Effect of Microencapsulated Basil Extract on Cream Cheese Quality and Stability
Abstract
1. Introduction
2. Results and Discussion
2.1. Total Polyphenol Content and Antioxidant Activity of Basil Extract
2.2. Antibacterial Activity of Basil Extract
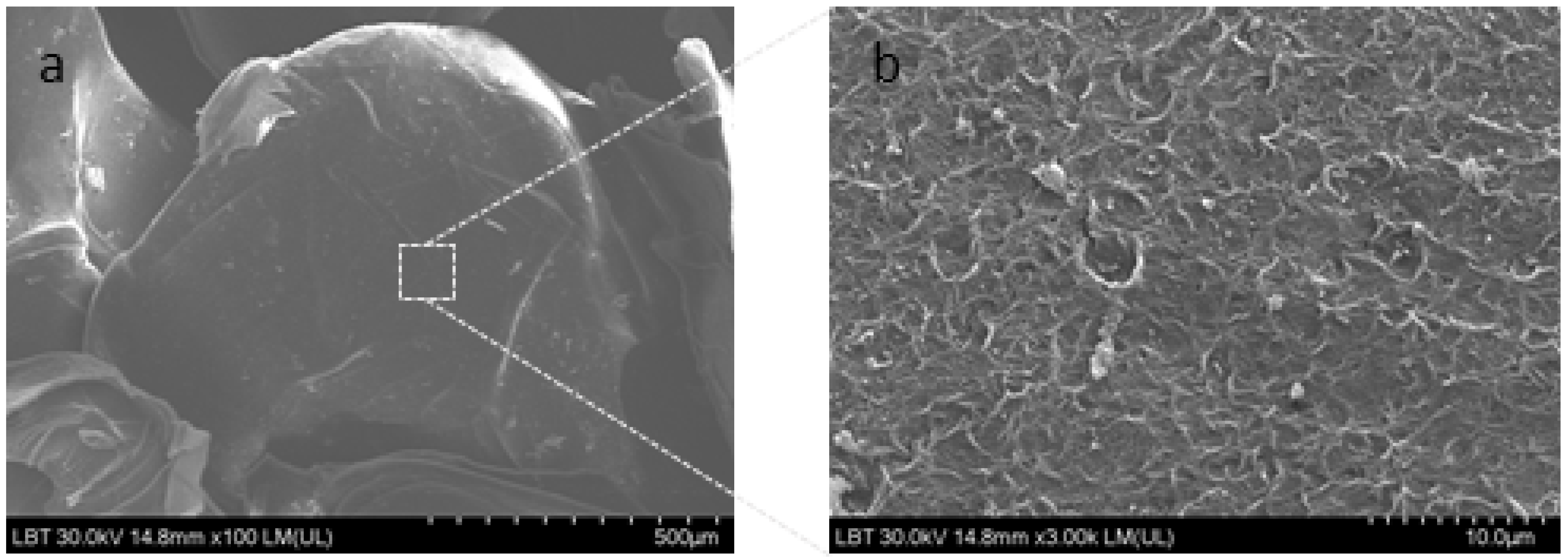
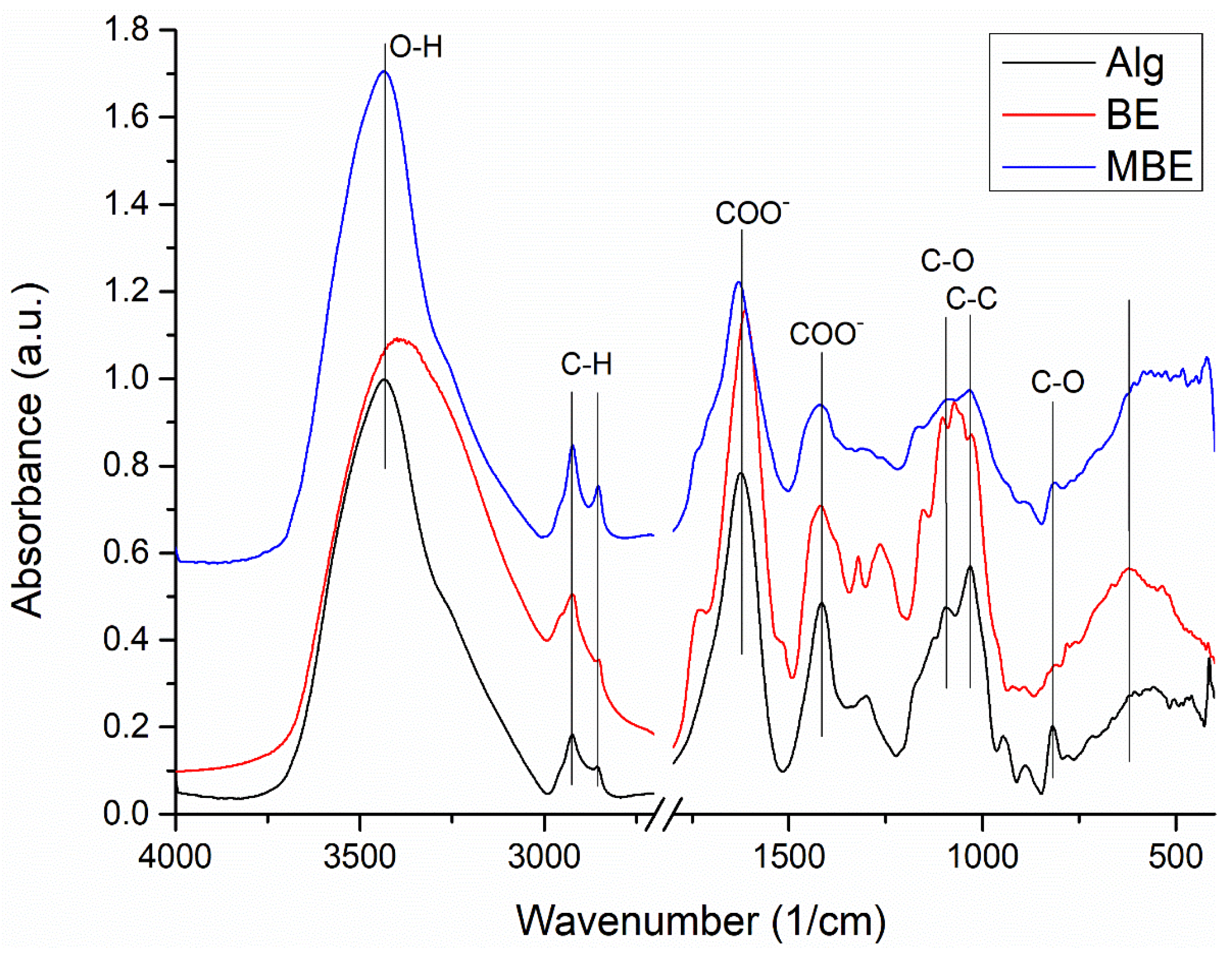
2.3. Microencapsulated Basil Extract Characterization
2.4. Cream Cheese Fortified with Microencapsulated Basil Extract Characterization
2.4.1. Physicochemical Analysis of the Cream Cheese Fortified with Microencapsulated Basil Extract
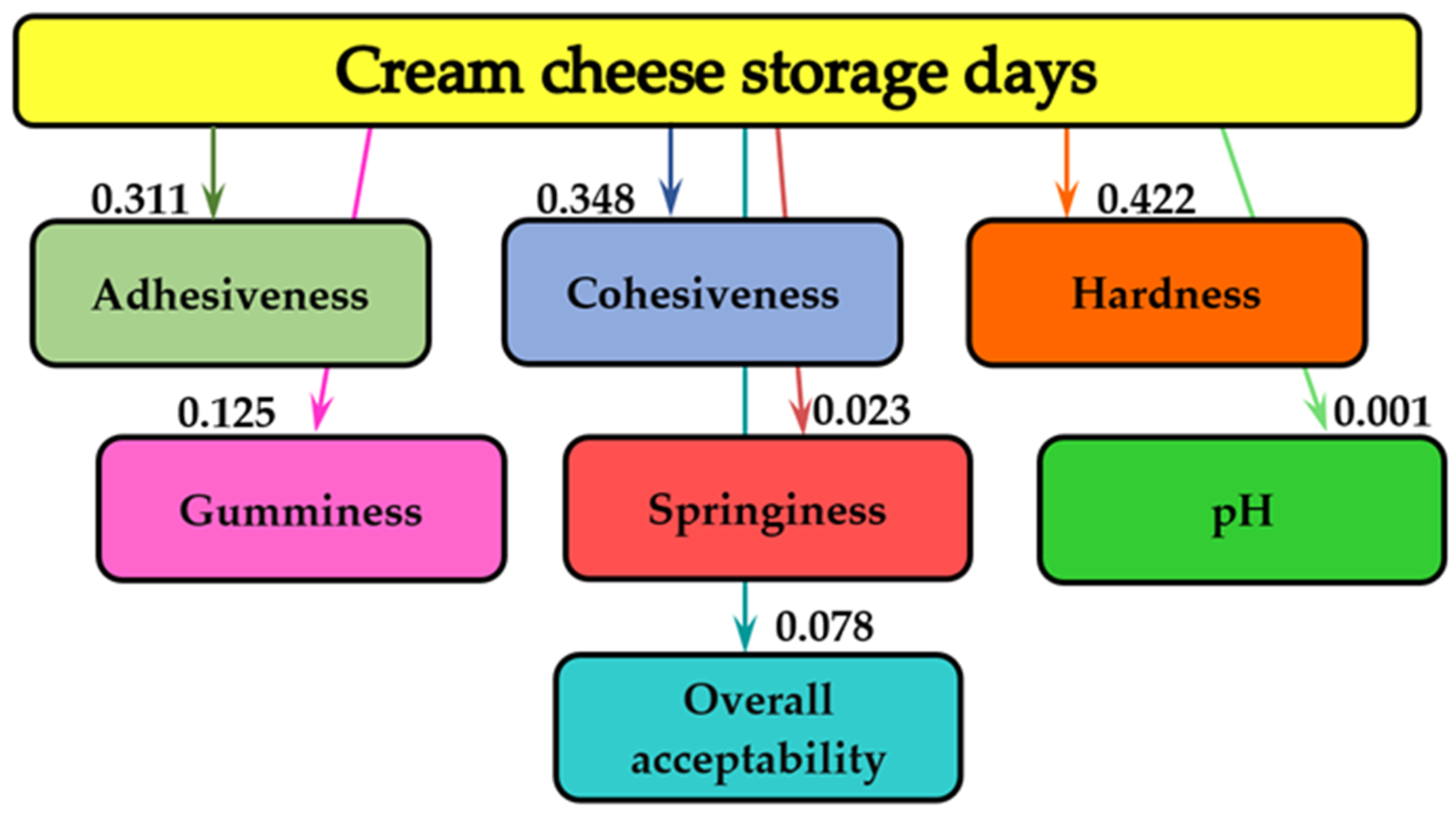
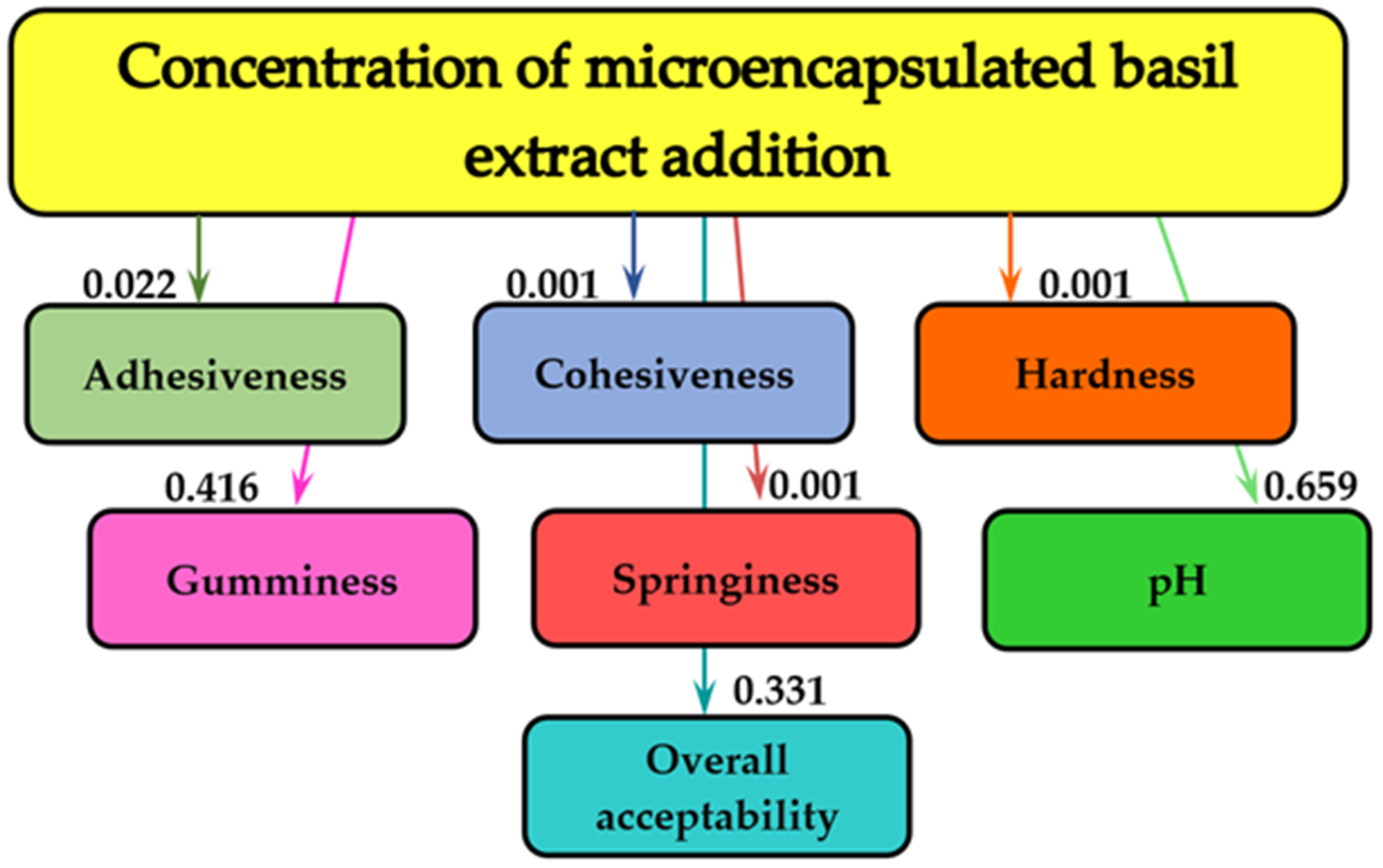
2.4.2. Evolution of the Cream Cheese Fortified with Microencapsulated Basil Extract Characteristics during Storage
2.5. Mathematical Modeling
3. Materials and Methods
3.1. Materials
3.2. Extraction and Characterization of Basil Extract
3.2.1. Preparation Basil Extract
3.2.2. Total Polyphenol Content
3.2.3. HPLC–DAD-ESI-MS Analysis of Polyphenols
3.2.4. Antioxidant Activity
3.2.5. Antimicrobial Activity
Test Microorganisms
Agar Well Diffusion Method
Minimal Inhibitory Concentration and Minimum Bactericidal Concentration
3.3. Preparation and Characterization of Microencapsulated Basil Extract
3.3.1. Preparation Microencapsulated Basil Extract
3.3.2. Physicochemical Analysis
3.3.3. Scanning Electron Microscope
3.3.4. Fourier-Transform Infrared Spectroscopy
3.3.5. Encapsulation Efficiency
3.4. Preparation and Characterization of Cream Cheese with Microencapsulated Basil Extract
3.4.1. Preparation Cream Cheese with Microencapsulated Basil Extract
3.4.2. Physicochemical Analysis
3.4.3. Sensory Analysis
3.4.4. Texture Profile Analysis
3.5. Mathematical Modeling
3.6. Statistical Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Moula Ali, M.A.; Sant’Ana, A.S.; Bavisetty, S.C.B. Sustainable preservation of cheese: Advanced technologies, physicochemical properties and sensory attributes. Trends Food Sci. Technol. 2022, 129, 306–326. [Google Scholar] [CrossRef]
- Costa, M.J.; Maciel, L.C.; Teixeira, J.A.; Vicente, A.A.; Cerqueira, M.A. Use of edible films and coatings in cheese preservation: Opportunities and challenges. Food Res. Int. 2018, 107, 84–92. [Google Scholar] [CrossRef] [PubMed]
- Weragama, D.; Weerasingha, V.; Jayasumana, L.; Adikari, J.; Vidanarachchi, J.K.; Priyashantha, H. The physicochemical, microbiological, and organoleptic properties and antioxidant activities of cream cheeses fortified with dried curry leaves (Murraya koenigii L.) powder. Food Sci. Nutr. 2021, 9, 5774–5784. [Google Scholar] [CrossRef] [PubMed]
- Monnet, C.; Landaud, S.; Bonnarme, P.; Swennen, D. Growth and adaptation of microorganisms on the cheese surface. FEMS Microbiol. Lett. 2014, 263, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Popescu, L. Effects of natural bioactive compounds on microbial safety and quality of dairy products. J. Eng. Sci. 2021, 2, 147–158. [Google Scholar] [CrossRef]
- Nájera, A.I.; Nieto, S.; Barron, L.J.R.; Albisu, M. A Review of the preservation of hard and semi-hard cheeses: Quality and safety. Int. J. Environ. Res. Public Health 2021, 18, 9789. [Google Scholar] [CrossRef] [PubMed]
- Christaki, S.; Moschakis, T.; Kyriakoudi, A.; Biliaderis, C.G.; Mourtzinos, I. Recent advances in plant essential oils and extracts: Delivery systems and potential uses as preservatives and antioxidants in cheese. Trends Food Sci. Technol. 2021, 116, 264–278. [Google Scholar] [CrossRef]
- Tarchoune, I.; Sgherri, C.; Izzo, R.; Lachaâl, M.; Navari-Izzo, F.; Ouerghi, Z. Changes in the antioxidative systems of Ocimum basilicum L. (cv. Fine) under different sodium salts. Acta Physiol. Plant 2012, 34, 1873–1881. [Google Scholar] [CrossRef]
