Biomimetic Guided Bi2WO6/Bi2O3 Vertical Heterojunction with Controllable Microstructure for Efficient Photocatalysis
Abstract
1. Introduction
2. Results and Discussion
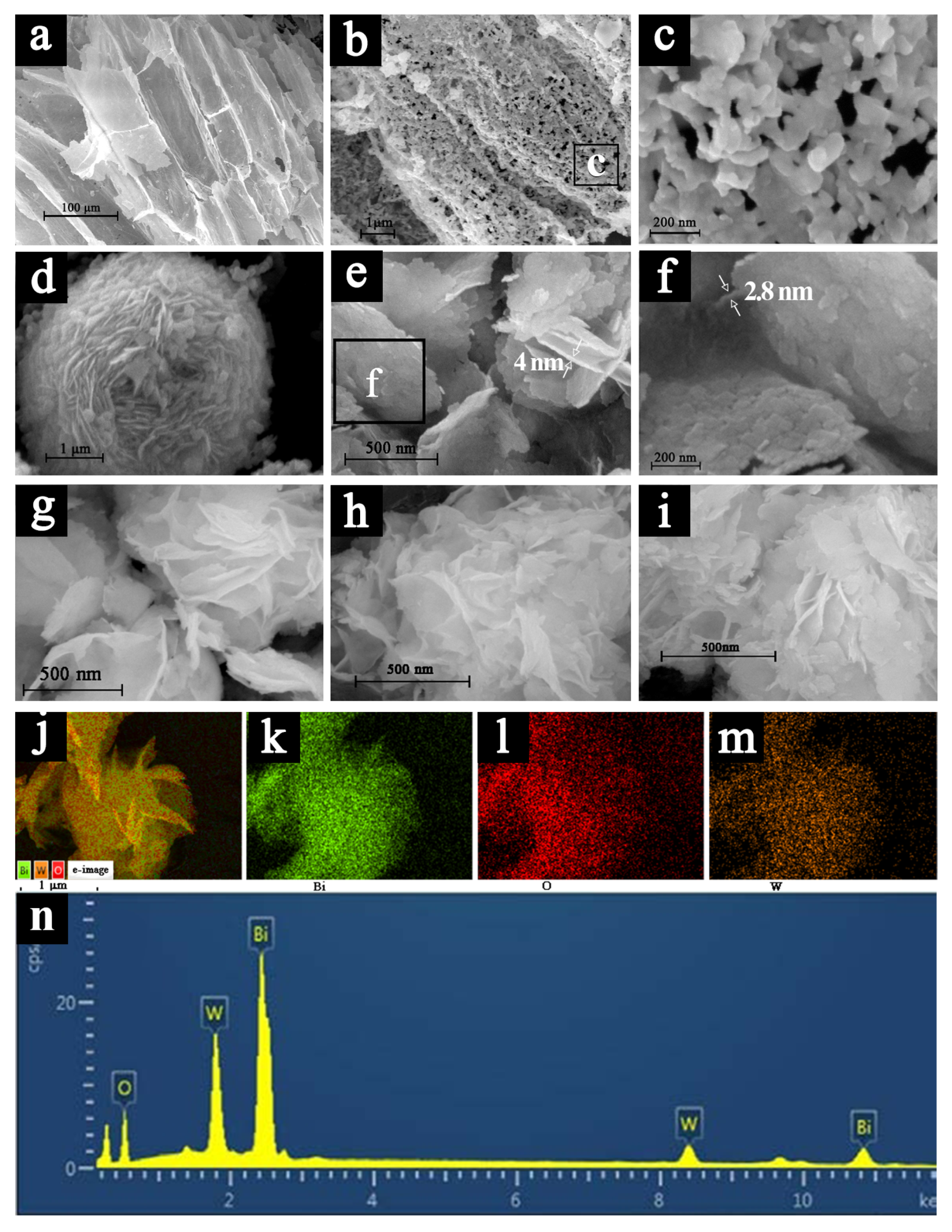
2.1. Composition and Morphology
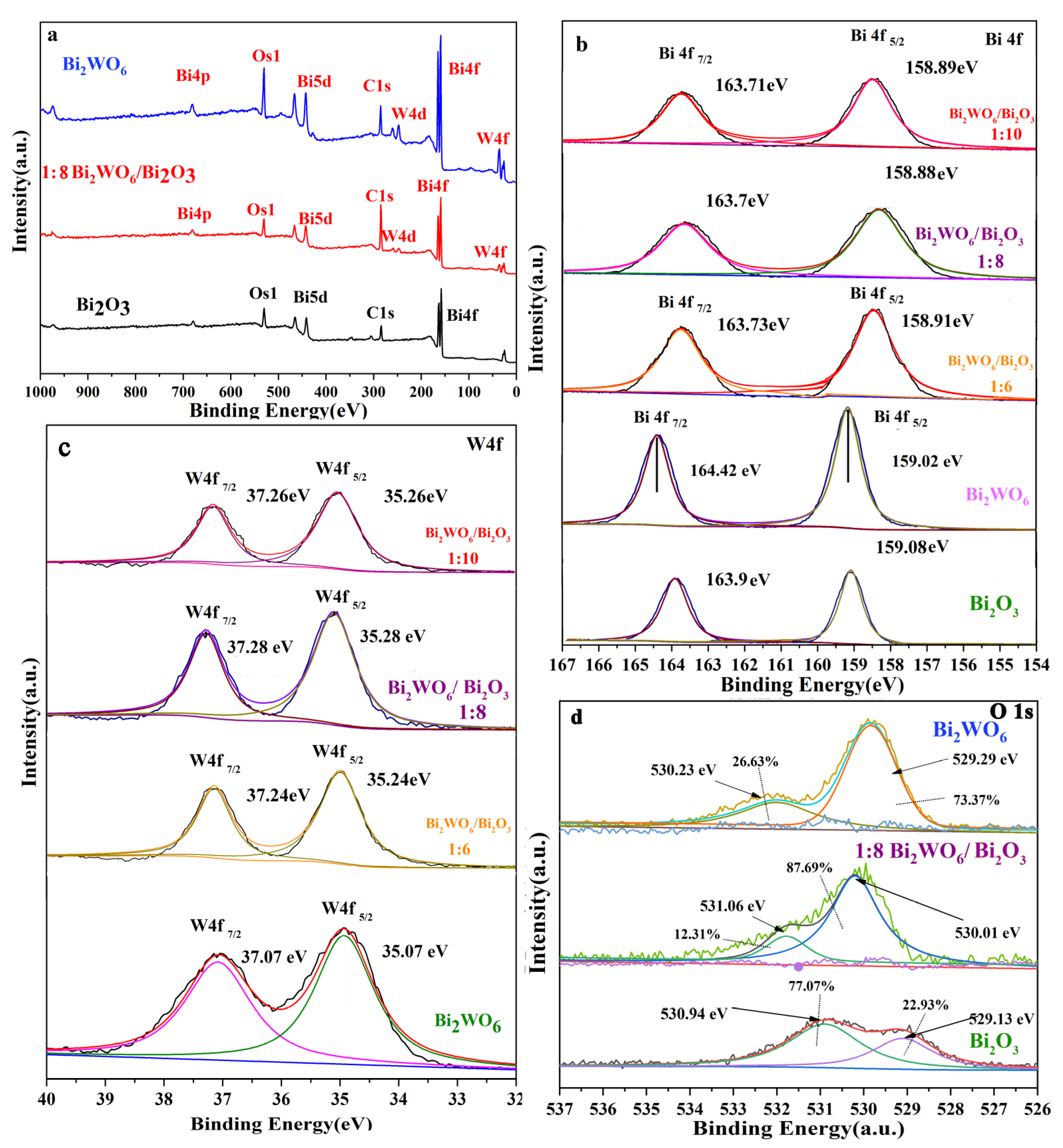
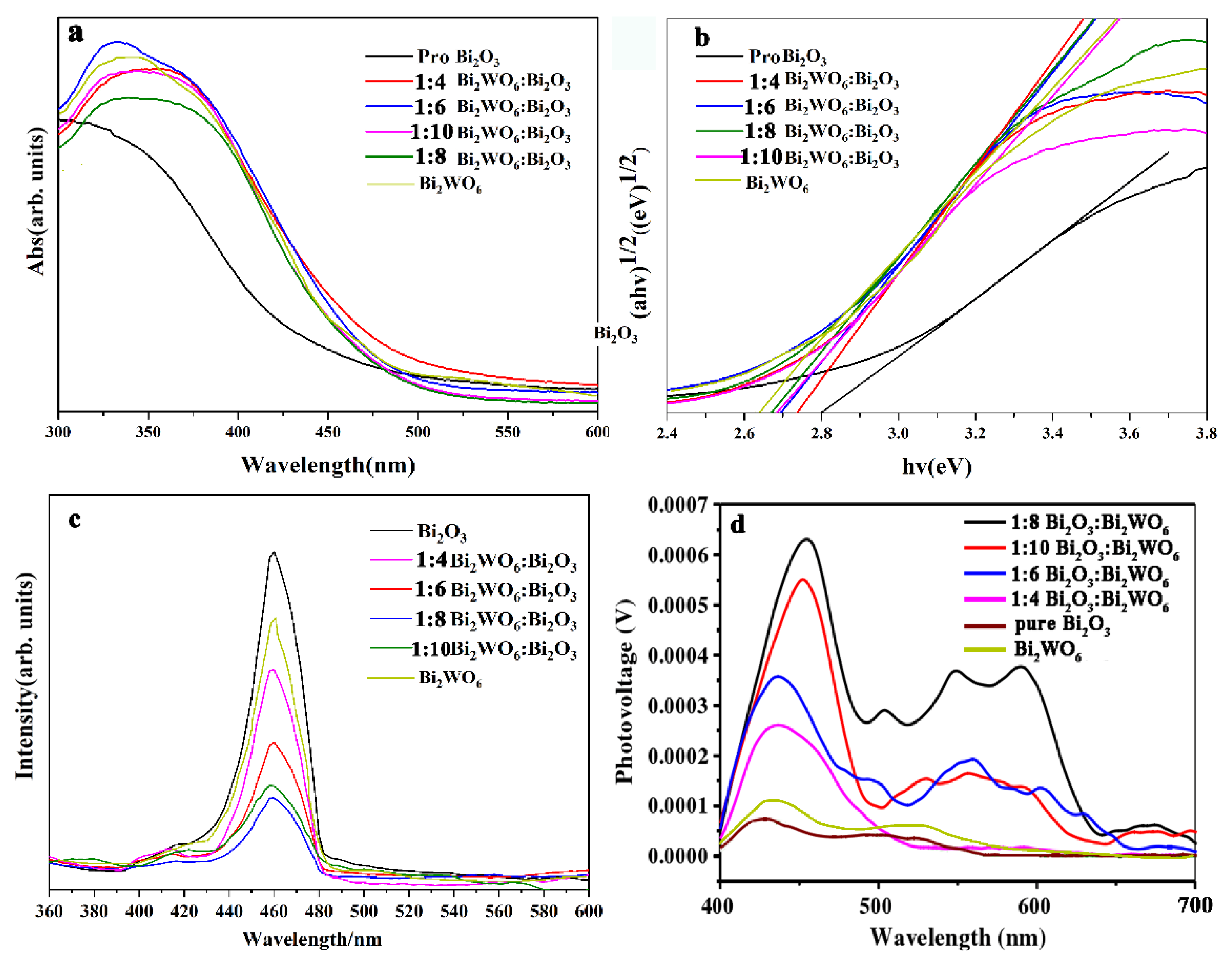
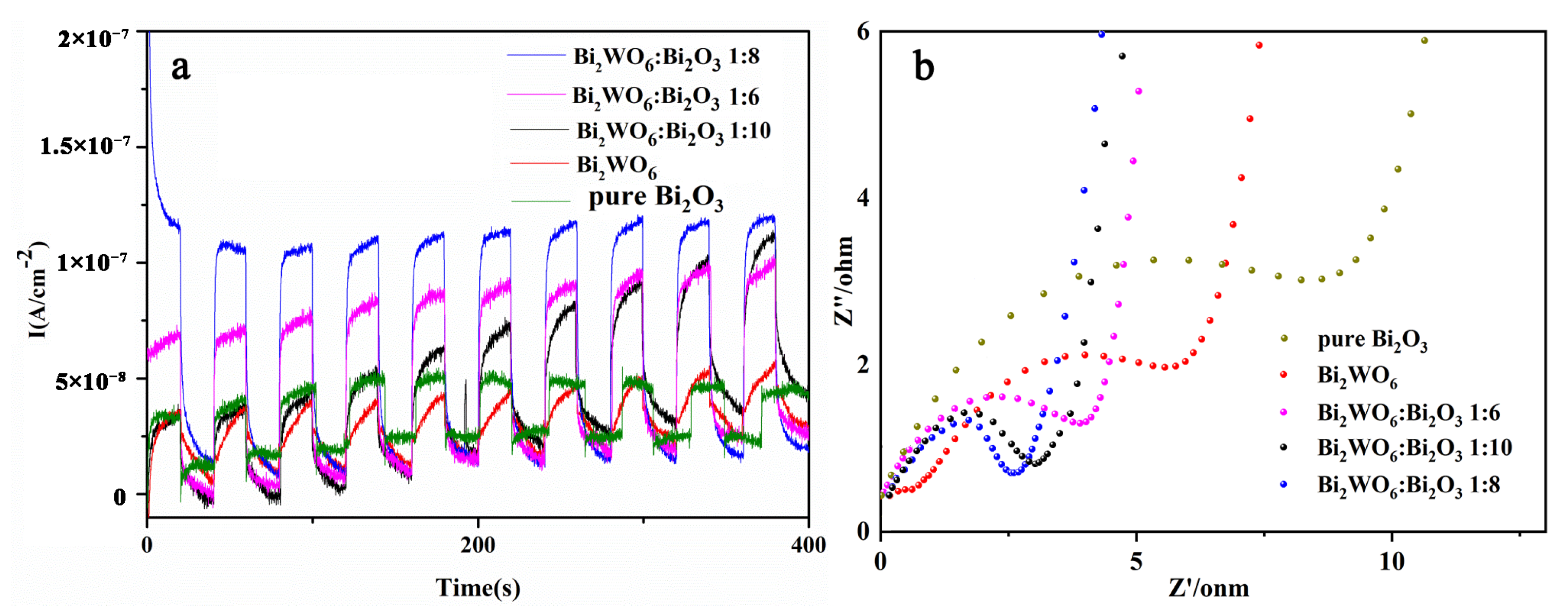
2.2. Spectroscopy and Electrochemical Characterization
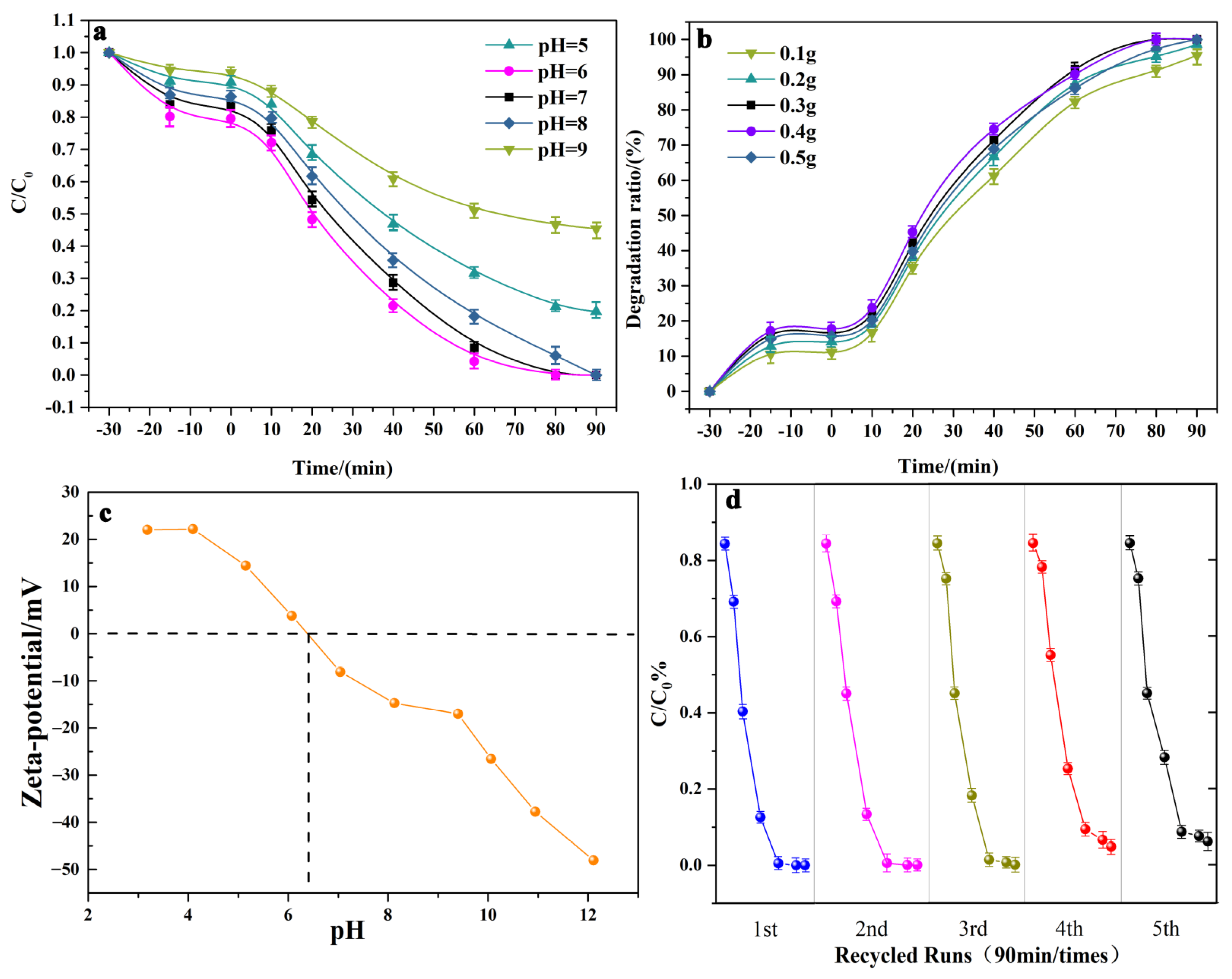
2.3. Photocatalytic Degradation of Tetracycline
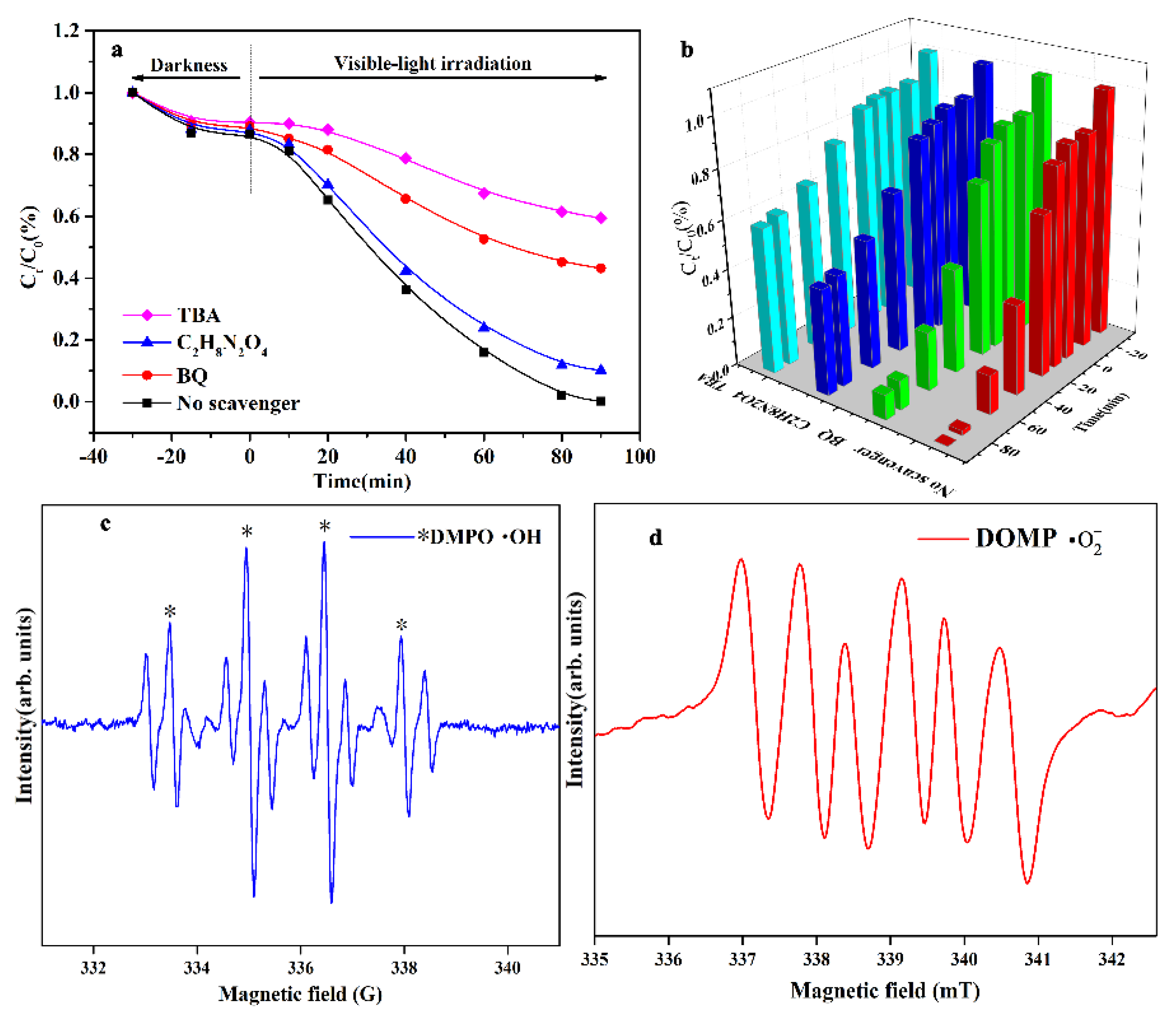
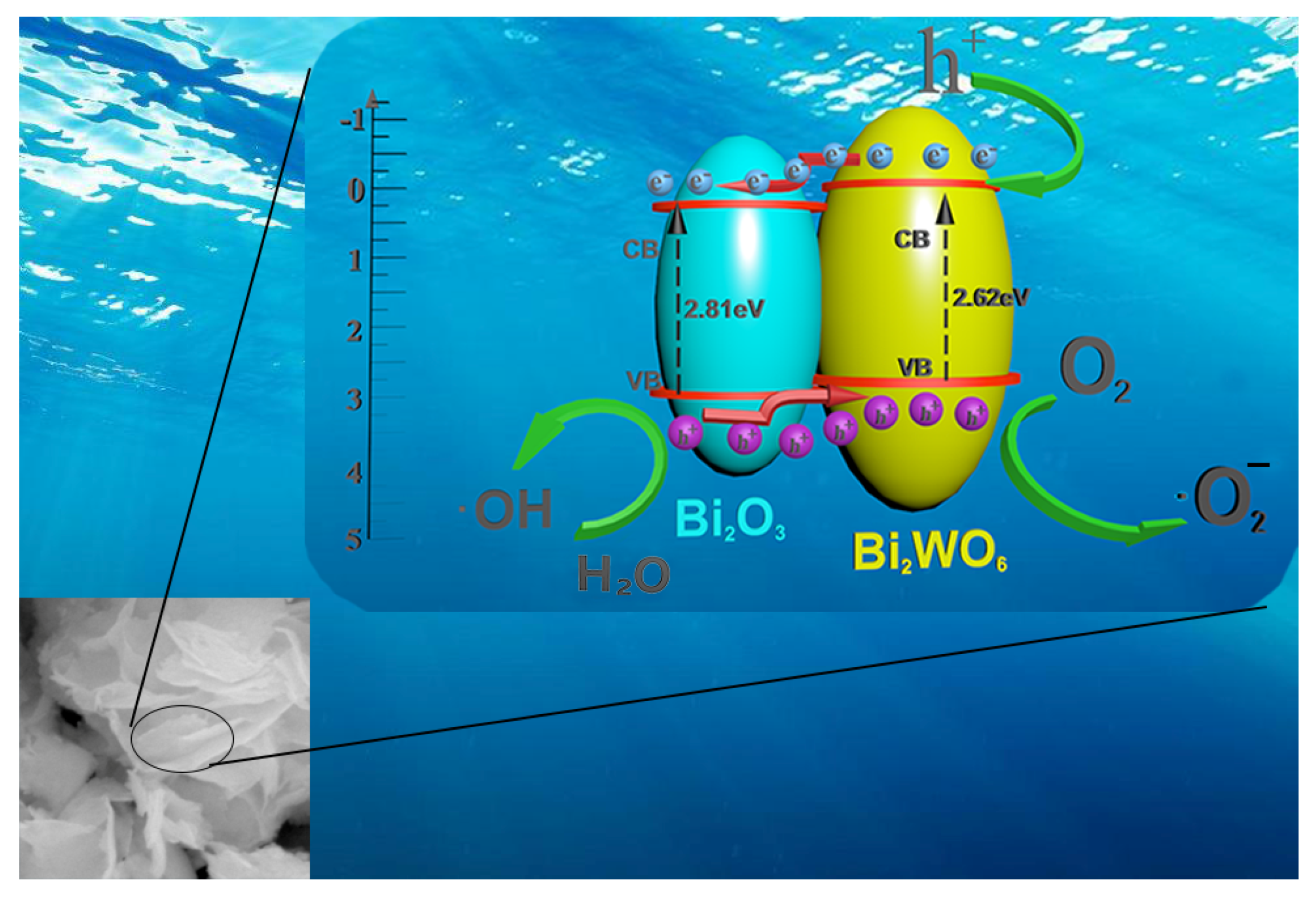
2.4. Mechanisms
3. Conclusions
4. Materials and Methods
4.1. Chemicals
4.2. Material Synthesis
4.2.1. Pretreatment of Sunflower Stalk
4.2.2. Preparation of Bi2O3 by Biological Template
4.2.3. Preparation of Bi2WO6/Bi2O3
4.3. Characterization
4.4. Catalytic Performance Testing of Materials
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Pan, M.; Lyu, T.; Zhan, L.; Matamoros, V.; Angelidaki, I.; Cooper, M.; Pan, G. Mitigating antibiotic pollution using cyanobacteria: Removal efficiency, pathways and metabolism. Water Res. 2021, 190, 116735. [Google Scholar] [CrossRef]
- Li, Y.; Lin, X.; Zhang, C.; Zhuang, Q.; Dong, W. Polydopamine magnetic microspheres grafted with sulfonic acid groups for efficient adsorption of tetracycline. Colloids Surf. A Physicochem. Eng. Asp. 2021, 628, 127263. [Google Scholar] [CrossRef]
- Wang, Z.; Wang, H.; Wang, Z.; Huang, D.; Qin, H.; He, Y.; Chen, M.; Zeng, G.; Xu, P. Ferrocene modified g-C3N4 as a heterogeneous catalyst for photo-assisted activation of persulfate for the degradation of tetracycline. Colloids Surf. Physicochem. Eng. Asp. 2021, 626, 127024. [Google Scholar] [CrossRef]
