Effect of Ag Modification on the Structure and Photocatalytic Performance of TiO2/Muscovite Composites
Abstract
1. Introduction
2. Results and Discussion
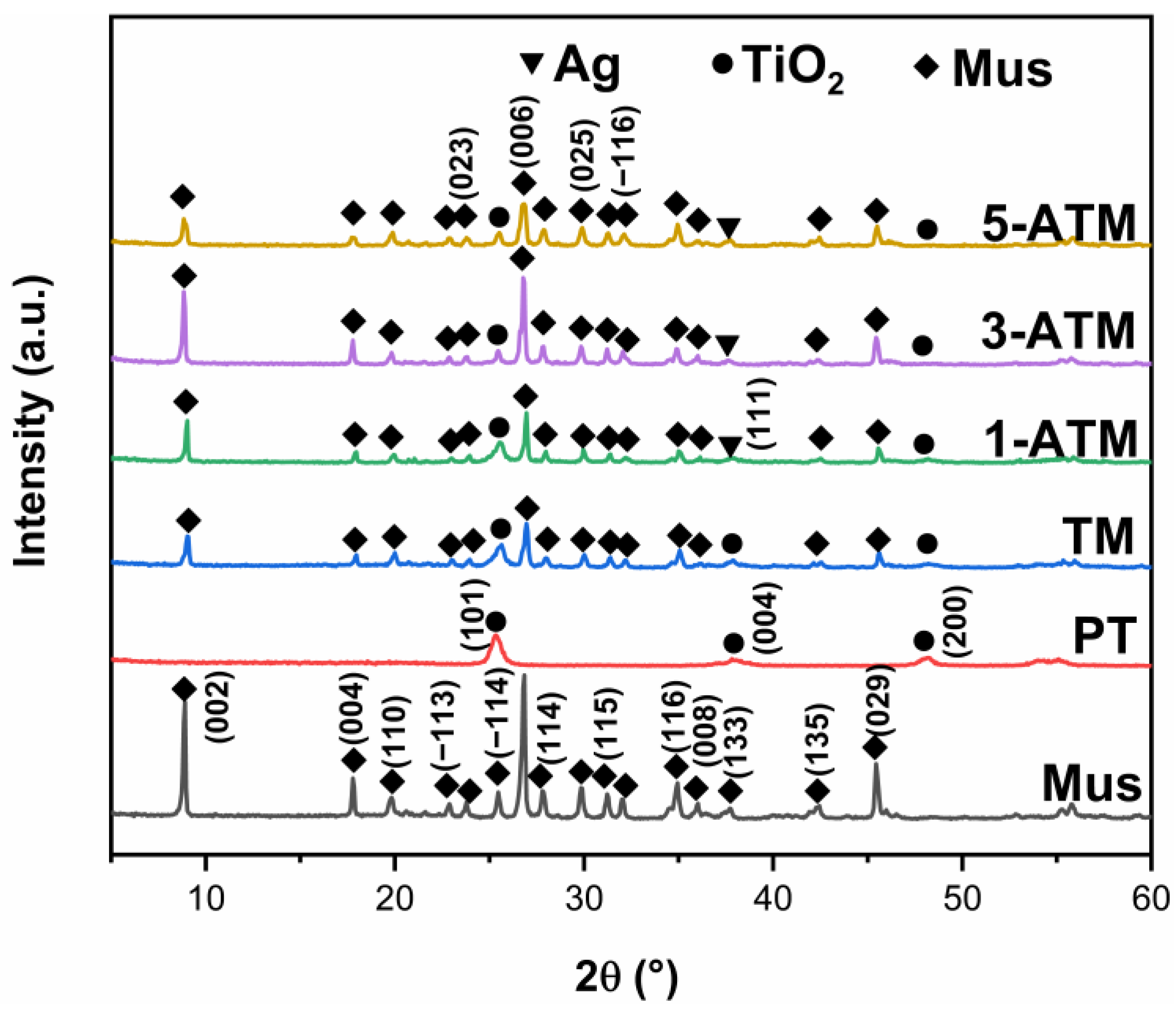
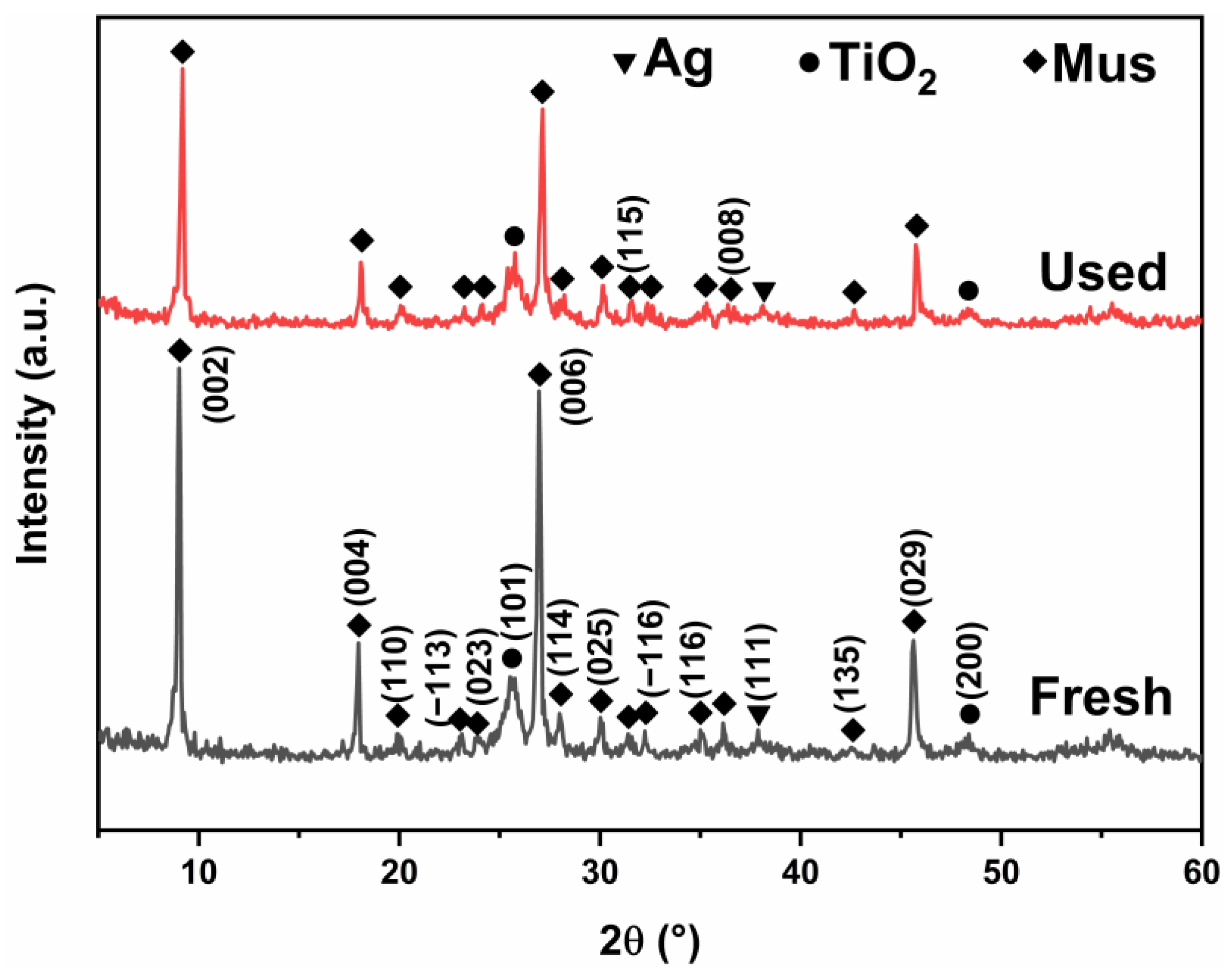
2.1. Phase Composition
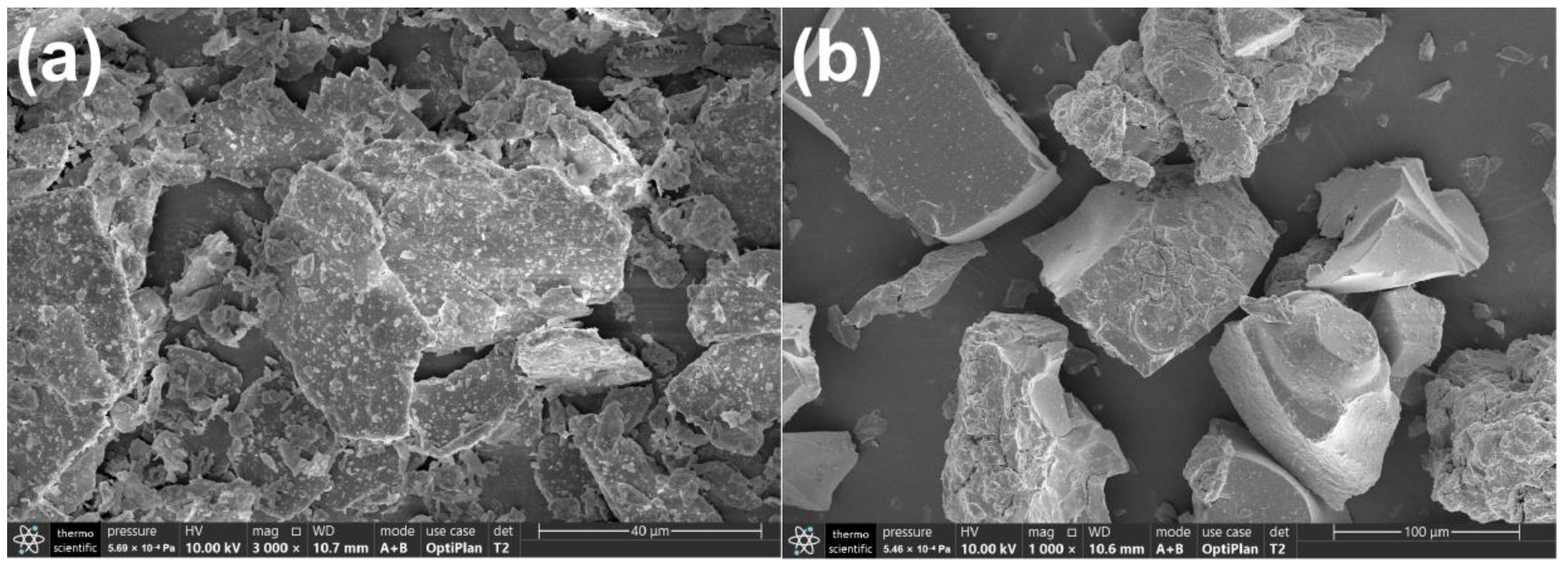
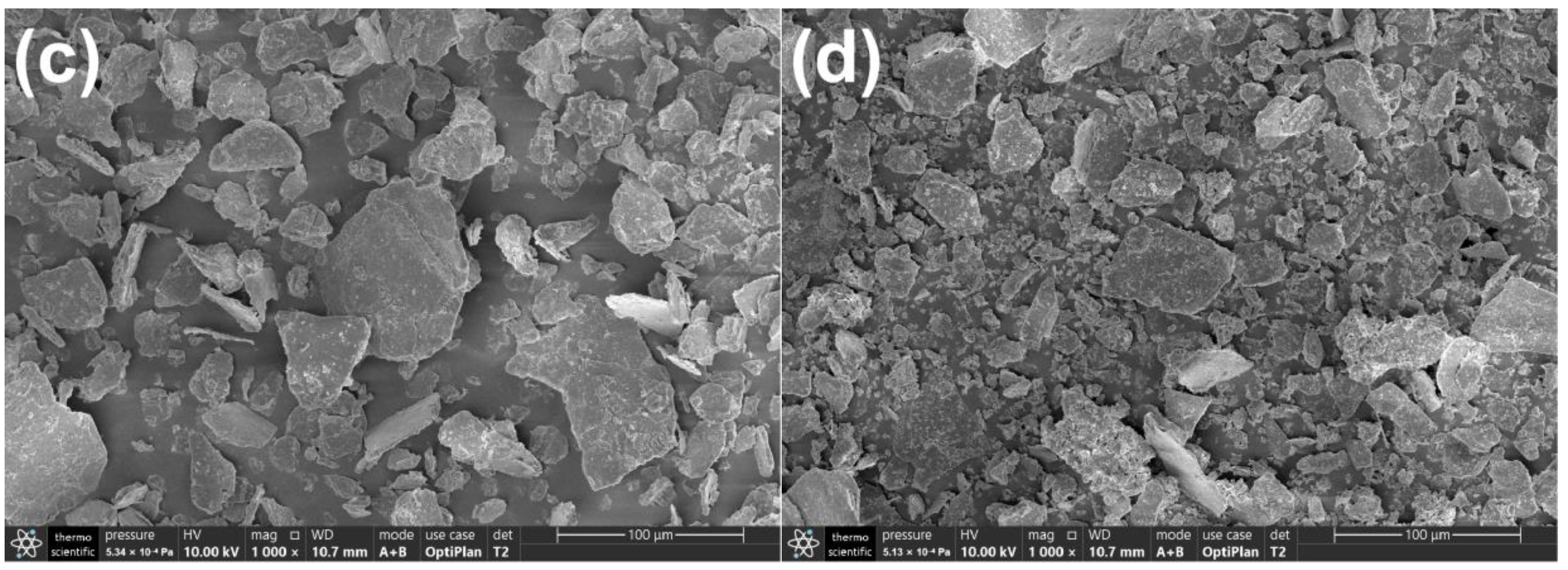
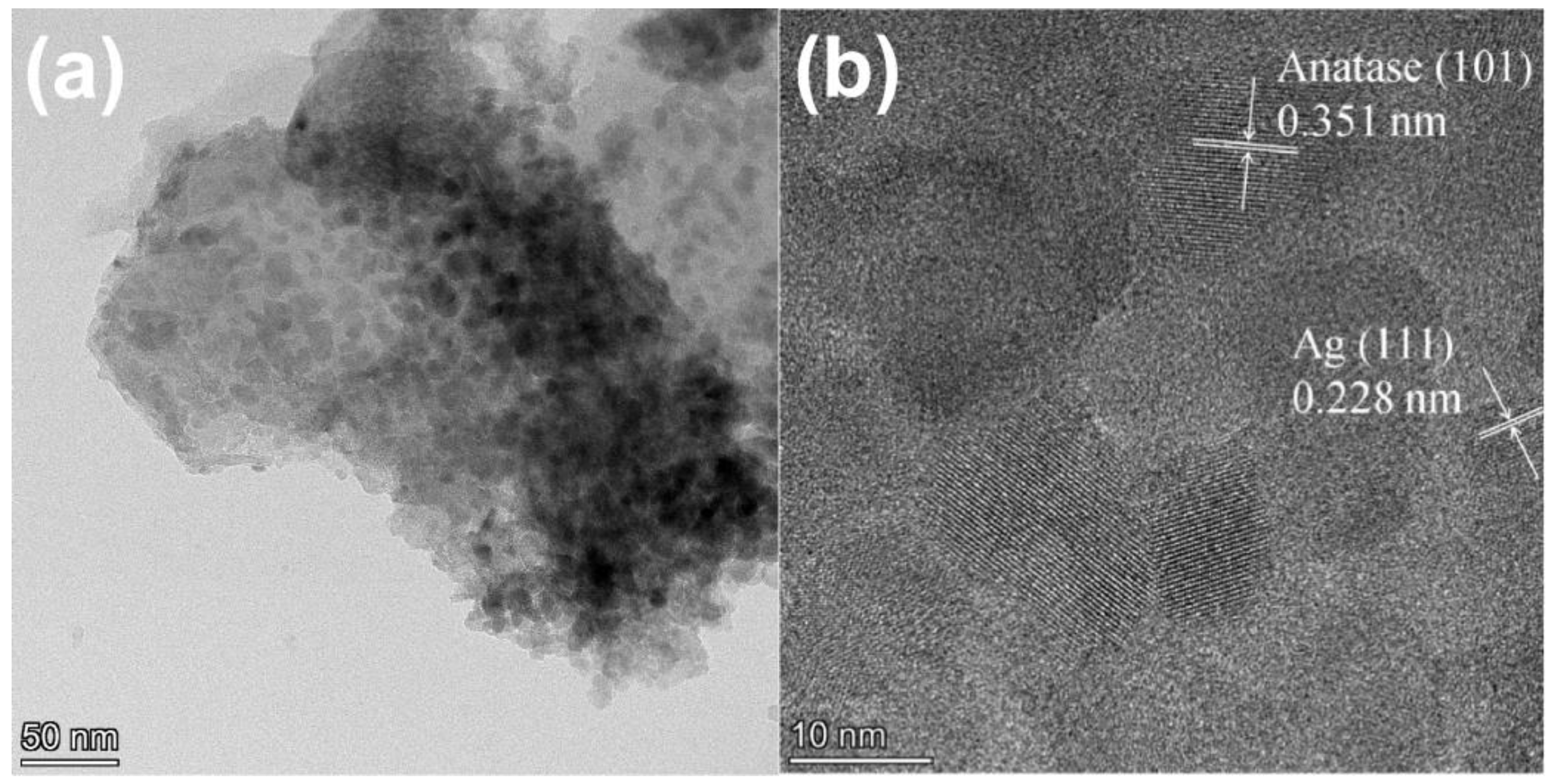
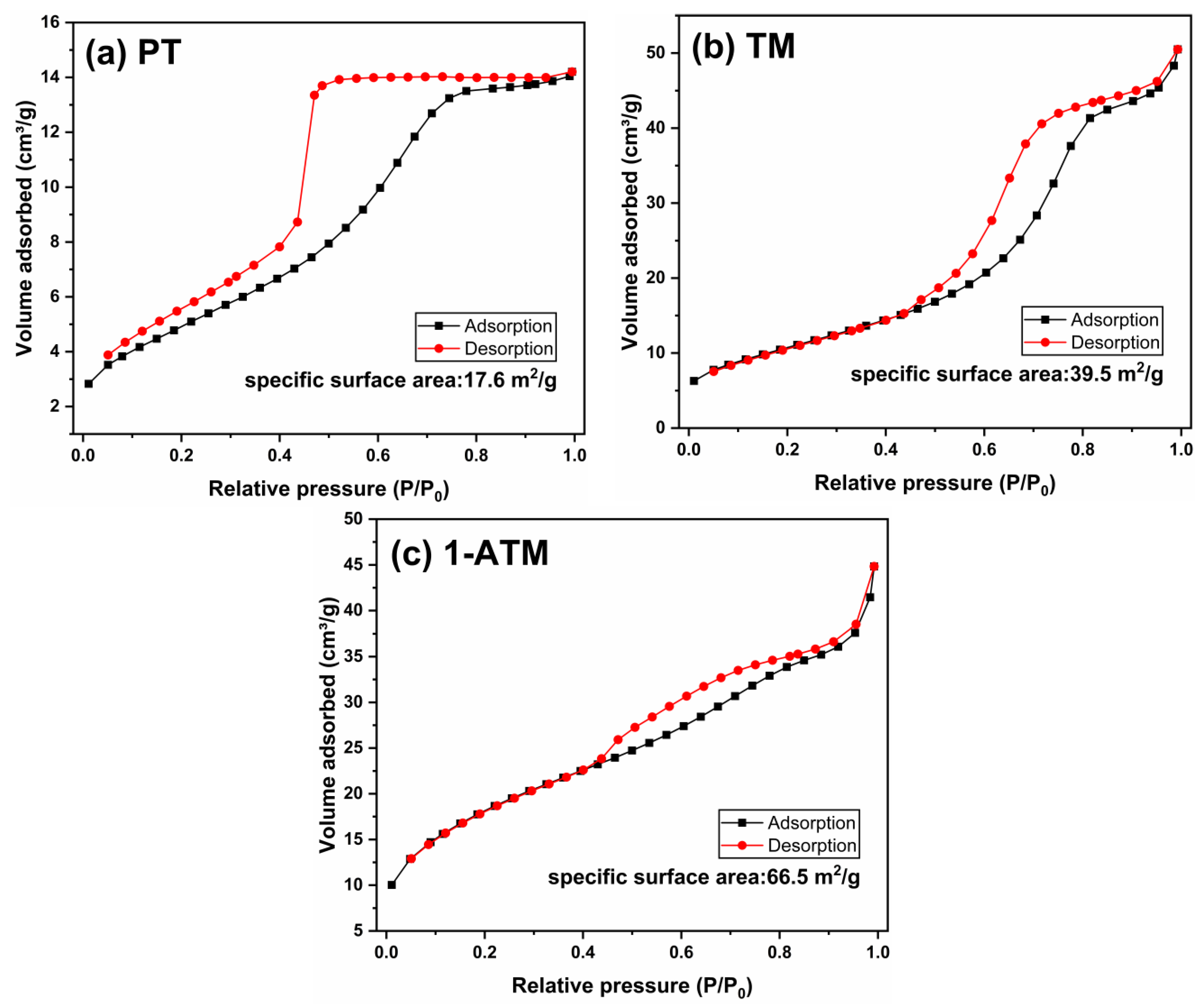
2.2. Morphology and BET Surface Area
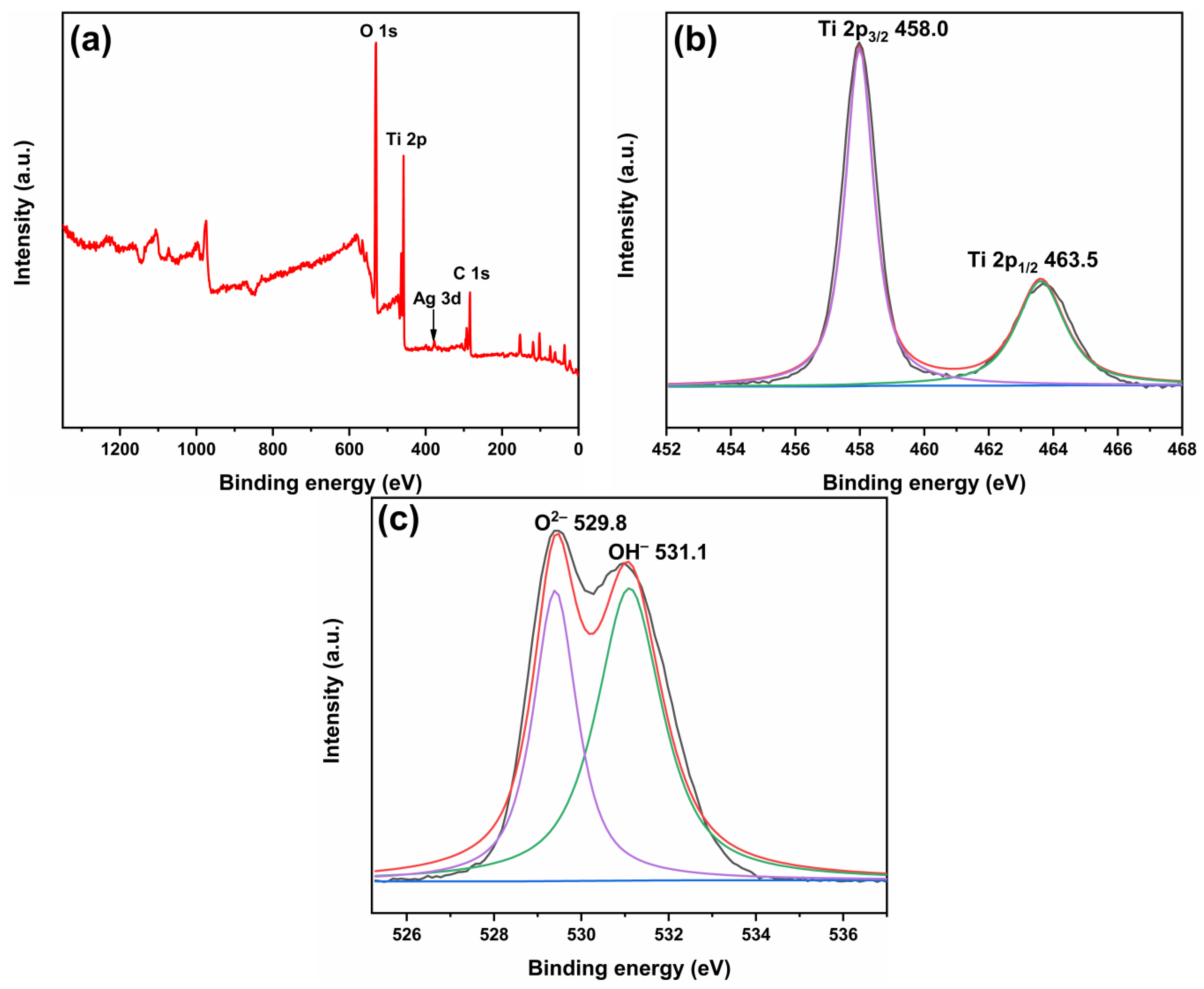
2.3. Element Valence State
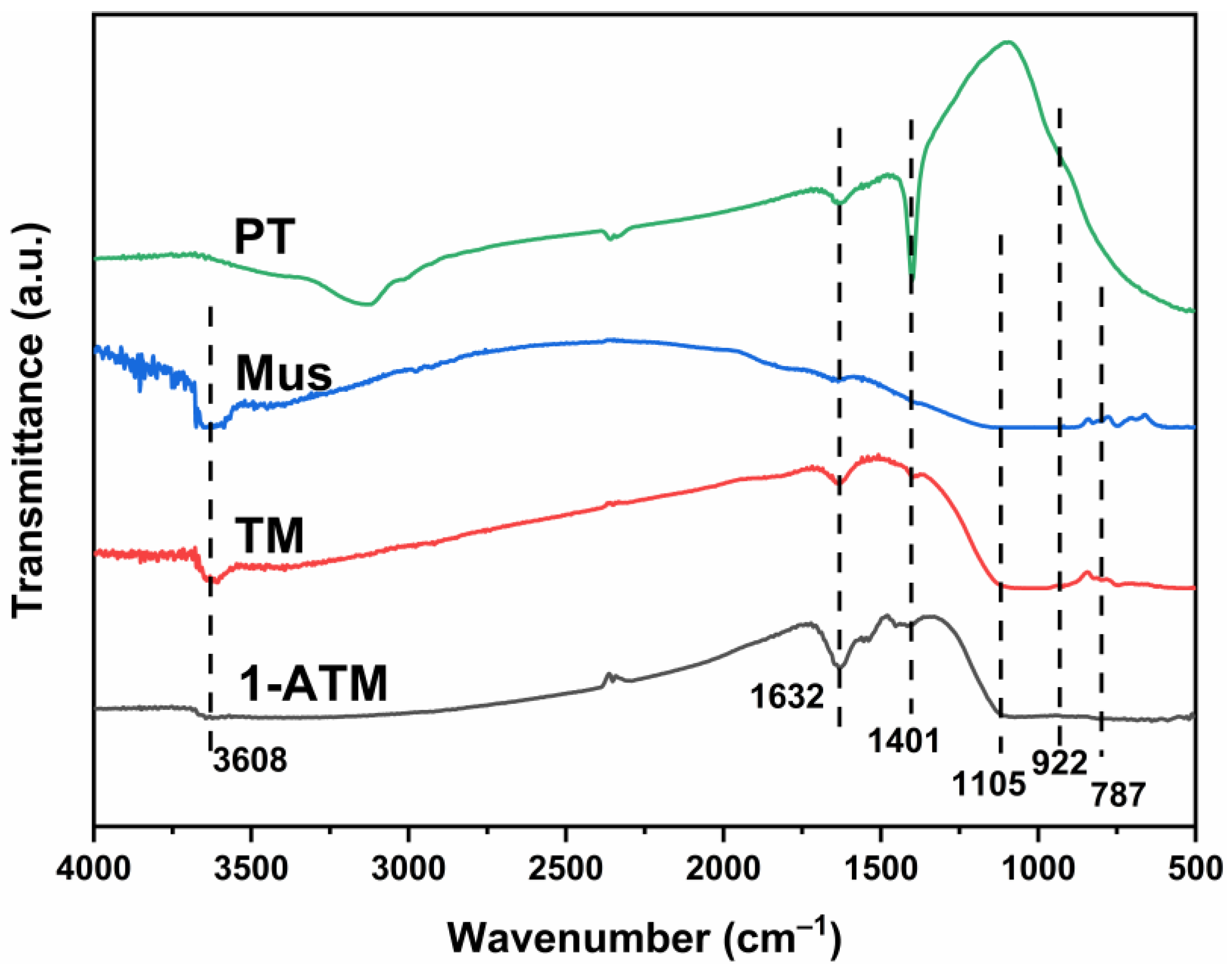
2.4. FTIR Analysis
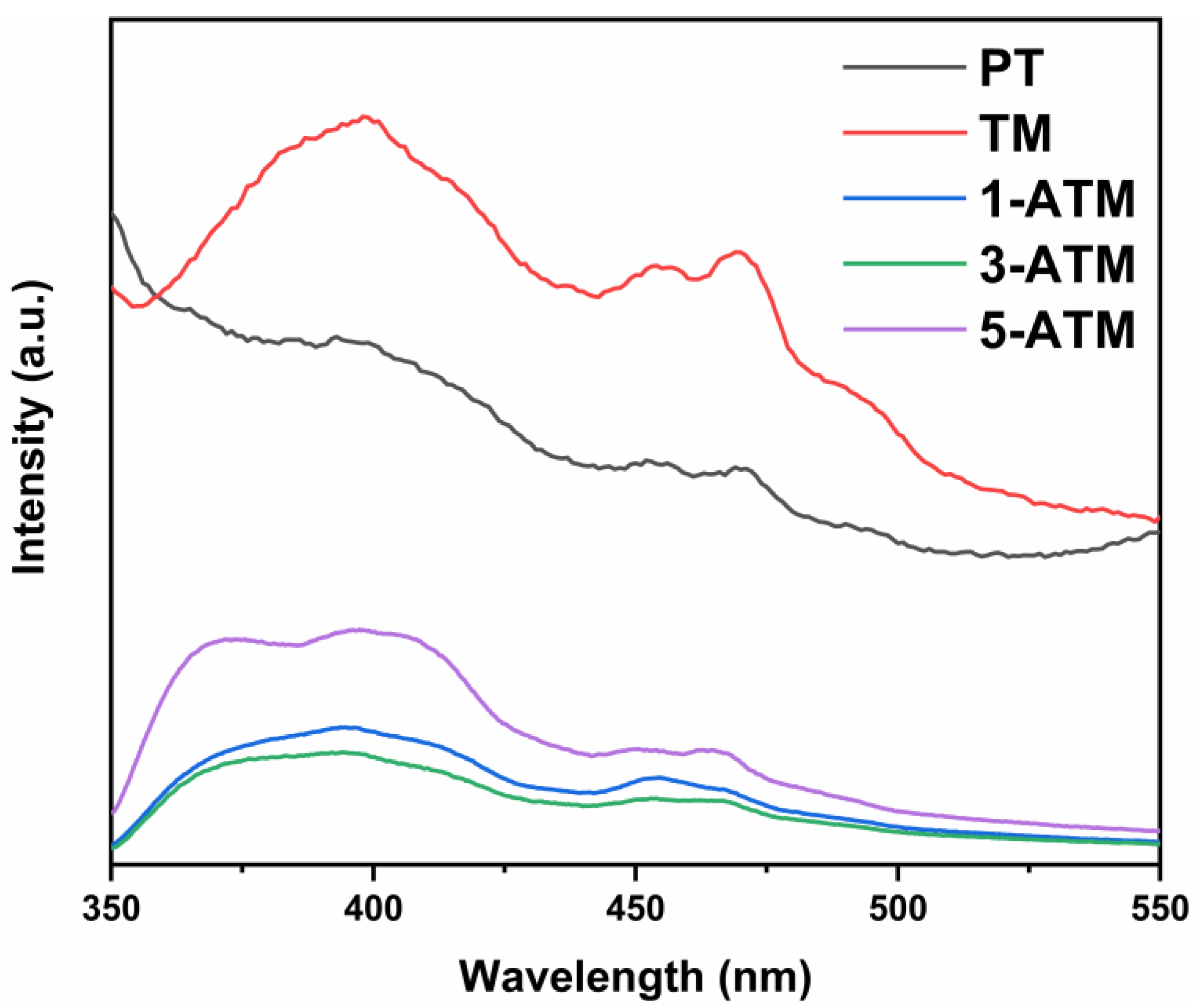
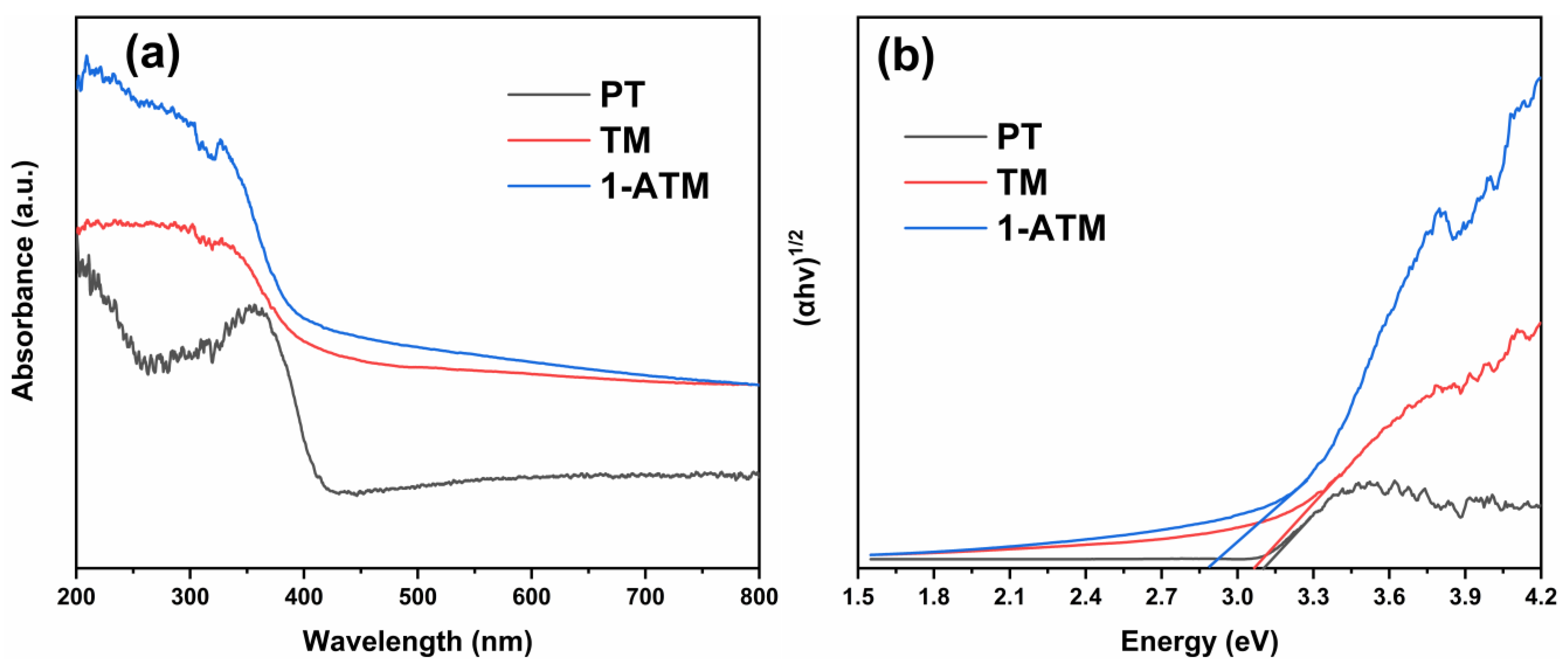
2.5. Optical Property
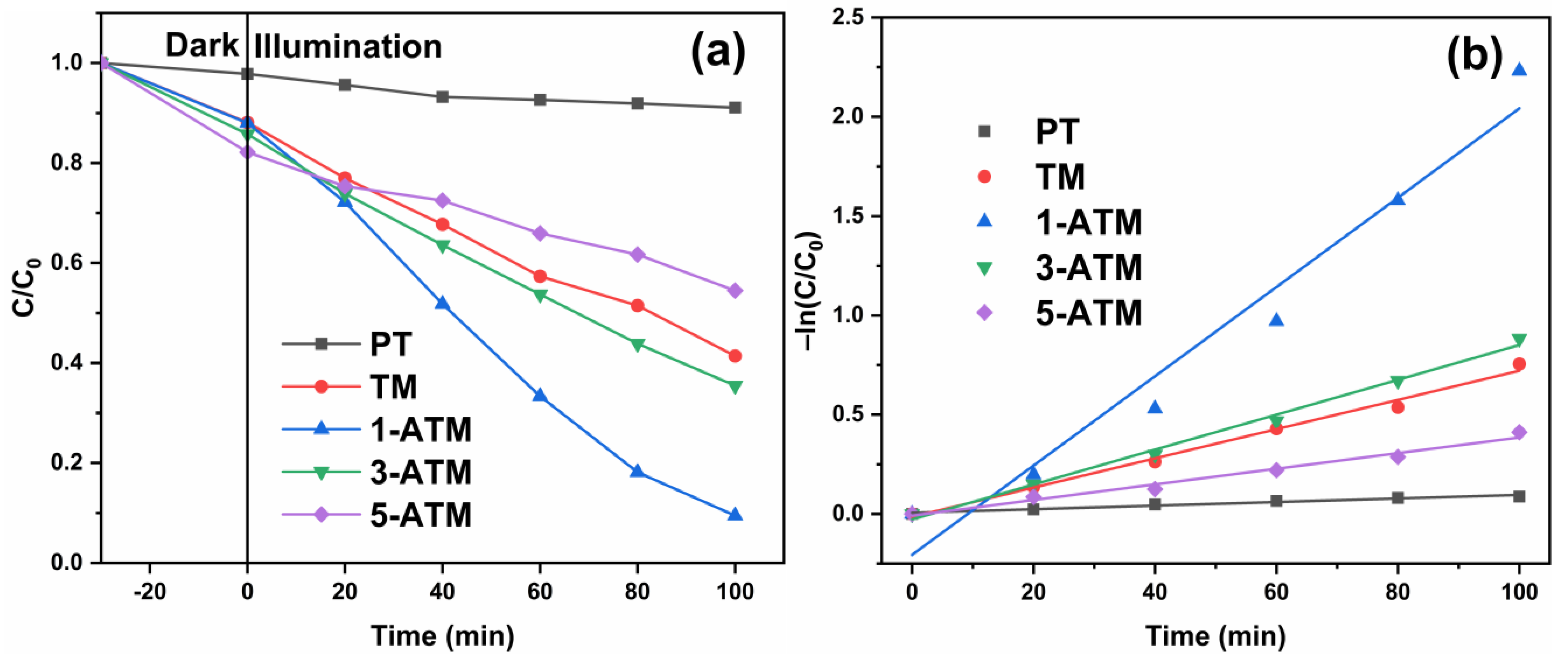
2.6. Photocatalytic Performance
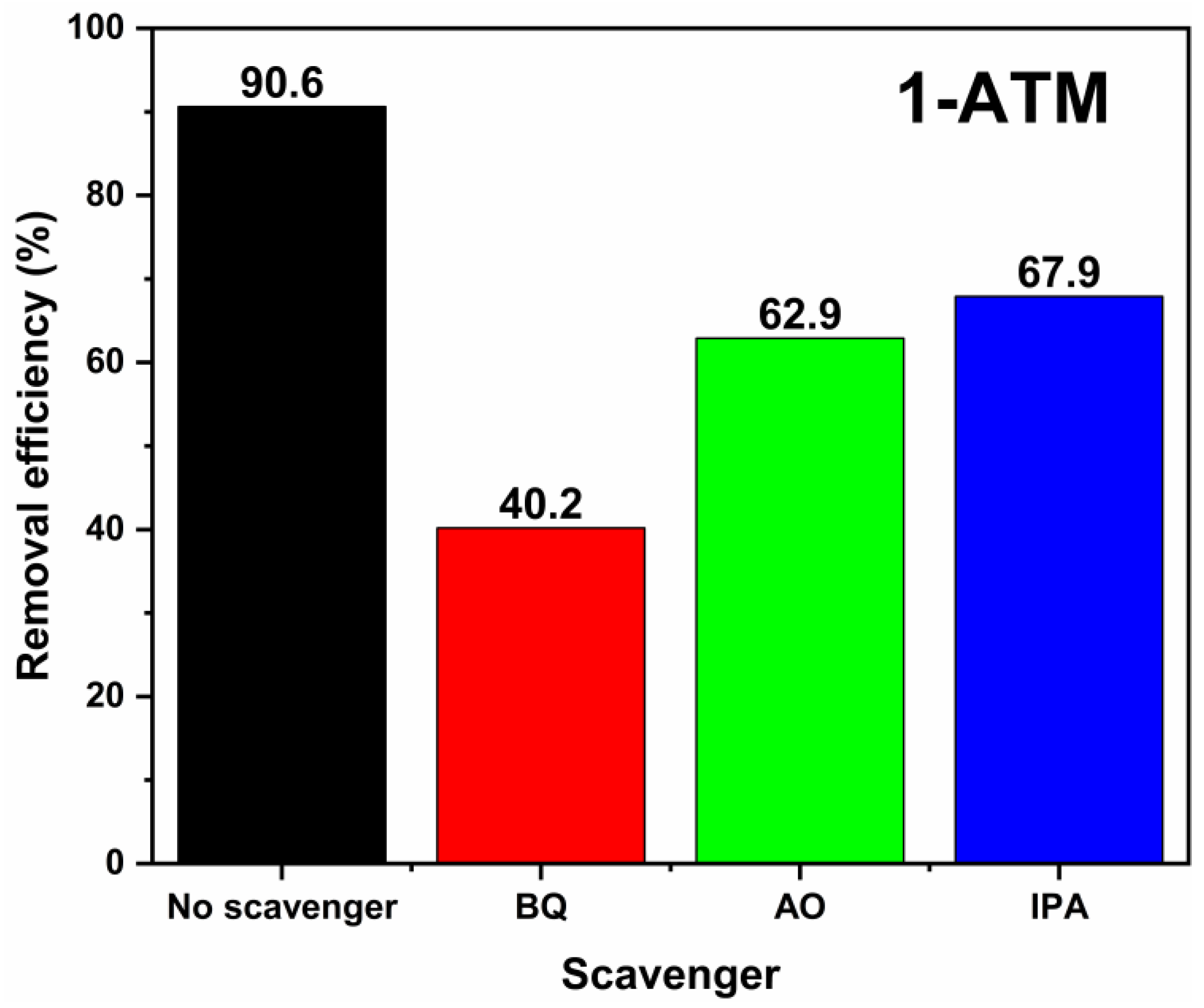
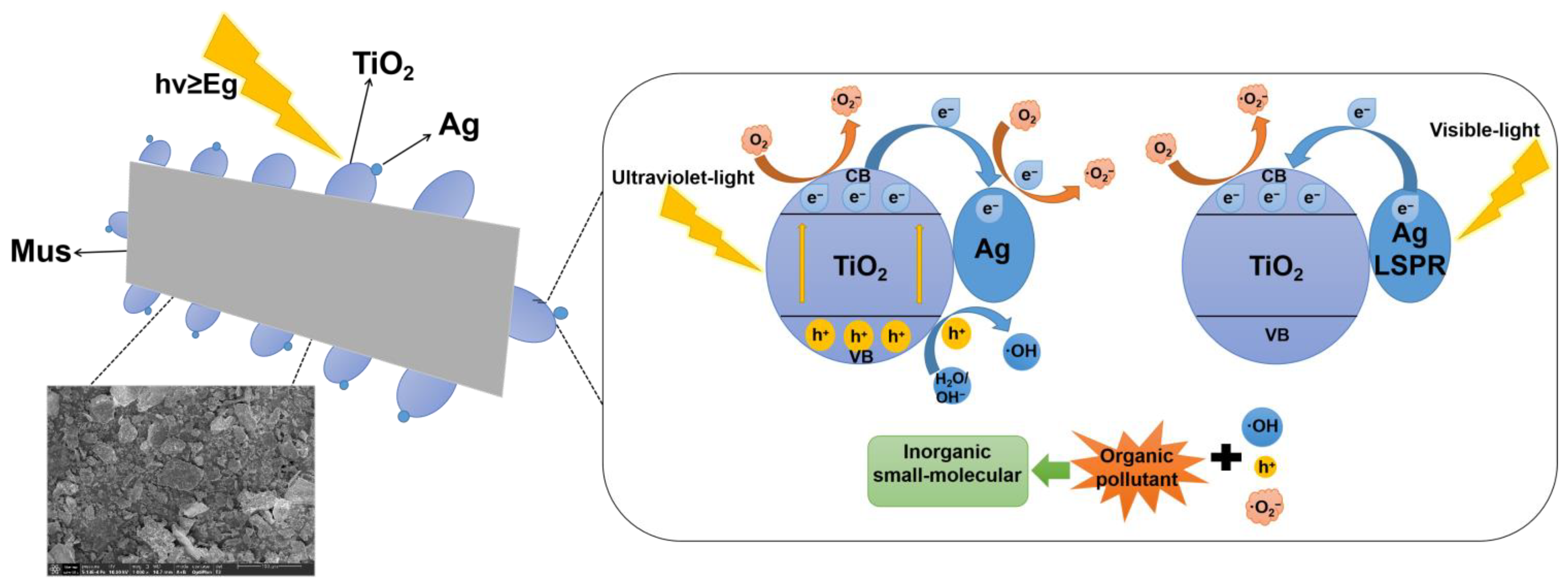
2.7. Photocatalytic Degradation Mechanism
3. Materials and Methods
3.1. Sample Preparation
3.2. Sample Characterization
3.3. Photocatalysis Experiment
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Chen, Y.M.; Pu, S.L.; Wang, D.Y.; Zhang, Y.C.; Wan, G.J.; Zhao, Q.; Sun, Y. Facile synthesis of AgBr@ZIF-8 hybrid photocatalysts for degradation of Rhodamine B. J. Solid State Chem. 2023, 321, 123857. [Google Scholar] [CrossRef]
- Nazir, M.A.; Najam, T.; Jabeen, S.; Wattoo, M.A.; Bashir, M.S.; Shah, S.S.A.; Rehman, A.U. Facile synthesis of Tri-metallic layered double hydroxides (NiZnAl-LDHs): Adsorption of Rhodamine-B and methyl orange from water. Inorg. Chem. Commun. 2022, 145, 110008. [Google Scholar] [CrossRef]
- Zhu, X.D.; Pei, L.X.; Zhu, R.R.; Jiao, Y.; Tang, R.Y.; Feng, W. Preparation and characterization of Sn/La co-doped TiO2 nanomaterials and their phase transformation and photocatalytic activity. Sci. Rep. 2018, 8, 12387. [Google Scholar] [CrossRef]
- Cadenbach, T.; Benitez, M.J.; Tirado, S.A.; Herrera, V.O.; Debut, A.; Vizuete, K. Adsorption enhanced photocatalytic degradation of Rhodamine B using GdxBi1-xFeO3@SBA-15 (x = 0, 0.05, 0.10, 0.15) nanocomposites under visible light irradiation. Ceram. Int. 2021, 47, 29139–29148. [Google Scholar] [CrossRef]
