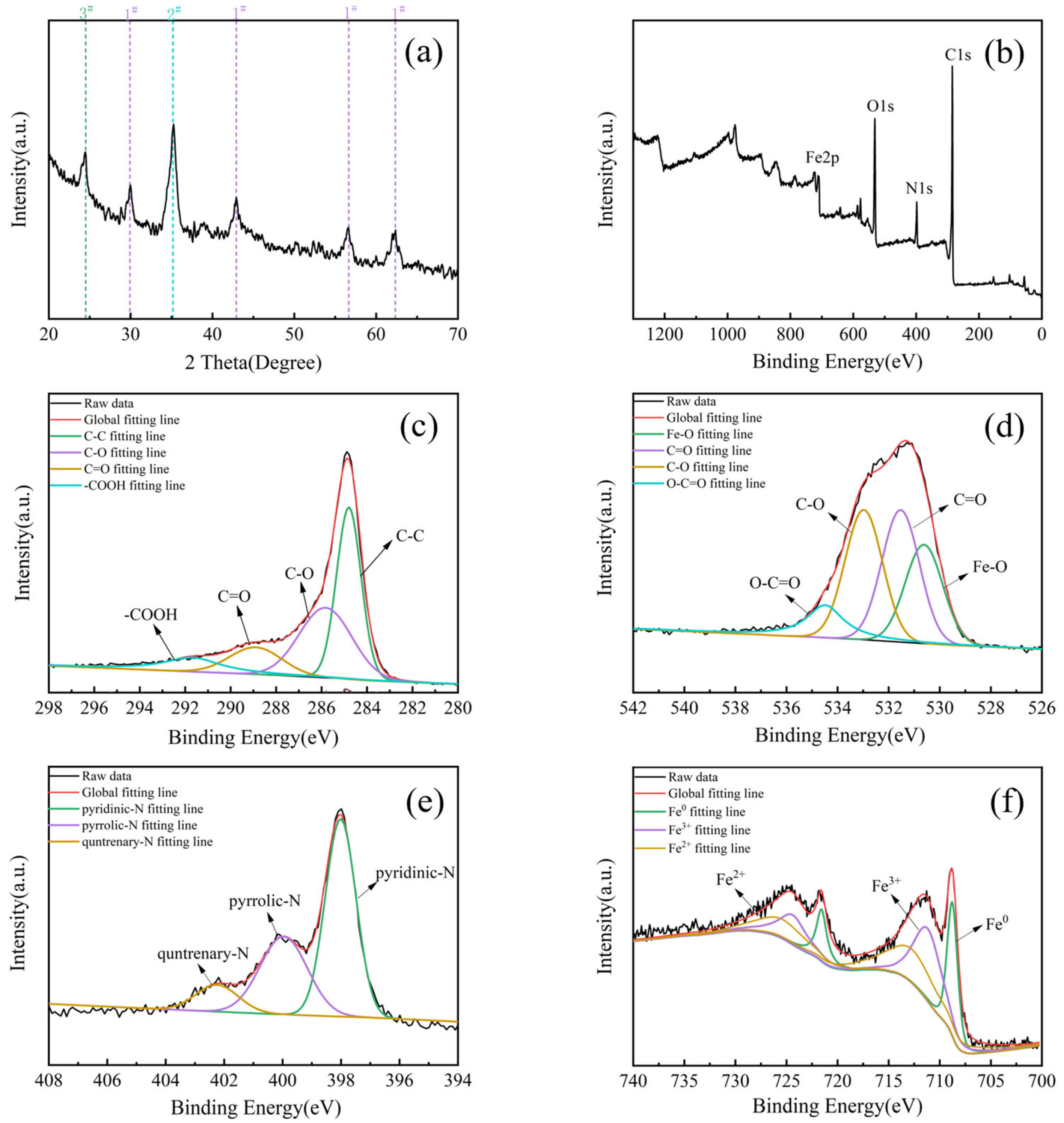
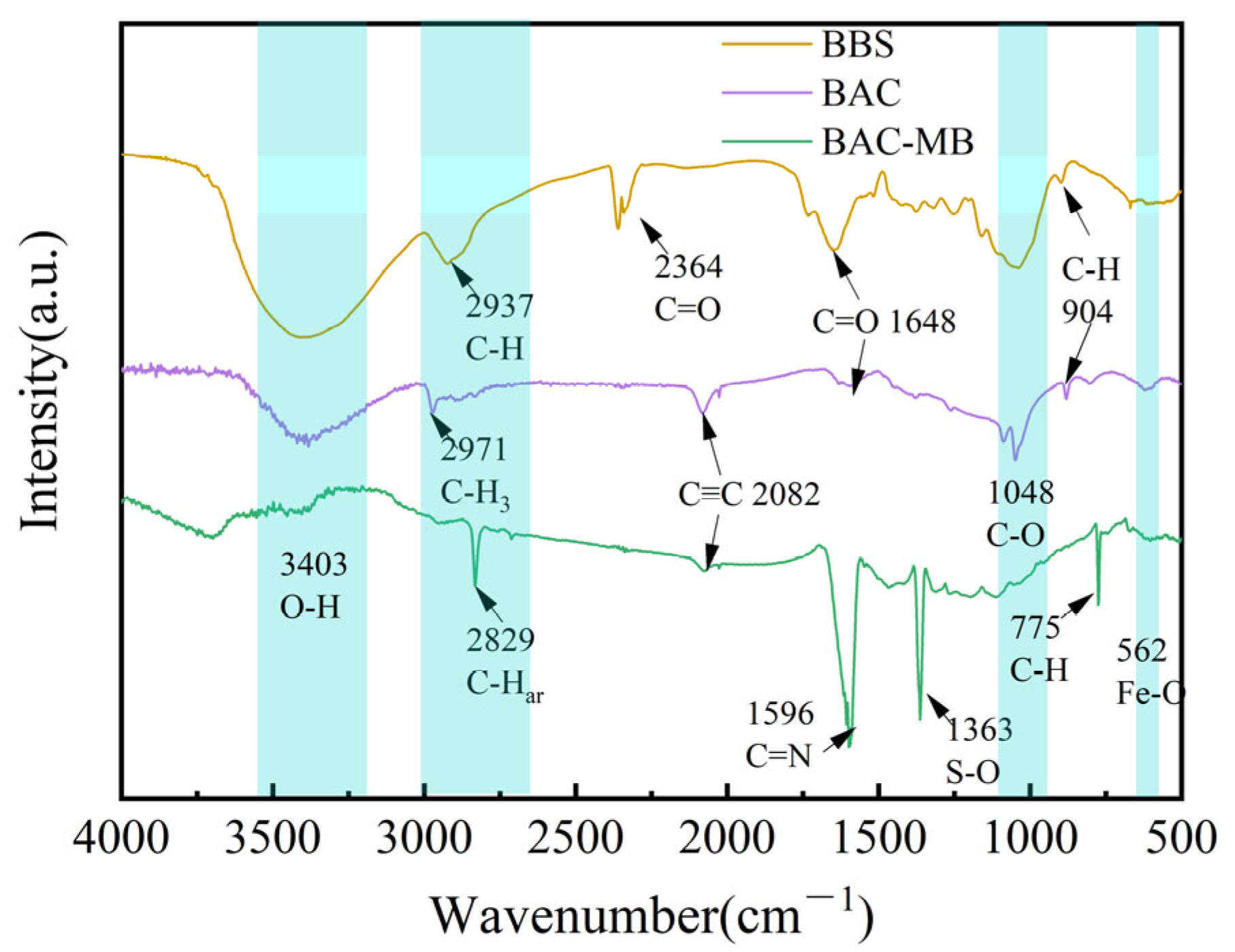
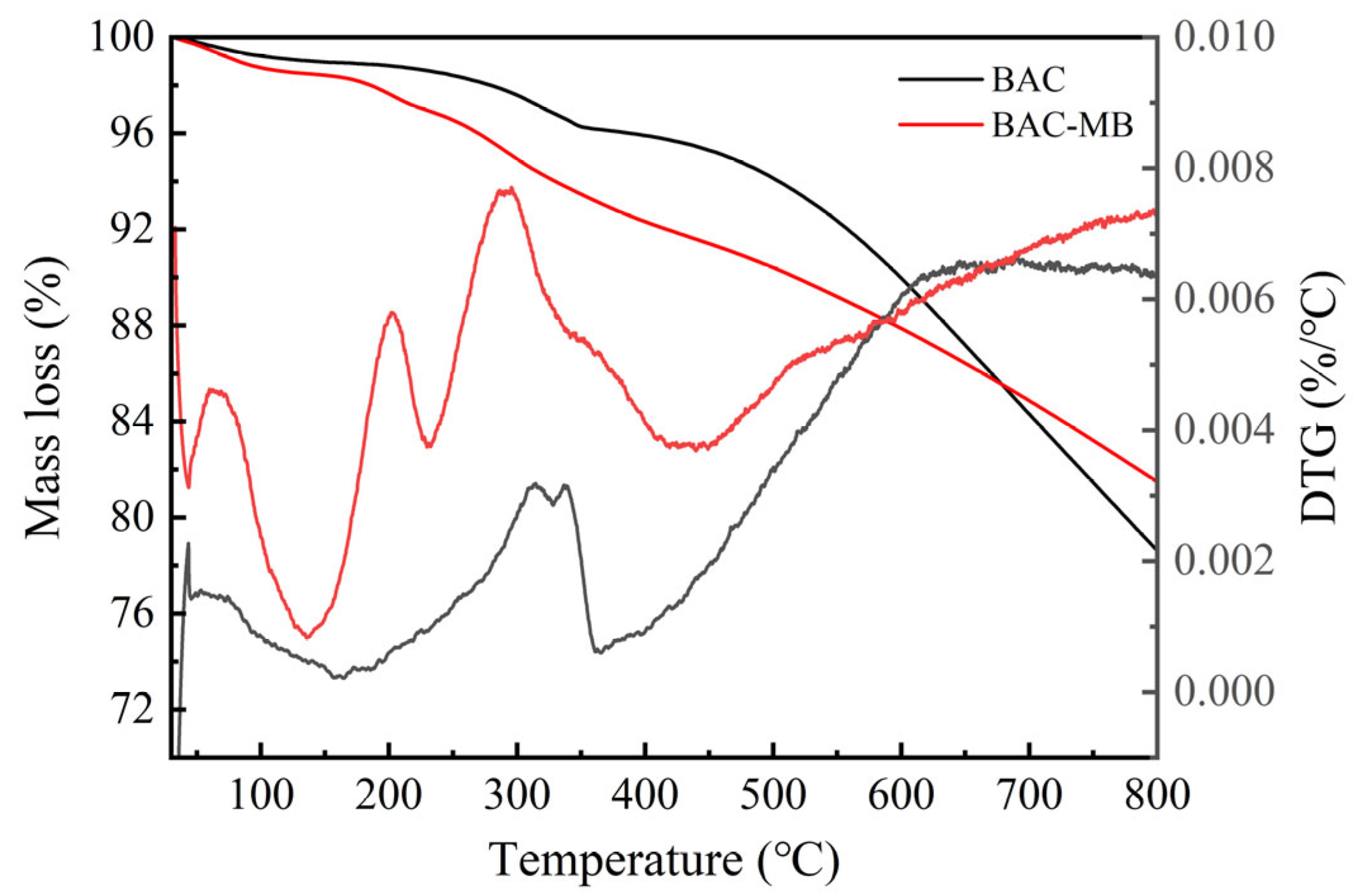
2.3. Characterizations of Optimized BAC
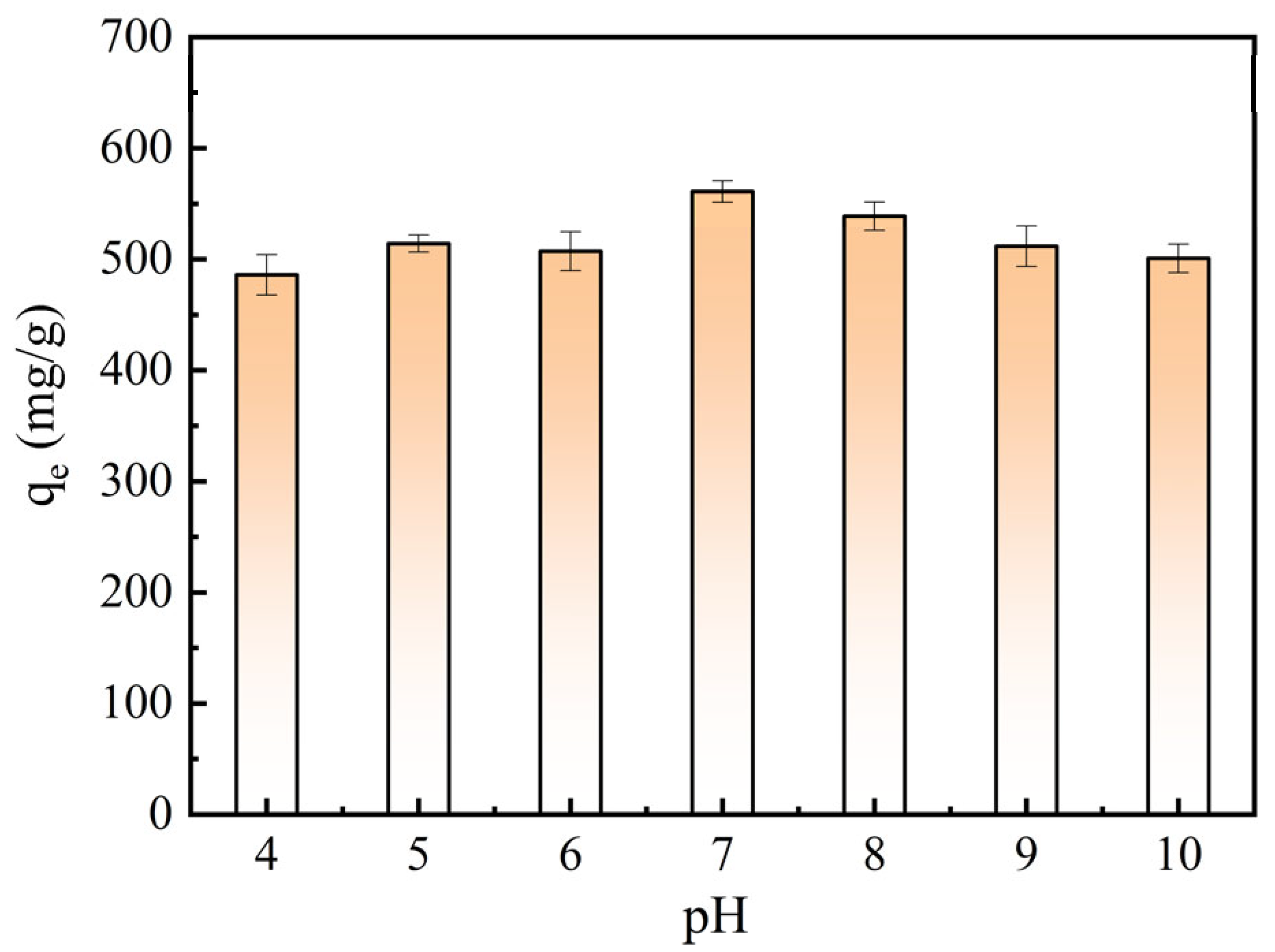
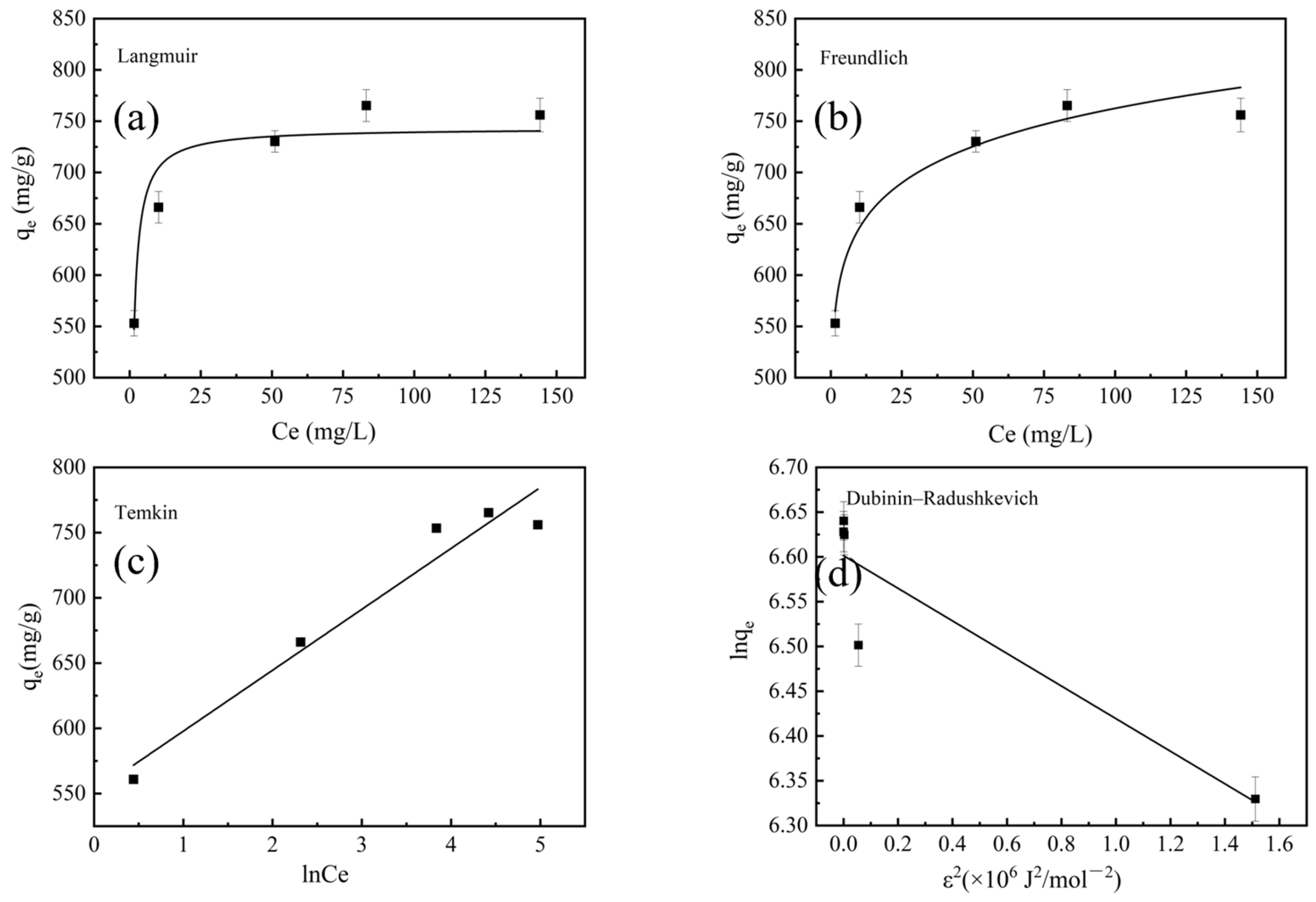
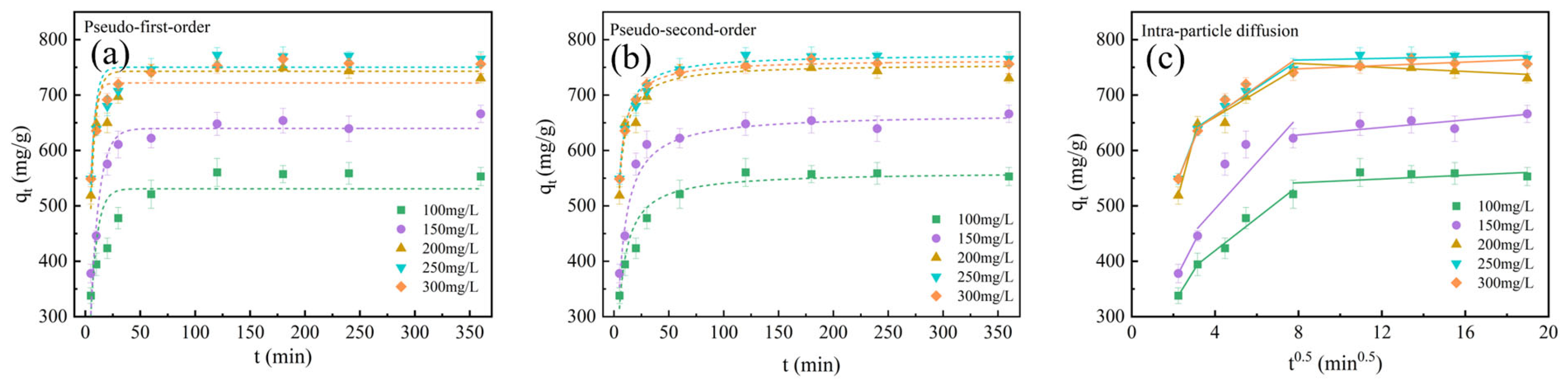
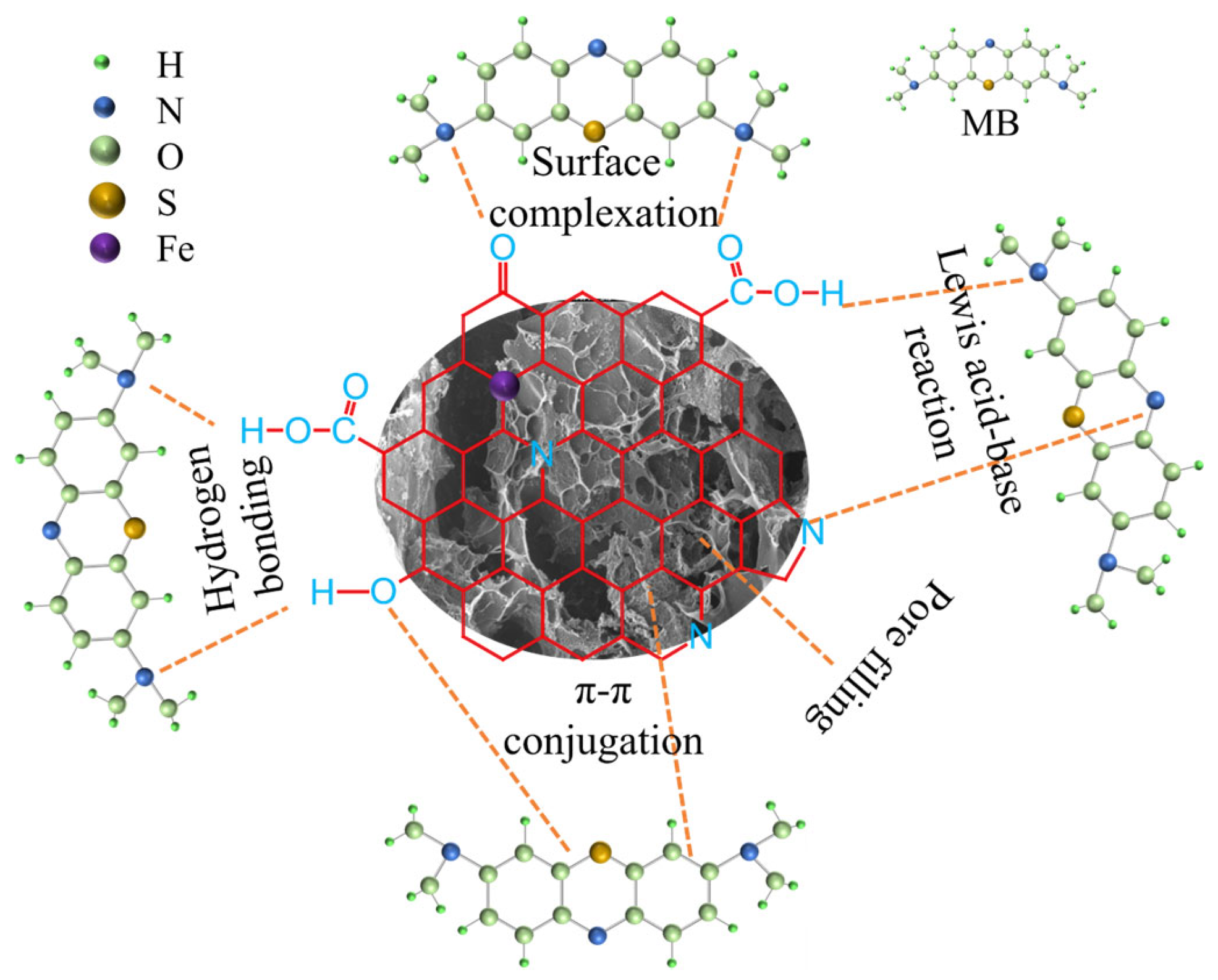
2.4. Adsorption Properties of Optimized BAC
3. Materials and Methods
3.1. Materials
3.2. Preparation and Characterization of BACs
3.3. Adsorption Performances of Methylene Blue (MB)
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Yadav, S.K.; Dhakate, S.R.; Singh, B.P. Carbon nanotube incorporated eucalyptus derived activated carbon-based novel adsorbent for efficient removal of methylene blue and eosin yellow dyes. Bioresour. Technol. 2022, 344, 126231. [Google Scholar] [CrossRef] [PubMed]
- Tran, T.H.; Le, H.H.; Pham, T.H.; Nguyen, D.T.; La, D.D.; Chang, S.W.; Lee, S.M.; Chung, W.J.; Nguyen, D.D. Comparative study on methylene blue adsorption behavior of coffee husk-derived activated carbon materials prepared using hydrothermal and soaking methods. J. Environ. Chem. Eng. 2021, 9, 105362. [Google Scholar] [CrossRef]
- Katheresan, V.; Kansedo, J.; Lau, S.Y. Efficiency of various recent wastewater dye removal methods: A review. J. Environ. Chem. Eng. 2018, 6, 4676–4697. [Google Scholar] [CrossRef]
- Wang, Q.; Lai, Z.; Luo, C.; Zhang, J.; Cao, X.; Liu, J.; Mu, J. Honeycomb-like activated carbon with microporous nanosheets structure prepared from waste biomass cork for highly efficient dye wastewater treatment. J. Hazard. Mater. 2021, 416, 125896. [Google Scholar] [CrossRef] [PubMed]
- Rajeswari, A.; Vismaiya, S.; Pius, A. Preparation, characterization of nano ZnO-blended cellulose acetate-polyurethane membrane for photocatalytic degradation of dyes from water. Chem. Eng. J. 2017, 313, 928–937. [Google Scholar] [CrossRef]
- Gokce, Y.; Yaglikci, S.; Yagmur, E.; Banford, A.; Aktas, Z. Adsorption behaviour of high performance activated carbon from demineralised low rank coal (rawdon) for methylene blue and phenol. J. Environ. Chem. Eng. 2021, 9, 104819. [Google Scholar] [CrossRef]