- Xu, S.; Jin, Y.; Li, R.; Shan, M.; Zhang, Y. Amidoxime modified polymers of intrinsic microporosity/alginate composite hydrogel beads for efficient adsorption of cationic dyes from aqueous solution. J. Colloid Interface Sci. 2022, 607, 890–899. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Yuan, X.; Li, X.; Jiang, L.; Wang, H. Burgeoning prospects of biochar and its composite in persulfate-advanced oxidation process. J. Hazard. Mater. 2021, 409, 124893. [Google Scholar] [CrossRef] [PubMed]
- Ji, H.; Du, P.; Zhao, D.; Li, S.; Sun, F.; Duin, E.C.; Liu, W. 2D/1D graphitic carbon nitride/titanate nanotubes heterostructure for efficient photocatalysis of sulfamethazine under solar light: Catalytic “hot spots” at the rutile–anatase–titanate interfaces. Appl. Catal. B Environ. 2020, 263, 118357. [Google Scholar] [CrossRef]
- Zhu, C.; Lee, S.C.; Chen, H.; Yue, H.; Rueping, M. Reductive Cross-Coupling of α-Oxy Halides Enabled by Thermal Catalysis, Photocatalysis, Electrocatalysis, or Mechanochemistry. Angew. Chem. Int. Ed. 2022, 61, e202204212. [Google Scholar] [CrossRef]
- Bibi, S.; Ahmad, A.; Anjum, M.A.R.; Haleem, A.; Siddiq, M.; Shah, S.S.; Kahtani, A.A. Photocatalytic degradation of malachite green and methylene blue over reduced graphene oxide (rGO) based metal oxides (rGO-Fe3O4/TiO2) nanocomposite under UV-visible light irradiation. J. Environ. Chem. Eng. 2021, 9, 105580. [Google Scholar] [CrossRef]
- Yang, Y.; Deng, Q.; Zhang, Y. Comparative study of low-index {1 0 1}-TiO2, {0 0 1}-TiO2, {1 0 0}-TiO2 and high-index {2 0 1}-TiO2 on glyphosate adsorption and photo-degradation. Chem. Eng. J. 2019, 360, 1247–1254. [Google Scholar] [CrossRef]