- Zhang, J.Y.; Tong, H.J.; Pei, W.K.; Liu, W.H.; Shi, F.Y.; Li, Y.; Huo, Y.N. Integrated photocatalysis-adsorption-membrane separation in rotating reactor for synergistic removal of RhB. Chemosphere 2021, 270, 129424. [Google Scholar] [CrossRef]
- Kundu, B.K.; Han, G.Q.; Sun, Y.J. Derivatized benzothiazoles as two-photon-absorbing organic photosensitizers active under near infrared light irradiation. J. Am. Chem. Soc. 2023, 145, 3535–3542. [Google Scholar] [CrossRef] [PubMed]
- Nazir, M.A.; Najam, T.; Shahzad, K.; Wattoo, M.A.; Hussain, T.; Tufail, M.K.; Shah, S.S.A.; Rehman, A.U. Heterointerface engineering of water stable ZIF-8@ZIF-67: Adsorption of rhodamine B from water. Surf. Interfaces 2022, 34, 102324. [Google Scholar] [CrossRef]
- Jamshaid, M.; Nazir, M.A.; Najam, T.; Shah, S.S.A.; Khan, H.M.; Rehman, A.U. Facile synthesis of Yb3+-Zn2+ substituted M type hexaferrites: Structural, electric and photocatalytic properties under visible light for methylene blue removal. Chem. Phys. Lett. 2022, 805, 139939. [Google Scholar] [CrossRef]
- Yuan, L.; Geng, Z.Y.; Zhang, S.; Xu, J.K.; Guo, F.; Kundu, B.K.; Han, C. Efficient all-in-one removal of total chromium over nonconjugated polymer-inorganic ZnIn2S4 semiconductor hybrid. J. Colloid Interface Sci. 2022, 628, 100–108. [Google Scholar] [CrossRef]
- Alkorbi, A.S.; Javed, H.M.A.; Hussain, S.; Lati, S.; Mahr, M.S.; Mustafa, M.S.; Alsaiari, R.; Alhemiary, N.A. Solar light-driven photocatalytic degradation of methyl blue by carbon-doped TiO2 nanoparticles. Opt. Mater. 2022, 127, 112259. [Google Scholar] [CrossRef]
- Tashkandi, N.Y.; Albukhari, S.M.; Ismail, A.A. Mesoporous TiO2 enhanced by anchoring Mn3O4 for highly efficient photocatalyst toward photo-oxidation of ciprofloxacin. Opt. Mater. 2022, 127, 112274. [Google Scholar] [CrossRef]
- Ortelli, S.; Vespignani, M.; Zanoni, I.; Blosi, M.; Vineis, C.; Piancastelli, A.; Baldi, G.; Dami, V.; Albonetti, S.; Costa, A.L. Design of TiO2-surfactin hybrid systems with multifunctional properties. Molecules 2023, 28, 1863. [Google Scholar] [CrossRef]
- Lu, N.; Cai, J.Z.; Niu, B.L.; Zhou, Y.; Zhao, G.H. Preferential removal of phthalic esters by photocatalysis on selective TiO2. Chem. Eng. J. 2023, 460, 141735. [Google Scholar]
