Silver Nanoparticles Loaded on Chitosan-g-PVA Hydrogel for the Wound-Healing Applications
Abstract
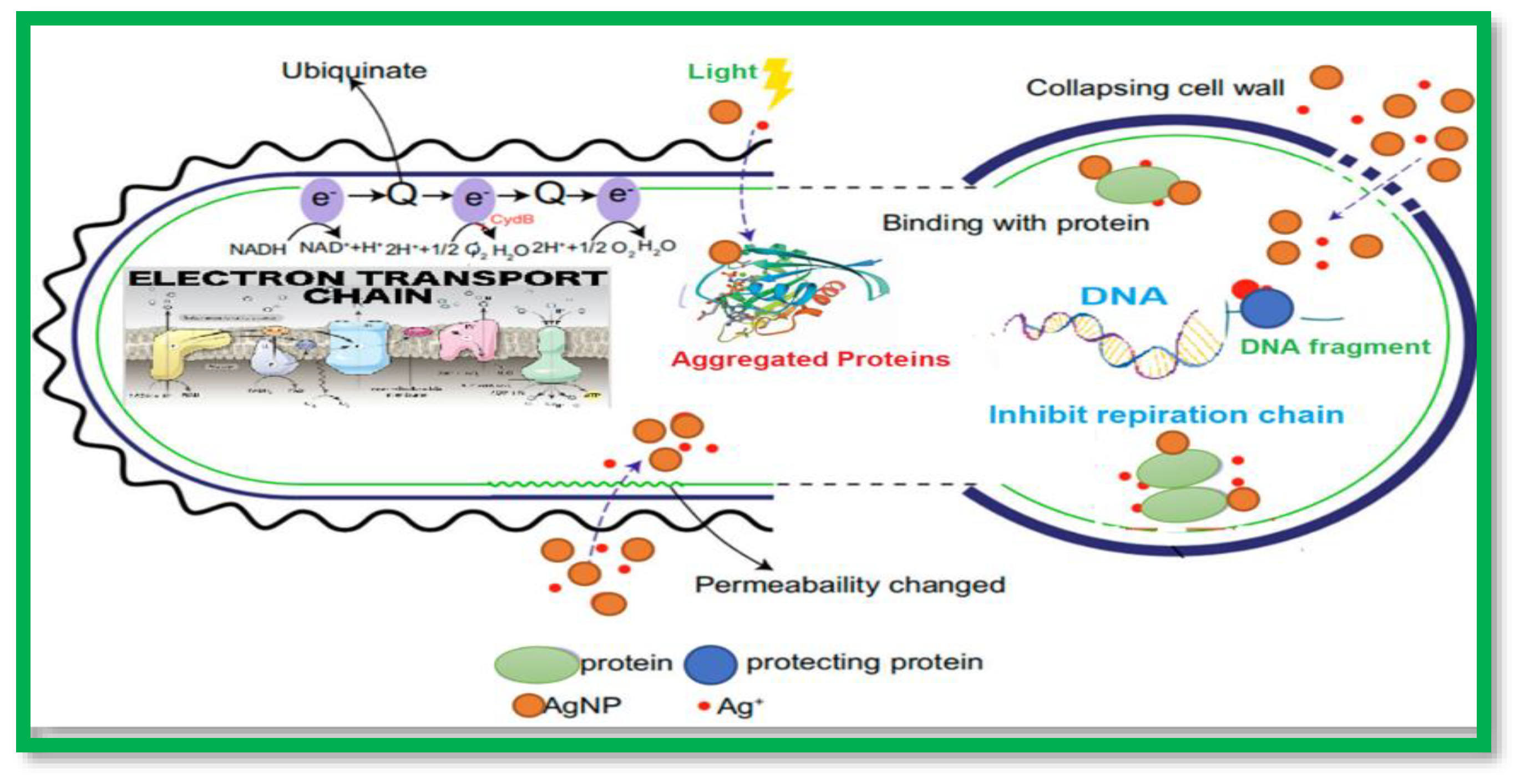
1. Introduction
2. Results and Discussion
2.1. Characterization of the Prepared AgNPs
2.2. Hydrogel Antimicrobial Activity
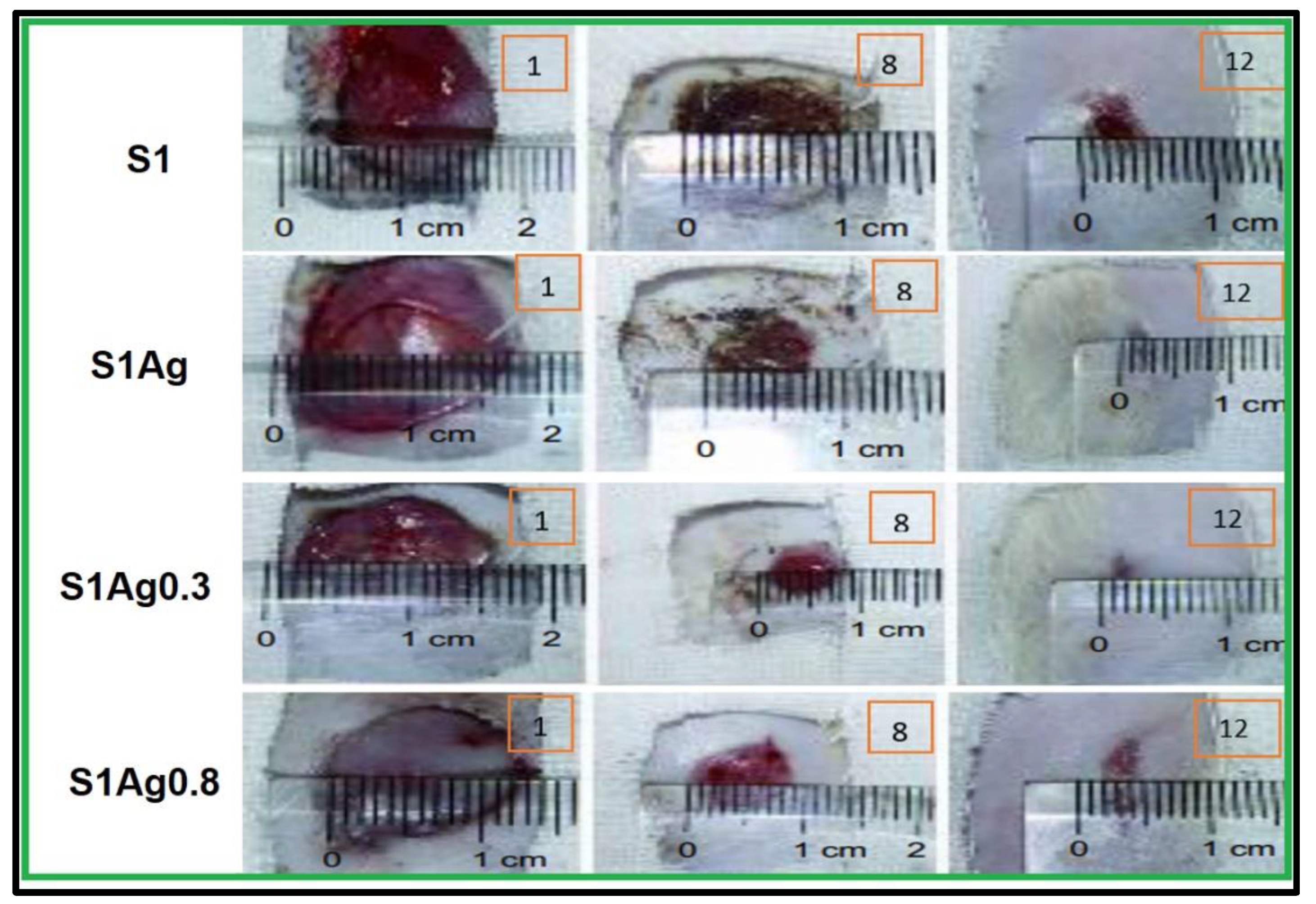
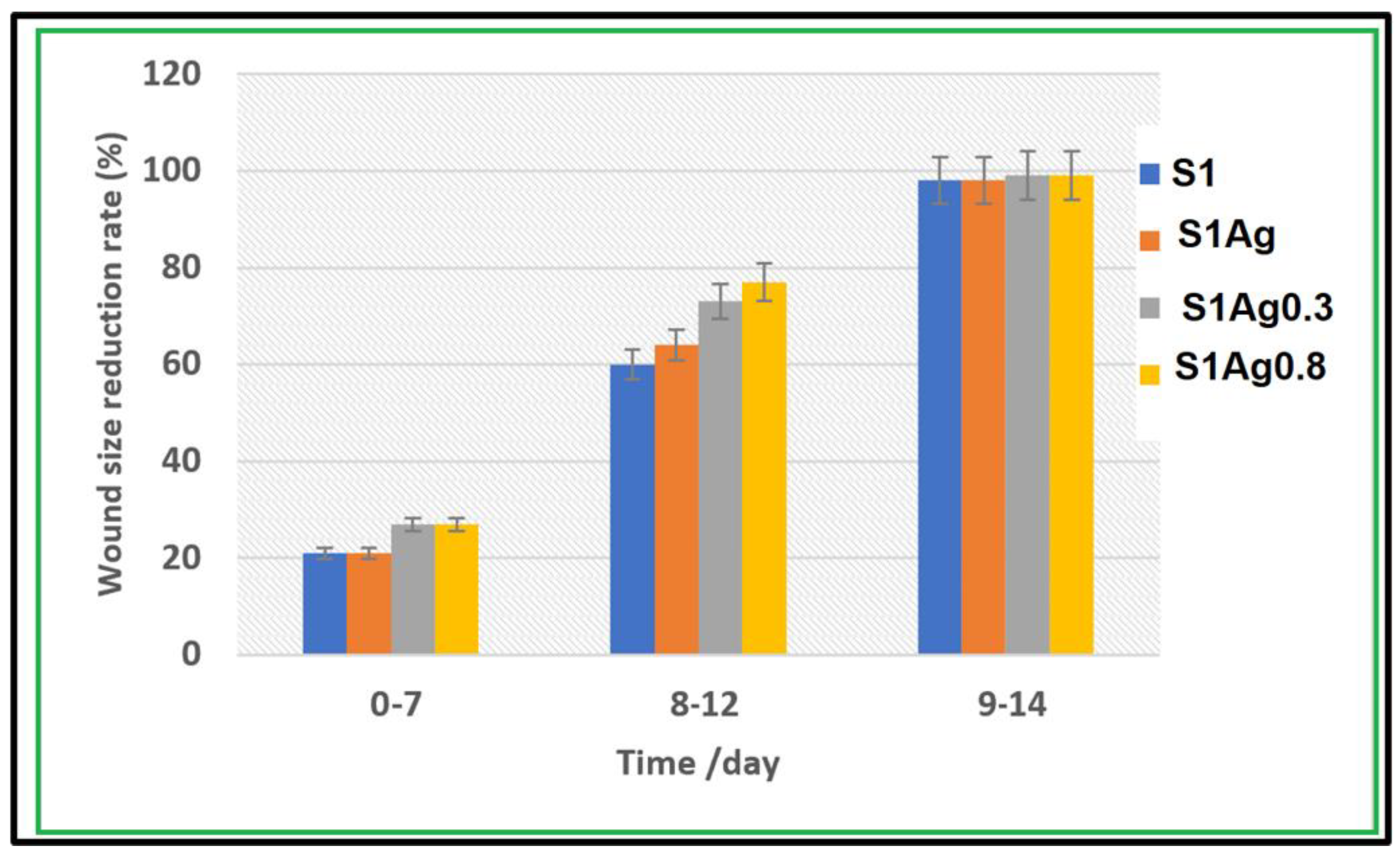
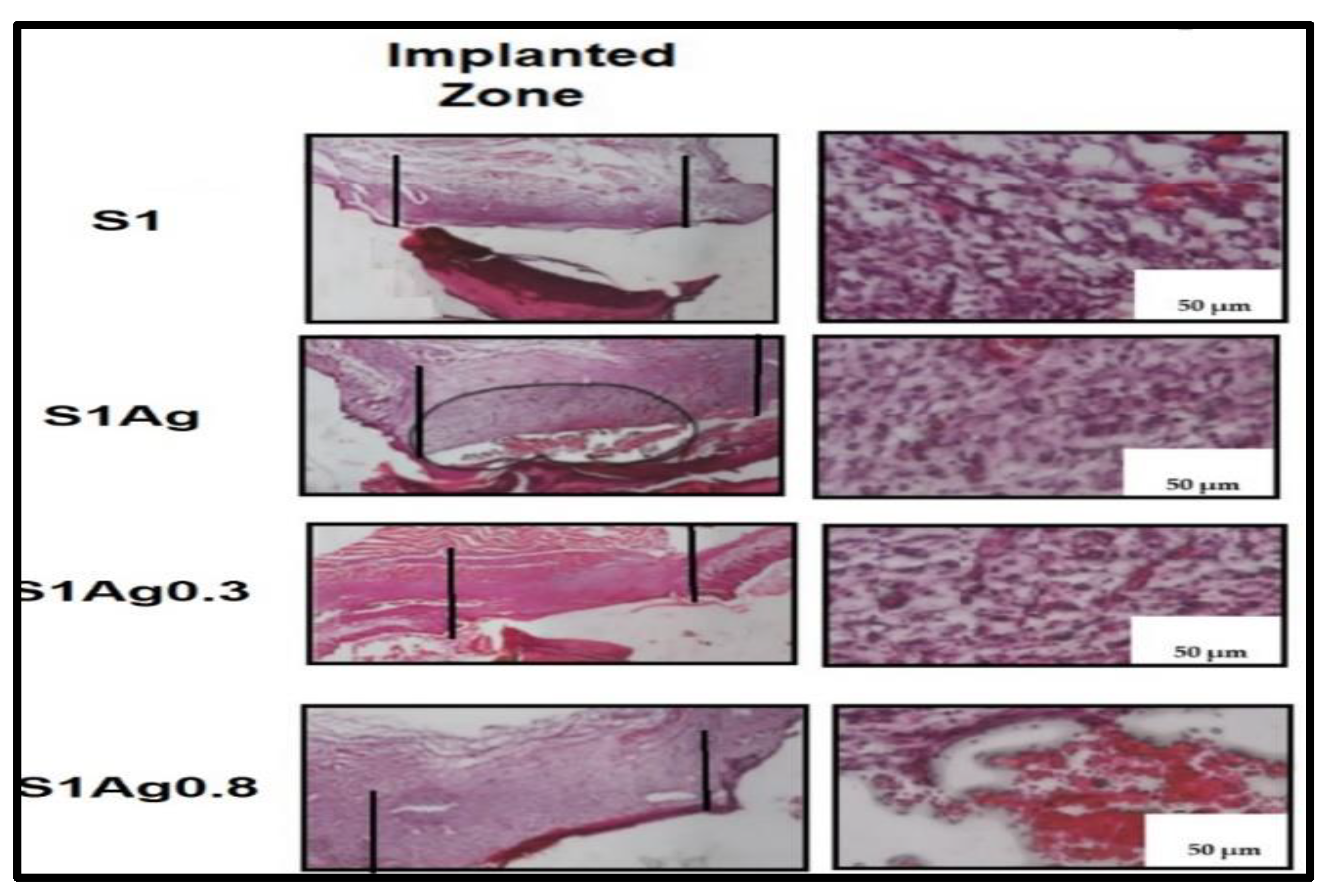
2.3. In Vivo Skin-Wound Healing
3. Methods
3.1. Ethical Considerations
3.2. Experimental Animals and Housing Conditions
3.3. Test Strains/Tissue Cell Line
3.4. Green’s Synthesis Method for AgNPs
3.5. Synthesis of Chitosan Grafted PVA/AgNPs Hydrogels
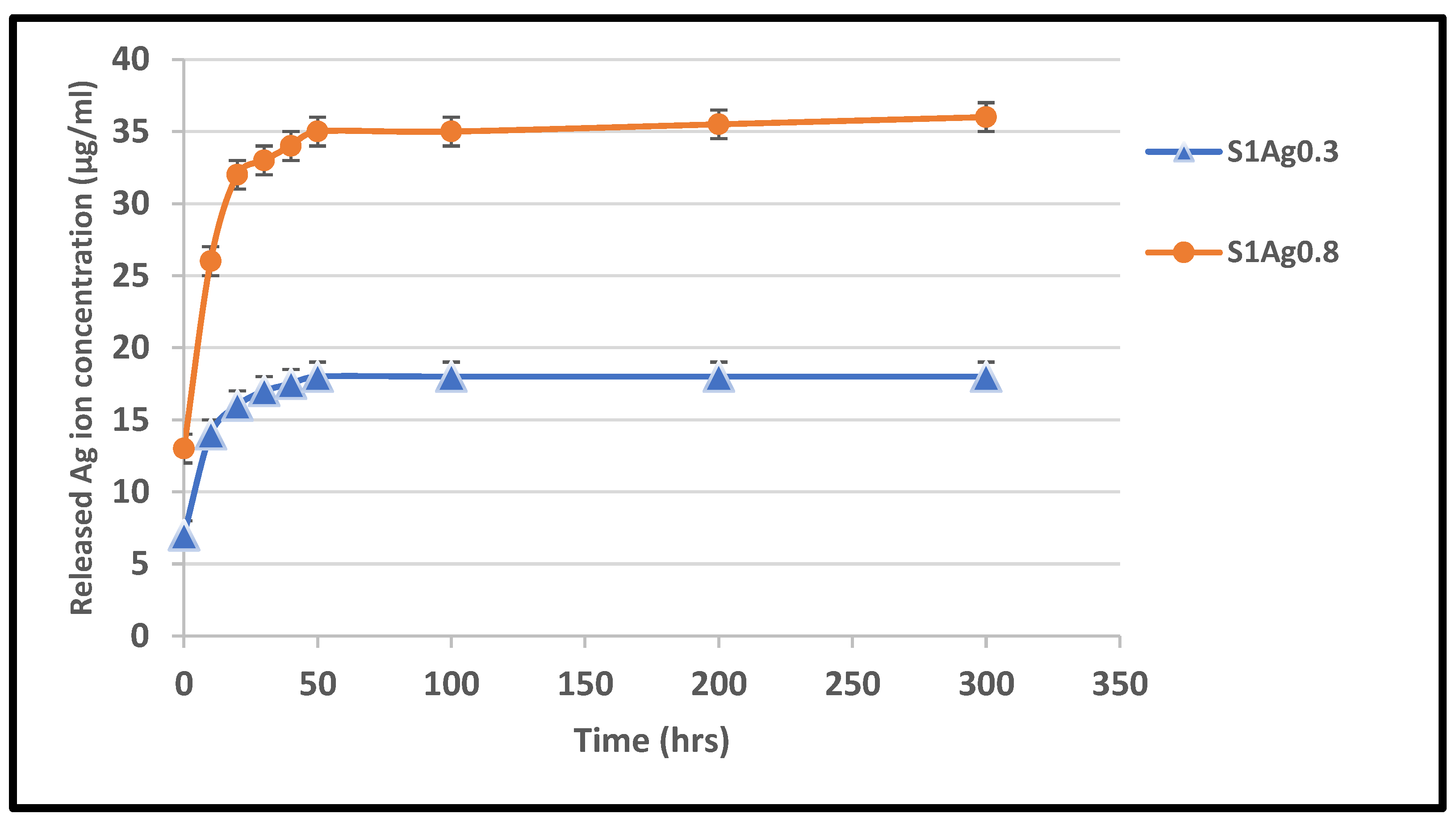
3.6. Release of Silver Ions
3.7. Characterization Using TEM and FTIR
3.8. Statistical Analysis
3.9. In Vitro Antimicrobial Assessment Using Agar Diffusion Test
3.10. Human Skin Fibroblast Cells Cytotoxicity Evaluation
3.11. In Vivo Study and Histological Examination
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Jie, L.; Wenqi, J.; Qianyue, X.; Yongjie, Z. Progress in Antibacterial Hydrogel Dressing. Gels 2022, 8, 503. [Google Scholar]
- Priya, D.; Dominik, B.; Ramya, D.; Daniil, K.; Bozena, H.; Marta, K.; Carlos, F.; Branislav, R.; Hoai, N.; Awais, F.; et al. Silver Nanomaterials for Wound Dressing Applications. Pharmaceutics 2020, 12, 821. [Google Scholar]