- Ihaddaden, S.; Aberkane, D.; Boukerroui, A.; Robert, D. Removal of methylene blue (basic dye) by coagulation-flocculation with biomaterials (bentonite and opuntia ficus indica). J. Water Process. Eng. 2022, 49, 102952. [Google Scholar] [CrossRef]
- Shu, Z.; Wu, H.; Lin, H.; Li, T.; Liu, Y.; Ye, F.; Mu, X.; Li, X.; Jiang, X.; Huang, J. Decolorization of Remazol Brilliant Blue r using a novel acyltransferase-ISCO (in situ chemical oxidation) coupled system. Biochem. Eng. J. 2016, 115, 56–63. [Google Scholar] [CrossRef]
- Hu, Y.; Chen, D.; Wang, S.; Zhang, R.; Wang, Y.; Liu, M. Activation of peroxymonosulfate by nitrogen-doped porous carbon for efficient degradation of organic pollutants in water: Performance and mechanism. Sep. Purif. Technol. 2022, 280, 119791. [Google Scholar] [CrossRef]
- Paredes-Laverde, M.; Salamanca, M.; Diaz-Corrales, J.D.; Flórez, E.; Silva-Agredo, J.; Torres-Palma, R.A. Understanding the removal of an anionic dye in textile wastewaters by adsorption on ZnCl2 activated carbons from rice and coffee husk wastes: A combined experimental and theoretical study. J. Environ. Chem. Eng. 2021, 9, 105685. [Google Scholar] [CrossRef]
- Yan, J.; Zuo, X.; Yang, S.; Chen, R.; Cai, T.; Ding, D. Evaluation of potassium ferrate activated biochar for the simultaneous adsorption of copper and sulfadiazine: Competitive versus synergistic. J. Hazard. Mater. 2022, 424, 127435. [Google Scholar] [CrossRef]
- Qu, J.; Wu, Z.; Liu, Y.; Li, R.; Wang, D.; Wang, S.; Wei, S.; Zhang, J.; Tao, Y.; Jiang, Z.; et al. Ball milling potassium ferrate activated biochar for efficient chromium and tetracycline decontamination: Insights into activation and adsorption mechanisms. Bioresour. Technol. 2022, 360, 127407. [Google Scholar] [CrossRef]
- Yin, Z.; Xu, S.; Liu, S.; Xu, S.; Li, J.; Zhang, Y. A novel magnetic biochar prepared by K2FeO4-promoted oxidative pyrolysis of pomelo peel for adsorption of hexavalent chromium. Bioresour. Technol. 2020, 300, 122680. [Google Scholar] [CrossRef]
- Tu, W.; Liu, Y.; Xie, Z.; Chen, M.; Ma, L.; Du, G.; Zhu, M. A novel activation-hydrochar via hydrothermal carbonization and KOH activation of sewage sludge and coconut shell for biomass wastes: Preparation, characterization and adsorption properties. J. Colloid Interface Sci. 2021, 593, 390–407. [Google Scholar] [CrossRef]
