Cornus mas L. Extract Targets the Specific Molecules of the Th17/Treg Developmental Pathway in TNBS-Induced Experimental Colitis in Rats
Abstract
1. Introduction
2. Results
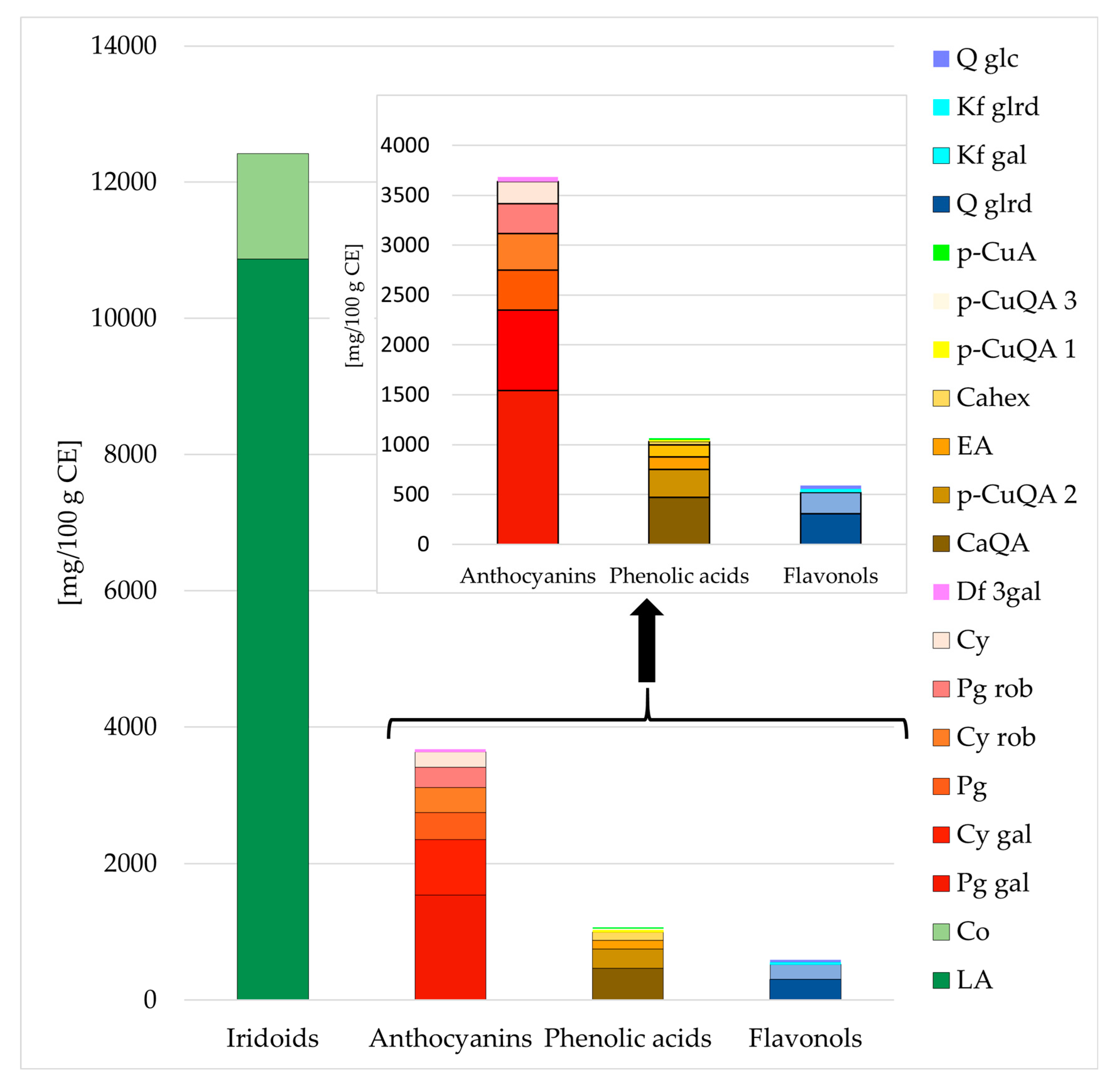
2.1. Chemical Characterization of Cornelian Cherry Iridoid-Polyphenolic Extract
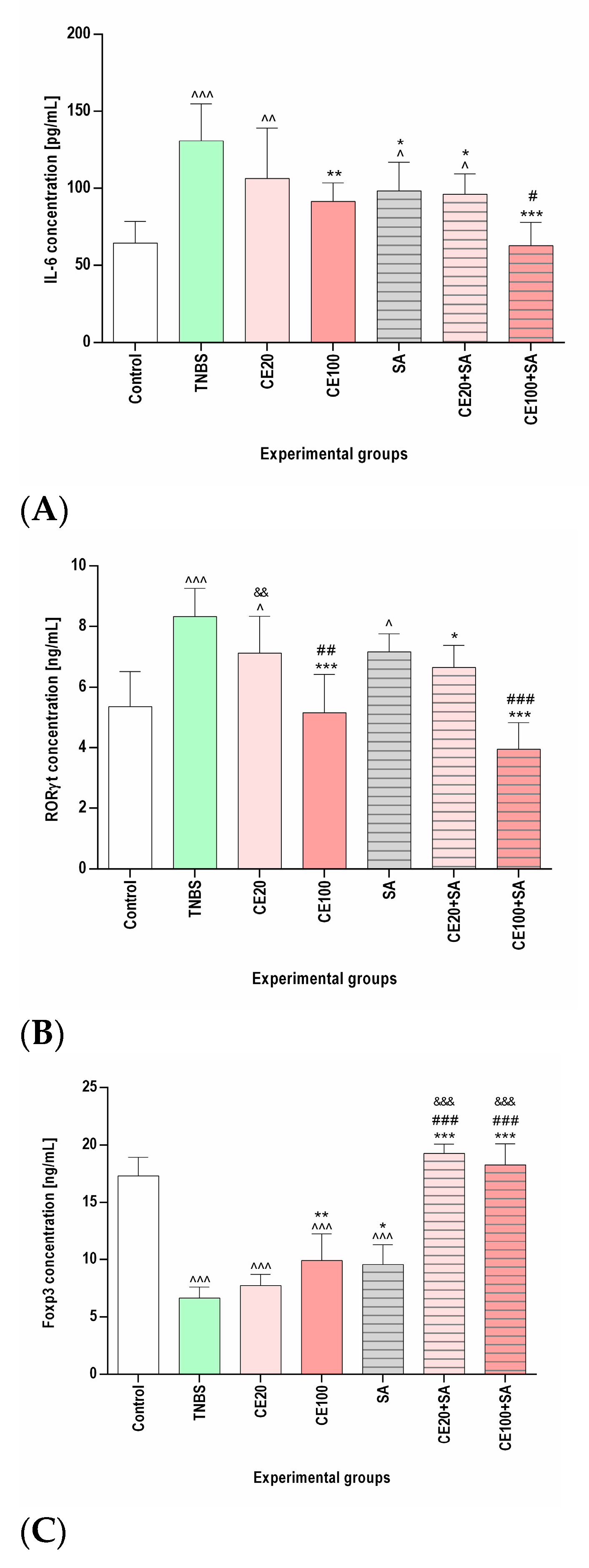
2.2. The Effects of the Cornelian Cherry Iridoid-Polyphenolic Extract on the Concentration of IL-6, RORγt, Total STAT3, p-STAT3, and Foxp3 in the Colon Tissues
2.3. The Effects of Sulfasalazine Alone or in Combination with Cornelian Cherry Iridoid-Polyphenolic Extract on the Concentration of IL-6, RORγt, Total STAT3, p-STAT3, and Foxp3 in the Colon Tissues
2.4. The Effects of the Cornelian Cherry Iridoid-Polyphenolic Extract on PIAS3 Expression in the Colon Tissues
2.5. The Effects of Sulfasalazine Alone or in Combination with Cornelian Cherry Iridoid-Polyphenolic Extract on PIAS3 Expression in the Colon Tissues
3. Discussion
4. Materials and Methods
4.1. In Vivo Experiment, Colon Tissue Samples Collection
4.2. Plant Material and Extraction
4.3. Chemical Analysis of Cornelian Cherry Iridoid-Polyphenolic Extract
4.4. Preparation of Colon Tissue Homogenates
4.5. IL-6, RORγt, Total STAT3, Phosphorylated STAT3 (p-STAT3), and Foxp3 Quantification by Enzyme-Linked Immunosorbent Assay (ELISA)
4.6. RNA Isolation, Quantification and Reverse Transcription
4.7. Quantitative Real-Time Polymerase Chain Reaction (qPCR)
4.8. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Gálvez, J. Role of Th17 Cells in the Pathogenesis of Human IBD. ISRN Inflamm. 2014, 2014, 928461. [Google Scholar] [CrossRef] [PubMed]
- Ananthakrishnan, A.N.; Kaplan, G.G.; Ng, S.C. Changing Global Epidemiology of Inflammatory Bowel Diseases: Sustaining Health Care Delivery into the 21st Century. Clin. Gastroenterol. Hepatol. 2020, 18, 1252–1260. [Google Scholar] [CrossRef] [PubMed]
- Lönnfors, S.; Vermeire, S.; Greco, M.; Hommes, D.; Bell, C.; Avedano, L. IBD and Health-Related Quality of Life—Discovering the True Impact. J. Crohns Colitis 2014, 8, 1281–1286. [Google Scholar] [CrossRef] [PubMed]
- Seyedian, S.S.; Nokhostin, F.; Malamir, M.D. A Review of the Diagnosis, Prevention, and Treatment Methods of Inflammatory Bowel Disease. J. Med. Life 2019, 12, 113–122. [Google Scholar] [CrossRef]