- Feng, X.T.; Gu, L.F.; Wang, N.Y.; Pu, Q.S.; Liu, G.L. Fe/N co-doped nano-TiO2 wrapped mesoporous carbon spheres for synergetically enhanced adsorption and photocatalysis. J. Mater. Sci. Technol. 2023, 135, 54–64. [Google Scholar] [CrossRef]
- Hu, X.L.; Li, C.Q.; Song, J.Y.; Zheng, S.L.; Sun, Z.M. Hierarchical assembly of visible-light-driven Bi2MoO6/TiO2/sepiolite composite for effective formaldehyde removal. Appl. Clay Sci. 2022, 227, 106590. [Google Scholar] [CrossRef]
- Zhang, J.J.; Tan, H.B.; Deng, X.F.; Li, M.G.; Jian, S.W.; Li, G.Y. Preparation of organic montmorillonite supported TiO2 and its application in methylene blue removal. Constr. Build. Mater. 2022, 341, 127762. [Google Scholar] [CrossRef]
- Li, Z.Y.; He, J.L.; Ma, H.F.; Zang, L.H.; Li, D.; Guo, S.Z.; Ci, Y.H. Preparation of heterogeneous TiO2/g-C3N4 with a layered mosaic stack structure by use of montmorillonite as a hard template approach: TC degradation, kinetic, mechanism, pathway and DFT investigation. Appl. Clay Sci. 2021, 207, 106107. [Google Scholar] [CrossRef]
- Chkirida, S.; Zari, N.; Achour, R.; Hassoune, H.; Lachehab, A.; Qaiss, A.E.K.; Bouhfid, R. Highly synergic adsorption/photocatalytic efficiency of Alginate/Bentonite impregnated TiO2 beads for wastewater treatment. J. Photochem. Photobiol. A 2021, 412, 113215. [Google Scholar] [CrossRef]
- Pan, X.F.; Gao, H.L.; Lu, Y.; Wu, C.Y.; Wu, Y.D.; Wang, X.Y.; Pan, Z.Q.; Dong, L.; Song, Y.H.; Cong, H.P.; et al. Transforming ground mica into high-performance biomimetic polymeric mica film. Nat. Commun. 2018, 9, 2974. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Sun, H.J.; Peng, T.J.; You, H.; Qin, Y.T.; Zeng, L. Effects of muscovite matrix on photocatalytic degradation in TiO2/muscovite nanocomposites. Appl. Clay Sci. 2019, 179, 105155. [Google Scholar] [CrossRef]
- Wang, N.; Zhao, Z.T.; Liu, L.; Xing, J.J. Preparation of muscovite/tungsten-doped TiO2 composites for the efficient photocatalytic degradation of methyl orange under simulated solar light irradiation. Inorg. Chem. Commun. 2022, 138, 109285. [Google Scholar] [CrossRef]