- Zhu, Q.; Hailili, R.; Xin, Y.; Zhou, Y.; Huang, Y.; Pang, X.; Zhang, K.; Robertson, P.K.J.; Bahnemann, D.W.; Wang, C. Efficient full spectrum responsive photocatalytic NO conversion at Bi2Ti2O7: Co-effect of plasmonic Bi and oxygen vacancies. Appl. Catal. B Environ. 2022, 319, 121888. [Google Scholar] [CrossRef]
- Liu, T.; Hao, L.; Zhang, Y.; Hu, Y.; Zhao, Q.; Lu, Y.; Ping, X. Anodized Bi2O3 film prepared in NaOH and oxalic acid and the photocatalytic activity in organic dye degradation. J. Mater. Sci. Mater. Electron. 2020, 31, 10846–10854. [Google Scholar] [CrossRef]
- Huang, R.Z.; Wei, Y.Y.; Gao, T.F.; Li, C.M.; Jiang, C.H. Structure and electronic properties of δ-Bi2O3 tuned by vacancy and doping: A first-principles study. Ceram. Int. 2021, 47, 205–213. [Google Scholar] [CrossRef]
- Shawky, A.; Tashkandi, N.Y. Visible-light photooxidation of ciprofloxacin utilizing metal oxide incorporated sol-gel processed La-doped NaTaO3 nanoparticles: A comparative study. Environ. Res. 2022, 213, 113718. [Google Scholar] [CrossRef] [PubMed]
- Rao, F.; Zhu, G.; Wang, M.; Zubairu, S.M.; Peng, J.; Gao, J.; Hojamberdiev, M. Constructing the Pd/PdO/β-Bi2O3 microspheres with enhanced photocatalytic activity for Bisphenol A degradation and NO removal. J. Chem. Technol. Biotechnol. 2020, 95, 862–874. [Google Scholar] [CrossRef]
- Zahid, A.H.; Han, Q. A review on the preparation, microstructure, and photocatalytic performance of Bi2O3in polymorphs. Nanoscale 2021, 13, 17687–17724. [Google Scholar] [CrossRef]
- Haleem, A.; Shafiq, A.; Chen, S.-Q.; Nazar, M. A Comprehensive Review on Adsorption, Photocatalytic and Chemical Degradation of Dyes and Nitro-Compounds over Different Kinds of Porous and Composite Materials. Molecules 2023, 28, 1081. [Google Scholar] [CrossRef]