- Tianwen, W.; Fang, Z.; Rui, Z.; Can, W.; Kehui, H.; Yi, S.; Constantinus, P.; Amin, S.; Lei, N. Polyvinyl Alcohol/Sodium Alginate Hydrogels Incorporated with Silver Nanoclusters via Green Tea Extract for Antibacterial Applications. Des. Monomers Poly. 2020, 23, 118–133. [Google Scholar]
- Ziai, Y.; Petronella, F.; Rinoldi, C.; Nakielski, P.; Zakrzewska, A.; Kowalewski, T.A.; Augustyniak, W.; Li, X.; Calogero, A.; Sabała, I.; et al. Chameleon-inspired multifunctional plasmonic nanoplatforms for biosensing applications. NPG Asia Mater. 2022, 14, 1. [Google Scholar] [CrossRef]
- Khalil, A.M.; Rabie, S.T. Antimicrobial behavior and photostability of polyvinyl chloride/1-vinylimidazole nanocomposites loaded with silver or copper nanoparticles. J. Vinyl Addit. Technol. 2017, 23, E25–E33. [Google Scholar] [CrossRef]
- Beyene, H.D.; Werkneh, A.A.; Bezabh, H.K.; Ambaye, T.G. Synthesis paradigm and applications of AgNPs (AgNPs), a review. Sustain. Mater. Technol. 2017, 13, 18–23. [Google Scholar]
- Alqanoo, A.A.; Ahmed, N.M.; Hashim, M.R.; Almessiere, M.A.; Taya, S.A.; Zyoud, S.H. Silver nanowires assisted porous silicon for high photodetector sensitivity using surface plasmonic phenomena. Sens. Actuators A Phys. 2022, 347, 113942. [Google Scholar] [CrossRef]