- Szandruk-Bender, M.; Merwid-Ląd, A.; Wiatrak, B.; Danielewski, M.; Dzimira, S.; Szkudlarek, D.; Szczukowski, Ł.; Świątek, P.; Szeląg, A. Novel 1,3,4-Oxadiazole Derivatives of Pyrrolo[3,4-d]Pyridazinone Exert Anti-Inflammatory Activity without Acute Gastrotoxicity in the Carrageenan-Induced Rat Paw Edema Test. J. Inflamm. Res. 2021, 14, 5739–5756. [Google Scholar] [CrossRef]
- Yan, J.-B.; Luo, M.-M.; Chen, Z.-Y.; He, B.-H. The Function and Role of the Th17/Treg Cell Balance in Inflammatory Bowel Disease. J. Immunol. Res. 2020, 2020, 8813558. [Google Scholar] [CrossRef]
- Noack, M.; Miossec, P. Th17 and Regulatory T Cell Balance in Autoimmune and Inflammatory Diseases. Autoimmun. Rev. 2014, 13, 668–677. [Google Scholar] [CrossRef]
- Yagil, Z.; Nechushtan, H.; Kay, G.; Yang, C.M.; Kemeny, D.M.; Razin, E. The Enigma of the Role of Protein Inhibitor of Activated STAT3 (PIAS3) in the Immune Response. Trends Immunol. 2010, 31, 199–204. [Google Scholar] [CrossRef]
- Chang, Y.; Zhai, L.; Peng, J.; Wu, H.; Bian, Z.; Xiao, H. Phytochemicals as Regulators of Th17/Treg Balance in Inflammatory Bowel Diseases. Biomed. Pharm. 2021, 141, 111931. [Google Scholar] [CrossRef]
- Fasching, P.; Stradner, M.; Graninger, W.; Dejaco, C.; Fessler, J. Therapeutic Potential of Targeting the Th17/Treg Axis in Autoimmune Disorders. Molecules 2017, 22, 134. [Google Scholar] [CrossRef]
- Szandruk-Bender, M.; Wiatrak, B.; Dzimira, S.; Merwid-Ląd, A.; Szczukowski, Ł.; Świątek, P.; Szeląg, A. Targeting Lineage-Specific Transcription Factors and Cytokines of the Th17/Treg Axis by Novel 1,3,4-Oxadiazole Derivatives of Pyrrolo[3,4-d]Pyridazinone Attenuates TNBS-Induced Experi-Mental Colitis. Int. J. Mol. Sci. 2022, 23, 9897. [Google Scholar] [CrossRef]
- Szandruk-Bender, M.; Rutkowska, M.; Merwid-Ląd, A.; Wiatrak, B.; Szeląg, A.; Dzimira, S.; Sobieszczańska, B.; Krzystek-Korpacka, M.; Kucharska, A.Z.; Matuszewska, A.; et al. Cornelian Cherry Iridoid-Polyphenolic Extract Improves Mucosal Epithelial Barrier Integrity in Rat Experimental Colitis and Exerts Antimicrobial and Antiadhesive Activities In Vitro. Oxid. Med. Cell. Longev. 2020, 2020, 7697851. [Google Scholar] [CrossRef]
- Dziąbowska-Grabias, K.; Sztanke, M.; Zając, P.; Celejewski, M.; Kurek, K.; Szkutnicki, S.; Korga, P.; Bulikowski, W.; Sztanke, K. Antioxidant Therapy in Inflammatory Bowel Diseases. Antioxidants 2021, 10, 412. [Google Scholar] [CrossRef] [PubMed]