- Nadeem, H.R.; Akhtar, S.; Sestili, P.; Ismail, T.; Neugart, S.; Qamar, M.; Esatbeyoglu, T. Toxicity, Antioxidant Activity, and Phytochemicals of Basil (Ocimum basilicum L.) Leaves Cultivated in Southern Punjab, Pakistan. Foods 2022, 11, 1239. [Google Scholar] [CrossRef] [PubMed]
- Leri, M.; Scuto, M.; Ontario, M.L.; Calabrese, V.; Calabrese, E.J.; Bucciantini, M.; Stefani, M. Healthy effects of plant polyphenols: Molecular mechanisms. Int. J. Mol. Sci. 2020, 21, 1250. [Google Scholar] [CrossRef]
- Fratianni, F.; Nazzaro, F.; Marandino, A.; Fusco, M.R.; Coppola, R.; Feo, V.D.; Martino, L.D. Biochemical composition, antimicrobial activities, and anti–quorum-sensing activities of ethanol and ethyl acetate extracts from Hypericum connatum Lam. (Guttiferae). J. Med. Food 2013, 16, 454–459. [Google Scholar] [CrossRef]
- Eghbal, N.; Liao, W.; Dumas, E.; Azabou, S.; Dantigny, P.; Gharsallaoui, A. Microencapsulation of natural food antimicrobials: Methods and applications. Appl. Sci. 2022, 12, 3837. [Google Scholar] [CrossRef]
- Mehta, N.; Kumar, P.; Verma, A.K.; Umaraw, P.; Kumar, Y.; Malav, O.P.; Sazili, A.Q.; Domínguez, R.; Lorenzo, J.M. Microencapsulation as a Noble Technique for the Application of Bioactive Compounds in the Food Industry: A Comprehensive Review. Appl. Sci. 2022, 12, 1424. [Google Scholar] [CrossRef]
- Haratifar, S.; Corredig, M. Interactions between tea catechins and casein micelles and the impact on renneting functionality. Food Chem. 2014, 143, 27–32. [Google Scholar] [CrossRef]
- Massounga Bora, A.F.; Ma, S.; Li, X.; Liu, L. Application of microencapsulation for the safe delivery of green tea polyphenols in food systems: Review and recent advances. Food Res. Int. 2018, 105, 241–249. [Google Scholar] [CrossRef]
- Soltanzadeh, M.; Peighambardoust, S.H.; Ghanbarzadeh, B.; Mohammadi, M.; Lorenzo, J.M. Chitosan nanoparticles as a promising nanomaterial for encapsulation of pomegranate (Punica granatum L.) peel extract as a natural source of antioxidants. Nanomaterials 2021, 11, 1439. [Google Scholar] [CrossRef]
- Gouvea, F.D.S.; Rosenthal, A.; Ferreira, E.H.D.R. Plant extract and essential oils added as antimicrobials to cheeses: A review. Ciência Rural 2017, 47, e20160908. [Google Scholar] [CrossRef]
- Saikia, S.; Mahnot, N.K.; Mahanta, C.L. Optimisation of phenolic extraction from Averrhoa carambola pomace by response surface methodology and its microencapsulation by spray and freeze drying. Food Chem. 2015, 171, 144–152. [Google Scholar] [CrossRef] [PubMed]
- Farrag, A.F.; Zahran, H.; Al-Okaby, M.F.; El-Sheikh, M.M.; Soliman, T.N. Physicochemical properties of white soft cheese supplemented with encapsulated olive phenolic compounds. Egypt. J. Chem. 2020, 63, 2921–2931. [Google Scholar] [CrossRef]
- Caleja, C.; Ribeiro, A.; Barros, L.; Barreira, J.C.M.; Antonio, A.L.; Oliveira, M.P.P.; Barreiro, M.F.; Ferreira, I.C.F.R. Cottage cheeses functionalized with fennel and chamomile extracts: Comparative performance between free and microencapsulated forms. Food Chem. 2016, 199, 720–726. [Google Scholar] [CrossRef] [PubMed]
- Soliman, T.N.; Mohammed, D.M.; El-Messery, T.M.; Elaaser, M.; Zaky, A.A.; Eun, J.-B.; Shim, J.-H.; El-Said, M.M. Microencapsulation of plant phenolic extracts using complex coacervation incorporated in ultrafiltered cheese against AlCl3-induced neuroinflammation in rats. Front. Nutr. 2022, 9, 929977. [Google Scholar] [CrossRef] [PubMed]
- El-Messery, T.M.; El-Said, M.M.; Shahein, N.M.; El-Din, H.F.; Farrag, A. Functional yoghurt supplemented with extract orange peel encapsulated using coacervation technique. Pak. J. Biol. Sci. 2019, 22, 231–238. [Google Scholar] [CrossRef]