- Gao, Q.; Ni, L.; He, Y.; Hou, Y.; Hu, W.; Liu, Z. Effect of hydrothermal pretreatment on deashing and pyrolysis characteristics of bamboo shoot shells. Energy 2022, 247, 123510. [Google Scholar] [CrossRef]
- Mi, B.; Wang, J.; Xiang, H.; Liang, F.; Yang, J.; Feng, Z.; Zhang, T.; Hu, W.; Liu, X.; Liu, Z.; et al. Nitrogen self-doped activated carbons derived from bamboo shoots as adsorbent for methylene blue adsorption. Molecules 2019, 24, 3012. [Google Scholar] [CrossRef]
- Gao, Q.; Xiang, H.; Ni, L.; Hou, Y.; He, Y.; Feng, Z.; Yang, J.; Hu, W.; Liu, Z. Nitrogen self-doped activated carbons with narrow pore size distribution from bamboo shoot shells. Colloids Surfaces A 2021, 629, 127408. [Google Scholar] [CrossRef]
- Ma, D.; Yang, Y.; Liu, B.; Xie, G.; Chen, C.; Ren, N.; Xing, D. Zero-valent iron and biochar composite with high specific surface area via K2Feo4 fabrication enhances sulfadiazine removal by persulfate activation. Chem. Eng. J. 2021, 408, 127992. [Google Scholar] [CrossRef]
- Thi Minh Tam, N.; Liu, Y.; Bashir, H.; Yin, Z.; He, Y.; Zhou, X. Efficient removal of diclofenac from aqueous solution by potassium ferrate-activated porous graphitic biochar: Ambient condition influences and adsorption mechanism. Int. J. Environ. Res. Public Health 2020, 17, 291. [Google Scholar] [CrossRef]
- Yi, Y.; Huang, Z.; Lu, B.; Xian, J.; Tsang, E.P.; Cheng, W.; Fang, J.; Fang, Z. Magnetic biochar for environmental remediation: A review. Bioresour. Technol. 2020, 298, 122468. [Google Scholar] [CrossRef]
- Zhang, Y.; Wang, R.; Qiu, G.; Jia, W.; Guo, Y.; Guo, F.; Wu, J. Synthesis of porous material from coal gasification fine slag residual carbon and its application in removal of methylene blue. Molecules 2021, 26, 6116. [Google Scholar] [CrossRef] [PubMed]
- Gao, Q.; Feng, Z.; He, Y.; Hou, Y.; Ren, H.; Su, M.; Ni, L.; Liu, Z. Pyrolysis self-activation: An environmentally friendly method to transform biowaste into activated carbon for arsenic removal. Bioresour. Technol. 2023, 368, 128353. [Google Scholar] [CrossRef] [PubMed]