- Chen, X.Y.; Liu, E.D.; Zhang, D.F.; Zhang, Z.D.; Dong, Q.; Zhang, X.Y.; Ding, X.Y.; Chen, S.H.; Zhu, X.D. Synthesis of novel muscovite loaded nano Ag/Cu2-x-FexO composites with excellent visible-light responsive photocatalysis. Opt. Mater. 2022, 124, 112002. [Google Scholar] [CrossRef]
- Zhang, Y.; Wang, T.; Zhou, M.; Wang, Y.; Zhang, Z.M. Hydrothermal preparation of Ag-TiO2 nanostructures with exposed {001}/{101} facets for enhancing visible light photocatalytic activity. Ceram. Int. 2017, 43, 3118–3126. [Google Scholar] [CrossRef]
- Mishra, S.; Chakinala, N.; Chakinala, A.G.; Surolia, P.K. Photocatalytic degradation of methylene blue using monometallic and bimetallic Bi-Fe doped TiO2. Catal. Commun. 2022, 171, 106518. [Google Scholar] [CrossRef]
- Akhter, P.; Nawaz, S.; Shafiq, I.; Nazir, A.; Shafique, S.; Jamil, F.; Park, Y.K.; Hussain, M. Efficient visible light assisted photocatalysis using ZnO/ TiO2 nanocomposites. Mol. Catal. 2023, 535, 112896. [Google Scholar] [CrossRef]
- Bamola, P.; Sharma, M.; Dwivedi, C.; Singh, B.; Ramakrishna, S.; Dalapati, G.K.; Sharma, H. Interfacial interaction of plasmonic nanoparticles (Ag, Au) decorated floweret TiO2 nanorod hybrids for enhanced visible light driven photocatalytic activity. Mater. Sci. Eng. B 2021, 273, 115403. [Google Scholar] [CrossRef]
- Zhang, Z.; Yates, J.T. Band bending in semiconductors: Chemical and physical consequences at surfaces and interfaces. Chem. Rev. 2012, 112, 5520–5551. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.L.; Zhang, L.S.; Chen, Z.G.; Hu, J.Q.; Li, S.J.; Wang, Z.H.; Liu, J.S.; Wang, X.C. Semiconductor heterojunction photocatalysts: Design, construction, and photocatalytic performances. Chem. Soc. Rev. 2014, 43, 5234–5244. [Google Scholar] [CrossRef]
- Chen, Q.F.; Wang, K.Y.; Gao, G.M.; Ren, J.Z.; Duan, R.; Fang, Y.F.; Hu, X. Singlet oxygen generation boosted by Ag-Pt nanoalloy combined with disordered surface layer over TiO2 nanosheet for improving the photocatalytic activity. Appl. Surf. Sci. 2021, 538, 147944. [Google Scholar] [CrossRef]
- Bai, S.; Jiang, J.; Zhang, Q.; Xiong, Y.J. Steering charge kinetics in photocatalysis: Intersection of materials syntheses, characterization techniques and theoretical simulations. Chem. Soc. Rev. 2015, 44, 2893. [Google Scholar] [CrossRef]