- Nguyen, V.T.; Nguyen, N.Q.; Thi, N.Q.N.; Thi, C.Q.N.; Truc, T.T.; Nghi, P.T.B. Studies on chemical, polyphenol content, flavonoid content, and antioxidant activity of sweet basil leaves (Ocimum basilicum L.). IOP Conf. Ser. Mater. Sci. Eng. 2021, 1092, 012083. [Google Scholar] [CrossRef]
- Prinsi, B.; Morgutti, S.; Negrini, N.; Faoro, F.; Espen, L. Insight into composition of bioactive phenolic compounds in leaves and flowers of green and purple basil. Plants 2019, 9, 22. [Google Scholar] [CrossRef]
- Aburigal, Y.A.A.; Mirghani, M.E.S.; Elmogtaba, E.Y.; Sirible, A.A.M.; Hamza, N.B.; Hussein, I.H. Total phenolic content and antioxidant capacity of basil (Ocimum basilicum L.) leaves from different locations. Int. Food Res. J. 2017, 24, S378–S381. [Google Scholar]
- Zaman, G.; Farooq, U.; Bajwa, M.N.; Jan, H.; Shah, M.; Ahmad, R.; Andleeb, A.; Drouet, S.; Hano, C.; Abbasi, B.H. Effects of yeast extract on the production of phenylpropanoid metabolites in callus culture of purple basil (Ocimum Basilicum L. var purpurascens) and their in-vitro evaluation for antioxidant potential. Plant Cell Tissue Organ Cult. 2022, 150, 543–553. [Google Scholar] [CrossRef]
- Romano, R.; De Luca, L.; Aiello, A.; Pagano, R.; Di Pierro, P.; Pizzolongo, F.; Masi, P. Basil (Ocimum basilicum L.) Leaves as a Source of Bioactive Compounds. Foods 2022, 11, 3212. [Google Scholar] [CrossRef]
- Khatib, S.; Harnafi, M.; Touiss, I.; Bekkouch, O.; Milenkovic, D.; Amrani, S.; Harnafi, H. HPLC–DAD profiling of a phenolic extract from Moroccan sweet Basil and its application as oxidative stabilizer of sunflower oil. Chem. Pap. 2021, 75, 1907–1917. [Google Scholar] [CrossRef]
- Ghasemzadeh, A.; Ashkani, S.; Baghdadi, A.; Pazoki, A.; Jaafar, H.Z.E.; Rahmat, A. Improvement in Flavonoids and Phenolic Acids Production and Pharmaceutical Quality of Sweet Basil (Ocimum basilicum L.) by Ultraviolet-B Irradiation. Molecules 2016, 21, 1203. [Google Scholar] [CrossRef] [PubMed]
- Vlaicu, P.A.; Untea, A.E.; Turcu, R.P.; Saracila, M.; Panaite, T.D.; Cornescu, G.M. Nutritional composition and bioactive compounds of basil, thyme and sage plant additives and their functionality on broiler thigh meat quality. Foods 2022, 11, 1105. [Google Scholar] [CrossRef] [PubMed]
- Chulova, M.N.; Vrancheva, R.Z.; Stoyanova, M.A.; Pavlov, A.I. Antioxidant activity and phenolic profile of extracts of basil. Sci. Work. Univ. Food Technol. 2016, 63, 178–186. [Google Scholar]
- Willis, K.J. State of the World’s Plants 2017. Report; Royal Botanics Gardens Kew: London, UK, 2017; 100p, ISBN 9781842466476. [Google Scholar]
- Khameneh, B.; Iranshahy, M.; Soheili, V.; Bazzaz, B.S.F. Review on plant antimicrobials: A mechanistic viewpoint. Antimicrob. Resist. Infect. Control 2019, 8, 118. [Google Scholar] [CrossRef]
- Ghendov-Mosanu, A.; Cojocari, D.; Balan, G.; Patras, A.; Lung, I.; Soran, M.-L.; Opriş, O.; Cristea, E.; Sturza, R. Chemometric Optimization of Biologically Active Compounds Extraction from Grape Marc: Composition and Antimicrobial Activity. Molecules 2022, 27, 1610. [Google Scholar] [CrossRef] [PubMed]
- Ghendov-Moșanu, A.; Cojocari, D.; Balan, G.; Sturza, R. Antimicrobial activity of rose hip and hawthorn powders on pathogenic bacteria. J. Eng. Sci. 2018, 25, 100–107. [Google Scholar] [CrossRef]
- Sandulachi, E.; Cojocari, D.; BALAN, G.; Popescu, L.; Ghendov-Moșanu, A.; Sturza, R. Antimicrobial effects of berries on Listeria monocytogenes. Food Nutr. Sci. 2020, 11, 873–886. [Google Scholar] [CrossRef]
- Macari, A.; Sturza, R.; Lung, I.; Soran, M.-L.; Opris, O.; Balan, G.; Ghendov-Mosanu, A.; Vodnar, D.C.; Cojocari, D. Antimicrobial effects of basil, summer savory and tarragon lyophilized extracts in cold storage sausages. Molecules 2021, 26, 6678. [Google Scholar] [CrossRef] [PubMed]
- Sturza, R.; Sandulachi, E.; Cojocari, D.; Balan, G.; Popescu, L.; Ghendov-Mosanu, A. Antimicrobial properties of berry powders in cream cheese. J. Eng. Sci. 2019, 3, 125–136. [Google Scholar] [CrossRef]