- Mohammadlou, M.; Maghsoudi, H.; Jafarizadeh-Malmiri, H. A review on green silver nanoparticles based on plants: Synthesis, potential applications, and eco-friendly approach. Int. Food Res. J. 2016, 23, 446–463. [Google Scholar]
- Nune, S.K.; Chanda, N.; Shukla, R.; Katti, K.; Kulkarni, R.R.; Thilakavathy, S.; Mekapothula, S.; Kannan, R.; Katti, K.V. Green nanotechnology from tea: Phytochemicals in tea as building blocks for production of biocompatible gold nanoparticles. J. Mater. Chem. 2009, 19, 2912–2929. [Google Scholar] [CrossRef]
- Gaggìa, F.; Baffoni, L.; Galiano, M.; Nielsen, D.S.; Jakobsen, R.R.; Castro-Mejía, J.L.; Bosi, S.; Truzzi, F.; Musumeci, F.; Dinelli, G.; et al. Kombucha beverage from green, black and rooibos teas: A comparative study looking at microbiology, chemistry and antioxidant activity. Nutrients 2018, 11, 1. [Google Scholar] [CrossRef]
- Graham, H.N. Green tea composition, consumption, and polyphenol chemistry. Prev. Med. 1992, 21, 334–350. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Xu, Y.; Lei, Y.; Wen, X.; Liang, J. Tourmaline nanoparticles modifying hemostatic property of chtosan/polyvinyl alcohol hydrogels. Mater. Lett. 2022, 324, 132718. [Google Scholar] [CrossRef]