- Ji, X.; Wang, Q.; Lu, J.-F.; Zhang, D. Construction of a novel Ca2Bi2O5/α-Bi2O3 semiconductor heterojunction for enhanced visible photocatalytic application. Ceram. Int. 2020, 46, 13630–13640. [Google Scholar] [CrossRef]
- Nurlan, N.; Akmanova, A.; Han, S.; Lee, W. Enhanced reduction of aqueous bromate by catalytic hydrogenation using the Ni-based Metal-organic framework Ni(4,4′-bipy)(1,3,5-BTC) with NaBH4. Chem. Eng. J. 2021, 414, 128860. [Google Scholar] [CrossRef]
- Jiang, Z.; Wang, B.; Li, Y.; Chan, H.S.; Sun, H.; Wang, T.; Li, H.; Yuan, S.; Leung, M.K.H.; Lu, A.; et al. Solar-light-driven rapid water disinfection by ultrathin magnesium titanate/carbon nitride hybrid photocatalyst: Band structure analysis and role of reactive oxygen species. Appl. Catal. B Environ. 2019, 257, 117898. [Google Scholar] [CrossRef]
- Zuarez-Chamba, M.; Rajendran, S.; Herrera-Robledo, M.; Priya, A.K.; Navas-Cárdenas, C. Bi-based photocatalysts for bacterial inactivation in water: Inactivation mechanisms, challenges, and strategies to improve the photocatalytic activity. Environ. Res. 2022, 209, 112834. [Google Scholar] [CrossRef]
- Chakraborty, A.; Samriti; Ruzimuradov, O.; Gupta, R.K.; Cho, J.; Prakash, J. TiO2 nanoflower photocatalysts: Synthesis, modifications and applications in wastewater treatment for removal of emerging organic pollutants. Environ. Res. 2022, 212, 113550. [Google Scholar] [CrossRef]
- Yuting, H.; Zuping, Y.; Jixing, Z.; Sigeng, Z.; Lu, L.; Wei, F. Hierarchical Porous ZnO Nanomaterial Synthesized with Corn Straw as Biological Templates and Its Photocatalytic Performance. Gāoděng Xuéxiào Huàxué Xuébaò 2018, 39, 2031–2038. [Google Scholar] [CrossRef]
- Kong, S.; An, Z.; Zhang, W.; An, Z.; Yuan, M.; Chen, D. Preparation of hollow flower-like microspherical β-Bi2O3/BiOCL heterojunction and high photocatalytic property for tetracycline hydrochloride degradation. Nanomaterials 2019, 10, 57. [Google Scholar] [CrossRef]