- Boyapati, R.K.; Kalla, R.; Satsangi, J.; Ho, G.-T. Biomarkers in Search of Precision Medicine in IBD. Am. J. Gastroenterol. 2016, 111, 1682–1690. [Google Scholar] [CrossRef] [PubMed]
- Garrido-Mesa, N.; Algieri, F.; Rodríguez Nogales, A.; Gálvez, J. Functional Plasticity of Th17 Cells: Implications in Gastrointestinal Tract Function. Int. Rev. Immunol. 2013, 32, 493–510. [Google Scholar] [CrossRef] [PubMed]
- Patel, D.D.; Kuchroo, V.K. Th17 Cell Pathway in Human Immunity: Lessons from Genetics and Therapeutic Interventions. Immunity 2015, 43, 1040–1051. [Google Scholar] [CrossRef] [PubMed]
- Zhao, J.; Lu, Q.; Liu, Y.; Shi, Z.; Hu, L.; Zeng, Z.; Tu, Y.; Xiao, Z.; Xu, Q. Th17 Cells in Inflammatory Bowel Disease: Cytokines, Plasticity, and Therapies. J. Immunol. Res. 2021, 2021, 8816041. [Google Scholar] [CrossRef]
- Zhou, L.; Ivanov, I.I.; Spolski, R.; Min, R.; Shenderov, K.; Egawa, T.; Levy, D.E.; Leonard, W.J.; Littman, D.R. IL-6 Programs T(H)-17 Cell Differentiation by Promoting Sequential Engagement of the IL-21 and IL-23 Pathways. Nat. Immunol. 2007, 8, 967–974. [Google Scholar] [CrossRef]
- Rébé, C.; Végran, F.; Berger, H.; Ghiringhelli, F. STAT3 Activation. JAKSTAT 2013, 2, e23010. [Google Scholar] [CrossRef]
- Korn, T.; Bettelli, E.; Oukka, M.; Kuchroo, V.K. IL-17 and Th17 Cells. Annu. Rev. Immunol. 2009, 27, 485–517. [Google Scholar] [CrossRef]
- Ueno, A.; Ghosh, A.; Hung, D.; Li, J.; Jijon, H. Th17 Plasticity and Its Changes Associated with Inflammatory Bowel Disease. World J. Gastroenterol. 2015, 21, 12283–12295. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.O.; Nurieva, R.; Martinez, G.J.; Kang, H.S.; Chung, Y.; Pappu, B.P.; Shah, B.; Chang, S.H.; Schluns, K.S.; Watowich, S.S.; et al. Molecular Antagonism and Plasticity of Regulatory and Inflammatory T Cell Programs. Immunity 2008, 29, 44–56. [Google Scholar] [CrossRef] [PubMed]
- Abraham, C.; Dulai, P.S.; Vermeire, S.; Sandborn, W.J. Lessons Learned From Trials Targeting Cytokine Pathways in Patients With Inflammatory Bowel Diseases. Gastroenterology 2017, 152, 374–388.e4. [Google Scholar] [CrossRef] [PubMed]
