Evaluation of Antibacterial Activity of Thiourea Derivative TD4 against Methicillin-Resistant Staphylococcus aureus via Destroying the NAD+/NADH Homeostasis
Abstract
1. Introduction
2. Results
2.1. Synthesis of Thiourea Derivatives
2.2. Screening and Antimicrobial Spectrum of Thiourea Derivatives
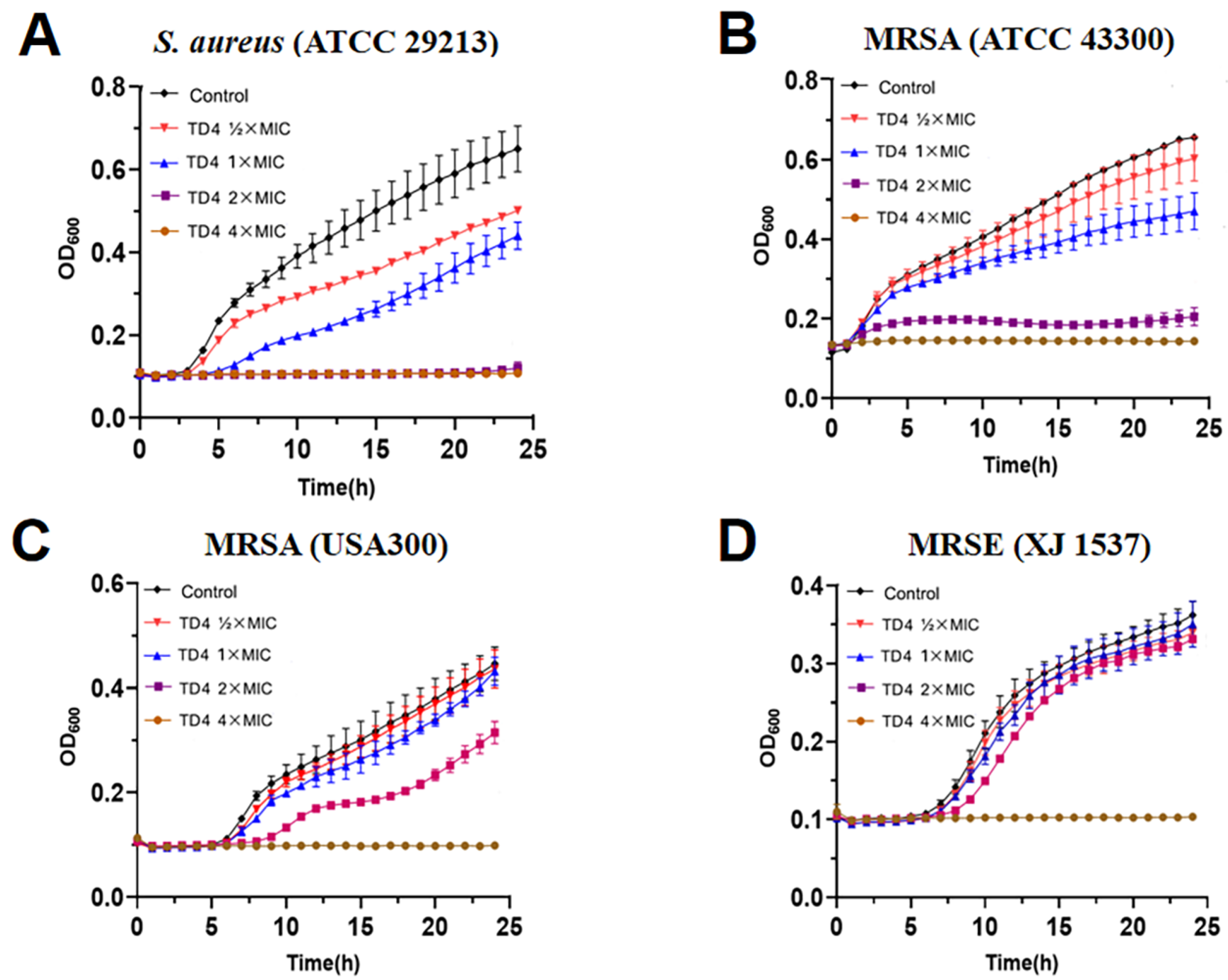
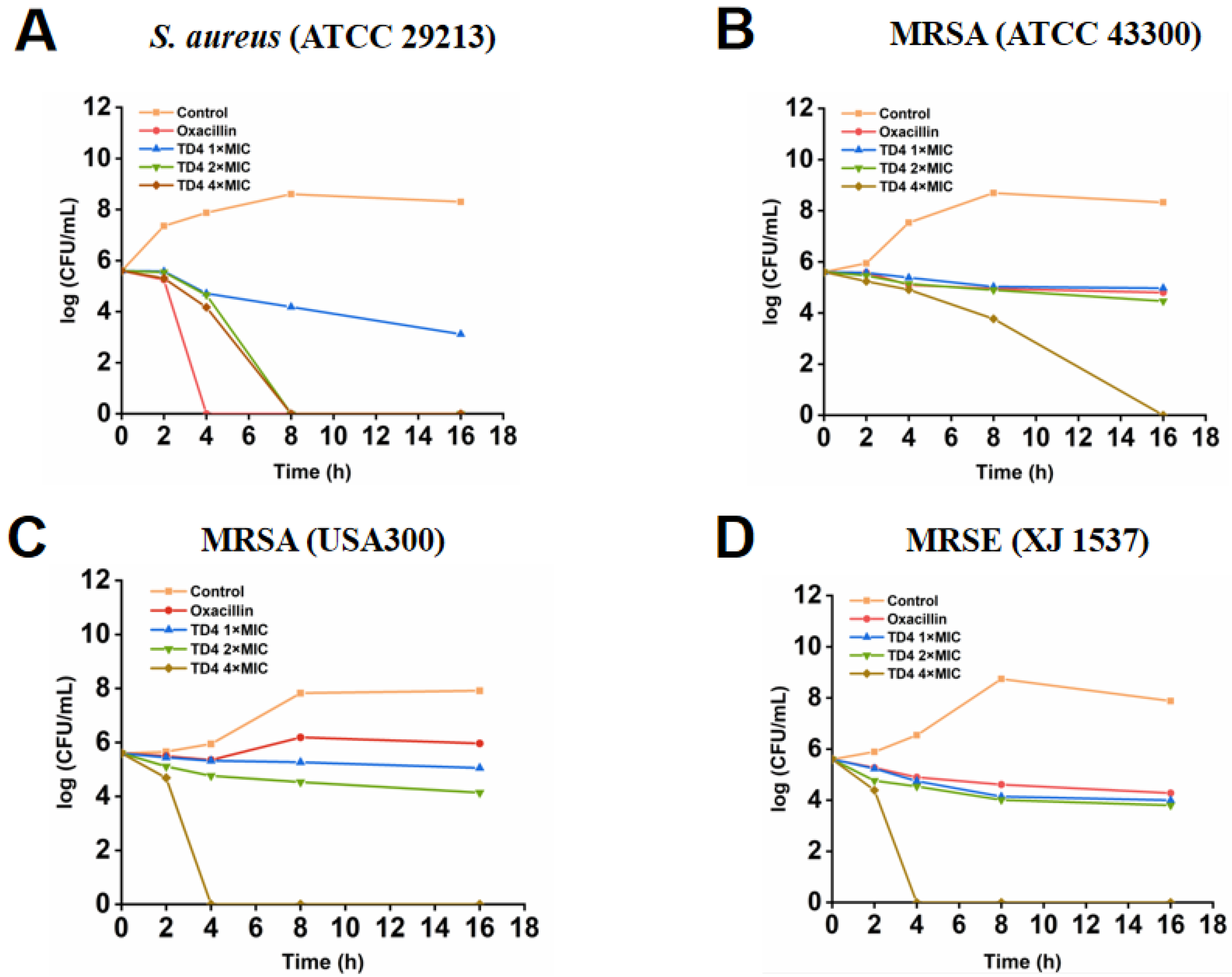
2.3. Inhibitory Activity of TD4 on MRSA Growth Curves and Bacterial Colonies
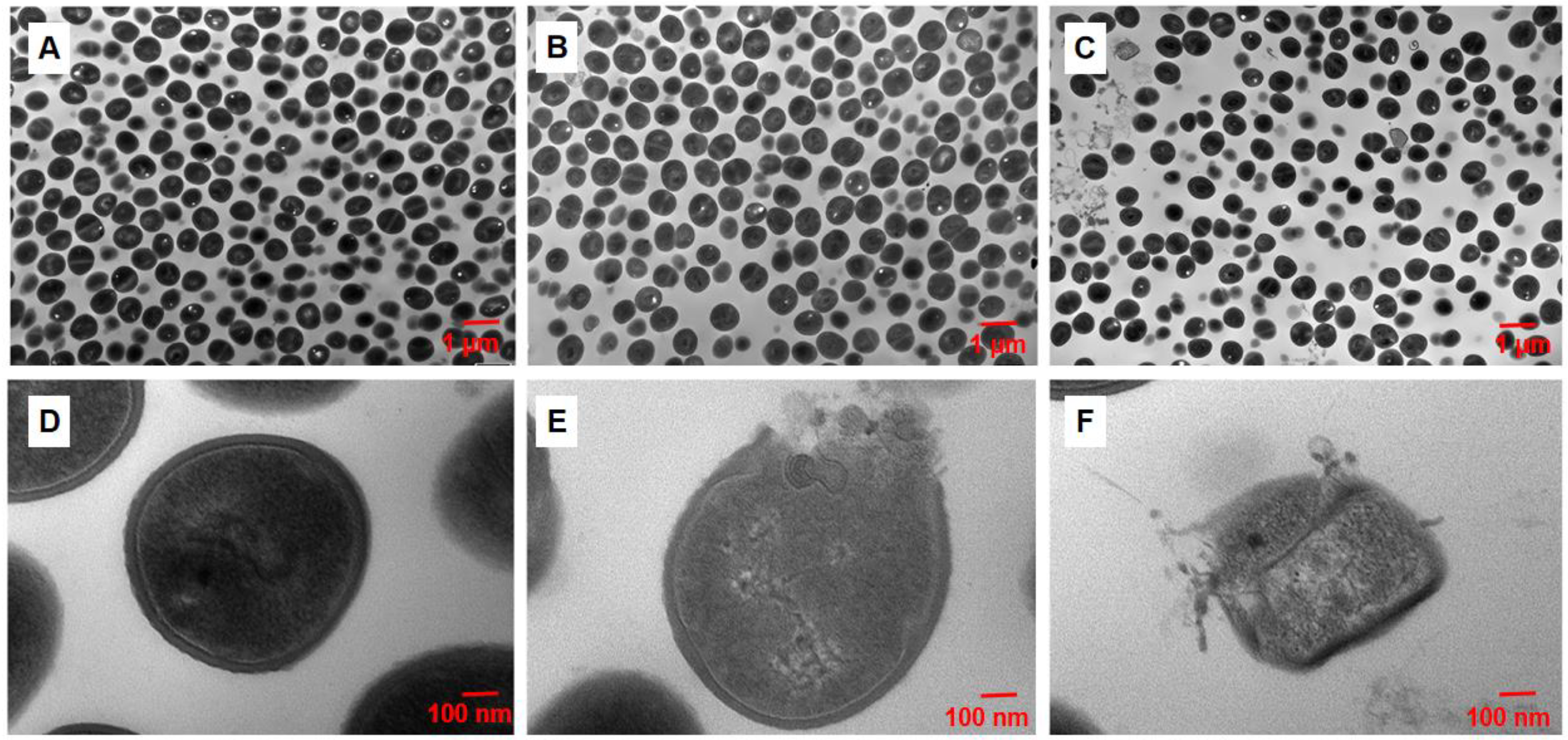
2.4. Disruption of TD4 on the Integrity of MRSA Cell Wall
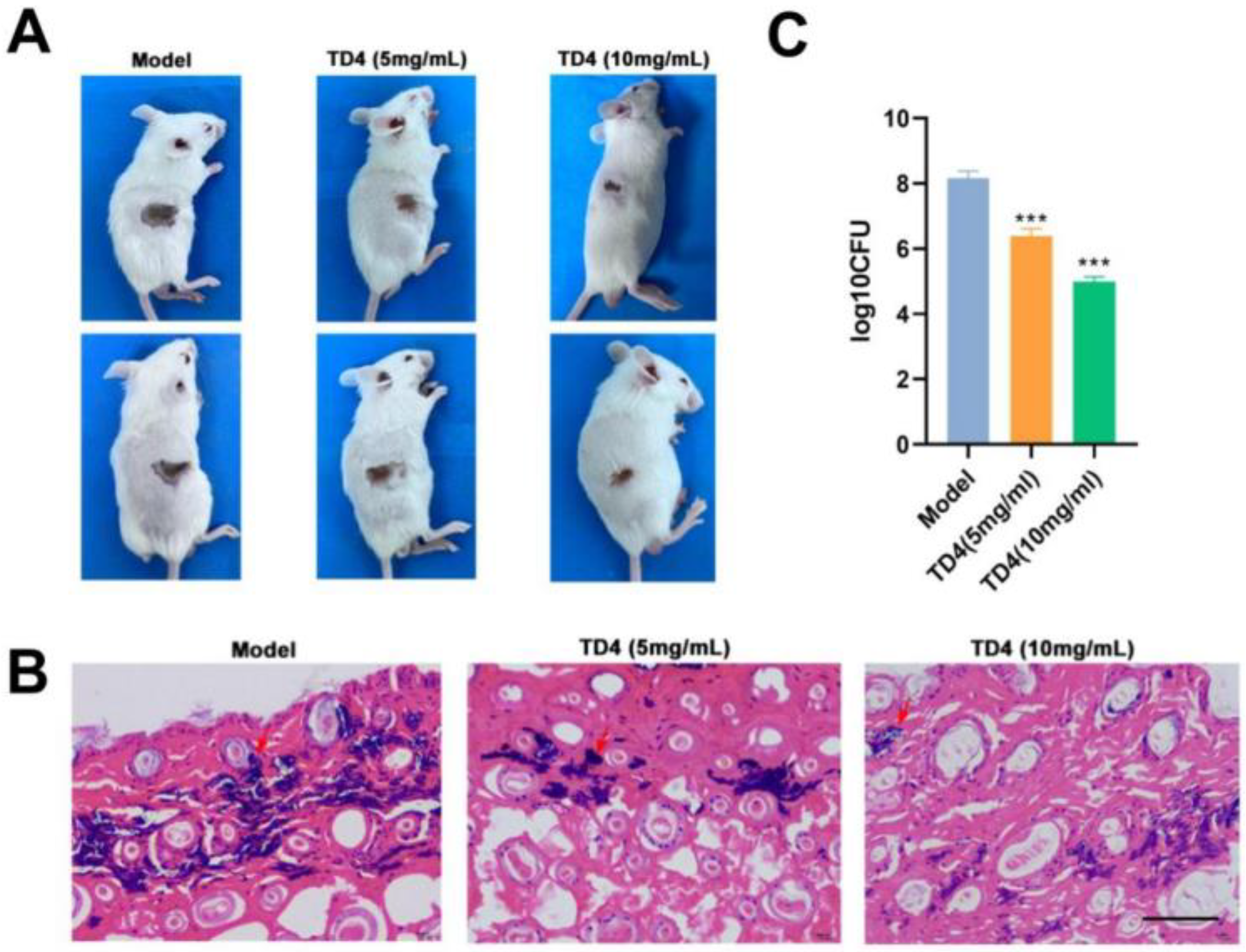
2.5. Therapeutic Efficiency of TD4 on MRSA-Induced Skin Infection
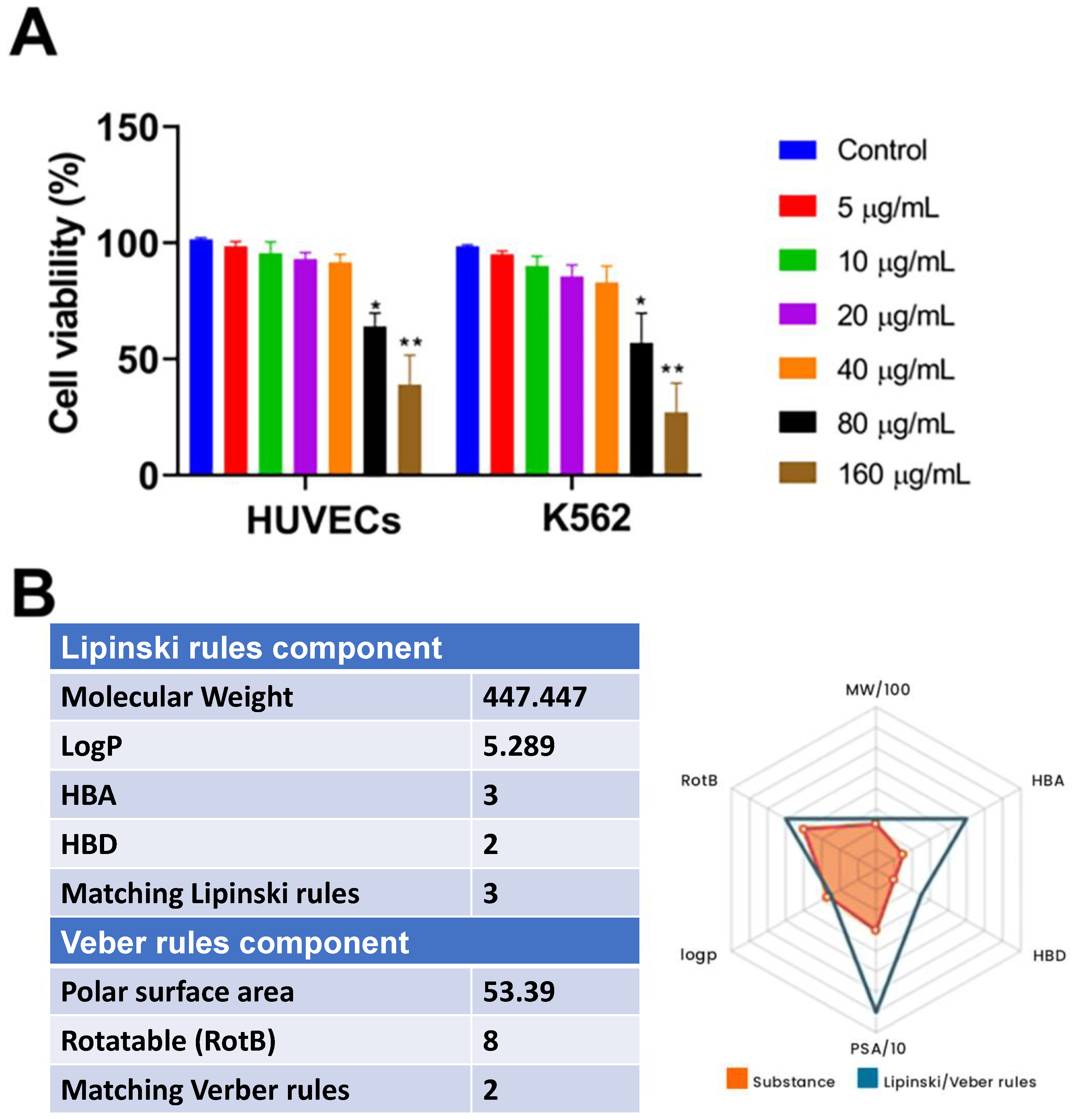
2.6. Cytotoxicity and Drug-Likeness Evaluation of TD4
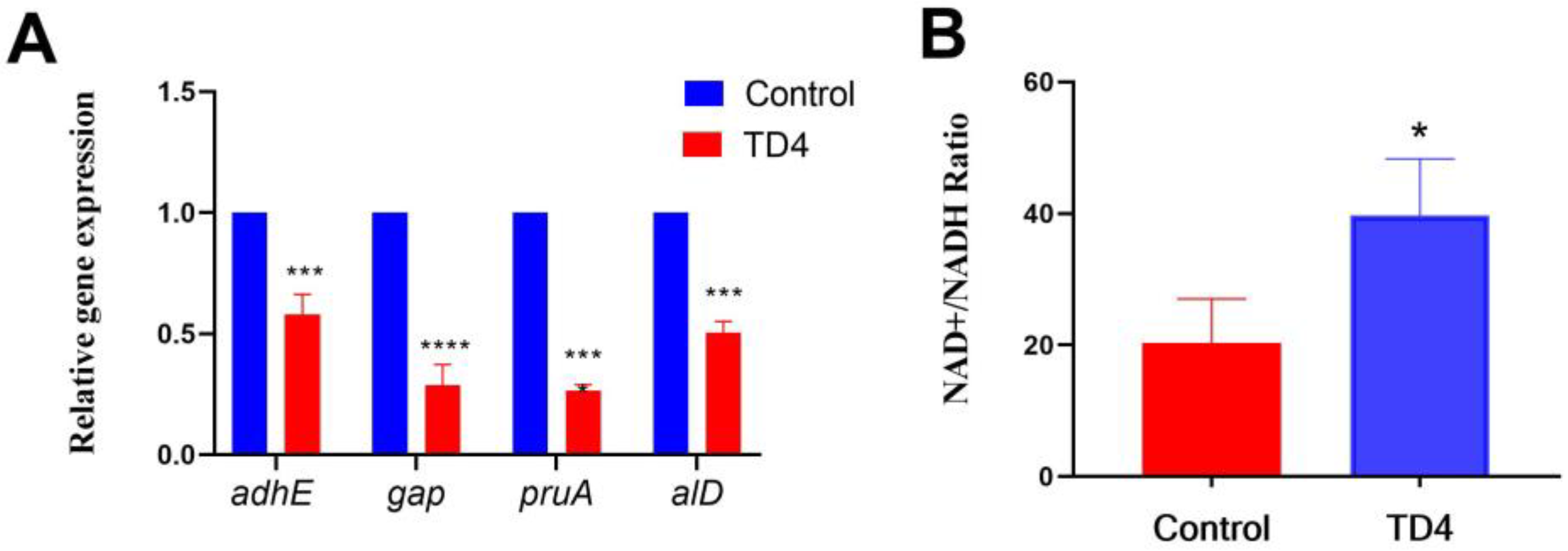
2.7. TD4 Disrupted Alanine Dehydrogenase-Dependent NAD+/NADH Homeostasis
3. Materials and Methods
3.1. Bacterial Strains, Cells, and Animals
3.2. Determination of Minimum Inhibitory Concentration
3.3. Bacterial Growth Curve Assay
3.4. In Vitro Time–Kill Curve Assay
3.5. Establishment of Bacterial Skin Infection Mouse Model
3.6. Electron Microscopy Observation
3.7. Cytotoxicity Test
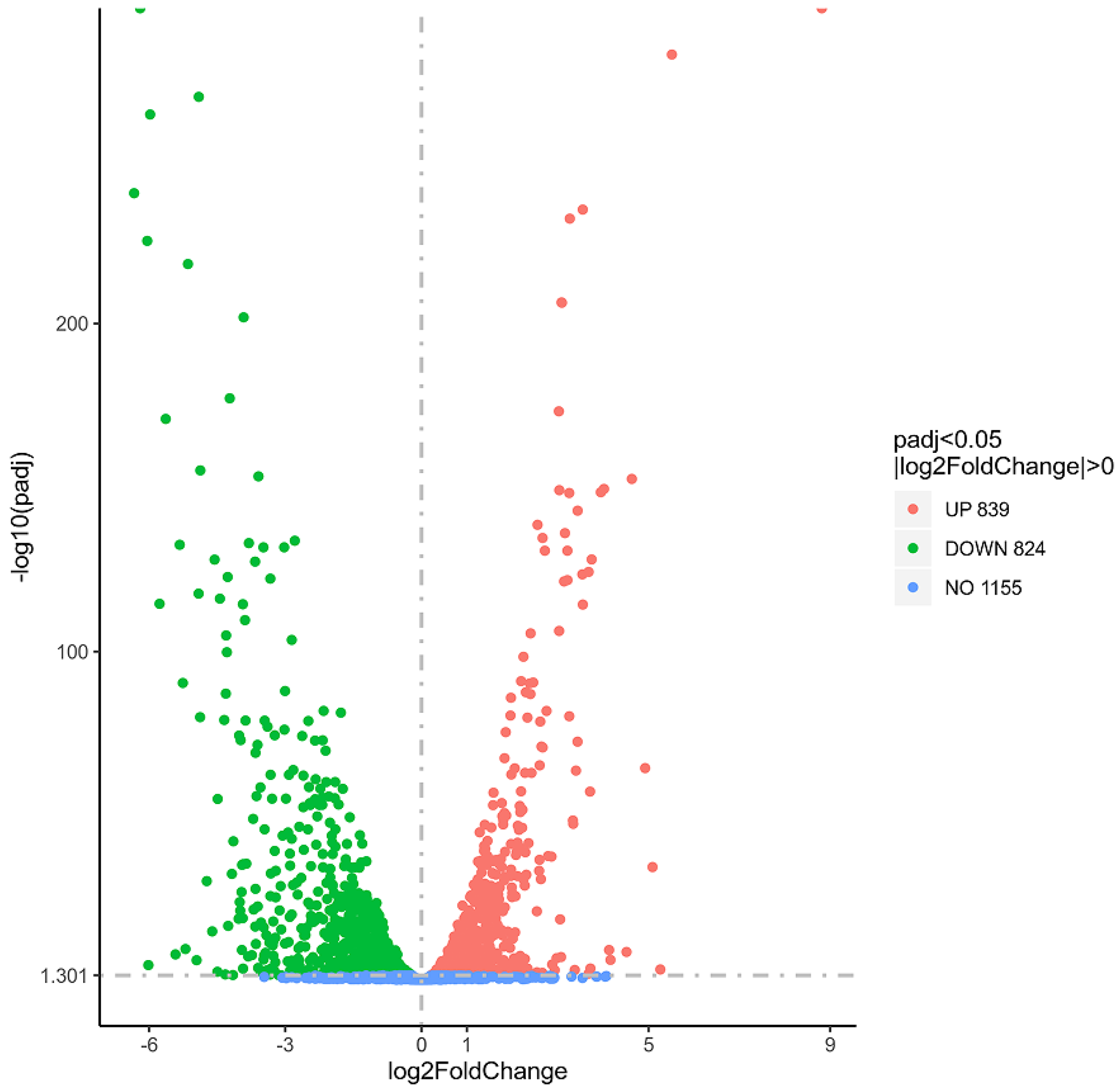
3.8. Transcriptome Analysis
3.9. Real-Time PCR
3.10. NAD+/NADH Ratio Measurement
3.11. Statistical Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ahmad-Mansour, N.; Loubet, P.; Pouget, C.; Dunyach-Remy, C.; Sotto, A.; Lavigne, J.P.; Molle, V. Staphylococcus aureus toxins: An update on their pathogenic properties and potential treatments. Toxins 2021, 13, 677. [Google Scholar] [CrossRef] [PubMed]