- Chen, J.; Zhong, J.; Li, J.; Qiu, K. Boosted photocatalytic removal of tetracycline on S-scheme Bi12O17Cl2/α-Bi2O3 heterojunctions with rich oxygen vacancies. Appl. Surf. Sci. 2021, 563, 150246. [Google Scholar] [CrossRef]
- Zheng, S.; Ding, B.; Qian, X.; Yang, Y.; Mao, L.; Zheng, S.; Zhang, J. High efficiency degradation of tetracycline and rhodamine B using Z-type BaTiO3/γ-Bi2O3 heterojunction. Sep. Purif. Technol. 2022, 278, 119666. [Google Scholar] [CrossRef]
- Yang, X.; Zhang, Y.; Wang, Y.; Xin, C.; Zhang, P.; Liu, D.; Mamba, B.B.; Kefeni, K.K.; Kuvarega, A.T.; Gui, J. Hollow β-Bi2O3@CeO2 heterostructure microsphere with controllable crystal phase for efficient photocatalysis. Chem. Eng. J. 2020, 387, 124100. [Google Scholar] [CrossRef]
- Chen, J.; Tang, T.; Feng, W.; Liu, X.; Yin, Z.; Zhang, X.; Chen, J.; Cao, S. Large-Scale Synthesis of p–n Heterojunction Bi2O3/TiO2 Nanostructures as Photocatalysts for Removal of Antibiotics under Visible Light. ACS Appl. Nano Mater. 2022, 5, 1296–1307. [Google Scholar] [CrossRef]
- Bahnemann, D.W.; Kholuiskaya, S.N.; Dillert, R.; Kulak, A.I.; Kokorin, A.I. Photodestruction of dichloroacetic acid catalyzed by nano-sized TiO2 particles. Appl. Catal. B Environ. 2002, 36, 161–169. [Google Scholar] [CrossRef]
- Diebold, U. The surface science of titanium dioxide. Surf. Sci. Rep. 2003, 48, 53–229. [Google Scholar] [CrossRef]
- Zhu, D.; Zhou, Q. Novel Bi2WO6 modified by N-doped graphitic carbon nitride photocatalyst for efficient photocatalytic degradation of phenol under visible light. Appl. Catal. B Environ. 2020, 268, 118426. [Google Scholar] [CrossRef]