- Masood, N.; Ahmed, R.; Tariq, M.; Ahmed, Z.; Masoud, M.S.; Ali, I.; Asghar, R.; Andleeb, A.; Hasan, A. Silver nanoparticle impregnated chitosan-PEG hydrogel enhances wound healing in diabetes induced rabbits. Int. J. Pharm. 2019, 559, 23–36. [Google Scholar] [CrossRef] [PubMed]
- Nie, L.; Wang, C.; Hou, R. Preparation and characterization of dithiol-modified graphene oxide nanosheets reinforced alginate nanocomposite as bone scaffold. SN Appl. Sci. 2019, 1, 545. [Google Scholar] [CrossRef]
- El Sayed, M.; Mohsen, D.; Al Bazedi, G. Antimicrobial: A Comparative Study of Silver Nanoparticles and Chitosan Hydrogel Effect on Pathogenic. E. Coli. Res. J. Pharma Bio. Chem. Sci. 2017, 8, 956–967. [Google Scholar]
- Nguyen, H.; Kim, H.; Song, Y.; Lee, B. Nano Ag loaded PVA nano-fibrous mats for skin applications. J. Biomed. Mat. Res. Part B Appl. Biomat. 2011, 96, 225–233. [Google Scholar] [CrossRef]
- Abdelgawad, M.; Hudson, M.; Rojas, J. Antimicrobial wound dressing nanofiber mats from multicomponent (chitosan/silver-NPs/polyvinyl alcohol) systems. Carbohydr. Polym. 2014, 100, 166–178. [Google Scholar] [CrossRef]
- Jung, W.K.; Koo, H.C.; Kim, W.K.; Shin, S.; Kim, S.H.; Park, Y.H. Antibacterial activity and mechanism of action of the silver ion in Staphylococcus aureus and Escherichia coli. Appl. Environ. Microbiol. 2008, 74, 2171–2178. [Google Scholar] [CrossRef]