- Chang, J.; Lee, R.E.; Lee, W. A pursuit of Staphylococcus aureus continues: A role of persister cells. Arch. Pharm. Res. 2020, 43, 630–638. [Google Scholar] [CrossRef]
- Bronesky, D.; Wu, Z.; Marzi, S.; Walter, P.; Geissmann, T.; Moreau, K.; Vandenesch, F.; Caldelari, I.; Romby, P. Staphylococcus aureus RNAIII and its regulon link quorum sensing, stress responses, metabolic adaptation, and regulation of virulence gene expression. Annu. Rev. Microbiol. 2016, 70, 299–316. [Google Scholar] [CrossRef] [PubMed]
- Cheung, G.Y.C.; Bae, J.S.; Otto, M. Pathogenicity and virulence of Staphylococcus aureus. Virulence 2021, 12, 547–569. [Google Scholar] [CrossRef]
- Kwiecinski, J.M.; Horswill, A.R. Staphylococcus aureus bloodstream infections: Pathogenesis and regulatory mechanisms. Curr. Opin. Microbiol. 2020, 53, 51–60. [Google Scholar] [CrossRef] [PubMed]
- Suresh, M.K.; Biswas, R.; Biswas, L. An update on recent developments in the prevention and treatment of Staphylococcus aureus biofilms. Int. J. Med. Microbiol. 2019, 309, 1–12. [Google Scholar] [CrossRef]
- Liu, Y.; Zhang, J.; Ji, Y. Environmental factors modulate biofilm formation by Staphylococcus aureus. Sci. Prog. 2020, 103, 36850419898659. [Google Scholar] [CrossRef]