- Deba, F.; Xuan, T.D.; Yasuda, M.; Tawata, S. Chemical composition and antioxidant, antibacterial and antifungal activities of the essential oils from Bidens pilosa Linn. var. Radiata. Food Control 2008, 19, 346–352. [Google Scholar] [CrossRef]
- Inouye, S.; Uchida, K.; Takizawa, T.; Yamaguchi, H.; Abe, S. Evaluation of the effect of terpenoid quinones on Trichophyton mentagrophytes by solution and vapor contact. J. Infect. Chemother. 2006, 12, 100–104. [Google Scholar] [CrossRef] [PubMed]
- Adigüzel, A.; Medine, G.; Meryem, B.; Hatice, U.T.C.; Fikrettin, A.; Üsa, K. Antimicrobial effects of Ocimum basilicum (Labiatae) extract. Turk. J. Biol. 2005, 29, 155–160. [Google Scholar]
- Petchsomrit, A.; Sermkaew, N.; Wiwattanapatapee, R. Effect of alginate and surfactant on physical properties of oil entrapped alginate bead formulation of curcumin. Int. J. Pharm. Pharm. Sci. 2013, 7, 864–868. [Google Scholar]
- Yang, N.; Wang, R.; Rao, P.; Yan, L.; Zhang, W.; Wang, J.; Chai, F. The fabrication of calcium alginate beads as a green sorbent for selective recovery of Cu(Ⅱ) from metal mixtures. Crystals 2019, 9, 255. [Google Scholar] [CrossRef]
- Sarker, B.; Papageorgiou, D.G.; Silva, R.; Zehnder, T.; Gul-E-Noor, F.; Bertmer, M.; Kaschta, J.; Chrissafis, K.; Detscha, R.; Boccaccini, A.R. Fabrication of alginate–gelatin crosslinked hydrogel microcapsules and evaluation of the microstructure and physico-chemical properties. J. Mater. Chem. B 2014, 2, 1470–1482. [Google Scholar] [CrossRef] [PubMed]
- Derkach, S.R.; Voron’ko, N.G.; Sokolan, N.I.; Kolotova, D.S.; Kuchina, Y.A. Interactions between gelatin and sodium alginate: UV and FTIR studies. J. Dispers. Sci. Technol. 2019, 41, 690–698. [Google Scholar] [CrossRef]
- Sukardi; Pulungan, M.H.; Purwaningsih, I.; Sita, P.F. Extraction of phenolic compounds from basil (Ocimum americanum L.) leaves with pretreatment using pulsed electric field (PEF). IOP Conf. Ser. Earth Environ. Sci. 2020, 475, 012056. [Google Scholar] [CrossRef]
- Tomé, A.C.; da Silva, F.A. Alginate based encapsulation as a tool for the protection of bioactive compounds from aromatic herbs. Food Hydrocoll. Health 2022, 2, 100051. [Google Scholar] [CrossRef]
- Balabanova, T.; Petkova, N.; Ivanova, M.; Panayotov, N. Design of Labneh cheese fortified with alginate-encapsulated pepper (Capsicum annuum) extracts. Emir. J. Food Agric. 2020, 32, 559–566. [Google Scholar] [CrossRef]
- Azarashkan, Z.; Motamedzadegan, A.; Saraei, A.G.-H.; Biparva, P.; Rahaiee, S. Investigation of the physicochemical, antioxidant, rheological, and sensory properties of ricotta cheese enriched with free and nano-encapsulated broccoli sprout extract. Food Sci. Nutr. 2022, 10, 4059–4072. [Google Scholar] [CrossRef]
- Hala, M.; Ebtisam, E.; Sanaa, I.; Badran, M.; Marwa, A.; Said, M. Manufacture of low fat UF-soft cheese supplemented with rose- mary extract (as natural antioxidant). J. Am. Sci. 2010, 6, 570–579. [Google Scholar]
- Soodam, K.; Ong, L.; Powell, I.B.; Kentish, S.E.; Gras, S.L. Effect of rennet on the composition, proteolysis and microstructure of reduced-fat Cheddar cheese during ripening. Dairy Sci. Technol. 2015, 95, 665–686. [Google Scholar] [CrossRef]
- Artiga-Artigas, M.; Acevedo-Fani, A.; Martín-Belloso, O. Improving the shelf life of low-fat cut cheese using nanoemulsion- based edible coatings containing oregano essential oil and mandarin fiber. Food Control 2017, 76, 1–12. [Google Scholar] [CrossRef]
- Pérez-Soto, E.; Cenobio-Galindo, A.D.J.; Espino-Manzano, S.O.; Franco-Fernández, M.J.; Ludeña-Urquizo, F.E.; Jiménez-Alvarado, R.; Zepeda-Velázquez, A.P.; Campos-Montiel, R.G. The addition of microencapsulated or Nanoemulsified bioactive compounds influences the antioxidant and antimicrobial activities of a fresh cheese. Molecules 2021, 26, 2170. [Google Scholar] [CrossRef] [PubMed]