- Almawgani, A.H.; Suthar, B.; Bhargava, A.; Taya, S.A.; Daher, M.G.; Wu, F.; Colak, I. Sucrose concentration detector based on a binary photonic crystal with a defect layer and two nanocomposite layers. Z. Nat. A 2022, 77, 909–919. [Google Scholar] [CrossRef]
- Alotaibi, A.; Alsaleh, N.; Aljasham, A.; Tawfik, E.; Almutairi, M.; Assiri, M.; Alkholief, M.; Almutairi, M. Silver Nanoparticle-Based Combinations with Antimicrobial Agents against Antimicrobial-Resistant Clinical Isolates. Antibiotics 2022, 11, 1219. [Google Scholar] [CrossRef]
- Zhao, Y.; Yang, L.; Xu, M.; Wang, H.; Gao, X.; Niu, B.; Li, W. Gallic acid functionalized chitosan immobilized nanosilver for modified chitosan/poly (vinyl alcohol) composite film. Int. J. Biol. Macromol. 2022, 222, 2987–3000. [Google Scholar] [CrossRef] [PubMed]
- Qi, J.; Zhang, J.; Jia, H.; Guo, X.; Yue, Y.; Yuan, Y.; Yue, T. Synthesis of silver/Fe3O4@ chitosan@ polyvinyl alcohol magnetic nanoparticles as an antibacterial agent for accelerating wound healing. Int. J. Biol. Macromol. 2022, 221, 1404–1414. [Google Scholar] [CrossRef] [PubMed]