- Turner, N.A.; Kuinkel, B.K.S.; Maskarinec, S.A.; Eichenberger, E.M.; Shah, P.P.; Carugati, M.; Holland, T.L.; Fowler, V.G., Jr. Methicillin-resistant Staphylococcus aureus: An overview of basic and clinical research. Nat. Rev. Microbiol. 2019, 17, 203–218. [Google Scholar] [CrossRef]
- Chalmers, S.J.; Wylam, M.E. Methicillin-resistant Staphylococcus aureus infection and treatment options. Methods. Mol. Biol. 2020, 2069, 229–251. [Google Scholar]
- Ju, Y.; An, Q.; Zhang, Y.; Sun, K.; Bai, L.; Luo, Y. Recent advances in Clp protease modulation to address virulence, resistance and persistence of MRSA infection. Drug. Discov. Today 2021, 26, 2190–2197. [Google Scholar] [CrossRef]
- Maalik, A.; Rahim, H.; Saleem, M.; Fatima, N.; Rauf, A.; Wadood, A.; Malik, M.I.; Ahmed, A.; Rafique, H.; Zafar, M.N.; et al. Synthesis, antimicrobial, antioxidant, cytotoxic, antiurease and molecular docking studies of N-(3-trifluoromethyl) benzoyl-N’-aryl thiourea derivatives. Bioorg. Chem. 2019, 88, 102946. [Google Scholar] [CrossRef] [PubMed]
- Canudo-Barreras, G.; Ortego, L.; Izaga, A.; Marzo, I.; Herrera, R.P.; Gimeno, M.C. Synthesis of new thiourea-metal complexes with promising anticancer properties. Molecules 2021, 26, 6891. [Google Scholar] [CrossRef] [PubMed]
- Li, R.; Cen, B.; Duan, W.G.; Lin, G.S. Synthesis, antifungal activity and 3D-QSAR study of novel anisaldehyde-derived amide-thiourea compounds. Chem. Biodivers. 2022, 19, e202101025. [Google Scholar] [CrossRef] [PubMed]
- Ronchetti, R.; Moroni, G.; Carotti, A.; Gioiello, A.; Camaioni, E. Recent advances in urea- and thiourea-containing compounds: Focus on innovative approaches in medicinal chemistry and organic synthesis. RSC. Med. Chem. 2021, 12, 1046–1064. [Google Scholar] [CrossRef]