- Popescu, L.; Ceșco, T.; Gurev, A.; Ghendov-Mosanu, A.; Sturza, R.; Tarna, R. Impact of apple pomace powder on the bioactivity, and the sensory and textural characteristics of yogurt. Foods 2022, 11, 3565. [Google Scholar] [CrossRef] [PubMed]
- Bulgaru, V.; Popescu, L.; Netreba, N.; Ghendov-Mosanu, A.; Sturza, R. Assessment of quality indices and their influence on the texture profile in the dry-aging process of beef. Foods 2022, 11, 1526. [Google Scholar] [CrossRef]
- Ghendov-Mosanu, A.; Cristea, E.; Patras, A.; Sturza, R.; Padureanu, S.; Deseatnicova, O.; Turculet, N.; Boestean, O.; Niculaua, M. Potential application of hippophae rhamnoides in wheat bread production. Molecules 2020, 25, 1272. [Google Scholar] [CrossRef] [PubMed]
- Ghendov-Mosanu, A.; Cristea, E.; Patras, A.; Sturza, R.; Niculaua, M. Rose Hips, a valuable source of antioxidants to improve gingerbread characteristics. Molecules 2020, 25, 5659. [Google Scholar] [CrossRef] [PubMed]
- Ivanova, V.; Stefova, M.; Chinnici, F. Determination of the polyphenol contents in Macedonian grapes and wines by standardized spectrophotometric methods. J. Serb. Chem. Soc. 2010, 75, 45–59. [Google Scholar] [CrossRef]
- Brand-Williams, W.; Cuvelier, M.E.; Berset, C. Use of a free radical method to evaluate antioxidant activity. LWT―Food Sci. Technol. 1995, 28, 25–30. [Google Scholar] [CrossRef]
- Thaipong, K.; Boonprakob, U.; Crosby, K.; Cisneros-Zevallos, L.; Byrne, D.H. Comparison of ABTS, DPPH, FRAP, and ORAC assays for estimating antioxidant activity from guava fruit extracts. J. Food Compos. Anal. 2006, 19, 669–675. [Google Scholar] [CrossRef]
- Magaldi, S.; Mata-Essayag, S.; de Capriles, C.H. Well diffusion for antifungal susceptibility testing. Int. J. Infect. Dis. 2004, 8, 39–45. [Google Scholar] [CrossRef] [PubMed]
- Valgas, C.; De Souza, S.M.; Smânia, E.F.A. Screening methods to determine antibacterial activity of natural products. Braz. J. Microbiol. 2007, 38, 369–380. [Google Scholar] [CrossRef]
- Weinstein, M.P.; Patel, J.B.; Burnham, C.-A.; Campeau, S.; Conville, P.S.; Doern, C.; Eliopoulos, G.M.; Galas, M.F.; Humphries, R.M.; Jenkins, S.G.; et al. M07-A10 Methods for Dilution Antimicrobial Susceptibility Tests for Bacteria that Grow Aerobically, Approved Standard, 10th ed.; Clinical and Laboratory Standards Institute: Wayne, PA, USA, 2015. [Google Scholar]
- Rijo, P.; Matias, D.; Fernandes, A.S.; Simões, M.F.; Nicolai, M.; Reis, C.P. Antimicrobial plant extracts encapsulated into polymeric beads for potential application on the skin. Polymers 2014, 6, 479–490. [Google Scholar] [CrossRef]
- Nwabor, O.F.; Singh, S.; Marlina, D.; Voravuthikunchai, S.P. Chemical characterization, release, and bioactivity of Eucalyptus camaldulensis polyphenols from freeze-dried sodium alginate and sodium carboxymethyl cellulose matrix. Food Qual. Saf. 2020, 4, 203–212. [Google Scholar] [CrossRef]
- Surini, S.; Nursatyani, K.; Ramadon, D. Gel formulation containing microcapsules of grape seed oil (Vitis vinifera L.) for skin moisturizer. J. Young Pharm. 2018, 10, 41. [Google Scholar] [CrossRef]
- Machado, A.R.; Silva, P.M.P.; Vicente, A.A.; Souza-Soares, L.A.; Pinheiro, A.C.; Cerqueira, M.A. Alginate Particles for Encapsulation of Phenolic Extract from Spirulina sp. LEB-18: Physicochemical Characterization and Assessment of In Vitro Gastrointestinal Behavior. Polymers 2022, 14, 4759. [Google Scholar] [CrossRef] [PubMed]
- ISO 1211:2010|IDF 1:2010; Milk—Determination of Fat Content—Gravimetric Method (Reference Method). International Organization for Standardization: Geneva, Switzerland, 2010.