- He, W.; Sun, Y.; Jiang, G.; Huang, H.; Zhang, X.; Dong, F. Activation of amorphous Bi2WO6 with synchronous Bi metal and Bi2O3 coupling: Photocatalysis mechanism and reaction pathway. Appl. Catal. B Environ. 2018, 232, 340. [Google Scholar] [CrossRef]
- Zheng, Y.; Zhou, T.; Zhao, X.; Pang, W.K.; Gao, H.; Li, S.; Zhou, Z.; Liu, H.; Guo, Z. Atomic Interface Engineering and Electric-Field Effect in Ultrathin Bi2MoO6 Nanosheets for Superior Lithium Ion Storage. Adv. Mater. 2017, 29, 1700396. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Wang, H.; Xie, J.; Hou, J.; Song, X.; Dionysiou, D.D. Bi2WO6-TiO2/starch composite films with Ag nanoparticle irradiated by γ-ray used for the visible light photocatalytic degradation of ethylene. Chem. Eng. J. 2021, 421, 129986. [Google Scholar] [CrossRef]
- Nie, J.; Zhu, G.; Zhang, W.; Gao, J.; Zhong, P.; Xie, X.; Huang, Y.; Hojamberdiev, M. Oxygen vacancy defects-boosted deep oxidation of NO by β-Bi2O3/CeO2-δ p-n heterojunction photocatalyst in situ synthesized from Bi/Ce(CO3)(OH) precursor. Chem. Eng. J. 2021, 424, 130327. [Google Scholar] [CrossRef]
- Hao, Y.-j.; Li, F.-t.; Chen, F.; Chai, M.-j.; Liu, R.-h.; Wang, X.-j. In situ one-step combustion synthesis of Bi2O3/Bi2WO6 heterojunctions with notable visible light photocatalytic activities. Mater. Lett. 2014, 124, 1–3. [Google Scholar] [CrossRef]
- Bing, X.; Li, J.; Liu, J.; Cui, X.; Ji, F. Biomimetic synthesis of Bi2O3/Bi2WO6/MgAl-CLDH hybrids from lotus pollen and their enhanced adsorption and photocatalysis performance. J. Photochem. Photobiol. A Chem. 2018, 364, 449–460. [Google Scholar] [CrossRef]
- Zhang, Z.; Lin, Y.; Liu, F. Preparation, crystallization and properties of Bi2WO6 nanoparticles. Colloids Surf. A Physicochem. Eng. Asp. 2020, 590, 124493. [Google Scholar] [CrossRef]