- Alfuraydi, R.T.; Alminderej, F.M.; Mohamed, N.A. Evaluation of Antimicrobial and Anti-Biofilm Formation Activities of Novel Poly (vinyl alcohol) Hydrogels Reinforced with Crosslinked Chitosan and Silver Nano-Particles. Polymers 2022, 14, 1619. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Mao, J.; Guo, Z.; Hu, Y.; Wang, S. Polyvinyl alcohol/carboxymethyl chitosan hydrogel loaded with silver nanoparticles exhibited antibacterial and self-healing properties. Int. J. Biol. Macromol. 2022, 220, 211–222. [Google Scholar] [CrossRef]
- Chelu, M.; Moreno, J.C.; Atkinson, I.; Cusu, J.P.; Rusu, A.; Bratan, V.; Aricov, L.; Anastasescu, M.; Seciu-Grama, A.-M.; Musuc, A.M. Green synthesis of bioinspired chitosan-ZnO-based polysaccharide gums hydrogels with propolis extract as novel functional natural biomaterials. Int. J. Biol. Macromol. 2022, 211, 410–424. [Google Scholar] [CrossRef] [PubMed]
- Sun, Q.; Cai, X.; Li, J. Green synthesis of silver nanoparticles using tea leaf extract and evaluation of their stability and antibacterial activity. Colloids Surf. A Physicochem. Eng. Asp. 2014, 444, 226–231. [Google Scholar] [CrossRef]
- Tan, D.; Nguyen, N.; Khanh, L.; Anh, H.; Thanh, N.; Hieu, M.; Van, T.; Thi, H.; Thi, N. In Vivo Study of the Antibacterial Chitosan/Polyvinyl Alcohol Loaded with Silver Nanoparticle Hydrogel for Wound Healing Applications. Inter. J. Polym. Sci. 2019, 2019, 7382717. [Google Scholar]