- ISO 5534:2004|IDF 4:2004; Cheese and Processed Cheese—Determination of the Total Solids Content (Reference Method). International Organization for Standardization: Geneva, Switzerland, 2004.
- ISO 8968-1:2014|IDF 20-1:2014; Milk and Milk Products—Determination of Nitrogen Content—Part 1: Kjeldahl Principle and Crude Protein Calculation. International Organization for Standardization: Geneva, Switzerland, 2014.
- ISO 22935-3:2009|IDF 99-3:2009; Milk and Milk Products—Sensory Analysis—Part 3: Guidance on a Method for Evaluation of Compliance with Product Specifications for Sensory Properties by Scoring. International Organization for Standardization: Geneva, Switzerland, 2009.
- ISO 8586:2012; Sensory Analysis—General Guidelines for the Selection, Training and Monitoring of Selected Assessors and Expert Sensory Assessors. International Organization for Standardization: Geneva, Switzerland, 2012.
- Decision of the Government of the Republic of Moldova No. 158 of 07.03.2019 Regarding the Approval of the Quality Requirements for Milk and Dairy Products. 2019. Available online: https://www.legis.md/cautare/getResults?doc_id=113282&lang=ro (accessed on 1 February 2023).
- Paninski, L. Estimation of entropy and mutual information. Neural Comput. 2003, 15, 1191–1253. [Google Scholar] [CrossRef]
| Indices | Quantity |
|---|---|
| Total Polyphenol Content (Folin–Ciocalteu), mg GAE/g DW | 26.18 ± 0.21 |
| Epigallocatechin, mg/g DW | 0.72 ± 0.09 |
| Chicoric acid, mg/g DW | 1.30 ± 0.13 |
| Querectin-rutinoside, mg/g DW | 0.75 ± 0.02 |
| Luteolin-glucoside, mg/g DW | 0.85 ± 0.05 |
| Dehydrodiferulic acid, mg/g DW | 3.10 ± 0.26 |
| Rosmarinic acid, mg/g DW | 13.81 ± 0.57 |
| Methyl-rosmarinate, mg/g DW | 17.08 ± 0.39 |
| Carnosol, mg/g DW | 4.78 ± 0.06 |
| Rosmadial, mg/g DW | 6.45 ± 0.01 |
| Not identified | 8.46 ± 0.17 |
| Antioxidant activity (DPPH), mM Trolox/g DW | 644.75 ± 21.37 |
| Antioxidant activity (ABTS), mM Trolox/g DW | 8.95 ± 0.03 |
| Test Strains | Zone of Inhibition (mm) * | Basil Extract, mg/mL | |
|---|---|---|---|
| MIC | MBC/MFC | ||
| Gram-positive bacteria | |||
| Bacillus cereus | 11.0 ± 0.5 b | 22.5 ± 1.5 b | 22.5 ± 1.5 a |
| Enterococcus faecalis | 8.3 ± 0.5 a | 45.0 ± 0.0 c | 90.0 ± 0.0 c |
| Staphylococcus aureus | 26.3 ± 0.1 e | 11.2 ± 0.6 a | 45.0 ± 1.3 b |
| Geobacillus stearothermophilus | 15.3 ± 0.5 d | 11.2 ± 1.0 a | 22.5 ± 1.0 a |
| Gram-negative bacteria | |||
| Escherichia coli | 8.3 ± 0.5 a | 22.5 ± 1.3 b | 45.0 ± 1.7 b |
| Acinetobacter baumannii | 12.0 ± 0.6 b,c | 11.2 ± 0.8 a | 45.0 ± 1.5 b |
| Salmonella Abony | 8.3 ± 0.4 a | 45.0 ± 0.0 c | 45.0 ± 2.0 b |
| Yeast | |||
| Candida albicans | 11.0 ± 0.7 b | 22.5 ± 1.0 b | 22.5 ± 1.3 a |
| Parameters | Samples | ||||
|---|---|---|---|---|---|
| CC | 0.3% CCMBE | 0.6% CCMBE | 0.9% CCMBE | 1.2% CCMBE | |
| Dry matter, % | 34.32 ± 0.02 a | 34.50 ± 0.01 b | 34.69 ± 0.02 c | 34.89 ± 0.03 d | 35.09 ± 0.03 e |
| Protein content, % | 5.82 ± 0.0 e | 5.78 ± 0.0 d | 5.77 ± 0.01 c,d | 5.75 ± 0.01 b,c | 5.73 ± 0.01 a,b |
| Fat content, % | 23.04 ± 0.0 e | 22.93 ± 0.01 d | 22.86 ± 0.01 c | 22.79 ± 0.01 b | 22.72 ± 0.02 a |
| Sensory Properties | Storage Time, Day | Samples | ||||
|---|---|---|---|---|---|---|
| CC | 0.3% CCMBE | 0.6% CCMBE | 0.9% CCMBE | 1.2% CCMBE | ||
| Appearance | 1 | 5.00 ± 0.0 b | 5.00 ± 0.0 b | 5.00 ± 0.0 b | 5.00 ± 0.0 b | 5.00 ± 0.0 b |
| 7 | 5.00 ± 0.0 b | 5.00 ± 0.0 b | 5.00 ± 0.0 b | 5.00 ± 0.0 b | 5.00 ± 0.0 b | |
| 14 | 5.00 ± 0.0 b | 5.00 ± 0.0 b | 5.00 ± 0.0 b | 5.00 ± 0.0 b | 5.00 ± 0.0 b | |
| 21 | 5.00 ± 0.0 b | 5.00 ± 0.0 b | 5.00 ± 0.0 b | 5.00 ± 0.0 b | 5.00 ± 0.0 b | |
| 28 | 3.53 ± 0.01 a | 3.60 ± 0.1 a | 5.00 ± 0.0 b | 5.00 ± 0.0 b | 5.00 ± 0.0 b | |
| Texture | 1 | 5.00 ± 0.0 c | 5.00 ± 0.0 c | 5.00 ± 0.0 c | 5.00 ± 0.0 c | 5.00 ± 0.0 c |