- Thomas, S.J.; Balónová, B.; Jr, J.C.; Wass, M.N.; Serpell, C.J.; Blight, B.A.; Martin, M. Thiourea and guanidine compounds and their iridium complexes in drug-resistant cancer cell lines: Structure-activity relationships and direct luminescent imaging. ChemMedChem 2020, 15, 349–353. [Google Scholar] [CrossRef]
- Ghosh, A.K.; Brindisi, M. Urea derivatives in modern drug discovery and medicinal chemistry. J. Med. Chem. 2020, 63, 2751–2788. [Google Scholar] [CrossRef]
- Kumari, S.; Carmona, A.V.; Tiwari, A.K.; Trippier, P.C. Amide bond bioisosteres: Strategies, synthesis, and successes. J. Med. Chem. 2020, 63, 12290–12358. [Google Scholar] [CrossRef]
- Sun, C.P.; Zhang, X.Y.; Morisseau, C.; Hwang, S.H.; Zhang, Z.J.; Hammock, B.D.; Ma, X.C. Discovery of soluble epoxide hydrolase inhibitors from chemical synthesis and natural products J. Med. Chem. 2021, 64, 184–215. [Google Scholar] [CrossRef]
- Herrera, R.P.; Sgarzani, V.; Bernardi, L.; Ricci, A. Catalytic enantioselective Friedel-Crafts alkylation of indoles with nitroalkenes by using a simple thiourea organocatalyst. Angew. Chem. Int. Ed. 2005, 44, 6576–6579. [Google Scholar] [CrossRef]
- Sibi, P.M.; Kennosuke, I. Organocatalysis in conjugate amine additions synthesis of â-amino acid derivatives. J. Am. Chem. Soc. 2007, 129, 8064–8065. [Google Scholar] [CrossRef]