- El Sayed, M.; Sorour, M.; Abd El Moneem, N.; Talaat, H.; Shalaan, H.; El Marsafy, S. Characterization of hydrogel synthesized from natural polysaccharides blend grafted acrylamide using microwave (MW) and ultraviolet (UV) techniques. Starch 2013, 5, 172–178. [Google Scholar]
- Nguyen, T.; Huynh, C.; Niem, V.; Toi, V.; Quyen, T.; Hai, N.; Anh, M. Microwave-Assisted Synthesis of Chitosan/Polyvinyl Alcohol Silver nanoparticles Gel for Wound Dressing Applications. Int. J. Polym. Sci. 2016, 2016, 1584046. [Google Scholar]
- Foroushani, P.H.; Rahmani, E.; Alemzadeh, I.; Vossoughi, M.; Pourmadadi, M.; Rahdar, A.; Díez-Pascual, A.M. Curcumin sustained release with a hybrid chitosan-silk fibroin nanofiber containing silver nanoparticles as a novel highly efficient antibacterial wound dressing. Nanomaterials 2022, 12, 3426. [Google Scholar] [CrossRef]
- Ziai, Y.; Rinoldi, C.; Nakielski, P.; De Sio, L.; Pierini, F. Smart plasmonic hydrogels based on gold and silver nanoparticles for biosensing application. Curr. Opin. Biomed. Eng. 2022, 24, 100413. [Google Scholar] [CrossRef]
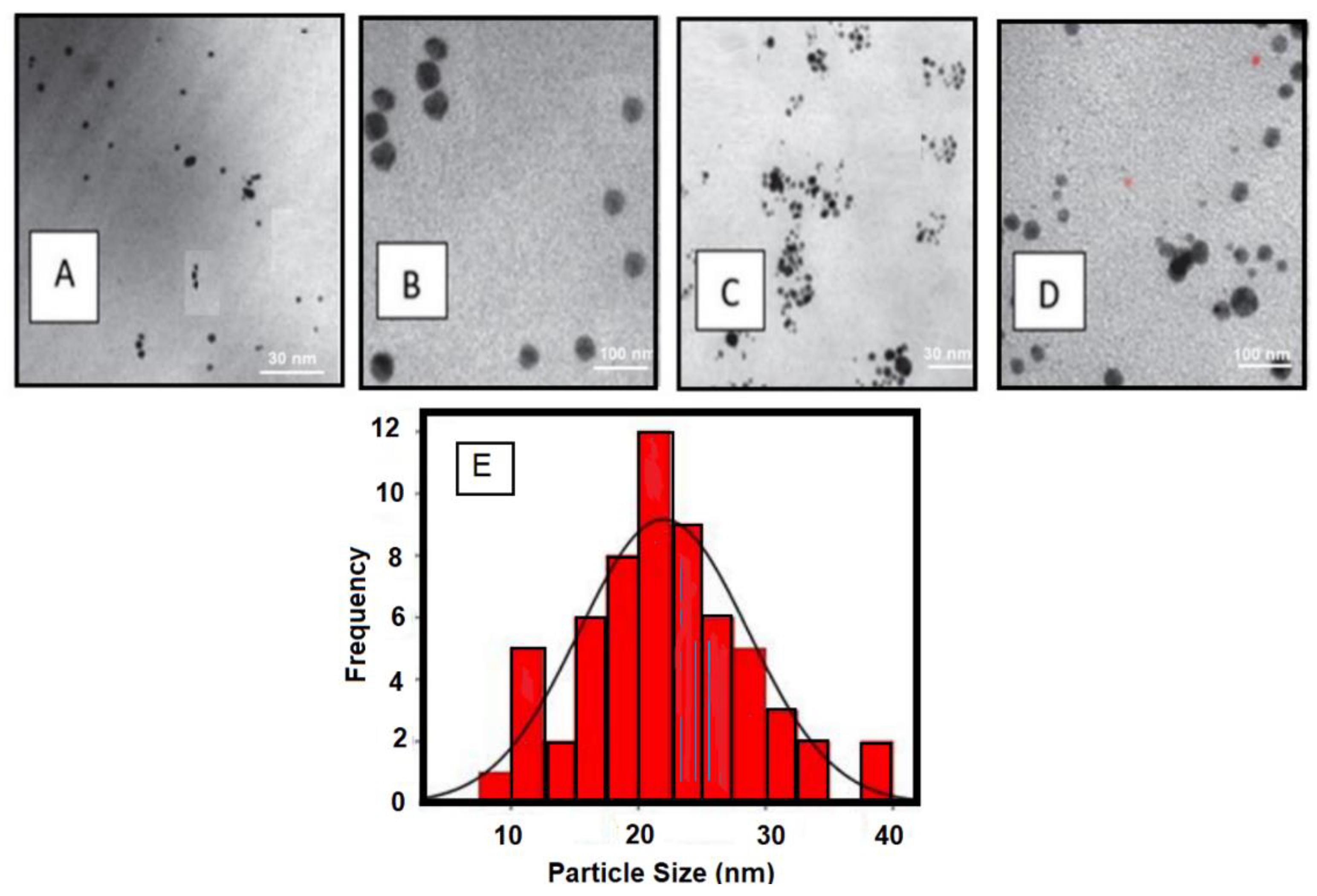




| No. | Green Tea Extract Volume “mL” | AgNPs (0.1 M) “mL” | Nanoparticle Diameter “nm” |