- Sun, Z.G.; Liu, C.Y.; Li, X.S.; Fang, Y.R.; Zhu, X.B.; Zhu, A.M. Semi-transparent nanofilms of plasmonic Au/TiO2 for visible-light photocatalysis. Mater. Chem. Phys. 2022, 280, 125773. [Google Scholar] [CrossRef]
- Zhu, X.D.; Qin, F.Q.; Xia, Y.W.; Yang, D.X.; Feng, W.; Jiao, Y. Three-phase mixed titania powder modified by silver and silver chloride with enhanced photocatalytic activity under UV–visible light. Nanomaterials 2022, 12, 1599. [Google Scholar] [CrossRef] [PubMed]
- Michalska, M.; Pavlovský, J.; Lemański, K.; Małecka, M.; Ptak, M.; Novák, V.; Kormunda, M.; Matějka, V. The effect of surface modification with Ag nanoparticles on 21 nm TiO2: Anatase/rutile material for application in photocatalysis. Mater. Today Chem. 2022, 26, 101123. [Google Scholar] [CrossRef]
- Yaacob, N.; Ismail, A.F.; Sean, G.P.; Nazri, N.A.M. Structural and photocatalytic properties of co-doped hybrid ZrO2-TiO2 photocatalysts. SN Appl. Sci. 2019, 1, 252. [Google Scholar] [CrossRef]
- Zhu, X.D.; Qin, F.Q.; Xia, Y.W.; Zhong, Y.Y.; Zhang, X.P.; Feng, W.; Jiao, Y. Synthesis of Ag@AgCl modified anatase/rutile/brookite mixed phase TiO2 and their photocatalytic property. Nanotechnol. Rev. 2022, 11, 2916–2927. [Google Scholar] [CrossRef]
- Ma, H.C.; Zheng, W.B.; Yan, X.; Li, S.Z.; Zhang, K.; Liu, G.J.; Jiang, L. Polydopamine-induced fabrication of Ag-TiO2 hollow nanospheres and their application in visible-light photocatalysis. Colloids Surf. A 2020, 586, 124283. [Google Scholar] [CrossRef]
- Tian, M.Q.; Wang, J.J.; Sun, R.J.; Lu, D.Z.; Li, N.; Liu, T.J.; Yao, M.; Zhang, G.Q.; Li, L.B. Facile synthesis of rod-like TiO2-based composite loaded with g-C3N4 for efficient removal of high-chroma organic pollutants based on adsorption-photocatalysis mechanism. Inorg. Chem. Commun. 2022, 141, 109517. [Google Scholar] [CrossRef]
- Ren, Y.W.; Xing, S.; Wang, J.; Liang, Y.; Zhao, D.Y.; Wang, H.L.; Wang, N.; Jiang, W.W.; Wu, S.M.; Liu, S.M.; et al. Weak-light-driven Ag-TiO2 photocatalyst and bactericide prepared by coprecipitation with effective Ag doping and deposition. Opt. Mater. 2022, 124, 111993. [Google Scholar] [CrossRef]