- Schneider, J.F.; Lauber, M.B.; Markus, V.; Kratzer, D.; Jan, P. Readily available hydrogen bond catalysts for the asymmetric transfer hydrogenation of nitroolefins. Org. Biomol. Chem. 2011, 9, 4323–4327. [Google Scholar] [CrossRef] [PubMed]
- Tan, W.; Wei, J.P.; Jiang, X.F. Thiocarbonyl surrogate via combination of sulfur and chloroform for thiocarbamide and oxazolidinethione construction. Org. Lett. 2017, 19, 2166–2169. [Google Scholar] [CrossRef]
- Zhu, J.C.; Cui, D.X.; Li, Y.D.; He, J.X.; Chen, W.P.; Wang, P.G. Enantioselective amination of nitroolefins under base-free and water-rich conditions using chiral bifunctional phase-transfer catalysts. Org. Biomol. Chem. 2018, 16, 3012–3017. [Google Scholar] [CrossRef] [PubMed]
- Khan, S.; Singh, N.; Saleem, K. Synthesis, characterization and in vitro antibacterial activity of thiourea and urea derivatives of steroids. Eur. J. Med. Chem. 2008, 43, 2272–2277. [Google Scholar] [CrossRef]
- Cui, P.; Li, X.; Zhu, M.; Wang, B.; Liu, J.; Chen, H. Design, synthesis and antibacterial activities of thiouracil derivatives containing acyl thiourea as SecA inhibitors. Bioorg. Med. Chem. Lett. 2017, 27, 2234–2237. [Google Scholar] [CrossRef]
- Mayer, C.; Kluj, R.; Mühleck, M.; Walter, A.; Unsleber, S.; Hottmann, I.; Borisova, M. Bacteria’s different ways to recycle their own cell wall. Int. J. Med. Microbiol. 2019, 309, 151326. [Google Scholar] [CrossRef]
- Horne, J.E.; Brockwell, D.J.; Radford, S.E. Role of the lipid bilayer in outer membrane protein folding in Gram-negative bacteria. J. Biol. Chem. 2020, 295, 10340–10367. [Google Scholar] [CrossRef]