- Buchele, V.; Konein, P.; Vogler, T.; Kunert, T.; Enderle, K.; Khan, H.; Büttner-Herold, M.; Lehmann, C.H.K.; Amon, L.; Wirtz, S.; et al. Th17 Cell-Mediated Colitis Is Positively Regulated by Interferon Regulatory Factor 4 in a T Cell-Extrinsic Manner. Front. Immunol. 2020, 11, 590893. [Google Scholar] [CrossRef]
- Neurath, M.F. Cytokines in Inflammatory Bowel Disease. Nat. Rev. Immunol. 2014, 14, 329–342. [Google Scholar] [CrossRef]
- Yamada, A.; Arakaki, R.; Saito, M.; Tsunematsu, T.; Kudo, Y.; Ishimaru, N. Role of Regulatory T Cell in the Pathogenesis of Inflammatory Bowel Disease. World J. Gastroenterol. 2016, 22, 2195–2205. [Google Scholar] [CrossRef]
- Yang, J.; Sundrud, M.S.; Skepner, J.; Yamagata, T. Targeting Th17 Cells in Autoimmune Diseases. Trends Pharmacol. Sci. 2014, 35, 493–500. [Google Scholar] [CrossRef]
- Park, B.V.; Pan, F. The Role of Nuclear Receptors in Regulation of Th17/Treg Biology and Its Implications for Diseases. Cell Mol. Immunol. 2015, 12, 533–542. [Google Scholar] [CrossRef]
- Min, H.-K.; Choi, J.; Lee, S.-Y.; Seo, H.-B.; Jung, K.; Na, H.S.; Ryu, J.-G.; Kwok, S.-K.; Cho, M.-L.; Park, S.-H. Protein Inhibitor of Activated STAT3 Reduces Peripheral Arthritis and Gut Inflammation and Regulates the Th17/Treg Cell Imbalance via STAT3 Signaling in a Mouse Model of Spondyloarthritis. J. Transl. Med. 2019, 17, 18. [Google Scholar] [CrossRef]
- Sozański, T.; Kucharska, A.; Dzimira, S.; Magdalan, J.; Szumny, D.; Matuszewska, A.; Nowak, B.; Piórecki, N.; Szeląg, A.; Trocha, M. Loganic Acid and Anthocyanins from Cornelian Cherry (Cornus mas L.)Fruits Modulate Diet-Induced Atherosclerosis and Redox Status in Rabbits. Adv. Clin. Exp. Med. 2018, 27, 1505–1513. [Google Scholar] [CrossRef]
- Danielewski, M.; Matuszewska, A.; Nowak, B.; Kucharska, A.Z.; Sozański, T. The Effects of Natural Iridoids and Anthocyanins on Selected Parameters of Liver and Cardiovascular System Functions. Oxidative Med. Cell. Longev. 2020, 2020, e2735790. [Google Scholar] [CrossRef] [PubMed]
- Dalmasso, G.; Nguyen, H.T.T.; Faïs, T.; Massier, S.; Barnich, N.; Delmas, J.; Bonnet, R. Crohn’s Disease-Associated Adherent-Invasive Escherichia Coli Manipulate Host Autophagy by Impairing SUMOylation. Cells 2019, 8, E35. [Google Scholar] [CrossRef] [PubMed]