- Zhu, X.D.; Liu, H.; Wang, J.; Dai, H.L.; Bai, Y.; Feng, W.; Han, S.H. Investigation of photocatalytic activity of Ag-rutile heterojunctions. Micro Nano Lett. 2020, 15, 1130–1133. [Google Scholar] [CrossRef]
- Zhu, X.D.; Qin, F.Q.; He, L.L.; Jiao, Y.; Feng, W. Enhanced photocatalytic activity of anatase/rutile heterojunctions by lanthanum and tin co-doping. Int. J. Mol. Sci. 2022, 23, 11339. [Google Scholar] [CrossRef]
- Sun, Y.; Gao, Y.; Zeng, J.Y.; Guo, J.; Wang, H. Enhancing visible-light photocatalytic activity of Ag-TiO2 nanowire composites by one-step hydrothermal process. Mater. Lett. 2020, 279, 128506. [Google Scholar] [CrossRef]
- Liang, C.X.; Li, C.T.; Zhu, Y.C.; Du, X.Y.; Zeng, Y.F.; Zhou, Y.H.; Zhao, J.G.; Li, S.H.; Liu, X.; Yu, Q.; et al. Light-driven photothermal catalysis for degradation of toluene on CuO/TiO2 Composite: Dominating photocatalysis and auxiliary thermalcatalysis. Appl. Surf. Sci. 2022, 601, 154144. [Google Scholar] [CrossRef]
- Zhu, X.D.; Zhu, R.R.; Pei, L.X.; Liu, H.; Xu, L.; Wang, J.; Feng, W.; Jiao, Y.; Zhang, W.M. Fabrication, characterization, and photocatalytic activity of anatase/rutile/SnO2 nanocomposites. J. Mater. Sci. Mater. Electron. 2019, 30, 21210–21218. [Google Scholar] [CrossRef]
- Zhou, Z.Y.; Zhu, Y.M.; Jin, J.P.; Han, Y.X.; Bai, Z.; Tang, Z.D. Enhanced vanadium extraction from Muscovite-type Vanadium-bearing shale by suspension oxidation roasting pretreatment-acid leaching. Sep. Purif. Technol. 2023, 309, 123066. [Google Scholar] [CrossRef]
- Bao, N.; Wei, Z.T.; Ma, Z.H.; Liu, F.; Yin, G.B. Si-doped mesoporous TiO2 continuous fibers: Preparation by centrifugal spinning and photocatalytic properties. J. Hazard. Mater. 2010, 174, 129–136. [Google Scholar] [CrossRef]
- Peng, F.P.; Ni, Y.R.; Zhou, Q.; Kou, J.H.; Lu, C.H.; Xu, Z.Z. New g-C3N4 based photocatalytic cement with enhanced visible-light photocatalytic activity by constructing muscovite sheet/SnO2 structures. Constr. Build. Mater. 2018, 179, 315–325. [Google Scholar] [CrossRef]