- Qu, D.; Hou, Z.; Li, J.; Luo, L.; Su, S.; Ye, Z.; Bai, Y.; Zhang, X.; Chen, G.; Li, Z.; et al. A new coumarin compound DCH combats methicillin-resistant Staphylococcus aureus biofilm by targeting arginine repressor. Sci. Adv. 2020, 6, 9597. [Google Scholar] [CrossRef]
- Yadav, A.K.; Cava, A.E.F. Bacterial strategies to preserve cell wall integrity against environmental threats. Front. Microbiol. 2018, 9, 2064. [Google Scholar] [CrossRef]
- Hatlen, T.J.; Miller, L.G. Staphylococcal skin and soft tissue infections. Infect. Dis. Clin. 2021, 35, 81–105. [Google Scholar] [CrossRef] [PubMed]
- Hindy, J.R.; Haddad, S.; Kanj, S. New drugs for methicillin-resistant Staphylococcus aureus skin and soft tissue infections. Curr. Opin. Infect. Dis. 2022, 35, 112–119. [Google Scholar] [CrossRef] [PubMed]
- Afzal, M.; Shafeeq, S.; Kuipers, O.P. NADH-mediated gene expression in Streptococcus pneumoniae and role of Rex as a transcriptional repressor of the Rex-regulon. Front Microbiol. 2018, 9, 1300. [Google Scholar] [CrossRef] [PubMed]
- Yu, M.X.; Hou, Y.C.; Cheng, M.L.; Liu, Y.S.; Ling, C.S.; Zhai, D.S.; Zhao, H.; Li, Y.Y.; Chen, Y.M.; Xue, X.Y.; et al. Antibacterial activity of squaric amide derivative SA2 against methicillin-resistant Staphylococcus aureus. Antibiotics 2022, 11, 1497. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Sauve, A.A. Assays for determination of cellular and mitochondrial NAD+ and NADH content. Methods Mol. Biol. 2021, 2310, 271–285. [Google Scholar] [PubMed]