- Abiodun, O.O.; Rodríguez-Nogales, A.; Algieri, F.; Gomez-Caravaca, A.M.; Segura-Carretero, A.; Utrilla, M.P.; Rodriguez-Cabezas, M.E.; Galvez, J. Antiinflammatory and Immunomodulatory Activity of an Ethanolic Extract from the Stem Bark of Terminalia catappa L. (Combretaceae): In Vitro and in Vivo Evidences. J. Ethnopharmacol. 2016, 192, 309–319. [Google Scholar] [CrossRef]
- Kang, S.-H.; Jeon, Y.-D.; Moon, K.-H.; Lee, J.-H.; Kim, D.-G.; Kim, W.; Myung, H.; Kim, J.-S.; Kim, H.-J.; Bang, K.-S.; et al. Aronia Berry Extract Ameliorates the Severity of Dextran Sodium Sulfate-Induced Ulcerative Colitis in Mice. J. Med. Food 2017, 20, 667–675. [Google Scholar] [CrossRef] [PubMed]
- Zahouani, Y.; Ben Rhouma, K.; Kacem, K.; Sebai, H.; Sakly, M. Aqueous Leaf Extract of Pistacia Lentiscus Improves Acute Acetic Acid-Induced Colitis in Rats by Reducing Inflammation and Oxidative Stress. J. Med. Food 2021, 24, 697–708. [Google Scholar] [CrossRef]
- Marín, M.; María Giner, R.; Ríos, J.-L.; Carmen Recio, M. Intestinal Anti-Inflammatory Activity of Ellagic Acid in the Acute and Chronic Dextrane Sulfate Sodium Models of Mice Colitis. J. Ethnopharmacol. 2013, 150, 925–934. [Google Scholar] [CrossRef] [PubMed]
- Saadatdoust, Z.; Pandurangan, A.K.; Ananda Sadagopan, S.K.; Mohd Esa, N.; Ismail, A.; Mustafa, M.R. Dietary Cocoa Inhibits Colitis Associated Cancer: A Crucial Involvement of the IL-6/STAT3 Pathway. J. Nutr. Biochem. 2015, 26, 1547–1558. [Google Scholar] [CrossRef]
- Biagioli, M.; Carino, A.; Fiorucci, C.; Annunziato, G.; Marchianò, S.; Bordoni, M.; Roselli, R.; Giorgio, C.D.; Castiglione, F.; Ricci, P.; et al. The Aryl Hydrocarbon Receptor (AhR) Mediates the Counter-Regulatory Effects of Pelargonidins in Models of Inflammation and Metabolic Dysfunctions. Nutrients 2019, 11, 1820. [Google Scholar] [CrossRef]
- Gan, Y.; Fu, Y.; Yang, L.; Chen, J.; Lei, H.; Liu, Q. Cyanidin-3-O-Glucoside and Cyanidin Protect Against Intestinal Barrier Damage and 2,4,6-Trinitrobenzenesulfonic Acid-Induced Colitis. J. Med. Food 2020, 23, 90–99. [Google Scholar] [CrossRef]
- Kim, Y.-J.; Ju, J.; Song, J.-L.; Yang, S.; Park, K.-Y. Anti-Colitic Effect of Purple Carrot on Dextran Sulfate Sodium (DSS)-Induced Colitis in C57BL/6J Mice. Prev. Nutr. Food Sci. 2018, 23, 77–83. [Google Scholar] [CrossRef]
- Xia, Y.; Tian, L.-M.; Liu, Y.; Guo, K.-S.; Lv, M.; Li, Q.-T.; Hao, S.-Y.; Ma, C.-H.; Chen, Y.-X.; Tanaka, M.; et al. Low Dose of Cyanidin-3-O-Glucoside Alleviated Dextran Sulfate Sodium-Induced Colitis, Mediated by CD169+ Macrophage Pathway. Inflamm. Bowel Dis. 2019, 25, 1510–1521. [Google Scholar] [CrossRef] [PubMed]