| 7 | 5.00 ± 0.0 c | 5.00 ± 0.0 c | 5.00 ± 0.0 c | 5.00 ± 0.0 c | 5.00 ± 0.0 c | |
| 14 | 5.00 ± 0.0 c | 5.00 ± 0.0 c | 5.00 ± 0.0 c | 5.00 ± 0.0 c | 5.00 ± 0.0 c | |
| 21 | 5.00 ± 0.0 c | 5.00 ± 0.0 c | 5.00 ± 0.0 c | 5.00 ± 0.0 c | 5.00 ± 0.0 c | |
| 28 | 4.42 ± 0.01 a | 4.69 ± 0.01 b | 5.00 ± 0.0 c | 5.00 ± 0.0 c | 5.00 ± 0.0 c | |
| Odor | 1 | 5.00 ± 0.0 e | 5.00 ± 0.0 e | 5.00 ± 0.0 e | 5.00 ± 0.0 e | 4.62 ± 0.02 d |
| 7 | 5.00 ± 0.0 e | 5.00 ± 0.0 e | 5.00 ± 0.0 e | 5.00 ± 0.0 e | 4.50 ± 0.01 c | |
| 14 | 5.00 ± 0.0 e | 5.00 ± 0.0 e | 5.00 ± 0.0 e | 5.00 ± 0.0 e | 4.42 ± 0.01 b,c | |
| 21 | 5.00 ± 0.0 e | 5.00 ± 0.0 e | 5.00 ± 0.0 e | 5.00 ± 0.0 e | 4.12 ± 0.01 a | |
| 28 | 4.48 ± 0.1 b | 4.62 ± 0.01 d | 5.00 ± 0.0 e | 5.00 ± 0.0 e | 4.10 ± 0.01 a | |
| Taste | 1 | 5.00 ± 0.0 h | 5.00 ± 0.0 h | 5.00 ± 0.0 h | 5.00 ± 0.0 h | 4.20 ± 0.01 e |
| 7 | 5.00 ± 0.0 h | 5.00 ± 0.0 h | 5.00 ± 0.0 h | 5.00 ± 0.0 h | 4.18 ± 0.01 d,e | |
| 14 | 5.00 ± 0.0 h | 5.00 ± 0.0 h | 5.00 ± 0.0 h | 5.00 ± 0.0 h | 4.10 ± 0.02 d | |
| 21 | 4.32 ± 0.01 f | 4.56 ± 0.01 g | 5.00 ± 0.0 h | 5.00 ± 0.0 h | 4.00 ± 0.01 c | |
| 28 | 3.60 ± 0.02 a,b | 3.68 ± 0.01 b | 5.00 ± 0.0 h | 5.00 ± 0.0 h | 4.00 ± 0.01 c | |
| Overall acceptance | 1 | 5.00 ± 0.0 h | 5.00 ± 0.0 h | 5.00 ± 0.0 h | 5.00 ± 0.0 h | 4.71 ± 0.01 d,e |
| 7 | 5.00 ± 0.0 h | 5.00 ± 0.0 h | 5.00 ± 0.0 h | 5.00 ± 0.0 h | 4.67 ± 0.01 d,e | |
| 14 | 5.00 ± 0.0 h | 5.00 ± 0.0 h | 5.00 ± 0.0 h | 5.00 ± 0.0 h | 4.63 ± 0.01 d | |
| 21 | 4.83 ± 0.01 f | 4.89 ± 0.01 g | 5.00 ± 0.0 h | 5.00 ± 0.0 h | 4.53 ± 0.01 c | |
| 28 | 4.01 ± 0.01 a | 4.15 ± 0.01 b | 5.00 ± 0.0 h | 5.00 ± 0.0 h | 4.53 ± 0.01 c | |
| Storage Time, Days | Samples | ||||
|---|---|---|---|---|---|
| CC | 0.3% CCMBE | 0.6% CCMBE | 0.9% CCMBE | 1.2% CCMBE | |
| 1 | 5.41 ± 0.0 h | 5.35 ± 0.01 g | 5.31 ± 0.01 f | 5.30 ± 0.02 e,f | 5.26 ± 0.01 d |
| 7 | 5.41 ± 0.0 h | 5.34 ± 0.01 g | 5.30 ± 0.01 e,f | 5.28 ± 0.01 d,e | 5.24 ± 0.02 c,d |
| 14 | 5.35 ± 0.01 g | 5.31 ± 0.01 f | 5.30 ± 0.01 e,f | 5.27 ± 0.02 d,e | 5.21 ± 0.01 b,c |
| 21 | 5.24 ± 0.01 c,d | 5.27 ± 0.01 d,e | 5.28 ± 0.01 d,e | 5.26 ± 0.01 d | 5.21 ± 0.01 b,c |
| 28 | 5.12 ± 0.01 a | 5.19 ± 0.01 b | 5.27 ± 0.01 d,e | 5.25 ± 0.01 c,d | 5.20 ± 0.01 b |
| Texture Parameters | Storage Time, Day | Samples | ||||
|---|---|---|---|---|---|---|
| CC | 0.3% CCMBE | 0.6% CCMBE | 0.9% CCMBE | 1.2% CCMBE | ||
| Hardness, g | 1 | 1914.1 ± 43.5 b | 1914.8 ± 34.1 b | 1913.5 ± 28.6 b | 1891.2 ± 10.2 b | 1439.8 ± 42.3 a |
| 7 | 2951.8 ± 60.2 e | 2568.8 ± 25.2 d | 2476.1 ± 25.4 d | 2405.1 ± 27.8 c | 1960.9 ± 52.3 b | |
| 14 | 4123.5 ± 32.5 h,i | 2807.2 ± 19.7 e | 2637.3 ± 32.5 d | 2506.9 ± 31.5 d | 2034.01 ± 39.6 b | |
| 21 | 4180.3 ± 22 i | 3254.3 ± 26.8 f | 2962.4 ± 31.6 e | 2960.8 ± 26.8 e | 2502.2 ± 44.3 d | |
| 28 | 2424.6 ± 17.4 c | 3512.01 ± 35.6 g | 3280.9 ± 18.8 f | 3225.6 ± 34.4 f | 3016.9 ± 36.8 e,f | |
| Springiness, % | 1 | 1.001 ± 0.0001 b | 1.000 ± 0.0001 a | 1.000 ± 0.0001 a | 1.002 ± 0.0001 c | 1.000 ± 0.0001 a |
| 7 | 1.000 ± 0.0001 a | 1.001 ± 0.0001 b | 1.000 ± 0.0001 a | 1.001 ± 0.0001 b | 1.002 ± 0.0001 c | |
| 14 | 1.001 ± 0.0001 b | 1.001 ± 0.0001 b | 1.001 ± 0.0001 b | 1.002 ± 0.0001 c | 1.000 ± 0.0001 a | |
| 21 | 1.001 ± 0.0001 b | 1.001 ± 0.0001 b | 1.001 ± 0.0001 b | 1.001 ± 0.0001 b | 1.000 ± 0.0001 a | |
| 28 | 1.001 ± 0.0001 b | 1.001 ± 0.0001 b | 1.001 ± 0.0001 b | 1.001 ± 0.0001 b | 1.001 ± 0.0001 b | |
| Cohesiveness, % | 1 | 0.462 ± 0.008 h | 0.476 ± 0.004 i | 0.489 ± 0.009 i | 0.534 ± 0.004 j | 0.568 ± 0.002 k |
| 7 | 0.214 ± 0.005 b,c | 0.295 ± 0.004 d | 0.315 ± 0.007 e | 0.390 ± 0.004 g | 0.386 ± 0.006 f,g | |
| 14 | 0.148 ± 0.006 a | 0.241 ± 0.007 c | 0.313 ± 0.009 e | 0.360 ± 0.009 f | 0.367 ± 0.008 f | |