- Wang, H.; Wu, Y.; Feng, M.; Tu, W.; Xiao, T.; Xiong, T.; Ang, H.; Yuan, X.; Chew, J.W. Visible-light-driven removal of tetracycline antibiotics and reclamation of hydrogen energy from natural water matrices and wastewater by polymeric carbon nitride foam. Water Res. 2018, 144, 215–225. [Google Scholar] [CrossRef]
- Wang, J.; Tang, L.; Zeng, G.; Deng, Y.; Liu, Y.; Wang, L.; Zhou, Y.; Guo, Z.; Wang, J.; Zhang, C. Atomic scale g-C3N4/Bi2WO6 2D/2D heterojunction with enhanced photocatalytic degradation of ibuprofen under visible light irradiation. Appl. Catal. B Environ. 2017, 209, 285–294. [Google Scholar] [CrossRef]
- Ma, D.; Wu, J.; Gao, M.; Xin, Y.; Ma, T.; Sun, Y. Fabrication of Z-scheme g-C3N4/RGO/Bi2WO6 photocatalyst with enhanced visible-light photocatalytic activity. Chem. Eng. J. 2016, 290, 136–146. [Google Scholar] [CrossRef]
- Zhang, L.; Jaroniec, M. Toward designing semiconductor-semiconductor heterojunctions for photocatalytic applications. Appl. Surf. Sci. 2018, 430, 2–17. [Google Scholar] [CrossRef]
- Gong, Y.; Wu, Y.; Xu, Y.; Li, L.; Li, C.; Liu, X.; Niu, L. All-solid-state Z-scheme CdTe/TiO2 heterostructure photocatalysts with enhanced visible-light photocatalytic degradation of antibiotic waste water. Chem. Eng. J. 2018, 350, 257–267. [Google Scholar] [CrossRef]
- Peng, D.; Zou, Z.; Long, F.; He, J.; Zhang, T. Solid state synthesis of nonstoichiometric Bi2WO6/Bi2O3 composites as visible-light photocatalyst. Ionics 2016, 22, 2347–2353. [Google Scholar] [CrossRef]
- Ponnaiah, S.K.; Periakaruppan, P.; Vellaichamy, B.; Nagulan, B. Efficacious separation of electron−hole pairs in CeO2-Al2O3 nanoparticles embedded GO heterojunction for robust visible-light driven dye degradation. J. Colloid Interface Sci. 2017, 512, 219. [Google Scholar] [CrossRef] [PubMed]