- Mechnou, I.; Meskini, S.; Mourtah, I.; Lebrun, L.; Hlaibi, M. Use of phosphorus-doped microporous carbon from olive mill wastewater for effective removal of crystal violet and methylene blue. J. Clean Prod. 2023, 393, 136333. [Google Scholar] [CrossRef]
- Mechnou, I.; Mourtah, I.; Raji, Y.; Chérif, A.; Lebrun, L.; Hlaibi, M. Effective treatment and the valorization of solid and liquid toxic discharges from olive oil industries, for sustainable and clean production of bio-coal. J. Clean Prod. 2021, 288, 125649. [Google Scholar] [CrossRef]
- Wu, G.; Zhang, X.; Hui, H.; Yan, J.; Zhang, Q.; Wan, J.; Dai, Y. Adsorptive removal of aniline from aqueous solution by oxygen plasma irradiated bamboo based activated carbon. Chem. Eng. J. 2012, 185, 201–210. [Google Scholar] [CrossRef]
- Cagnon, B.; Py, X.; Guillot, A.; Stoeckli, F. The effect of the carbonization/activation procedure on the microporous texture of the subsequent chars and active carbons. Microporous Mesoporous Mater. 2003, 57, 273–282. [Google Scholar] [CrossRef]
- Bouchelta, C.; Medjram, M.S.; Zoubida, M.; Chekkat, F.A.; Ramdane, N.; Bellat, J. Effects of pyrolysis conditions on the porous structure development of date pits activated carbon. J. Anal. Appl. Pyrolysis 2012, 94, 215–222. [Google Scholar] [CrossRef]
- Zhang, M.M.; Song, Z.X.; Liu, H.; Ma, T.J. Biomass-derived highly porous nitrogen-doped graphene orderly supported NiMn2O4 nanocrystals as efficient electrode materials for asymmetric supercapacitors. Appl. Surf. Sci. 2019, 507, 145065. [Google Scholar] [CrossRef]
- Ma, Y.; Li, M.; Li, P.; Yang, L.; Wu, L.; Gao, F.; Qi, X.; Zhang, Z. Hydrothermal synthesis of magnetic sludge biochar for tetracycline and ciprofloxacin adsorptive removal. Bioresour. Technol. 2021, 319, 124199. [Google Scholar] [CrossRef]
- Sun, Y.; Wang, T.; Han, C.; Lv, X.; Bai, L.; Sun, X.; Zhang, P. Facile synthesis of Fe-modified lignin-based biochar for ultra-fast adsorption of methylene blue: Selective adsorption and mechanism studies. Bioresour. Technol. 2022, 344, 126186. [Google Scholar] [CrossRef]