- Samarpita, S.; Ganesan, R.; Rasool, M. Cyanidin Prevents the Hyperproliferative Potential of Fibroblast-like Synoviocytes and Disease Progression via Targeting IL-17A Cytokine Signalling in Rheumatoid Arthritis. Toxicol. Appl. Pharm. 2020, 391, 114917. [Google Scholar] [CrossRef] [PubMed]
- Wei, C.-B.; Tao, K.; Jiang, R.; Zhou, L.-D.; Zhang, Q.-H.; Yuan, C.-S. Quercetin Protects Mouse Liver against Triptolide-Induced Hepatic Injury by Restoring Th17/Treg Balance through Tim-3 and TLR4-MyD88-NF-ΚB Pathway. Int. Immunopharmacol. 2017, 53, 73–82. [Google Scholar] [CrossRef] [PubMed]
- Morris, G.P.; Beck, P.L.; Herridge, M.S.; Depew, W.T.; Szewczuk, M.R.; Wallace, J.L. Hapten-Induced Model of Chronic Inflammation and Ulceration in the Rat Colon. Gastroenterology 1989, 96, 795–803. [Google Scholar] [CrossRef]
- Kucharska, A.Z.; Szumny, A.; Sokół-Łętowska, A.; Piórecki, N.; Klymenko, S.V. Iridoids and Anthocyanins in Cornelian Cherry (Cornus mas L.) Cultivars. J. Food Compos. Anal. 2015, 40, 95–102. [Google Scholar] [CrossRef]
- Kucharska, A.Z.; Sokół-Łętowska, A.; Oszmiański, J.; Piórecki, N.; Fecka, I. Iridoids, Phenolic Compounds and Antioxidant Activity of Edible Honeysuckle Berries (Lonicera caerulea var. kamtschatica sevast.). Molecules 2017, 22, 405. [Google Scholar] [CrossRef]

| Parameter | Group | Value | p-Value (vs. Control) | p-Value (vs. TNBS) | p-Value (vs. SA) | p-Value (vs. CE100) |
|---|---|---|---|---|---|---|
| Total STAT3 (ng/mL) | Control | 43.61 ± 10.01 | - | 0.0015 | NS | NS |
| TNBS | 64.05 ± 10.48 | 0.0015 | - | NS | NS | |
| CE20 | 61.39 ± 13.01 | 0.0114 | NS | NS | NS | |
| CE100 | 49.71 ± 8.08 | NS | NS | NS | - | |
| SA | 55.47 ± 9.82 | NS | NS | - | NS | |
| CE20 + SA | 59.03 ± 8.38 | 0.0414 | NS | NS | NS | |
| CE100 + SA | 39.32 ± 4.71 | NS | <0.0001 | 0.0283 | NS | |
| p-STAT3 (ng/mL) | Control | 9.32 ± 1.75 | - | <0.0001 | <0.0001 | 0.0033 |
| TNBS | 23.06 ± 5.50 | <0.0001 | - | 0.0167 | <0.0001 | |
| CE20 | 19.30 ± 2.23 | <0.0001 | NS | NS | NS | |
| CE100 | 15.04 ± 2.81 | 0.0033 | <0.0001 | NS | - | |
| SA | 18.06 ± 2.61 | <0.0001 | 0.0167 | - | NS | |
| CE20 + SA | 17.49 ± 1.51 | <0.0001 | 0.0061 | NS | NS | |