|---|---|---|---|
| 1 | 15 | 1 | Mean = 22.31 nm SD = 8.36 Counted = 32 particles |
| 2 | 10 | 1 | |
| 3 | 5 | 1 | |
| 4 | 3 | 1 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Aldakheel, F.M.; Mohsen, D.; El Sayed, M.M.; Alawam, K.A.; Binshaya, A.S.; Alduraywish, S.A. Silver Nanoparticles Loaded on Chitosan-g-PVA Hydrogel for the Wound-Healing Applications. Molecules 2023, 28, 3241. https://doi.org/10.3390/molecules28073241
Aldakheel FM, Mohsen D, El Sayed MM, Alawam KA, Binshaya AS, Alduraywish SA. Silver Nanoparticles Loaded on Chitosan-g-PVA Hydrogel for the Wound-Healing Applications. Molecules. 2023; 28(7):3241. https://doi.org/10.3390/molecules28073241
Chicago/Turabian StyleAldakheel, Fahad M., Dalia Mohsen, Marwa M. El Sayed, Khaled Ali Alawam, AbdulKarim S. Binshaya, and Shatha A. Alduraywish. 2023. "Silver Nanoparticles Loaded on Chitosan-g-PVA Hydrogel for the Wound-Healing Applications" Molecules 28, no. 7: 3241. https://doi.org/10.3390/molecules28073241
APA StyleAldakheel, F. M., Mohsen, D., El Sayed, M. M., Alawam, K. A., Binshaya, A. S., & Alduraywish, S. A. (2023). Silver Nanoparticles Loaded on Chitosan-g-PVA Hydrogel for the Wound-Healing Applications. Molecules, 28(7), 3241. https://doi.org/10.3390/molecules28073241