- Machado, N.C.F.; de Jesus, L.A.M.; Pinto, P.S.; de Paula, F.G.F.; Alves, M.O.; Mendes, K.H.A.; Mambrini, R.V.; Barrreda, D.; Rocha, V.; Santamaría, R.; et al. Waste-polystyrene foams-derived magnetic carbon material for adsorption and redox supercapacitor applications. J. Clean Prod. 2021, 313, 127903. [Google Scholar] [CrossRef]
- Molina-Sabio, M.; De Castro, M.C.M.; Martinez-Escandell, M.; Rodríguez-Reinoso, F. Preparation of high metal content nanoporous carbon. Fuel Process. Technol. 2013, 115, 115–121. [Google Scholar] [CrossRef]
- Li, S.; Yang, F.; Li, J.; Cheng, K. Porous biochar-nanoscale zero-valent iron composites: Synthesis, characterization and application for lead ion removal. Sci. Total Environ. 2020, 746, 141037. [Google Scholar] [CrossRef]
- Lian, F.; Cui, G.; Liu, Z.; Duo, L.; Zhang, G.; Xing, B. One-step synthesis of a novel N-doped microporous biochar derived from crop straws with high dye adsorption capacity. J. Environ. Manag. 2016, 176, 61–68. [Google Scholar] [CrossRef]
- Luo, S.; Lu, T.; Peng, L.; Shao, J.; Zeng, Q.; Gu, J. Synthesis of nanoscale zero-valent iron immobilized in alginate microcapsules for removal of Pb(Ⅱ) from aqueous solution. J. Mater. Chem. A 2014, 2, 15463. [Google Scholar] [CrossRef]
- Liu, Y.; Jiang, W.; Li, S.; Cheng, Z.P.; Song, D.; Zhang, X.J.; Li, F.S. Attachment of magnetic nanoparticles on carbon nanotubes using oleate as an interlinker molecule. Mater. Chem. Phys. 2009, 116, 438–441. [Google Scholar] [CrossRef]
- Chen, H.; Zhang, Y.J.; He, P.Y.; Li, C.J.; Liu, L.C. Facile synthesis of cost-effective iron enhanced hetero-structure activated carbon/geopolymer composite catalyst for nh3-scr: Insight into the role of iron species. Appl. Catal. A Gen. 2020, 605, 117804. [Google Scholar] [CrossRef]
- Sun, Z.; Mao, Y.; Liu, S.; Zhang, H.; Xu, Y.; Geng, R.; Lu, J.; Huang, S.; Yuan, Q.; Zhang, S.; et al. Effect of pretreatment with phanerochaete chrysosporium on physicochemical properties and pyrolysis behaviors of corn stover. Bioresour. Technol. 2022, 361, 127687. [Google Scholar] [CrossRef]
- Zhao, H.; Lang, Y. Behaviors and mechanisms of CIP and OFL adsorption by magnetic biochar. Environ. Sci. 2018, 39, 3729–3735. [Google Scholar] [CrossRef]