| Compound | MIC(µg/mL) | |
|---|---|---|
| S. aureus (ATCC 29213) | MRSA (USA 300) | |
| TD1 | 4 | 8 |
| TD2 | 8 | 8 |
| TD3 | 2 | 16 |
| TD4 | 2 | 4 |
| TD5 | 4 | 32 |
| TD6 | 32 | 32 |
| TD7 | 16 | 16 |
| TD8 | 8 | 16 |
| Oxacillin | 2 | >256 |
| Ceftazidime | 4 | >256 |
| Microorganism | MIC (µg/mL) |
|---|---|
| G+ Bacteria | |
| Staphylococcus aureus (MSSA, ATCC 29213) | 2 |
| Staphylococcus aureus (MRSA, ATCC 43300) | 8 |
| Staphylococcus aureus (MRSA, VISA, Mu50) | 4 |
| Staphylococcus aureus (MRSA, USA 300) | 4 |
| Staphylococcus aureus (MRSA, clinical strain XJ 26) | 8–16 |
| Staphylococcus aureus (MRSA, clinical strain XJ 216) | 8–16 |
| Staphylococcus aureus (MRSA, clinical strain XJ 317) | 8–16 |
| Methicillin-resistant Staphycoccus epidermidis (clinical strain XJ 1537) | 8 |
| Enterococcus faecalis (ATCC 29212) | 4 |
| Vancomycin resistant Enterococcus (clinical strain XJ 21) | 8–16 |
| Vancomycin resistant Enterococcus (clinical strain XJ 22) | 8–16 |
| Vancomycin resistant Enterococcus (clinical strain XJ 23) | 8–16 |
| G− Bacteria | |
| Escherichia coli (ATCC 25922) | >256 |
| Multi-drug resistant clinical strain (XJ 74283) | >256 |
| Multi-drug resistant (SL 1344) | >256 |
| Acinetobacter baumannii (ATCC 19606) | >256 |
| Pseudomonas aeruginosa (ATCC 27853) | >256 |
| Multi-drug resistant clinical strain (XJ 75315) | >256 |
| Klebsiella pneumoniae (ATCC 700603) | >256 |
| Klebsiella pneumoniae (ATCC 13885) | >256 |