- Jia, T.K.; Fu, F.; Yu, D.S.; Cao, J.L.; Sun, G. Facile synthesis and characterization of N-doped TiO2/C nanocomposites with enhanced visible-light photocatalytic performance. Appl. Surf. Sci. 2018, 430, 438–447. [Google Scholar] [CrossRef]
- Deng, J.L.; Gao, J.; Liu, M.; Zheng, L.C.; Wang, Y.F.; Wang, Y.Q.; Chen, C.Z.; Li, Y.; He, G.; Liu, Y. Construction of Z-scheme TiO2/Ag/ZIF-8 nanorod array film with boosting photocatalytic and photoelectrochemical properties. J. Alloys Compd. 2023, 932, 167680. [Google Scholar] [CrossRef]
- Lam, S.M.; Sin, J.C.; Abdullah, A.Z.; Mohamed, A.R. Investigation on visible-light photocatalytic degradation of 2,4-dichlorophenoxyacetic acid in the presence of MoO3/ZnO nanorod composites. J. Mol. Catal. A Chem. 2013, 370, 123–131. [Google Scholar] [CrossRef]
- Li, J.L.; Zhang, M.; Guan, Z.J.; Li, Q.Y.; He, C.Q.; Yang, J.J. Synergistic effect of surface and bulk single-electron-trapped oxygen vacancy of TiO2 in the photocatalytic reduction of CO2. Appl. Catal. B-Environ. 2017, 206, 300–307. [Google Scholar] [CrossRef]
- Yang, Q.L.; Hu, M.Y.; Guo, J.; Ge, Z.H.; Feng, J. Synthesis and enhanced photocatalytic performance of Ag/AgCl/TiO2 nanocomposites prepared by ion exchange method. J. Mater. 2018, 4, 402–411. [Google Scholar] [CrossRef]
- Li, J.R.; Jin, Z.Z.; Zhang, Y.M.; Liu, D.; Ma, A.J.; Sun, Y.M.; Li, X.Y.; Cai, Q.; Gui, J.Z. Ag-induced anatase-rutile TiO2-x heterojunction facilitating the photogenerated carrier separation in visible-light irradiation. J. Alloys Compd. 2022, 909, 164815. [Google Scholar] [CrossRef]
- Wei, Z.D.; Xu, M.Q.; Liu, J.Y.; Guo, W.Q.; Jiang, Z.; Shangguan, W.F. Simultaneous visible-light-induced hydrogen production enhancement and antibiotic wastewater degradation using MoS2@ZnxCd1-xS: Solid-solution-assisted photocatalysis. Chin. J. Catal. 2020, 41, 103–113. [Google Scholar] [CrossRef]
- Reddy, N.K.; Reddy, K.T.R. Optical behaviour of sprayed tin sulphide thin films. Mater. Res. Bull. 2006, 41, 414–422. [Google Scholar] [CrossRef]
- Zhu, X.D.; Xu, H.Y.; Yao, Y.; Liu, H.; Wang, J.; Pu, Y.; Feng, W.; Chen, S.H. Effects of Ag0-modification and Fe3+-doping on the structural, optical and photocatalytic properties of TiO2. RSC Adv. 2019, 9, 40003–40012. [Google Scholar] [CrossRef] [PubMed]