- Cha, J.S.; Jang, S.; Lam, S.S.; Kim, H.; Kim, Y.; Jeon, B.; Park, Y. Performance of CO2 and Fe-modified lignin char on arsenic (Ⅴ) removal from water. Chemosphere 2021, 279, 130521. [Google Scholar] [CrossRef]
- Ma, Y.; Yang, L.; Wu, L.; Li, P.; Qi, X.; He, L.; Cui, S.; Ding, Y.; Zhang, Z. Carbon nanotube supported sludge biochar as an efficient adsorbent for low concentrations of sulfamethoxazole removal. Sci. Total Environ. 2020, 718, 137299. [Google Scholar] [CrossRef] [PubMed]
- Mechnou, I.; Meskini, S.; El Ayar, D.; Lebrun, L.; Hlaibi, M. Olive mill wastewater from a liquid biological waste to a carbon/oxocalcium composite for selective and efficient removal of methylene blue and paracetamol from aqueous solution. Bioresour. Technol. 2022, 365, 128162. [Google Scholar] [CrossRef] [PubMed]
- Tang, L.; Yu, J.; Pang, Y.; Zeng, G.; Deng, Y.; Wang, J.; Ren, X.; Ye, S.; Peng, B.; Feng, H. Sustainable efficient adsorbent: Alkali-acid modified magnetic biochar derived from sewage sludge for aqueous organic contaminant removal. Chem. Eng. J. 2018, 336, 160–169. [Google Scholar] [CrossRef]
- Li, Y.; Taggart, M.A.; Mckenzie, C.; Zhang, Z.; Lu, Y.; Pap, S.; Gibb, S. Utilizing low-cost natural waste for the removal of pharmaceuticals from water: Mechanisms, isotherms and kinetics at low concentrations. J. Clean Prod. 2019, 227, 88–97. [Google Scholar] [CrossRef]
- Zhou, Y.; Liu, X.; Xiang, Y.; Wang, P.; Zhang, J.; Zhang, F.; Wei, J.; Luo, L.; Lei, M.; Tang, L. Modification of biochar derived from sawdust and its application in removal of tetracycline and copper from aqueous solution: Adsorption mechanism and modelling. Bioresour. Technol. 2017, 245, 266–273. [Google Scholar] [CrossRef]
- Mi, B.; Chen, X.; Jiang, C.; Wang, J.; Chen, X.; Zhang, B.; Liu, X.; Liu, Z.; Fei, B. Nitrogen-doped porous carbon derived from bamboo shoot as solid base catalyst for knoevenagel condensation and transesterification reactions. Catalysts 2018, 8, 232. [Google Scholar] [CrossRef]
- Yang, S.; Guo, Z.; Sheng, G.; Wang, X. Application of a novel plasma-induced CD/MWCNT/iron oxide composite in zinc decontamination. Carbohydr. Polym. 2012, 90, 1100–1105. [Google Scholar] [CrossRef]