- Zhou, J.; Wei, B.; Yao, Z.; Lin, H.; Tan, R.; Chen, W.; Guo, X. Preparation of hollow SiC spheres with biological template and research on its wave absorption properties. J. Alloys Compd. 2020, 819, 153021. [Google Scholar] [CrossRef]
- Zhang, L.; Pfund, B.; Wenger, O.S.; Hu, X. Oxidase-Type C−H/C−H Coupling Using an Isoquinoline-Derived Organic Photocatalyst. Angew. Chem. Int. Ed. 2022, 61, e202202649. [Google Scholar] [CrossRef]
- Chen, L.; Chen, C.; Yang, Z.; Li, S.; Chu, C.; Chen, B. Simultaneously Tuning Band Structure and Oxygen Reduction Pathway toward High-Efficient Photocatalytic Hydrogen Peroxide Production Using Cyano-Rich Graphitic Carbon Nitride. Adv. Funct. Mater. 2021, 31, 2105731. [Google Scholar] [CrossRef]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sun, Y.; Jia, Z.; Wan, N.; Feng, W. Biomimetic Guided Bi2WO6/Bi2O3 Vertical Heterojunction with Controllable Microstructure for Efficient Photocatalysis. Molecules 2023, 28, 3123. https://doi.org/10.3390/molecules28073123
Sun Y, Jia Z, Wan N, Feng W. Biomimetic Guided Bi2WO6/Bi2O3 Vertical Heterojunction with Controllable Microstructure for Efficient Photocatalysis. Molecules. 2023; 28(7):3123. https://doi.org/10.3390/molecules28073123
Chicago/Turabian StyleSun, Yuanbo, Ziang Jia, Ning Wan, and Wei Feng. 2023. "Biomimetic Guided Bi2WO6/Bi2O3 Vertical Heterojunction with Controllable Microstructure for Efficient Photocatalysis" Molecules 28, no. 7: 3123. https://doi.org/10.3390/molecules28073123
APA StyleSun, Y., Jia, Z., Wan, N., & Feng, W. (2023). Biomimetic Guided Bi2WO6/Bi2O3 Vertical Heterojunction with Controllable Microstructure for Efficient Photocatalysis. Molecules, 28(7), 3123. https://doi.org/10.3390/molecules28073123