- Yang, Z.L.; Lu, J.; Ye, W.C.; Yu, C.S.; Chang, Y.L. Preparation of Pt/TiO2 hollow nanofibers with highly visible light photocatalytic activity. Appl. Surf. Sci. 2017, 392, 472–480. [Google Scholar] [CrossRef]
- Gao, X.T.; Zhang, S.; Liu, J.C.; Xu, S.Q.; Li, Z.H. Enhanced active oxidative species generation over Fe-doped defective TiO2 nanosheets for boosted photodegradationt. Rsc Adv. 2020, 10, 40619–40624. [Google Scholar] [CrossRef]
- Zhu, X.D.; Wang, J.; Yang, D.X.; Liu, J.W.; He, L.L.; Tang, M.; Feng, W.; Wu, X.Q. Fabrication, characterization and high photocatalytic activity of Ag-ZnO heterojunctions under UV-visible light. Rsc Adv. 2021, 11, 27257–27266. [Google Scholar] [CrossRef]
- Celebi, N.; Aydin, M.Y.; Soysal, F.; Ciftci, Y.O.; Salimi, K. Ligand-free fabrication of Au/TiO2 nanostructures for plasmonic hot-electron-driven photocatalysis: Photoelectrochemical water splitting and organic-dye degredation. J. Alloys Compd. 2021, 860, 157908. [Google Scholar] [CrossRef]
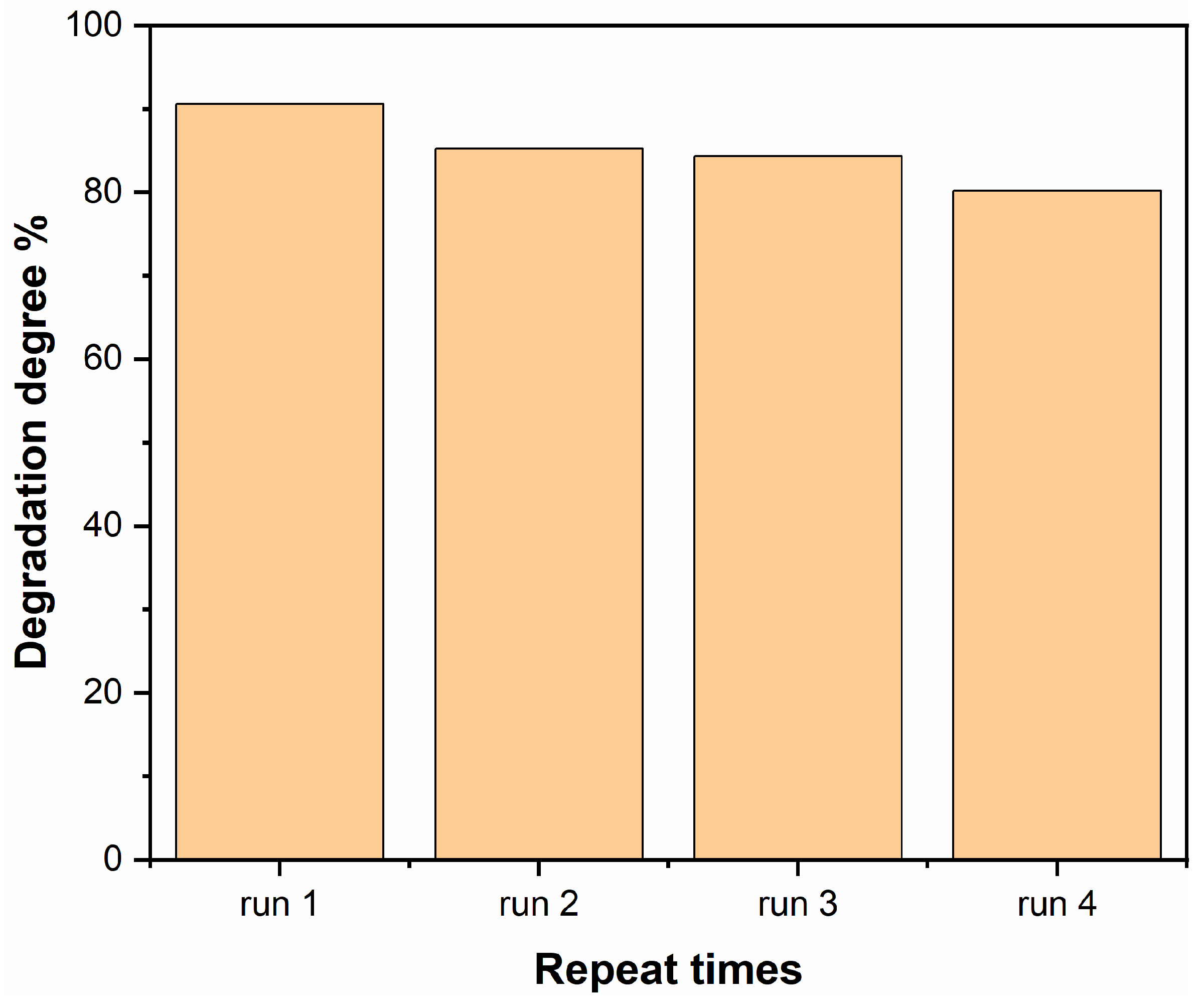

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Qin, F.; Zhang, L.; Luo, Y.; He, L.; Lu, S.; Xu, L.; Zhu, X.; Feng, W. Effect of Ag Modification on the Structure and Photocatalytic Performance of TiO2/Muscovite Composites. Molecules 2023, 28, 3187. https://doi.org/10.3390/molecules28073187
Qin F, Zhang L, Luo Y, He L, Lu S, Xu L, Zhu X, Feng W. Effect of Ag Modification on the Structure and Photocatalytic Performance of TiO2/Muscovite Composites. Molecules. 2023; 28(7):3187. https://doi.org/10.3390/molecules28073187
Chicago/Turabian StyleQin, Fengqiu, Ling Zhang, Yuhao Luo, Lili He, Shiji Lu, Li Xu, Xiaodong Zhu, and Wei Feng. 2023. "Effect of Ag Modification on the Structure and Photocatalytic Performance of TiO2/Muscovite Composites" Molecules 28, no. 7: 3187. https://doi.org/10.3390/molecules28073187
APA StyleQin, F., Zhang, L., Luo, Y., He, L., Lu, S., Xu, L., Zhu, X., & Feng, W. (2023). Effect of Ag Modification on the Structure and Photocatalytic Performance of TiO2/Muscovite Composites. Molecules, 28(7), 3187. https://doi.org/10.3390/molecules28073187