- Fan, S.; Wang, Y.; Wang, Z.; Tang, J.; Tang, J.; Li, X. Removal of methylene blue from aqueous solution by sewage sludge-derived biochar: Adsorption kinetics, equilibrium, thermodynamics and mechanism. J. Environ. Chem. Eng. 2017, 5, 601–611. [Google Scholar] [CrossRef]
- Zou, C.; Wu, Q.; Gao, Z.; Xu, Z.; Nie, F. The magnetic porous biochar prepared by K2FeO4-promoted oxidative pyrolysis of bagasse for adsorption of antibiotics in the aqueous solution. Biomass Convers. Bior. 2022, 3, 1–17. [Google Scholar] [CrossRef]
- Li, L.; Wu, M.; Song, C.; Liu, L.; Gong, W.; Ding, Y.; Yao, J. Efficient removal of cationic dyes via activated carbon with ultrahigh specific surface derived from vinasse wastes. Bioresour. Technol. 2021, 322, 124540. [Google Scholar] [CrossRef]
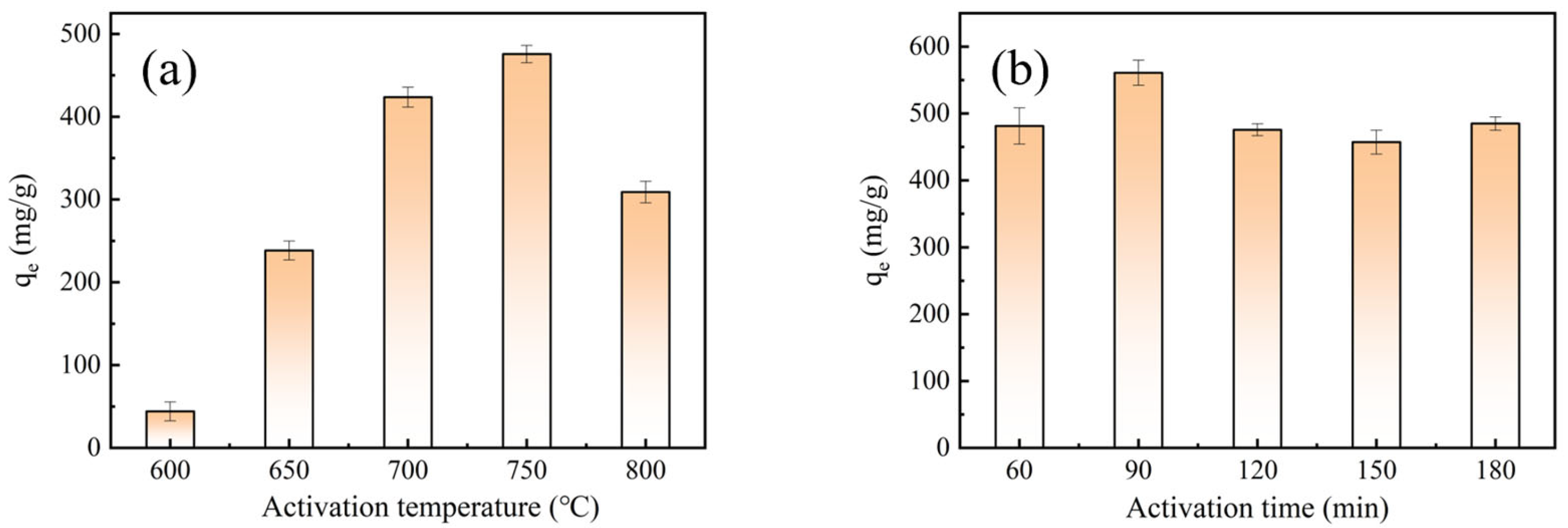
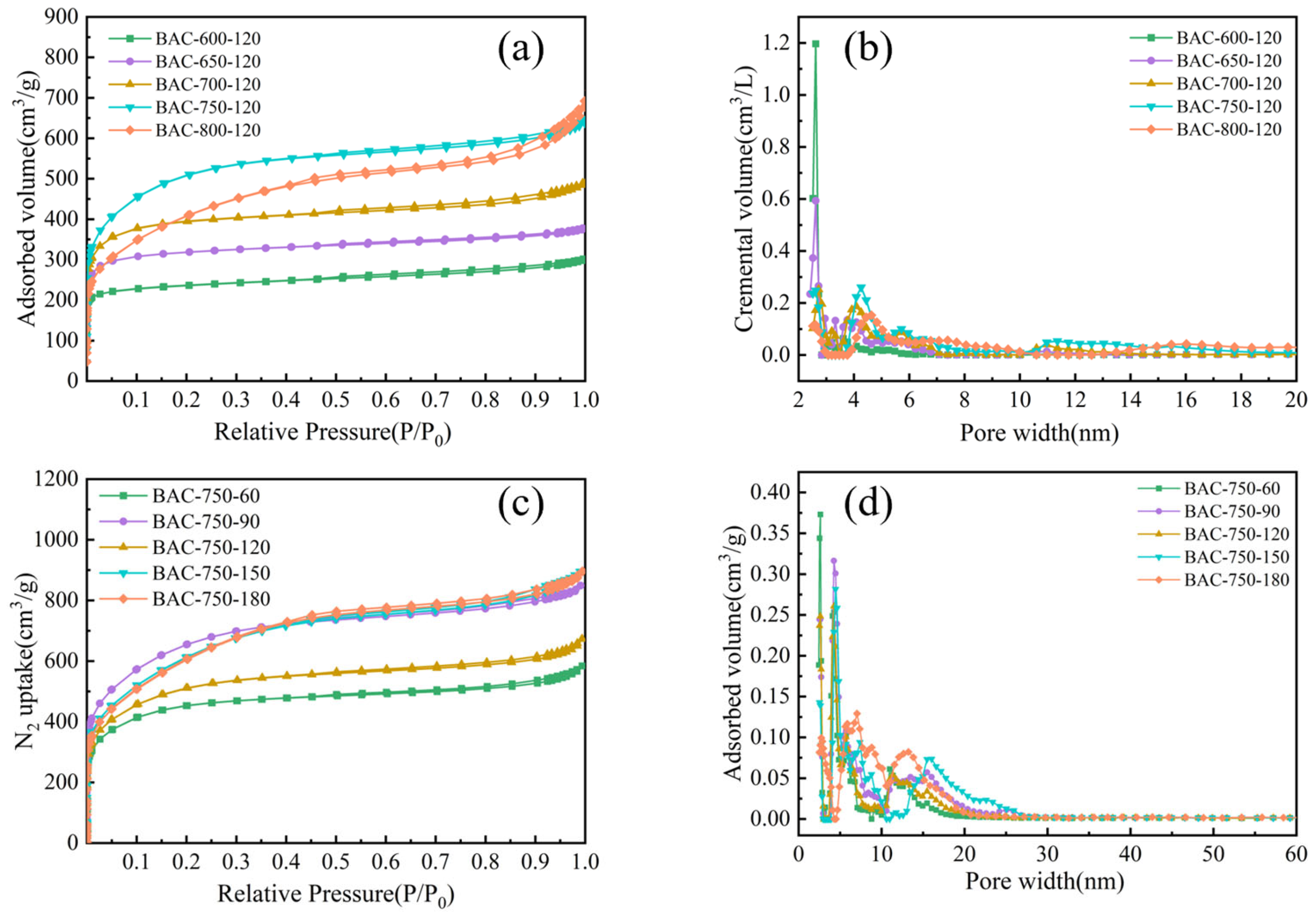

| Samples | SBET (cm2/g) | Vtot (cm3/g) | Vmic (cm3/g) | Vmic/Vtot (%) |
|---|---|---|---|---|
| BAC-600-120 | 919.9 | 0.45 | 0.31 | 69.08 |
| BAC-650-120 | 1243.0 | 0.59 | 0.44 | 73.95 |
| BAC-700-120 | 1469.2 | 0.76 | 0.55 | 72.04 |
| BAC-750-120 | 1835.1 | 1.04 | 0.67 | 64.23 |
| BAC-800-120 | 1450.7 | 1.11 | 0.38 | 34.59 |
| BAC-750-60 | 1649.0 | 0.90 | 0.62 | 68.14 |
| BAC-750-90 | 2327.7 | 1.31 | 0.84 | 63.82 |
| BAC-750-20 | 1835.1 | 1.04 | 0.67 | 64.23 |
| BAC-750-150 | 2169.1 | 1.38 | 0.67 | 48.23 |
| BAC-750-180 | 1729.0 | 0.87 | 0.65 | 74.62 |
| Isotherms | Parameters | ||
|---|---|---|---|
| Langmuir | Qm (mg/g) | KL (L/mg) RL | R2 |
| 745.23 | 1.79, 7.63–2.56 × 10−3 | 0.9414 | |
| Freundlich | KF ((mg/g) (L/mg)) 1/n | 1/n | R2 |
| 546.85 | 0.072 | 0.9664 | |
| Temkin | KT | B | R2 |
| 135.26 | 46.683 | 0.9511 | |
| Dubinin–Radushkevich | K (mol2 kJ2) | E (kJ/mol) | R2 |
| 0.1823 | 1.656 | 0.8454 | |
| Kinetics | Parameters | C0 (mg/g) | ||||
|---|---|---|---|---|---|---|
| 100 | 150 | 200 | 250 | 300 | ||
| qe,exp (mg/g) | 560.94 | 666.12 | 753.45 | 765.32 | 756.09 | |
| Pseudo-first-order kinetic model | k1 (min−1) | 0.17 | 0.13 | 0.22 | 0.24 | 0.28 |
| qe,cal (mg/g) | 530.89 | 639.49 | 742.94 | 750.18 | 721.86 | |
| R2 | 0.7626 | 0.9223 | 0.8364 | 0.8661 | 0.9295 | |