| CE100 + SA | 8.96 ± 1.69 | NS | <0.0001 | <0.0001 | 0.0017 | |
| p-STAT3/total STAT3 ratio | Control | 0.22 ± 0.07 | - | 0.0113 | NS | NS |
| TNBS | 0.36 ± 0.08 | 0.0113 | - | NS | NS | |
| CE20 | 0.33 ± 0.11 | NS | NS | NS | NS | |
| CE100 | 0.31 ± 0.05 | NS | NS | NS | - | |
| SA | 0.34 ± 0.10 | NS | NS | - | NS | |
| CE20 + SA | 0.30 ± 0.05 | NS | NS | NS | NS | |
| CE100 + SA | 0.23 ± 0.06 | NS | 0.0201 | NS | NS |
| Symbol | Gene Name | Accession No. | Primer Sequence 5′→ 3′ | Amp. Size |
|---|---|---|---|---|
| Gapdh | Glyceraldehyde-3-phosphate dehydrogenase | NM_017008.4 | F: tgactctacccacggcaagttcaa R: acgacatactcagcaccagcatca | 141 bp |
| Pias3 | Protein inhibitor of activated STAT3 | NM_031784.2 | F: ttcgctggcaggaacaagag R: gggcgcagctagacttgag | 79 bp |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Szandruk-Bender, M.; Nowak, B.; Merwid-Ląd, A.; Kucharska, A.Z.; Krzystek-Korpacka, M.; Bednarz-Misa, I.; Wiatrak, B.; Szeląg, A.; Piórecki, N.; Sozański, T. Cornus mas L. Extract Targets the Specific Molecules of the Th17/Treg Developmental Pathway in TNBS-Induced Experimental Colitis in Rats. Molecules 2023, 28, 3034. https://doi.org/10.3390/molecules28073034
Szandruk-Bender M, Nowak B, Merwid-Ląd A, Kucharska AZ, Krzystek-Korpacka M, Bednarz-Misa I, Wiatrak B, Szeląg A, Piórecki N, Sozański T. Cornus mas L. Extract Targets the Specific Molecules of the Th17/Treg Developmental Pathway in TNBS-Induced Experimental Colitis in Rats. Molecules. 2023; 28(7):3034. https://doi.org/10.3390/molecules28073034
Chicago/Turabian StyleSzandruk-Bender, Marta, Beata Nowak, Anna Merwid-Ląd, Alicja Z. Kucharska, Małgorzata Krzystek-Korpacka, Iwona Bednarz-Misa, Benita Wiatrak, Adam Szeląg, Narcyz Piórecki, and Tomasz Sozański. 2023. "Cornus mas L. Extract Targets the Specific Molecules of the Th17/Treg Developmental Pathway in TNBS-Induced Experimental Colitis in Rats" Molecules 28, no. 7: 3034. https://doi.org/10.3390/molecules28073034
APA StyleSzandruk-Bender, M., Nowak, B., Merwid-Ląd, A., Kucharska, A. Z., Krzystek-Korpacka, M., Bednarz-Misa, I., Wiatrak, B., Szeląg, A., Piórecki, N., & Sozański, T. (2023). Cornus mas L. Extract Targets the Specific Molecules of the Th17/Treg Developmental Pathway in TNBS-Induced Experimental Colitis in Rats. Molecules, 28(7), 3034. https://doi.org/10.3390/molecules28073034