| 21 | 0.199 ± 0.007 b | 0.204 ± 0.009 b | 0.245 ± 0.006 c | 0.282 ± 0.006 d | 0.307 ± 0.005 e | |
| 28 | 0.327 ± 0.007 e | 0.202 ± 0.005 b | 0.210 ± 0.005 b,c | 0.247 ± 0.005 c | 0.279 ± 0.007 d | |
| Adhesiveness, g⋅s | 1 | 2252.0 ± 26.7 c,d | 2217.0 ± 31.2 c | 2216.2 ± 29.7 c | 2134.5 ± 26.3 c | 1648.3 ± 35.4 a |
| 7 | 3589.8 ± 30.2 h | 3048.8 ± 25.7 f | 2914.6 ± 32.3 f | 2772.6 ± 34.7 e | 2276.0 ± 18.0 d | |
| 14 | 4833.9 ± 5.2 k | 3175.4 ± 34.8 g | 3104.8 ± 24.8 f,g | 2821.7 ± 29.8 e,f | 2336.0 ± 22.4 d | |
| 21 | 4903.0 ± 18.6 k | 3895.1 ± 28.6 i | 3606.3 ± 27.5 h | 3125.6 ± 25.5 f,g | 2841.6 ± 32.6 e,f | |
| 28 | 1929.6 ± 35.6 b | 4079.9 ± 31.9 j | 3916.5 ± 31.7 i | 3841.4 ± 31.3 i | 3318.4 ± 25.1 g | |
| Gumminess, % | 1 | 884.3 ± 6.7 i | 888.8 ± 10.2 i | 897.8 ± 9.7 i | 935.7 ± 10.9 j | 1009.9 ± 7.6 l |
| 7 | 631.7 ± 8.5 a,b | 756.9 ± 11.3 e | 757.8 ± 9.6 e | 927.2 ± 9.6 j | 980.0 ± 11.5 k,l | |
| 14 | 618.5 ± 8.6 a,b | 673.7 ± 9.1 c | 752.6 ± 9.4 e | 902.5 ± 11.7 i,j | 923.7± 10.5 j | |
| 21 | 611.9 ± 9.2 a | 663.9 ± 10.6 b,c | 725.8 ± 11.5 d,e | 834.9 ± 9.2 g,h | 899.1± 11.3 i | |
| 28 | 792.8 ± 10.1 f | 634.9 ± 11.9 a,b | 717.1 ± 10.1 d | 819.3 ± 10.3 g | 843.4± 12.4 g,h | |
| Compound | Max Absorbtion, nm | Retention Time, min | m/z [M+H]+ | Polyphenol Classes |
|---|---|---|---|---|
| Epigallocatechin | 280 | 13.17 | 306 | Flavavol |
| Chicoric acid | 330 | 14.38 | 475 | Hydroxycinnamic acid |
| Querectin-rutinoside | 355, 260 | 15.73 | 611 | Flavonol |
| Luteolin-glucoside | 350, 260 | 16.14 | 449 | Flavone |
| Dehydrodiferulic acid | 330 | 19.34 | 386, 194 | Hydroxycinnamic acid |
| Rosmarinic acid | 330 | 20.09 | 360 | Hydroxycinnamic acid |
| Methyl-rosmarinate | 330 | 22.39 | 375 | Hydroxycinnamic acid |
| Carnosol | 330, 270 | 23.17 | 331 | Phenolic terpene |
| Rosmadial | 330 | 23.56 | 345 | Phenolic terpene |
| Not identified | 270 | 24.75 | 624, 249 |
| Sensory Properties | Description |
|---|---|
| Appearance | Soft, creamy, clean, without curdling, white to slightly yellow color in the case of plain cream cheese, or characteristic of the added ingredients in the case of flavored cream cheese. |
| Texture | Fine, creamy paste |
| Odor | Characteristic odor of lactic fermentation in the case of plain cream cheese, and the specific odor of the used ingredients in the case of flavored cream cheese, without any foreign odor. |
| Taste | Characteristic of lactic fermentation in the case of plain cream cheese, or specific to the used ingredients in the case of flavored cream cheese, without any foreign taste. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Popescu, L.; Cojocari, D.; Lung, I.; Kacso, I.; Ciorîţă, A.; Ghendov-Mosanu, A.; Balan, G.; Pintea, A.; Sturza, R. Effect of Microencapsulated Basil Extract on Cream Cheese Quality and Stability. Molecules 2023, 28, 3305. https://doi.org/10.3390/molecules28083305
Popescu L, Cojocari D, Lung I, Kacso I, Ciorîţă A, Ghendov-Mosanu A, Balan G, Pintea A, Sturza R. Effect of Microencapsulated Basil Extract on Cream Cheese Quality and Stability. Molecules. 2023; 28(8):3305. https://doi.org/10.3390/molecules28083305
Chicago/Turabian StylePopescu, Liliana, Daniela Cojocari, Ildiko Lung, Irina Kacso, Alexandra Ciorîţă, Aliona Ghendov-Mosanu, Greta Balan, Adela Pintea, and Rodica Sturza. 2023. "Effect of Microencapsulated Basil Extract on Cream Cheese Quality and Stability" Molecules 28, no. 8: 3305. https://doi.org/10.3390/molecules28083305
APA StylePopescu, L., Cojocari, D., Lung, I., Kacso, I., Ciorîţă, A., Ghendov-Mosanu, A., Balan, G., Pintea, A., & Sturza, R. (2023). Effect of Microencapsulated Basil Extract on Cream Cheese Quality and Stability. Molecules, 28(8), 3305. https://doi.org/10.3390/molecules28083305