| Pseudo-second-order kinetic-model | k2 (g/mg/min) | 4.54 × 10−4 | 3.38 × 10−4 | 6.28 × 10−4 | 6.12 × 10−4 | 6.64 × 10−4 |
| qe,cal (mg/g) | 561.79 | 666.67 | 756.18 | 773.87 | 764.38 | |
| R2 | 0.9455 | 0.9937 | 0.9371 | 0.9902 | 0.9992 | |
| Intra-particle diffusion kinetic-model | k1 (g/mg/min−1/2) | 61.063 | 73.342 | 139.330 | 96.851 | 93.682 |
| C1 | 201.362 | 213.897 | 207.245 | 332.194 | 339.125 | |
| R12 | 1 | 1 | 1 | 1 | 1 | |
| k2 (g/mg/min−1/2) | 37.526 | 35.017 | 22.961 | 23.143 | 21.950 | |
| C2 | 268.519 | 380.975 | 565.232 | 572.379 | 582.382 | |
| R22 | 0.9854 | 0.7005 | 0.9265 | 0.9734 | 0.8674 | |
| k3 (g/mg/min−1/2) | −0.656 | 3.111 | −1.466 | 1.378 | 1.335 | |
| C3 | 566.824 | 604.468 | 763.829 | 746.593 | 736.569 | |
| R32 | 0.7966 | 0.65626 | 0.51949 | 0.30059 | 0.41108 | |
| ΔH (kJ/mol) | ΔS (kJ/mol/k) | ΔG (kJ/mol) | |||
|---|---|---|---|---|---|
| 288 K | 298 K | 308 K | 318 K | ||
| 13.31 | 48.72 | −6.28 | −13.58 | −17.10 | −21.20 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
He, Y.; Ni, L.; Gao, Q.; Ren, H.; Su, M.; Hou, Y.; Liu, Z. Activated Carbon with Ultrahigh Specific Surface Derived from Bamboo Shoot Shell through K2FeO4 Oxidative Pyrolysis for Adsorption of Methylene Blue. Molecules 2023, 28, 3410. https://doi.org/10.3390/molecules28083410
He Y, Ni L, Gao Q, Ren H, Su M, Hou Y, Liu Z. Activated Carbon with Ultrahigh Specific Surface Derived from Bamboo Shoot Shell through K2FeO4 Oxidative Pyrolysis for Adsorption of Methylene Blue. Molecules. 2023; 28(8):3410. https://doi.org/10.3390/molecules28083410
Chicago/Turabian StyleHe, Yuyu, Liangmeng Ni, Qi Gao, Hao Ren, Mengfu Su, Yanmei Hou, and Zhijia Liu. 2023. "Activated Carbon with Ultrahigh Specific Surface Derived from Bamboo Shoot Shell through K2FeO4 Oxidative Pyrolysis for Adsorption of Methylene Blue" Molecules 28, no. 8: 3410. https://doi.org/10.3390/molecules28083410
APA StyleHe, Y., Ni, L., Gao, Q., Ren, H., Su, M., Hou, Y., & Liu, Z. (2023). Activated Carbon with Ultrahigh Specific Surface Derived from Bamboo Shoot Shell through K2FeO4 Oxidative Pyrolysis for Adsorption of Methylene Blue. Molecules, 28(8), 3410. https://doi.org/10.3390/molecules28083410