| Multi-drug resistant clinical (ATCC 75297) | >256 |
| Gene ID | Gene Name | log2Fold Change |
|---|---|---|
| USA 300_RS00795 | adhE | −6.19 |
| USA 300_RS07360 | alD | −6.03 |
| USA 300_RS13865 | pruA | −2.11 |
| USA 300_RS08940 | gap | −3.80 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hou, Y.; Zhu, S.; Chen, Y.; Yu, M.; Liu, Y.; Li, M. Evaluation of Antibacterial Activity of Thiourea Derivative TD4 against Methicillin-Resistant Staphylococcus aureus via Destroying the NAD+/NADH Homeostasis. Molecules 2023, 28, 3219. https://doi.org/10.3390/molecules28073219
Hou Y, Zhu S, Chen Y, Yu M, Liu Y, Li M. Evaluation of Antibacterial Activity of Thiourea Derivative TD4 against Methicillin-Resistant Staphylococcus aureus via Destroying the NAD+/NADH Homeostasis. Molecules. 2023; 28(7):3219. https://doi.org/10.3390/molecules28073219
Chicago/Turabian StyleHou, Yachen, Sikai Zhu, Yamiao Chen, Moxi Yu, Yongsheng Liu, and Mingkai Li. 2023. "Evaluation of Antibacterial Activity of Thiourea Derivative TD4 against Methicillin-Resistant Staphylococcus aureus via Destroying the NAD+/NADH Homeostasis" Molecules 28, no. 7: 3219. https://doi.org/10.3390/molecules28073219
APA StyleHou, Y., Zhu, S., Chen, Y., Yu, M., Liu, Y., & Li, M. (2023). Evaluation of Antibacterial Activity of Thiourea Derivative TD4 against Methicillin-Resistant Staphylococcus aureus via Destroying the NAD+/NADH Homeostasis. Molecules, 28(7), 3219. https://doi.org/10.3390/molecules28073219






